Abstract
The link between tobacco abuse and cancer is well-established. However, emerging data indicate that toxins in tobacco smoke cause cellular injury due to enhanced toxic/metabolic effects of metabolites, disruption of intracellular signaling mechanisms, and formation of DNA, protein, and lipid adducts that impair function and promote oxidative stress and inflammation. These effects of smoking, which are largely non-carcinogenic, can be produced by tobacco-specific nitrosamines and their metabolites. These factors could account for the increased rates of neurodegeneration and insulin resistance diseases among smokers. Herein, we review nicotine and tobacco-specific nitrosamine metabolism, mechanisms of adduct formation, DNA damage, mutagenesis, and potential mechanisms of disease.
Keywords: Tobacco-specific nitrosamine, NNK, Smoking, Nicotine, Carcinogenesis, Adduct, Neurodegeneration, Diabetes, Tobacco
Overview of tobacco, tobacco-specific nitrosamines, and disease
The public health problem
Globally, tobacco and alcohol are the most widely abused legal drugs and their associated public health costs are vast and burdensome to society. Tobacco abuse eventually kills 50 % of its users, including nearly six million people per year worldwide, over five million of which are direct consumers, while the remainder are largely victims of second-hand exposures. In the USA, smoking kills over 430,000 people per year. Sadly, market forces drive tobacco consumption in low- and middle-income countries, a phenomenon that could catapult tobacco’s annual mortality to over eight million by 2030 [99]. Although lung and other cancers tend to be the main focus of tobacco-associated health concerns, other significant and often fatal consequences of tobacco abuse include emphysema, cardiovascular and cerebrovascular disease, aging, asthma, chronic obstructive pulmonary disease (COPD), diabetes, and fetal developmental abnormalities [91]. Another important fact that must be incorporated into the equation for societal and healthcare costs is that among adolescents and young people, tobacco abuse is often a gateway to other drugs that could be even more addictive and destructive to human life.
Tobacco’s use and abuse are linked to nicotine’s stimulating and addictive properties. Nicotine is released by all manners of tobacco consumption including smoking, chewing, sucking, snuff dipping (placing a pinch of snuff between the cheek and gum), and snusing (placing moist snuff under upper lip). In addition, electronic (“e”) cigarettes are a rapidly growing source of nicotine exposure. Smoking and consumption of smokeless tobacco pose health risks due to concomitant exposures to toxic alkaloid metabolites of nicotine and tobacco-specific nitrosamines. Tobacco-specific nitrosamines are highly carcinogenic and form bulky adducts with DNA and hemoglobin [38]. Tobacco-specific nitrosamines are detectable in saliva, and their metabolites are measurable in urine.
Carcinogenesis and genotoxicity
Tobacco’s role in cancer was confirmed by the Working Group of the International Agency for Research on Cancer (IARC), whose report highlighted experimental data showing that exposures to whole cigarette smoke induce malignant neoplasms of the respiratory tract in hamsters and rats and that direct skin and pulmonary exposures to smoke cause skin and lung cancers, respectively [37]. In humans, the main cause of lung cancer is tobacco smoking, and lung cancer risk and mortality correlate with smoking duration and dose, i.e., pack-years. In addition, smoking and smokeless tobacco exposures increase carcinogenesis of the upper respiratory and digestive tracts (oral, oropharyngeal, hypopharyngeal, laryngeal, and oesophageal), bladder, pancreas, renal pelvis, liver, large bowel, and stomach [91]. However, with regard to many non-pulmonary malignancies, tobacco-specific nitrosamines seem to function as carcinogenic co-factors along with heavy alcohol abuse.
Besides nicotine and tobacco-specific nitrosamine exposures, smoking causes tissue buildup of tar, a harmful, highly toxic, partially combusted particulate, resinous substance. Tar contains most of the carcinogenic and genotoxic substances in tobacco smoke [37]. Tar damages and kills ciliated respiratory epithelial cells, impairing their ability to trap airborne toxic particulate matter. Consequently, tar descends into the lower respiratory tract and alveoli where it exerts its carcinogenic effects. Moreover, tar is responsible for toxic lung injury, tooth blackening and rotting, gum damage, and desensitization of taste buds. It is not yet known whether health threats are lowered in individuals who consume cigarettes that contain substantially lower levels of tar and nicotine [37].
Non-carcinogenic effects—links to insulin resistance diseases
Besides cancer, epidemiological studies suggest cigarette smoking is associated with cardiovascular, metabolic, and reproductive diseases. Hypertension is a major contributor to cardiovascular morbidity and mortality. Cigarette smoking greatly increases the risk of hypertension, ultimately resulting in coronary heart disease, stroke, and cardiac failure [41]. Moreover, experimental and clinical studies suggest that smoking decreases insulin sensitivity and results in glucose and lipid metabolism disorders such as hyperglycemia and dyslipidemia [100]. Diabetic patients who smoke need larger insulin doses to achieve similar metabolic control to that of non-smoker diabetics [49]. Smoking exacerbates non-alcoholic fatty liver disease in obese rats by increasing oxidative stress and apoptosis in the liver [4]. Tobacco use also has detrimental effects on female and male fertility. Smoking before and during pregnancy is a major cause of reduced fertility as well as maternal, fetal, and infant morbidity and mortality [91]. Although these epidemiological and experimental studies show causal associations between smoking and a range of diseases, the mechanism of nitrosamine action behind these effects remains to be elucidated.
The long-term adverse effects of smoking on brain structure and function are under investigation. However, several studies provide evidence that heavy cigarette smoking adversely affects neurocognitive function [17] and brain white matter structure (neuroimaging) [94], suggesting that tobacco and its toxic metabolites may cause neurodegeneration. Several neuroimaging studies have shown smoking-related brain abnormalities in humans [13, 18, 19, 46], and meta-analysis revealed smoking-related gray matter loss in the anterior cingulate, prefrontal cortex, and cerebellum [59]. Furthermore, there is some evidence that chronic smoking causes frontal and temporal lobe atrophy with volume loss in gray matter structures. The main limitation of these works is that most studies have been under-powered due to heterogeneous levels and durations of tobacco smoke exposure and inability to correlate long-term biomarkers of smoking with brain atrophy and cognitive impairment. Future studies should determine the agents in tobacco smoke that mediate neurodegeneration and cognitive impairment, as that information could be used to develop assays that monitor exposure and early stage effects of neurodegeneration. The present review focuses on mechanisms of tobacco exposure-mediated cellular injury and disease.
Nicotine
Bio-distribution and addiction
Tobacco contains several alkaloids including nicotine, nornicotine, anabasine, anatabine, and myosmine. Nicotine is the only tobacco component responsible for addiction and the main alkaloid present in both unburned tobacco and tobacco smoke. Nicotine comprises between 1 % and 2 % of unburned tobacco. Nicotine inhaled with smoke or released during smokeless tobacco or e-cigarette consumption rapidly enters the circulation and achieves plasma concentrations of about 15 ng/mL [37]. Nicotine then quickly distributes to various organs, including liver, kidney, spleen, lung, and brain [43]. Nicotine crosses all membranes, including skin and placenta. It accumulates in fetal serum and amniotic fluid and is detectable in breast milk of smoking mothers [10]. Moreover, during development, inadequate detoxifying mechanisms render fetuses and infants more vulnerable to the adverse effects of nicotine.
Within 10 or 20 s after tobacco smoke is inhaled, nicotine crosses the blood–brain-barrier. Selectively high nicotine uptake in the brain [43] could account for nicotine’s prominent neurobehavioral effects. Nicotine binds to nicotinic cholinergic receptors containing alpha7 subunits (nAChR-alpha7), which are ligand-gated ion channels, stabilizing their open state and thereby allowing influx of cations, including calcium, potassium, and sodium ions. Nicotine stimulation of nAChR releases neurotransmitters such as dopamine, norepinephrine, serotonin, and gamma-amino butyric acid (GABA), resulting in feelings of well-being, relaxation, calmness, alertness, and euphoria, improved learning, memory, and information processing, and suppression of appetite, pain, and anxiety [22].
