Abstract
The antioxidant activities and total phenolic content of fermented Salvia miltiorrhiza with fungus Geomyces luteus were investigated. The results revealed that G. luteus fermentation could significantly improve the antioxidant activity and total phenolic content of S. miltiorrhiza. The main antioxidant constituents were characterized by spectroscopic analysis as salvianolic acids. High-performance liquid chromatography (HPLC) quantification also showed the enhanced content of salvianolic acid B after fermentation. The present study suggests that G. luteus fermentations are effective in the S. miltiorrhiza salvianolic acids’ enrichment process.
Keywords: Salvia miltiorrhiza, Geomyces luteus, Salvianolic acids, Antioxidant, Fermentation
1. Introduction
Salvia miltiorrhiza Bunge (Labiatae), known as Danshen and first recorded in Shennong Bencaojing (an ancient herbalism in China), has long been used to treat cerebrovascular and cardiovascular diseases. It is listed in the Chinese Pharmacopeia. Modern pharmacological studies revealed that S. miltiorrhiza could activate blood circulation to dissipate blood stasis, alleviate pain, and treat inflammatory diseases (Zhou et al., 2005), which may be because of its large number of bioactive ingredients, including diterpene, chinone, and phenolic acids (Li, 1997). Salvianolic acids, the main polyphenolic acids in S. miltiorrhiza (Sun et al., 2009), produce a variety of pharmacological activities such as reducing atherosclerosis, inhibiting formation of low-density lipoprotein, and prohibiting the aggregation of platelets, which has long stimulated scientists’ strong interest (Yang et al., 2010). Plant phenolics, particularly phenolic acids, are known to be potent antioxidants, and can effectively scavenge free radicals and cut off radical chain reactions (Duan et al., 2006). Previous studies revealed that a certain amount of phenolics possessing significant antioxidant activity exist in S. miltiorrhiza (Zhang et al., 2010), and these be vital contributors to the pharmacological activity of S. miltiorrhiza.
Traditional Chinese medicine (TCM) has played a significant role in healing for a very long time, but recently scientific evidence regarding the better utilization of the resources of TCM has been needed. Fermented traditional medicine (FTM) has garnered attention for its enhanced biological activity compared to traditional medicine (Wei et al., 2007; Okabe et al., 2011). Several studies using single herbs and fungi support the availability and efficacy of FTM. Chen and Chen (2000) had reported that yeast elicitors increased the cryptotanshinone production of the S. miltiorrhiza extracts. Coprinus comatus fermentation enhanced the anti-inflammatory properties of Sophora flavescens (Han et al., 2011).
In the present study, S. miltiorrhiza was fermented with Geomyces luteus and 19 other fungi. The antioxidant activities of fermented S. miltiorrhiza were evaluated with 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) assays. Subsequently, the total phenolic contents (TPCs) of fermented S. miltiorrhiza were measured. Extensive spectroscopic analysis (ultraviolet (UV), infrared (IR), and nuclear magnetic resonance (NMR)) and the high-performance liquid chromatography (HPLC) method were employed for the characterization and quantitative analysis of the main antioxidant constituents of fermented S. miltiorrhiza. This paper explores a new method to enrich the salvianolic acids in S. miltiorrhiza by microorganism fermentation.
2. Materials and methods
2.1. Materials, chemicals, and instruments
Trolox, salvianolic acid B, ABTS, and DPPH were obtained from J&K Chemical (Beijing, China). Gallic acid and rutin were purchased from Aladdin-Reagent (Shanghai, China). Deuterated methanol (CD3OD) and dimethylsulfoxide (DMSO) were obtained from Sigma-Aldrich (Shanghai, China). Deionized water (resistivity ≥18.25 MΩ/cm) was acquired with a Youpu purity water system (Chengdu, China). Acetonitrile and CH3OH (HPLC grade) were supplied by Fisher Scientific (NJ, USA). All other reagents were of analytical grade.
Roots of S. miltiorrhiza were obtained from Kunming City, China in October 2013. The sample was authenticated by Professor Shu-gang LU at the School of Life Science in Yunnan University. A voucher specimen (No. DS01) has been deposited in the Key Laboratory of Medicinal Chemistry for Natural Resources, Yunnan University, China.
