Abstract
Eye and ear employ specialized glutamatergic synapses that feature an elaborate electron‐dense projection—the synaptic ribbon. Despite major efforts, the function of the synaptic ribbon has remained enigmatic, because its brick‐stone‐like core‐component RIBEYE has remained hard to crack genetically. In an elegant study, Maxeiner et al (2016) genetically deleted RIBEYE in mice. This abolished retinal ribbons and impaired exocytosis at the presynaptic active zone of bipolar cells.
Subject Categories: Neuroscience
Coding of light and sound in our eyes and ears features amazing performance still unparalleled by even the smartest technology. For example, these sensory organs process light and pressure waves spanning many orders of magnitude in real time for hours with great temporal fidelity. Like in a photocamera, the eye deals with different light intensities with changing the size of the pupil. However, different from the chip of a camera, the retina implements several mechanism of light adaptation on various timescales. Beyond adaptation at the stage of phototransduction, processing at specialized glutamatergic synapses of the retina contributes to such adaptation and in addition detects contrast (e.g., Jackman et al, 2009; Oesch & Diamond, 2011).
The benefits of using chemical synapses as a dynamic and tunable system to code sensory stimuli across the large range of behaviorally relevant light and sound intensities offer one intuitive explanation for why nature evolved the eye and the ear to employ synaptic pre‐processing rather than direct action potential coding as is done in the primary sensory cells of the somatosensory and olfactory systems. However, this also comes at a prize: The metabolic costs are increased, and the indefatigable release of transmitter at rates of hundreds of Hertz per second required evolution to come up with a specialized synaptic machinery. Most notably, active zones of the afferent glutamatergic synapses in the eye and ear feature an elaborate electron‐dense multiprotein complex—the synaptic ribbon that is decorated by a halo of synaptic vesicles.
The synaptic ribbon is an enigmatic nanomachine thought to relate to the high rate of transmission of sensory information. The ribbon is primarily composed of the protein RIBEYE of which the A‐domain is unique but the B‐domain is transcribed from the same gene as the transcriptional corepressor C‐terminal binding protein 2 (CtBP2; Schmitz et al, 2000). RIBEYE forms aggregates (Schmitz et al, 2000) via multiple self‐interactions (Magupalli et al, 2008) but testing the function of RIBEYE has remained challenging (e.g., Wan et al, 2005) as genetic interference needs to conserve the function of CtBP2 that is essential for survival. Therefore, alternative approaches have been taken to study the role of the synaptic ribbon for sensory processing in the eye and ear such as genetic disruption of bassoon that anchors the ribbon to the active zone (Dick et al, 2003; Khimich et al, 2005) and photoablation (Snellman et al, 2011). These studies indicated a role of the ribbon in promoting a large readily releasable pool of vesicles at the active zone and facilitating its replenishment, both working in favor of high rates of synchronous and sustained transmission. However, uncertainties remained: Bassoon disruption also reduced the Ca2+ current in hair cells (Khimich et al, 2005), and photoablation might also affect proteins other than RIBEYE, for example at the arciform density underneath the ribbon.
Maxeiner and colleagues, in this issue of The EMBO Journal, now succeeded to genetically disrupt RIBEYE and to abolish retinal ribbons without much reduction in the abundance of CtBP2 or of active zone proteins in the retina. Moreover, except for some bipolar cell dendritic sprouting into the outer nuclear layer as also found in bassoon mutant mice (Dick et al, 2003), retinal morphology seemed unaltered. While Ca2+ channels clusters extend along the base of the horseshoe‐shaped rod photoreceptor ribbon in wild type, they seemed disintegrated into smaller spots reminiscent of findings in bassoon‐deficient hair cells. Membrane proximal vesicles, thought to constitute the readily releasable pool of vesicles, were reduced at the ribbonless rod photoreceptor synapse.
Figure 1. Genetic manipulation of the ribbon synapse of retinal rod photoreceptor.