Nicotine neuroprotection
Nicotine has demonstrated neuroprotective effects, functioning through nAChR to increase dopamine release in nigral dopaminergic neurons [74, 75, 89] and counteract adverse effects of reduced dopamine release in the neostriatum [40]. These actions account for nicotine’s amelioration of motor symptoms in Parkinson’s disease [98] and epidemiologic data showing lower rates of Parkinson’s and other neurodegenerative diseases among smokers [69, 74, 75, 98]. The findings that prior nicotine exposures reduce glutamate-[1], NMDA-[50], kainate-[77], and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [76]-mediated neurotoxicity and improve neuronal survival further support the concept that nicotine is neuroprotective. Additional avenues of nicotine neuroprotection include, alteration of dopamine receptor dynamics rather than prevention of neuronal loss [21], induction of tyrosine hydroxylase plasticity via stimulation of dopamine synthetic pathways [92], and enhancement of long-term potentiation in hippocampal neurons by increasing NMDA receptor responses [104]. It is noteworthy that impairments in nAChR expression or function are linked to aging and aging-associated neurodegeneration [12, 69, 98].
Mechanistically, nicotine’s stimulatory effects on nAChR and synaptic transmission can occur via calcium influx which induces vesicular neurotransmitter release [22]. Consequently, nicotine initiates synaptic potentiation by enhancing glutamatergic excitation and decreasing GABA inhibition on dopaminergic neurons [70]. However, after chronic exposure, the protective effects of nicotine can be independent of calcium flux or accumulation, as occurs with respect to NMDA damage [72], indicating that long-term neuroprotection utilizes different mechanisms. For example, nicotine stimulation can promote cell growth and survival by activating nAChRs via PI3K-Akt [89] and reducing oxidative stress-induced apoptosis in astrocytes [48].
Nicotine and neurodegeneration
Following short-term exposure, toxic levels of nicotine can cause ganglionic blockade leading to bradycardia, hypotension, and depression [6]. Although many positive nicotine effects occur with short-term stimulation, evidence suggests that chronic continuous exposures can be harmful to degenerating nigro-striatal dopamine neurons and worsen choline acetyltransferase dysfunction in limbic [20] and forebrain [7] circuitry. In addition, while low-dose cigarette smoke exposures have been touted as protective against toxin-induced Parkinson’s disease, high doses are not [60]. Another study showed that chronic tobacco smoke exposure exacerbates amyloid pathology in an Alzheimer’s transgenic mouse model [54]. Two important concepts must be considered with respect to potential negative effects of nicotine exposure: metabolism and stage of development. With prolonged or continuous exposures, accumulation of cotinine, a stable toxic metabolite of nicotine, can cause tissue injury leading to glia activation [11]. Unlike nicotine, cotinine is not neuroprotective [61]. Age-related sensitivity to nicotine is well-documented with respect to its adverse effects on dopaminergic [71] and seratonergic [102] systems following adolescent or prenatal exposures. In contrast to the stimulatory effects in adults, nicotine exposures during adolescence increase hippocampal apoptosis with loss of neurons and glia [58] and damage 5HT projections [102]. It would be of interest to determine if development- or age-related sensitivities to nicotine are mediated by enhanced metabolism to cotinine and other stable toxic metabolites.
Nicotine metabolism
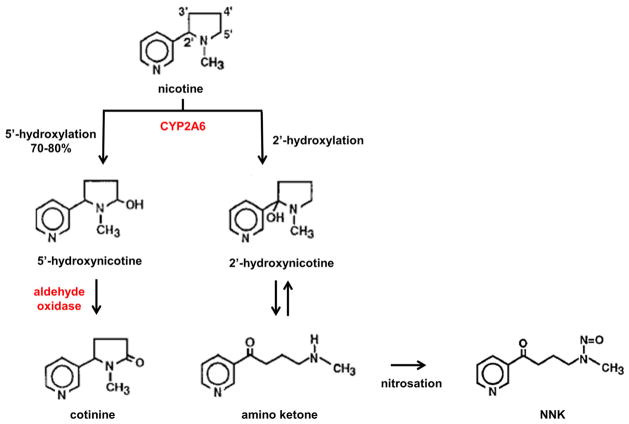
Experimental animal studies have shown that nicotine is rapidly metabolized by cytochrome P450 (CYP) in liver, lung, kidney, nasal mucosa, and brain [93]. In liver, 70–80 % of nicotine is 5′-hydroxylated to 5′-hydroxynicotine, mainly by CYP2A6, and lesser extents by CYP2B6 and CYP2D6. The hydroxyl intermediate is then oxidized by aldehyde oxidase to form cotinine, which is further metabolized by glucuronidation and excreted in the urine. However, CYP2A6 can also hydroxylate nicotine, yielding 2′-hydroxynicotine, which spontaneously forms an amino ketone (4-(methylamino)-1-(3-pyridyl)-1-butanone) intermediate. Nitrosation of amino ketone produces the tobacco pro-carcinogen, nitrosamine ketone (NNK) [29] (Fig. 1). Since the dominant CYP2A6-generated metabolite of nicotine is cotinine, whose t1/2 is ~16 h compared with ~2 h for nicotine, and whose plasma levels are considerably higher than those of nicotine (275 versus 15 ng/mL) [37], cotinine is used as an indicator of environmental exposure to tobacco smoke.
Fig. 1.
Nicotine hydroxylation pathways in human liver. CYP2A6 can hydroxylate nicotine at either the 5′ or 2′ position. Hydroxylation at the 5′ position generates the 5′-hydroxynicotine intermediate, which is then oxidized to form cotinine. Cotinine is excreted in urine. Hydroxylation at the 2′ position spontaneously forms an amino ketone intermediate whose subsequent nitrosation produces the tobacco pro-carcinogen, nicotine-derived nitrosamine ketone (NNK)
The less frequent CYP2A6-induced 2′-hydroxylation of nicotine is problematic because the tertiary amine structure of amino ketone can react with nitrosating agents and produce stable chemicals known as nitrosamines. For example, nitrites present in saliva can nitrosate tobacco and tobacco smoke alkaloids to produce nitrosamines [30]. Nitrosation reactions occur by replacement of N–H with N–N=O in the case of secondary amines or via oxidative cleavage of carbon-nitrogen bonds of tertiary amines [28]. Nitrosation of nicotine yields the pro-carcinogenic nitrosamines, NNK, nitrosamino aldehyde (NNA), and N′-nitrosonornicotine (NNN). These points raise concerns about the safety and health effects of long-term, unregulated e-cigarette consumption.
Tobacco-specific nitrosamines
Tobacco-specific nitrosa mines, in clud ing 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone or nicotine-derived NNK, NNN, nitrosaminoaldehyde (NNAL), N-nitrosoanatabine (NAT), N-nitrosoanabasine (NAB), iso-NNAL, and iso-N-nitrosamino acids (iso-NNAC), are non-volatile, present in unburned tobacco leaves and tobacco smoke, and pro-carcinogenic. Therefore, both smoked and smokeless tobacco products contain nitrosamines. NNK, NNN, and NNAL have the most potent pro-carcinogenic activities. Nitrosamines exist in unburned tobacco leaves because they are produced with processing, i.e., curing, fermentation, and aging. For example, high levels of NNK (0.42 μg/g) are present in Nicotiana tabacum leaves grown in the USA. In addition, high levels of NNK, NNN, and NAT are detectable in tobacco smoke [26] and environments polluted by tobacco smoke [30] and therefore could contribute to carcinogenesis via second-hand exposures. Although nitrosamines induce tumors in various organs throughout the body, including esophagus, lung, liver, pancreas, and bladder, chemical structure and animal species are important factors dictating their targets. For example, lung is the major organ for NNK-mediated carcinogenesis [73]. In F344 rats, NNK is far more potent than N-nitrosodimethylamine (NDMA) in causing lung and nasal cavity tumors [32]. NNK’s substantial potency as a pro-carcinogen is further demonstrated by the development of respiratory tract and lung tumors in Syrian gold hamsters or A/J mice following a single dose of NNK (~1 mg) [27]. In humans, NNAL and NNAL-glucuronide levels are reliable indices of NNK exposure. Among current light or non-smokers (<10 cigarettes/day), moderate smokers (10–<20/day), and heavy smokers (20+/day) in a Shanghai Cohort Study from 1986 to 2008, total urinary NNAL concentrations (geometric mean (95 % CI)) in femtomole per milligram creatinine were 210 (158–280), 372 (308–449), and 475 (404–559), respectively [105].
