Abstract
Soil salinity is becoming the key constraints factor to agricultural production. Therefore, the plant especially the crops possessing capacities of salt tolerance will be of great economic significance. The adaptation or tolerance of plant to salinity stress involves a series of physiological, metabolic and molecular mechanisms. Halophytes are the kind of organisms which acquire special salt tolerance mechanisms to respond to the salt tress and ensure normal growth and development under saline conditions in their lengthy evolutionary adaptation, so understanding how halophytes respond to salinity stress will provide us with methods and tactics to foster and develop salt resistant varieties of crops. The strategies in physiological and molecular level adopted by halophytes are various including the changes in photosynthetic and transpiration rate, the sequestration of Na+ to extracellular or vacuole, the regulation of stomata aperture and stomatal density, the accumulation and synthesis of the phytohormones as well as the relevant gene expression underlying these physiological traits, such as the stress signal transduction, the regulation of the transcription factors, the activation and expression of the transporter genes, the activation or inhibition of the synthetases and so on. This review focuses on the research advances of the regulating mechanisms in halophytes from physiological to molecular, which render the halophytes tolerance and adaption to salinity stress.
Keywords: Halophytes, Physiological traits, Salt tolerance, Signal transduction, Soil salinity, Transcription factor.
1. INTRODUCTION
As sessile organisms, plants in their whole life cycle suffer from diverse adverse environments such as drought, salinity, chilling, high temperature, pest etc. which are also the key constraints factors for agriculture production. However, salinity is the most extensive and common unfriendly condition among them. As we all know, soil salinity is turning into a severe problem for agriculture in worldwide, devouring as much as 7% of the total land and the proportion is growing all the time [1-3]. That is our arable land is lessening little by little.
Adding to the gradual diminishing land are the increasing population and global climate change. The population is projected to reach at about 9.5 billion by 2050, which means that the agriculture production must double by then [4, 5]. Generally, the excessive salt comes from the seawater and the irrigation water which carry trace amount of NaCl [6-8]. Thus the continuous sea-level rising resulted from Global Warming compounds the situation further especially for the coastal lowlands [9, 10]. It is estimated by the United Nations Food and Agriculture Organization that the salinized land has already reaches up to 4 million square kilometers [11]. In this context we are facing a daunting task.
Salt accumulation in arable soil hinders the plant to take up water and leads to the enrichment of Na+ and Cl- in plant. As a result the excess saline ions disrupt homeostasis of water potential and ion distribution at the cellular and whole plant levels [12]. Decreased photosynthesis, disordered metabolic processes, growth arrest, crop yield and even death come along [13, 14]. Therefore, as fertile soil becomes salinization, the agricultural production declines. Of course the plants are not merely to suffer the adverse impact passively. To the contrary, they can enact a battery of strategies to mitigate the harmful effects actively. For example, the plant is able to relief the osmotic stress by reducing water loss and reinforcing water absorption [15, 16]. Furthermore, by means of compartmentalizing the Na+ into vacuoles or excluding out of the leaf tissues, the plant can oppose the harmful effects of the Na+ ions stress [17]. Even so, duo to the majority of the crops are glycophyte, salt stress is still the key negative factor that reduces crop yield annually. Therefore, the plant especially the crops possessing capacities of salt tolerance will be of great economic significance and the salt tolerance should be an important-agronomical trait for crops [2, 18]. Scientists have long been devoting to engineering crops with salt tolerance, while the achievement is not optimistic enough. So in breeding salt resistant crops and developing saline agriculture we still have a long way to go. Thankfully, the existences of the salt tolerant plants, halophytes, which are capable of surviving under high salt environment, render us with a shimmer of hope. They can act as an excellent resource for the identification of our desired traits and promote the development of new crop system.
Presently, there is only about 1% percent in the land plant that is able to grow under coastal or saline conditions. Halophytes are the organisms having given morphological, anatomical and physiological characteristics to survive under high salt environment [3]. The salt tolerance of plant is simple and clear, while the concrete definition of halophyte remains problematic duo to the wide variety of the salt stress environments [19, 20]. For instance, halophytes are defined by Chapman as the plants which are able to complete their life cycle in the living environment with salt concentrations exceeding 0.5% NaCl [21]. In his opinion, the euhalophytes should be restricted to the plants which are able to grow well under more than 0.5% NaCl conditions. Stocker proposed that the halophyte should be the plants which are exposed to salt stress at least during a period of their life that is lethal for the large proportion of the plants [22]. According to their salinity tolerance, Braun-Blanquet classified the halophytes into oligo-, meso- and euhalophytes [23]. While Flowers et al. gave the definition of the halophyte on the basis that they were the organisms which were able to complete their life cycle under 200mM NaCl or more [3, 18]. Compared to the complicated classifications, we are more interested in how halophytes manage to survive and develop under the harmful conditions. What are the mechanisms adopted by the halophyte to cope with the salt stress. As early as middle twenties century, scientists had already realized that the osmotic pressure of cells must be high enough to allow water uptake in a saline environment [23-26].
Modern technology has speeded up our realization of the salt-resistant mechanisms and we have also recognized that there exists more than one mechanism that entrusts the halophytes salt tolerance, while these are far from enough. As is mentioned above, the serious situation compels us to understand the tolerance mechanisms in molecular, physiological and ecological further in halophytes. Only in this way can we be able to utilize the knowledge learned from halophytes to guide the better performance of the crops in saline oil. With the help of the extremophiles, we can try to introduce the salt tolerance genes and mechanisms into traditional crops to improve their adaptive capacity to environment. Therefore, in this review we will sum up the latest research advances in the regulating mechanisms in halophytes at physiological, ecological and molecular levels, which render the halophytes tolerance and adaption to salinity stress. Meanwhile, we must realize that some halophytes have the potential to be domesticated to crops directly, providing us with food, fiber, fodder and industrial materials.