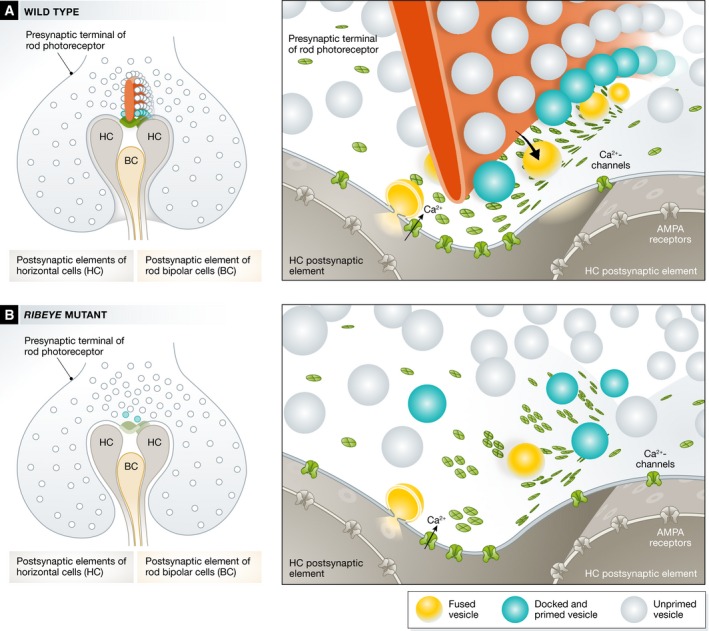
Photoreceptor synapses with horizontal cells (HC) and bipolar cells (BC) show a presynaptic electron‐dense horseshoe‐shaped ribbon that tethers synaptic vesicles of which the lowest rows are thought to reside within 30 nm from Ca2+ channels at the active zone and are thought to be primed for fusion upon Ca2+ influx (A). Genetic disruption of RIBEYE (B) causes a loss of the synaptic ribbon and a mislocalization of the Ca2+ channels at the active zone likely leading to impaired coupling of Ca2+ influx and exocytosis.
Paired pre‐ and postsynaptic recordings from the ribbon synapse between rod bipolar cells and AII amacrine cells were used to characterize the effects of RIBEYE deletion on synaptic transmission with great resolution. This analysis demonstrated that Ca2+ influx was unchanged but RIBEYE/the ribbon is required for both phasic and sustained synaptic transmission. Spontaneous release was preserved but displayed a higher sensitivity to the slow Ca2+ chelators EGTA, which is indicative of an impaired spatial coupling between Ca2+ channels and vesicular release sites. The data collectively indicate that RIBEYE/the ribbon promotes a large complement of vesicular release sites and their replenishment. In addition, the ribbon seems involved in establishing the tight coupling of Ca2+ channels and release sites at this synapse. Future work will be required to analyze the topographies of Ca2+ channels and vesicular release sites and to study their coupling during presynaptic voltage‐clamp stimulation, which is thought to involve control of vesicle exocytosis at each site by a Ca2+ nanodomain generated by few nearby Ca2+ channel(s) (Brandt et al, 2005; Jarsky et al, 2010).
See also: S Maxeiner et al (May 2016)
References
- Brandt A, Khimich D, Moser T (2005) Few CaV1. 3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J Neurosci 25: 11577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermüller J, Weiler R, Garner CC, Gundelfinger ED, Brandstätter JH (2003) The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron 37: 775–786 [DOI] [PubMed] [Google Scholar]
- Jackman SL, Choi S‐Y, Thoreson WB, Rabl K, Bartoletti TM, Kramer RH (2009) Role of the synaptic ribbon in transmitting the cone light response. Nat Neurosci 12: 303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Tian M, Singer JH (2010) Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci 30: 11885–11895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, tom Dieck S, Egner A, Gundelfinger ED, Moser T (2005) Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature 434: 889–894 [DOI] [PubMed] [Google Scholar]
- Magupalli VG, Schwarz K, Alpadi K, Natarajan S, Seigel GM, Schmitz F (2008) Multiple RIBEYE‐RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons. J Neurosci 28: 7954–7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxeiner S, Luo F, Tan A, Schmitz F, Südhof TC (2016) How to make a synaptic ribbon: RIBEYE deletion abolishes ribbons in retinal synapses and disrupts neurotransmitter release. EMBO J 35: 1098–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch NW, Diamond JS (2011) Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat Neurosci 14: 1555–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F, Königstorfer A, Südhof TC (2000) RIBEYE, a component of synaptic ribbons: a protein's journey through evolution provides insight into synaptic ribbon function. Neuron 28: 857–872 [DOI] [PubMed] [Google Scholar]
- Snellman J, Mehta B, Babai N, Bartoletti TM, Akmentin W, Francis A, Matthews G, Thoreson W, Zenisek D (2011) Acute destruction of the synaptic ribbon reveals a role for the ribbon in vesicle priming. Nat Neurosci 14: 1135–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Almers W, Chen W (2005) Two ribeye genes in teleosts: the role of Ribeye in ribbon formation and bipolar cell development. J Neurosci 25: 941–949 [DOI] [PMC free article] [PubMed] [Google Scholar]


