Abstract
Cardiac ankyrin repeat protein (CARP) not only serves as an important component of muscle sarcomere in the cytoplasm, but also acts as a transcription co-factor in the nucleus. Previous studies have demonstrated that CARP is up-regulated in some cardiovascular disorders and muscle diseases; however, its role in these diseases remains controversial now. In this review, we will discuss the continued progress in the research related to CARP, including its discovery, structure, and the role it plays in cardiac development and heart diseases.
Keywords: Cardiac ankyrin repeat protein (CARP), Cardiovascular disease, Cardiac development
1. Introduction
Cardiac ankyrin repeat protein (CARP) was first discovered in the nucleus as a transcription co-factor to regulate cardiac gene expression in 1995 (Chu et al., 1995). Subsequently, it was also found to be distributed in sarcomere I-band interacting with the giant elastic protein titin (Miller et al., 2003). Since its discovery, CARP has elicited significant interest, especially in cardiovascular and muscle diseases. The expression of CARP was increased in several diseases. Moreover, CARP mutation was found in dilated cardiomyopathy and hypertrophic cardiomyopathy, which suggests its role in diagnosis and prognosis of these diseases. However, the specific role of CARP in the progress of these diseases is still inconsistent now, which implies that the discrepancy will be crucial to deepen our understanding of CARP and open new avenues for cardiovascular diseases therapy. In this study, we primarily review the structure and role of CARP in cardiac development and heart diseases.
2. Discovery of CARP
CARP was originally identified, in 1995, as a cytokine-inducible transcription factor through screening of a complementary DNA (cDNA) library prepared from interleukin-1α and tumor necrosis factor-α-stimulated human dermal microvascular endothelial cells designated as C-193 (Chu et al., 1995), and the location and sequence of C-193 and its compiled protein structure were first determined. Subsequently in 1997, rat CARP was independently isolated by three labs (Baumeister et al., 1997; Jeyaseelan et al., 1997; Zou et al., 1997), and rabbit cDNA was then cloned and its characterization was determined in 1999 (Aihara et al., 1999). In order to characterize the factors that regulate the expression of ventricular myosin light chain-2 (MLC-2v) gene, Zou et al. (1997) isolated the YB-1-associated nuclear factor from the neonatal rat cardiomyocyte cDNA library by performing a yeast-two-hybrid screening, and designated the results as CARP because of the repeated ankyrin protein domain and its exclusive expression in the heart. Jeyaseelan et al. (1997) found this protein in their search for additional cardiac-specific molecules that mediate the toxic effect of doxorubicin (DOX; adriamycin), hence they called this protein cardiac adriamycin-responsive protein, whose expression is down-regulated in response to adriamycin. At the same time, the muscle ankyrin repeat protein (MARP) was cloned as a gene induced in the denervated skeletal muscle of adult rats, and proved to be a rodent homologue of the human’s C-193 and identical to rat’s CARP (Baumeister et al., 1997). However, MARP was later identified as a family composed of three proteins: ankyrin repeat domain-containing protein 2 (ANKRD2), CARP, and the diabetes-related ankyrin repeat protein (DARP), which exert their functions together in the muscle (Miller et al., 2003). Accumulating data showed that ANKRD2 was primarily expressed in the skeletal muscle (Singal and Iliskovic, 1998; Tsukamoto et al., 2002; Miller et al., 2003), while CARP was primarily expressed in the heart (Baumeister et al., 1997; Jeyaseelan et al., 1997; Zou et al., 1997; Boengler et al., 2003; Miller et al., 2003) and DARP was expressed in both tissues (Ikeda et al., 2003; Barash et al., 2004). These represent homologous ankyrin repeat proteins, and interact together in the muscles. Recently, sheep homolog of the CARP gene was also cloned and characterized (Ma et al., 2013).
3. CARP gene and protein structure
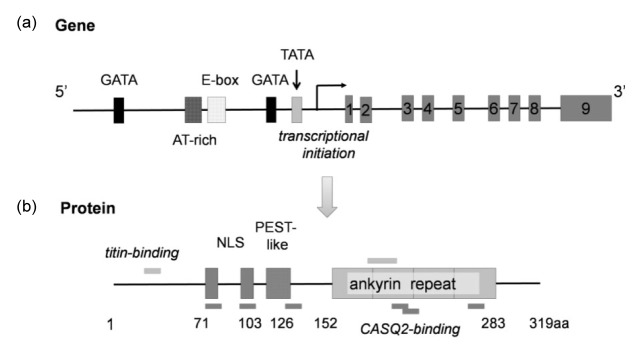
CARP is encoded by the ankyrin repeat domain 1 gene (ankrd1), localized in human chromosome 10q23.31 and chromosome 19C2 in mice. Ankrd1 sequence is highly conserved among different mammalian species, with nine exons and several canonical response elements in the 5'-untranslated region, including the GATA-box, AT-rich, E-box, and TATA-box (Fig. 1). The cDNA of the human ankrd1 is 1901 base pairs long and encodes 319 amino acids (aa) with a molecular weight of 36 kDa (Chu et al., 1995; Jeyaseelan et al., 1997; Zou et al., 1997).
Fig. 1.