2. THE IONS TRANSMEMBRANE TRANSPORTATION
Generally the series of the harmful effects caused by salt stress mainly derive from two aspects the osmotic stress and the ion imbalance both of which are associated with ion, thus ion is the key node of the salt resistant [12, 27]. The regulation of the ions transmembrane transportation in cell primarily involves the compartmentalization of ion into vacuole and the secretion out of cells through plasma membrane [1, 4, 6]. The compartmentalization of Na+ into vacuole is a common strategy in ion regulation found in both halophyte and nonhalophyte (glycophyte). However, the smart of the halophytes reflects in that they not only can sequester the excess sodium into vacuole more efficiently but also use the vast amount of the sodium as a cheap osmolyte to maintain low water potentials under salt stress. Therefore, one of the hallmarks of halophytes is their high efficient vacuolar Na+ sequestration. For instance, the research on suspension-cultured cells of the halophyte Sonneratia alba versus the glycophyte Oryza sativa suggested that the halophyte Sonneratia alba was much more efficient in transporting Na+ into vacuole than Oryza sativa [28]. The transcriptome analysis of halophyte Kosteletzkya virginica and Suaeda fruticosa also showed that the ion transporters were synthesized and accumulated under salt stress [29, 30]. Sesuvium portulacastrum is a typical halophyte with optimal growth at 200-300mM NaCl and the X-ray microanalysis on it also showed that Na+ was mainly compartmentalized into cell vacuole under salinity [17]. A similar result was also acquired from another halophyte, quinoa (Chenopodium quinoa). By controling the activity of slow (SV) and fast (FV) tonoplast channels, it succeeded in locking most of the accumulated Na+ in vacuole [31].
For the compartmentalization of the excess ions, the roles of the transporters are indispensable. The accumulation of the ions in vacuole is only a physiological response of the halophyte, while from the molecular point of view the fundamental reason is the synthesis and activation of the transporters in tonoplast and plasma membrane. Up to now, SOS1 and NHXs are the two kinds of well-known transporters for Na+ in plasma membrane and tonoplast respectively [1, 4, 32-36]. In addition, the in-depth and systematic researches into them are plentiful and a large number of reports indicate that the halophytes have greater abilities to sequester the Na+ into vacuoles. For example, at the end of the twenties century Barkla and Glenn had already put forward that halophytes were more able to sequester Na+ into vacuoles because of their constitutive expression of tonoplast Na+/H+ antiporters and rapid activation of their activities under salt stress conditions [37, 38]. The research on five halophytes (Sarcocornia fruticosa, Inula crithmoides, Plantago crassifolia, Juncus maritimus and J. acutus) also showed that the pivotal tolerance mechanisms involving the concentration regulating of Na+, Cl- and osmolyte were constitutive [2, 39]. To the contrary, the expressions of such transporters in glycophytes are slight and must be activated by NaCl [40]. On the other hand a study that compared the differences in proton pumping and Na+/H+ exchange at the leaf cell tonoplast between a halophyte Salicornia dolichostachya and glycophyte Spinacia oleracea implied that the S. dolichostachya were more efficient in retaining Na+ in the vacuole, preventing the Na+ reflux [41].
Meanwhile, the scientists in salt resistant crops breeding are also aware of this, so by overexpressing these transporters to improve the salt tolerance of crops becomes attractive. Many studies have focused on this field, while the results are not optimistic enough and only a few have acquired comforting outcomes [42]. Although some scientists have already point out the possible reasons of the failure such as the activities of the tonoplast H+-ATPase also should be increased at the same time [43]. Thus, we should recognize that salt tolerance is a complex trait involving many different genes and various biochemical and physiological mechanisms, so the specific and more mechanisms and researches are still needed. On the other hand, the studies around plasma membrane transporter SOS1 have never been interrupted, especially since the clarification of the SOS signaling transduction [33, 44]. The expression and activation of the SOS regulation system under salt stress are undoubted and the transgenetic engineering on it has already achieved some success [45, 46].
However, more halophytes are likely to depend on NHXs to compartment the ions into vacuole, as is another wise choice of halophytes. Halophytes choose to make full use of the excess inorganic ions (K+, Na+, Cl+) to maintain osmotic and turgor pressure under saline condition to guarantee the normal life activity [38, 47, 48], while to the contrary, the non-halophytes mainly rely on the synthesis of the compatible organic solute. Hence for some euhalophytes (for example Chenopodiaceae) the activity of SOS1 stays the same or even inhibited under salt stress to increase the accumulation of the inorganic ions in cells [49, 50]. It was discovered that under saline conditions, the inorganic ions constitute 80%-95% of the cell sap osmotic pressure in halophytes [38, 51], while for non-halophytes such as the crops wheat and barley the ration is only at about 50-70% [52, 53]. This refers to an important alternative mechanism for salt resistance, and that is the osmotic adjustment which will be discussed in more detail in the following. In addition, in recent years, the functions of the Na+/K+ transporter HKTs aroused extensive concern. The identified HKTs so far are mainly act as Na+ selective transport and Na+/K+ co-transport [54, 55]. As we all know, the Na+/K+ ratio is a key index in measuring plant salt-resistance ability, so it is not difficult to understand that the HKTs must play important roles in salt resistant. However, the studies of HKTs in halophytes have not been reported so far, they might be a new breakthrough in understanding the mechanisms of salt-resistance in halophytes in the near future.
3. THE SYNTHESIS AND ACCUMULATION OF OSMOTIC SUBSTANCES
As is mentioned and discussed above, the accumulated vast amount of ions in vacuole in halophytes serve as cheap inorganic osmolytes to maintain low water potentials under salt stress. Similarly, the roles of the organic osmolytes for salt resistance can`t be ignored and sometimes they might be the principal factors. In the first place, they are needed to maintain a steady osmotic pressure in cytoplasm, especially when large amount of inorganic ions in halophytes are sequestrated into vacuole [56]. Secondly, the organic osmotica such as proline, glycine bataine can protect the macromolecules such as enzymes, nucleic acid from inactivation, degeneration and degradation directly [57, 58]. The proteins are easily to become degeneration once the cell suffered from stress conditions, while the organic osmolytes can act as molecular chaperones to protect the proteins [59]. And not only that, they can also help to keep the cell structure stable especially the plasma membrane. The stability of the plasma membrane under salt stress is the determinant of the cell [60]. The halophytes are good at taking advantages of these osmotica and have an enormous ability to produce these osmolytes. Recently, the proteomic studies on halophytes give the best proofs. The comparative proteomics of Thellungiella revealed that it is the hyperactive starch and sucrose metabolism that ensures it with an extraordinary ability to compartmentalize Na+ into vacuole and accumulate proline and soluble sugars. Alongside, the morphological study on it also demonstrated that it was able to accumulate more chloroplasts and starch grains than glycophytes [61]. This phenomenon also evidences that the halophytes opt to protect the photosynthetic components on priority under high salinity. The same discovery was also found in the proteomic study of the true halophyte S. portulacastrum. Its carbohydrate metabolism and energy production-related enzymes were induced obviously upon salinity. In addition, the whole plant coordination of S. portulacastrum under high salinity including the changes of cells and chloroplasts, the more accumulated starch grains, the accumulation of the soluble sugar and proline were another successful strategy worth learning [17].
