Abstract
Objectives
This study evaluated the effects of different surface conditioning methods on the bond strength of orthodontic brackets to glazed full-zirconia surfaces.
Materials and Methods
Glazed zirconia (except for the control, Zirkonzahn Prettau) disc surfaces were pre-treated: PO (control), polishing; BR, bur roughening; PP, cleaning with a prophy cup and pumice; HF, hydrofluoric acid etching; AA, air abrasion with aluminum oxide; CJ, CoJet-Sand. The surfaces were examined using profilometry, scanning electron microscopy, and electron dispersive spectroscopy. A zirconia primer (Z-Prime Plus, Z) or a silane primer (Monobond-S, S) was then applied to the surfaces, yielding 7 groups (PO-Z, BR-Z, PP-S, HF-S, AA-S, AA-Z, and CJ-S). Metal bracket-bonded specimens were stored in water for 24 hr at 37℃, and thermocycled for 1,000 cycles. Their bond strengths were measured using the wire loop method (n = 10).
Results
Except for BR, the surface pre-treatments failed to expose the zirconia substructure. A significant difference in bond strengths was found between AA-Z (4.60 ± 1.08 MPa) and all other groups (13.38 ± 2.57 - 15.78 ± 2.39 MPa, p < 0.05). For AA-Z, most of the adhesive remained on the bracket.
Conclusions
For bracket bonding to glazed zirconia, a simple application of silane to the cleaned surface is recommended. A zirconia primer should be used only when the zirconia substructure is definitely exposed.
Keywords: Adhesive, Bonding, Orthodontic brackets, Zirconia
Introduction
Direct bonding in orthodontics has improved esthetics, decreased gingival inflammation and enamel decalcification, and made the placement of orthodontic appliances more comfortable for patients and orthodontists.1 With the increased demands for adult orthodontic treatment and growing popularity of esthetic dentistry, clinicians are often faced with the problem of bonding orthodontic brackets to different types of restorations as well as to the enamel.2 Based on this trend, bond strength of brackets to esthetic pontic materials,3 temporary polycarbonate crowns,4 or ceramic surfaces including porcelain5,6 has undergone evaluation. Numerous options to improve bracket bonding to such substrates have been suggested, generally combinations of mechanical (e.g., surface roughening by air-particle abrasion, bur grinding, or hydrofluoric acid etching (HF) and chemical (e.g., primer application) conditioning methods.7
Recently, zirconia, specifically yttria-stabilized tetragonal zirconia polycrystal (Y-TZP), has become favored as a core material mainly due to its improved fracture resistance by transformation toughening.8,9 However, clinical trials have shown high rate of porcelain veneer fracture, probably due to the mismatch between the thermal expansion coefficients of the zirconia and the veneered porcelain.10 To reduce these failures, anatomically contoured (full-contour) zirconia-based dental prostheses have also been developed. The elimination of the veneering porcelain layer has improved the clinical success and reliability of zirconia restorations.11
In practice, many full-contour zirconia crowns are glazed and stained superficially during fabrication to improve their esthetic properties.12 Hence, the strategy of orthodontic bracket bonding to glazed zirconia restorations should target two different bonding substrates: the thin porcelain glaze layer and the zirconia substructure. For bonding to porcelain, application of silane primer after HF produces a high resin bond strength by forming a covalent bond with both the ceramic and the resin.13,14 For bonding to zirconia, in contrast, different approaches, such as air-particle abrasion and the use of zirconia primers or adhesive luting cements, are needed because zirconia is not readily etched by hydrofluoric acid due to its high crystalline content and such silane chemistry is ineffective with zirconia.8,15 A review of the relevant literature indicates that little research has been carried out with respect to surface conditioning protocols for bonding of orthodontic brackets to glazed zirconia. It may be necessary to determine adequate surface conditioning methods to ensure a clinically acceptable bond strength of brackets to glazed full-contour zirconia.
The purpose of this study was to test the effect of different combinations of surface pre-treatments and primer applications on the shear bond strength of brackets bonded to glazed full-contour zirconia. The null hypothesis tested was that there is no difference among shear bond strengths of the groups tested.
