Abstract
Urachal carcinoma (UC) is a rare tumor mainly affecting middle-aged males. Metastases occur most frequently in lymph nodes and the lungs. There are no standard adjuvant and metastatic treatments. We report the case of a 36-year-old female with UC treated with partial cystectomy who relapsed 3 years after surgery with left choroidal, lung, mediastinal lymph node, right adrenal, mammary, and bone metastases as well as peritoneal carcinomatosis. She obtained a partial response after 10 cycles of chemotherapy with a modified docetaxel, cisplatin and 5-fluorouracil (mTPF) regimen. This is the first report on the use of the mTPF regimen in UC and on the existence of choroidal, adrenal, and mammary metastases.
Key words: Urachal carcinoma; Choroidal, adrenal, and mammary metastases; Modified docetaxel, cisplatin and 5-fluorouracil regimen
Background
The urachus is a hollow musculofibrous band. Its internal stratum has a secretory activity. It connects the dome of the bladder to the umbilicus and is an embryological remnant of the allantoic diverticulum, which appears at the sixteenth day of life from the cloaca. At the fifth month, in most of cases, the allantoic diverticulum is obstructed, but in some people, it does not close well, leading to benign pathologies. There are 2 theories concerning the development of malignant disease: the dysplastic theory (inclusion of digestive germ cells during embryogenesis) and the metaplastic theory (transformation of the intern stratum due to irritative factors such as infections or reflux).
Urachal carcinomas (UC) represent about 0.2% of all bladder tumors. Most of them (93%) are adenocarcinomas, and 48% produce mucin [1]. All UC express CK20 and 50% CK7 [2]. Most patients are male, and the median age at diagnosis ranged from 50 to 58 years in previous studies [1, 3, 4]. Five-year overall survival is only 20% in patients with lymph node or distant metastases and 45% in patients with localized disease [1]. Recent studies show a median overall survival according to the TNM score ranging from 6.2 to 10.8 years for stages I and II, 2.8 years for stage III, and only 1 year for metastatic disease [4, 5]. Lymph node, lung, peritoneal, omental, mesenteric, brain, and liver metastases are the most frequent ones [5].
Case Presentation
A 31-year-old female presented with hematuria in July 2010. Clinical examination was normal. Ultrasound and a computed tomography (CT) scan were performed, showing a 2-cm intravesical mass on the bladder dome. In September 2010, cystoscopy revealed a right lesion on the bladder dome (fig. 1). Transurethral resection of the bladder tumor confirmed a well-differentiated adenocarcinoma, with an immunohistochemistry profile in favor of an urachal tumor. The patient was pregnant, and a therapeutic abortion was performed at 12 weeks of amenorrhea. The colonoscopy, gastroscopy and bone scans were normal.
Fig. 1.
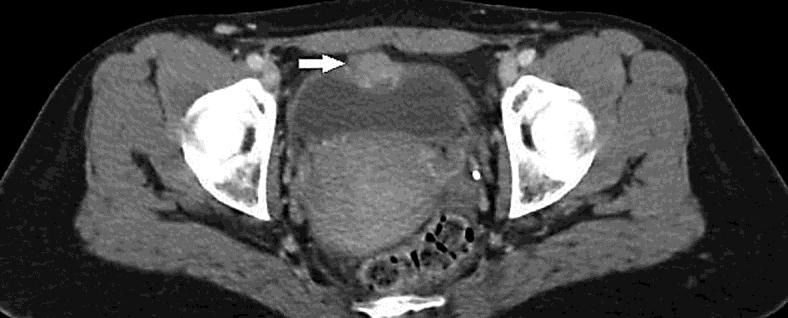
CT scan in September 2010. Intravesical mass on the bladder dome.
She had a partial cystectomy with excision of the urachus and umbilicus as well as bilateral pelvic lymph node dissection in October 2010. The anatomopathological examination confirmed an urachal adenocarcinoma, mucinous subtype. The 2.5-cm tumor infiltrated the entire bladder wall up to the fat, with perineural and vascular invasion (PT3b pN0 R0). The tumor was CK7 negative, CK20 positive, and ACE positive. The positron emission tomography (PET) scan performed in January 2011 was normal, so she was monitored every 3 months first and then every 6 months until May 2014, with no evidence of disease. The patient got pregnant during this follow-up and gave birth in February 2014.