As a matter of fact, besides sugars the osmotica also include the quaternary amino acid derivatives, tertiary amines, sulfonium compounds and so on, most of which are the secondary metabolites [4, 42]. Generally, the secondary metabolites include sugars (simple and complex), quaternary ammonium compounds, polyols, amino acid derivatives and antioxidants [62, 63]. Halophytes have long been known for the ability to produce secondary metabolites, so making full use of the secondary metabolites is another wisdom action of halophytes. Therefore, the production of the secondary metabolites has great significance. Firstly, the accumulation of the secondary metabolites can act as osmotica to resist the high salt conditions. Secondly, some given secondary metabolites can be turned into functional foods [64]. Last but not least, some secondary metabolites can help to eliminate the reactive oxygen produced under adverse environment [65]. Studies have discovered that they protect the cells from oxidative stress through eliminating ROS directly or stabilizing the antioxidant enzymes [66, 67]. In some cases, the osmotica were discovered to play an important role in signaling transduction and gene expression regulation. Thus we can say that the osmotica have great significance in salt resistance.
However, on the other hand the multiple functions of the osmolytes in halophytes need a more stringent coordination control to ensure that the osmolytes are not produced immoderately. Some smarter halophytes also know that the synthesis of the organic osmolytes is an energy demanding process that is why most of the halophytes select to accumulate the inorganic ions into vacuole to maintain osmotic pressure instead of the de novo synthesis of the organic osmolytes. Yet the glycophytes are inclined to dependent on the synthesis of the organic osmotica both in vacuole and cytosol [68]. Although the organic osmolytes are obligatory in cytosol, for a mature plant cell the vacuole occupies almost the whole cell volume (for example approximately 73-99% of the cell volume in barley cells) [69]. This means that only a small absolute increase in cellular osmolyte contents will suffice for osmotic balance and then many more remaining organics can participate in metabolism to supply the plant with energy and nutrition. Therefore, from this point of view the halophytes are also sensible, worthy of our learning. What is more, in some special halophytes such as vetiver grass [70], quinoa [71] and Limonium latifolium [72], the concentrations of the organic osmolytes were found to be much lower than that of the inorganic ones under NaCl treatment. In a word, the coordination regulation of the synthesis and accumulation of the osmolytes including inorganic and organic osmolytes demonstrated the attractions of the halophytes in salt resistant again, many more painstaking researches on them are imperative. Although by overexpressing some given metabolism related enzymes of the osmolytes to increase the concentrations of the osmotica in trangenetic plant has got some success in improving salt resistance [42, 73-75], while many more tests have not yet. The behaviors of the halophytes tell us that the complex and coordination of the osmoregulatory regulation mechanisms, therefore the breeding of a promising salt-resistant crop is indeed a complicated and systematic engineering.
4. THE ADAPTIVE MECHANISMS OF THE PHOTOSYNTHESIS IN HALOPHYTES
Under salt stress the stomatal conductance is always decreased in order to reduce the water loss, so the available CO2 for carbon fixation is also limited, resulted in a reduced photosynthesis [76]. Meanwhile, the enzymes involved in photosynthetic carbon metabolism and components in photosynthetic electron transport are affects by salt stress directly [77]. Hence the reduction in photosynthesis is the most common negative result of high salinity. When the photosynthetic rate is restricted under salt stress, the demand for light energy reduces. In this case, the excessive reducing power which is the main source of the reactive oxygen species (ROS) begins to accumulate and cause harm to intracellular components, for example the photosystem II (PSII) [78, 79]. The studies on glycophytes Cyperus longus and halophytes Spartina versicolor revealed that the differences at the PSII activities between them are evident. The PSII activity in glycophytes decreased drastically under salt stress, while to the contrary the halophytes did no displayed distinct difference [80]. And in addition, a closer look into the deeper mechanisms in halophytes informed us that the PSs of the halophytes were so strong that they were still able to absorb light even under high Na+ concentrations [81]. The halophytes do have greater ability under saline conditions. Porteresia coarctate which is the halophytic relative of rice is better than rice in protecting the photosynthetic components under salt stress [82, 83]. Similarly, the proteomics study between wheat and its halophytic relative indicated that the relative was able to accumulate more CP24 protein precursor to stabilize PSII under 200 mM NaCl treatment [84]. In addition, as mentioned above the accumulation of the osmolytes under salt stress also can protect the photosynthetic compartments from salt stress. In halophytes, betain is produced enormously in the photosynthetic compartments to balance and resist the osmotic stress imposed by salinity stress, while in glycophytes this strategy is not able to come into play [80].
Another brilliant of the halophytes with respect to salt resistance in photosynthesis is the transformation of the carbon assimilation under salt stress. It was discovered that some special halophytes have the ability to change the carbon assimilation pathways. In generally, they opt to cease the C3 carbon assimilation pathway and enter into the C4 pathway or even Crassulacean Acid Metabolism (CAM) pathway during the salt stress. We mentioned earlier that the decrease of the stomatal conductance hindered the CO2 supply, so the photorespiration rate which was another ROS resource would increase in C3 plant [85]. The CAM plants are able to accumulate vast amount of CO2 by opening stomata in night and maintain normal photosynthesis in daytime with closed stoma to cope with low water potential under salt stress. In 1999, the halophyte Atriplex lentiformis was reported to be able to shift from C3 to C4 pathway in carbon assimilation to respond the salinity stress [86]. Moreover, in M. crystallinum the production of the H2O2 declined with the expression of the CAM under 400 mM NaCl [87]. To sum up, the photosynthetic adaptive mechanisms in halophytes are effective and in time. Both the salt-resistant photosynthetic system components and the conversion from C3 to C4 carbon assimilation were the special mechanisms of halophytes suggesting that they had stronger adaptation to salt stress.
5. OTHER MECHANISMS IN HALOPHYTES
5.1. Salt Gland Or Bladder
Some evident adaptations of halophytes can be observed directly and one of the distinct anatomical features of the halophyte is the production of the salt gland, but not all. It was estimated that about 50% halophytes possess salt bladder [3]. The salt gland located at the leaf surface is needed for the secretion strategy exclusive to halophytes [80]. The existence and function of the salt bland should be the most studied resistant mechanism in halophytes [88]. As everyone knows, the function of the salt gland is to secret the excessive Na+ out of the cells directly to reduce the negative effects [89]. Therefore, salt gland is the highly advantageous for plants growing in saline conditions. For example, it was reported that the epidermal bladder cell (EBC) was 1000-fold larger than the common epidermic cells in volume, meaning that it had 1000-fold Na+ excretion ability [89]. Generally, the salt bladders are regarded as the derivative of the trichomes, glandular hairs, thorns and surface glands [90]. As a matter of fact, the cereal crops also have the similar structures, yet these structures in crops do not big enough to have the similar functions in halophytes [91]. So modifying the number, size and shape of these structures in crops might be a practicable and promising method to improve the Na+ excretion ability in traditional cereal crops [47]. Meanwhile we have to recognize that the molecular mechanisms underlying the formation of the salt gland, the pumping of the Na+ into the salt gland and the genes involved in the epidermal cell patterning were far from clear enough. The scanty researches were coming from Arabidopsis [92], so we have to make a study carefully on the halophytes and learn from them.
