Abstract
Two innovative studies recently identified functional lymphatic structures in the meninges that may influence the development of HIV-associated neurological disorders (HAND). Until now, blood vessels were assumed to be the sole transport system by which HIV-infected monocytes entered the brain by bypassing a potentially hostile blood-brain-barrier through inflammatory-mediated semi-permeability. A cascade of specific chemokine signals promote monocyte migration from blood vessels to surrounding brain tissues via a well-supported endothelium, where the cells differentiate into tissue macrophages capable of productive HIV infection. Lymphatic vessels on the other hand are more loosely organized than blood vessels. They absorb interstitial fluid from bodily tissues where HIV may persist and exchange a variety of immune cells (CD4+ T-cells, monocytes, macrophages and dendritic cells) with surrounding tissues through discontinuous endothelial junctions. We propose that the newly discovered meningeal lymphatics are key to HIV migration among viral reservoirs and brain tissue during periods of undetectable plasma viral loads due to suppressive combinational antiretroviral therapy, thus redefining the migration process in terms of a blood-lymphatic transport system.
Keywords: meninges, HIV brain infection, lymphatic system, neurological disease, macrophage-targeted therapy
Mini-review
An elegant study recently published by Louveau et al. convincingly demonstrated, for the first time, well-organized and functional lymphatic vessels lining the dural sinuses of the meninges (Louveau et al. 2015). The vessels were connected to deep cervical lymph nodes, expressed all the markers for endothelial cells and carried fluid containing monocytes and macrophages (the primary immune cells within the brain) and T-cells. The authors suggested that, in light of these data, “a reassessment of basic assumptions in neuroimmunology” was warranted that could “shed new light on the etiology of neuroinflammatory and neurodegenerative diseases associated with immune system dysfunction.” Simultaneously, Aspelund and colleagues defined a similar lymphatic vessel network in the dura mater of the mouse brain (Aspelund et al. 2015). We whole-heartedly agree with their assessments and are eager to note with particular interest the implications for the development of HIV-associated neurocognitive diseases (HAND) occurring in patients on otherwise efficacious virally suppressive combinational antiretroviral therapy (cART), including both severe forms of HIV-associated dementia (HAD) as well as milder pathologies such as asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disease (MND) (Antinori et al. 2007). We propose that the newly discovered relationship of the meninges to the lymphatic system provides a key – and previously unrecognized – mechanism by which HIV-infected macrophages can surreptitiously and continually migrate between the brain and the lymphatic system in the face of cART and contribute to the development of HAND-related disorders.
HIV is undoubtedly neurovirulent and neuropathogenic (Harezlak et al. 2011). While cART has greatly reduced the incidence of HAD, approximately 50% of HIV+ patients still develop some degree of HAND that can reoccur over the course of their lifespan (Heaton et al. 2010). The mechanisms behind this pathology are unclear, given that the vast majority of patients on therapy experience well controlled plasma viral loads to undetectable levels. HIV enters the brain during both early and late infection (Burdo et al. 2013; Strickland et al. 2014; Kim et al. 2003; Fischer-Smith et al. 2001). Once the brain is infected, HIV variants that are genetically distinct from plasma or other anatomical sites may appear (Korber et al. 1994; Salemi et al. 2005). When observed, this viral compartmentalization could suggest the selection of brain-adapted HIV over time. Another hypothesis is that a subset of cells migrating from the periphery carry a subpopulation of virus to the brain (Williams and Burdo 2012)(Lamers et al. 2010). Several questions regarding HIV infection in the brain remain elusive, including the impact of cART on brain viral evolution, whether HAND is caused directly by HIV-infected brain macrophages or if HAND is the result of chronic systemic immune activation. While cART penetration will reduce cerebral spinal fluid virus loads (Eggers et al. 2003; Antinori et al. 2002), it does not necessarily improve HAND, and in some cases HAND becomes worse with cART initiation (Cespedes and Aberg 2006).
