Abstract
It has been shown recently that neutrophils are able to produce IL-22 and IL-17, which differentially regulate the pathogenesis of inflammatory bowel disease (IBD). However, it is still largely unknown how the neutrophil production of IL-22 and IL-17 is regulated, and their role in the pathogenesis of IBD. In this study, we found that IL-23 promoted neutrophil production of IL-17 and IL-22. IL-23 stimulated the neutrophil expression of IL-23 receptor as well as rorc and ahr. RORγt and AhR differentially regulated IL-23 induction of neutrophil IL-17 and IL-22. Additionally, IL-23 induced the activation of mTOR in neutrophils. Blockade of mTOR pathway inhibited IL-23-induced expression of rorc and ahr as well as IL-17 and IL-22 production. By utilizing a microbiota antigen specific T-cell mediated colitis model, we demonstrated that depletion of neutrophils, as well as blockade of IL-22, resulted in a significant increase in the severity of colitis, thereby indicating a protective role of neutrophils and IL-22 in chronic colitis. Collectively, our data revealed that neutrophils negatively regulate microbiota antigen specific T cell induction of colitis, and IL-23 induces neutrophil production of IL-22 and IL-17 through induction of rorc and ahr, which is mediated by mTOR pathway.
Introduction
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn's disease (CD), represent chronic or recurring inflammation in the gastrointestinal tract. The pathogenesis of IBD is considered multifactorial, involving compromise of the intestinal epithelial barrier and dysregulation of immune responses to commensal bacteria (1). As an essential component of the innate immune system, neutrophils are implicated in the pathogenesis of IBD by virtue of the high neutrophilic influx in the inflamed intestinal tissue of IBD patients (2). Emerging evidence highlights the greater value of neutrophil-derived products such as fecal calprotectin (S100A8/S100A9) than the traditional inflammatory marker C-reactive protein (CRP) in the assessment of disease activity in clinical practice (3-5). Although intense efforts have been made to elucidate the role of neutrophils in IBD, conflicting data have been reported in different animal models that mimic IBD. In the dextran sodium sulfate (DSS)-induced colitis model, the ablation of neutrophils accentuated colitis, likely indicating a potential beneficial role of neutrophils (4). Furthermore, the mice with chronic granulomatous disease contain neutrophils with impaired functions, and these mice showed poor inflammatory resolution and succumbed rapidly during TNBS-induced colitis (7, 8). This finding has been elaborated as a protective function of neutrophils by generating the “inflammatory hypoxia” microenvironment in the inflamed tissue as a way to promote intestinal epithelial healing through HIF-1 induction (6). In contrast, it has also been reported that specific inhibition of neutrophils by monoclonal antibody attenuates DSS-induced colitis in rats (7), and the influx of neutrophils into the intestines leads to massive migration of neutrophils across intestinal epithelium into the intestinal lumen, thereby weakening the cell-to-cell junctions and increasing epithelial permeability, which results in subsequent bacteria translocation and water influx that worsens inflammation (10, 11). Notably, all animal models used in previous reports were acute colitis models, i.e. DSS- or TNBS-induced colitis, and thus, it is still unclear how neutrophils regulate chronic colitis with characteristics like those found in human patients with IBD.
Recently, neutrophils have been identified as an important source of both IL-17 and IL-22 (9, 10). IL-22, a member of the IL-10 cytokine family, specifically targets epithelial cells based on the expression pattern of the IL-22 receptor (IL-22R) (11). IL-22 has been shown to promote barrier defense and wound healing by inducing intestinal epithelial cell secretion of antimicrobial proteins, epithelial cell differentiation, and goblet cell activation (12). IL-17 is a critical component of mucosal immune defense (13, 14). Although detrimental effects of IL-17 have been implied during colitis (15, 16), its protective function has also been demonstrated recently (17, 18). Notably, IL-22-producing neutrophils are reported protective during acute colitis, in that colitis was alleviated in DSS-treated IL-22−/− mice receiving neutrophils from wide-type mice, whereas neutrophils from IL-22−/− mice exhibited little effect on disease progression (10). On the other hand, IL-17-producing neutrophils aid in bactericidal defense during fungal infection (9, 19, 20). However, it is unclear how neutrophil production of IL-22 and IL-17 are regulated and their roles in the pathogenesis of chronic colitis must yet be clarified. We report here that IL-23 primarily promotes neutrophil production of IL-17 and IL-22 by regulating RORγt and AhR through activation of mTOR. Furthermore, neutrophils protect the intestines from chronic intestinal inflammation, possibly by producing IL-22.
Materials and Methods
Mice
C57BL/6 (B6) mice and Rag2−/− mice were obtained from the Jackson Laboratory. CBir1 flagellin-specific TCR transgenic (CBir1-Tg) mice (21) were bred in the Animal Facilities at University of Texas Medical Branch and maintained there. All animal work was reviewed and approved by the Institutional Animal Care and Use Committees of the UTMB.
Antibody and reagents
Neutralizing antibodies to Ly6G (1A8), Thy1.2 (30H12), IFNg (XMG1.2), and IL-4 (11B11) were purchased from Bio X Cell (West Lebanon, NH). Neutralizing antibody to mouse IL-22 (8E11.9) was provided by Genentech (South San Francisco, CA). Thioglycollate broth was purchased from Sigma-Aldrich (St. Louis, MO). RORγt inhibitor was provided by Bristol Myers Squibb. AhR inhibitor, CH-223191 was purchased from Sigma-Aldrich, and mTOR inhibitor rapamycin and AZD8055 from Selleck Chemicals (Houston TX). PI3K inhibitor LY294002, was purchased from Sigma-Aldrich. STAT3 inhibitor HJC0152 was kindly provided Dr. Jia Zhou at University of Texas Medical Branch. Antibodies against phosphorylated mTOR (S2448), phosphorylated 4E-BP1 (T70), phosphorylated STAT3 (P65), phosphorylated NF-kB (Y705), β-actin and HRP-conjugated anti-rabbit secondary antibody were obtained from Cell Signaling Technology (Danvers, MA) for western blot analysis. The following antibodies were used for flow cytometry: PerCP/Cy5.5-IL-17 (TC11-18H10.1), FITC-CD11b (M1/70), PE/Cy7- Ly6G (1A8), were from Biolegend (San Diego, CA). PE- IL-22 (1H8PWSR), APC- IL-10 (JES5-16E3), from eBioscience (San Diego, CA). Foxp3 Perm/ Fix Kit for intracellular permeabilization from eBioscience and Live/Dead Fixable Dead Cell Stain Kit from Life Technologies (Carlsbad, CA). Golgi Stop was purchased from BD Biosciences (San Diego, CA).