5.2. Stomatal Density
Stoma is the main channel for water loss and CO2 diffusion in plants, so it is the stoma that controls the rate of the water loss and the CO2 assimilation. The stomatal density and the stomatal conductance are the two activities of stoma to regulate the water and gas circulation. The stomatal conductance is a dynamic process regulated by the osmotic conditions of the guard cells. In glycophytes, the stomatal conductance declined with the decreased water potential resulted from salinity stress [93], while this behavior was not suitable for halophytes. In halophytes the stomatal conductance was controlled by the coordination regulation of a series processes, thus the activity of the stoma was very different for some halophyte species and more studies were needed in this aspect [51, 94].
However the situation of the stomatal density seems simple and clear. Under the condition of the salt stress, the halophytes choose to decrease the stomatal density to reduce the water loss. For instance, the halophyte quinoa was discovered to reduce stomatal density at about 30% under salinity [47, 95], similarly in an amaranth species the stomatal density decreased with the increase in the salt concentration [96]. The same conclusion was also acquired in highly salt-tolerant halophytes including Distichlis spicata [97], Suaeda maritima [98], Kochia prostrate [99] and Aeloropus lagopodies and Lasiurus scindicus [100]. Maricle et al. also reported that the salt marsh plant had lower stomatal densities than the fresh water ones [101]. In addition, some further researches demonstrated that the reduction in stomatal density may help to optimize the water use efficiency (WUE) in halophytes under salt stress [102]. Hence, by regulating the stomatal density to improve the salt resistance is another strategy which we can learn from the halophytes.
5.3. Na+/Ca2+ Converse Transport Mechanism
The Na+/Ca2+exchanger-like proteins were able to transport Ca2+ bidirectionally, working conversely to the transportation of Na+ [103]. The turbulence of the Ca2+ concentration under salt stress has long been discovered and the concentration can rise rapidly within seconds after exposure to NaCl [104], therefore the Ca2+ and Ca2+ channel play important roles in salt stress response. The comparative study between the halophyte Sonneratia alba and the glycophyte Oryza sativa revealed that there was a marked decrease in the Ca concentration in cytoplasmic matrix and the vacuolar lumen upon exposed to 50 mM NaCl in Sonneratia alba, contrary to the concentrations of the Na and the Cl. For the case of Oryza sativa, not only the concentrations of Ca in vacuole and cytoplasm were not as high as that in Sonneratia alba but also no marked changes in the concentration in Ca upon salt stress [28]. This suggested that the halophyte has specific Na+/Ca2+ converse transportation mechanism to coordinate the Na and Ca concentrations to adapt the severe adverse environment.
5.4. Succulence
Although the salt gland or bladder is a high-efficient strategy for halophytes to resist the excessive salt stress, but not all the halophytes have this structure. So some other strategies are adopted by the halophytes that do not rely on the glands or bladders to regulate ion concentrations. There is a kind of halophytes called succulent halophytes including two types: stem-succulent and fleshy succulent leaves. Different from the others, succulent halophytes accumulate a great amount of ions in their cells or tissues instead of discharging or compartmentalization [105]. The prominent feature of them is to resist the ions toxicity by developing succulence. Sometimes, the contents of the salt ions can reach up to 60% of the leaf dry weight [106]. Suaeda is a representative of the succulent halophytes with highly succulent leaves, thus it is able to adapt to the high salinity without the participation of the salt gland [3, 107]. In addition several other succulent halophytes belonging to the Amaranthaceae were also reported, including the Suaeda and Salicornia [10, 62, 108, 109]. The other typical representative is S. portulacastrum, which is a euhalophyte belonging to Aizoaceae [103]. This plant is regarded as a salt accumulator, accumulating lots of salts in cells or tissues with its succulent leaves [17]. Therefore, this kind of halophytes is considered to be the perfect candidate for the phytoremediation, which is a biological method to improve the salt-affected soils [110].
6. CONCLUSION
As described above, the halophytes have excellent adaptabilities to high salinity in both cell and the whole plant levels which were summarized in (Fig. 1). On the one hand, they have evolved some special adaptive mechanisms such as salt gland, succulent leaves, while what is more important is that the halophytes were better at taking full advantages of the common mechanisms which are also the strategies taken by glycophytes including the compartmentalization of the ions, the accumulation of the osmolytes. Therefore, when we are busy in exploring the novel mechanisms utilized in halophytes, researches in-depth in the common mechanisms can`t be ignored at the same time. Although the researches on halophytes have lasted for more than 100 years, some basic mechanisms are still unclear. In recent years the developments of the genomics and the proteomics have promoted our understanding on halophytes greatly. They tell us that the plant salt tolerance is a complex response involving the whole plant, rather than the action of a single gene or protein. This explains why the manipulation of a single gene or protein through genetic engineering to improve the salt tolerance of a glyocphyte always can`t acquired the ideal result. In addition, the new concept halophytic crops attract more and more attentions. Namely, the halophyte itself can be used for fodder, biofuel, industry, ornament or even food. In this way, the domestication of the halophytes to crops is also a good choice. In brief, the physiological, biochemical and molecular studies on halophytes open their salt-tolerant door, thus we are able to learn more strategies to develop crops with enhanced salt tolerance allowing the crops to be able to develop normally in saline lands in future.
Fig. (1).
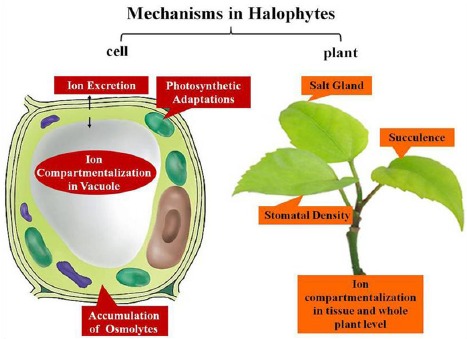
The mechanisms in halophytes from cells to whole plant level.