Materials and Methods
Specimen preparation and surface pre-treatments
Disc-shaped zirconia specimens (Zirkonzahn Prettau, Zirkonzahn GMBH, Gais, Italy) with a diameter of 10 mm and a thickness of 1 mm were fabricated (sintered by firing at 1,600℃ for 2 hours) and the surfaces were glazed with Initial IQ Lustre Pastes NF (GC America Inc., Alsip, IL, USA). The specimen preparation and glazing procedures were carried out by the same well-trained dental technician in accordance with the manufacturers' instructions. Unglazed zirconia served as the control bonding substrate in this study.
The surface was ultrasonically cleaned in isopropyl alcohol, air-dried, and subjected to one of the following pre-treatments (Table 1): PO (control), the unglazed surfaces were polished with silicon carbide paper up to #2000;8 BR, the glazed surfaces were gently roughened using a diamond bur (TR-19 [grit size: 106 - 125 µm], Mani Inc., Tochigi, Japan) in high-speed handpiece;4 PP, cleaned for 10 seconds with a rubber prophy cup and fluoride-free pumice; HF, etched with 4% hydrofluoric acid gel (Bisco Inc., Schaumburg, IL, USA, lot no: 1200011101) for 5 minutes, washed thoroughly with water to remove the residual acid, and air-dried;7 AA, air-particle abrasion was performed with an intraoral air abrasion device (Microetcher IIA, Danville, San Ramon, CA, USA) filled with 30 µm aluminum oxide particles from a distance of approximately 10 mm at a pressure of 0.25 MPa for 15 seconds;7,8 CJ, the tribochemical silica coating was applied using 30 µm CoJet-Sand (3M ESPE, Seefeld, Germany) in the same device under the same conditions as AA.7,16
Table 1. Surface conditioning (pre-treatment + primer application) methods to glazed zirconia specimens.
| Group code | Pre-treatment | Applied primer |
|---|---|---|
| PO-Z | Polishing unglazed (control) zirconia surface with silicon carbide paper | Z-Prime Plus* |
| BR-Z | Roughening with a diamond bur (grit size: 106 - 125 µm) | Z-Prime Plus |
| PP-S | Cleaning with a rubber prophy cup and pumice | Monobond-S† |
| HF-S | 4% hydrofluoric acid etching | Monobond-S |
| AA-S | Air-particle abrasion with 30 µm aluminum oxide particles | Monobond-S |
| AA-Z | Air-particle abrasion with 30 µm aluminum oxide particles | Z-Prime Plus |
| CJ-S | Air-particle abrasion with 30 µm silicon dioxide particles (CoJet-Sand) | Monobond-S |
PO, polishing unglazed zirconia; BR, bur roughening; PP, cleaning with a prophy cup and pumice; HF, hydrofluoric acid etching; AA, air-particle abrasion with aluminum oxide; CJ, silicon dioxide (CoJet-Sand) particles; Z, Z-Prime Plus; S, Monobond-S.
*1 - 2 coats were applied and dried with an air syringe for 3 - 5 sec.
†Applied with a brush, allowed to react for 60 sec, then dispersed with a strong stream of air.
Surface characterization
To examine the glaze layer, the glazed (not pre-treated) zirconia specimens were embedded in epoxy resin (Epofix, Struers, Copenhagen, Denmark) for cross-sectioning using a low-speed diamond saw (IsoMet Low Speed Saw, Buehler, Lake Bluff, IL, USA) under continuous water irrigation. The surface was ground, polished, and observed with a field emission-scanning electron microscope (FE-SEM, JSM-6700F, Jeol, Tokyo, Japan) after platinum sputter-coating.
The 6 pre-treated zirconia surfaces were investigated using SEM and additionally electron dispersive spectroscopy (EDS, Incax-sight, Oxford Instruments, High Wycombe, UK) to identify the chemical composition.
Surface roughness
The surface roughness Ra of pre-treated specimens (n = 10 per group) was measured using a previously calibrated profilometer (Surftest SV-400, Mitutoyo Corp., Kawasaki, Japan) at a stylus speed of 0.1 mm/sec, a cutoff of 0.8 mm, and a range of 600 µm. The Ra of each specimen was recorded as the average of the five readings.