2.2. Microorganisms and fermentation
G. luteus and 19 other fungi (Talaromyces purpurogenus, Penicillium vancouverense, Alternaria tenuissima, Chaetomium globosum, Penicillium swiecickii, Arthrinium phaeospermum, Stagonosporopsis cucurbitacearum, Arthrinium arundinis, Scytalidium lignicola, Fusarium oxysporum, Clonostachys rogersoniana, Alternaria compacta, Cladosporium cladosporioides, Arthrinium sacchari, Stagonosporopsis chrysanthemi, Botrytis cinerea, Fusarium avenaceum, Helminthosporium maydis, Trichophyton verrucosum) were inoculated in potato dextrose agar slant media in a constant temperature incubator at 28.0 °C for 7 d. Dried and powdered roots of S. miltiorrhiza (5.0 g) were added to 150 ml Erlenmeyer flask to form the solid fermentation culture medium. A total of 11 ml distilled water was added to the medicine to achieve a moisture circumstance, and autoclave-sterilized at 120 °C for 30 min. Then the mature-inoculated slants were added and incubated with S. miltiorrhiza at 28.0 °C for a month, together with a blank fermentation (without added microbe). Each of the samples had 3 other parallel controls.
The original material and microorganism-fermented S. miltiorrhiza were immersed in 50 ml of 80% (v/v) CH3OH-H2O for 24 h, and the extracts were filtered after ultrasonic treatment for 30 min. The extraction was repeated 3 times. The extracts of the samples and parallel controls were concentrated under vacuum to dryness using a rotary evaporator at 60 °C (Heidolph, Germany). The non-fermented S. miltiorrhiza (original material) and S. miltiorrhiza fermented with G. luteus were respectively obtained as NFS (2.5015 g) and GFS (0.7030 g).
2.3. DPPH assay
The DPPH assay was performed according to the method described by He et al. (2015). Briefly, DPPH• solution (3.9 ml, 0.075 mmol/L) was mixed with 0.1 ml of the sample at gradient concentrations (10, 20, 25, 30, 35, 40, 50, and 60 μg/ml). The mixture was shaken and rested at 25 °C for 30 min. The absorbance was determined at 515 nm. The DPPH• scavenging effect was calculated using the following equation: inhibition=(1−A s/A o)×100%, where A s is the absorbance of the sample mixture and A o is the absorbance of the mixture without the sample. The 50% absorbance reduction (IC50) was estimated based on a curve relating concentration to the absorbance of a sample. Rutin was used as a positive reference.
2.4. ABTS assay
The ABTS assay was performed according to the method described by Re et al. (1999). Briefly, 7 mmol/L ABTS solution was mixed with 2.5 mmol/L K2S2O8 solution and incubated in the dark for 12‒16 h to achieve an ABTS+• solution. The ABTS+• solution was diluted with phosphate buffer saline (PBS; 200 mmol/L, pH=7.4) to acquire an initial absorbance of 0.70±0.02 at 734 nm. The 100-μl sample extract was added to 3.9 ml of ABTS+• working solution. After resting at room temperature for 6 min, the absorbance was measured at 734 nm. A standard curve was acquired using Trolox solution at different concentrations in the PBS buffer solution. The ABTS+• scavenging effect was evaluated using the following equation: inhibition=(1−A's/A'o)×100%, where A's is the absorbance of the sample solution mixed with ABTS+• and A'o is the absorbance of blank reaction where the sample was replaced by the PBS buffer solution. The antioxidant capacity was obtained by the standard curve and expressed as Trolox equivalent antioxidant capacity (TEAC). Rutin was used as reference standard.
2.5. Total phenolic content
The TPC was measured by the Folin-Ciocalteau method (Gursoy et al., 2009). Folin-Ciocalteau reagent was prepared by the method described by Huang et al. (2005). Briefly, Folin-Ciocalteau reagent (4.5 ml) was mixed with a 1.0-ml proper concentration sample. After 5 min, 3.0 ml of 7.5% (0.075 g/ml) Na2CO3 was added. After resting at room temperature for 30 min, the absorbance was measured at 765 nm. Gallic acid was used as a standard to prepare for a calibration curve (4, 8, 12, 16, 20, and 24 μg). The TPC was expressed as mg gallic acid equivalents/g dry extract (mg GAE/g Ex).