ACKNOWLEDGEMENTS
This work was jointly supported by by the Innovative Team Construction Project for Utilization of Jujube Germplasm Resources and Variety Breeding (2014CC006), the National Natural Science Foundation of China (41171216), Jiangsu Agricultural Science and Technology Independent Innovation Project [CX (15)1005], Yantai Double–hundred High–end Talent Plan (XY–003–02) and 135 Development Plan of YIC-CAS.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCEs
- 1.Deinlein U., Stephan A.B., Horie T., Luo W., Xu G., Schroeder J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19(6):371–379. doi: 10.1016/j.tplants.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flowers T.J., Muscolo A. Introduction to the Special Issue: Halophytes in a changing world. AoB Plants. 2015;7:231–245. doi: 10.1093/aobpla/plv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flowers T.J., Colmer T.D. Salinity tolerance in halophytes. New Phytol. 2008;179(4):945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- 4.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 5.Hajkowicz S., Cook H., Littleboy A. Our future world: global megatrends that will change the way we live. https://publications.csiro.au/rpr/download?pid=csiro:EP126135&dsid=DS2 . 2012.
- 6.Flowers T., Yeo A. Breeding for salinity resistance in crop plants: where next? Funct. Plant Biol. 1995;22(6):875–884. [Google Scholar]
- 7.Tester M., Davenport R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. (Lond.) 2003;91(5):503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rengasamy P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010;37(7):613–620. doi: 10.1071/FP09249. [DOI] [Google Scholar]
- 9.Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005;167(3):645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 10.Rozema J., Flowers T. Ecology. Crops for a salinized world. Science. 2008;322(5907):1478–1480. doi: 10.1126/science.1168572. [DOI] [PubMed] [Google Scholar]
- 11.FAO. Terrastat Database. www.fao.org/agl/agl1/terrastat .
- 12.Zhu J-K. Plant salt tolerance. Trends Plant Sci. 2001;6(2):66–71. doi: 10.1016/S1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003;6(5):441–445. doi: 10.1016/S1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 14.Cabot C., Sibole J.V., Barceló J., Poschenrieder C. Lessons from crop plants struggling with salinity. Plant Sci. 2014;226:2–13. doi: 10.1016/j.plantsci.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Han B., Wang T., Chen S., Li H., Zhang Y., Dai S. Mechanisms of plant salt response: insights from proteomics. J. Proteome Res. 2012;11(1):49–67. doi: 10.1021/pr200861w. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson N.J., Urwin P.E. The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot. 2012;63(10):3523–3543. doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- 17.Yi X., Sun Y., Yang Q., Guo A., Chang L., Wang D., Tong Z., Jin X., Wang L., Yu J., Jin W., Xie Y., Wang X. Quantitative proteomics of Sesuvium portulacastrum leaves revealed that ion transportation by V-ATPase and sugar accumulation in chloroplast played crucial roles in halophyte salt tolerance. J. Proteomics. 2014;99:84–100. doi: 10.1016/j.jprot.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Flowers T.J., Colmer T.D. Plant salt tolerance: adaptations in halophytes. Ann. Bot. (Lond.) 2015;115(3):327–331. doi: 10.1093/aob/mcu267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huchzermeyer B., Flowers T. Putting halophytes to work–genetics, biochemistry and physiology. Funct. Plant Biol. 2013;40(9):v–viii. doi: 10.1071/FPv40n9_FO. [DOI] [PubMed] [Google Scholar]
- 20.Breckle S.-W. Salinity, halophytes and salt affected natural ecosystems. Salinity: environment-plants-molecules. 2002:53–72. [Google Scholar]
- 21.Chapman V. The new perspective in the halophytes. Rev. Bio. 1942;17(4):291–311. doi: 10.1086/394660. [DOI] [Google Scholar]
- 22.Stocker O. Das halophytenproblem. Ergeb. Biol. 1928;3:265–353. doi: 10.1007/978-3-642-91065-4_4. [DOI] [Google Scholar]
- 23.Braun-Blanquet J., Bharucha F., Meier H. Zur Frage der physiologischen Trockenheit der Salzboeden. Berichte der Schweizerischen Botanischen Gesellschaft. 1931;40:33–39. [Google Scholar]
- 24.Montfort C. Physiologische und pflanzengeographische Seesalzwirkungen: Einfluβ ausgeglichener Salzlösungen auf Mesophyll-und Schlieszellen; Kritik der Iljinschen Hypothese der Salzbeständigkeit. Jahrbuch der wissenschaftlichen Botanik. 1926;65:502–550. [Google Scholar]
- 25.Yan K., Shao H., Shao C., Chen P., Zhao S., Brestic M., Chen X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 2013;35(10):2867–2878. doi: 10.1007/s11738-013-1325-7. [DOI] [Google Scholar]
- 26.Steiner M. Zur Ökologie der Salzmarschen der nordöstlichen Vereinigten Staaten von Nordamerika. Jb. wiss. Bot. 1934;81:94–202. [Google Scholar]
- 27.Askari H., Edqvist J., Hajheidari M., Kafi M., Salekdeh G.H. Effects of salinity levels on proteome of Suaeda aegyptiaca leaves. Proteomics. 2006;6(8):2542–2554. doi: 10.1002/pmic.200500328. [DOI] [PubMed] [Google Scholar]
- 28.Hayatsu M., Suzuki S., Hasegawa A., Tsuchiya S., Sasamoto H. Effect of NaCl on ionic content and distribution in suspension-cultured cells of the halophyte Sonneratia alba versus the glycophyte Oryza sativa. J. Plant Physiol. 2014;171(15):1385–1391. doi: 10.1016/j.jplph.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Tang X., Wang H., Shao C., Shao H. Global Gene Expression of Kosteletzkya virginica Seedlings Responding to Salt Stress. PLoS One. 2015;10(4):e0124421. doi: 10.1371/journal.pone.0124421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diray-Arce J., Clement M., Gul B., Khan M.A., Nielsen B.L. Transcriptome assembly, profiling and differential gene expression analysis of the halophyte Suaeda fruticosa provides insights into salt tolerance. BMC Genomics. 2015;16:353. doi: 10.1186/s12864-015-1553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonales-Alatorre E., Shabala S., Chen Z., Pottosin I.I. Reduced tonoplast FV and SV channels activity is essential for conferring salinity tolerance in a facultative halophyte, Chenopodium quinoa. Plant Physiol. 2013;113:216–232. doi: 10.1104/pp.113.216572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J-K. Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol. 2000;124(3):941–948. doi: 10.1104/pp.124.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji H., Pardo J.M., Batelli G., Van Oosten M.J., Bressan R.A., Li X. The Salt Overly Sensitive (SOS) pathway: established and emerging roles. Mol. Plant. 2013;6(2):275–286. doi: 10.1093/mp/sst017. [DOI] [PubMed] [Google Scholar]