Neutrophil isolation and culture
The neutrophils were prepared from the peritoneal cavity as we previously described (22). Briefly, C57BL/6 mice were injected with 1ml of 3% thioglycollate broth, and the peritoneal cavity was washed with cold PBS buffer containing 5% FBS 5 hours later. Neutrophils were separated from other cell types in the peritoneal exudate by using 50% Percoll (Sigma-Aldrich). Cell viability (>95%) was validated by trypan blue staining, and flow cytometry was performed to confirm neutrophil purity (>95%). For bone marrow neutrophil collection, murine femurs were obtained under aseptic conditions and flushed by cold PBS. Neutrophils were then isolated from bone marrow cells by using anti-mouse Ly6G-magnetic sorting (Miltenyi Biotec).
Real-Time Quantitative Reverse Transcription PCR
RNA was extracted with TRIzol (Life Technologies; Carlsbad, CA) and quantified for cDNA synthesis. Quantitative PCR reactions were performed by using TaqMan Gene Expression Assays (Life Technologies). Predesigned primers and probes for il17 (Mm00439618_m1), il22 (Mm00444241_m1),,rorc (Mm01261022_m1), ahr (Mm00478932_m1), Hnf4α (Mm01247712_m1) and gapdh (Mm99999915_g1) were purchased from Applied Biosystems and data were normalized to gapdh mRNA expression. Aliquots of PCR products were visualized under UV by electrophoresis on 1.5% agarose gels.
Induction of colitis
CD4+ T cells were isolated from the spleens of CBir1-Tg mice by using anti-mouse CD4-magnetic beads (BD Biosciences) as previously described (23). To generate Th17 cells, 0.2×106 CD4+ CBirl-Tg T cells were cultured with the same number of irradiated splenocytes in the presence of 10ng/ml TGFβ1, 20ng/ml IL-6, 10μg/ml anti-IFNγ (XMG1.2), and 10μg/ml anti-IL-4 (12B11) for 5 days. The polarized Th17 cells were validated by FACS staining. Then, 2×106 Th17 cells were transferred i.v. into the recipient Rag−/− mice. Mice were monitored by weight weekly and sacrificed either when body weight reached 80% of initial weight or at the end of 6 weeks. As previously described (18), 2% DSS (MP Biomedicals) was administrated to Rag−/− mice for seven days, followed by three days of fresh water. Mice were monitored by weight daily and sacrificed either when body weight reached 80% of initial weight or at the end of day 10.
Preparation of lamina propria cells
To isolate lamina propria cells, intestines were opened and cleaned of feces. Intestines were chopped and incubated with 50mM EDTA for 40 minutes. Remaining tissue was incubated with 0.5g/ml Collagenase IV and 5 μg/ml DNase I (Sigma-Aldrich) for 2 rounds of 30 minutes. Lamina propria cells were isolated from the interface of a 40%/75% Percoll interface.
Ex vivo colon organ culture and ELISAs
Colons were open and cleaned of fecal matter and contaminates. Then, 3mm biopsies from the ascending colon were placed into RPMI (10% FBS, HEPES, penicillin–streptomycin, 2-mecapto- ethanol, and sodium pyruvate) (Invitrogen, Carlsbad, CA, USA) cultured at 37°C with 5% CO2. Supernatants were harvested after one day, and the concentration of cytokine was determined by ELISA as previously described (18). Mouse IL-6, IL-17(A), IL-22, TNFα and IFNγ ELISA kits (BD Pharmingen; San Diego, CA) were used in the present study according to the manufacturer's protocols.
Histopathologic assessment
At necropsy, cecum and colon were harvested and washed with PBS. Swiss rolls were prepared. Tissues were fixed in 10% buffered formalin and embedded in paraffin. Then, 5μm sections were sliced, stained with H&E, and histological scoring was blindly performed by an experienced pathologist using a modified scoring system reported previously (24). The scoring includes the following aspects: lesion in intestinal crypts; goblet cell condition; crypt exudate; inflammatory cell infiltration; and tissue inflammatory condition. The damages were estimated and scored from 0 to 3, representative of intensity, i.e., absent, mild, moderate, or severe, respectively. The final colitis severity score was calculated by taking both intensity and extent of lesion into account.
Statistical analysis
To assess an effect of a given sample size, power analysis was performed by using preliminary data sets. For comparison between samples, levels of significance were determined by appropriate statistical analysis based on whether the data were normally distributed and the number of tested groups for comparison. All statistical analysis was performed in Prism 5.0 (Graphpad Software; San Diego, CA). Results are shown as mean ± s.e.m. *p < 0.05; **p < 0.01; ***p < 0.001.
Results
1. IL-23 stimulates neutrophil production of IL-17 and IL-22
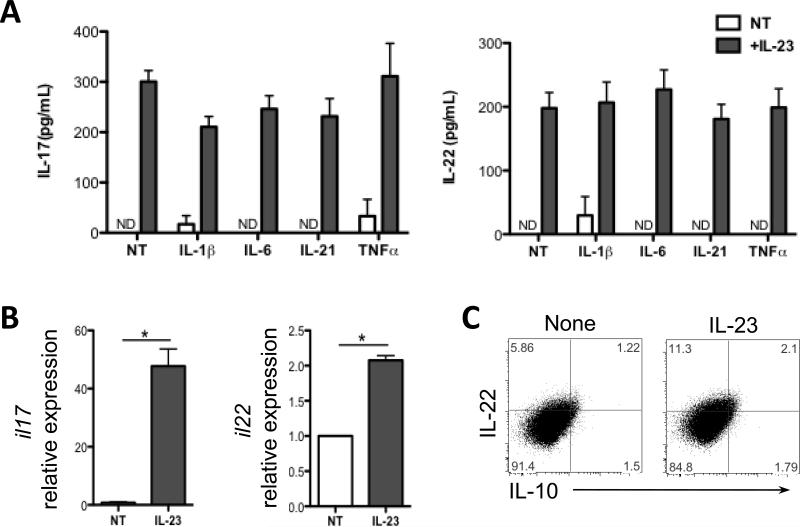
IL-23 has been consistently shown able to stimulate IL-22 production in γδ T cells, dendritic cells (DCs) and type 3 innate lymphoid cells (ILC3s). IL-6, IL-21, TNFα, and IL-1β have also been able to stimulate IL-17 and/or IL-22 production at different levels in ILCs and several CD4+ T cell subtypes (9, 25). Although IL-23 itself induces little IL-17 or IL-22 in CD4+ T cells, it can greatly augment IL-17 and IL-22 production in Th17 cells (26). In a recent report, colonic neutrophils isolated from mice with DSS-induced colitis mice have been shown to produce both IL-17 and IL-22 after IL-23 treatment. Colon tissue of IL-23a−/− mice revealed a significant decrease in IL-22 levels compared to those in wide-type mice (10). However, the cytokines, which stimulate neutrophil production of IL-17 and/or IL-22, have not been carefully elucidated. The short half-life of neutrophils makes it hard to isolate neutrophils from intestines, as it requires a long processing time, and thus most neutrophils isolated would have died by the end of preparation. We thus use peritoneal neutrophils as they are ready to be prepared in large quantities in our in vitro studies (22). We stimulated peritoneal neutrophils with IL-23, IL-6, IL-21, TNFα, or IL-1β to assess which cytokine(s) trigger IL-17 and IL-22 production in neutrophils. A portion of cells was stained for CD11b and Ly6G to assure that the purity of neutrophils population (CD11b+Ly6G+) is higher than 95% (Fig. S1). The levels of IL-17 and IL-22 in the supernatant were determined via enzyme-linked immunosorbent assay (ELISA). Consistent with a previous report, treatment with IL-23 alone markedly enhanced production of IL-17 and IL-22 at levels of both protein and mRNA (Fig. 1A and 1B). In contrast, IL-1β, IL-6, IL-21, and TNFα had little or no effect on the production of IL-17 and IL-22 when administered either alone or in combination with IL-23 (Fig. 1A), indicating a critical role of IL-23 in inducing IL-17 and IL-22 production in neutrophils. As recent evidence indicates that there are different subsets of neutrophils, to evaluate whether the same or different neutrophils produce IL-17 or IL-22, we used flow cytometry to identify the subsets of IL-17- and IL-22-producing neutrophils after IL-23 stimulation. As shown in Figure 1C, increased amount of IL-22-producing CD11b+Ly6G+ neutrophils were found relative to untreated cells. Interestingly, we found a slight increased number of IL-10+ neutrophils in the presence of IL-23.