2.6. Spectroscopic analysis
UV spectra of samples (0.05 mg/ml in 50% (v/v) CH3OH-H2O) were acquired with a Shimadzu UV-Vis 2550 spectrometer (Kyoto, Japan) within the wavelength range of 200 to 600 nm. IR spectra of solid dry samples (0.5 mg) were acquired with a Nicolet Avatar 360 FT-IR spectrometer (Madison, USA) with KBr pellets within the range of 400–4000 cm−1. 1H and 13C NMR spectra were collected using a Bruker AM-500 spectrometer (Karlsruhe, Germany) in CD3OD-D2O (1:1, v/v).
2.7. HPLC analysis
The quantifications of crude extracts of GFS and NFS were achieved by an HPLC system. NFS (2.00 mg/ml), GFS (1.00 mg/ml), and salvianolic acid B (0.20 mg/ml) were dissolved with 50% CH3OH-H2O. Before injection, the samples were passed through a filter (0.45 μm). Mobile phase A was 0.1% aqueous formic acid, and mobile phase B was acetonitrile. The recorded HPLC spectrogram was linearly graduated: 0–10.0 min, 10.0%–20.0% (v/v) solvent B; 10.0–27.0 min, 20.0%–33.0% solvent B; 27.0–30.0 min, 33.0%–70.0% solvent B; 30.0–50.0 min, 70.0%–85.0% solvent B. The column temperature was 20 °C, the flow-rate was 1.0 ml/min, the injection volume was 10 μl, and the UV detection wavelength was 280 nm (Qu et al., 2007).
2.8. Statistical analysis
The results were expressed as the mean±standard deviation (SD) of three replicates for each sample. Statistical analyses were performed with SPSS Version 19.0 (SPSS Inc., Chicago, IL, USA). A significant difference was assessed at a level of P<0.05.
3. Results and discussion
3.1. Antioxidant activity
Two different models of S. miltiorrhiza, DPPH and ABTS assays, were used to assess the antioxidant activity of extracts of S. miltiorrhiza fermented with 20 fungi. As observed in Table 1, the GFS (IC50, (25.0±0.6) μg/ml) exhibited higher DPPH• scavenging capacity than NFS (IC50, (30.9±1.1) μg/ml), but less than the positive control rutin (IC50, (4.32±0.15) μg/ml), which implied that the DPPH• scavenging effect of the S. miltiorrhiza was increased after fermentation by G. luteus.
Table 1.
Total phenolic contents and antioxidant activities
| Sample | TPC (mg GAE/g Ex) | IC50 of DPPH• (μg/ml) | TEAC of ABTS+• (μmol Trolox/g Ex) |
| NFS | 87.9±3.8 | 30.9±1.1 | 435.1±15.3 |
| GFS | 167.8±4.6 | 25.0±0.6 | 1006.6±23.6 |
| Rutin | 4.32±0.15 | 4546.8±79.5 |
Each value is presented as mean±SD (n=3)
The antioxidant activity of extracts of fermented S. miltiorrhiza was also determined as Trolox equivalents using the ABTS+• assay. As shown in Table 1, the TEACs of ABTS+• of NFS, GFS, and rutin were (435.1±15.3), (1006.6±23.6), and (4546.8±79.5) μmol Trolox/g Ex, respectively. Similar to the results of the DPPH• scavenging effect, the higher ABTS+• scavenging activity was obtained after fermentation by G. luteus. In addition, the results indicated that the GFS showed an ABTS value more than twice as high as that of NFS. In conclusion, the above methods gave similar results that the fermentation by G. luteus could effectively enhance the antioxidant activity of S. miltiorrhiza.
3.2. Total phenolic content
Previous studies have revealed that phenolics are the major bioactive components in S. miltiorrhiza (Zhao et al., 2006). In this research, 20 fermentation extracts of S. miltiorrhiza with different fungi were screened for their ability to improve the phenolic content, and the G. luteus fermentation extract of S. miltiorrhiza showed notable improvement in phenolic content. The results revealed that GFS possessed the highest TPC ((167.8±4.7) mg GAE/g Ex), which was about twice as high as that of NFS ((87.9±3.8) mg GAE/g Ex), indicating that some biotransformation might be taking place to improve hydroxyls in the chemical constituents of S. miltiorrhiza.