- 34.Blumwald E., Poole R.J. Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol. 1985;78(1):163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez-Rosales M.P., Jiang X., Gálvez F.J., Aranda M.N., Cubero B., Venema K. Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol. 2008;179(2):366–377. doi: 10.1111/j.1469-8137.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang H., Tang X., Shao C., Shao H., Wang H. Molecular cloning and bioinformatics analysis of a new plasma membrane Na(+)/H(+) antiporter gene from the halophyte Kosteletzkya virginica. Scientific World J. 2014;2014:141675. doi: 10.1155/2014/141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barkla B.J., Zingarelli L., Blumwald E., Smith J. Tonoplast Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L. Plant Physiol. 1995;109(2):549–556. doi: 10.1104/pp.109.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glenn E.P., Brown J.J., Blumwald E. Salt tolerance and crop potential of halophytes. Crit. Rev. Plant Sci. 1999;18(2):227–255. doi: 10.1016/S0735-2689(99)00388-3. [DOI] [Google Scholar]
- 39.Kosová K., Vítámvás P., Urban M.O., Prášil I.T. Plant proteome responses to salinity stress–comparison of glycophytes and halophytes. Funct. Plant Biol. 2013;40(9):775–786. doi: 10.1071/FP12375. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H-X., Blumwald E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat. Biotechnol. 2001;19(8):765–768. doi: 10.1038/90824. [DOI] [PubMed] [Google Scholar]
- 41.Katschnig D., Jaarsma R., Almeida P., Rozema J., Schat H. Differences in proton pumping and Na/H exchange at the leaf cell tonoplast between a halophyte and a glycophyte. AoB Plants. 2014;6:plu023. doi: 10.1093/aobpla/plu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang X., Mu X., Shao H., Wang H., Brestic M. Global plant-responding mechanisms to salt stress: physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015;35(4):425–437. doi: 10.3109/07388551.2014.889080. [DOI] [PubMed] [Google Scholar]
- 43.Shabala S., Cuin T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008;133(4):651–669. doi: 10.1111/j.1399-3054.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 44.Belver A., Olías R., Huertas R., Rodríguez-Rosales M.P. Involvement of SlSOS2 in tomato salt tolerance. Bioengineered. 2012;3(5):298–302. doi: 10.4161/bioe.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Q., Chen Z-Z., Zhou X-F., Yin H-B., Li X., Xin X-F., Hong X-H., Zhu J-K., Gong Z. Overexpression of SOS (Salt Overly Sensitive) genes increases salt tolerance in transgenic Arabidopsis. Mol. Plant. 2009;2(1):22–31. doi: 10.1093/mp/ssn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janicka-Russak M., Kabała K., Wdowikowska A., Kłobus G. Modification of plasma membrane proton pumps in cucumber roots as an adaptation mechanism to salt stress. J. Plant Physiol. 2013;170(10):915–922. doi: 10.1016/j.jplph.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Shabala S. Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. (Lond.) 2013;112(7):1209–1221. doi: 10.1093/aob/mct205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu H., Shabala L., Liu X., Azzarello E., Zhou M., Pandolfi C., Chen Z.H., Bose J., Mancuso S., Shabala S. Linking salinity stress tolerance with tissue-specific Na(+) sequestration in wheat roots. Front. Plant Sci. 2015;6:71. doi: 10.3389/fpls.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv S., Jiang P., Chen X., Fan P., Wang X., Li Y. Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol. Biochem. 2012;51:47–52. doi: 10.1016/j.plaphy.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Ushakova S., Kovaleva N., Gribovskaya I., Dolgushev V., Tikhomirova N. Effect of NaCl concentration on productivity and mineral composition of Salicornia europaea as a potential crop for utilization NaCl in LSS. Adv. Space Res. 2005;36(7):1349–1353. doi: 10.1016/j.asr.2004.09.017. [DOI] [Google Scholar]
- 51.Shabala S., Mackay A. Ion transport in halophytes. Adv. Bot. Res. 2011;57:151–187. doi: 10.1016/B978-0-12-387692-8.00005-9. [DOI] [Google Scholar]
- 52.Chen Z., Pottosin I.I., Cuin T.A., Fuglsang A.T., Tester M., Jha D., Zepeda-Jazo I., Zhou M., Palmgren M.G., Newman I.A., Shabala S. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007;145(4):1714–1725. doi: 10.1104/pp.107.110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cuin T.A., Parsons D., Shabala S. Wheat cultivars can be screened for NaCl salinity tolerance by measuring leaf chlorophyll content and shoot sap potassium. Funct. Plant Biol. 2010;37(7):656–664. doi: 10.1071/FP09229. [DOI] [Google Scholar]
- 54.Rubio F., Gassmann W., Schroeder J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270(5242):1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 55.Mäser P., Hosoo Y., Goshima S., Horie T., Eckelman B., Yamada K., Yoshida K., Bakker E.P., Shinmyo A., Oiki S., Schroeder J.I., Uozumi N. Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc. Natl. Acad. Sci. USA. 2002;99(9):6428–6433. doi: 10.1073/pnas.082123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo L., Shi D., Wang D. The Key Physiological Response to Alkali Stress by the Alkali‐Resistant Halophyte Puccinellia tenuiflora is the Accumulation of Large Quantities of Organic Acids and into the Rhyzosphere. J. Agron. Crop Sci. 2010;196(2):123–135. doi: 10.1111/j.1439-037X.2009.00397.x. [DOI] [Google Scholar]
