Abstract
Unlike Elizabethkingia meningoseptica, the clinical importance of E. anophelis is poorly understood. We determined the clinical and molecular epidemiology of bacteremia caused by Elizabethkingia-like species from five regional hospitals in Hong Kong. Among 45 episodes of Elizabethkingia-like bacteremia, 21 were caused by Elizabethkingia, including 17 E. anophelis, three E. meningoseptica and one E. miricola; while 24 were caused by other diverse genera/species, as determined by 16S rRNA gene sequencing. Of the 17 cases of E. anophelis bacteremia, 15 (88%) were clinically significant. The most common diagnosis was pneumonia (n = 5), followed by catheter-related bacteremia (n = 4), neonatal meningitis (n = 3), nosocomial bacteremia (n = 2) and neutropenic fever (n = 1). E. anophelis bacteremia was commonly associated with complications and carried 23.5% mortality. In contrast, of the 24 episodes of bacteremia due to non-Elizabethkingia species, 16 (67%) were clinically insignificant. Compared to non-Elizabethkingia bacteremia, Elizabethkingia bacteremia was associated with more clinically significant infections (P < 0.01) and positive cultures from other sites (P < 0.01), less polymicrobial bacteremia (P < 0.01), and higher complication (P < 0.05) and mortality (P < 0.05) rates. Elizabethkingia bacteremia is predominantly caused by E. anophelis instead of E. meningoseptica. Elizabethkingia bacteremia, especially due to E. anophelis, carries significant morbidity and mortality, and should be considered clinically significant unless proven otherwise.
The genus Elizabethkingia comprises aerobic, non-fermenting, non-motile and non-spore-forming gram-negative rods that were previously named Flavobacterium or belonged to CDC group IIa and later reclassified as Chryseobacterium in 19941. In 2005, Chryseobacterium meningosepticum and C. miricola were transferred to a new genus, Elizabethkingia, on the basis of combined phenotypic and phylogenetic characteristics2. The genus comprises three medically important species, Elizabethkingia anophelis, E. meningoseptica and E. miricola. A novel species, E. endophytica, isolated from sweet corn, was also recently proposed3. E. meningoseptica, previously named Flavobacterium meningosepticum or C. meningosepticum, is the best known species among the genus. E. meningoseptica is a causative agent of nosocomial infections especially in immunocompromised patients, as well as neonatal meningitis and sepsis4. Besides soil, fresh water and plants, the bacterium can be found in hospital environments and may contaminate flushing solutions and medical devices5. Infections caused by E. meningoseptica can be difficult to treat and carry high mortalities, which may be partly explained by their intrinsic multidrug resistance towards commonly used antibiotics such as β-lactams and aminoglycosides6. Therefore, accurate diagnosis is important to guide appropriate antibiotic regimens which often consist of a combination of ciprofloxacin or rifampicin with piperacillin-tazobactam or vancomycin.
In contrast to E. meningoseptica, the epidemiology and pathogenicity of E. anophelis and E. miricola were less well understood. E. miricola, originally named C. miricola when first isolated from condensed water obtained from the Russian space station, Mir, only rarely causes nosocomial infections in human7,8. On the other hand, E. anophelis was first isolated from midgut of the mosquito Anopheles gambiae in 20119. Soon after its discovery, it was reported to cause neonatal meningitis in the Central African Republic and a nosocomial outbreak in an intensive-care unit in Singapore10,11. The first discovery of E. anophelis from mosquito gut has raised suspicion on mosquitoes as the source of neonatal meningitis cases in Africa10. However, our recent report on E. anophelis meningitis in two neonates and chorioamnionitis in a neonate’s mother in Hong Kong suggested that mosquitoes were unlikely the vehicles of transmission12. Since the transmission route was initially obscure, draft genome sequencing was performed and showed evidence for perinatal vertical transmission from a mother to her neonate12. The ultimate resolution power of genome sequencing also enabled species confirmation and discrimination from the phenotypically similar species, E. meningoseptica12.
Since E. anophelis was commonly misidentified as E. meningoseptica in previous reports10,11,12,13, we hypothesize that many previously described E. meningoseptica isolates were actually E. anophelis and that E. anophelis may account for a significant proportion of Elizabethkingia infections. To better understand the epidemiology and clinical disease spectrum of E. anophelis and Elizabethkingia as a whole, we determined the clinical and molecular epidemiology of bacteremia caused by Elizabethkingia-like species from five regional hospitals in Hong Kong. All bacteremia episodes caused by Elizabethkingia-like species identified by conventional phenotypic tests from 2004 to 2013 during the study period were included. For the 45 episodes of Elizabethkingia-like bacteremia identified, 16S rRNA gene sequencing was performed for species identification and clinical characteristics and outcomes were analyzed.