The improvement of phenolic content in S. miltiorrhiza might be responsible for the increase in antioxidant activity. Previous studies have demonstrated the positive correlation between antioxidant constituents and phenolic content in different plant extracts (Socha et al., 2009), which suggested that the increased antioxidant activity might result from the increasing of phenolic content after fermentation.
3.3. Characterization of the antioxidant compounds of S. miltiorrhiza fermented with G. luteus
The UV-Vis spectrum was used for the characterizations of NFS and GFS (0.05 mg/ml in H2O). As shown in Fig. 1a, the absorption peak at maximal wavelength (λ max) 285 nm was the signal of π→π* electron transition of phenolic acid (Fang and Tan, 2005; Zhang et al., 2009). The Fig. 1b shows that the absorbance (λ max=285 nm) of GFS was much higher than that of NFS, indicating the higher content of phenolic acid in GFS, which was consistent with the results of the TPC and antioxidant activity.
Fig. 1.
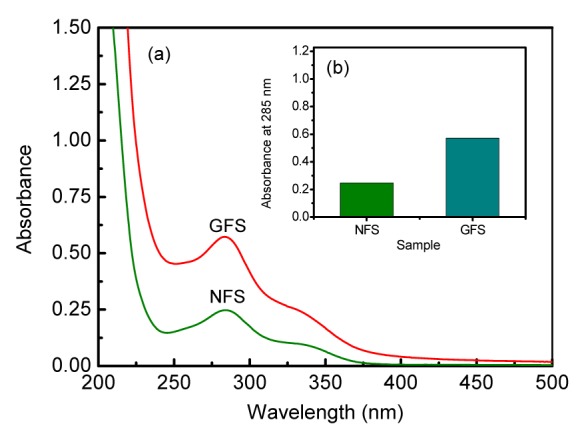
UV-Vis spectra (a) and the absorbance at 285 nm (b) of NFS and GFS
To clarify more biotransformation details of S. miltiorrhiza caused by G. luteus fermentation, the IR spectrum was used for the chemical characterization of GFS compared with NFS. The IR spectrum (Fig. 2) revealed that the band at 1042 cm−1 was the change angle vibration of O–H and the stretching vibration of C–O–C (Chen et al., 2012). The absorption peak at 1042 cm−1 weakened significantly after fermentation, indicating that the hydrolyzation of glucoside and metabolism of saccharides might take place in fermentation processing.
Fig. 2.
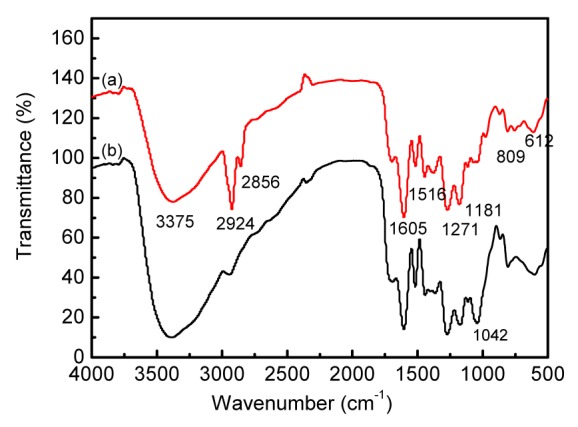
FT-IR spectra of extracts of GFS (a) and NFS (b)
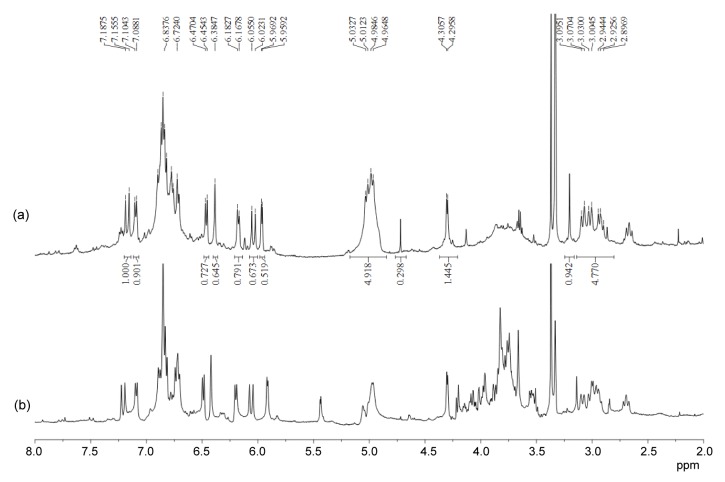