- 57.Wang J., Meng Y., Li B., Ma X., Lai Y., Si E., Yang K., Xu X., Shang X., Wang H., Wang D. Physiological and proteomic analyses of salt stress response in the halophyte Halogeton glomeratus. Plant Cell Environ. 2015;38(4):655–669. doi: 10.1111/pce.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slama I., Abdelly C., Bouchereau A., Flowers T., Savouré A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. (Lond.) 2015;115(3):433–447. doi: 10.1093/aob/mcu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holthauzen L.M., Auton M., Sinev M., Rösgen J. Protein stability in the presence of cosolutes. Methods Enzymol. 2011;492:61–125. doi: 10.1016/B978-0-12-381268-1.00015-X. [DOI] [PubMed] [Google Scholar]
- 60.Ignatova Z., Gierasch L.M. Effects of osmolytes on protein folding and aggregation in cells. Methods Enzymol. 2007;428:355–372. doi: 10.1016/S0076-6879(07)28021-8. [DOI] [PubMed] [Google Scholar]
- 61.Wang X., Chang L., Wang B., Wang D., Li P., Wang L., Yi X., Huang Q., Peng M., Guo A. Comparative proteomics of Thellungiella halophila leaves from plants subjected to salinity reveals the importance of chloroplastic starch and soluble sugars in halophyte salt tolerance. Mol. Cell. Proteomics. 2013;12(8):2174–2195. doi: 10.1074/mcp.M112.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ventura Y., Sagi M. Halophyte crop cultivation: the case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013;92:144–153. doi: 10.1016/j.envexpbot.2012.07.010. [DOI] [Google Scholar]
- 63.Parvaiz A., Satyawati S. Salt stress and phyto-biochemical responses of plants-a review. Plant Soil Environ. 2008;54(3):89. [Google Scholar]
- 64.Buhmann A., Papenbrock J. An economic point of view of secondary compounds in halophytes. Funct. Plant Biol. 2013;40(9):952–967. doi: 10.1071/FP12342. [DOI] [PubMed] [Google Scholar]
- 65.Hasegawa P.M., Bressan R.A., Zhu J-K., Bohnert H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;51(1):463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- 66.Jithesh M.N., Prashanth S.R., Sivaprakash K.R., Parida A.K. Antioxidative response mechanisms in halophytes: their role in stress defence. J. Genet. 2006;85(3):237–254. doi: 10.1007/BF02935340. [DOI] [PubMed] [Google Scholar]
- 67.Uzilday B., Ozgur R., Sekmen A.H., Yildiztugay E., Turkan I. Changes in the alternative electron sinks and antioxidant defence in chloroplasts of the extreme halophyte Eutrema parvulum (Thellungiella parvula) under salinity. Ann. Bot. (Lond.) 2015;115(3):449–463. doi: 10.1093/aob/mcu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raven J.A. Tansley review No. 2. New Phytol. 1985;101(1):25–77. doi: 10.1111/j.1469-8137.1985.tb02816.x. [DOI] [PubMed] [Google Scholar]
- 69.Winter H., Robinson D.G., Heldt H.W. Subcellular volumes and metabolite concentrations in barley leaves. Planta. 1993;191(2):180–190. doi: 10.1007/BF00199748. [DOI] [Google Scholar]
- 70.Zhou Q., Yu B. Accumulation of inorganic and organic osmolytes and their role in osmotic adjustment in NaCl-stressed vetiver grass seedlings. Russ. J. Plant Physiol. 2009;56(5):678–685. doi: 10.1134/S1021443709050148. [DOI] [Google Scholar]
- 71.Hariadi Y., Marandon K., Tian Y., Jacobsen S-E., Shabala S. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J. Exp. Bot. 2011;62(1):185–193. doi: 10.1093/jxb/erq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gagneul D., Aïnouche A., Duhazé C., Lugan R., Larher F.R., Bouchereau A. A reassessment of the function of the so-called compatible solutes in the halophytic plumbaginaceae Limonium latifolium. Plant Physiol. 2007;144(3):1598–1611. doi: 10.1104/pp.107.099820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garg A.K., Kim J-K., Owens T.G., Ranwala A.P., Choi Y.D., Kochian L.V., Wu R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA. 2002;99(25):15898–15903. doi: 10.1073/pnas.252637799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Z., Qi X., Wang Z., Li P., Wu C., Zhang H., Zhao Y. Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana enhances tolerance to high salinity and osmotic stresses. Plant Physiol. Biochem. 2013;69:82–89. doi: 10.1016/j.plaphy.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 75.Székely G., Abrahám E., Cséplo A., Rigó G., Zsigmond L., Csiszár J., Ayaydin F., Strizhov N., Jásik J., Schmelzer E., Koncz C., Szabados L. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008;53(1):11–28. doi: 10.1111/j.1365-313X.2007.03318.x. [DOI] [PubMed] [Google Scholar]
- 76.Ozgur R., Uzilday B., Sekmen A.H., Turkan I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013;40(9):832–847. doi: 10.1071/FP12389. [DOI] [PubMed] [Google Scholar]
- 77.Tuteja N. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 2007;428:419–438. doi: 10.1016/S0076-6879(07)28024-3. [DOI] [PubMed] [Google Scholar]
- 78.Megdiche W., Hessini K., Gharbi F., Jaleel C.A., Ksouri R., Abdelly C. Photosynthesis and photosystem 2 efficiency of two salt-adapted halophytic seashore Cakile maritima ecotypes. Photosynthetica. 2008;46(3):410–419. doi: 10.1007/s11099-008-0073-1. [DOI] [Google Scholar]
- 79.Qiu N., Lu Q., Lu C. Photosynthesis, photosystem II efficiency and the xanthophyll cycle in the salt‐adapted halophyte Atriplex centralasiatica. New Phytol. 2003;159(2):479–486. doi: 10.1046/j.1469-8137.2003.00825.x. [DOI] [PubMed] [Google Scholar]
- 80.Duarte B., Sleimi N., Caçador I. Biophysical and biochemical constraints imposed by salt stress: learning from halophytes. Front. Plant Sci. 2014;5:746. doi: 10.3389/fpls.2014.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rabhi M., Castagna A., Remorini D., Scattino C., Smaoui A., Ranieri A., Abdelly C. Photosynthetic responses to salinity in two obligate halophytes: Sesuvium portulacastrum and Tecticornia indica. S. Afr. J. Bot. 2012;79:39–47. doi: 10.1016/j.sajb.2011.11.007. [DOI] [Google Scholar]
- 82.Sengupta S., Majumder A.L. Insight into the salt tolerance factors of a wild halophytic rice, Porteresia coarctata: a physiological and proteomic approach. Planta. 2009;229(4):911–929. doi: 10.1007/s00425-008-0878-y. [DOI] [PubMed] [Google Scholar]
- 83.Sengupta S., Majumder A.L. Porteresia coarctata (Roxb.) Tateoka, a wild rice: a potential model for studying salt-stress biology in rice. Plant Cell Environ. 2010;33(4):526–542. doi: 10.1111/j.1365-3040.2009.02054.x. [DOI] [PubMed] [Google Scholar]
