Abstract
Ovarian follicular cysts are anovulatory follicular structures that lead to infertility. Hormones play key roles in the formation and persistence of cysts. Inhibins are heterodimeric gonadal glycoprotein hormones that belong to the transforming growth factor-β superfamily. These hormones suppress the secretion of follicle-stimulating hormone. In this report, partial fragment of inhibin-α (INHA) subunit gene of Large White pig was detected from the genomic DNA by polymerase chain reaction. The sequence showed a 283 bp fragment insertion/deletion (I/D) polymorphism in INHA subunit gene. A total of 49 Large White sows with cystic follicles and 152 normal sows were screened for this polymorphism. The relationship of INHA I/D polymorphisms with follicular cysts was investigated. The distribution of I/D was significantly different between cystic and normal sows, thereby suggesting that the INHA subunit gene might be a potential biological marker for breeding programs in pig.
Keywords: follicular cyst, inhibin α, polymorphism, swine
Inhibins are heterodimeric gonadal glycoprotein hormones belonging to the growth factor-β superfamily. These hormones are called inhibins for their ability to suppress follicle-stimulating hormone (FSH) synthesis and secretion from anterior pituitary via negative feedback [10]. Inhibins are composed of a common inhibin-α subunit (INHA, 14 kDa) and one of two inhibin-β subunits (INHβA, 18 kDa; or INHβB, 18 kDa).
Previous studies have demonstrated that inhibins are associated with the balance of the endocrine in the hypothalamo-hypophyseal-gonadal axis [7] and are essential for animal reproduction. In females, ovaries are the main source of inhibins. Gonadotropes are the main target cells of inhibin [1]. Besides inhibiting FSH secretion via endocrine, inhibins also exert local action through autocrine and paracrine pathways in reproductive tissues [17]. These hormones play key roles in folliculogenesis, oocyte maturation and embryo development in females [16] by regulating granulosa cell proliferation and development. In in vitro cultured goose granulosa cells, in which inhibin-α subunit gene expression was down-regulated by RNAi, the apoptosis and proliferation indices were significantly higher than in the control groups; the G1 phase percentage decreased, and a corresponding increase in the S phase occurred [3]. Immunization against inhibin can increase the ovulation rate [13] and litter size [12] in domestic animals.
The important roles of INHA gene make it a strong candidate gene in mammalian reproduction. In this study, a new insertion/deletion (I/D) polymorphism of the pig INHA gene was detected by polymerase chain reaction (PCR). The relationship between the polymorphisms of INHA gene and follicular cysts was investigated in sows.
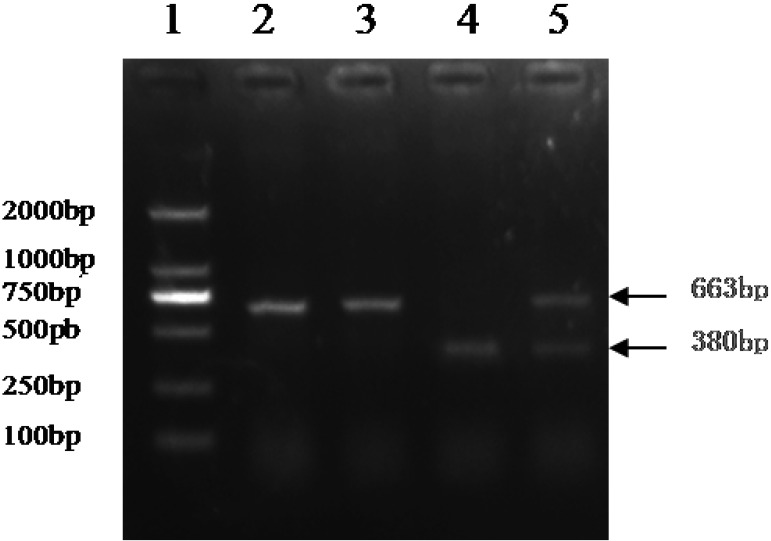
Ovaries from 49 Large White sows with cystic follicle and 152 normal sows in the age range of 5–6 months old were collected from a local slaughterhouse. The normal ovaries and follicular cysts were identified through the size of follicular diameter combined with the histological structure and hormone changes, as described in our previous research [16]. Cystic follicles were greater than 21 mm in diameter and were characterized by fluid-filled structures with a smooth and thin wall in the absence of corpus luteum. Genomic DNA was extracted from samples of ovary tissues (30 mg) by using a Multisource Genomic DNA Miniprep Kit (AXYGEN, Hangzhou, China). The