Bracket bonding
From preliminary tests, the sample size (n = 10) was determined with a power analysis to provide statistical significance at 80% power. Prior to pre-treatments, the specimens were embedded in round silicone rubber molds using acrylic resin, ensuring the surface to be bonded remained uncovered. The surface was ultrasonically cleaned in isopropyl alcohol, air-dried, and subjected to the pretreatments described above. Z-Prime Plus (Z, Bisco Inc., lot no: 1200011069) or Monobond-S (S, Ivoclar Vivadent, Schaan, Liechtenstein, lot no: P57807) was applied to the pre-treated surfaces according to the manufacturers' instructions, yielding 7 groups (PO-Z, BR-Z, PP-S, HF-S, AA-S, AA-Z, and CJ-S, n = 10 per group). The pre-treatment and primer application procedures are summarized in Table 1.
Stainless steel brackets designed for mandibular incisors (Gemini series, 3M Unitek, Monrovia, CA, USA) were bonded to the surfaces using a light-curing orthodontic bonding system (Transbond XT, 3M Unitek). The average surface of the orthodontic bracket base was 10.5 mm2. The excess material was removed from the bracket margin with a hand scaler and light-cured from the mesial and distal aspects for 5 seconds each (total = 10 seconds) using a light-emitting diode curing unit (Bluephase G2, Ivoclar Vivadent) with an output intensity of 1,200 mW/cm2 (high mode) as measured with a built-in radiometer. The specimens were stored in water for 24 hours at 37℃ and then thermocycled at 5 and 55℃ for 1,000 cycles.17,18
Shear bond strength testing
Shear bond strength tests were performed by the wire loop method previously described by Mojtahedzadeh et al.19 The bonded specimens were mounted in a jig attached to a universal testing machine (3343, Instron Inc., Canton, MA, USA). A 0.018 × 0.025 inch stainless steel wire was engaged under tie wings. The shear load (pull of the steel wire) was applied at a crosshead speed of 0.5 mm/min until failure. The results were calculated in MPa.
After debonding, the residual composite on the zirconia surface was assessed based on an adhesive remnant index (ARI) score under an optical microscope (SMZ800, Nikon Corp., Tokyo, Japan) at ×10 magnification. Each specimen was scored according to the amount of composite remaining on the surface as follows: 0 = no composite remaining, 1 = less than 50% of composite remaining, 2 = more than 50% of composite remaining, and 3 = all composite remaining, with a distinct impression of the bracket base.20
Statistical analysis
