Abstract
Oils of plant origin have been predominantly used for food-based applications. Plant oils not only represent a non-polluting renewable resource but also provide a wide diversity in fatty acids (FAs) composition with diverse applications. Besides being edible, they are now increasingly being used in industrial applications such as paints, lubricants, soaps, biofuels etc. In addition, plants can be engineered to produce fatty acids which are nutritionally beneficial to human health. Thus these oils have potential to 1) substitute ever increasing demand of non –renewable petroleum sources for industrial application and 2) also spare the marine life by providing an alternative source to nutritionally and medically important long chain polyunsaturated fatty acids or ‘Fish oil’. The biochemical pathways producing storage oils in plants have been extensively characterized, but the factors regulating fatty acid synthesis and controlling total oil content in oilseed crops are still poorly understood. Thus understanding of plant lipid metabolism is fundamental to its manipulation and increased production. This review on oils discusses fatty acids of nutritional and industrial importance, and approaches for achieving future designer vegetable oil for both edible and non-edible uses. The review will discuss the success and bottlenecks in efficient production of novel FAs in non-native plants using genetic engineering as a tool.
Keywords: Omega-3 fatty acids, Hydroxy fatty acids, Stearidonic acid, γ-linolenic acid, Oleic acids, Erucic acid.
1. INTRODUCTION
Oils have a variety of uses besides edible applications. There is now an increasing evidence that fatty acids (FAs) play a crucial role in human nutrition that include therapeutic and prophylactic prevention of diseases, in growth and development of human embryo, brain function and provide protection against many serious diseases such as cardiovascular, inflammation etc. Many FAs are now known to have anticancer potential. Importance of role of fats and fatty acids in human nutrition is gaining attention as more and more research is being done. Besides an essential component of human diet, FAs also find importance in various industrial applications such as soaps and detergents, cosmetics, lubricants, ink, varnish, paints etc. Thus an ever expanding market exists for oilseed crops from both nutritional and industrial perspectives. In addition, plants produce a wide variety of fatty acids with different structures that confer unique physico-chemical properties on them and make them useful.
With increasing petroleum prices and depleting natural resources, there is a longstanding need to explore and develop new sources of fatty acids of both industrial and nutritional importance. With advancement of understanding the steps in metabolic pathways in the synthesis of fatty acids, attempts have been intensified in engineering the pathways for the production of useful and/or novel fatty acids in a cost effective way. Designer oils that preferentially produce these fatty acids can be created and will be economically feasible and competitive to petroleum based products.
2. BIOSYNTHESIS OF FATTY ACIDS AND TRIACYLGLYCEROLS
Normally plants produce FAs which may have zero to three double bonds. These commonly found usual FAs include palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), and linolenic acid (18:3). In oilseed plants, these fatty acids are predominantly stored as triacylglycerols (TAG) which is the major storage form in seed. These lipids may be stored in cotyledon or endosperm which are used to supply energy during germination. In addition to TAG, fatty acids also exist in the form of wax esters, for example jojoba fruit (Simmondsia chinensis).
The fatty acids are synthesized in plastids from acetyl-CoA as starting substrate and on acyl carrier protein (ACP) (Fig. 1). The fatty acids are then removed from ACP by action of enzyme, thioesterase. Free fatty acids move to cytosol where they are further incorporated into acyl-CoA pool and/or phosphatidylcholine (PC) pool, which then undergo modifications such as desaturation or hydroxylation, epoxylation etc. and their inclusion into TAG takes place. These later processes occur in endoplasmic reticulum (ER) of plant cells [1, 2]. TAGs are the major storage form found in seeds. It is synthesized in ER, using acyl-CoA and glycerol-3-phosphate as substrates by the Kennedy pathway. The first enzyme is glycerol-3-phosphate acyltransferase (GPAT), that acylates sn-1 position of glycerol backbone to form lysophosphatidic acid (LPA). The second enzyme of the pathway is lysophosphatidic acid acyltransferase (LPAAT) that acylates at the sn-2 position to form phosphatidic acid (PA), which is then converted into diacylglycerol (DAG) by the enzyme phosphatidic acid phosphatase (PAP). Another acyltransferase, diacylglycerol acyltransferase (DGAT), forms TAG from DAG using an acyl-CoA as a substrate [1, 2].
Fig. (1).