DNA quality and concentration were determined using a NanoDropTM ND-2000 UV-Vis spectrometer (Thermo Fisher Scientific, Wilmington, DE, U.S.A.). Primers (F:5′- CCCGTGTCCTCACGGATACCAGT-3′; R:5′-GTGCTGGGACGGCCGGAATAC-3′) that amplified this 663 bp (or 380 bp) fragment of the partial intron containing 283 bp I/Dfragment were designed according to the porcine INHA gene sequence (GenBank ID: 397386) using Primer 5.0. PCR was performed in reaction mixtures containing 100 ng DNA, 2.5 µl of 10 × buffer, 5 pmol of each primer, 2 µl of 2.5 mM dNTPs and 2 units of pfu DNA polymerase (Promega, Madison, WI, U.S.A.) at a final volume of 25 µl. The conditions for amplification were as follows: 4 min at 95°C, followed by 35 cycles of 30 sec at 95°C, 30 sec at 59°C, 30 sec at 72°C and finally, 10 min at 72°C. PCR products were identified on 1.5% agarose gel electrophoresis to confirm I/D variants based on their size differences (Fig. 1). A polymorphism with one fragment (663 bp in length) was characterized as I/I. A polymorphism with one fragment of 380 bp length was characterized as D/D. A polymorphism with both fragments was characterized as I/D. Subsequently, PCR products with I/I and D/D fragments were amplified using pfu DNA polymerase (Promega), purified using Spin Column DNA Gel Extraction Kit (Sangon Biotech, Shanghai, China), cloned into bacterial plasmid by using the pMD-18T vector (TaKaRa, Tokyo, Japan) and sequenced using ABI 3730X DNA sequence at the BGI (BGI, Tianjin, China).
Fig. 1.
PCR fragments of homozygous and heterozygous genotypes of INHA insertion (I) and deletion (D) allele polymorphism on agarose gel. Lane 1=molecular markers (2,000, 1,000, 750, 500, 250, 100 bp); lanes 2 and 3 =I/I genotype; lane 4 =D/D genotype; lane 5= I/D genotype.
According to the sequencing results, a fragment of I/D (283 bp in length) from nt 2495 to nt 2777 was present in Large White sows (Fig. 2). Numbering is scored relative to the first nucleotide (+1) of the start codon in the sequencer or analyzer of the INHA gene in pig. This I/D polymorphism was located within the intron.
Fig. 2.
Sequences of the deletion fragment of the INHA gene of pig. Upper line was the sequence of the gene of INHA published on NCBI (GenBank: No. 397386). Numbering is scored relative to first nucleotide (+1) of start codon in sequence of INHA gene in pig; Lower line was the sequence of the PCR product of D/D fragments.
Hardy–Weinberg equilibrium for genotypic distribution was investigated by using the Chi-Square test. A deviation from the Hardy–Weinberg equilibrium for the INHA I/D polymorphism was observed in the normal and cystic groups. Genotype and allele frequencies between cystic and normal subjects were compared by Pearson’s Chi-Square test, and the results are summarized in Table 1. The genotype distributions of the I/D polymorphism in the cystic groups were significantly different from those in the normal group. The II, ID and DD rates for the cystic and normal groups (P=0.03) were 63.3%, 20.4% and 16.3% and 45.4%, 17.8% and 36.8%, respectively. The allele I frequency in cystic groups was higher than in the normal group (73.5% vs. 54.3%). The strength of the relationship between genotype and cyst presence was measured using odd ratio (OR). The results suggested that I/D polymorphism was associated with the presence of cysts. Sows with DD genotype (OR=0.483, confidence interval=0.249–0.937, P=0.031) have lower risk of developing follicular cysts than sows with other genotypes.