Statistical analyses was carried out using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA) at a level of significance of α = 0.05. The Shapiro-Wilks normality test and Levene's variance homogeneity test were applied to the surface roughness and bond strength data. As the roughness data were normally distributed but showed inhomogeneity of variances between groups (p < 0.001), they were analyzed using Welch's variance-weighted one-way analysis of variance (ANOVA) and the Games-Howell post hoc test. The bond strength data, which met both the normality and variance homogeneity assumptions, were analyzed using one-way ANOVA and Tukey post hoc test. The ARI scores were tabulated and analyzed using the Fisher's exact test.
Results
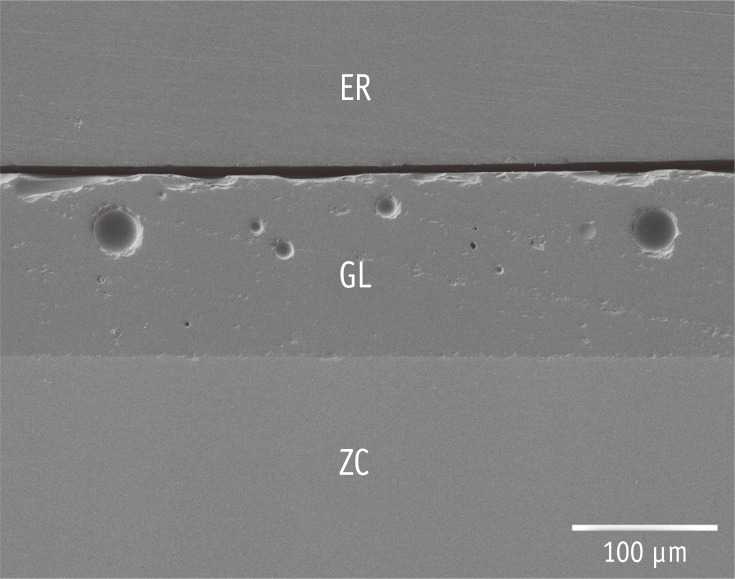
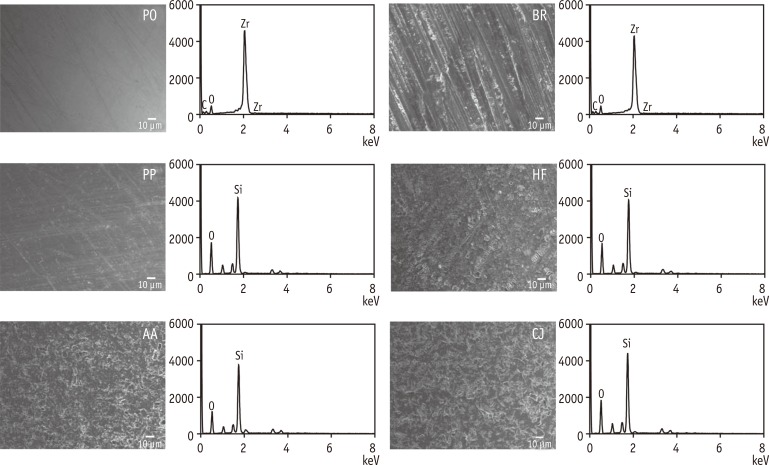
Figure 1 shows the representative cross-sectional SEM image of the glazed (not pre-treated) zirconia. The thickness of the glaze layer was found to be approximately 0.1 mm. The topographic SEM images of the pre-treated surfaces and their corresponding EDS spectra are presented in Figure 2. The surface roughness is also presented in Table 2. BR showed the roughest surface among the groups, followed by AA and CJ, which shared significantly similar Ra values (p = 0.283). HF exhibited a roughened surface with some striation formation. The EDS spectrum of BR showed Zr peaks (no Si peaks), whereas those of HF, AA, and CJ exhibited Si peaks (no Zr peaks).
Figure 1. Representative cross-sectional SEM image of the glazed full-zirconia ceramic (original magnification ×200, bar represents 100 µm). ER, epoxy resin for embedding; GL, glaze layer; ZC, zirconia ceramic.
Figure 2. Topographic SEM images and corresponding EDS spectra of the surfaces subjected to 6 different pre-treatments. Note that only the PO and BR spectra show Zr peaks. PO, polishing unglazed zirconia; BR, bur roughening; PP, cleaning with a prophy cup and pumice; HF, hydrofluoric acid etching; AA, air-particle abrasion with aluminum oxide; CJ, silicon dioxide [CoJet-Sand] particles.
Table 2. Ra surface roughness (µm) after surface pre-treatment.
| Surface pre-treatment | n | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| PO (polishing unglazed zirconia) | 10 | 0.06a | 0.02 | 0.05 | 0.09 |
| BR (bur roughening) | 10 | 1.25e | 0.24 | 0.85 | 1.64 |
| PP (cleaning) | 10 | 0.24b | 0.05 | 0.16 | 0.34 |
| HF (hydrofluoric acid etching) | 10 | 0.32c | 0.05 | 0.24 | 0.39 |
| AA (alumina air-abrasion) | 10 | 0.51d | 0.09 | 0.39 | 0.67 |
| CJ (CoJet-Sand air-abrasion) | 10 | 0.60d | 0.08 | 0.40 | 0.69 |
Welch's variance-weighted one-way ANOVA showed a significant difference in mean Ra value among the groups (p < 0.001); the Games-Howell post hoc test showed that means with the same superscripted letter were not significantly different (p > 0.05).
SD, standard deviation; Min, minimum value; Max, maximum value.
Table 3 presents the mean shear bond strength values, standard deviations, and ranges of all groups. ANOVA and Tukey post hoc test revealed a significant difference in values between AA-Z (4.60 ± 1.08 MPa) and the remaining groups (ranging from 13.38 ± 2.57 to 15.78 ± 2.39 MPa, p < 0.05).