In May 2014, she noticed visual disturbances. Clinical examination was normal except for a bilateral visual acuity of 7/10. There was no suspicious skin lesion. The ophthalmologist found a hyperechoic lesion on B-scan ultrasonography of 7 × 2 mm in the left eye, without signs of pigmentation or exudative retinal detachment and without any choroidal excavation (fig. 2). These clinical and acoustic features were atypical for any of the following diagnoses: achromic choroidal nevi, malignant melanoma, or choroidal metastasis. The patient was breastfeeding, so the angiography was only performed in October 2014. An increase in the lesion size (9 mm) with subretinal pigmentation and exudative retinal detachment was found, which was suggestive of a choroidal metastasis.
Fig. 2.
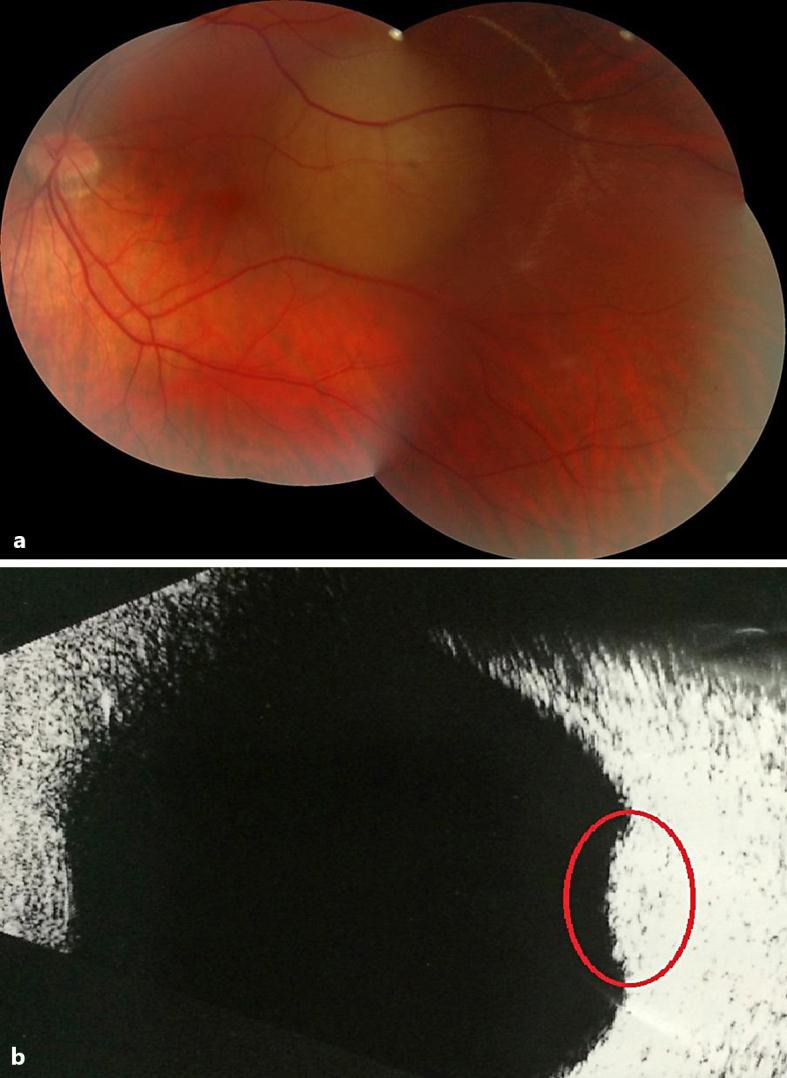
Left fundus (a) and left B-scan ultrasonography (b) in May 2014. Nonpigmented choroidal lesion located in the posterior pole that results in an echogenic choroidal mass (circle) without choroidal excavation.
In October 2014, a PET scan showed hypermetabolic mediastinal lymph nodes and pulmonary and right adrenal lesions.
In November and December 2014, the fine-needle aspirate of mediastinal lymph nodes, the multiple lung microbiopsies, and the bronchoalveolar lavage fluid showed only inflammatory cells, without dysplasia or active tumor. The patient refused surgical lung biopsy.
In December, the ophthalmologist found a second choroidal lesion located in the inferior periphery of the fundus, whereas the posterior pole tumor showed a marked growth in size with subretinal pigmented spicules. Multiple achromic choroidal masses developing quite rapidly were strong arguments for the diagnosis of choroidal metastases and were not relevant for choroidal primary melanoma. She received ocular intensity-modulated radiotherapy (50 Gy) in February and March 2015.