Results
Identification of Elizabethkingia-like bacteremia by 16S rRNA gene sequencing
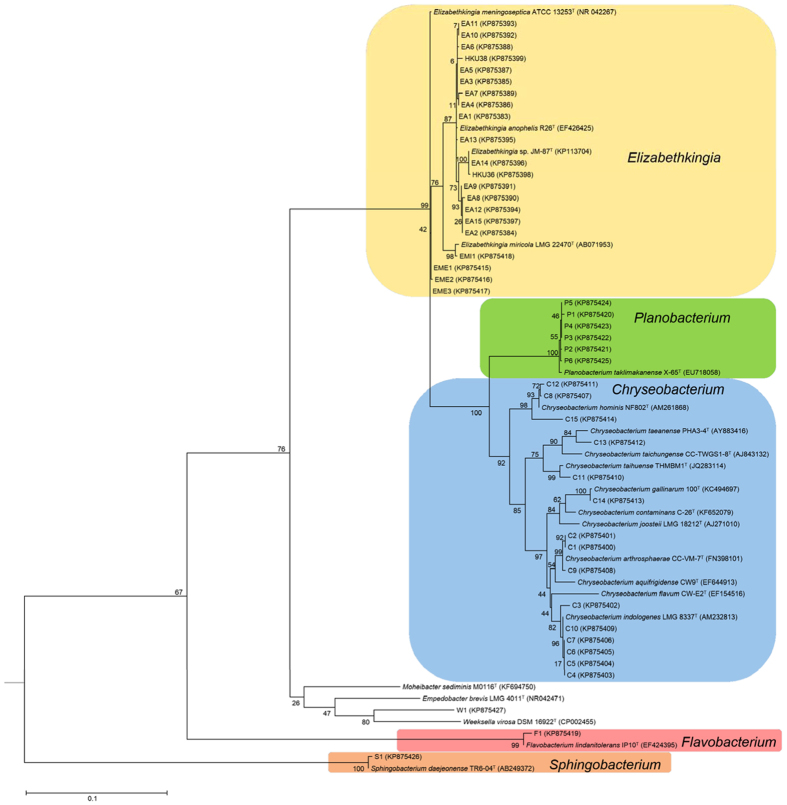
Twenty-one of the 45 episodes of Elizabethkingia-like bacteremia were caused by Elizabethkingia, while 24 episodes were caused by diverse genera/species including Chryseobacterium (n = 15), Flavobacterium (n = 1), Planobacterium (n = 6), Sphingobacterium (n = 1) and Weeksella-like species (n = 1) according to 16S rRNA gene analysis (Fig. 1 and see Supplementary Table 1). Of the 21 episodes of Elizabethkingia bacteremia, 17 were caused by E. anophelis (99.0–99.9% nucleotide identity to E. anophelis type strain R26T), three by E. meningoseptica (99.4–99.8% nucleotide identity to E. meningoseptica type strain ATCC 13253T) and one by E. miricola (99.5% nucleotide identity to E. miricola type strain LMG 22470T).
Figure 1. Phylogenetic tree showing the relationship of the 45 Elizabethkingia-like blood culture isolates to related bacterial species using 16S rRNA gene sequence analysis.
The tree was constructed by maximum likelihood method using General Time Reversible model and Escherichia coli (CP010304) as the root. A total of 1325 nucleotide positions were included in the analysis. Bootstrap values were calculated from 1000 replicates. The scale bar indicates the number of substitutions per site. Names and accession numbers are given as cited in GenBank database.
Among the 24 episodes of non-Elizabethkingia bacteremia, 15 were caused by Chryseobacterium species, among which two (C13 and C15) represented two potentially novel Chryseobacterium species (≤98.4% nucleotide identity to existing Chryseobacterium species). One episode was caused by another potentially novel species most closely related to Weeksella (91.9% nucleotide identity to Weeksella virosa DSM 16922T). The other eight episodes were caused by Planobacterium (n = 6), Flavobacterium (n = 1) and Sphingobacterium (n = 1) species respectively.
Clinical characteristics of patients with Elizabethkingia-like bacteremia
The clinical characteristics of the 45 episodes of Elizabethkingia-like bacteremia were summarized in Tables 1, 2, 3. Of the 17 episodes of E. anophelis bacteremia, the male:female ratio was 11:6, with a median age of 58 years (range, 1 day to 104 years) (Table 2). Most patients had underlying diseases. Except two patients with pseudobacteremia, most cases were associated with clinically significant bacteremia, with 12 cases being hospital-acquired and three community-acquired. The most common diagnosis was pneumonia (n = 5), followed by catheter-related bacteremia (n = 4), neonatal meningitis (n = 3), nosocomial bacteremia (n = 2) and neutropenic fever (n = 1). Among the five cases of pneumonia, three were community-acquired, including one in a 51-year-old previously healthy man (case EA10). All three cases of neonatal meningitis were hospital-acquired. Details of two (HKU36 and HKU38) of the three neonatal meningitis cases have been reported previously12. They were included because they fell into the inclusion criteria during the study period. The source of E. anophelis in the two cases of nosocomial bacteremia and one case of neutropenic fever was obscure, although severe mucositis was present in the latter. Three of the 17 patients had multiple positive blood cultures. E. anophelis was concomitantly isolated from other body sites in seven patients, including cerebrospinal fluid (n = 3), respiratory samples (n = 3) and catheter tip (n = 1). Most patients survived, but four patients died despite appropriate antibiotics given in two of the four patients. Complications as a result of E. anophelis bacteremia included acute pulmonary edema, congestive heart failure, multi-organ failure, disseminated intravascular coagulation, septic shock, haematemesis, acute renal failure, metabolic acidosis and intraventricular haemorrhage. Various antibiotic regimens as single drug or combinations were used for empirical or definitive treatment, including quinolones, penicillins, cephalosporins, carbapenems, vancomycin and rifampicin. Removal of catheter was often required in catheter-related bacteremia cases in addition to antibiotic treatment.
Table 1. Characteristics of the 45 patients with bacteremia caused Elizabethkingia-like organisms.
| Characteristics | Number of patients (%) |
P-valuec | |
|---|---|---|---|
| Elizabethkingiabacteremia (n = 21) | Non-Elizabethkingiabacteremia (n = 24) | ||
| Sex (male:female) | 11:10 | 13:11 | 0.90 |
| Underlying diseases | 19 (90.5) | 24 (100) | 0.12 |
| Hospital- vs community-acquireda | 15:4 | 7:1 | 0.60 |
| Diagnosisb | |||
| Biliary tract infection | 2 (9.5) | 0 (0) | 0.12 |
| Catheter-related bacteremia | 5 (23.8) | 1 (4.2) | 0.05 |
| Community-acquired pneumonia | 3 (14.3) | 0 (0) | 0.06 |
| Neonatal meningitis | 3 (14.3) | 0 (0) | 0.06 |
| Neutropenic fever | 1 (4.8) | 2 (8.3) | 0.63 |
| Nosocomial bacteremia | 3 (14.3) | 5 (20.8) | 0.57 |
| Nosocomial pneumonia | 2 (9.5) | 0 (0) | 0.12 |
| Primary bacteremia | 0 (0) | 1 (4.2) | 0.34 |
| Pseudobacteremia | 2 (9.5) | 16 (76.1) | 0.00009 |
| >1 positive blood cultures | 3 (14.3) | 1 (4.2) | 0.23 |
| Polymicrobial bacteremia | 0 (0) | 10 (41.7) | 0.0008 |
| Positive cultures from other sites | 8 (38.1) | 1 (4.2) | 0.005 |
| Complications | 7 (33.3) | 1 (4.2) | 0.01 |
| Attributable mortality | 4 (19.0) | 0 (0) | 0.025 |
aexcluding pseudobacteremia.
bThe percentages add up to more than 100% because some patients have more than one diagnosis.
cby Chi-square test.