Table 3. Shear bond strength values (MPa) for each group.
| Group | n | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| PO (polishing unglazed zirconia)-Z* | 10 | 13.38a | 2.57 | 9.75 | 18.22 |
| BR (bur roughening)-Z | 10 | 15.48a | 3.15 | 10.68 | 19.92 |
| PP (cleaning)-S† | 10 | 14.90a | 2.75 | 10.93 | 18.91 |
| HF (hydrofluoric acid etching)-S | 10 | 15.24a | 3.36 | 8.49 | 20.16 |
| AA (alumina air-abrasion)-S | 10 | 15.78a | 2.39 | 11.23 | 19.02 |
| AA (alumina air-abrasion)-Z | 10 | 4.60b | 1.08 | 2.76 | 5.98 |
| CJ (CoJet-Sand air-abrasion)-S | 10 | 14.81a | 2.91 | 10.01 | 19.75 |
*Z-Prime Plus; †Monobond-S.
One-way ANOVA showed a significant difference in mean shear bond strength among the groups (p < 0.001); the Tukey post hoc test showed that means with the same superscripted letter were not significantly different (p > 0.05).
SD, standard deviation; Min, minimum value; Max, maximum value.
Table 4 presents the residual composite on the surfaces as evaluated by ARI scores. The Fisher's exact test indicated significant differences among the groups (p < 0.001). For AA-Z, most of the adhesive remained on the bracket (scores 0 and 1), indicating failure at the ceramic-adhesive interface. Except for AA-Z, most of the adhesive remained on the ceramic surfaces (scores 2 or 3), indicating failure at the bracket-adhesive interface. No ceramic fractures were observed in this study.
Table 4. Distribution of ARI scores for each group.
| Group | n | ARI score‡ | Fisher's exact test | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| PO (polishing unglazed zirconia)-Z* | 10 | 0 | 3 | 2 | 5 | p < 0.001 |
| BR (bur roughening)-Z | 10 | 0 | 1 | 2 | 7 | |
| PP (cleaning)-S† | 10 | 0 | 4 | 2 | 4 | |
| HF (hydrofluoric acid etching)-S | 10 | 0 | 3 | 2 | 5 | |
| AA (alumina air-abrasion)-S | 10 | 0 | 2 | 1 | 7 | |
| AA (alumina air-abrasion)-Z | 10 | 8 | 2 | 0 | 0 | |
| CJ (CoJet-Sand air-abrasion)-S | 10 | 0 | 1 | 1 | 8 | |
*Z-Prime Plus; †Monobond-S.
‡ARI, adhesive remnant index, 0, No composite left on surface; 1, less than half of the composite left on surface; 2, more than half of the composite left on surface; 3, all composite left on surface, with a distinct impression of the bracket mesh. No ceramic fractures were observed in this study.
Discussion
In this study, 6 experimental (glazed, pre-treated, and primer-applied) groups were tested to simulate clinical situations (Table 1). Bond strength of orthodontic brackets should be high enough to resist accidental detachment during treatment but also low enough that excessive force need not be applied during debonding at the end of the treatment.7 Thus, 6 to 8 MPa has been suggested as clinically adequate bond strength for metal bracket to enamel.7,20 In this study, except for AA-Z, the mean shear bond strengths of metal brackets to glazed zirconia surfaces submitted to 5 different surface conditioning procedures fell within this range or exceeded these limits and therefore could be considered sufficient for clinical applications (Table 3). Thus, the null hypothesis was partially rejected. BR produced effective bond strength with the subsequent application of a zirconia primer (Z-Prime Plus), whereas PP, HF, AA, and CJ performed well with silane (Monobond-S).
It has been reported that a combination of air-particle abrasion and resin composites with organophosphate ester monomers results in a relatively strong and durable bond to zirconia.15 Another approach to chemical bonding with zirconia is to use various primers, which seems more suitable for use with non-adhesive orthodontic bonding systems such as Transbond XT. Among various zirconia primers, Z-Prime Plus, which is based on organophosphate/carboxylic acid monomers, has been reported to be highly effective in achieving durable resin bonding to air-abraded zirconia surface.15,21 The EDS analysis showed that only bur grinding easily removed the thin (approximately 0.1 mm, Figure 1) glaze layer and exposed the zirconia substructure (Figure 2). A high bond strength (15.48 ± 3.15 MPa) for BR-Z indicates that the application of the zirconia primer to exposed and roughened zirconia by diamond bur grinding is effective for orthodontic bracket bonding. In this study, PO (control) showed comparable shear bond strength (13.38 ± 2.57 MPa), suggesting that the primer works well chemically even with a polished zirconia surface.