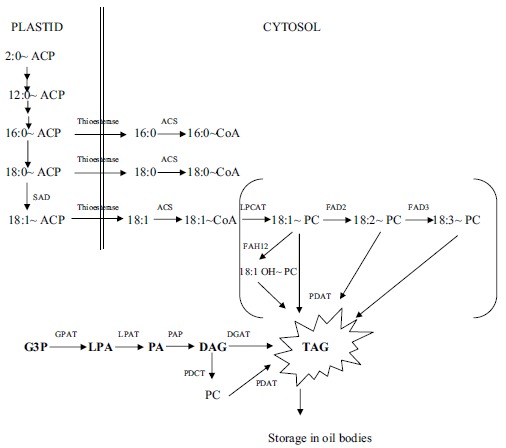
Biosynthesis of commonly found fatty acids in plants. ACP: Acyl carrier protein, SAD: Stearoyl ACP desaturase, CoA: Coenzyme A, PC: Phosphatidylcholine, FAH12: Fatty acid hydroxylase 12, FAD2: Fatty acid desaturase 2, FAD3: Fatty acid desaturase 3, G3P: Glyceraldehyde-3-phosphate, LPA : Lysophosphatidic acid, PA: Phosphatidic acid, PC : Phosphatidylcholine, DAG: Diacylglycerol, TAG: Triacylglycerol, PDAT: Phospholipid: diacylglycerol acyltransferase, DGAT: acyl-CoA: Diacylglycerol acyltransferase, GPAT: acyl-CoA: Glyceraldehyde-3-phosphate acyltransferase, LPAT: Lysophosphatidic acid acyltransferase, LPCAT : Lysophosphatidic acid acyltransferase, PAP: Phosphatidic acid phosphatase, ACS: Acyl-CoA synthetase.
Vegetable oils constitutes an important component of human diet. Major edible vegetable oils in terms of production include from soybean, canola, sunflower and peanut. They are source of edible FAs (saturated, monounsaturated or polyunsaturated), which play an important role in cellular metabolism as a way to store energy and also by providing energy when required. FAs are known to play an important role in cell division and growth. They are an integral component of cell membranes, hormones, neurotransmitters etc. Intake of different fatty acids has a direct influence on human health. For example, increased intake of saturated fatty acids has been linked to cardiovascular diseases. Thus it is considered desirable to have diets low in saturated fatty acids. Besides these, some very long chain-polyunsaturated fatty acid (VLC-PUFA; C20-C22) such as arachidonic acid (ARA; 20:4), eicosapentaenoic acid (EPA; 20:5) and docosahexaenoic acid (DHA; 22:6), which are usually derived from marine resources, have been shown to play an important role in human nutrition.
Long-chain polyunsaturated fatty acids (LC-PUFAs) play a variety of roles in human nutrition. Based on the position of the first double bond, PUFA are of two types: omega-3 and omega-6 fatty acids. Of PUFA, linolenic acid (LA; 18:2) is a major omega-6 fatty acid while α-linolenic acid (ALA; 18:3), is a major omega-3 fatty acid. These fatty acids are synthesized by higher plants. Oleic acid is converted into LA by ∆12-desaturase and LA, is then converted to ALA by ∆15-desaturase. However, human body cannot synthesize these fatty acids de novo. Thus, these are essential fatty acids that need to be supplemented regularly in diet [3, 4]. The major source of these fatty acids in human diet is marine fishes. These fishes feed on other marine organisms such as algae and diatoms which are the primary source of these LC-PUFAs [5]. In human body, LA and ALA can be further metabolized to form longer chain fatty acids which play crucial role in human growth and development. The linoleic acid is converted into arachidonic acid (ARA; 20:4), and α-linolenic acid into eicosapentaenoic acid (EPA; 20:5), and docosahexaenoic acid (DHA; 22:6) [3, 6-8]. However, these cannot be efficiently made by human body and need to be regularly supplemented to the diet [3, 9]. Though VLC-PUFAs cannot be synthesized by higher plants, there are some plants reported to produce stearidonic acid (SDA; 18:4) and γ-linolenic acid (GLA; 18:3), which are intermediates in the pathway of synthesis of these VLC-PUFA and have similar health benefits [10-14].
Different pathways of VLC-PUFAs have been identified in different organisms [7, 8, 15, 16]. In the conventional or Δ6-desaturation pathway (Fig. 2), linoleic acid is first converted into γ-linolenic acid (GLA; 18:3) by Δ6-desaturase [15]. The same enzyme also converts ALA into stearidonic acid (SDA; 18:4). The next step involves the synthesis of dihomo-γ-linolenic acid (DGLA; 20:3) and eicosatetraenoic acid (ETA; 20:4) by C2 elongation. In the final step, Δ5-desaturase generates ARA (20:4) and EPA (20:5), respectively. EPA is further converted into DHA, via C2 elongation by enzyme Δ5-elongase, followed by desaturation by Δ4-specific desaturase. Pathway leading to the synthesis of DHA also varies in some organisms. The other pathway that has been characterized is Δ9-pathway or also known as alternative pathway. This pathway is found in Tetrahymena pyroformis, Pavlova sp. Isochrysis sp. etc, where LA and ALA undergo elongation by Δ9-elongase enzyme to give eicosadienoic acid and ETA, respectively. A specific Δ8-desaturase acts on these substrates to form DGLA and ETA, respectively and as in the conventional Δ6-pathway mentioned above, Δ5-desaturase converts these fatty acids into ARA and EPA [7, 8].