Table 2. Clinical characteristics of patients with Elizabethkingia bacteremia.
| Case/strain no. | Sex | Age | Underlying diseases | Diagnosis | Community- or hospital-acquired | No. of positive blood cultures (concomitant isolates) | Other culture-positive specimens | Antibiotic treatment | Complications/outcomee |
|---|---|---|---|---|---|---|---|---|---|
| E. anophelis | |||||||||
| EA1 | M | 54 | DM, hyperlipidemia, ischemic cardiomyopathy | Nosocomial pneumonia | HA | 1 | None | Piperacillin-tazobactam | Acute pulmonary edema, CHF, survived |
| EA2 | M | 65 | HT, DM, CAD, PVD, RAS, hyperlipidemia, carotid stenosis | Pseudobacteremia | NA | 1 | None | Cloxaciillin and levofloxacin, then amoxicillin-clavulanate | Survived |
| EA3 | F | 1m | Prematurity, twin, RDS, PDA | Catheter-related bacteremia | HA | 3 | None | Vancomycin, cefoperazone-sulbactam | Multi-organ failure, died |
| EA4 | F | 8d | Imperforated anus, rectovaginal fistula | Neonatal meningitis | HA | 2 | CSF | Vancomycin and rifampicin | Survived |
| EA5 | M | 65 | COPD, CAD, CHF, AF, CRHD, right hip AVN | Community-acquired pneumonia | CA | 1 | Sputum | Ciprofloxacin | Survived |
| EA6 | M | 37 | Dilated cardiomyopathy, CAD | Nosocomial bacteremia | HA | 1 | None | Ciprofloxacin | DIC, survived |
| EA7 | F | 64 | HT, hyperlipidemia, stage 4 DLBC lymphoma | Neutropenic fever, severe mucositis | HA | 2 | None | Meropenem | Septic shock, haematemesis, died |
| EA8 | M | 68 | HT, CRF, AAA | Nosocomial bacteremia | HA | 1 | Tracheal aspirate | Levofloxacin and vancomycin | Acute renal failure, died |
| EA9 | M | 73 | CA hypopharynx, CA ampulla of Vater | Pseudobacteremia | NA | 1 | None | Cefuroxime | Survived |
| EA10 | M | 51 | None | Community-acquired pneumonia | CA | 1 | Sputum | Ampicillin-sulbactam, then ciprofloxacin | Survived |
| EA11 | F | 104 | HT, CAD, CVA, nephrotic syndrome | Community-acquired pneumonia | CA | 1 | None | Amoxicillin-clavulanate | Died |
| EA12 | M | 59 | obesity, DM, CAD | Catheter-related bacteremia, drip site cellulitis | HA | 1 | None | Levofloxacin | Survived |
| EA13 | F | 35 | Epilepsy, HT, ESRF on HD | Catheter-related bacteremia | HA | 1 | None | Levofloxacin | Survived |
| EA14 | M | 58 | CRHD with AVR, ESRF on HD | Catheter-related bacteremia | HA | 1 | Catheter tip | Piperacillin-tazobactam, then levofloxacin | Septic shock, survived |
| EA15 | M | 88 | COPD, old PTB | Nosocomial (aspiration) pneumonia | HA | 1 | None | Levofloxacin | Survived |
| HKU36 | M | 21d | None | Neonatal meningitis | HA | 1 | CSF | Vancomycin, piperacillin and rifampicin | Survived |
| HKU38 | F | 1d | Apnea of prematurity | Neonatal meningitis | HA | 1 | CSF | Vancomycin, piperacillin-tazobactam and rifampicin | metabolic acidosis, IVH, survived |
| E. meningoseptica | |||||||||
| EME1 | M | 51 | Asthma, HT, scoliosis, MSSA infective spondylitis | Nosocomial bacteremia | HA | 1 | None | Ticarcillin-clavulanate | Survived |
| EME2 | M | 52 | Biliary pancreatitis, RPC, cholecystectomy, cirrhosis with biliary stent | Post-cholangiogram acute cholangitis | HA | 1 | None | Levofloxacin and metronidazole | Survived |
| EME3 | F | 89 | HT, AF, painless obstructive jaundice on palliative stenting | Biliary sepsis | CA | 1 | None | Levofloxacin | Survived |
| E. miricola | |||||||||
| EMI1 | M | 54 | DM, hyperlipidemia, ischemic cardiomyopathy | Catheter-related bacteremia | HA | 1 | CVP catheter | Levofloxacin | Survived |
aAAA, abdominal aortic aneurysm; AF, atrial fibrillation; AVN, avascular necrosis; AVR, atrial valve replacement; CA, carcinoma; CA, community-acquired; CAD, coronary artery disease; CHF, congestive heart failure; COPD; chronic obstructive pulmonary disease; CRF, chronic renal failure; CRHD, chronic rheumatic heart disease; CSF, cerebrospinal fluid; CVA, cerebrovascular accident; CVP, central venous pressure; DIC, disseminated intravascular coagulation; DLBC, diffuse large B cell; DM, diabetes mellitus; ESRF, end-stage renal failure; HA, hospital-acquired; HD, hemodialysis; HT, hypertension; IVH, intraventricular hemorrhage; MSSA, methicillin-sensitive Staphylococcus aureus; NA, not applicable; PDA, patent ductus arteriosus; PTB, pulmonary tuberculosis; PVD, peripheral vascular disease; RAS, renal artery stenosis; RDS, respiratory distress syndrome; RPC, recurrent pyogenic cholangitis.