HIV productively infects dendritic cells, macrophages, and CD4+ T-cells (Kedzierska and Crowe 2002; Campbell et al. 2014; Teleshova et al. 2003; Alexaki et al. 2008). Monocytes, which are macrophage precursors, can carry HIV to tissues, but the degree that they can replicate the virus prior to macrophage differentiation remains debated (Kedzierska and Crowe 2002). Although cART effectively reduces overall plasma viral loads to undetectable levels, usually the virus quickly rebounds as a viable replicating population once therapy is removed (Rothenberger et al. 2015). The location of cellular HIV reservoirs is a matter of considerable interest and controversy (Le Douce et al. 2010; Pierson et al. 2000; Svicher et al. 2014; Coleman and Wu 2009). Latently infected and long-lived resting memory CD4+ T-cells are presumed to act as the primary reservoir of residual virus due to their relatively high levels of infectivity during natural infection and capacity to remain in a non-dividing state for an extended period. These cells also have the advantage of being readily isolated from peripheral blood, easy to culture, and infect in vitro, and therefore have been extensively studied. Tissue macrophages, which can originate during embryonic development (Epelman et al. 2014) or are derived from circulating monocytes, can also harbor and readily replicate HIV during cART (Lamers et al. 2012; Le Douce et al. 2010; McGrath 1996; Moir and Fauci 2010). Furthermore, the HIV Nef protein enhances macrophage tissue infiltration and could contribute to the accumulation of macrophages in anatomical sites of some HIV-infected patients (Verollet et al. 2015). However, the low frequency of HIV-infected macrophages, combined with the fact that they rarely circulate in blood, renders this a difficult cell population to study. Macrophages are distinct from activated CD4+ T-cells in that they can live for months or even years, they show a much lower intra-cellular concentration of antiviral drugs compared to other HIV-target cells (Abbas et al. 2015), and they are less prone to viral-activated cell death (Swingler et al. 2007; Crowe et al. 2003). At end-stage HIV disease, when viral loads can be very high and T-cell levels plummet, most HIV is coming from a productive macrophage reservoir (Igarashi et al. 2001). The penetration of drugs deep into macrophages is likely much lower than T-cells (Gavegnano and Schinazi 2009). Furthermore, macrophages are efficient at cell-to-cell transfer of HIV RNA to other neighboring macrophages and T-cells (Duncan et al. 2014), which allows the virus to by-pass critical stages of assembly, budding, cell-receptor attachment and entry that are targeted by the immune system and/or therapy. Therefore, although the absolute numbers of infected macrophages is probably much lower than T-cells (Rothenberger et al. 2015), they have the potential to harbor persistent infection for a long time in the face of cART.
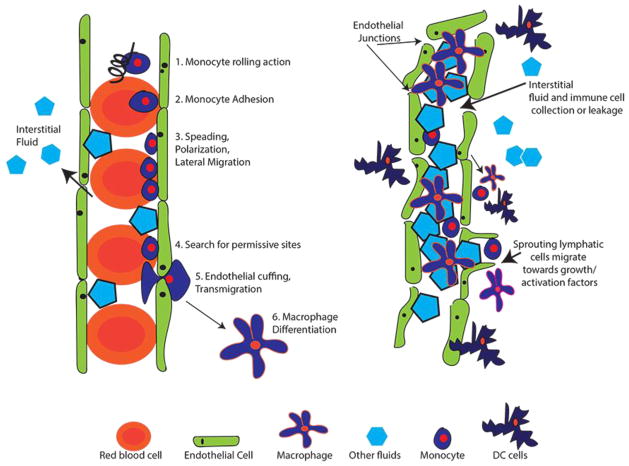
Productive HIV infection in the brain is found within the inflammatory infiltrate, which predominantly consists of macrophages (Williams et al. 2001; Fischer-Smith et al. 2008). Until now, the only mechanism for HIV to enter and infect brain macrophages was assumed to be via migrating blood HIV+ monocytes through a complex process (Williams et al. 2012). The blood vessel endothelium (BVE) is tightly supported on a basement membrane located in the arachnoid layers of the brain. Monocytes migrate through the intact BVE (diapedesis), which requires a multifaceted series of signaling events (Kamei and Carman 2010) (Figure 1), beginning with interactions of cell-adhesion molecules that cause a monocyte rolling action. Monocyte rolling accelerates their responses to the chemokines presented on the surface of the BVE. Next, there is an interaction between monocyte integrin receptors with their endothelial ligands that causes monocytes to mobilize and undergo an actin-dependent spreading, polarization and lateral migration on the luminal surface of the endothelium. Finally, monocytes seek permissive sites where they protrude and eventually migrate between the tightly packed endothelial cells (pericellular diapedesis) or through a transcellular pore within an endothelial cell (transcellular diapedesis)(Kamei and Carman 2010) to an interstitial tissue compartment where they differentiate into varied macrophage types depending on the cytokines they encounter (i.e. activated or regulatory), and varied morphology depending on their anatomical location (i.e. Kuppfer cells in the liver or alveolar macrophages in the lung).