Structure of the CARP gene (ankrd1) and translated protein
(a) Ankrd1 sequence has 9 exons and several canonical response elements in the 5'-untranslated region, including the GATA-box, AT-rich, E-box, and TATA-box. (b) The CARP protein has 319 amino acids (aa) consisting of nuclear localization signals (NLSs) (71‒80 aa, 94‒103 aa), PEST-like regions (108‒126 aa), and four ankyrin-like repeats (152‒283 aa). CARP also contains six CASQ-2-binding sites and two titin-binding sites
The CARP protein consists of the nuclear localization signals (NLSs) (71‒80 aa, 94‒103 aa), PEST-like region (108‒126 aa), four ankyrin-like repeats (152‒283 aa), and multiple consensus protein phosphorylation sites (Chu et al., 1995; Jeyaseelan et al., 1997; Zou et al., 1997). The PEST-like region, enriched with proline (P), glutamic acid (E), serine (S), and threonine (T), is involved in rapid mRNA and protein degradation (Rogers et al., 1986). Therefore, it has been expressed in many short-lived proteins, such as G1 cyclins (Evans et al., 1983), c-myc, c-fos (Rogers et al., 1986), and p53 (Gronostajski et al., 1984). Ankyrin repeat protein is a 33-aa sequence motif, mediating protein-protein interactions. It has been identified in many proteins, including cyclin-dependent kinase inhibitors, cytoskeletal organizers, transcriptional regulators, and developmental regulators (Sedgwick and Smerdon, 1999). CARP also contains six calsequestrin-2 (CASQ-2)-binding sites (Torrado et al., 2005) and two titin-binding sites as shown in Fig. 1.
4. Function of CARP
CARP has been identified in both nucleus and cytoplasm, and plays different roles in different subcellular localizations and different cell types. Currently, the role of CARP is primarily characterized in cardiac and muscle tissues.
4.1. A component of muscle sarcomere
CARP is found in the sarcomeric I-band binding to the titin-N2A element as a member of the titin mechanosensory unit (Miller et al., 2003). The giant protein titin, also known as connectin, is anchored in the Z-disk and extends to the M-line region of the sarcomere (Fig. 2). It provides a structural framework through the association with other proteins of the sarcomere, keeps the thick filament centered in the sarcomere during activation, and functions as a molecular spring in the muscle sarcomere, which is involved in myocyte stress-sensing signaling (Labeit et al., 1997; Granzier and Labeit, 2004). CARP plays a crucial role in maintaining sarcomeric integrity, myofibrillar signaling, and stretch sensing in the heart, interacting with other sarcomeric proteins including myopalladin (Bang et al., 2001) and cardiac CASQ-2 (Miller et al., 2004; Torrado et al., 2005). Furthermore, CARP is also expressed in the nucleus function as a transcription co-factor; this dual localization may mediate the communication between the sarcomere and nucleus, transforming the muscle stretch signal to the gene transcription. However, the mechanisms of this process are not currently clear.
Fig. 2.
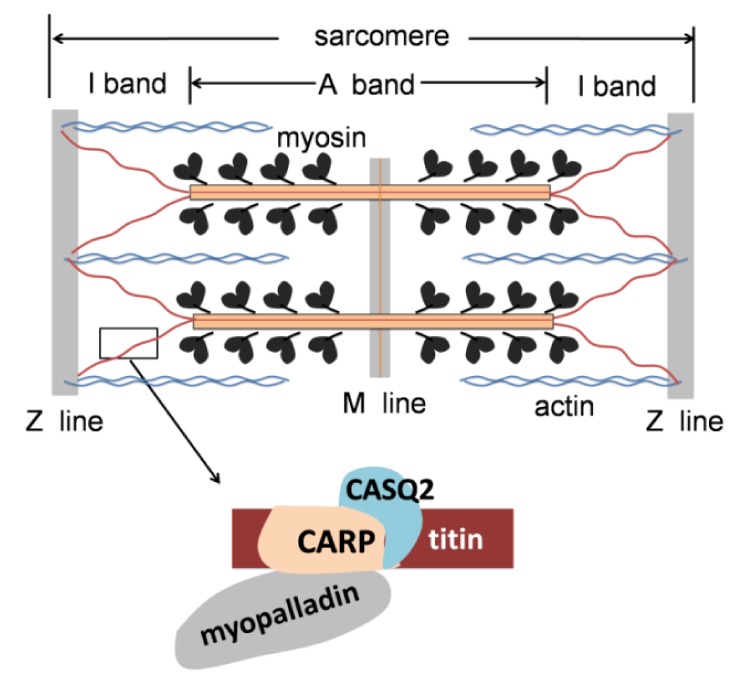
CARP in sarcomere
CARP is bound to the titin-N2A elements in the sarcomeric I-band, interacting with CASQ2 and myopalladin
4.2. Nuclear transcriptional co-factor that negatively regulates cardiac gene expression and cardiac morphogenesis
CARP was initially discovered as a nuclear transcriptional co-factor, which can negatively regulate several cardiac-specific gene expressions, including MLC-2v, atrial natriuretic factor (ANF), and cardiac troponin C (cTnC) (Zou et al., 1997). All of these are cardiac early genes in heart development, and CARP was discovered very early in E7.5 (7.5 d related to the presence of a vaginal plug in females, indicating that the mating occurred), suggesting its role in cardiogenesis. However, contradictory results revealed differences in the MLC-2v and cTnC transcriptions in the muscle LIM protein deficient (MLP−/−) mice, characterized by high levels of CARP (Arber et al., 1997). Therefore, further investigations are required to clarify this issue.