At the end of February 2015, she felt nodes in her right breast (fig. 3a). Magnetic resonance imaging (MRI) showed more than 10 nodules in each breast (the biggest ones were 33 × 14 and 20 × 10 mm). The biopsies confirmed UC metastasis. The initial histological pattern and the biopsy of the metastasis were compared and confirmed the same morphology. The immunohistochemical analysis of the metastatic lesion was ER–, PR–, HER2–, CK7–, CK20+, and ACE+, identical to the primary tumor. A primary breast adenocarcinoma diagnosis was excluded.
Fig. 3.
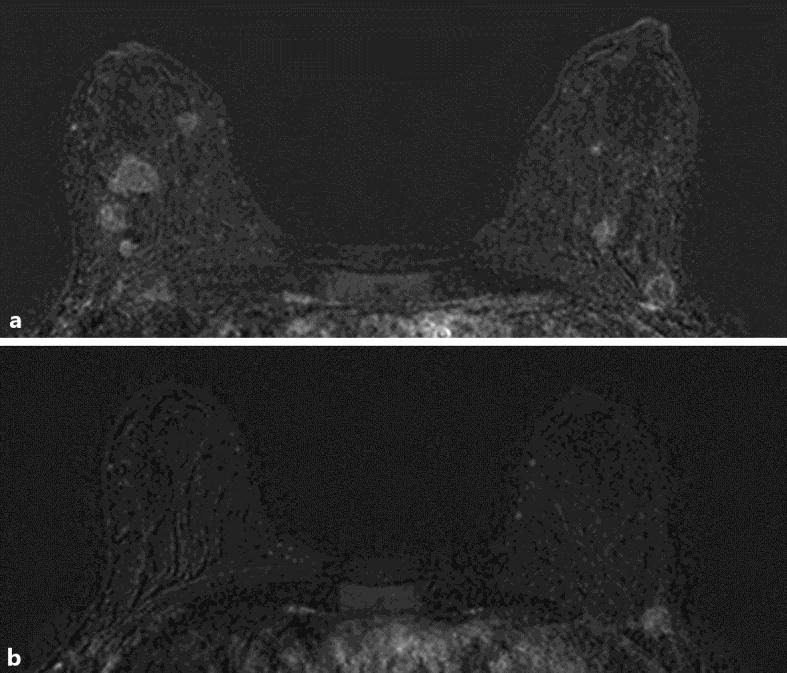
Breast MRI with multiple nodes in February 2015 (a) and at the end of the treatment in May 2015 (b).
A PET scan showed peritoneal carcinomatosis, multiple mammary metastases, a pathological fracture of L1, an increase in the mediastinal lymph nodes, and pulmonary and right adrenal metastases.
She received chemotherapy from April to September 2015 with the modified docetaxel, cisplatin and 5-fluorouracil (mTPF) regimen every 14 days: docetaxel 40 mg/m2 + cisplatin 40 mg/m2 + 5-FU bolus 400 mg/m2 and 2,000 mg/m2 over 48 h. She was included in a clinical trial (PROFILER) to analyze the molecular profile of the tumor. The results were obtained 3 months after the beginning of the chemotherapy, and 2 mutations of PIK3CA and 1 mutation of TP53 were found.
She showed partial clinical and radiological response after 10 cycles with disappearance of the retinal detachment, partial regression of the choroidal metastases, persistence of only 2 lesions in the right breast (8 and 4 mm) and 2 in the left breast (11 and 6 mm) on MRI (fig. 3b), and global stability on the CT scan. The patient is now monitored every 3 months.
Conclusions
We report the first case of a 36-year-old woman with urachal adenocarcinoma who relapsed with left choroidal, lung, mediastinal lymph node, right adrenal, mammary, and bone metastases as well as peritoneal carcinomatosis 3 years after partial cystectomy.
The evolution of the disease was extremely fast and explosive, and the choroidal, adrenal, and mammary metastases were very unusual localizations. Uveal metastases (UM) are rare (4–8% of all visceral tumors). The choroid is the most common site for UM (88%), with an average of 2 tumors per eye. UM develop from a primary cancer of the breast in almost half of the patients (47%). The other primary cancers are lung (21%), gastrointestinal tract (4%), kidney (2%), skin (2%), prostate (2%) tumors [6]. The frequency of adrenal metastases is not well known. In a study analyzing 464 patients with metastatic disease in the adrenal glands over a 30-year period in Hong Kong, 90% of the metastatic adrenal tumors were carcinomas and 56% of these were adenocarcinomas. The most common primary sites were the lung (35%), stomach (14%), esophagus (12%), and liver/bile ducts (10%) [7]. Mammary metastases are rare too. They often develop from cutaneous melanomas (31%) and lung cancers (25%). The other primaries are non-Hodgkin lymphoma (15%), soft tissue sarcoma (12%), colon (6%), endometrial, ovary, and bladder cancers [8]. There is only one case report in the literature with both mammary and ocular metastases, and the primary cancer was a mucinous adenocarcinoma of the rectum [9].