Table 1. Frequency distribution of INHA I/D alleles and genotypes in cystic sows and normal group No. (%).
| Variables | All sows (n=201) | Cystic (n=49) | Normal (n=152) | P value | OR (95 CI) |
|---|---|---|---|---|---|
| II | 100 (49.8) | 31 (63.3) | 69 (45.4) | 0.03a) | 0.483 (0.249–0.937) |
| ID | 37 (18.4) | 10 (20.4) | 27 (17.8) | ||
| DD | 64 (31.8) | 8 (16.3) | 56 (36.8) | ||
| I | 237 (59.0) | 72 (73.5) | 165 (54.3) | 0.001 | 0.429 (0.259–0.708) |
| D | 165 (41.0) | 26 (26.5) | 139 (45.7) |
P value assessed using Pearson’s chi-square test (two-tailed) compared with normal group. OR: odds ratio; CI: 95% confidence interval. a) Recessive genetic model: II versus I/D+DD.
During antral follicle development in normal pig, increased pulsatile secretion of LH and FSH stimulates estradiol secretion in the ovary. FSH and estradiol could increase LH receptor expression level in the ovary [5, 8], thereby leading to the stimulation of ovulation through the LH pathway. However, the concentration of estradiol in cystic follicles was significantly lower than in normal large follicles. The markedly low concentration of estradiol in follicles might decrease their responsiveness to LH. Expression level of 3β-hydroxysteroid dehydrogenase (3β-HSD) decreased in theca cells of ovary with cystic follicles. 3β-HSD is essential for the biosynthesis of mineralocorticoid, glucocorticoid and reproductive steroid hormones. Activin reduced the expression level of 3β-HSD mRNA, whereas inhibin A significantly increased the 3β-HSD expression level in ovarian theca cells [20]. Our previous studies showed that expression levels of inhibin α subunit and β-glycan were lower in cystic follicles than in normal large follicles.
Under normal physiological conditions, inhibins suppress FSH synthesis and secretion. Thus, the reduction in the concentration of the INHA subunit results in the elevation of FSH levels. FSH secretion disorder is among the main factors that can lead to cyst formation [19]. However, low concentration of inhibin in serum did not increase FSH synthesis in pig with follicular cysts. Follicular cystic cattle induced with estradiol and progesterone showed clinical and histological characteristics that are similar to those in cattle with spontaneously occurring cysts [9]. In this cyst model, the mean FSH concentrations in the cystic group were lower than in the normal group, but the difference was not statistically significant [4]. Similar to induced cysts, the serum FSH concentration in sows with cystic follicle (46.8867 ± 1.9575 mIU/ml) was lower than in sows with large follicles (6–8 mm) (56.9714 ± 16.5193 mIU/ml), but the difference was not statistically significant [18].
In another study, we found that expressions of betaglycan mRNA and protein in granulosa cells increased in response to exogenous FSH application compared with the control. Therefore, synthesis of FSH is insufficient in the pituitary, and unovulated follicles could become cystic, because of decreased expression levels of inhibin α and betaglycan [21]. INHA gene mutations may influence the bioactivity of inhibin. A 769 G/A mutation of human INHA could impair the cleavage of the mature peptide, dimer formation or binding of inhibin to its receptor, thereby resulting in the inability to activate the subsequent signal transduction pathway [14]. Thus, the ability to downregulate the FSH level through negative feedback was affected. Furthermore, INHA also exerts local action in folliculogenesis by regulating the granulosa cell proliferation and apoptosis through the autocrine and paracrine pathways [16, 17]. In cystic follicles, the expression level of β-glycan, which is the co-receptor of inhibin, was significantly lower than in normal follicles [21]. These results indicated that the inhibin-β-glycan signaling pathway was disrupted in cystic follicles.