Fig. (2).
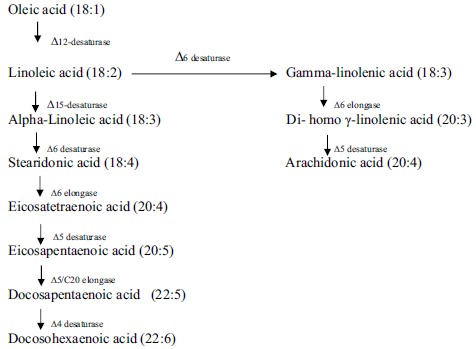
Conversion of the linoleic acid to arachidonic acid (ARA) and α-linolenic acid to eicosapentaenoic acid (EPA) / Docosohexaenoic acid (DHA).
3. BENEFICIAL EFFECTS OF OMEGA-3 FATTY ACIDS
As mentioned earlier, PUFA or more specifically the omega-3 fatty acids find more importance as human dietary supplement. Omega-3 fatty acids have been shown to decrease the risk of cardiovascular diseases or occurrence of type-2 diabetes (17,18,19). There is increasing evidence that omega-3 fatty acids also play a helpful role in treating ailments like depression- a widespread problem [20]. DHA has shown to improve sensitivity of brain tumor cells to anticancer drug, etoposide (VP16) and therapy [21]. The fatty acids are very crucial for retina and neural development, and for overall fetal development [19, 22]. Thus milk powders are being fortified or supplemented with DHA and ARA for brain development in infants [22]. Omega-6 fatty acids such as γ-linolenic acid and dihomo-γ-linolenic acid (DGLA) have shown anticancer activities and have inhibitory effect on proliferation of cells [23].
Due to their health benefits, it is recommended to take omega-3 FAs as regular dietary supplement. On an average an adult needs to consume 250–2000 mg per day of EPA + DHA (FAO) [9]. There is therefore a growing demand for these fatty acids. Currently, as stated earlier the major source of VLC-PUFA is fish oil which is largely derived from marine resources. Marine fishes feed on marine microalgae like diatoms that are primary source of PUFA. The high demand of these FAs is leading to overfishing. Also, there are concerns of environmental pollutants [8]. Other sources such as aquaculture itself is dependent on fish meal as a source for feed to maintain levels of PUFA in cultured fishes. Thus, fish oil is not able to meet the current demand for omega-3 fatty acids, necessitating development of alternative and sustainable sources. In addition, linseed oil which is rich in omega-3 FA is also being promoted as a nutritional supplement.
In order to gain benefits of PUFA, one has to consider the intake of omega-3 FA in relation to omega-6 fatty acid consumption. This is because conversion of ALA to EPA and DHA also depends on the amount of intake of linoleic acid. It has been observed that increasing the ratio of intake of linoleic acid to α-linolenic acid competitively reduces the conversion of ALA to longer chain omega-3 fatty acid [22, 24]. There is a competition between omega-6 and omega-3 fatty acids for desaturation by the same Δ6-desaturase i.e. LA to GLA and ALA into SDA. A ratio of 2:1 to 6:1 of omega-6 and omega-3 fatty acids is considered good and recommended, possibly improving cardiovascular health, asthma etc [25, 26]. This can be achieved by dietary intake of oils which are rich in α-linolenic acid such as flaxseed, walnut or monounsaturated rich vegetable oils such as olive oil. There are plants like Camelina sativa having very high content of α-linolenic acid.
4. FATTY ACIDS OF INDUSTRIAL IMPORTANCE
In addition to usual fatty acids discussed earlier, certain plants also synthesize several other FAs which deviate significantly in their physico-chemical properties. They are often termed “unusual fatty acids” (UFA) and may differ in carbon chain length, number and position of double bond, or they may have different functional groups such as hydroxy, epoxy, conjugated or acetylenic bonds etc. These UFAs are also stored mainly in TAG fraction inside seed. There are different types of unusual fatty acids reported that find their use in various applications. Unusual fatty acids have distinct chemical and physical properties which make them useful in industrial applications such as soaps, plastics, nylon, lubricants, paints, coatings and adhesives. For example, α-eleostearic acid found in tung oil has applications in paints and printing inks. Lauric acid, which is derived from coconut, is used for making soaps and detergents. Calendic acid is another type of UFA produced via desaturation of linoleic acid and is an important component of marigold seed oil. Besides these, another important industrial application of vegetable oil is as biofuel. A huge market therefore exists for these fatty acids. Normally the raw material for these applications is derived from petroleum-based resources. But due to depleting oil reserves and concerns for environmental pollution, there is an ensuing need of developing an alternative source.