The EDS analysis indicated that the bonding substrate in most of the 6 experimental groups (except for BR) was virtually the porcelain glaze layer (Figure 2). Despite the inconvenience of its intraoral manipulation, hydrofluoric acid etching followed by silane application to silicabased ceramic such as porcelain has been reported to be an efficient conditioning method.17,22 Strong acids such as 9.6% hydrofluoric acid are commonly used to etch porcelain.4 However, highly corrosive hydrofluoric acid can cause severe trauma to soft tissues and tooth substance when used intraorally.4,13 In this study, therefore, a low concentration (4%) of hydrofluoric acid was used with a longer application time (5 minutes). Despite a significantly larger Ra value than PP (Table 2), HF showed a relatively smooth surface structure with some striation formation (Figure 2), rather than the amorphous spongy-like microstructure found on the conventional veneering ceramic surface.23 This reduced etching effect may be attributable to the use of a low hydrofluoric acid concentration.23 In terms of bond strength, there was no significant difference between HF and PP (Table 3). These findings suggest that chemical bonding by silane treatment exceeds the mechanical retention provided by hydrofluoric acid, although both steps contribute to final bond strength.6
In this study, air-particle abrasion was performed with 30 µm aluminum oxide particles (used for intraoral) on the glazed zirconia, producing a randomized roughened glazing porcelain surfaces (Figure 2).4 Although AA produced a significantly rougher surface than HF, no gain in bond strength was observed even when silane was subsequently applied (AA-S and HF-S), confirming that chemical adhesion by silane treatment might have a greater effect than surface roughening (Tables 2 and 3).6 To simulate a possible clinical situation in which a zirconia primer is applied to glazed full-contour zirconia restorations, Z-Prime Plus was applied to the air-abraded surface (AA-Z), yielding a poor shear bond strength (4.60 ± 1.08 MPa). This finding indicates that acidic monomer-containing primer is incompatible with porcelain even though the surface has been significantly roughened (AA vs. PP).13,15 The surface of glazed full-contour zirconia restorations might be altered or removed mainly by the dentist's proximal contact adjustment procedures before insertion or by occlusal wear within the first six months after insertion.24 The glazed layer over the labial/buccal surface of a glazed zirconia restoration may also be worn off by long-term tooth brushing. As shown in AA-Z, a zirconia primer should not be used in clinical practice when the porcelain glaze layer appears to remain (Table 3). When it is unclear whether the surface to be bonded with bracket is a porcelain glazed layer or zirconia substructure, thoroughly exposing the zirconia by bur grinding and then applying a zirconia primer would be preferable.
It has been reported that tribochemical silica coating with subsequent silanization enhances the bond between the ceramic surfaces and the resin composite, the silica layer left on the ceramic surface by such coating providing a basis for silane.7 In terms of bond strength, the current study found no additional effect of this treatment compared to air-particle abrasion (Table 3). As the porcelain glaze layer was already rich in Si, such tribochemical silica coating did not considerably increase the amount of silanereactive Si (Figure 2).
Since no recommendations or standards for glazing dental zirconia are found in the literature at present,25 the quality (particularly the thickness) of the glaze layer may vary depending on the glazing technique used. Nonetheless, the silane chemistry remains effective on porcelain glaze layer irrespective of thickness.13 In this study, brackets for the mandibular incisors were used because their flat bases ensured optimal adaptation to the flat glazed or unglazed zirconia surface,7 but only metal brackets were employed. The use of ceramic or polycarbonate orthodontic brackets is more desirable on zirconia-based restorations in esthetic terms. Thus, the bond strength of such tooth-colored brackets to zirconia ceramic should be further investigated.
In this study, shear bond strength was tested using the wire loop method. Mojtahedzadeh et al.19 suggested that this method might more closely reproduce clinical loads than the shear blade method. In addition, thermocycling (1,000 cycles) was applied prior to debonding to approximate clinical reality.17 When the specimens were stored only in water without thermocycling, the bond strengths to ceramics and the incidence of cohesive ceramic fractures were found to be excessively high.19 In this study, no ceramic fractures were found, probably indicating that the exposure to water and temperature changes involved in thermocycling mainly affected the bond of the resin composite to the metal bracket base (Table 4).18 However, de Oliveira et al.26 reported that the bracket bond strength to enamel was significantly improved after thermocycling. Further studies are required to determine whether longer thermocycling regimens are needed.