Other studies suggest that ovarian cysts should be classified as a quantitative trait disorder [15]. The INHA gene was identified in one of the quantitative trait loci for ovulation rate in pig [10]. Linkage disequilibrium may occur between the INHA gene mutations detected in this study and other genes located near the INHA loci.
In summary, we identified a new I/D of INHA polymorphism in pig by using PCR. I/D polymorphism is significantly associated with the presence of follicular cysts. Sows with I allele have a higher risk of developing follicular cysts. These findings may provide a novel biological marker and promising genetic therapy candidates for ovarian cysts in pigs, which would greatly benefit pig breeding programs. However, ovarian cysts may be induced by other factors, such as age, nutrition, and season of the year [6, 11], and some cysts may disappear approximately 1week after formation [2]. More studies are needed to confirm the relationship between the formation of ovarian cysts and genetic markers in other breeds.
Acknowledgments
This research was supported by the State Key Development Program of Basic Research (973 Program) of China (No.2011CB944203), Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, No. IRT1248), The National Natural Science Foundation of China (31301224, 31372308, 31301969) and the Changchun Science and Pillar program in Modern Agriculture (13NK25).
References
- 1.Bilezikjian L. M., Blount A. L., Donaldson C. J., Vale W. W.2006. Pituitary actions of ligands of the TGF-beta family: activins and inhibins. Reproduction 132: 207–215. doi: 10.1530/rep.1.01073 [DOI] [PubMed] [Google Scholar]
- 2.Castagna C. D., Peixoto C. H., Bortolozzo F. P., Wentz I., Neto G. B., Ruschel Fc. 2004. Ovarian cysts and their consequences on the reproductive performance of swine herds. Anim. Reprod. Sci. 81: 115–123. doi: 10.1016/j.anireprosci.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 3.Chen F., Jiang X., Chen X., Liu G., Ding J.2007. Effects of downregulation of inhibin alpha gene expression on apoptosis and proliferation of goose granulosa cells. J. Genet. Genomics 34: 1106–1113. doi: 10.1016/S1673-8527(07)60126-X [DOI] [PubMed] [Google Scholar]
- 4.Cook D. L., Parfet J. R., Smith C. A., Moss G. E., Youngquist R. S., Garverick H. A.1991. Secretory patterns of LH and FSH during development and hypothalamic and hypophysial characteristics following development of steroid-induced ovarian follicular cysts in dairy cattle. J. Reprod. Fertil. 91: 19–28. doi: 10.1530/jrf.0.0910019 [DOI] [PubMed] [Google Scholar]
- 5.Erickson G. F., Wang C., Hsueh A. J.1979. FSH induction of functional LH receptors in granulosa cells cultured in a chemically defined medium. Nature 279: 336–338. doi: 10.1038/279336a0 [DOI] [PubMed] [Google Scholar]
- 6.Gherpelli M., Tarocco C.1996. A study on the incidence and clinical evolution of the ovarian cysts in the sow. p. 587. Proc 14th IPVS Congress, Bologna, Italy.