More exploration was performed to identify and characterize the major components of GFS and NFS by NMR spectroscopy (Pieri et al., 2011). The NMR spectra of GFS indicated the presence of the characteristic signals of salvianolic acids (Figs. 3 and 4). In its 1H NMR spectrum (Fig. 3a), δ H 6.0 (d, 1H, J=16 Hz) and 7.1 (d, 1H, J=16 Hz) were assigned to the trans double bond protons of H-7 and H-8, respectively, in unit A of the salvianolic acids (Fig. 5). Its 13C NMR spectrum (Fig. 4a) showed the signals of C-7 and C-8 in unit C at δ C 87.8 and 57.7, respectively. The signals at δ C 38.0, 37.5, 77.8, and 77.1 were assigned to C-7 and C-8 in units B and D, respectively. The chemical shifts at δ C 143.7–148.6 were the typical signals of C-3 and C-4 in units A, B, C, and D, while the signals of δ C 168.9–176.3 were ascribed to C-9 in units A, B, C, and D.
Fig. 3.
1H NMR spectra (400 MHz, CD3OD-D2O) of extracts of GFS (a) and NFS (b) at the same concentration of 20 mg/ml (ppm: δ, 10−6)
Fig. 4.
13C NMR spectra (500 MHz, CD3OD-D2O) of extracts of GFS (a) and NFS (b) at the same concentration of 50 mg/ml (ppm: δ, 10−6)
Fig. 5.
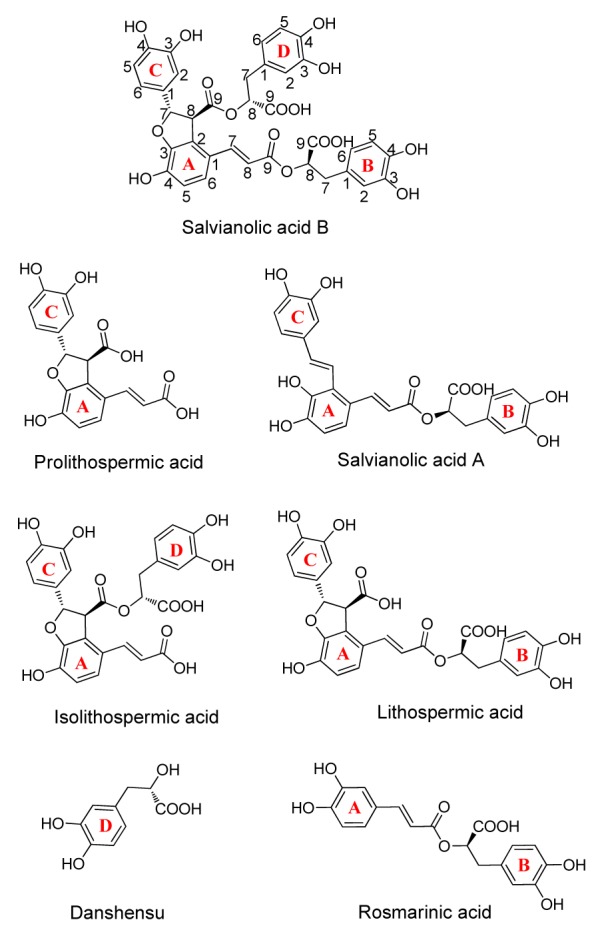
Structures of salvianolic acids
Therefore it can be concluded that GFS was mainly composed of salvianolic acids, including salvianolic acids A and B, prolithospermic acid, isolithospermic acid, lithospermic acid, danshensu, and so on (Fig. 5). Similarly, the NMR spectra of NFS (Figs. 3b and 4b) showed the presence of the signals of salvianolic acids. However, the NMR spectra of NFS revealed the presence of the obvious saccharide signals at δ H 3.5–4.2, 5.1 and δ C 62.2–78.0, 93.2–105.0, which were not observed in the NMR spectra of GFS. These results implied that G. luteus fermentation caused the deglycosylation of phenolic glycosides in S. miltiorrhiza, which led to the improvement of the TPC and antioxidant activity.