Figure 1. Blood vs. Lymphatic Vessel Architecture.
Panel A. Schematic outlining the steps required for monocyte migration through a blood vessel until macrophage differentiation. Endothelial cells (green) are tightly connected to restrict flow in or out of the vessel. As blood moves through vessels, pressure difference allows some fluid to moves outside of vessels, collecting in tissues (interstitial fluid). Monocytes (dark blue) migrate due out of vessels due to chemokine signals presented in the surrounding environment. Panel B. Lymphatic vessels collect excess interstitial fluid and immune cells for collection and processing in lymph nodes. Fluid and cellular exchange can also occur. Activated macrophages in the surrounding tissues can encourage lymphatic vessel growth.
The recent discovery of lymphatic vessels in the meninges opens up an unexplored route for HIV+ monocytes or HIV+-differentiated tissue macrophages collected in lymph from other anatomical sites to migrate to and from brain tissue. Monocytes and macrophages are heterogeneous populations, and the possibility of an HIV+ subpopulation selectively using this route is of great interest. Lymphatic vessels are dynamic structures that continually adapt to their environment and are distributed throughout the body where they closely interact with the circulatory system and all anatomical tissues. While they are associated with drainage of excess tissue fluids to lymph nodes, lymphatic vessels are also conduits for immune cell trafficking and profoundly influence the immune system by manipulating inflammatory processes (Kataru et al. 2014; Kuan et al. 2015). Unlike blood vessels, the endothelium of lymphatic vessels is more loosely constructed, built from a single layer of lymphatic endothelial cells that includes numerous discontinuous endothelial junctions, which allow for rapid absorption of fluids as well as the mobilization of immune cells to and from sites of inflammation (Figure 1)(Kuan et al. 2015). The meningeal lymphatic vessels discovered by Louveau et al. were defined as initial lymphatics, which differ from collecting lymphatics due to overlapping flaps at borders of oak leaf-shaped endothelial cells that form discontinuous button-like junctions, whereas collecting lymphatics have continuous, zipper-like junctions at cell borders without openings (Baluk et al. 2007). While initial lymphatic vessels absorb fluids from anatomical sites where HIV-infected tissue macrophages potentially reside, their inherent permeability permits the distribution of lymph components to dendritic cells and macrophages in other adjacent tissues. Moreover, the lymphatic network is linked to spleen, lymph nodes and adipose tissue, all of which are known to contain large reservoirs of HIV target cells (Couturier et al. 2015; van't Wout et al. 1998; Svicher et al. 2014).
The timing of HIV infection in the brain has been studied using a phylogenetic approach and an in-depth molecular clock analysis in autopsy tissues from HIV+ patients and in Rhesus macaques infected with Simian Immunodeficiency Virus (SIV). While HIV/SIV appears to enter the brain via monocyte trafficking in the early stages of infection (Kim et al. 2003; Williams and Burdo 2012), the virus also appears to reseed the brain at the onset of AIDS (Fischer-Smith et al. 2008). Intermixing of HIV in brain and peripheral tissues has been occasionally observed (Liu et al. 2000; Wang et al. 2001; Lamers et al. 2010); however, most HIV phylogenetic studies have demonstrated a large degree of HIV brain compartmentalization with respect to HIV populations in peripheral tissues (Haggerty and Stevenson 1991; Salemi et al. 2007; van't Wout et al. 1998; Wong et al. 1997). In our work, we also found that deep brain tissue HIV was significantly compartmentalized with respect to plasma HIV; however we further determined that the meninges contained virus populations similar to both brain and peripheral tissues (Lamers et al. 2011a; Lamers et al. 2010; Lamers et al. 2011b; Salemi et al. 2005). This observation led us to further explore viral gene flow patterns between brain regions, the meninges and peripheral tissues in five patient autopsies (Lamers et al. 2011a). In all five patients we found evidence of gene flow from deep brain tissues to the meninges, and some evidence of gene flow from meninges to the brain. These results highlighted the potential importance of the meninges in delivering virus to and from the deep brain tissue, but could not explain the mechanism by which the virus was migrating.