4.3. Enhancing neovascularization
CARP mRNA and protein were found to be dramatically up-regulated in excisional wounds (Shi et al., 2005) and femoral ligation models (Boengler et al., 2003), suggesting their roles in the process of angiogenesis and arteriogenesis. Additionally, CARP was also found to be expressed in the endothelial cells and smooth muscle cells (SMCs) of the collateral artery, even the inflammatory and epithelial cells within the wound. CARP overexpression could induce neovascularization and increase blood perfusion in vivo, and promote human umbilical vein endothelial cell (HUVEC) survival and migration in vitro. These mechanisms may be due to their transcriptional regulatory abilities that activate the expression of the classic angiogenic factors (vascular endothelial growth factor, hepatic growth factor, fibroblast growth factor, etc.) or inhibit the expression of angiogenic inhibitors, or paracrine effects through activating non-vascular cell types such as fibroblasts, leukocytes, or keratinocytes (Shi et al., 2005). CARP may be a new target for stimulating neovascularization in ischemia tissue and wound healing. The mechanisms of CARP-induced neovascularization need to be further clarified.
5. CARP and diseases
5.1. Cardiovascular disease
CARP expression is increased in human heart failure and in different animal models of cardiac hypertrophy. The role of CARP is primarily studied in cardiovascular and muscular diseases.
5.1.1. Cardiac hypertrophy
Cardiac hypertrophy is an adaptive response of increased heart afterload, characterized by an increase in cardiomyocyte size and enhanced cardiac fibroblast synthesis (Frey and Olson, 2003). There is now a wealth of evidence indicating that CARP expression can be markedly induced by various hypertrophic stimuli and in distinct animal models of hypertrophy, including constriction of the abdominal aorta, spontaneously in hypertensive rats and Dahl salt-sensitive rats (Aihara et al., 2000a). Hypertrophic agonists activated p38 and Rac1 expression in mitogen-activated protein kinase (MAPK) pathways, which transcriptionally activate CARP expression through binding the muscle-CAT (M-CAT) elements in the promoter sequence. Of additional note, CARP can be rapidly induced and sustained in cardiac hypertrophy, which is different from other transiently increased hypertrophy-induced transcription factors (c-fos, c-jun, c-myc, and egr-1) (Chien et al., 1993; Sadoshima and Izumo, 1997).
However, CARP overexpression experiments were primarily performed in vitro by transfecting the CARP gene to cardiomyocyte until Song et al. (2012) first generated a cardiac-specific CARP-overexpressing transgenic (CARP Tg) mice, in which they found no differences in the heart function compared with wild-type littermate. They discovered that CARP Tg mice developed less hypertrophy than wild-type mice in cardiac hypertrophy models, including transverse aortic constriction (TAC) and isoproterenol, and concluded that CARP could attenuate cardiac hypertrophy mediated by inhibition of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) and transforming growth factor β (TGF-β) pathways and then decrease fibrosis deposition in the heart. Conversely, Bang et al. (2014) found that CARP is not essential for normal cardiac development and functions in basal conditions and in response to mechanical pressure overload; Chen et al. (2014) demonstrated that CARP promoted cardiomyocyte hypertrophy through calcineurin accumulation.
By analyzing 384 hypertrophic cardiomyopathy (HCM) patients, Arimura et al. (2009) detected three ankrd1 missense mutations (Pro52Ala, Thr123Met, Ile280Val) in HCM patients, and all mutations showed increased binding of CARP to both titin and myopalladin. These findings suggest that the binding of sarcomeric CARP to titin and myopalladin plays a pivotal role in maintaining the cardiac function. Subsequently, Crocini et al. (2013) investigated the effects of HCM-associated mutations on contraction ability after gene transfer in engineered heart tissues, providing evidence that CARP mutations influenced myocyte contractions through different mechanisms.
5.1.2. Dilated cardiomyopathy
Ankrd1 was identified as a new gene associated with dilated cardiomyopathy (DCM) by two labs independently (Duboscq-Bidot et al., 2009; Moulik et al., 2009). Genetic and functional analyses in cardiomyocytes demonstrated that ankrd1 mutations can impair CARP’s nuclear function. Similarly, three missense heterozygous ankrd1 mutations (P105S, V107L, M184I) were discovered in 4 DCM patients after screening for mutations of ankrd1 in 208 DCM patients (Moulik et al., 2009). While performing in vitro functional assays, Moulik et al. (2009) found that the CARP mutations altered CARP-associated protein interactions and expressions of proteins involved in key cellular pathways, such as cell cycle (p53, myogenin), apotosis (p53), growth (TGF-β, early growth response protein 1), and calcium signaling proteins (troponin T, CASQ2) when compared with wild-type CARP. These mutations resulted in disruption of the normal cardiac stretch-based signaling, providing a new pathway associated with DCM in addition to the abnormalities in structural components of the sarcomere and cytoskeleton. However, because their experiments were performed using in vitro models, these findings still need to be evaluated in animal models.
5.1.3. Adriamycin (doxorubicin)-induced cardiomyopathy
Adriamycin (DOX) is an effective chemotherapeutic agent used frequently to treat many human neoplasms, including breast cancer, leukemia, and sarcomas (Bristow et al., 1978). However, severe cardiotoxicity of DOX limits its clinical use (Steinherz et al., 1991; Singal and Iliskovic, 1998). The proposed mechanism for DOX-induced cardiomyopathy is the production of reactive oxygen species in cardiomyocyte mitochondria (Doroshow et al., 1980; Yen et al., 1996; Zhou et al., 2001). The characteristic features of DOX-induced cardiomyopathy are the loss of myofibrils and the vacuolization of cardiac myocytes (Singal and Iliskovic, 1998).