NNK metabolism
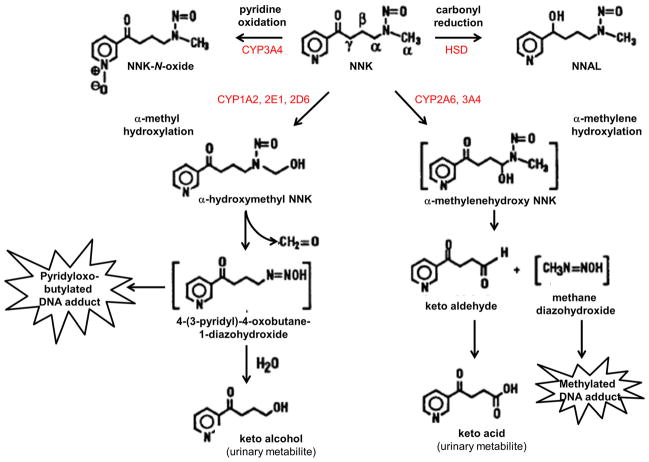
Mechanistically, tobacco-specific nitrosamines cause tissue injury and malignant transformation by forming adducts with DNA. These processes require metabolic activation of the nitrosamines. Six different pathways regulate NNK metabolism. In vitro experiments showed that NNK metabolism can be activated via (1) reduction of the carbonyl group, (2) oxidation of the pyridine nitrogen, (3) α-hydroxylation of the methylene carbon adjacent to the N-nitroso nitrogen, (4) α-hydroxylation of methyl carbons adjacent to the N-nitroso nitrogen, (5) dinitrosation, and (6) ADP adduct formation. In vivo studies confirmed NNK metabolism via carbonyl reduction, pyridine oxidation, and α-hydroxylation but not dinitrosation or ADP adduct formation [26].
Carbonyl reduction
Carbonyl reduction of NNK to yield NNAL is the dominant pathway for NNK metabolism in several species and tissues. 11β-Hydroxysteroid dehydrogenase (EC 1.1.1.146) is one such carbonyl reductase enzyme [52] (Fig. 2). Carbonyl reductase-mediated metabolism of NNK is reflected by elevated tissue levels of NNAL following NNK or tobacco smoke exposure. Tissue- and species-specific carbonyl reductase activation was demonstrated by the findings of increased NNAL levels in rat intestine and pancreas, rodent (rat, mouse, and hamster) and human livers, and human lung, erythrocytes, but not in rat nasal mucosa following NNK or tobacco smoke exposures either in vivo [34, 83] or in vitro [2, 67]. NNAL is further metabolized by glucuronidation, resulting in increased levels of NNAL-glucuronide in urine [31]. NNAL and NNAL-glucuronide are reliable and robust indices of NNK exposures in humans. The efficiency of NNK detoxification via carbonylation and glucuronidation correlates with tissue expression levels and gene polymorphisms [51]. Another potentially important factor is the rate in which NNAL is oxidized back to NNK. Therefore, although NNAL lacks carcinogenic activity, in some cell types and perhaps under certain conditions such as inefficient NNK carbonylation/NNAL-glucuronidation, its partial oxidation back to NNK could contribute to tumorigenesis.
Fig. 2.
NNK metabolism. In humans, NNK is metabolized through four different pathways: (1) carbonyl reduction, yielding 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL); (2) pyridine ring oxidation, yielding NNK-N-oxide; (3) α-hydroxylation of methyl carbons adjacent to the N-nitroso nitrogen, producing pyridyloxobutylated DNA adduct; and (4) α-hydroxylation of the methylene carbon adjacent to the N-nitroso nitrogen, producing a methylated DNA adduct [26]
Pyridine oxidation
CYP2B1 and CYP3A4 metabolize NNK to NNK-N-oxide via oxidation of the pyridine nitrogen. This reaction occurs in microsomes of rat and mouse lungs [85], but not livers or nasal mucosa [25, 66], and accounts for less than 10 % of NNK metabolism, based on the levels of NNK-oxide detected in urine [34]. A specific role for CYP2B1 in pyridine oxidation of NNK was strongly suggested by its isolation and detection in rat liver, catalytic activity, and metabolism of NNK in human kidney cells transfected with the corresponding cDNA [23], induction in rat livers that were pretreated with phenobarbital [25], and decreased oxidative metabolism of NNK in rat liver, lung, and nasal microsomes that were treated with phenethyl isothiocyanate (PEITC), a chemical inhibitor of CYP2B1 [84]. In human liver microsomes, CYP3A4 rather than CYP2B1 mediates pyridine oxidation of NNK [88] (Fig. 2).
α-Methyl hydroxylation
NNK can be metabolized by hydroxylation at the methyl group adjacent to N-nitroso, resulting in the formation of α-hydroxymethyl-NNK. α-Hydroxymethyl-NNK decomposes to formaldehyde and 4-(3-pyridyl)-4-oxobutane-1-diazohydroxide. Reaction of 4-(3-pyri-dyl)-4-oxobutane-1-diazohydroxide with water yields keto alcohol, which is detectable in urine (Fig. 2) [26]. Alternatively, the 4-(3-pyridyl)-4-oxobutane-1-diazohydroxide NNK metabolite can pyridyloxobutylate DNA and thereby promote carcinogenesis. Two additional pathways for α-hydroxymethyl-NNK metabolism include glucuronidation with formation of α-hydroxymethyl-glucuronide, which is detectable in rat hepatocytes and urine [56], and denitrosation with formation of myosmine [9].
From the above discussion and Fig. 2, it is evident that α-methyl hydroxylation of NNK is one of the main pathways for NNK-mediated DNA damage and carcinogenesis. Correspondingly, the high levels of α-methyl hydroxylation activity in rodent lungs correlate with their high levels of susceptibility to NNK-induced lung cancers [66, 84]. Several CYP isoforms catalyze α-methyl hydroxylation of NNK, but in human liver, CYP1A2 is dominant, while CYP2A6, CYP2D6, CYP2B7, CYP2E1, CYP2F1, CYP3A4, and CYP3A5 have varied roles [62, 83]. The contributions of individual CYP isoforms to α-methyl hydroxylation of NNK have been demonstrated using in vivo and in vitro studies. For example, CYP1A2, CYP2A1, and CYP3A antibody binding was shown to inhibit keto alcohol formation, while enzyme inducers increased keto alcohol generation in NNK-exposed rats [25].
α-Methylene hydroxylation
Hydroxylation of NNK can also occur at its methylene carbon, which yields α-methylenehydroxy-NNK. α-Methylenehydroxy-NNK is an unstable intermediate that rapidly decomposes to methane diazohydroxide and keto aldehyde (Fig. 2). Oxidation of keto aldehyde results in keto acid formation which is detectable in urine and the principal urinary metabolite of NNK in rodents and primates [66]. α-Methylene hydroxylation of NNK significantly contributes to carcinogenesis because DNA methylation by methane diazohydroxide leads to formation of 7-methyl guanine and O6-methyl guanine adducts [15]. CYP2B1, CYP1A2, and CYP3A mediate α-methyl hydroxylation of NNK in rat liver, lung, and nasal mucosa [23, 25, 84], while CYP2A6 and CYP3A4 mediate α-methyl hydroxylation of NNK in human liver [62]. Chemical inhibitors of P450 isoforms decrease α-methylene hydroxylation of NNK, whereas chronic NNK exposures result in increased keto aldehyde binding to P450 catalytic sites [87]. In essence, both α-methyl and α-methylene hydroxylations of NNK mediate carcinogenesis by causing pyridyloxobutylated or methylated DNA adducts to be formed. In vitro studies demonstrated tissue- and species-specific differences in dominance of either or neither pathway [66, 84, 88], which could account for the nature of DNA damage and signatures of NNK-mediated carcinogenesis.
Denitrosation
Denitrosation of NNK results in the formation of an α-carbon radical, followed by elimination of nitric oxide and formation of an imine. Imines are ketone- and aldehyde-related functional groups or chemicals that contain a carbon-nitrogen double bond with the nitrogen attached to an organic group or hydrogen atom. Although imines could potentially be hydrolyzed to keto aldehyde and myosmine, these products have not been detected. On the other hand, nitrite does form in NNK-treated rat liver microsomes, indicating that denitrosation of NNK occurs, at least in vitro. P450s could mediate NNK denitrosation; however, additional research is needed to identify the specific isoforms involved [9, 26].