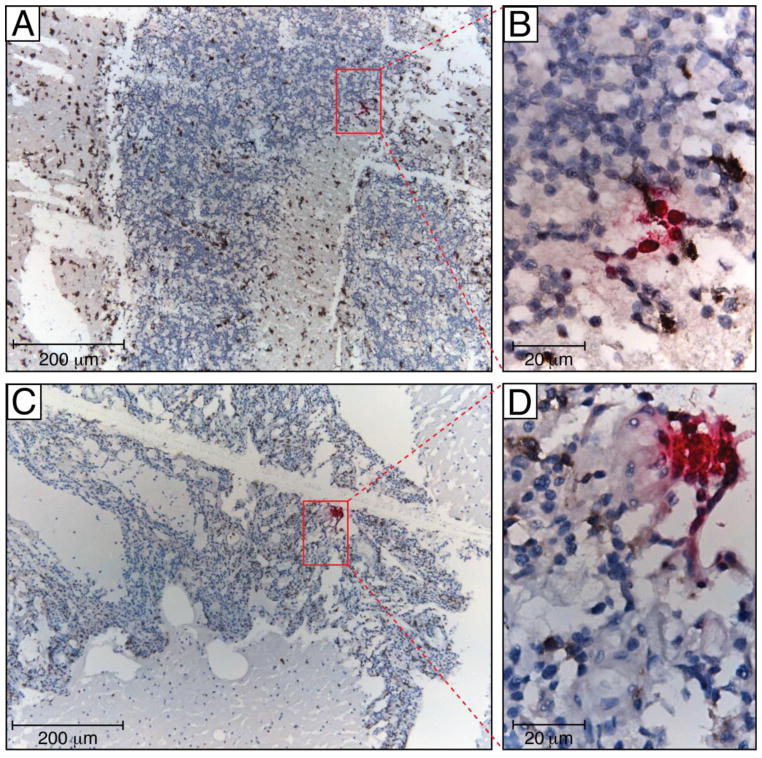
We have previously identified HIV-infected macrophages lining vessel walls within meningeal tissues in pre-ART patient autopsy tissues using immunohistochemistry for HIV p24 (Figure 2)(Lamers et al. 2012). Although HIV is found in much lower frequency in brain tissues from patients on ART, we have successfully identified HIV-positive cells in cerebellum tissue that are surrounded by infiltrating macrophages using RNAscope, a novel next generation in situ hybridization technique developed by Advanced Cell Diagnostics that employs a unique “double Z” probe design, which greatly increases signal-to-noise ratio with visualization of single RNA transcripts. (Figure 3). Preliminary HIV sequencing data indicated that the virus derived from this patient’s brain showed no signs of compartmentalization, originated from the patient’s lymph node, and included historical as well as recently migrated and clonally expanding virus (manuscript in preparation). Further experiments are underway to confirm these findings in other patients. These evolutionary patterns were entirely different from pre-ART studies, suggesting a different HIV migratory pathway to the brain in ART-treated patients. The studies of Louveau et al. and Aspelund et al. thus provide a realistic mechanism (the meningeal lymphatics) by which HIV can migrate into the brain via infected macrophages during ART in which plasma and CSF viral load is undetectable.
Figure 2. HIV lining a vessel in the meninges.
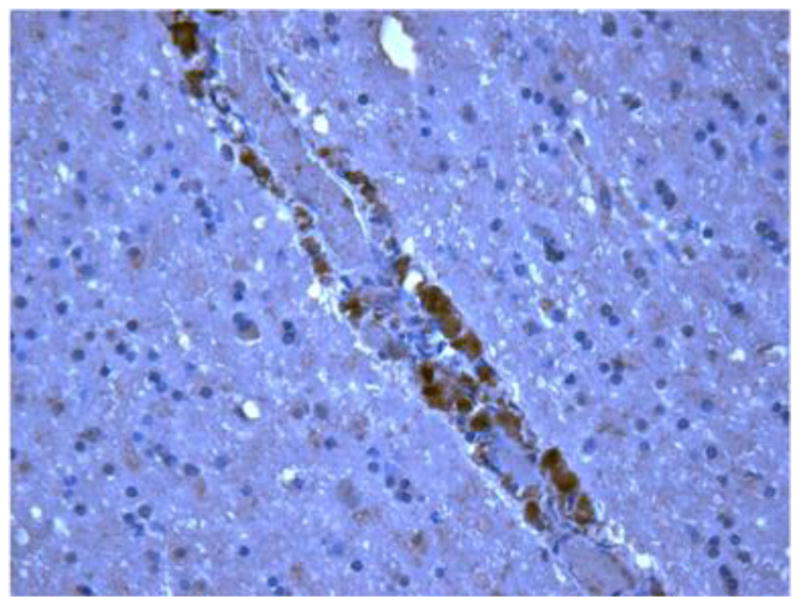
Tissue was stained using anti-p24 to identify infiltrating macrophages productively infected with HIV DNA. Images are shown at 400X.