- 7.Guthrie H. D., Ireland J. L., Good T. E., Ireland J. J.1997. Expression of different molecular mass forms of inhibin in atretic and nonatretic follicles during the early luteal phase and altrenogest-synchronized follicular phase in pigs. Biol. Reprod. 56: 870–877. doi: 10.1095/biolreprod56.4.870 [DOI] [PubMed] [Google Scholar]
- 8.Ikeda S., Nakamura K., Kogure K., Omori Y., Yamashita S., Kubota K., Mizutani T., Miyamoto K., Minegishi T.2008. Effect of estrogen on the expression of luteinizing hormone-human chorionic gonadotropin receptor messenger ribonucleic acid in cultured rat granulosa cells. Endocrinology 149: 1524–1533. doi: 10.1210/en.2007-1163 [DOI] [PubMed] [Google Scholar]
- 9.Kesler D. J., Garverick H. A.1982. Ovarian cysts in dairy cattle: a review. J. Anim. Sci. 55: 1147–1159. [DOI] [PubMed] [Google Scholar]
- 10.Ling N., Ying S. Y., Ueno N., Shimasaki S., Esch F., Hotta M., Guillemin R.1986. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature 321: 779–782. doi: 10.1038/321779a0 [DOI] [PubMed] [Google Scholar]
- 11.Liptrap R. M., Doble E.1981. Relationship of prostaglandin F2alpha to cystic ovarian follicles in the sow. Br. Vet. J. 137: 289–299. [DOI] [PubMed] [Google Scholar]
- 12.Medan M. S., Watanabe G., Sasaki K., Nagura Y., Sakaime H., Fujita M., Sharawy S., Taya K.2003. Ovarian and hormonal response of female goats to active immunization against inhibin. J. Endocrinol. 177: 287–294. doi: 10.1677/joe.0.1770287 [DOI] [PubMed] [Google Scholar]
- 13.Naqvi S. M. K., Joshi A., Gulyani R., Saha S., Manik R. S., Palta P.2009. Increase in ovulation rate by active immunization against bovine inhibin-based synthetic peptides in a non-prolific sheep breed. Small Rumin. Res. 85: 70–73. doi: 10.1016/j.smallrumres.2009.07.009 [DOI] [Google Scholar]
- 14.Shelling A. N., Burton K. A., Chand A. L., van Ee C. C., France J. T., Farquhar C. M., Milsom S. R., Love D. R., Gersak K., Aittomäki K., Winship I. M.2000. Inhibin: a candidate gene for premature ovarian failure. Hum. Reprod. 15: 2644–2649. doi: 10.1093/humrep/15.12.2644 [DOI] [PubMed] [Google Scholar]
- 15.Simpson J.1992. Elucidating the genetics of polycystic ovary syndrome. pp. 59–77. In: Polycystic Ovary Syndrome (Dunaif, A., Givens, J. R., Haseltine, F. P. and Merriam, G. R. eds.), Blackwell Scientific Publication, Oxford. [Google Scholar]
- 16.Sirotkin A. V.2011. Cytokines: signaling molecules controlling ovarian functions. Int. J. Biochem. Cell Biol. 43: 857–861. doi: 10.1016/j.biocel.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 17.Stenvers K. L., Findlay J. K.2010. Inhibins: from reproductive hormones to tumor suppressors. Trends Endocrinol. Metab. 21: 174–180. doi: 10.1016/j.tem.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 18.Sun Y. L., Ping Z. G., Li C. J., Sun Y. F., Yi K. L., Chen L., Li X. Y., Wang X. L., Zhou X.2011. Comparative proteomic analysis of follicular fluids from normal and cystic follicles in sows. Reprod. Domest. Anim. 46: 889–895. doi: 10.1111/j.1439-0531.2011.01760.x [DOI] [PubMed] [Google Scholar]
- 19.Tsilchorozidou T., Overton C., Conway G. S.2004. The pathophysiology of polycystic ovary syndrome. Clin. Endocrinol. (Oxf.) 60: 1–17. doi: 10.1046/j.1365-2265.2003.01842.x [DOI] [PubMed] [Google Scholar]
- 20.Young J. M., McNeilly A. S.2012. Inhibin removes the inhibitory effects of activin on steroid enzyme expression and androgen production by normal ovarian thecal cells. J. Mol. Endocrinol. 48: 49–60. doi: 10.1530/JME-11-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Li C., Li H., Ma W., Chen S., Zhao Y., Rao J., Zhou X.2015. Downregulation of the expression of inhibin α subunit and betaglycan in porcine cystic follicles. J. Vet. Med. Sci. [Epub ahead of print]. doi: 10.1292/jvms.14-0617 [DOI] [PMC free article] [PubMed] [Google Scholar]