ADP adduct formation
The ADP adducts, (NNK)ADPH and (NNK)ADP+, are products of NNK metabolism. They are detectable in rat pancreatic and liver microsomes, and their formation is catalyzed by microsomal NAD glycohydrolase. Missing elements in this equation are whether or not ADP adducts are formed in vivo and what role they play in carcinogenesis [67].
DNA adducts from NNK metabolism
DNA pyridyloxobutyl adducts
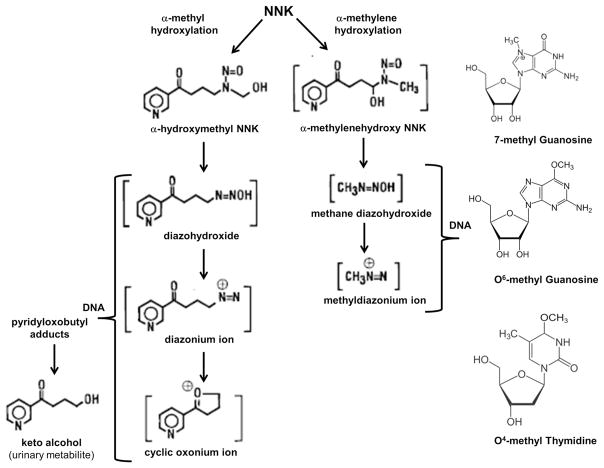
NNK metabolism through α-methyl hydroxylation produces DNA pyridyloxobutyl adducts (Fig. 3). Due to instability of α-hydroxymethyl-NNK, the first product of NNK α-methyl hydroxylation, investigators used an alternative approach that involved solvolysis of 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone (NNKOAc; NNK precursor) to examine DNA pyridyloxobutylation in detail. Those studies demonstrated that α-hydroxymethyl-NNK decomposes to diazohydroxide, followed by diazonium ion, and then cyclic oxonium ion, and that each of these metabolites can form DNA adducts. Since the adducts cause keto alcohol to be released [90] (Fig. 3), they are referred to as “keto alcohol-releasing adducts” [26]. Instability of these adducts has precluded their characterization by HPLC. Instead, keto alcohol release is used as the marker or index of DNA pyridyloxobutylation. Keto alcohol-releasing adducts have been detected in rat and mouse lungs and livers, and in rat nasal mucosa, and have significant roles in NNK-mediated tumor induction [55, 64, 86, 90]. The only structurally identified DNA adduct produced by methyl hydroxylation of NNK is the O6-pyridyloxobutyl adduct, which is formed by reaction of DNA with NNKOAc [95]. Moreover, pyridyloxobutylated adducts containing poly(dGdC) have reported to inhibit O6-alkylguanine DNA alkyltransferase (AGT), which repairs O6-methyl guanine [65]. In rat nasal mucosa, DNA methylation was observed in greater amounts than pyridyloxobutylation, which could be explained by potential glucuronidation of hydroxymethyl-NNK [26, 90].
Fig. 3.
DNA adduct formation from NNK. NNK can be metabolized via α-methyl or α-methylene hydroxylation. α-Methyl hydroxylation yields diazohydroxide, followed by diazonium ion, and then cyclic oxonium ion. Each of these metabolites can form O6-pyridyloxobutyl DNA adducts, releasing keto alcohol as a urinary metabolite and marker of DNA pyridyloxobutylation. α-Methylene hydroxylation of NNK sequentially produces α-methylenehydroxy NNK, methane diazohydroxide, and methyldiazonium ion. These products can react with DNA and yield 7-methyl guanine, O6-methyl guanine, or O4-methyl thymine adducts
DNA methyl adducts
NNK metabolism via α-methylene hydroxylation sequentially produces α-methylenehydroxy NNK, methane diazohydroxide, and methyldiazonium ion, each of which can react with DNA and yield 7-methyl guanine, O6-methyl guanine, and O4-methyl thymine adducts (Fig. 3). Methylated DNA adducts have been detected in rat, hamster, mouse, and human tissues in association with tobacco smoking or NNK exposures. Target organs/tissues include lung, nasal mucosa, and oral tissue [24]. In rat lung, liver, and nasal mucosa and A/J mouse lung, the dominant adduct is 7-methyl guanine, followed by O6-methyl guanine and then O4-methyl thymine [55, 64, 86]. In human lungs, 7-methyldeoxyguanine levels were found to be higher than pyridyloxobutylated adducts [42], and smoking was associated with significantly increased levels of 7-methyl guanine, reflecting contributions of NNK in DNA adduct formation [68]. Methyl-DNA adducts are also detectable in rat and hamster livers following NNK exposure and metabolism. O6-methyl guanine was found to be important in NNK-induced lung carcinogenesis in mice [64] but not hamsters [47], and O6-methyl guanine repair by AGT occurred at a faster rate in the rats compared with hamsters. These findings highlight the species and tissue difference in the nature, levels, and consequences of NNK-induced DNA adduct formation, emphasizing the need to understand a broader range of molecular and cellular responses, to NNK and other tobacco-specific nitrosamine exposures, as well as host and co-factor variables that influence utilization of different NNK metabolic pathways.
Single-strand DNA breaks
Exposure to tobacco smoke increases production of reactive oxygen species (ROS) which leads to oxidative DNA damage [44]. These effects are partly due to NNK-induced 8-hydroxyguanine (8-OH-Gua) adducts which are pro-mutagenic. Correspondingly, peripheral blood leukocyte levels of 8-OHdG are significantly higher among smokers compared to non-smokers [3]. Experimentally, NNK induction of 8-oxo-2′deoxyguanosine (8-oxo-dG) adducts was demonstrated in fetal liver after transplacental NNK exposure in pregnant mice [81]. NNK-induced DNA damage is mediated by radical attack leading to single-strand breaks in DNA. Correspondingly, NNK-mediated DNA damage is attenuated by treatment with oxygen radical scavengers (superoxide dismutase, catalase, mannitol) [96], and adduct formation can be reduced by treatment with green tea or epigallocatechin gallate, its major antioxidant [101]. In addition to adducts, hydroxyl radicals and other reactive oxygen species produced during NNK metabolism may also cause DNA damage since genotoxicity is reduced by superoxide dismutase and catalase treatments [96] (Fig. 4).
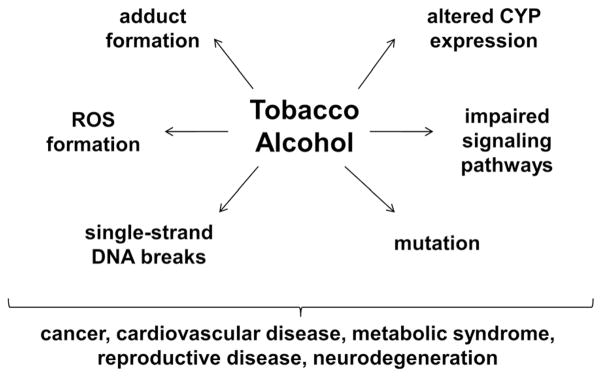
Fig. 4.
Potential shared mechanisms of tobacco and alcohol-mediated diseases. Tobacco-specific nitrosamines, such as NNK, lead to formation of DNA, hemoglobin, and lipid adducts, impaired intracellular signaling through PI3K-Akt, Erk-MAP kinase, and IGF-1R, mutagenesis, altered CYP 450 expression/activity, accumulation of reactive oxygen species (ROS), and stress-induced single stranded DNA breaks. Alcohol exposures can have the same effects, either directly or via acetaldehyde generation, leading to impaired signaling, macromolecular adduct formation, increased oxidative stress, and altered CYP expression. Consequences include malignant transformation or enhanced growth of malignant neoplastic cells, cardiovascular disease, e.g., atherosclerosis, dysregulated metabolism, e.g., insulin resistance and metabolic syndrome, reproductive diseases, and neurodegeneration
Hemoglobin binding of NNK
Most of the investigations have focused on DNA adducts and DNA damage-mediated by NNK, its metabolites, and other tobacco-specific nitrosamines. However, there is convincing evidence that NNK also forms adducts with hemoglobin following metabolic activation via α-methylene or α-methyl hydroxylation. α-Methylene hydroxylation of NNK results in globin methylation, while α-methyl hydroxylation results in globin pyridyloxobutylation [8]. Pyridyloxobutylated hemoglobin adducts are specific to NNK and NNN. In support of these observations, keto alcohol-releasing adducts were formed by treatment of hemoglobin with NNK or NNKOAc [63]. In addition, reductions in keto alcohol-releasing product effectuated by treatment with phenethyl isothiocyanate significantly reduced NNK-mediated lung tumorigenesis [33]. Although hemoglobin keto alcohol-releasing adducts could potentially serve as non-invasive biomarkers of cancer risk in people exposed to NNK/tobacco smoke, their exceedingly low levels in smokers render this goal challenging for the near future.