Figure 1. IL-23 stimulates neutrophil production of IL-17 and IL-22.
(A) Peritoneal neutrophils were treated with IL-1β (20ng/ml), IL-6 (30ng/ml), IL-21 (20ng/ml), or TNFα (20ng/ml), alone or in combination with IL-23 (20ng/ml) for 24 hours. IL-17 and IL-22 production were measured from the supernatant by ELISA. (B) Neutrophils were stimulated with IL-23 for 6 hrs. mRNA for il17 and il22 were determined by qRT-PCR and normalized against gapdh. The relative expression of IL-17 and IL-22 untreated neutrophils was arbitrarily set to 1.0. IL-17 and IL-22 expression was compared between the IL-23-treated and untreated neutrophils. *p<0.05. NT, no treatment. Data are representative of 3 independent experiments. (C) Neutrophils were stimulated with IL-23 (20ng/ml) in the presence of Golgi Stop for 12 hours. Cytokine expression was measured by flow cytometry. FACS plots are representative of 3 independent experiments.
2. IL-23 induces IL-23R expression on neutrophils
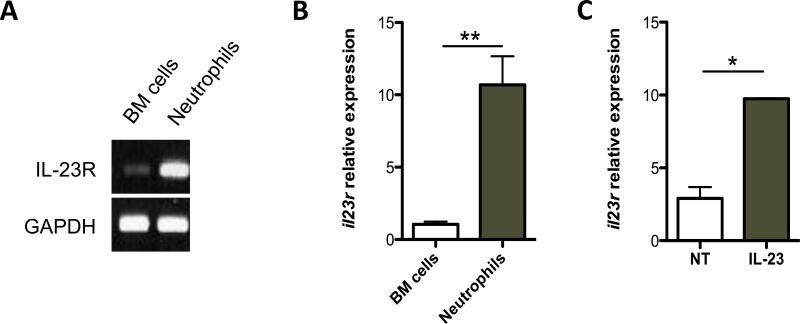
IL-23 receptor (IL-23R) mRNA has been recently found to be expressed on naïve neutrophils in bone marrow (BM) (9). Neutrophils also appear to express IL-23R after migration into the inflamed colon (10). The isolated neutrophils are more activated than those from BM after migrating from blood vessels to peritoneal cavity in response to thioglycollate, while BM neutrophils are naïve. To understand the regulation of neutrophil IL-23R expression, we examined the expression of IL-23R in peritoneal neutrophils. As shown in Fig. 2A and B, IL-23R was expressed in the peritoneal neutrophils at a higher level compared to that in BM cells (Fig. 2A and 2B). The peritoneal neutrophils can further respond to stimulants and elicit more exaggerated response. Accordingly, we found that IL-23 further stimulated IL-23R expression in the peritoneal neutrophils (Fig. 2C). Together, these data suggest that, unlike T cells, naïve neutrophils constantly express a basal amount of IL-23R and the expression of IL-23R increases as the cells become activated.
Figure 2. IL-23 induces IL-23R expression on neutrophils.
2μg RNA from freshly isolated peritoneal neutrophils and bone marrow cells was used to analyze IL-23R expression by reverse transcription–PCR (RT–PCR) (A) and real-time PCR (B). Gapdh was used as a housekeeping gene. Il23r expression values were normalized to Gapdh expression. The relative expression of il23r of BM cells was arbitrarily set to 1.0. il23r expression was compared between the BM cells and peritoneal neutrophils. **p<0.01. (C) Neutrophils were treated with 20ng/ml IL-23 for 6 hours. mRNA for Il23r was determined by qRT-PCR and normalized against gapdh. Data are reflective of 3 independent experiments. *p<0.05. NT, no treatment.