This study suggests that prior to orthodontic bracket bonding, a simple surface cleaning of the zirconia glaze layer with a prophy cup and pumice followed by silane application suffices. Additional roughening of the glaze layer did not improve the shear bond strength of the brackets. Moreover, a roughened surface can be expected to promote bacterial accumulation if it is not well-covered by a bracket after bonding.27 Such simple surface cleaning may be replaced by phosphoric acid conditioning, which produces no morphological changes to porcelain, only removing its smear layer.6 Nonetheless, the former would be a safer choice of surface cleaning than the latter (i.e., intraoral use of an acid). Recently, multi-function primer for working on all types of restoration surfaces and universal adhesives for treatment of tooth surfaces as well as restoration surfaces have been launched. These materials were not tested in this study; further tests should be performed to prove their effectiveness in orthodontic bracket bonding to glazed zirconia.
Conclusions
It was found that the most simple and effective surface conditioning method for orthodontic bracket bonding to glazed zirconia was a silane application to the cleaned surface in the clinic.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2061732). The authors are grateful to Mr. Jong-Beop Song (MyeongMoon Dental Co., Ltd., Daegu, Korea) for preparing the zirconia specimens used in this study.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Lifshitz AB, Cárdenas M. A comparison between the shear bond strength of brackets bonded to glazed and deglazed porcelain surfaces with resin-reinforced glassionomer cement and a bis-GMA resin adhesive. World J Orthod. 2006;7:134–137. [PubMed] [Google Scholar]
- 2.Trakyali G, Malkondu O, Kazazoğlu E, Arun T. Effects of different silanes and acid concentrations on bond strength of brackets to porcelain surfaces. Eur J Orthod. 2009;31:402–406. doi: 10.1093/ejo/cjn118. [DOI] [PubMed] [Google Scholar]
- 3.Maryanchik I, Brendlinger EJ, Fallis DW, Vandewalle KS. Shear bond strength of orthodontic brackets bonded to various esthetic pontic materials. Am J Orthod Dentofacial Orthop. 2010;137:684–689. doi: 10.1016/j.ajodo.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Blakey R, Mah J. Effects of surface conditioning on the shear bond strength of orthodontic brackets bonded to temporary polycarbonate crowns. Am J Orthod Dentofacial Orthop. 2010;138:72–78. doi: 10.1016/j.ajodo.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Abu Alhaija ES, Al-Wahadni AM. Shear bond strength of orthodontic brackets bonded to different ceramic surfaces. Eur J Orthod. 2007;29:386–389. doi: 10.1093/ejo/cjm032. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Zeng J, Wang S, Yang Z, Huang Q, Chen P, Zhou S, Liu X. Influence of surface treatments on the shear bond strength of orthodontic brackets to porcelain. Appl Surf Sci. 2008;255:416–418. [Google Scholar]
- 7.Özcan M, Vallittu PK, Peltomäki T, Huysmans MC, Kalk W. Bonding polycarbonate brackets to ceramic: effects of substrate treatment on bond strength. Am J Orthod Dentofacial Orthop. 2004;126:220–227. doi: 10.1016/j.ajodo.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Kim MJ, Kim YK, Kim KH, Kwon TY. Shear bond strengths of various luting cements to zirconia ceramic: surface chemical aspects. J Dent. 2011;39:795–803. doi: 10.1016/j.jdent.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Kasraei S, Rezaei-Soufi L, Heidari B, Vafaee F. Bond strength of resin cement to CO2 and Er:YAG laser-treated zirconia ceramic. Restor Dent Endod. 2014;39:296–302. doi: 10.5395/rde.2014.39.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komine F, Strub JR, Matsumura H. Bonding between layering materials and zirconia frameworks. Jpn Dent Sci Rev. 2012;48:153–161. [Google Scholar]