Role of NNK in carcinogenesis via mutagenesis versus signal transduction
NNK activation and metabolism in lung and liver lead to formation of reactive metabolites that can mutate oncogenes such as KRas and p53. For example, O6-methylguanine induces KRas GGT→GAT mutations that are found in mouse lung tumors caused by NNK exposure [16]. In humans, the scenario is far more complex because tobacco smoke contains many toxins and carcinogens, including poly-aromatic hydrocarbons, aromatic amines, oxygen radicals, and α,β-aldehydes that also cause DNA damage and mutations [57].
Besides mutagenesis, NNK exposures can modulate signal transduction networks, including activation of the PI3K-Akt pathway which promotes cell growth, proliferation, survival, migration, and metabolism. In this regard, NNK treatment of A/J mice and lung cancers from smokers were found to be associated with increased Akt activity [97]. In addition, nicotine stimulates lung cancer cell growth, suppresses apoptosis, and increases MAP kinase signaling networks [35]. Extended cell survival due to increased Akt activation could enable the accumulation of DNA adducts that promote mutagenesis and carcinogenesis. Like nicotine, NNK exhibits high affinity binding for nAChR (alpha7) [39] and activates voltage-gated calcium channels, leading to calcium influx and stimulation of growth-promoting signal transduction pathways [80]. NNK binding to AChR(alpha7) increases Erk1, Erk2, and DNA synthesis [79], enhancing growth of malignant neoplastic cells [103]. Finally, there is evidence that NNK can activate upstream mediators of growth and metabolism by enhancing expression and signaling through the insulin-like growth factor type 1 receptor (IGF-IR) [82]. Conceivably, overexpression and activation of IGF-IR in various cancers, including lung [78], are mediated by chronic NNK/tobacco nitrosamines, which confer cell proliferation, survival, and metastatic growth advantages to the tumors [5].
NNK metabolism and disease-potential alcohol-tobacco interactive effects
Altered expression and function of enzymes that metabolize both tobacco nitrosamines and alcohol could impact risk for smoking-related diseases among heavy drinkers. For example, CYP2B6 and CYP2E1 were both found to be expressed at higher levels in the postmortem brains from alcoholics and smokers versus non-alcoholics and non-smokers. Mechanistically, elevated levels of CYP2B6 activity in brain could alter responses to centrally acting drugs, increase susceptibility to neurotoxins and xenobiotics, and increase nicotine tolerance [53]. Increased levels of brain CYP2E1 could increase drug metabolism and risk for neurotoxicity due to metabolic activation of neurotoxins [36]. Furthermore, since CYP2E1 is involved in α-methyl hydroxylation of NNK, elevated levels of CYP2E1 could stimulate hydroxylation of NNK at the α-methyl group leading to the formation of pyridyloxobutylated DNA adducts (Fig. 2). NDMA, another potent pro-carcinogen found in preserved and processed foods [14], also requires α-hydroxylation by CYP2E1 to form methanediazonium ion, which interacts with DNA-forming O6-methylguanine DNA adducts [45]. Therefore, tobacco and alcohol stimulation of CYP2E1 activity could further increase risk for carcinogenesis via NDMA activation.
Conclusions
Tobacco nitrosamines play an important role in the pathogenesis of cancer. As a result of metabolic activation, NNK forms DNA adducts and generates hydroxyl radicals or other reactive oxygen species that can further damage DNA and lead to single-strand breaks. In addition to DNA damage, reactive oxygen species increase oxidative stress, lipid peroxidation, and formation of protein adducts. Recent studies suggest that interactions between NNK and signaling growth pathways such as PI3K and MAPK could potentially alter downstream responses to insulin and IGF-1 signaling. In this regard, we recently showed that chronic low-level NNK exposures cause steatohepatitis with perturbations in insulin/IGF-1 signaling through Akt as well as increased levels of lipid and DNA adducts [106]. Impaired signaling together with DNA damage and oxidative stress could be responsible for the persistent activation of pro-inflammatory cytokines, insulin resistance, and hepatic fibrogenesis [106]. Therefore, the links between tobacco smoke exposure and type 2 diabetes mellitus or Alzheimer’s disease, which are associated with impairments in insulin and IGF-1 signaling through Akt, could be explained on the basis of NNK-mediated disruption of intracellular signaling and buildup of adduct that damage protein and lipid functions.
Acknowledgments
This study was supported by F32AA024018, AA-11431 and AA-12908 from the National Institutes of Health.
References
- 1.Akaike A, Tamura Y, Yokota T, Shimohama S, Kimura J. Nicotine-induced protection of cultured cortical neurons against N-methyl-D-aspartate receptor-mediated glutamate cytotoxicity. Brain Res. 1994;644:181–187. doi: 10.1016/0006-8993(94)91678-0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KE, Hammons GJ, Kadlubar FF, Potter JD, Kaderlik KR, Ilett KF, Minchin RF, Teitel CH, Chou HC, Martin MV, et al. Metabolic activation of aromatic amines by human pancreas. Carcinogenesis. 1997;18:1085–1092. doi: 10.1093/carcin/18.5.1085. [DOI] [PubMed] [Google Scholar]
- 3.Asami S, Hirano T, Yamaguchi R, Tomioka Y, Itoh H, Kasai H. Increase of a type of oxidative DNA damage, 8-hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer Res. 1996;56:2546–2549. [PubMed] [Google Scholar]
- 4.Azzalini L, Ferrer E, Ramalho LN, Moreno M, Dominguez M, Colmenero J, Peinado VI, Barbera JA, Arroyo V, Gines P, et al. Cigarette smoking exacerbates nonalcoholic fatty liver disease in obese rats. Hepatology (Baltimore, Md) 2010;51:1567–1576. doi: 10.1002/hep.23516. [DOI] [PubMed] [Google Scholar]
- 5.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 6.Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- 7.Carlson J, Noguchi K, Ellison G. Nicotine produces selective degeneration in the medial habenula and fasciculus retroflexus. Brain Res. 2001;906:127–134. doi: 10.1016/s0006-8993(01)02570-7. [DOI] [PubMed] [Google Scholar]
- 8.Carmella SG, Hecht SS. Formation of hemoglobin adducts upon treatment of F344 rats with the tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and n′-nitrosonornicotine. Cancer Res. 1987;47:2626–2630. [PubMed] [Google Scholar]
- 9.Castonguay A, Pepin P, Briere N. Modulation of 4-(methylnitrosamino)-1-(3-pyridyl)-1 butanone demethylation and denitrosation by rat liver microsomes. Cancer Lett. 1991;59:67–74. doi: 10.1016/0304-3835(91)90137-7. [DOI] [PubMed] [Google Scholar]
- 10.Dahlstrom A, Lundell B, Curvall M, Thapper L. Nicotine and cotinine concentrations in the nursing mother and her infant. Acta Paediatr Scand. 1990;79:142–147. doi: 10.1111/j.1651-2227.1990.tb11430.x. [DOI] [PubMed] [Google Scholar]
- 11.Dalgic A, Okay O, Helvacioglu F, Daglioglu E, Akdag R, Take G, Belen D. Tobacco-induced neuronal degeneration via cotinine in rats subjected to experimental spinal cord injury. J Neurol Surg A Cent Eur Neurosurg. 2013;74:136–145. doi: 10.1055/s-0033-1337607. [DOI] [PubMed] [Google Scholar]
- 12.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 13.Das D, Cherbuin N, Anstey KJ, Sachdev PS, Easteal S. Lifetime cigarette smoking is associated with striatal volume measures. Addict Biol. 2012;17:817–825. doi: 10.1111/j.1369-1600.2010.00301.x. [DOI] [PubMed] [Google Scholar]