Figure 3. RNAscope of cerebellum in a patient who died with no detectable viral load.
HIV vRNA (coding RNA+, fuchsia) was detected by RNAscope, a novel next generation in situ hybridization technique developed by Advanced Cell Diagnostic, using a HIV gag-pol probe in formalin-fixed and paraffin-embedded cerebellum tissue samples. The RNAscope assay was followed by colorimetric IHC for macrophage markers using mouse mAb to CD163 (Novocastra) and CD68 (Dako) (both brown), and nuclei were counterstained with hematoxylin. To confirm the specificity of in situ hybridization, we used lymph node tissue samples from HIV-negative individual (not shown). Human peptidyl-prolyl cis-trans isomerase B encoded by PPIB gene was detected with the Hs-PPIB probe in the HeLa cell control (ACD) and served as a RNAscope positive control (not shown). Tissue sections were analyzed with a Leica DM6000 B microscope equipped with a Leica DFC 500 camera. Red oblongs in panels A and C outline areas represented in panels B and D, correspondently. Scale bars: 200 μm (A, C) and 20 μm (B, D)
Lymphatic anatomical sites are known sites of viral persistence and a source of rebound virus after cART (Rothenberger et al. 2015). Adipose tissue, which is intimately associated with vessel growth and surrounds all lymph nodes, may be abnormally distributed due to HIV infection of adipose macrophages (Shikuma et al. 2014) and may also act as a persistent HIV reservoir during cART (Couturier et al. 2015). The finding of a direct route between persistently infected lymphatic tissues to the meninges, where HIV-infected monocytes or macrophages could potentially migrate through endothelial junctions, has clear implications for HIV persistence, the establishment of HIV reservoirs during cART, and the development of the spectrum of neurological disorders associated with HIV infection. In addition, these pathways may be implicated in AIDS-related pathologies other than HAND, including as a metastatic pathway for tumor cells. Further studies are needed for defining the role of the meningeal lymphatic system as a primary mechanism of transport for HIV+ immune cells to the brain.
Acknowledgments
MM, EM, CAS are funded by National Cancer Institutes grant #CA181255. SLL, RR, MM are funded by the National Institutes of Mental Health grant #MH100984. SLL, DJN, MS are funded by the National Institutes of Neurological Disorders and Stroke grant #NS063897. LCH is funded by the National Institutes of Mental Health grant #MH104141
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Abbas W, Tariq M, Iqbal M, Kumar A, Herbein G. Eradication of HIV-1 from the macrophage reservoir: an uncertain goal? Viruses. 2015;7(4):1578–1598. doi: 10.3390/v7041578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Current HIV research. 2008;6(5):388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Giancola ML, Grisetti S, Soldani F, Alba L, Liuzzi G, Amendola A, Capobianchi M, Tozzi V, Perno CF. Factors influencing virological response to antiretroviral drugs in cerebrospinal fluid of advanced HIV-1-infected patients. AIDS. 2002;16(14):1867–1876. doi: 10.1097/00002030-200209270-00003. [DOI] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204(10):2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254(1):102–113. doi: 10.1111/imr.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS. 2014 doi: 10.1097/QAD.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cespedes MS, Aberg JA. Neuropsychiatric complications of antiretroviral therapy. Drug Saf. 2006;29(10):865–874. doi: 10.2165/00002018-200629100-00004. [DOI] [PubMed] [Google Scholar]
- Coleman CM, Wu L. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology. 2009;6:51. doi: 10.1186/1742-4690-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier J, Suliburk JW, Brown JM, Luke DJ, Agarwal N, Yu X, Nguyen C, Iyer D, Kozinetz CA, Overbeek PA, Metzker ML, Balasubramanyam A, Lewis DE. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS. 2015;29(6):667–674. doi: 10.1097/QAD.0000000000000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74(5):635–641. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- Duncan CJ, Williams JP, Schiffner T, Gartner K, Ochsenbauer C, Kappes J, Russell RA, Frater J, Sattentau QJ. High-multiplicity HIV-1 infection and neutralizing antibody evasion mediated by the macrophage-T cell virological synapse. Journal of virology. 2014;88(4):2025–2034. doi: 10.1128/JVI.03245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers C, Hertogs K, Sturenburg HJ, van Lunzen J, Stellbrink HJ. Delayed central nervous system virus suppression during highly active antiretroviral therapy is associated with HIV encephalopathy, but not with viral drug resistance or poor central nervous system drug penetration. AIDS. 2003;17(13):1897–1906. doi: 10.1097/01.aids.0000076273.54156.8f. [DOI] [PubMed] [Google Scholar]