Table 3. Clinical characteristics of patients with non-Elizabethkingia bacteremia.
| Case/ strain no. | Sex | Age | Underlying diseases | Diagnosis | Community- or hospital-acquired | No. of positive blood cultures (concomitant isolates) | Other culture-positive specimens | Treatment + removal of catheter | Complications + outcome |
|---|---|---|---|---|---|---|---|---|---|
| Potentially novel Chryseobacterium species | |||||||||
| C13 | M | 64 | Metastatic pancreatic carcinoma, sepsis | Postmortem pseudobacteremia | NA | 1 | None | NA | Died (non-attributable) |
| C15 | M | 68 | Head injury | Pseudobacteremia | NA | 1 (Acinetobacter sp., Enterococcus faecium) | No | Amoxicillin-clavulanate | Survived |
| Chryseobacterium arthrosphaerae | |||||||||
| C1 | M | 82 | HT, gout, BPH, bilateral hydronephrosis and hydroureter | Nosocomial bacteremia | HA | 1 | None | Piperacillin-tazobactam, then levofloxacin | Survived |
| C2 | F | 48 | Stage IV DLBC lymphoma | Catheter-related bacteremia, neutropenic fever | HA | 6 (Klebsiella pneumoniae, Acinetobacter baumanii) | Central catheter | Imipenem-cilastatin and amikacin | Septic shock, survived |
| C9 | M | 56 | COPD, pneumothorax | Nosocomial bacteremia | HA | 1 | None | Ticarcillin-clavulanate, levofloxacin | Survived |
| Chryseobacterium gallinarum | |||||||||
| C14 | M | 1 | Cow milk allergy | Nosocomial bacteremia | HA | 1 | None | Piperacillin-tazobactam | Survived |
| Chryseobacterium hominis | |||||||||
| C8 | F | 76 | DM, HT, CVA, CHF, AAA, PVD, hyperlipidemia | Pseudobacteremia | NA | 1 | None | None | Survived |
| C12 | F | 51 | AML | Neutropenic fever | HA | 1 (Moraxella sp.) | None | Piperacillin-tazobactam, vancomycin | Survived |
| Chryseobacterium indologenes | |||||||||
| C3 | F | 58 | Hyperlipidemia, IgA nephropathy, Multiple myeloma | Pseudobacteremia | NA | 1 (Stenotrophomonas maltophila, Commamonas sp,, Ralstonia sp.) | None | Levofloxacin, piperacillin-tazobactam | Survived |
| C4 | F | 49 | DM, CRF, bilateral hydronephrosis, perinephric and psoas abscess | Pseudobacteremia | NA | 1 (Enterococcus facaelis, A. baumanii, Streptococcus mitis) | None | Cefuroxime, ampicillin, levofloxacin | Survived |
| C5 | M | 78 | HT, gout, CA caecum with metastases, thyroidectomy | Pseudobacteremia | NA | 1 (A. baumanii) | None | Amoxicillin-clavulanate, ciprofloxacin | Survived |
| C6 | F | 57 | CA rectum, mania, small bowel obstruction | Pseudobacteremia | NA | 1 (E. facaelis, A. baumanii) | None | Amoxicillin-clavulanate | Survived |
| C7 | M | 84 | HT, AF,CRHD, CVA, gout, BPH COPD | Pseudobacteremia | NA | 1 | None | Amoxicillin-clavulanate | Survived |
| C10 | F | 56 | Acute encephalopathy, aspiration pneumonia, sepsis | Postmortem pseudobacteremia | NA | 1 (Pseuodmonas putida, Bacillus sp., CNS, MSSA, non-hemolytic Streptococcus) | None | NA | Died (non-attributible) |
| Chryseobacterium taihuense | |||||||||
| C11 | M | 85 | DM, DU, BPH | Pseudobacteremia | NA | 1 | None | Amoxicillin-clavulanate | Survived |
| Flavobacterium lindanitolerans | |||||||||
| F1 | F | 30 | SLE, lupus nephritis | Nosocomial (post-transfusion) bacteremia | HA | 1(Bacillus sp.) | None | Levofloxacin | Survived |
| Planobacterium taklimakanense | |||||||||
| P1 | M | 90 | CAD, COPD, DM, BPH | Primary bacteremia | CA | 1 | None | Ceftriaxone | Survived |
| P2 | M | 1d | RDS, congenital pneumonia | Pseudobacteremia | NA | 1 | None | Penicillin and netilmicin | Survived |
| P3 | F | 99 | COPD, CVA, intertrochanteric fracture | Pseudobacteremia | NA | 1 | None | Amoxicillin-clavulanate | Survived |
| P4 | M | 56 | COPD, BPH, PTB | Pseudobacteremia | NA | 1 | None | Levofloxacin | Survived |
| P5 | M | 52 | NPC, thyroidectomy, hypothyroidism | Nosocomial (post-transfusion) bacteremia | HA | 1 | None | Piperacillin-tazobactam | Survived |
| P6 | F | 1m | Sepsis, hypoglycemia | Pseudobacteremia | NA | 1 | None | Cefotaxime | Survived |
| Sphingobacterium daejeonense | |||||||||
| S1 | M | 94 | COPD, cor pulmonale, BPH, gout, Shy-Drager syndrome, CAD | Pseudobacteremia | NA | 1 (K. pneumoniae) | None | Cotrimoxazole | Survived |
| Potentially novel Weeksella-related species | |||||||||
| W1 | F | 18d | RSV pneumonia | Pseudobacteremia | NA | 1 | None | Ampicillin, netilmicin and erythromycin | Survived |
aAAA, abdominal aortic aneurysm; AF, atrial fibrillation; AML, acute myeloid leukemia; BPH, benign prostatic hyperplasia; CA, carcinoma; CA, community-acquired; CAD, coronary artery disease; CHF, congestive heart failure; CNS, coagulase-negative Staphylococcus; COPD; chronic obstructive pulmonary disease; CRF, chronic renal failure; CRHD, chronic rheumatic heart disease; CVA, cerebrovascular accident; DLBC, diffuse large B cell; DM, diabetes mellitus; DU, Duodenal ulcer; HA, hospital-acquired; HT, hypertension; MSSA, methicillin-sensitive Staphylococcus aureus; NA, not applicable; NPC, nasopharyngeal carcinoma; PTB, pulmonary tuberculosis; PVD, peripheral vascular disease; RDS, respiratory distress syndrome; RSV, respiratory syncytial virus; SLE, systemic lupus erythematosus.