- 14.de la Monte SM, Neusner A, Chu J, Lawton M. Epidemiological trends strongly suggest exposures as etiologic agents in the pathogenesis of sporadic Alzheimer’s disease, diabetes mellitus, and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;17:519–529. doi: 10.3233/JAD-2009-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devereux TR, Anderson MW, Belinsky SA. Factors regulating activation and DNA alkylation by 4-(n-methyl-n-nitrosamino)-1-(3-pyridyl)-1-butanone and nitrosodimethylamine in rat lung and isolated lung cells, and the relationship to carcinogenicity. Cancer Res. 1988;48:4215–4221. [PubMed] [Google Scholar]
- 16.Devereux TR, Anderson MW, Belinsky SA. Role of ras protooncogene activation in the formation of spontaneous and nitrosamine-induced lung tumors in the resistant C3H mouse. Carcinogenesis. 1991;12:299–303. doi: 10.1093/carcin/12.2.299. [DOI] [PubMed] [Google Scholar]
- 17.Durazzo TC, Gazdzinski S, Meyerhoff DJ. The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol Alcohol. 2007;42:174–185. doi: 10.1093/alcalc/agm020. [DOI] [PubMed] [Google Scholar]
- 18.Durazzo TC, Mattsson N, Weiner MW. Alzheimer’s disease neuroimaging, I. Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimers Dement. 2014;10:S122–S145. doi: 10.1016/j.jalz.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritz HC, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K, Hosten N, Lotze M. Current smoking and reduced gray matter volume—a voxel-based morphometry study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2594–2600. doi: 10.1038/npp.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuxe K, Rosen L, Lippoldt A, Andbjer B, Hasselrot U, Finnman UB, Agnati LF. Chronic continuous infusion of nicotine increases the disappearance of choline acetyl-transferase immunoreactivity in the cholinergic cell bodies of the medial septal nucleus following a partial unilateral transection of the fimbria fornix. Clin Investig. 1994;72:262–268. doi: 10.1007/BF00180037. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Montes JR, Boronat-Garcia A, Lopez-Colome AM, Bargas J, Guerra-Crespo M, Drucker-Colin R. Is nicotine protective against Parkinson’s disease? An experimental analysis. CNS Neurol Disord Drug Targets. 2012;11:897–906. doi: 10.2174/1871527311201070897. [DOI] [PubMed] [Google Scholar]
- 22.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 23.Guo Z, Smith TJ, Ishizaki H, Yang CS. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by cytochrome P450IIB1 in a reconstituted system. Carcinogenesis. 1991;12:2277–2282. doi: 10.1093/carcin/12.12.2277. [DOI] [PubMed] [Google Scholar]
- 24.Guo Z, Smith TJ, Thomas PE, Yang CS. Metabolic activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone as measured by DNA alkylation in vitro and its inhibition by isothiocyanates. Cancer Res. 1991;51:4798–4803. [PubMed] [Google Scholar]
- 25.Guo Z, Smith TJ, Thomas PE, Yang CS. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by inducible and constitutive cytochrome P450 enzymes in rats. Arch Biochem Biophys. 1992;298:279–286. doi: 10.1016/0003-9861(92)90124-f. [DOI] [PubMed] [Google Scholar]
- 26.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 27.Hecht SS, Adams JD, Numoto S, Hoffmann D. Induction of respiratory tract tumors in Syrian golden hamsters by a single dose of 4-(methylnitrosamino)-1-(3-pyri-dyl)-1-butanone (NNK) and the effect of smoke inhalation. Carcinogenesis. 1983;4:1287–1290. doi: 10.1093/carcin/4.10.1287. [DOI] [PubMed] [Google Scholar]
- 28.Hecht SS, Chen CB, Ornaf RM, Jacobs E, Adams JD, Hoffmann D. Reaction of nicotine and sodium nitrite: formation of nitrosamines and fragmentation of the pyrrol-idine ring. J Org Chem. 1978;43:72–76. doi: 10.1021/jo00395a017. [DOI] [PubMed] [Google Scholar]
- 29.Hecht SS, Hochalter JB, Villalta PW, Murphy SE. 2′-hydroxylation of nicotine by cytochrome P450 2A6 and human liver microsomes: formation of a lung carcinogen precursor. Proc Natl Acad Sci USA. 2000;97:12493–12497. doi: 10.1073/pnas.220207697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9:875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- 31.Hecht SS, Spratt TE, Trushin N. Absolute configuration of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol formed metabolically from 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis. 1997;18:1851–1854. doi: 10.1093/carcin/18.9.1851. [DOI] [PubMed] [Google Scholar]
- 32.Hecht SS, Trushin N, Castonguay A, Rivenson A. Comparative tumorigenicity and DNA methylation in F344 rats by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N-nitrosodimethylamine. Cancer Res. 1986;46:498–502. [PubMed] [Google Scholar]
- 33.Hecht SS, Trushin N, Rigotty J, Carmella SG, Borukhova A, Akerkar S, Desai D, Amin S, Rivenson A. Inhibitory effects of 6-phenylhexyl isothiocyanate on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolic activation and lung tumorigenesis in rats. Carcinogenesis. 1996;17:2061–2067. doi: 10.1093/carcin/17.9.2061. [DOI] [PubMed] [Google Scholar]
- 34.Hecht SS, Young R, Chen CB. Metabolism in the F344 rat of 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco-specific carcinogen. Cancer Res. 1980;40:4144–4150. [PubMed] [Google Scholar]
- 35.Heusch WL, Maneckjee R. Signalling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis. 1998;19:551–556. doi: 10.1093/carcin/19.4.551. [DOI] [PubMed] [Google Scholar]
- 36.Howard LA, Miksys S, Hoffmann E, Mash D, Tyndale RF. Brain CYP2E1 is induced by nicotine and ethanol in rat and is higher in smokers and alcoholics. Br J Pharmacol. 2003;138:1376–1386. doi: 10.1038/sj.bjp.0705146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.International Agency for Research on Cancer (IARC), I.A.f.o.C. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, tobacco smoking. 1986. [Google Scholar]
- 38.International Agency for Research on Cancer (IARC), I.A.f.o.C. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, smokeless tobacco and some tobacco-specific N-nitrosamines. 2007. [Google Scholar]
- 39.Improgo MR, Tapper AR, Gardner PD. Nicotinic acetylcholine receptor-mediated mechanisms in lung cancer. Biochem Pharmacol. 2011;82:1015–1021. doi: 10.1016/j.bcp.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Janson AM, Meana JJ, Goiny M, Herrera-Marschitz M. Chronic nicotine treatment counteracts the decrease in extracellular neostriatal dopamine induced by a unilateral transection at the mesodiencephalic junction in rats: a micro-dialysis study. Neurosci Lett. 1991;134:88–92. doi: 10.1016/0304-3940(91)90515-u. [DOI] [PubMed] [Google Scholar]
- 41.Kannel WB, Higgins M. Smoking and hypertension as predictors of cardiovascular risk in population studies. J Hypertens Suppl. 1990;8:S3–S8. [PubMed] [Google Scholar]
- 42.Kato S, Bowman ED, Harrington AM, Blomeke B, Shields PG. Human lung carcinogen-DNA adduct levels mediated by genetic polymorphisms in vivo. J Natl Cancer Inst. 1995;87:902–907. doi: 10.1093/jnci/87.12.902. [DOI] [PubMed] [Google Scholar]
- 43.Kemp PM, Sneed GS, George CE, Distefano RF. Postmortem distribution of nicotine and cotinine from a case involving the simultaneous administration of multiple nicotine transdermal systems. J Anal Toxicol. 1997;21:310–313. doi: 10.1093/jat/21.4.310. [DOI] [PubMed] [Google Scholar]