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008;14(4):318–326. doi: 10.1080/13550280802132857. 902189571 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L'Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7(6):528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Gavegnano C, Schinazi RF. Antiretroviral therapy in macrophages: implication for HIV eradication. Antivir Chem Chemother. 2009;20(2):63–78. doi: 10.3851/IMP1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty S, Stevenson M. Predominance of distinct viral genotypes in brain and lymph node compartments of HIV-1-infected individuals. Viral Immunol. 1991;4(2):123–131. doi: 10.1089/vim.1991.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Alger J, Singer E, Campbell T, Yiannoutsos C, Cohen R, Navia B. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, Brown CR, Endo Y, Buckler-White A, Plishka R, Bischofberger N, Hirsch V, Martin MA. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):658–663. doi: 10.1073/pnas.021551798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei M, Carman CV. New observations on the trafficking and diapedesis of monocytes. Curr Opin Hematol. 2010;17(1):43–52. doi: 10.1097/MOH.0b013e3283333949. [DOI] [PubMed] [Google Scholar]
- Kataru RP, Lee YG, Koh GY. Interactions of immune cells and lymphatic vessels. Adv Anat Embryol Cell Biol. 2014;214:107–118. doi: 10.1007/978-3-7091-1646-3_9. [DOI] [PubMed] [Google Scholar]
- Kedzierska K, Crowe SM. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem. 2002;9(21):1893–1903. doi: 10.2174/0929867023368935. [DOI] [PubMed] [Google Scholar]
- Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol. 2003;74(5):650–656. doi: 10.1189/jlb.0503207. jlb.0503207 [pii] [DOI] [PubMed] [Google Scholar]
- Korber BT, Kunstman KJ, Patterson BK, Furtado M, McEvilly MM, Levy R, Wolinsky SM. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. Journal of virology. 1994;68(11):7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, Childs EW, Platt AM, Jakubzick CV, Mason RJ, Gashev AA, Nussenzweig M, Swartz MA, Dustin ML, Zawieja DC, Randolph GJ. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. Journal of immunology. 2015;194(11):5200–5210. doi: 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers SL, Fogel GB, Singer EJ, Salemi M, Nolan DJ, Huysentruyt LC, McGrath MS. HIV-1 Nef in macrophage-mediated disease pathogenesis. Int Rev Immunol. 2012;31(6):432–450. doi: 10.3109/08830185.2012.737073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers SL, Gray RR, Salemi M, Huysentruyt LC, McGrath MS. HIV-1 phylogenetic analysis shows HIV-1 transits through the meninges to brain and peripheral tissues. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011a;11(1):31–37. doi: 10.1016/j.meegid.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers SL, Poon AF, McGrath MS. HIV-1 nef protein structures associated with brain infection and dementia pathogenesis. PloS one. 2011b;6(2):e16659. doi: 10.1371/journal.pone.0016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers SL, Salemi M, Galligan DC, Morris A, Gray R, Fogel G, Zhao L, McGrath MS. Human immunodeficiency virus-1 evolutionary patterns associated with pathogenic processes in the brain. J Neurovirol. 2010;16(3):230–241. doi: 10.3109/13550281003735709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douce V, Herbein G, Rohr O, Schwartz C. Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology. 2010;7:32. doi: 10.1186/1742-4690-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tang XP, McArthur JC, Scott J, Gartner S. Analysis of human immunodeficiency virus type 1 gp160 sequences from a patient with HIV dementia: evidence for monocyte trafficking into brain. J Neurovirol. 2000;6(Suppl 1):S70–81. [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath MS. T-cells and macrophages in HIV disease. Clin Rev Allergy Immunol. 1996;14(4):359–366. doi: 10.1007/BF02771752. [DOI] [PubMed] [Google Scholar]