All the four patients with E. meningoseptica or E. miricola bacteremia also had underlying disease and clinically significant infections (Table 2). Two patients with E. meningoseptica bacteremia had biliary tract infections, while one had nosocomial bacteremia. Interestingly, the only episode of E. miricola bacteremia (case EMI1) was detected in the same patient with E. anophelis nosocomial pneumonia (case EA1) which occurred more than one month previously during the same admission and was complicated by heart failure and acute pulmonary edema requiring intubation and inotropes. Five weeks after successful treatment with piperacillin-tazobactam, the patient developed another episode of bacteremia caused by E. miricola which was also isolated from central venous catheter. The patient recovered with levofloxacin and catheter removal.
In contrast, 16 of the 24 patients with bacteremia due to non-Elizabethkingia species had pseudobacteremia (Table 3). The other eight patients were diagnosed to have nosocomial bacteremia (three cases), transfusion-related bacteremia (two cases), catheter-related bacteremia (one case), neutropenic fever (one case) and sepsis (one case). Except for a case of community-acquired sepsis in a 90-year-old man (case P1), all other cases were hospital-acquired. Only one episode of catheter-related bacteremia was complicated by septic shock, where C. arthrosphaerae was isolated from six blood cultures. All the eight patients survived.
Comparison between Elizabethkingia and non-Elizabethkingia cases showed that clinically significant infections were more common in patients with Elizabethkingia bacteremia than those with non-Elizabethkingia bacteremia (i.e. fewer pseudobacteremia cases were observed) (P < 0.01). Moreover, patients with Elizabethkingia bacteremia had more positive cultures from other sites (P < 0.05) and lower incidence of polymicrobial bacteremia (P < 0.01). They also showed higher complication (P < 0.05) and mortality (P < 0.05) rates than those with with non-Elizabethkingia bacteremia (Table 1).
Microbiological characteristics of Elizabethkingia isolates
All the 21 Elizabethkingia isolates were non-motile, oxidase-positive, catalase-positive, indole-positive, non-glucose-fermenting, Gram-negative bacilli. Growth on MacConkey agar, citrate utilization, urea hydrolysis and fermentation of cellobiose and melibiose fermentation were variable. All 21 isolates were identified as E. meningoseptica by Vitek 2 GNI system with 91–99% confidence. All 21 isolates were susceptible to ciprofloxacin, cefoperazone-sulbactam and vancomycin, but were resistant or intermediate-resistant to imipenem, amikacin, gentamicin and tobramycin. Susceptibilities to ceftazidime, piperacillin, rifampicin and cotrimoxazole were variable (see Supplementary Table 2).
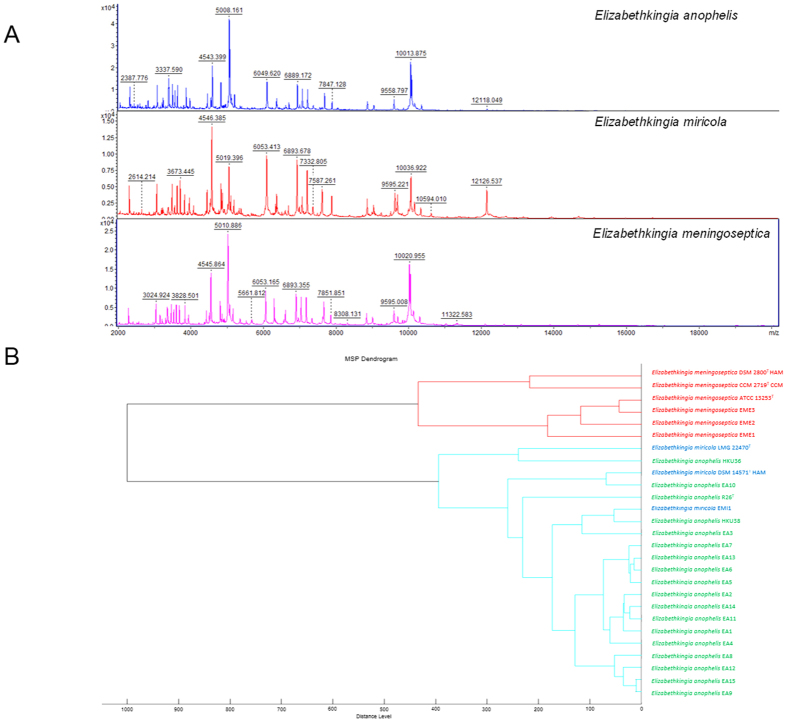
MALDI-TOF MS using Reference Library Biotyper v3.1.2.0 (Bruker Daltonik, Germany) failed to identify the 17 E. anophelis isolates (10 misidentified as E. meningoseptica with score 2.073 to 2.403, two identified as Elizabethkingia species with score 1.952–1.971 and five unidentified with score 1.32 to 1.42 to E. meningoseptica). When the database was expanded with inclusion of mass spectra from seven E. anophelis isolates, all the other 10 E. anophelis isolates were correctly identified as E. anophelis with score 2.321 to 2.634. The three E. meningoseptica and one E. miricola strains were correctly identified by the Bruker reference library (see Supplementary Table 2). Hierarchical cluster analysis showed that the protein mass spectra of E. anophelis and E. miricola were clustered together but formed a distinct branch from E. meningoseptica (Fig. 2).
Figure 2. Results of MALDI-TOF MS identification of the 21 Elizabethkingia strains.
In panel (A), representative MALDI-TOF MS spectra of the three Elizabethkingia species are shown. In panel (B), dendrogram was generated from hierarchical clustering of MALDI-TOF MS spectra of 21 Elizabethkingia isolates and reference strains of E. meningoseptica and E. miricola, using ClinProTools 3.0 (Bruker Daltonics, Germany). Distances are displayed in relative units.