Previous studies demonstrated that DOX also depleted GATA4 expression in cardiomyocytes, and preservation of GATA4 levels prevented DOX-induced cardiomyocyte death (Kim et al., 2003; Aries et al., 2004). CARP was once called the cardiac adriamycin-responsive protein, because of the fact that its expression is down-regulated in response to adriamycin (Jeyaseelan et al., 1997), which was confirmed in subsequent studies (Aihara et al., 2000b). Of note, CARP was reported as a downstream target of GATA4 (Kuo et al., 1999; Kim et al., 2003; Chen et al., 2012). Therefore, GATA4 and CARP would be therapeutic targets in DOX-induced cardiomyopathy.
5.1.4. Cardiac ischemia injury and myocardial apoptosis
Hypoxia and ischemia/reperfusion (I/R) injuries in neonatal rat cardiomyocytes and I/R rat hearts can induce apoptosis-related gene GADD153 overexpression, resulting in the down-regulation of CARP (Han et al., 2005; Lee et al., 2009). These studies demonstrated that hypoxia could down-regulate CARP expression in cardiomyocytes through GADD153. Conversely, CARP was found to be significantly increased in the swine model of transient ischemia (Depre et al., 2001). These studies consistently suggested the protective role of CARP in cardiac ischemia injuries. However, a recent study revealed that overexpression of CARP enhanced cardiomyocyte apoptosis by promoting p53 activation and mitochondrial dysfunction in rodents (Shen et al., 2015). The exact role of CARP on myocardial apoptosis needs further studies to clarify the initial results.
5.1.5. Heart failure
CARP mRNA and protein levels were markedly increased in the canine model of pacing-induced heart failure and human heart failure due to dilated or ischemic cardiomyopathy; however, it should be noted that this study only examined left ventricle-derived specimens (Zolk et al., 2002). Subsequently, overcoming this limitation, it was shown that CARP down-regulated in atria and up-regulated in ventricles were evident in diastolic heart failure, while systolic heart failure results in up-regulation in both atria and ventricles occurring in the pig heart failure model. Interestingly, CARP presented a left-right asymmetric distribution with protein levels higher in the left as compared to the right ventricle (Torrado et al., 2004; 2006). It still obscures the role and mechanisms of CARP asymmetric distribution in heart failure.
5.1.6. Atherosclerosis
It was recently reported that CARP was involved in inhibition of atherosclerotic lesion formation (de Waard et al., 2003). CARP expression was observed in endothelial cells and quiescent intimal SMCs in human plaque. CARP-expressing SMCs are different from medical activated SMCs. However, CARP is not identified in quiescent SMCs in healthy vessels. Furthermore, TGF-β could activate CARP expression to inhibit the proliferation of vascular smooth muscle cells (VSMCs) (Kanai et al., 2001). Collectively, these data suggest that CARP might be involved in the transition of activated SMCs into quiescent SMCs, thereby inhibiting the plaque progress.
5.2. Muscle disease
In the skeletal muscle, CARP expression is found to be low under basal conditions but could be induced in several circumstances such as exercise, muscular atrophy, amyotrophic lateral sclerosis (ALS), and other muscle pathologies (Carson et al., 2002; Tsukamoto et al., 2002; Nakada et al., 2003a; 2003b; Barash et al., 2004; Witt et al., 2004; Hentzen et al., 2006). In transient or definitive denervation-induced muscle atrophy and different muscular dystrophy models, the expression of CARP was persistently up-regulated, suggesting that it is a hub protein involved in the muscular pathological pathway. To further understand its contribution to muscle diseases, adenovirus fused with CARP coding sequence was injected into the tibial anterior muscle of normal mice. The results demonstrated no difference in muscle weight or histological appearance compared with the untreated mice, but slow-twitch fiber was reduced, suggesting that CARP overexpression in wild-type mice does not induce atrophy, but alters the fiber type composition (Laure et al., 2009). In clinical studies, it has been reported that in congenital myopathies, CARP was expressed in severely damaged myofibers, but not detected in the central core disease (Nakada et al., 2003a). These findings suggest that immunohistochemical evaluation of CARP may be helpful in the diagnosis of some congenital myopathies. Furthermore, CARP was found to be a sensitive and specific marker for rhabdomyosarcoma and it would be attributed in the diagnosis of rhabdomyosarcoma (Ishiguro et al., 2008). However, the mechanism of CARP up-regulation and its exact role in muscular diseases remain obscure, possibly becoming a target for muscular disease therapy.
5.3. Other diseases
CARP mutation was identified in the total anomalous pulmonary venous return disease, a congenital heart defect in which pulmonary veins fail to enter the left atrium and drain instead into the right atrium or one of its venous tributaries. The mutation affected PEST-motif in CARP protein, thus enhancing the stability of the CARP protein (Cinquetti et al., 2008). Increased CARP expression was also found in renal podocytes positively correlated with the severity of proteinuria in patients with lupus nephritis (Matsuura et al., 2007) and cisplatin resistance in ovarian cancer chemotherapy (Scurr et al., 2008), and CARP represents a novel target to sensitize tumors to platinum-based drugs (Lei et al., 2015). Gene expression profiling following a crushing injury of the peripheral and central dorsal root ganglion neurons suggests that CARP expression is necessary for nerve regeneration (Stam et al., 2007).