- 84.Peng Z., Wang M., Li F., Lv H., Li C., Xia G. A proteomic study of the response to salinity and drought stress in an introgression strain of bread wheat. Mol. Cell. Proteomics. 2009;8(12):2676–2686. doi: 10.1074/mcp.M900052-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foyer C.H., Noctor G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 2003;119(3):355–364. doi: 10.1034/j.1399-3054.2003.00223.x. [DOI] [Google Scholar]
- 86.Meinzer F.C., Zhu J. Efficiency of C4 photosynthesis in Atriplex lentiformis under salinity stress. Funct. Plant Biol. 1999;26(1):79–86. [Google Scholar]
- 87.Sunagawa H., Cushman J.C., Agarie S. Crassulacean acid metabolism may alleviate production of reactive oxygen species in a facultative CAM plant, the common ice plant Mesembryanthemum crystallinum L. Plant Prod. Sci. 2010;13(3):256–260. doi: 10.1626/pps.13.256. [DOI] [Google Scholar]
- 88.Rozema J., Gude H., Pollak G. An ecophysiological study of the salt secretion of four halophytes. New Phytol. 1981;89(2):201–217. doi: 10.1111/j.1469-8137.1981.tb07483.x. [DOI] [Google Scholar]
- 89.Shabala S., Bose J., Hedrich R. Salt bladders: do they matter? Trends Plant Sci. 2014;19(11):687–691. doi: 10.1016/j.tplants.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 90.Ishida T., Kurata T., Okada K., Wada T. A genetic regulatory network in the development of trichomes and root hairs. Annu. Rev. Plant Biol. 2008;59:365–386. doi: 10.1146/annurev.arplant.59.032607.092949. [DOI] [PubMed] [Google Scholar]
- 91.Baker D.A., Hall J.L. Solute transport in plant cells and tissues. Longman Scientific & Technical. 1988 [Google Scholar]
- 92.Glover B.J. Differentiation in plant epidermal cells. J. Exp. Bot. 2000;51(344):497–505. doi: 10.1093/jexbot/51.344.497. [DOI] [PubMed] [Google Scholar]
- 93.Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25(2):239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- 94.Redondo-Gómez S., Mateos-Naranjo E., Davy A.J., Fernández-Muñoz F., Castellanos E.M., Luque T., Figueroa M.E. Growth and photosynthetic responses to salinity of the salt-marsh shrub Atriplex portulacoides. Ann. Bot. (Lond.) 2007;100(3):555–563. doi: 10.1093/aob/mcm119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orsini F., Accorsi M., Gianquinto G., Dinelli G., Antognoni F., Carrasco K.B., Martinez E.A., Alnayef M., Marotti I., Bosi S. Beyond the ionic and osmotic response to salinity in Chenopodium quinoa: functional elements of successful halophytism. Funct. Plant Biol. 2011;38(10):818–831. doi: 10.1071/FP11088. [DOI] [PubMed] [Google Scholar]
- 96.Omamt E., Hammes P., Robbertse P. Differences in salinity tolerance for growth and water‐use efficiency in some amaranth (Amaranthus spp.) genotypes. N. Z. J. Crop Hortic. Sci. 2006;34(1):11–22. doi: 10.1080/01140671.2006.9514382. [DOI] [Google Scholar]
- 97.Kemp P.R., Cunningham G.L. Light, Temperature and Salinity Effects on Growth, Leaf Anatomy and Photosyntesis of Distichlis spicata (L.) Greene. Am. J. Bot. 1981;•••:507–516. doi: 10.2307/2443026. [DOI] [Google Scholar]
- 98.Flowers T. Physiology of halophytes. Plant Soil. 1985;89(1-3):41–56. doi: 10.1007/BF02182232. [DOI] [Google Scholar]
- 99.Karimi G., Ghorbanli M., Heidari H., Nejad R.K., Assareh M. The effects of NaCl on growth, water relations, osmolytes and ion content in Kochia prostrata. Biol. Plant. 2005;49(2):301–304. doi: 10.1007/s10535-005-1304-y. [DOI] [Google Scholar]
- 100.Naz N., Hameed M., Ashraf M., Al-Qurainy F., Arshad M. Relationships between gas-exchange characteristics and stomatal structural modifications in some desert grasses under high salinity. Photosynthetica. 2010;48(3):446–456. doi: 10.1007/s11099-010-0059-7. [DOI] [Google Scholar]
- 101.Maricle B.R., Koteyeva N.K., Voznesenskaya E.V., Thomasson J.R., Edwards G.E. Diversity in leaf anatomy, and stomatal distribution and conductance, between salt marsh and freshwater species in the C(4) genus Spartina (Poaceae). New Phytol. 2009;184(1):216–233. doi: 10.1111/j.1469-8137.2009.02903.x. [DOI] [PubMed] [Google Scholar]
- 102.Adolf V.I., Shabala S., Andersen M.N., Razzaghi F., Jacobsen S-E. Varietal differences of quinoa’s tolerance to saline conditions. Plant Soil. 2012;357(1-2):117–129. doi: 10.1007/s11104-012-1133-7. [DOI] [Google Scholar]
- 103.Wang D., Wang H., Han B., Wang B., Guo A., Zheng D., Liu C., Chang L., Peng M., Wang X. Sodium instead of potassium and chloride is an important macronutrient to improve leaf succulence and shoot development for halophyte Sesuvium portulacastrum. Plant Physiol. Biochem. 2012;51:53–62. doi: 10.1016/j.plaphy.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 104.Knight H., Trewavas A.J., Knight M.R. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12(5):1067–1078. doi: 10.1046/j.1365-313X.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- 105.Youssef A.M. Salt tolerance mechanisms in some halophytes from Saudi Arabia and Egypt. J. Agric. Biol. Sci. 2009;5(3):191–206. [Google Scholar]
- 106.Eshel A. Effects of NaCl and KCl on growth and ionic composition of the halophytic C4 succulent chenopods Salsola kali, Suaeda monoica and Suaeda aegyptiaca. Funct. Plant Biol. 1985;12(3):319–328. [Google Scholar]
- 107.Song J., Wang B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. (Lond.) 2015;115(3):541–553. doi: 10.1093/aob/mcu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Glenn E.P., Anday T., Chaturvedi R., Martinez-Garcia R., Pearlstein S., Soliz D., Nelson S.G., Felger R.S. Three halophytes for saline-water agriculture: An oilseed, a forage and a grain crop. Environ. Exp. Bot. 2013;92:110–121. doi: 10.1016/j.envexpbot.2012.05.002. [DOI] [Google Scholar]
- 109.Rozema J., Schat H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2013;92:83–95. doi: 10.1016/j.envexpbot.2012.08.004. [DOI] [Google Scholar]
- 110.Rabhi M., Ferchichi S., Jouini J., Hamrouni M.H., Koyro H-W., Ranieri A., Abdelly C., Smaoui A. Phytodesalination of a salt-affected soil with the halophyte Sesuvium portulacastrum L. to arrange in advance the requirements for the successful growth of a glycophytic crop. Bioresour. Technol. 2010;101(17):6822–6828. doi: 10.1016/j.biortech.2010.03.097. [DOI] [PubMed] [Google Scholar]