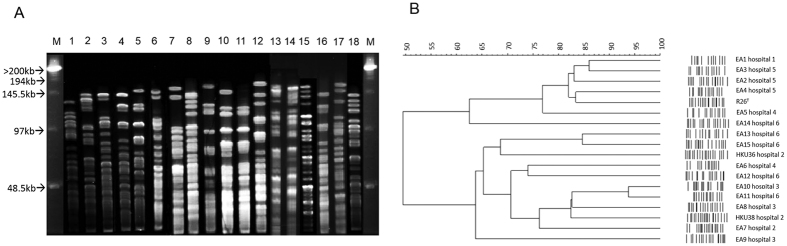
PFGE typing of E. anophelis
To determine the genetic relatedness of the 17 E. anophelis strains isolated from five different hospitals, PFGE was performed using the bacterial genomic and DNA restriction endonuclease XbaI. In general, the PFGE patterns among the 17 E. anophelis strains were distinct from each other and different from that of the type strain R26T. Dendrogram constructed using the PFGE images showed that some adjacently clustered strains were isolated from the same hospital (Fig. 3). For example, strains EA2, EA3 and EA4 were from hospital 5, while strains EA13 and EA15 were from hospital 6. However, no clear epidemiological linkage could be identified in terms of time and place of blood culture isolation. Specifically, strains EA2, EA3 and EA4 were isolated from different years. Strains EA13 and EA15 were isolated four months apart and the two patients were hospitalized in different wards of hospital 6.
Figure 3. Pulsed-field gel electrophoresis (PFGE) analysis of the 17 E. anophelis isolates and E. anophelis type strain R26T.
(lane 1 = EA1, lane 2 = EA2, lane 3 = EA3, lane 4 = EA4, lane 5 = EA5, lane 6 = EA6, lane 7 = EA7, lane 8 = EA8, lane 9 = EA9, lane 10 = EA10, lane 11 = EA11, lane 12 = EA12, lane 13 = EA13, lane 14 = EA14, lane 15 = EA15, lane 16 = HKU38, lane 17 = HKU36, lane 18 = R26T, M = lambda marker). In Panel (A), PFGE was performed using CHEF Mapper XA system (Bio-Rad) and restriction endonuclease XbaI. Results showed that the 17 isolates possessed distinct PFGE patterns. In Panel (B), dendrogram was constructed with PFGE data by similarity and clustering analysis using the Dice coefficient (1% tolerance and 0.5% optimization) and unweighted pair-group method using average linkages with GelCompar II.
Discussion
E. anophelis bacteremia should be considered clinically significant unless proven otherwise and should prompt appropriate antibiotic therapy. In contrast to the traditional belief that E. meningoseptica was the most important Elizabethkingia species associated with bacteremia, the present study showed that the majority of Elizabethkingia bacteremia cases were caused by E. anophelis. In particular, neonatal meningitis was associated exclusively with E. anophelis in this study. In the present series, E. anophelis bacteremia mainly occurred in neonates or adults with underlying medical illnesses, and was most commonly associated with pneumonia and hospital-acquired infections such as catheter-related bacteremia and neonatal meningitis. Notably, community-acquired pneumonia occurred in three patients including a healthy middle-aged adult, where the infective source remained obscure. Apart from the two previous reported cases of neonatal meningitis (HKU36 and HKU38) which were most likely acquired from maternal-to-infant transmission based on comparative genomics12, no obvious epidemiological linkage or genetic relatedness was identified among the other cases. Nevertheless, some potentially genetically related strains upon PFGE analysis may have circulated in the same hospital. Further studies should be performed to better understand the disease spectrum and transmission routes of E. anophelis.
E. anophelis bacteremia carries significant morbidity and mortality. Various complications were observed and four patients died, giving a mortality rate of 23.5%. This is in line with previous reports of E. anophelis neonatal meningitis being associated with poor outcomes10. Nevertheless, all the three present cases of neonatal meningitis were cured with early use of appropriate antibiotics12. Although E. anophelis usually confers resistance to multiple antibiotics such as ceftazidime, imipenem and aminoglycosides12, all the 17 isolates were susceptible to ciprofloxacin, cefoperazone-sulbactam and vancomycin, which should be considered in empirical treatment while awaiting susceptibility results. In cases of catheter-related bacteremia, infected catheters should be removed in addition to antibiotic treatment. Future prospective studies with population-based data should be performed to determine the prevalence or incidence of E. anophelis bacteremia.
E. meningoseptica and E. miricola appeared to be much less prevalent than E. anophelis, although similar studies in other countries are required to more accurately assess their relative importance. Similar to E. anophelis, E. meningoseptica and E. miricola bacteremia were associated with clinically significant infections. Besides one case of E. meningoseptica nosocomial bacteremia and one case of E. miricola catheter-related bacteremia, biliary tract infections were also noted in two cases of E. meningoseptica bacteremia. It remains to be determined if E. meningoseptica may have the propensity to cause biliary tract infections among the genus. Given their similar antibiotic susceptibility profiles to that of E. anophelis, ciprofloxacin, cefoperazone-sulbactam and vancomycin should also be included in treatment regimens for E. meningoseptica and E. miricola bacteremia.
In contrast to Elizabethkingia species, isolation of non-Elizabethkingia species from blood cultures should raise suspicion of their clinical significance. In this study, non-Elizabethkingia bacteremia is associated with higher incidence of pseudobacteremia and polymicrobial bacteremia than Elizabethkiniga bacteremia. In particular, all six isolates of C. indologenes and the three potentially novel species were associated with pseudobacteremia. Bacteremia caused by non-Elizabethkingia species is also associated with lower incidence of complications and mortality than Elizabethkingia bacteremia, suggesting that these bacterial species may be less virulent than Elizabethkingia. Moreover, these environmental, non-Elizabethkingia bacterial species may contaminate blood cultures when aseptic techniques during blood taking are breached. Careful clinical assessment is required to determine the clinical significance and the need for antibiotics when these bacteria are isolated from blood cultures.
Although the two E. anophelis strains, HKU36 and EA14, are phylogenetically genetically close to E. endophytica strain JM-87T, they should belong to E. anophelis instead of E. endophytica or a novel species. The two strains possessed 99.9% nucleotide identities to both the newly proposed species, E. endophytica strain JM-87T and E. anophelis R26T in their 16S rRNA gene sequences. Although strain JM-87T was reported to possess 51–52% similarities to E. anophelis R26T by DNA-DNA hybridization3, our previous study showed that the draft genome of strain HKU36 possessed 78.3% nucleotide identity to the genome of E. anophelis R26T by estimation of intergenomic distance12. Given the close relatedness of strains HKU36, EA14 and JM-87T in their 16S rRNA genes (Fig. 1), it is likely that the genome sequences of strain JM-87T and EA14 may also possess >70% identity to that of E. anophelis R26T. Since genome-based comparison can offer ultimate resolution for species delineation which is superior to traditional DNA-DNA hybridization methods, genome sequencing of strain JM-87T and related strains such as EA14 should be performed to more accurately define their taxonomic positions.