3. RORγt and AhR differentially regulate IL-17 and IL-22 production in neutrophils
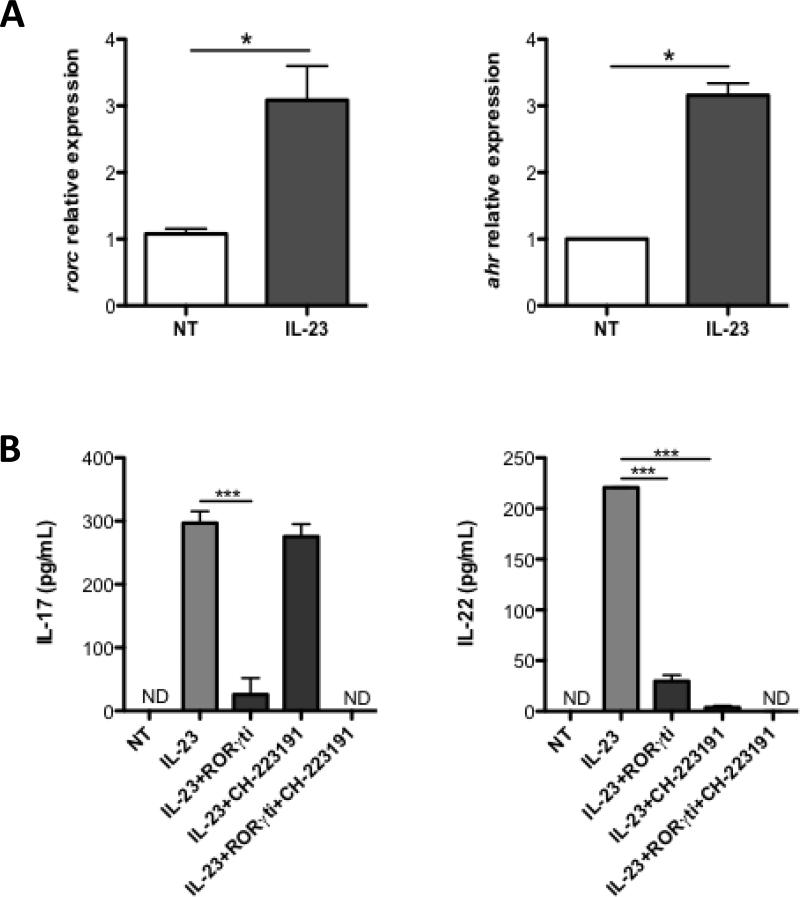
Retinoid-acid Receptor-related Orphan Receptor gamma t (RORγt) is an essential transcription factor mediating IL-17 production in Th17 cells (27). Moreover, it has been shown that RORγt is indispensable for IL-22 expression in ILC3 (28). The ligand-dependent transcription factor Aryl-hydrocarbon Receptor (AhR) also contributes to IL-17 and IL-22 expression in lymphoid cells (28–30). We then investigated whether RORγt and AhR are involved in IL-23-driven IL-17 and IL-22 production in neutrophils. As shown in Figure 3A, addition of IL-23 upregulated expression of transcripts encoding RORγt (rorc) and AhR (ahr) in neutrophils, indicating that IL-23 is capable of inducing both transcription factors. To investigate the role of RORγt and AhR in neutrophil IL-17 and IL-22 production, we treated peritoneal neutrophils with small molecule inhibitors against RORγt or AhR in the presence of IL-23. As shown in Figure 3B, inhibition of RORγt compromised both IL-17 and IL-22 production in IL-23-treated neutrophils, indicating that induction of both cytokines is RORγt-dependent (Fig. 3B). Interestingly, AhR inhibition only decreased IL-22 production with no detectable impact on IL-17 expression. Addition of both RORγt and AhR inhibitors further reduced IL-17 and IL-22 production, suggesting that RORγt and AhR act in a synergistic manner. Collectively, the data indicate that RORγt and AhR differentially regulate neutrophil production of IL-17 and IL-22.
Figure 3. RORγt and AhR differentially regulate IL-17 and IL-22 production in neutrophils.
(A) Neutrophils were treated with IL-23 (20ng/ml) for 6 hours. mRNA for Il23r was determined by qRT-PCR and normalized against gapdh. The relative expression of rorc and ahr of BM cells was arbitrarily set to 1.0 respectively. rorc and ahr expression was compared between the untreated and IL-23-treated neutrophils. *p<0.05; NT, no treatment. (B) Neutrophils were treated with IL-23 (20ng/ml), in addition to a RORγt inhibitor (1μM), and/or AhR inhibitor (3μM) respectively. IL-17 and IL-22 production were measured from supernatant by ELISA. Data are reflective of 4 independent experiments. *p<0.05, **p<0.01, ***p<0.001. NT, no treatment.
4. mTOR regulates IL-23-driven IL-17 and IL-22 production in neutrophils
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase that is crucial for cell growth and proliferation (31). Previous data indicate that mTOR is expressed in neutrophils and modulates neutrophil function (32), and therefore we investigated whether mTOR is involved in IL-23 induction of IL-17 and IL-22 in neutrophils. IL-23 treatment led to the phosphorylation of mTOR (Fig. 4A). Additionally, 4E-BP1, a major downstream effector molecule of mTOR complex 1 (mTORC1) was also activated after the treatment of IL-23, further confirming that IL-23 activates mTOR pathway. We then applied rapamycin, a well-established mTORC1 inhibitor to neutrophils in combination with IL-23. As expected, rapamycin greatly attenuated IL-23-induced phosphorylation of 4E-BP1 (Fig. 4B). To confirm these results, we adopted another mTOR inhibitor (AZD8055), which targets both mTORC1 and mTORC2 to neutrophils (33–35). Consistent with rapamycin, AZD8055 inhibited IL-23-induced phosphorylation of 4E-BP1 (Fig. 4B). To determine whether mTOR regulates IL-23-driven cytokine production in neutrophils, we first examined IL-17 and IL-22 gene transcription. The qRT-PCR showed markedly decreased IL-17 and IL-22 mRNA expression after blockade of mTOR signaling by rapamycin. We also demonstrated a similar effect of AZD8055 on IL-23-treated neutrophils, in that both IL-17 and IL-22 transcription were dramatically inhibited (Fig. 4C). Additionally, ELISA revealed that production of IL-17 and IL-22 under IL-23 stimulation was inhibited by both mTOR inhibitors respectively (Fig. 4D). In order to illustrate the molecular mechanisms as to how mTOR regulates IL-17 and IL-22 production, transcription of RORγt and AhR was examined in the presence of mTOR inhibitors. We found that the induction of both rorc and ahr by IL-23 was inhibited by rapamycin (Fig. 4E). Similarly, AZD8055 remarkably decreased rorc and ahr mRNA expression in IL-23-treated neutrophils. Taken together, these data demonstrated that IL-23 signals through the mTOR pathway to positively regulate the production of both IL-17 and IL-22 by upregulating RORγt and AhR expression in neutrophils.
Figure 4. mTOR regulates IL-23-driven IL-17 and IL-22 production in neutrophils.
(A) Peritoneal neutrophils were treated with IL-23 (20ng/ml) for the indicated time points. mTOR phosphorylation (Ser2448) was determined by Western blot. (B) Neutrophils were treated with IL-23 (20ng/ml) for 1 hour, in addition to rapamycin (2μM) or AZD8055 (1μM) respectively. Phosphorylated 4E-BP1 was detected by western blot, with β-actin as a loading control. (C) Neutrophils were treated with IL-23 (20ng/ml) for 6 hours. mRNA for Il17 and il22 were determined by qRT-PCR and normalized against gapdh. *p<0.05, **p<0.01. (D) Neutrophils were treated with IL-23 (20ng/ml), in the presence or absence of rapamycin (2μM,), or AZD8055 (1μM). IL-17 and IL-22 production were measured from supernatant by ELISA. *p<0.05, ***p<0.001. (E) mRNA for rorc and ahr were determined by qRT-PCR and normalized against gapdh. Data are reflective of 4 independent experiments. *p<0.05. NT, no treatment.