- Moir S, Fauci AS. Nef, macrophages and B cells: a highway for evasion. Immunol Cell Biol. 2010;88(1):1–2. doi: 10.1038/icb.2009.82. icb200982 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson T, McArthur J, Siliciano RF. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annual review of immunology. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- Rothenberger MK, Keele BF, Wietgrefe SW, Fletcher CV, Beilman GJ, Chipman JG, Khoruts A, Estes JD, Anderson J, Callisto SP, Schmidt TE, Thorkelson A, Reilly C, Perkey K, Reimann TG, Utay NS, Nganou Makamdop K, Stevenson M, Douek DC, Haase AT, Schacker TW. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(10):E1126–1134. doi: 10.1073/pnas.1414926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemi M, Burkhardt BR, Gray RR, Ghaffari G, Sleasman JW, Goodenow MM. Phylodynamics of HIV-1 in lymphoid and non-lymphoid tissues reveals a central role for the thymus in emergence of CXCR4-using quasispecies. PloS one. 2007;2(9):e950. doi: 10.1371/journal.pone.0000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemi M, Lamers SL, Yu S, de Oliveira T, Fitch WM, McGrath MS. Phylodynamic analysis of human immunodeficiency virus type 1 in distinct brain compartments provides a model for the neuropathogenesis of AIDS. Journal of virology. 2005;79(17):11343–11352. doi: 10.1128/JVI.79.17.11343-11352.2005. 79/17/11343 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma CM, Gangcuangco LM, Killebrew DA, Libutti DE, Chow DC, Nakamoto BK, Liang CY, Milne CI, Ndhlovu LC, Barbour JD, Shiramizu BT, Gerschenson M. The role of HIV and monocytes/macrophages in adipose tissue biology. Journal of acquired immune deficiency syndromes. 2014;65(2):151–159. doi: 10.1097/01.qai.0000435599.27727.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland SL, Rife BD, Lamers SL, Nolan DJ, Veras NM, Prosperi MC, Burdo TH, Autissier P, Nowlin B, Goodenow MM, Suchard MA, Williams KC, Salemi M. Spatiotemporal Dynamics of SIV Brain Infection in CD8+ Lymphocyte-Depleted Rhesus Macaques with NeuroAIDS. The Journal of general virology. 2014 doi: 10.1099/vir.0.070318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svicher V, Ceccherini-Silberstein F, Antinori A, Aquaro S, Perno CF. Understanding HIV compartments and reservoirs. Curr HIV/AIDS Rep. 2014;11(2):186–194. doi: 10.1007/s11904-014-0207-y. [DOI] [PubMed] [Google Scholar]
- Swingler S, Mann AM, Zhou J, Swingler C, Stevenson M. Apoptotic killing of HIV-1-infected macrophages is subverted by the viral envelope glycoprotein. PLoS pathogens. 2007;3(9):1281–1290. doi: 10.1371/journal.ppat.0030134. 07-PLPA-RA-0294 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleshova N, Frank I, Pope M. Immunodeficiency virus exploitation of dendritic cells in the early steps of infection. J Leukoc Biol. 2003;74(5):683–690. doi: 10.1189/jlb.0403178. [DOI] [PubMed] [Google Scholar]
- van't Wout AB, Ran LJ, Kuiken CL, Kootstra NA, Pals ST, Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. Journal of virology. 1998;72(1):488–496. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verollet C, Souriant S, Bonnaud E, Jolicoeur P, Raynaud-Messina B, Kinnaer C, Fourquaux I, Imle A, Benichou S, Fackler OT, Poincloux R, Maridonneau-Parini I. HIV-1 reprograms the migration of macrophages. Blood. 2015;125(10):1611–1622. doi: 10.1182/blood-2014-08-596775. [DOI] [PubMed] [Google Scholar]
- Wang TH, Donaldson YK, Brettle RP, Bell JE, Simmonds P. Identification of shared populations of human immunodeficiency virus type 1 infecting microglia and tissue macrophages outside the central nervous system. Journal of virology. 2001;75(23):11686–11699. doi: 10.1128/JVI.75.23.11686-11699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91(3):401–415. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Burdo TH. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J Neuroimmune Pharmacol. 2012;7(2):363–371. doi: 10.1007/s11481-011-9330-3. [DOI] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193(8):905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Ignacio CC, Torriani F, Havlir D, Fitch NJ, Richman DD. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. Journal of virology. 1997;71(3):2059–2071. doi: 10.1128/jvi.71.3.2059-2071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]