3.4. Quantification of GFS and NFS
The chromatographic profiles of GFS and NFS are shown in Fig. 6. The HPLC quantitative data were acquired from the calibration curve. The content of salvianolic acid B in GFS ((95.2±2.5) mg/g Ex) is nearly twice as high as that in NFS ((59.0±1.2) mg/g Ex). The improvement of salvianolic acid B after fermentation may account for the increased TPC of S. miltiorrhiza. The antioxidant activity of salvianolic acid B was determined using DPPH assay with rutin as the positive control. Salvianolic acid B exhibited significantly high DPPH• scavenging activity (IC50, 2.71 μmol/L) and was more effective than rutin (IC50, 7.08 μmol/L), which indicated that the enrichment of salvianolic acid B had a significant contribution to the improvement of the antioxidant abilities.
Fig. 6.
HPLC chromatograms of NFS (a) and GFS (b) at the concentrations of 2 and 1 mg/ml, respectively
4. Conclusions
G. luteus fermentation could significantly improve the antioxidant activity and TPC of S. miltiorrhiza, suggesting that G. luteus fermentation was effective in S. miltiorrhiza processing. This might be attributed to the deglycosylation of phenolic glycosides in S. miltiorrhiza. The main antioxidant constituents were characterized by spectroscopic analysis as salvianolic acids, and the content of salvianolic acid B improved. This study is the first report on the enrichment of salvianolic acids by microbial fermentation.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81460648) and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13095), China
Compliance with ethics guidelines: Yun XING, Le CAI, Tian-peng YIN, Yang CHEN, Jing YU, Ya-rong WANG, and Zhong-tao DING declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Chen H, Chen F. Effects of yeast elicitor on the growth and secondary metabolism of a high-tanshinone-producing line of the Ti transformed Salvia miltiorrhiza cells in suspension culture. Process Biochem. 2000;35(8):837–840. (Available from: http://dx.doi.org/10.1016/S0032-9592(99)00146-6) [Google Scholar]
- 2.Chen Y, Mao W, Yang Y, et al. Structure and antioxidant activity of an extracellular polysaccharide from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Carbohydr Polym. 2012;87(1):218–226. doi: 10.1016/j.carbpol.2011.07.042. (Available from: http://dx.doi.org/10.1016/j.carbpol.2011.07.042) [DOI] [PubMed] [Google Scholar]
- 3.Duan XJ, Zhang WW, Li XM, et al. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata . Food Chem. 2006;95(1):37–43. (Available from: http://dx.doi.org/10.1016/j.foodchem.2004.12.015) [Google Scholar]
- 4.Fang XS, Tan XM. The qualitative analysis and quantitative analysis of purification of salvianolic acids by macroreticular resin. China J Chin Mater Med. 2005;30(17):1331–1334. (in Chinese) [PubMed] [Google Scholar]
- 5.Gursoy N, Sarikurkcu C, Cengiz M, et al. Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species. Food Chem Toxicol. 2009;47(9):2381–2388. doi: 10.1016/j.fct.2009.06.032. (Available from: http://dx.doi.org/10.1016/j.fct.2009.06.032) [DOI] [PubMed] [Google Scholar]
- 6.Han CC, Wei H, Guo JY. Anti-inflammatory effects of fermented and non-fermented Sophora flavescens: a comparative study. BMC Complement Altern Med. 2011;11(1):100. doi: 10.1186/1472-6882-11-100. (Available from: http://dx.doi.org/10.1186/1472-6882-11-100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Yin T, Chen Y, et al. Phenolic compounds and antioxidant activities of edible flowers of Pyrus pashia . J Funct Foods. 2015;17:371–379. (Available from: http://dx.doi.org/10.1016/j.jff.2015.05.045) [Google Scholar]