The present results confirmed our suspicion that E. anophelis was a previously under-reported bacterium which can be easily misidentified as E. meningoseptica12. Although E. anophelis was first discovered from mosquito gut10, we previously showed that maternal chorioamnionitis, instead of mosquitoes, was more likely the source of neonatal meningitis12. Similarly, mosquitoes are unlikely the route of transmission in other E. anophelis infections. We speculate that contaminated environments, such as infected catheters, are the source of infection in most cases, as in the case of previously described “E. meningoseptica” infections. Given their similar phenotypic characteristics, E. anophelis isolates from previous reports were often mistaken as E. meningoseptica initially10,11,12,14. Phenotypic tests, such as acid production from cellobiose and citrate utilization, previously reported as potentially useful for species discrimination12, were unlikely to be reliable. In our previous study, the three E. anophelis strains were also initially misidentified as E. meningoseptica even with MALDI-TOF MS, owing to the absence of E. anophelis spectra in commercial databases12. The 16S rRNA genes of E. anophelis possessed >98% nucleotide identity to those of E. meningoseptica and E. miricola, which should offer sufficient discriminative power12. However, some “E. meningoseptica” strains with 16S rRNA sequences deposited in GenBank, such as strains G3-1-08 and 50215,16, should belong to E. anophelis based on phylogenetic analysis12. These “misidentified” strains in GenBank may confuse the interpretation of 16S rRNA gene sequencing results and should be rectified12. While E. anophelis can be distinguished from E. meningoseptica by MALDI-TOF MS when the database is expanded with mass spectra from E. anophelis strains, E. anophelis and E. miricola appear to be indistinguishable from each other. With an expanded database using E. anophelis isolates, MALDI-TOF MS is the method of choice for rapid and accurate diagnosis of E. anophelis infections, which is crucial to better understand its epidemiology and clinical disease spectrum.
Methods
Ethics statement
The use of blood culture isolates and anonymous clinical data were approved by Institute Review Board, The University of Hong Kong/Hospital Authority, Hong Kong (reference UW 04-278 T/600). The methods and all experimental protocols were carried out in accordance with the approved guidelines. Since this study does not involve experimentation on human subjects or the use of tissue samples from human subjects, written informed consent has been waived by our institutional review board.
Settings and Patients
Patients were hospitalized in five regional hospitals located in different areas of Hong Kong from 2004 to 2013. To identify potential cases of Elizabethkingia bacteremia, all bacteremia episodes caused by oxidase-positive, non-glucose fermenters that were identified as Elizabethkingia, Flavobacterium or Chryseobacterium species by conventional phenotypic tests during the study period were included with clinical data analyzed17. Bacteremia was categorized as clinically significant or pseudobacteremia (contamination of blood culture) by clinical and laboratory criteria18. The criteria include the patient’s clinical presentation, physical examination findings, body temperature at the time of the blood culture, leukocyte and differential cell counts, imaging or operative results, histopathological findings, number of positive blood cultures out of the total number performed, and response to treatment.
Bacterial isolates
Collection of clinical specimens, bacterial cultures and conventional phenotypic identification were performed according to standard protocols19. Two of the 45 Elizabethkingia-like isolates from two neonates (HKU36 and HKU38) have been reported previously12. The same isolate recovered from the same patient was counted only once.
16S rRNA gene sequencing for species identification
The 45 blood culture isolates were subject to 16S rRNA gene sequencing according to previously published protocols with modifications, using primers LPW57 (5′-AGTTTGATCCTGGCTCAG-3′) and LPW58 (5′-AGGCCCGGGAACGTATTCAC-3′)12,20. The sequences of PCR products were compared to known gene sequences in GenBank by multiple sequence alignment using CLUSTAL_W in MEGA version 621. Phylogenetic tree was constructed by maximum likelihood method using MEGA version 621.
Statistical analysis
A comparison of characteristics was made between patients with Elizabethkingia and non-Elizabethkingia bacteremia using Chi-square test (IBM SPSS Statistics version 19). P < 0.05 was regarded as statistically significant.
Phenotypic characterization and matrix-assisted laser-desorption ionization-time-of-flight mass-spectrometry (MALDI-TOF MS) of Elizabethkingia isolates
The 21 Elizabethkinigia isolates were characterized by phenotypic tests, Vitek 2 GNI system (bioMérieux, France) and MALDI-TOF MS. MALDI-TOF MS was performed by ethanol formic acid extraction method as described previously, using Bruker Daltonics microflex LT system with Reference Library Biotyper v3.1.2.0 (Bruker Daltonik, Germany)22,23. Since E. anophelis is not included in the Bruker reference, mass spectra generated from seven E. anophelis strains with identity confirmed by 16S rRNA gene sequencing were later added to the database. Obtained spectra were subject to hierarchical cluster analysis as described previously24. Antibiotic susceptibility testing was performed by Kirby Bauer disk diffusion method with results interpreted according to Clinical and Laboratory Standards Institute for Staphylococcus aureus (vancomycin) and Pseudomonas aeruginosa (other drugs), because of the lack of interpretative criteria for Elizabethkingia25.
Pulsed-field gel electrophoresis (PFGE)
The 17 E. anophelis isolates were characterized by PFGE using CHEF Mapper XA system (Bio-Rad, CA, USA) and restriction endonuclease XbaI as described previously12,26. After PFGE, the gel was stained with ethidium bromide (1 μg/ml) for 30 minutes and the patterns of the genomic DNA digest were visualized with a UV transilluminator. Digital images were stored electronically as TIFF files and analyzed visually and with GelCompar II (version 3.0; Applied Maths, Kortrijk, Belgium), and represented by UPGMA method.
Nucleotide sequence accession number
The 16S rRNA gene sequences of the 45 blood culture isolates have been deposited in the GenBank sequence database under accession no. KP875383 to KP875427.
Additional Information
How to cite this article: Lau, S. K. P. et al. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci. Rep. 6, 26045; doi: 10.1038/srep26045 (2016).