5. PI3K regulates mTOR pathway to promote IL-17 and IL-22 production in neutrophils
PI3K has been shown to positively regulate mTOR activation (36). Based on the data that mTOR and its downstream proteins facilitate IL-23 signaling, we postulated that IL-23 regulates mTOR pathway through induction of PI3K. As shown in Figure 5A, IL-23 treatment enhanced the phosphorylation of PI3K in neutrophils. Addition of the PI3K antagonist LY294002 inhibited IL-23-induced PI3K phosphorylation and activation of 4E-BP1, suggesting that IL-23 activates the mTOR pathway through activation of PI3K. Moreover, treatment of neutrophils with LY294002 in the presence of IL-23 inhibited IL-17 and IL-22 production, indicating that stimulation of IL-17 and IL-22 production is mediated by PI3K pathway (Figs. 5B and 5C). LY294002 treatment also greatly decreased the transcription level of both rorc and ahr (Fig. 5D). Notably, IL-23 treatment also led to p38 mitogen-activated protein kinase (MAPK) phosphorylation, which was unaffected by treatment with either mTOR or PI3K inhibitor, confirming the specific inhibition of the selected pathways without compromising neutrophil viability (data not shown). Collectively, these data indicate that IL-23 stimulates neutrophil production of IL-17 and IL-22 through the activation of PI3K-mTOR pathway.
Figure 5. PI3K regulates mTOR activation and IL-17 and IL-22 production in neutrophils.
(A) Neutrophils were treated with IL-23 (20ng/ml) for 1 hour with or without Ly294002 (10μM). Phosphorylation of PI3K and 4E-BP1 were detected by western blot, with β-actin as a loading control. (B) Neutrophils were treated with IL-23 (20ng/ml) for 6 hours. mRNA for Il17 and il22 were determined by qRT-PCR and normalized against gapdh. *p<0.05. (C) Neutrophils were treated with IL-23 (20ng/ml) with or without Ly294002 (10uM) for 24 hrs. IL-17 and IL-22 production were measured from supernatant by ELISA. *p<0.05, **p<0.01. (D) mRNA for rorc and ahr were determined by qRT-PCR and normalized against gapdh. (E) Neutrophils were treated with IL-23 (20ng/ml) for 1 hour and phosphorylation of STAT3 and NF-kB were detected by western blot, with β-actin as a loading control. (F) Neutrophils were treated with IL-23 (20ng/ml) with or without HJC0152 (5uM) for 24 hrs. IL-17 and IL-22 production were measured from supernatant by ELISA. *p<0.05. Data are reflective of 4 independent experiments. *p<0.05. NT, no treatment.
Signal transducers and activator of transcription 3 (STAT3) has been implicated as a transcriptional regulator in T cell IL-17 and IL-22 production (37, 38). Earlier studies showed that STAT3 regulated neutrophil chemotaxis and migration (39). We then questioned whether STAT3 could also be involved in IL-23-induced IL-17 and IL-22 production in neutrophils. We cultured neutrophils with IL-23 in the presence or absence of STAT3 inhibitor HJC0152 (40). As expected, IL-23 was able to induce phosphorylation of STAT3, which was abrogated by HJC0152 (Fig. 5E). IL-17 and IL-22 production downstream of IL-23 signaling were reduced by addition of HJC0152 (Fig. 5F). IL-23 also activated NF-κB in neutrophils (Fig. 5E), and addition of NF-κB inhibitor inhibited IL-23-induced IL-17 and IL-22 (data not shown). Collectively, these data indicated that STAT3-NF-κB pathway also mediates IL-23 induction of IL-17 and IL-22 in neutrophils.
6. Neutrophils play a protective role in the pathogenesis of chronic colitis via IL-22
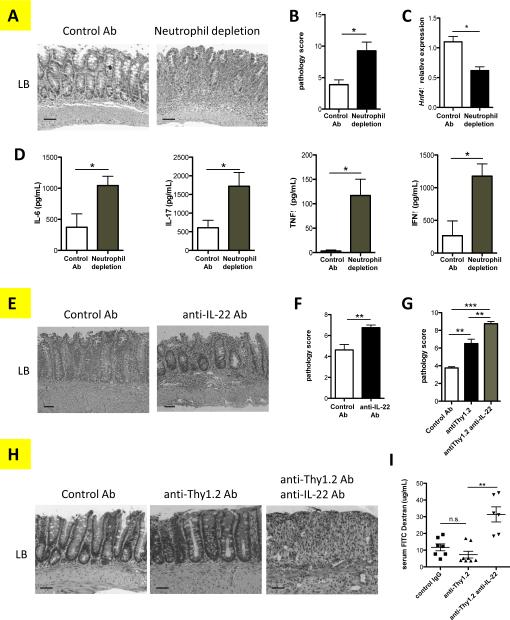
Thus far, our results in vitro have demonstrated the molecular mechanisms involved in the regulation of neutrophil cytokine production. We sought to determine the role of neutrophils in regulating the pathogenesis of chronic colitis. We previously established a microbiota antigen-specific model of colitis by adoptive transfer of CD4+ T cells from CBir1 TCR transgenic mice, which are specific for an immunodominant microbiota antigen CBir1 flagellin, into Rag−/− mice. The recipient mice developed colitis 4-6 weeks after cell transfer (41). A neutrophil-specific Ly6G-depleting antibody was administered i.p. twice per week to deplete neutrophils. Six weeks later, the histopathology was evaluated in large bowel (LB) and cecum. FACS staining confirmed that neutrophils were almost completely depleted in the mice administered with anti-Ly6G antibody (Fig. S2). Overall, in the absence of neutrophils, mice presented much more severe colitis than did the neutrophil-sufficient control group (Fig. 6A), as evidenced by destroyed colon structure and loss of goblet cells. This finding corresponded to the higher pathology score of both large bowel (LB) and cecum in mice with neutrophil depletion (Fig. 6B). The worsened disease was also associated with significantly decreased expression of hepatocyte nuclear factor 4alpha (Hnf4α), a critical regulator of tight junction proteins (42) (Fig. 6C) and increased amounts of pro-inflammatory cytokines in colon organ cultures (Fig. 6D). However, surface and intracellular staining of lamina propria cells of colitic mice revealed no significant difference of T cells in percentage and cytokine production (Fig. S3). Collectively, these data demonstrated that neutrophils protect the intestines from chronic inflammation in response to microbiota. As IL-22 has been shown to be an important cytokine that contributes to intestinal protection and tissue repair of the epithelial layer, we then investigated whether IL-22 protected the intestine in CBir1 T cell-mediated colitis. We administered a neutralizing antibody against IL-22 from the same day of T cell transfer and twice per week thereafter for 6 weeks. As shown in Figures 6E and 6F, blockade of IL-22 increased the severity of colitis in comparison with the mice giving control mAb. T cells and ILCs have been shown as cell sources of IL-22, in addition to neutrophils (43, 44). To elucidate the role of neutrophil-derived IL-22, independently of T cells or ILCs, we induced colitis in Rag−/− mice, which do not have T cells but with intact ILC3 and neutrophils, upon DSS insult. We then depleted ILCs by giving Rag−/− mice anti-Thy1.2 antibody (Fig. S4), thus, the only known resource of IL-22 will be neutrophils. Depletion of ILCs worsened the colitis in Rag−/− mice after feeding DSS. When treated with anti-IL-22 antibody, those mice developed even more severe colitis (Figs. 6G and 6H), as well as increased intestinal permeability as evidenced by FITC-dextran migration into the serum (Fig. 6I), demonstrating that neutrophil production of IL-22 at least partially protects the mice from colitis.