- 8.Huang DJ, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53(6):1841–1856. doi: 10.1021/jf030723c. (Available from: http://dx.doi.org/10.1021/jf030723c) [DOI] [PubMed] [Google Scholar]
- 9.Li LN. Water soluble active components of Salvia miltiorrhiza and related plants. J Chin Pharm Sci. 1997;6(2):57–64. (in Chinese) [Google Scholar]
- 10.Okabe Y, Shimazu T, Tanimoto H. Higher bioavailability of isoflavones after a single ingestion of aglycone-rich fermented soybeans compared with glucoside-rich non-fermented soybeans in Japanese postmenopausal women. J Sci Food Agric. 2011;91(4):658–663. doi: 10.1002/jsfa.4228. (Available from: http://dx.doi.org/10.1002/jsfa.4228) [DOI] [PubMed] [Google Scholar]
- 11.Pieri V, Belancic A, Morales S, et al. Identification and quantification of major steviol glycosides in Stevia rebaudiana purified extracts by 1H NMR spectroscopy. J Agric Food Chem. 2011;59(9):4378–4384. doi: 10.1021/jf104922q. (Available from: http://dx.doi.org/10.1021/jf104922q) [DOI] [PubMed] [Google Scholar]
- 12.Qu HB, Zhai XJ, Shao Q, et al. Simultaneous determination of seven active compounds in Radix Salviae miltiorrhizae by temperature-controlled ultrasound-assisted extraction and HPLC. Chromatographia. 2007;66(1-2):21–27. (Available from: http://dx.doi.org/10.1365/s10337-007-0244-4) [Google Scholar]
- 13.Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26(9-10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. (Available from: http://dx.doi.org/10.1016/S0891-5849(98)00315-3) [DOI] [PubMed] [Google Scholar]
- 14.Socha R, Juszczak L, Pietrzyk S, et al. Antioxidant activity and phenolic composition of herbhoneys. Food Chem. 2009;113(2):568–574. (Available from: http://dx.doi.org/10.1016/j.foodchem.2008.08.029) [Google Scholar]
- 15.Sun Y, Zhu H, Wang J, et al. Isolation and purification of salvianolic acid A and salvianolic acid B from Salvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. J Chromatogr B. 2009;877(8-9):733–737. doi: 10.1016/j.jchromb.2009.02.013. (Available from: http://dx.doi.org/10.1016/j.jchromb.2009.02.013) [DOI] [PubMed] [Google Scholar]
- 16.Wei QK, Chen TR, Chen JT. Using of Lactobacillus and Bifidobacterium to product the isoflavone aglycones in fermented soymilk. Int J Food Microbiol. 2007;117(1):120–124. doi: 10.1016/j.ijfoodmicro.2007.02.024. (Available from: http://dx.doi.org/10.1016/j.ijfoodmicro.2007.02.024) [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Wang S, Xie Y, et al. Effect of salvianolic acid B and paeonol on blood lipid metabolism and hemorrheology in myocardial ischemia rabbits induced by pituitruin. Int J Mol Sci. 2010;11(10):3696–3704. doi: 10.3390/ijms11103696. (Available from: http://dx.doi.org/10.3390/ijms11103696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang CG, Cui HM, Zhang QY, et al. The stability of salvianolic acid B of extraction of Salvia miltiorrhiza in different pH. Chin J Exp Tradit Med Formul. 2009;15(1): 3-5(in Chinese):3–5 (in Chinese). (Available from: http://dx.chinadoi.cn/10.3969/j.issn.1005-9903.2009.01.002) [Google Scholar]
- 19.Zhang Y, Li X, Wang Z. Antioxidant activities of leaf extract of Salvia miltiorrhiza Bunge and related phenolic constituents. Food Chem Toxicol. 2010;48(10):2656–2662. doi: 10.1016/j.fct.2010.06.036. (Available from: http://dx.doi.org/10.1016/j.fct.2010.06.036) [DOI] [PubMed] [Google Scholar]
- 20.Zhao GR, Xiang ZJ, Ye TX, et al. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng . Food Chem. 2006;99(4):767–774. (Available from: http://dx.doi.org/10.1016/j.foodchem.2005.09.002) [Google Scholar]
- 21.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45(12):1345–1359. doi: 10.1177/0091270005282630. (Available from: http://dx.doi.org/10.1177/0091270005282630) [DOI] [PubMed] [Google Scholar]