6. Conclusions
CARP is a multifunctional protein, which acts as a nucleus transcriptional co-factor negatively regulating cardiac genes and plays a significant role in different heart diseases, and is a component of sarcomere. Due to the important and multifunctional role of CARP, it could become a new diagnostic marker and therapeutic target of cardiovascular and muscle diseases after determining its role and understanding the mechanisms underlying the various roles it can assume.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31171392)
Compliance with ethics guidelines: Na ZHANG, Xiao-jie XIE, and Jian-an WANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Aihara Y, Kurabayashi M, Arai M, et al. Molecular cloning of rabbit CARP cDNA and its regulated expression in adriamycin-cardiomyopathy. Biochim Biophys Acta. 1999;1447(2-3):318–324. doi: 10.1016/s0167-4781(99)00171-2. (Available from: http://dx.doi.org/10.1016/S0167-4781(99)00171-2) [DOI] [PubMed] [Google Scholar]
- 2.Aihara Y, Kurabayashi M, Saito Y, et al. Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy: role of M-CAT element within the promoter. Hypertension. 2000;36(1):48–53. doi: 10.1161/01.hyp.36.1.48. (Available from: http://dx.doi.org/10.1161/01.HYP.36.1.48) [DOI] [PubMed] [Google Scholar]
- 3.Aihara Y, Kurabayashi M, Tanaka T, et al. Doxorubicin represses CARP gene transcription through the generation of oxidative stress in neonatal rat cardiac myocytes: possible role of serine/threonine kinase-dependent pathways. J Mol Cell Cardiol. 2000;32(8):1401–1414. doi: 10.1006/jmcc.2000.1173. (Available from: http://dx.doi.org/10.1006/jmcc.2000.1173) [DOI] [PubMed] [Google Scholar]
- 4.Arber S, Hunter JJ, Ross JJr, et al. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88(3):393–403. doi: 10.1016/s0092-8674(00)81878-4. (Available from: http://dx.doi.org/10.1016/S0092-8674(00)81878-4) [DOI] [PubMed] [Google Scholar]
- 5.Aries A, Paradis P, Lefebvre C, et al. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. PNAS. 2004;101(18):6975–6980. doi: 10.1073/pnas.0401833101. (Available from: http://dx.doi.org/10.1073/pnas.0401833101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arimura T, Bos JM, Sato A, et al. Cardiac ankyrin repeat protein gene (ANKRD1) mutations in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54(4):334–342. doi: 10.1016/j.jacc.2008.12.082. (Available from: http://dx.doi.org/10.1016/j.jacc.2008.12.082) [DOI] [PubMed] [Google Scholar]
- 7.Bang ML, Mudry RE, McElhinny AS, et al. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001;153(2):413–428. doi: 10.1083/jcb.153.2.413. (Available from: http://dx.doi.org/10.1083/jcb.153.2.413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang ML, Gu Y, Dalton ND, et al. The muscle ankyrin repeat proteins CARP, Ankrd2, and DARP are not essential for normal cardiac development and function at basal conditions and in response to pressure overload. PLoS ONE. 2014;9(4):e93638. doi: 10.1371/journal.pone.0093638. (Available from: http://dx.doi.org/10.1371/journal.pone.0093638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barash IA, Mathew L, Ryan AF, et al. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol. 2004;286(2):C355–C364. doi: 10.1152/ajpcell.00211.2003. (Available from: http://dx.doi.org/10.1152/ajpcell.00211.2003) [DOI] [PubMed] [Google Scholar]
- 10.Baumeister A, Arber S, Caroni P. Accumulation of muscle ankyrin repeat protein transcript reveals local activation of primary myotube endcompartments during muscle morphogenesis. J Cell Biol. 1997;139(5):1231–1242. doi: 10.1083/jcb.139.5.1231. (Available from: http://dx.doi.org/10.1083/jcb.139.5.1231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boengler K, Pipp F, Fernandez B, et al. Arteriogenesis is associated with an induction of the cardiac ankyrin repeat protein (carp) Cardiovasc Res. 2003;59(3):573–581. doi: 10.1016/s0008-6363(03)00511-x. (Available from: http://dx.doi.org/10.1016/S0008-6363(03)00511-X) [DOI] [PubMed] [Google Scholar]
- 12.Bristow MR, Billingham ME, Mason JW, et al. Clinical spectrum of anthracycline antibiotic cardiotoxicity. Cancer Treat Rep. 1978;62(6):873–879. [PubMed] [Google Scholar]
- 13.Carson JA, Nettleton D, Reecy JM. Differential gene expression in the rat soleus muscle during early work overload-induced hypertrophy. FASEB J. 2002;16(2):207–209. doi: 10.1096/fj.01-0544fje. (Available from: http://dx.doi.org/10.1096/fj.01-0544fje) [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Zhong L, Roush SF, et al. Disruption of a GATA4/Ankrd1 signaling axis in cardiomyocytes leads to sarcomere disarray: implications for anthracycline cardiomyopathy. PLoS ONE. 2012;7(4):e35743. doi: 10.1371/journal.pone.0035743. (Available from: http://dx.doi.org/10.1371/journal.pone.0035743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Shen L, Cao S, et al. Cytosolic CARP promotes angiotensin II- or pressure overload-induced cardiomyocyte hypertrophy through calcineurin accumulation. PLoS ONE. 2014;9(8):e104040. doi: 10.1371/journal.pone.0104040. (Available from: http://dx.doi.org/10.1371/journal.pone.0104040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chien KR, Zhu H, Knowlton KU, et al. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol. 1993;55:77–95. doi: 10.1146/annurev.ph.55.030193.000453. (Available from: http://dx.doi.org/10.1146/annurev.ph.55.030193.000453) [DOI] [PubMed] [Google Scholar]