Figure 6. Neutrophils and IL-22 protect the intestines from chronic inflammation.
2×106 CBir1 Th17 cells were i.v. transferred into Rag−/− mice. (A-C) Recipient mice were treated with anti-Ly-6G depleting antibody (4mg/kg) or control mAb twice per week. At week 6 post-Th17 cell transfer, (A) H&E staining was performed on colonic histopathology of control antibody-treated mice (left), and neutrophil-depleted mice (right) for (B) blinded histological scoring. N=4 mice per group. *p<0.05. (C) Tissue RNA was isolated from large bowel (LB). mRNA for Hnf4α was determined by qRT-PCR among two groups of mice and normalized against gapdh. *p<0.05. (D) IL-6, IL-17, TNFα, and IFNγ production were measured from the supernatant of colon tissue cultures from 4 mice per group by ELISA. Data are one representative of 3 independent experiments with similar results. *p<0.05. (E-F) CBir1 T cell recipient mice were treated with 6mg/kg of anti-IL-22 or control mAb twice per week. On week 6 post-Th17 cell transfer, H&E staining was performed and colonic histopathology determined. (E) Histopathology of control antibody-treated mice (left) and anti-IL-22 antibody-treated mice (right), and (F) histological scores. N=4 mice per group. Data are one representative of 2 independent experiments with similar results. *p<0.05, **p<0.01. (G-I) Rag−/− mice were fed in drinking water with 2% DSS for seven days, followed by three days of fresh water, and injected IgG control mAb, 20mg/kg anti-Thy1.2 mAb with or without 6mg/kg anti-IL-22 mAb, respectively. (G) Blinded histological scoring was examined, and (H) H&E staining was performed. (I) FITC-dextran level in plasma was determined. N=4 mice per group, Data are one representative of 2 independent experiments with similar results. n.s. indicates no significant difference *p < 0.01. Bars: (A and F) 100 μm; (H) 50 μm.
Discussion
Neutrophils have been demonstrated recently to produce both IL-17 and IL-22 under various inflammatory/infectious conditions (9, 10, 19, 45). However, it is still unclear how neutrophil production of these cytokines is regulated. Also remaining unclarified is the role of neutrophils and their production of these cytokines in the pathogenesis of chronic colitis. In the present study, we demonstrated that IL-23 induced neutrophil cytokine production by upregulating RORγt and AhR, which was mediated by the mTOR pathway. Furthermore, neutrophils protected the intestines from inflammation, possibly via the production of IL-22.
With more than 1014 commensal microbiota coexisting in the gut, the host immune system employs multiple strategies to maintain intestinal homeostasis (46). Among them, neutrophils are critical components of the mucosal immune defense. The infiltration of neutrophils in chronically inflamed tissues has long been considered to exacerbate inflammation and perpetuate disease. However, recent studies have also demonstrated a beneficial effect by the accumulated neutrophils for limiting inflammation, which may outweigh their drawbacks during acute colitis (6). Consistent with these findings, we demonstrated that the depletion of neutrophils leads to more severe inflammation and exacerbated colitis during chronic intestinal inflammation, indicating a protective role for neutrophils in chronic colitis (Fig. 6A-D). In a recent GWAS study, the gene encoding HNF4α has been reported as a susceptibility gene for UC (47). Our finding that decreased expression of HNF4α in intestines of neutrophil-depleted mice indicates that the absence of neutrophils is correlated to the enhanced gut epithelial permeability. Since neutrophils share many features with myeloid-derived suppressor cells (MDSC), Ly6G-depleting antibody may also abolish the potential suppressive role of MDSCs, although the concept and functional mechanisms of MDSCs are still not clear. Thus, it is warranted to investigate the functional relationship between neutrophils and MDSCs, and their relative contributions to the intestinal homeostasis.
Previous studies elaborated the protective effects of IL-22 on host bactericidal response, tissue regeneration, and wound healing. IL-22-producing neutrophils have been reported to promote antimicrobial peptide production of intestinal epithelial cells (10). Consistently, our data demonstrated a worsened chronic colitis in mice after blockade of IL-22 (Figs. 6E-F), indicating that neutrophils produce IL-22 as a possible way to resolve chronic colitis. T cells and ILCs have been implicated as cell sources for IL-22, in addition to neutrophils. Our data indicated that neutrophil production of IL-22 at least partially contributes to protection of the intestines from inflammation. Upon DSS insult, depletion of ILCs partially protected the Rag−/− mice, which do not have T cells, from colitis (Figs. 6G-I). This could be attributed to their production of IL-22, among other possible mechanisms. Interestingly, blockade of IL-22 in ILC-depleted Rag−/− mice further worsened the colitis, indicating that neutrophil-derived IL-22 protected the intestines from inflammation.
Despite the proinflammatory role of IL-17 in the pathogenesis of IBD highlighted in previous reports (3, 14), recent studies have also demonstrated a protective role of IL-17 in the maintenance of intestinal homeostasis through induction of intestinal epithelial cell expression of antimicrobial peptide and promoting intestinal IgA response against microbiota (18). The ubiquitous expression of IL-17 receptors on a variety of cell types suggests that neutrophils can also “cross talk” with lymphoid, myeloid, and stromal/mesenchymal cells, and exhibit effector function (48). Thus, in the intestines, IL-17 may also contribute to the protective effects of neutrophils (17). In other systems, while the beneficial role of IL-17-producing neutrophils been demonstrated during Aspergillus fumigatus infection (9, 19), neutrophil-derived IL-17 were not detectable in certain Candida albicans infection (20), indicating that neutrophil IL-17 production may be limited to certain conditions or infections.