- 44.Leanderson P. Cigarette smoke-induced DNA damage in cultured human lung cells. Ann N Y Acad Sci. 1993;686:249–259. doi: 10.1111/j.1749-6632.1993.tb39183.x. discussion 259–261. [DOI] [PubMed] [Google Scholar]
- 45.Lee VM, Keefer LK, Archer MC. An evaluation of the roles of metabolic denitrosation and alpha-hydroxylation in the hepatotoxicity of n-nitrosodimethylamine. Chem Res Toxicol. 1996;9:1319–1324. doi: 10.1021/tx960077u. [DOI] [PubMed] [Google Scholar]
- 46.Liao Y, Tang J, Liu T, Chen X, Hao W. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol. 2012;17:977–980. doi: 10.1111/j.1369-1600.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- 47.Liu L, Castonguay A, Gerson SL. Lack of correlation between DNA methylation and hepatocarcinogenesis in rats and hamsters treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis. 1992;13:2137–2140. doi: 10.1093/carcin/13.11.2137. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Zeng X, Hui Y, Zhu C, Wu J, Taylor DH, Ji J, Fan W, Huang Z, Hu J. Activation of alpha7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: implications for Parkinson’s disease. Neuropharmacology. 2015;91:87–96. doi: 10.1016/j.neuropharm.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 49.Madsbad S, McNair P, Christensen MS, Christiansen C, Faber OK, Binder C, Transbol I. Influence of smoking on insulin requirement and metabolic status in diabetes mellitus. Diabetes Care. 1980;3:41–43. doi: 10.2337/diacare.3.1.41. [DOI] [PubMed] [Google Scholar]
- 50.Marin P, Maus M, Desagher S, Glowinski J, Premont J. Nicotine protects cultured striatal neurones against N-methyl-D-aspartate receptor-mediated neurotoxicity. Neuroreport. 1994;5:1977–1980. doi: 10.1097/00001756-199410000-00035. [DOI] [PubMed] [Google Scholar]
- 51.Maser E. Stress, hormonal changes, alcohol, food constituents and drugs: factors that advance the incidence of tobacco smoke-related cancer? Trends Pharmacol Sci. 1997;18:270–275. doi: 10.1016/s0165-6147(97)01090-0. [DOI] [PubMed] [Google Scholar]
- 52.Maser E, Richter E, Friebertshauser J. The identification of 11 beta-hydroxysteroid dehydrogenase as carbonyl reductase of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Eur J Biochem. 1996;238:484–489. doi: 10.1111/j.1432-1033.1996.0484z.x. [DOI] [PubMed] [Google Scholar]
- 53.Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology. 2003;45:122–132. doi: 10.1016/s0028-3908(03)00136-9. [DOI] [PubMed] [Google Scholar]
- 54.Moreno-Gonzalez I, Estrada LD, Sanchez-Mejias E, Soto C. Smoking exacerbates amyloid pathology in a mouse model of Alzheimer’s disease. Nat Commun. 2013;4:1495. doi: 10.1038/ncomms2494. [DOI] [PubMed] [Google Scholar]
- 55.Murphy SE, Palomino A, Hecht SS, Hoffmann D. Dose–response study of DNA and hemoglobin adduct formation by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in F344 rats. Cancer Res. 1990;50:5446–5452. [PubMed] [Google Scholar]
- 56.Murphy SE, Spina DA, Nunes MG, Pullo DA. Glucuronidation of 4-((hydroxymethyl)nitrosamino)-1-(3-pyridyl)-1-butanone, a metabolically activated form of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, by phenobarbital-treated rats. Chem Res Toxicol. 1995;8:772–779. doi: 10.1021/tx00047a018. [DOI] [PubMed] [Google Scholar]
- 57.Nesnow S, Ross JA, Stoner GD, Mass MJ. Mechanistic linkage between DNA adducts, mutations in oncogenes and tumorigenesis of carcinogenic environmental polycyclic aromatic hydrocarbons in strain A/J mice. Toxicology. 1995;105:403–413. doi: 10.1016/0300-483x(95)03238-b. [DOI] [PubMed] [Google Scholar]
- 58.Oliveira-da-Silva A, Vieira FB, Cristina-Rodrigues F, Filgueiras CC, Manhaes AC, Abreu-Villaca Y. Increased apoptosis and reduced neuronal and glial densities in the hippocampus due to nicotine and ethanol exposure in adolescent mice. Int J Dev Neurosci. 2009;27:539–548. doi: 10.1016/j.ijdevneu.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 59.Pan P, Shi H, Zhong J, Xiao P, Shen Y, Wu L, Song Y, He G. Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci. 2013;34:813–817. doi: 10.1007/s10072-012-1256-x. [DOI] [PubMed] [Google Scholar]
- 60.Parain K, Hapdey C, Rousselet E, Marchand V, Dumery B, Hirsch EC. Cigarette smoke and nicotine protect dopaminergic neurons against the 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine parkinsonian toxin. Brain Res. 2003;984:224–232. doi: 10.1016/s0006-8993(03)03195-0. [DOI] [PubMed] [Google Scholar]
- 61.Parain K, Marchand V, Dumery B, Hirsch E. Nicotine, but not cotinine, partially protects dopaminergic neurons against MPTP-induced degeneration in mice. Brain Res. 2001;890:347–350. doi: 10.1016/s0006-8993(00)03198-x. [DOI] [PubMed] [Google Scholar]
- 62.Patten CJ, Smith TJ, Murphy SE, Wang MH, Lee J, Tynes RE, Koch P, Yang CS. Kinetic analysis of the activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by heterologously expressed human P450 enzymes and the effect of P450-specific chemical inhibitors on this activation in human liver microsomes. Arch Biochem Biophys. 1996;333:127–138. doi: 10.1006/abbi.1996.0373. [DOI] [PubMed] [Google Scholar]
- 63.Peterson LA, Carmella SG, Hecht SS. Investigations of metabolic precursors to hemoglobin and DNA adducts of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis. 1990;11:1329–1333. doi: 10.1093/carcin/11.8.1329. [DOI] [PubMed] [Google Scholar]
- 64.Peterson LA, Hecht SS. O6-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in a/j mouse lung. Cancer Res. 1991;51:5557–5564. [PubMed] [Google Scholar]
- 65.Peterson LA, Liu XK, Hecht SS. Pyridyloxobutyl DNA adducts inhibit the repair of o6-methylguanine. Cancer Res. 1993;53:2780–2785. [PubMed] [Google Scholar]
- 66.Peterson LA, Mathew R, Hecht SS. Quantitation of microsomal alpha-hydroxylation of the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1991;51:5495–5500. [PubMed] [Google Scholar]
- 67.Peterson LA, Ng DK, Stearns RA, Hecht SS. Formation of NADP(H) analogs of tobacco-specific nitrosamines in rat liver and pancreatic microsomes. Chem Res Toxicol. 1994;7:599–608. doi: 10.1021/tx00041a003. [DOI] [PubMed] [Google Scholar]
- 68.Petruzzelli S, Tavanti LM, Celi A, Giuntini C. Detection of N7-methyldeoxyguanosine adducts in human pulmonary alveolar cells. Am J Respir Cell Mol Biol. 1996;15:216–223. doi: 10.1165/ajrcmb.15.2.8703477. [DOI] [PubMed] [Google Scholar]
- 69.Picciotto MR, Zoli M. Nicotinic receptors in aging and dementia. J Neurobiol. 2002;53:641–655. doi: 10.1002/neu.10102. [DOI] [PubMed] [Google Scholar]
- 70.Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Placzek AN, Zhang TA, Dani JA. Age dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochem Pharmacol. 2009;78:686–692. doi: 10.1016/j.bcp.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prendergast MA, Harris BR, Mayer S, Holley RC, Hauser KF, Littleton JM. Chronic nicotine exposure reduces N-methyl-D-aspartate receptor-mediated damage in the hippocampus without altering calcium accumulation or extrusion: evidence of calbindin-D28K overexpression. Neuroscience. 2001;102:75–85. doi: 10.1016/s0306-4522(00)00450-4. [DOI] [PubMed] [Google Scholar]
- 73.Preusmann R, Stewart B. N-nitroso carcinogens. Searle. C. E; Washington, DC: 1984. [Google Scholar]
- 74.Quik M, Kulak JM. Nicotine and nicotinic receptors; relevance to Parkinson’s disease. Neurotoxicology. 2002;23:581–594. doi: 10.1016/s0161-813x(02)00036-0. [DOI] [PubMed] [Google Scholar]
- 75.Quik M, McIntosh JM. Striatal alpha6* nicotinic acetylcholine receptors: potential targets for Parkinson’s disease therapy. J Pharmacol Exp Ther. 2006;316:481–489. doi: 10.1124/jpet.105.094375. [DOI] [PubMed] [Google Scholar]