- 17.Chu W, Burns DK, Swerlick RA, et al. Identification and characterization of a novel cytokine-inducible nuclear protein from human endothelial cells. J Biol Chem. 1995;270(17):10236–10245. doi: 10.1074/jbc.270.17.10236. (Available from: http://dx.doi.org/10.1074/jbc.270.17.10236) [DOI] [PubMed] [Google Scholar]
- 18.Cinquetti R, Badi I, Campione M, et al. Transcriptional deregulation and a missense mutation define ANKRD1 as a candidate gene for total anomalous pulmonary venous return. Hum Mutat. 2008;29(4):468–474. doi: 10.1002/humu.20711. (Available from: http://dx.doi.org/10.1002/humu.20711) [DOI] [PubMed] [Google Scholar]
- 19.Crocini C, Arimura T, Reischmann S, et al. Impact of ANKRD1 mutations associated with hypertrophic cardiomyopathy on contraction parameters of engineered heart tissue. Basic Res Cardiol. 2013;108(3):349. doi: 10.1007/s00395-013-0349-x. (Available from: http://dx.doi.org/10.1007/s00395-013-0349-x) [DOI] [PubMed] [Google Scholar]
- 20.Depre C, Tomlinson JE, Kudej RK, et al. Gene program for cardiac cell survival induced by transient ischemia in conscious pigs. PNAS. 2001;98(16):9336–9341. doi: 10.1073/pnas.171297498. (Available from: http://dx.doi.org/10.1073/pnas.171297498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Waard V, van Achterberg TA, Beauchamp NJ, et al. Cardiac ankyrin repeat protein (CARP) expression in human and murine atherosclerotic lesions: activin induces CARP in smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23(1):64–68. doi: 10.1161/01.atv.0000042218.13101.50. (Available from: http://dx.doi.org/10.1161/01.ATV.0000042218.13101.50) [DOI] [PubMed] [Google Scholar]
- 22.Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980;65(1):128–135. doi: 10.1172/JCI109642. (Available from: http://dx.doi.org/10.1172/JCI109642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duboscq-Bidot L, Charron P, Ruppert V, et al. Mutations in the ANKRD1 gene encoding CARP are responsible for human dilated cardiomyopathy. Eur Heart J. 2009;30(17):2128–2136. doi: 10.1093/eurheartj/ehp225. (Available from: http://dx.doi.org/10.1093/eurheartj/ehp225) [DOI] [PubMed] [Google Scholar]
- 24.Evans T, Rosenthal ET, Youngblom J, et al. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. (Available from: http://dx.doi.org/10.1016/0092-8674(83)90420-8) [DOI] [PubMed] [Google Scholar]
- 25.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Ann Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. (Available from: http://dx.doi.org/10.1146/annurev.physiol.65.092101.142243) [DOI] [PubMed] [Google Scholar]
- 26.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res. 2004;94(3):284–295. doi: 10.1161/01.RES.0000117769.88862.F8. (Available from: http://dx.doi.org/10.1161/01.RES.0000117769.88862.F8) [DOI] [PubMed] [Google Scholar]
- 27.Gronostajski RM, Goldberg AL, Pardee AB. Energy requirement for degradation of tumor-associated protein p53. Mol Cell Biol. 1984;4(3):442–448. doi: 10.1128/mcb.4.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han XJ, Chae JK, Lee MJ, et al. Involvement of GADD153 and cardiac ankyrin repeat protein in hypoxia-induced apoptosis of H9c2 cells. J Biol Chem. 2005;280(24):23122–23129. doi: 10.1074/jbc.M501095200. (Available from: http://dx.doi.org/10.1074/jbc.M501095200) [DOI] [PubMed] [Google Scholar]
- 29.Hentzen ER, Lahey M, Peters D, et al. Stress-dependent and -independent expression of the myogenic regulatory factors and the MARP genes after eccentric contractions in rats. J Physiol. 2006;570(Pt 1):157–167. doi: 10.1113/jphysiol.2005.093005. (Available from: http://dx.doi.org/10.1113/jphysiol.2005.093005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda K, Emoto N, Matsuo M, et al. Molecular identification and characterization of a novel nuclear protein whose expression is up-regulated in insulin-resistant animals. J Biol Chem. 2003;278(6):3514–3520. doi: 10.1074/jbc.M204563200. (Available from: http://dx.doi.org/10.1074/jbc.M204563200) [DOI] [PubMed] [Google Scholar]
- 31.Ishiguro N, Motoi T, Araki N, et al. Expression of cardiac ankyrin repeat protein, CARP, in malignant tumors: diagnostic use of CARP protein immunostaining in rhabdomyosarcoma. Hum Pathol. 2008;39(11):1673–1679. doi: 10.1016/j.humpath.2008.04.009. (Available from: http://dx.doi.org/10.1016/j.humpath.2008.04.009) [DOI] [PubMed] [Google Scholar]