- 11.Guess PC, Schultheis S, Bonfante EA, Coelho PG, Ferencz JL, Silva NR. All-ceramic systems: laboratory and clinical performance. Dent Clin North Am. 2011;55:333–352. doi: 10.1016/j.cden.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Kim JW. Graded structures for damage resistant and aesthetic all-ceramic restorations. Dent Mater. 2009;25:781–790. doi: 10.1016/j.dental.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabianelli A, Pollington S, Papacchini F, Goracci C, Cantoro A, Ferrari M, van Noort R. The effect of different surface treatments on bond strength between leucite reinforced feldspathic ceramic and composite resin. J Dent. 2010;38:39–43. doi: 10.1016/j.jdent.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Dos Santos VH, Griza S, de Moraes RR, Faria-E-Silva AL. Bond strength of self-adhesive resin cements to composite submitted to different surface pretreatments. Restor Dent Endod. 2014;39:12–16. doi: 10.5395/rde.2014.39.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha JY, Son JS, Kim YK, Kim KH, Kwon TY. Effect of heat treatment of dental zirconia ceramic treated with three different primers on the bonding durability of resin cement. Macromol Res. 2013;21:71–77. [Google Scholar]
- 16.Shon WJ, Kim TW, Chung SH, Jung MH. The effects of primer precuring on the shear bond strength between gold alloy surfaces and metal brackets. Eur J Orthod. 2012;34:72–76. doi: 10.1093/ejo/cjq163. [DOI] [PubMed] [Google Scholar]
- 17.Zachrisson YO, Zachrisson BU, Büyükyilmaz T. Surface preparation for orthodontic bonding to porcelain. Am J Orthod Dentofacial Orthop. 1996;109:420–430. doi: 10.1016/s0889-5406(96)70124-5. [DOI] [PubMed] [Google Scholar]
- 18.Goracci C, Margvelashvili M, Giovannetti A, Vichi A, Ferrari M. Shear bond strength of orthodontic brackets bonded with a new self-adhering flowable resin composite. Clin Oral Investig. 2013;17:609–617. doi: 10.1007/s00784-012-0729-x. [DOI] [PubMed] [Google Scholar]
- 19.Mojtahedzadeh F, Akhoundi MS, Noroozi H. Comparison of wire loop and shear blade as the 2 most common methods for testing orthodontic shear bond strength. Am J Orthod Dentofacial Orthop. 2006;130:385–387. doi: 10.1016/j.ajodo.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Ryou DB, Park HS, Kim KH, Kwon TY. Use of flowable composites for orthodontic bracket bonding. Angle Orthod. 2008;78:1105–1109. doi: 10.2319/013008-51.1. [DOI] [PubMed] [Google Scholar]
- 21.Magne P, Paranhos MP, Burnett LH., Jr New zirconia primer improves bond strength of resin-based cements. Dent Mater. 2010;26:345–352. doi: 10.1016/j.dental.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Al-Hity R, Gustin MP, Bridel N, Morgon L, Grosgogeat B. In vitro orthodontic bracket bonding to porcelain. Eur J Orthod. 2012;34:505–511. doi: 10.1093/ejo/cjr043. [DOI] [PubMed] [Google Scholar]
- 23.Chaiyabutr Y, McGowan S, Phillips KM, Kois JC, Giordano RA. The effect of hydrofluoric acid surface treatment and bond strength of a zirconia veneering ceramic. J Prosthet Dent. 2008;100:194–202. doi: 10.1016/S0022-3913(08)60178-X. [DOI] [PubMed] [Google Scholar]
- 24.Preis V, Behr M, Hahnel S, Handel G, Rosentritt M. In vitro failure and fracture resistance of veneered and full-contour zirconia restorations. J Dent. 2012;40:921–928. doi: 10.1016/j.jdent.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Mitov G, Heintze SD, Walz S, Woll K, Muecklich F, Pospiech P. Wear behavior of dental Y-TZP ceramic against natural enamel after different finishing procedures. Dent Mater. 2012;28:909–918. doi: 10.1016/j.dental.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 26.de Oliveira AS, Mirapalhete RC, Amaral CC, de Moraes RR. A modified photoactivation protocol using two simultaneous light-curing units for bonding brackets to enamel. Braz Dent J. 2015;26:393–397. doi: 10.1590/0103-6440201300133. [DOI] [PubMed] [Google Scholar]
- 27.Ahn HB, Ahn SJ, Lee SJ, Kim TW, Nahm DS. Analysis of surface roughness and surface free energy characteristics of various orthodontic materials. Am J Orthod Dentofacial Orthop. 2009;136:668–674. doi: 10.1016/j.ajodo.2007.11.032. [DOI] [PubMed] [Google Scholar]