In agreement with previous studies (10, 49), our results showed that in response to stimulation of IL-23, but not IL-6 or other cytokines tested, neutrophils produce IL-17 and IL-22. Notably, we found a large subset of IL-22+ neutrophils after stimulation of IL-23 (Fig. 1C), which corresponds to large amounts of IL-22 and IL-17 in the neutrophil culture supernatant (Fig. 1A), as well as the increased expression of il22 and il17 mRNA expression (Fig. 1B). Also, a small proportion of neutrophils are also IL-10+. As IL-10 is a well-established anti-inflammatory cytokine, whether these IL-10+ neutrophils function as regulatory cells to inhibit colitis warrants further investigation. The neutrophils are notable for their unique secretion of pre-made granules containing cytokines and other molecules. Indeed, a recent study reported that neutrophils release IL-17 via a neutrophil extracellular trap (NET) (50). However, the signal pathways that regulate IL-17 and IL-22 production in neutrophils remain unknown. We demonstrated that unlike CD4+ T cell subtypes and ILC3, neutrophil production of IL-17 and IL-22 is strictly IL-23-dependent (Fig. 1A). Interestingly, IL-23 promotes the expression of its own receptor by neutrophils (Fig. 2), likely indicating a positive feedback to stabilize the neutrophil phenotype and contributing to constant IL-17 and IL-22 production. Furthermore, IL-23 induces the expression of RORγt and AhR (Fig. 3A), which differentially regulate neutrophil production of IL-17 and IL-22. Inhibition of RORγt inhibited IL-23-induced IL-17 and IL-22 production (Fig. 3B), whereas AhR inhibitor only elicited effect on IL-22 production in neutrophils. Blockade of both RORγt and AhR signaling led to complete abrogation of IL-17 and IL-22 production. Taken together, our results indicate that neutrophils utilize pathways similar to that of ILC3, in that AhR synergizes with RORγt for the induction of IL-22 but not IL-17. In addition, IL-23 activates STAT3 and NF-kB in neutrophils, which is also contributed to IL-23-induction of IL-17 and IL-22.
The critical role of the PI3K/mTOR pathway has been implicated in various cellular functions (51–53). Our data indicated that the mTOR pathway mediates IL-23 induction of the neutrophil production of IL-17 and IL-22, in that IL-23 activated mTOR pathway, and inhibition of mTOR severely compromised IL-17 and IL-22 production. It has recently been shown that mTOR induces NETosis after lipopolysaccharide (LPS) exposure. Thus, mTOR may facilitate neutrophil cytokine production in multiple aspects.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by NIH grant DK098370 and DK105585, and John Sealy Memorial Endowment Fund. FC is a recipient of the J.W. McLaughlin Predoctoral Fellowship, UTMB.
Abbreviations used
- IL
interleukin
- IBD
inflammatory bowel disease
Footnotes
Disclosures: The authors report no financial conflict of interests.
References
- 1.Strober W, Fuss I, Mannon P. Science in medicine The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 2007;117:514–21. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fournier BM, a Parkos C. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 3.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang R, Ito S, Nishio N, Cheng Z, Suzuki H, Isobe K. Up-regulation of Gr1+CD11b+ population in spleen of dextran sulfate sodium administered mice works to repair colitis. Inflamm. Allergy Drug Targets. 2011;10:39–46. doi: 10.2174/187152811794352114. [DOI] [PubMed] [Google Scholar]
- 5.de Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, Gresnigt MS, Begun J, Plantinga TS, Joosten L. a B., van der Meer JWM, Chamilos G, Netea MG, Xavier RJ, Dinarello C. a, Romani L, van de Veerdonk FL. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3526–31. doi: 10.1073/pnas.1322831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, Colgan SP. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natsui M, Kawasaki K, Takizawa H, Hayashi SI, Matsuda Y, Sugimura K, Seki K, Narisawa R, Sendo F, Asakura H. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. J. Gastroenterol. Hepatol. 1997;12:801–808. doi: 10.1111/j.1440-1746.1997.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 8.Kucharzik T, V Walsh S, Chen J, a Parkos C, Nusrat a. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 2001;159:2001–2009. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor PR, Roy S, Leal SM, Sun Y, Howell SJ, a Cobb B, Li X, Pearlman E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat. Immunol. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zindl CL, Lai J-F, Lee YK, Maynard CL, Harbour SN, Ouyang W, Chaplin DD, Weaver CT. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12768–73. doi: 10.1073/pnas.1300318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleicher L, de Moura PR, Watanabe L, Colau D, Dumoutier L, Renauld JC, Polikarpov I. Crystal structure of the IL-22/IL-22R1 complex and its implications for the IL-22 signaling mechanism. FEBS Lett. 2008;582:2985–2992. doi: 10.1016/j.febslet.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 12.Turner JE, Stockinger B, Helmby H. IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection. PLoS Pathog. 2013;9:1–7. doi: 10.1371/journal.ppat.1003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Boussiotis V. a. The role of IL-17 producing Foxp3+ CD4+ T cells in inlfammatory bowel disease and colon cancer. Clin. Immunol. 2014;148:1–13. doi: 10.1016/j.clim.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shabgah AG, Fattahi E, Shahneh FZ. Interleukin-17 in human inflammatory diseases. 2014:256–261. doi: 10.5114/pdia.2014.40954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm. Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 16.Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J. Immunol. 2011;186:6313–6318. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connor W, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, a Flavell R. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis Anthony. Changes. 2012;29:997–1003. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werner JL, Gessner M. a., Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, Brown GD, Steele C. Neutrophils produce interleukin 17A (IL-17A) in a Dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect. Immun. 2011;79:3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huppler AR, Verma AH, Conti HR, Gaffen SL. Neutrophils Do Not Express IL-17A in the Context of Acute Oropharyngeal Candidiasis. Pathog. (Basel, Switzerland) 2015;4:559–72. doi: 10.3390/pathogens4030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlsena ED, Liang Y, Shelitec TR, Walkerc DH, Melby PC, Soong L. Permissive and protective roles for neutrophils in leishmaniasis Eric. Laryngoscope. 2014:2–31. doi: 10.1111/cei.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cong Y, Weaver CT, Lazenby a, Elson CO. Colitis induced by enteric bacterial antigen-specific CD4+ T cells requires CD40-CD40 ligand interactions for a sustained increase in mucosal IL-12. J. Immunol. 2000;165:2173–2182. doi: 10.4049/jimmunol.165.4.2173. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal N, Oliver JR, Wagner FH, Lazenby AS, Elson CO, Weaver CT. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J. Exp. Med. 2002;195:71–84. doi: 10.1084/jem.2001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouyang W. Distinct roles of IL-22 in human psoriasis and inflammatory bowel disease. Cytokine Growth Factor Rev. 2010;21:435–441. doi: 10.1016/j.cytogfr.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical Regulation of Early Th17 Cell Differentiation by Interleukin-1 Signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Cella M, Colonna M. AHR and the transcriptional regulation of type-17/22 ILC. Front. Immunol. 2012;3:1–8. doi: 10.3389/fimmu.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld J-C, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 30.Abe H, Kimura A, Tsuruta S, Fukaya T, Sakaguchi R, Morita R, Sekiya T, Shichita T, Chayama K, Fujii-Kuriyama Y, Yoshimura A. Aryl hydrocarbon receptor plays protective roles in cona-induced hepatic injury by both suppressing IFN-γ expression and inducing IL-22. Int. Immunol. 2014;26:129–137. doi: 10.1093/intimm/dxt049. [DOI] [PubMed] [Google Scholar]