- 32.Jeyaseelan R, Poizat C, Baker RK, et al. A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem. 1997;272(36):22800–22808. doi: 10.1074/jbc.272.36.22800. (Available from: http://dx.doi.org/10.1074/jbc.272.36.22800) [DOI] [PubMed] [Google Scholar]
- 33.Kanai H, Tanaka T, Aihara Y, et al. Transforming growth factor-β/Smads signaling induces transcription of the cell type-restricted ankyrin repeat protein CARP gene through CAGA motif in vascular smooth muscle cells. Circ Res. 2001;88(1):30–36. doi: 10.1161/01.res.88.1.30. (Available from: http://dx.doi.org/10.1161/01.RES.88.1.30) [DOI] [PubMed] [Google Scholar]
- 34.Kim Y, Ma AG, Kitta K, et al. Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Mol Pharmacol. 2003;63(2):368–377. doi: 10.1124/mol.63.2.368. (Available from: http://dx.doi.org/10.1124/mol.63.2.368) [DOI] [PubMed] [Google Scholar]
- 35.Kuo H, Chen J, Ruiz-Lozano P, et al. Control of segmental expression of the cardiac-restricted ankyrin repeat protein gene by distinct regulatory pathways in murine cardiogenesis. Development. 1999;126(19):4223–4234. doi: 10.1242/dev.126.19.4223. [DOI] [PubMed] [Google Scholar]
- 36.Labeit S, Kolmerer B, Linke WA. The giant protein titin. Emerging roles in physiology and pathophysiology. Circ Res. 1997;80(2):290–294. doi: 10.1161/01.res.80.2.290. (Available from: http://dx.doi.org/10.1161/01.RES.80.2.290) [DOI] [PubMed] [Google Scholar]
- 37.Laure L, Suel L, Roudaut C, et al. Cardiac ankyrin repeat protein is a marker of skeletal muscle pathological remodelling. FEBS J. 2009;276(3):669–684. doi: 10.1111/j.1742-4658.2008.06814.x. (Available from: http://dx.doi.org/10.1111/j.1742-4658.2008.06814.x) [DOI] [PubMed] [Google Scholar]
- 38.Lee MJ, Kwak YK, You KR, et al. Involvement of GADD153 and cardiac ankyrin repeat protein in cardiac ischemia-reperfusion injury. Exp Mol Med. 2009;41(4):243–252. doi: 10.3858/emm.2009.41.4.027. (Available from: http://dx.doi.org/10.3858/emm.2009.41.4.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei Y, Henderson BR, Emmanuel C, et al. Inhibition of ANKRD1 sensitizes human ovarian cancer cells to endoplasmic reticulum stress-induced apoptosis. Oncogene. 2015;34(4):485–495. doi: 10.1038/onc.2013.566. (Available from: http://dx.doi.org/10.1038/onc.2013.566) [DOI] [PubMed] [Google Scholar]
- 40.Ma G, Wang H, Li Y, et al. Cloning, expression, and bioinformatics analysis of the sheep CARP gene. Mol Cell Biochem. 2013;378(1-2):29–37. doi: 10.1007/s11010-013-1590-1. (Available from: http://dx.doi.org/10.1007/s11010-013-1590-1) [DOI] [PubMed] [Google Scholar]
- 41.Matsuura K, Uesugi N, Hijiya N, et al. Upregulated expression of cardiac ankyrin-repeated protein in renal podocytes is associated with proteinuria severity in lupus nephritis. Hum Pathol. 2007;38(3):410–419. doi: 10.1016/j.humpath.2006.09.006. (Available from: http://dx.doi.org/10.1016/j.humpath.2006.09.006) [DOI] [PubMed] [Google Scholar]
- 42.Miller MK, Bang ML, Witt CC, et al. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol. 2003;333(5):951–964. doi: 10.1016/j.jmb.2003.09.012. (Available from: http://dx.doi.org/10.1016/j.jmb.2003.09.012) [DOI] [PubMed] [Google Scholar]
- 43.Miller MK, Granzier H, Ehler E, et al. The sensitive giant: the role of titin-based stretch sensing complexes in the heart. Trends Cell Biol. 2004;14(3):119–126. doi: 10.1016/j.tcb.2004.01.003. (Available from: http://dx.doi.org/10.1016/j.tcb.2004.01.003) [DOI] [PubMed] [Google Scholar]
- 44.Moulik M, Vatta M, Witt SH, et al. ANKRD1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J Am Coll Cardiol. 2009;54(4):325–333. doi: 10.1016/j.jacc.2009.02.076. (Available from: http://dx.doi.org/10.1016/j.jacc.2009.02.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakada C, Oka A, Nonaka I, et al. Cardiac ankyrin repeat protein is preferentially induced in atrophic myofibers of congenital myopathy and spinal muscular atrophy. Pathol Int. 2003;53(10):653–658. doi: 10.1046/j.1440-1827.2003.01541.x. (Available from: http://dx.doi.org/10.1046/j.1440-1827.2003.01541.x) [DOI] [PubMed] [Google Scholar]
- 46.Nakada C, Tsukamoto Y, Oka A, et al. Cardiac-restricted ankyrin-repeated protein is differentially induced in duchenne and congenital muscular dystrophy. Lab Invest. 2003;83(5):711–719. doi: 10.1097/01.lab.0000067484.35298.1a. [DOI] [PubMed] [Google Scholar]
- 47.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234(4774):364–368. doi: 10.1126/science.2876518. (Available from: http://dx.doi.org/10.1126/science.2876518) [DOI] [PubMed] [Google Scholar]
- 48.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Ann Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. (Available from: http://dx.doi.org/10.1146/annurev.physiol.59.1.551) [DOI] [PubMed] [Google Scholar]