The role of pregnancy in the occurrence of tumor appearance or relapse is unclear. Pregnancy induces a state of physiological immunosuppression but also immunological tolerance in order to protect the fetus from maternal immune response. It is often thought that pregnancy after cancer increases the risk of relapse, but in 2013, a multicenter retrospective cohort study analyzed the impact of pregnancy on disease-free survival in females with a history of breast cancer. Three hundred and thirty-three pregnant patients were matched with 874 nonpregnant patients, and no difference in disease-free survival was observed between the two groups in the ER-positive or the ER-negative cohorts. In conclusion, pregnancy after breast cancer does not seem to have an impact on the risk of breast cancer recurrence [10].
The patient now shows partial response after 10 cycles of chemotherapy with the mTPF regimen. This is the first report on the use of the mTPF regimen in UC. Since UC is of the enteric-type histologically, with the same mucin-producing component as gastric or colorectal adenocarcinomas, chemotherapy used to treat gastrointestinal tumors is often used for metastatic disease, and the choice is based on several case reports. Combinations of existing drugs (fluorouracil, taxanes, oxaliplatin and cisplatin, gemcitabine, capecitabine, irinotecan, and leucovorin) have already been tested, with variable response rates. The mTPF regimen could be a good strategy in metastatic UC considering that the TPF regimen is the standard first-line treatment for metastatic gastric cancer.
Statement of Ethics
Written informed consent for the publication of this paper was obtained from the patient.
Disclosure Statement
The authors declare that they have no conflicts of interest to disclose.
References
- 1.Bruins HM, Visser O, Ploeg M, Hulsbergen-van de Kaa CA, Kiemeney LA, Witjes JA. The clinical epidemiology of urachal carcinoma: results of a large, population based study. J Urol. 2012;188:1102–1107. doi: 10.1016/j.juro.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Paner GP, McKenney JK, Barkan GA, Yao JL, Frankel WL, Sebo TJ, et al. Immunohistochemical analysis in a morphologic spectrum of urachal epithelial neoplasms: diagnostic implications and pitfalls. Am J Surg Pathol. 2011;35:787–798. doi: 10.1097/PAS.0b013e3182189c11. [DOI] [PubMed] [Google Scholar]
- 3.Dhillon J, Liang Y, Kamat AM, Siefker-Radtke A, Dinney CP, Czerniak B, Guo CC. Urachal carcinoma: a pathologic and clinical study of 46 cases. Hum Pathol. 2015;46:1808–1814. doi: 10.1016/j.humpath.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, Li Y, Yu Z, et al. Investigating urachal carcinoma for more than 15 years. Oncol Lett. 2014;8:2279–2283. doi: 10.3892/ol.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina JR, Quevedo JF, Furth AF, Richardson RL, Zincke H, Burch PA. Predictors of survival from urachal cancer: a Mayo Clinic study of 49 cases. Cancer. 2007;110:2434–2440. doi: 10.1002/cncr.23070. [DOI] [PubMed] [Google Scholar]
- 6.Shields CL, Shields JA, Gross NE, et al. Survey of 520 eyes with uveal metastases. Ophtalmology. 1997;104:1265–1276. doi: 10.1016/s0161-6420(97)30148-1. [DOI] [PubMed] [Google Scholar]
- 7.Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002;56:95–101. doi: 10.1046/j.0300-0664.2001.01435.x. [DOI] [PubMed] [Google Scholar]
- 8.Wood B, Sterrett G, Frost F, Swarbrick N. Diagnosis of extramammary malignancy metastatic to the breast by fine needle biopsy. Pathology. 2008;40:345–351. doi: 10.1080/00313020801911520. [DOI] [PubMed] [Google Scholar]
- 9.Hisham RB, Thuaibah H, Gul YA. Mucinous adenocarcinoma of the rectum with breast and ocular metastases. Asian J Surg. 2006;29:95–97. doi: 10.1016/S1015-9584(09)60115-9. [DOI] [PubMed] [Google Scholar]
- 10.Azim HA, Jr, Kroman N, Paesmans M, Gelber S, Rotmensz N, Ameye L, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol. 2013;31:73–79. doi: 10.1200/JCO.2012.44.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]