- 76.Quik M, Parameswaran N, McCallum SE, Bordia T, Bao S, McCormack A, Kim A, Tyndale RF, Langston JW, Di Monte DA. Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. J Neurochem. 2006;98:1866–1875. doi: 10.1111/j.1471-4159.2006.04078.x. [DOI] [PubMed] [Google Scholar]
- 77.Riljak V, Milotova M, Jandova K, Pokorny J, Langmeier M. Morphological changes in the hippocampus following nicotine and kainic acid administration. Physiol Res. 2007;56:641–649. doi: 10.33549/physiolres.931048. [DOI] [PubMed] [Google Scholar]
- 78.Rotsch M, Maasberg M, Erbil C, Jaques G, Worsch U, Havemann K. Characterization of insulin-like growth factor I receptors and growth effects in human lung cancer cell lines. J Cancer Res Clin Oncol. 1992;118:502–508. doi: 10.1007/BF01225264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nature Reviews Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- 80.Sheppard BJ, Williams M, Plummer HK, Schuller HM. Activation of voltage-operated Ca2+-channels in human small cell lung carcinoma by the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Int J Oncol. 2000;16:513–518. doi: 10.3892/ijo.16.3.513. [DOI] [PubMed] [Google Scholar]
- 81.Sipowicz MA, Amin S, Desai D, Kasprzak KS, Anderson LM. Oxidative DNA damage in tissues of pregnant female mice and fetuses caused by the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (nnk) Cancer Lett. 1997;117:87–91. doi: 10.1016/s0304-3835(97)00208-5. [DOI] [PubMed] [Google Scholar]
- 82.Siwicky MD, Petrik JJ, Moorehead RA. The function of IGF-IR in NNK-mediated lung tumorigenesis. Lung Cancer. 2011;71:11–18. doi: 10.1016/j.lungcan.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 83.Smith TJ, Guo Z, Gonzalez FJ, Guengerich FP, Stoner GD, Yang CS. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human lung and liver microsomes and cytochromes P-450 expressed in hepatoma cells. Cancer Res. 1992;52:1757–1763. [PubMed] [Google Scholar]
- 84.Smith TJ, Guo Z, Hong JY, Ning SM, Thomas PE, Yang CS. Kinetics and enzyme involvement in the metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in microsomes of rat lung and nasal mucosa. Carcinogenesis. 1992;13:1409–1414. doi: 10.1093/carcin/13.8.1409. [DOI] [PubMed] [Google Scholar]
- 85.Smith TJ, Guo ZY, Thomas PE, Chung FL, Morse MA, Elkind K, Yang CS. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in mouse lung microsomes and its inhibition by isothiocyanates. Cancer Res. 1990;50:6817–6822. [PubMed] [Google Scholar]
- 86.Staretz ME, Foiles PG, Miglietta LM, Hecht SS. Evidence for an important role of DNA pyridyloxobutylation in rat lung carcinogenesis by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone: effects of dose and phenethyl isothiocyanate. Cancer Res. 1997;57:259–266. [PubMed] [Google Scholar]
- 87.Staretz ME, Koenig LA, Hecht SS. Effects of long term dietary phenethyl isothiocyanate on the microsomal metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F344 rats. Carcinogenesis. 1997;18:1715–1722. doi: 10.1093/carcin/18.9.1715. [DOI] [PubMed] [Google Scholar]
- 88.Staretz ME, Murphy SE, Patten CJ, Nunes MG, Koehl W, Amin S, Koenig LA, Guengerich FP, Hecht SS. Comparative metabolism of the tobacco-related carcinogens benzo[a]pyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and n′-nitrosonornicotine in human hepatic microsomes. Drug Metab Dispos. 1997;25:154–162. [PubMed] [Google Scholar]
- 89.Takeuchi H, Yanagida T, Inden M, Takata K, Kitamura Y, Yamakawa K, Sawada H, Izumi Y, Yamamoto N, Kihara T, et al. Nicotinic receptor stimulation protects nigral dopaminergic neurons in rotenone-induced Parkinson’s disease models. J Neurosci Res. 2009;87:576–585. doi: 10.1002/jnr.21869. [DOI] [PubMed] [Google Scholar]
- 90.Trushin N, Rivenson A, Hecht SS. Evidence supporting the role of DNA pyridyloxobutylation in rat nasal carcinogenesis by tobacco-specific nitrosamines. Cancer Res. 1994;54:1205–1211. [PubMed] [Google Scholar]
- 91.U.S. Department of Health and Human Services, P.H.S., Office of the Surgeon General. The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta, GA: 2014. [Google Scholar]
- 92.Urbanavicius J, Ferreira M, Costa G, Abin-Carriquiry JA, Wonnacott S, Dajas F. Nicotine induces tyrosine hydroxylase plasticity in the neurodegenerating striatum. J Neurochem. 2007;102:723–730. doi: 10.1111/j.1471-4159.2007.04560.x. [DOI] [PubMed] [Google Scholar]
- 93.Vahakangas K, Pelkonen O. Extrahepatic metabolism of nicotine and related compounds by P-450, in nicotine and related alkaloids: absorption-distribution-metabolism-excretion. Chapman & Hall; London: 1993. [Google Scholar]
- 94.Wang JJ, Durazzo TC, Gazdzinski S, Yeh PH, Mon A, Meyerhoff DJ. MRSI and DTI: a multimodal approach for improved detection of white matter abnormalities in alcohol and nicotine dependence. NMR Biomed. 2009;22:516–522. doi: 10.1002/nbm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L, Spratt TE, Liu XK, Hecht SS, Pegg AE, Peterson LA. Pyridyloxobutyl adduct o6-[4-oxo-4-(3-pyridyl)butyl] guanine is present in 4-acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone-treated DNA and is a substrate for o6-alkylguanine-DNA alkyltransferase. Chem Res Toxicol. 1997;10:562–567. doi: 10.1021/tx9602067. [DOI] [PubMed] [Google Scholar]
- 96.Weitberg AB, Corvese D. Oxygen radicals potentiate the genetic toxicity of tobacco-specific nitrosamines. Clin Genet. 1993;43:88–91. doi: 10.1111/j.1399-0004.1993.tb04455.x. [DOI] [PubMed] [Google Scholar]
- 97.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Whitehouse PJ, Kalaria RN. Nicotinic receptors and neurodegenerative dementing diseases: basic research and clinical implications. Alzheimer Dis Assoc Disord. 1995;9(Suppl 2):3–5. doi: 10.1097/00002093-199501002-00002. [DOI] [PubMed] [Google Scholar]
- 99.WHO. World Health Organization report on the global tobacco epidemic. 2013. [Google Scholar]
- 100.Xie XT, Liu Q, Wu J, Wakui M. Impact of cigarette smoking in type 2 diabetes development. Acta Pharmacol Sin. 2009;30:784–787. doi: 10.1038/aps.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu Y, Ho CT, Amin SG, Han C, Chung FL. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992;52:3875–3879. [PubMed] [Google Scholar]
- 102.Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- 103.Xue J, Yang S, Seng S. Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers. 2014;6:1138–1156. doi: 10.3390/cancers6021138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamazaki Y, Hamaue N, Sumikawa K. Nicotine compensates for the loss of cholinergic function to enhance long-term potentiation induction. Brain Res. 2002;946:148–152. doi: 10.1016/s0006-8993(02)02935-9. [DOI] [PubMed] [Google Scholar]
- 105.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary levels of the tobacco-specific carcinogen n′-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32:1366–1371. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zabala V, Tong M, Yu R, Ramirez T, Yalcin EB, Balbo S, Silbermann E, Deochand C, Nunez K, Hecht S, et al. Potential contributions of the tobacco nicotine-derived nitrosamine ketone (NNK) in the pathogenesis of steatohepatitis in a chronic plus binge rat model of alcoholic liver disease. Alcohol and alcoholism (Oxford, Oxfordshire) 2015;50:118–131. doi: 10.1093/alcalc/agu083. [DOI] [PMC free article] [PubMed] [Google Scholar]