- 49.Scurr LL, Guminski AD, Chiew YE, et al. Ankyrin repeat domain 1, ANKRD1, a novel determinant of cisplatin sensitivity expressed in ovarian cancer. Clin Cancer Res. 2008;14(21):6924–6932. doi: 10.1158/1078-0432.CCR-07-5189. (Available from: http://dx.doi.org/10.1158/1078-0432.CCR-07-5189) [DOI] [PubMed] [Google Scholar]
- 50.Sedgwick SG, Smerdon SJ. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci. 1999;24(8):311–316. doi: 10.1016/s0968-0004(99)01426-7. (Available from: http://dx.doi.org/10.1016/S0968-0004(99)01426-7) [DOI] [PubMed] [Google Scholar]
- 51.Shen L, Chen C, Wei X, et al. Overexpression of ankyrin repeat domain 1 enhances cardiomyocyte apoptosis by promoting p53 activation and mitochondrial dysfunction in rodents. Clin Sci. 2015;128(10):665–678. doi: 10.1042/CS20140586. (Available from: http://dx.doi.org/10.1042/CS20140586) [DOI] [PubMed] [Google Scholar]
- 52.Shi Y, Reitmaier B, Regenbogen J, et al. CARP, a cardiac ankyrin repeat protein, is up-regulated during wound healing and induces angiogenesis in experimental granulation tissue. Am J Pathol. 2005;166(1):303–312. doi: 10.1016/S0002-9440(10)62254-7. (Available from: http://dx.doi.org/10.1016/S0002-9440(10)62254-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. New Engl J Med. 1998;339(13):900–905. doi: 10.1056/NEJM199809243391307. (Available from: http://dx.doi.org/10.1056/NEJM199809243391307) [DOI] [PubMed] [Google Scholar]
- 54.Song Y, Xu J, Li Y, et al. Cardiac ankyrin repeat protein attenuates cardiac hypertrophy by inhibition of ERK1/2 and TGF-β signaling pathways. PLoS ONE. 2012;7(12):e50436. doi: 10.1371/journal.pone.0050436. (Available from: http://dx.doi.org/10.1371/journal.pone.0050436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stam FJ, MacGillavry HD, Armstrong NJ, et al. Identification of candidate transcriptional modulators involved in successful regeneration after nerve injury. Eur J Neurosci. 2007;25(12):3629–3637. doi: 10.1111/j.1460-9568.2007.05597.x. (Available from: http://dx.doi.org/10.1111/j.1460-9568.2007.05597.x) [DOI] [PubMed] [Google Scholar]
- 56.Steinherz LJ, Steinherz PG, Tan CT, et al. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266(12):1672–1677. (Available from: http://dx.doi.org/10.1001/jama.1991.03470120074036) [PubMed] [Google Scholar]
- 57.Torrado M, López E, Centeno A, et al. Left-right asymmetric ventricular expression of CARP in the piglet heart: regional response to experimental heart failure. Eur J Heart Fail. 2004;6(2):161–172. doi: 10.1016/j.ejheart.2003.11.004. (Available from: http://dx.doi.org/10.1016/j.ejheart.2003.11.004) [DOI] [PubMed] [Google Scholar]
- 58.Torrado M, Nespereira B, López E, et al. ANKRD1 specifically binds CASQ2 in heart extracts and both proteins are co-enriched in piglet cardiac Purkinje cells. J Mol Cell Cardiol. 2005;38(2):353–365. doi: 10.1016/j.yjmcc.2004.11.034. (Available from: http://dx.doi.org/10.1016/j.yjmcc.2004.11.034) [DOI] [PubMed] [Google Scholar]
- 59.Torrado M, Nespereira B, Bouzamayor Y, et al. Differential atrial versus ventricular ANKRD1 gene expression is oppositely regulated at diastolic heart failure. FEBS Lett. 2006;580(17):4182–4187. doi: 10.1016/j.febslet.2006.06.073. (Available from: http://dx.doi.org/10.1016/j.febslet.2006.06.073) [DOI] [PubMed] [Google Scholar]
- 60.Tsukamoto Y, Senda T, Nakano T, et al. Arpp, a new homolog of carp, is preferentially expressed in type 1 skeletal muscle fibers and is markedly induced by denervation. Lab Invest. 2002;82(5):645–655. doi: 10.1038/labinvest.3780459. [DOI] [PubMed] [Google Scholar]
- 61.Witt CC, Ono Y, Puschmann E, et al. Induction and myofibrillar targeting of CARP, and suppression of the Nkx2.5 pathway in the MDM mouse with impaired titin-based signaling. J Mol Biol. 2004;336(1):145–154. doi: 10.1016/j.jmb.2003.12.021. (Available from: http://dx.doi.org/10.1016/j.jmb.2003.12.021) [DOI] [PubMed] [Google Scholar]
- 62.Yen HC, Oberley TD, Vichitbandha S, et al. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest. 1996;98(5):1253–1260. doi: 10.1172/JCI118909. (Available from: http://dx.doi.org/10.1172/JCI118909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou S, Palmeira CM, Wallace KB. Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol Lett. 2001;121(3):151–157. doi: 10.1016/s0378-4274(01)00329-0. (Available from: http://dx.doi.org/10.1016/S0378-4274(01)00329-0) [DOI] [PubMed] [Google Scholar]
- 64.Zolk O, Frohme M, Maurer A, et al. Cardiac ankyrin repeat protein, a negative regulator of cardiac gene expression, is augmented in human heart failure. Biochem Biophys Res Commun. 2002;293(5):1377–1382. doi: 10.1016/S0006-291X(02)00387-X. (Available from: http://dx.doi.org/10.1016/S0006-291X(02)00387-X) [DOI] [PubMed] [Google Scholar]
- 65.Zou Y, Evans S, Chen J, et al. CARP, a cardiac ankyrin repeat protein, is downstream in the Nkx2-5 homeobox gene pathway. Development. 1997;124(4):793–804. doi: 10.1242/dev.124.4.793. [DOI] [PubMed] [Google Scholar]