Supplementary Material
Acknowledgments
We thank members of the Department of Microbiology, Queen Mary Hospital, Hong Kong for their technical support. This work was supported by Committee for Research and Conference Grant, Strategic Research Theme Fund, and University Development Fund, The University of Hong Kong; the Shaw Foundation; and donation from Ms. Eunice Lam.
Footnotes
Author Contributions S.K.P.L., W.N.C., G.C.S.L. and P.C.Y.W. wrote the manuscript. S.K.P.L., W.N.C., C.H.F., G.C.S.L., J.L.L.T. and J.H.K.C. analyzed data. W.N.C., C.H.F., S.O.T.C., G.C.S.L. and R.H.Y.N. performed experiments. S.K.P.L., R.H.Y.N., A.K.L.W., I.Y.Y.C., S.K.Y.C., D.C.L., R.A.L., C.W.S.T., K.S.C.F., T.L.Q. and P.C.Y.W. collected clinical samples and data.
References
- Vandamme P., Bernardet J.-F., Kersters S. K. & Holmes B. New perspectives in the classification of the Flavobacteria: description of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int. J. Syst. Evol. Microbiol. 44, 827–831 (1994). [Google Scholar]
- Kim K. K., Kim M. K., Lim J. H., Park H. Y. & Lee S. T. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int. J. Syst. Evol. Microbiol. 55, 1287–1293 (2005). [DOI] [PubMed] [Google Scholar]
- Kämpfer P., Busse H. J., McInroy J. A. & Glaeser S. P. Elizabethkingia endophytica sp. nov., isolated from Zea mays and emended description of Elizabethkingia anophelis Kämpfer et al. 2011. Int. J. Syst. Evol. Microbiol. 65, 2187–2193 (2015). [DOI] [PubMed] [Google Scholar]
- Bloch K. C., Nadarajah R. & Jacobs R. Chryseobacterium meningosepticum: an emerging pathogen among immunocompromised adults. Report of 6 cases and literature review. Medicine (Baltimore) 76, 30–41 (1997). [DOI] [PubMed] [Google Scholar]
- Weaver K. N. et al. Acute emergence of Elizabethkingia meningoseptica infection among mechanically ventilated patients in a long-term acute care facility. Infect. Control Hosp. Epidemiol. 31, 54–58 (2010). [DOI] [PubMed] [Google Scholar]
- Matyi S. A. et al. Draft genome sequences of Elizabethkingia meningoseptica. Genome Announc. 1, e00444–13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. et al. Chryseobacterium miricola sp. nov., a novel species isolated from condensation water of space station Mir. Syst. Appl. Microbiol. 26, 523–528 (2003). [DOI] [PubMed] [Google Scholar]
- Green O., Murray P. & Gea-Banacloche J. C. Sepsis caused by Elizabethkingia miricola successfully treated with tigecycline and levofloxacin. Diagn. Microbiol. Infect. Dis. 62, 430–432 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämpfer P. et al. Elizabethkingia anophelis sp. nov., isolated from the midgut of the mosquito Anopheles gambiae. Int. J. Syst. Evol. Microbiol. 61, 2670–2675 (2011). [DOI] [PubMed] [Google Scholar]
- Frank T. et al. First case of Elizabethkingia anophelis meningitis in the Central African Republic. Lancet 381, 1876 (2013). [DOI] [PubMed] [Google Scholar]
- Teo J. et al. First case of E anophelis outbreak in an intensive-care unit. Lancet 382, 855–856 (2013). [DOI] [PubMed] [Google Scholar]
- Lau S. K. et al. Evidence for Elizabethkingia anophelis transmission from mother to infant, Hong Kong. Emerg. Infect. Dis. 21, 232–241 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobossi-Serengbe G., Gody J. C., Beyam N. E. & Bercion R. First documented case of Chryseobacterium meningosepticum meningitis in Central African Republic. Med. Trop. (Mars) 66, 182–184 (2006). [PubMed] [Google Scholar]
- Balm M. N. et al. Bad design, bad practices, bad bugs: frustrations in controlling an outbreak of Elizabethkingia meningoseptica in intensive care units. J. Hosp. Infect. 85, 134–140 (2013). [DOI] [PubMed] [Google Scholar]
- Kajla M. K., Andreeva O., Gilbreath T. M. 3rd & Paskewitz S. M. Characterization of expression, activity and role in antibacterial immunity of Anopheles gambiae lysozyme c-1. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 155, 201–209 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick J., Constantinidou C., Pallen M. J., Oppenheim B. & Loman N. J. Draft genome sequence of Elizabethkingia meningoseptica isolated from a traumatic wound. Genome Announc. 2, e00355–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk W. K. et al. Inpatient emergencies encountered by an infectious disease consultative service. Clin. Infect. Dis. 26, 695–701 (1998). [DOI] [PubMed] [Google Scholar]
- Weinstein M. P. et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin. Infect. Dis. 24, 584–602 (1997). [DOI] [PubMed] [Google Scholar]
- Versalovic J. et al. Manual of Clinical Microbiology, 10th ed. (ASM Press, 2011). [Google Scholar]
- Lau S. K. P. et al. Eggerthella hongkongensis sp. nov. and Eggerthella sinensis sp. nov., two novel Eggerthella species, account for half of the cases of Eggerthella bacteremia. Diagn. Microbiol. Infect. Dis. 49, 255–263 (2004). [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A. & Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S. K. et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for rapid identification of Burkholderia pseudomallei: importance of expanding databases with pathogens endemic to different localities. J. Clin. Microbiol. 50, 3142–3143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S. K. P. et al. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for identification of bacteria that are difficult to identify in clinical laboratories. J. Clin. Pathol. 67, 361–366 (2014). [DOI] [PubMed] [Google Scholar]
- Tsang C. C. et al. Subcutaneous phaeohyphomycotic nodule due to Phialemoniopsis hongkongensis sp. nov. J. Clin. Microbiol. 52, 3280–3289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute. M02-A11: performance standards for antimicrobial disk susceptibility tests; approved standard, 11th edn. Wayne, Pa. (2012).
- Woo P. C. et al. & L. hongkongensis study group. Association of Laribacter hongkongensis in community-acquired gastroenteritis with travel and eating fish: a multicentre case-control study. Lancet 363, 1941–1947 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.