- 31.Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, Pulendran B, Hörl WH, Säemann MD, Weichhart T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J. Immunol. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 32.Itakura A, McCarty OJT. Pivotal role for the mTOR pathway in the formation of neutrophil extracellular traps via regulation of autophagy. Am. J. Physiol. Cell Physiol. 2013;305:C348–54. doi: 10.1152/ajpcell.00108.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu M, Huang H, Zhao R, Li P, Li M, Miao H, Chen N, Chen M. AZD8055 induces cell death associated with autophagy and activation of AMPK in hepatocellular carcinoma. Oncol. Rep. 2014;31:649–656. doi: 10.3892/or.2013.2890. [DOI] [PubMed] [Google Scholar]
- 34.Willems L, Chapuis N, Puissant a, Maciel TT, Green a S., Jacque N, Vignon C, Park S, Guichard S, Herault O, Fricot a, Hermine O, Moura IC, Auberger P, Ifrah N, Dreyfus F, Bonnet D, Lacombe C, Mayeux P, Bouscary D, Tamburini J. The dual mTORC1 and mTORC2 inhibitor AZD8055 has anti-tumor activity in acute myeloid leukemia. Leukemia. 2012;26:1195–1202. doi: 10.1038/leu.2011.339. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Li Y, Hu R, Li W, Qiu H, Cai H, Wang S. The mTOR inhibitor AZD8055 inhibits proliferation and glycolysis in cervical cancer cells. Oncol. Lett. 2013;5:717–721. doi: 10.3892/ol.2012.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis WJ, Lehmann PZ, Li W. Nuclear PI3K signaling in cell growth and tumorigenesis. Front. Cell Dev. Biol. Rev. 2015;3:1–14. doi: 10.3389/fcell.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirahara K, Ghoreschi K, Laurence A, Yang X, Kanno Y, Shea JJO. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 2010;21:425–434. doi: 10.1016/j.cytogfr.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Backert I, Koralov SB, Wirtz S, Kitowski V, Billmeier U, Hofmann K, Hildner K, Wittkopf N, Brecht K, Neurath MF, Becker C, Neufert C. STAT3 activation in Th17 and Th22 cells controls IL-22 mediated epithelial host defence during infectious colitis. JI . 2015 doi: 10.4049/jimmunol.1303076. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen-Jackson H, Panopoulos AD, Zhang H, Li HS, Watowich SS. STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction. Blood. 2010;115:3354–3363. doi: 10.1182/blood-2009-08-240317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Yang Z, Ding C, Chu L, Zhang Y, Terry K, Liu H, Shen Q, Zhou J. Discovery of O-Alkylamino Tethered Niclosamide Derivatives as Potent and Orally Bioavailable Anticancer Agents. ACS Med. Chem. Lett. 2013;4:180–185. doi: 10.1021/ml3003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. Interleukin-12 converts Foxp3+ regulatory T cells to interferonγ-producing Foxp3+ T cells that inhibit colitis. Gastroenterology. 2011;140:2031–2043. doi: 10.1053/j.gastro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: Lessons learned from animal models and human genetics. Front. Immunol. 2013;4:1–22. doi: 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goc J, Hepworth MR, Sonnenberg GF. Group 3 innate lymphoid cells: regulating host-commensal bacteria interactions in inflammation and cancer. Int. Immunol. 2015;28:43–52. doi: 10.1093/intimm/dxv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chewning JH, Weaver CT. Development and survival of Th17 cells within the intestines: the influence of microbiome- and diet-derived signals. J. Immunol. 2014;193:4769–77. doi: 10.4049/jimmunol.1401835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campillo-Gimenez L, Casulli S, Dudoit Y, Seang S, Carcelain G, Lambert-Niclot S, Appay V, Autran B, Tubiana R, Elbim C. Neutrophils in antiretroviral therapy-controlled HIV demonstrate hyperactivation associated with a specific IL-17/IL-22 environment. J. Allergy Clin. Immunol. 2014;134:1142–1152. e5. doi: 10.1016/j.jaci.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 46.Putignani L, Del Chierico F, Petrucca A, Vernocchi P, Dallapiccola B. The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatr. Res. 2014;76:2–10. doi: 10.1038/pr.2014.49. [DOI] [PubMed] [Google Scholar]
- 47.Consortium UIG, Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H, Nimmo ER, Massey D, Blaszczyk K, Elliott T, Cotterill L, Dallal H, Lobo AJ, Mowat C, Sanderson JD, Jewell DP, Newman WG, Edwards C, Ahmad T, Mansfield JC, Satsangi J, Parkes M, Mathew CG, Wellcome C, Trust Case Control. Donnelly P, Peltonen L, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Craddock N, Deloukas P, Duncanson A, Jankowski J, Markus HS, McCarthy MI, Palmer CN, Plomin R, Rautanen A, Sawcer SJ, Samani N, Trembath RC, Viswanathan AC, Wood N, Spencer CC, Bellenguez C, Davison D, Freeman C, Strange A, Langford C, Hunt SE, Edkins S, Gwilliam R, Blackburn H, Bumpstead SJ, Dronov S, Gillman M, Gray E, Hammond N, Jayakumar A, McCann OT, Liddle J, Perez ML, Potter SC, Ravindrarajah R, Ricketts M, Waller M, Weston P, Widaa S, Whittaker P, Attwood AP, Stephens J, Sambrook J, Ouwehand WH, McArdle WL, Ring SM, Strachan DP. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat. Genet. 2009;41:1330. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 49.Katayama M, Ohmura K, Yukawa N, Terao C, Hashimoto M, Yoshifuji H, Kawabata D, Fujii T, Iwakura Y, Mimori T. Neutrophils Are Essential As A Source Of Il-17 In The Effector Phase Of Arthritis. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keijsers RRMC, Hendriks AGM, van Erp PEJ, van Cranenbroek B, van de Kerkhof PCM, Koenen HJPM, Joosten I. In vivo induction of cutaneous inflammation results in the accumulation of extracellular trap-forming neutrophils expressing RORγt and IL-17. J. Invest. Dermatol. 2014;134:1276–84. doi: 10.1038/jid.2013.526. [DOI] [PubMed] [Google Scholar]
- 51.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cargnello M, Tcherkezian J, Roux PP. The expanding role of mTOR in cancer cell growth and proliferation. Mutagenesis. 2015;30:169–176. doi: 10.1093/mutage/geu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nardella C, Carracedo A, Alimonti A, Hobbs RM, Clohessy JG, Chen Z, Egia A, Fornari A, Fiorentino M, Loda M, Kozma SC, Thomas G, Cordon-Cardo C, Pandolfi PP. Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Sci. Signal. 2009;2:ra2. doi: 10.1126/scisignal.2000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.