Of all the unusual fatty acids, hydroxy fatty acids (HFA) are the most important from industrial standpoint. Ricinoleic acid is a kind of hydroxy-fatty acid produced by castor bean plants (Ricinus communis) of family Euphorbiaceae. It is produced by addition of hydroxyl group to oleic acid by enzyme oleate hydroxylase or fatty acid hydroxylase-12 (FAH12). Castor oil is composed of ~90% ricinoleic acid which is stored as TAGs in the seed. Ricinoleic acid has various industrial applications such as in nylons, paints, coatings, lubricants etc. Castor bean plants are the only commercial source for this fatty acid. However, one major drawback with castor plant is the presence of a protein toxin ricin, which is present in the endosperm of castor seed. Ricin has an enzymatic activity that catalyzes removal of an adenine moiety from a conserved specific region of 28S rRNA and thereby inhibiting protein synthesis by ribosomes containing depurinated 28S rRNA [27]. India is the largest producer of castor oil followed by China and Brazil. The other HFA is lesquerolic acid (20:1-OH), produced by lesquerella (Physaria fendleri), of Brassicaceae family which also has industrial applications.
Erucic acid is a very long chain fatty acid which finds its use in many industrial applications such as lubricant, plastic films, cosmetics etc. This fatty acid is produced from oleic acid through a series of reactions catalyzed by the enzyme fatty acid elongase, FAE1. It is normally produced in high levels in special cultivars of Brassica napus, also known as high erucic acid rapeseed (HEAR). Other sources include Crambe abyssinica which produces up to 55%–60% erucic acid in its seed oil. The Brassica species produce a maximum of 45-50% of erucic acid, which is usually incorporated at sn-1 and sn-3 position of TAG.
5. GENETIC ENGINEERING OF PLANTS FOR PRODUCTION OF NUTRITIONALLY IMPORTANT FATTY ACIDS
Recently, there has been an interest to genetically modify plants to produce fatty acids from nutritional standpoint as well. With the advent of genetic engineering technology, it is now possible to produce designer oils with desirable fatty acid composition. One of the major advantages of using genetic engineering is to produce nutritionally beneficial fatty acids, which are normally produced in non-cultivated plant species or derived from marine sources, in agriculturally amenable crops. Once these designer plants are created, production of these FAs can become economically viable and can substitute limited and overexploited resources like marine life. Following are some examples where genetic manipulations have been carried out keeping nutrition as a focal point.
6. PRODUCTION OF OMEGA-3 FAS
As discussed earlier, PUFAs have beneficial effect on human health, and are largely derived from marine sources such as fish oil. There is a huge gap in the current demand and supply of fish oil and there is a need to identify and develop alternative sources to produce these FAs. Cultivation of microalgae directly for production of PUFA has a potential. The microalgae such as Mortierella alpina that are the primary producer of PUFA have been used to derive PUFA for commercial production [28]. Production of PUFA have also been detected in various microalgae that include Phaedodactylum tricornutum, Fistulifera species [29]. However, many of these microalgae are not suitable for large scale production and the technology needs further optimization, so that they become economically viable and cost effective [30]. Use of iterative metabolic engineering also holds potential in accumulation of omega-3 FAs as seen in diatoms, Phaedodactylum tricornutum by expressing gene from another alga, Ostreococcus tauri [31]. Metabolic engineering of yeast and Yarrowia lipolytica have been done to obtain sustainable production of EPA (reviewed in [32]). Still the global demand far exceeds the supply from above mentioned sources including marine or aquaculture. Other alternative approach is to identify and develop unconventional sources for production of PUFAs. Biotechnology can provide us a mean to genetically engineer oilseed plants to produce these PUFAs, in an economically viable way.
7. TRANSGENIC PRODUCTION OF STEARIDONIC ACID AND γ- LINOLENIC ACID
Besides fish oil, humans can also consume flaxseed, walnut etc, which have very high levels of α-linolenic acid (18: 3). This ALA can be converted into EPA and DHA in the human body. However, it is more efficient to convert SDA to EPA than ALA to EPA and supports the suggestion that ∆6-desaturase activity is rate limiting [33] and provides a step which can be modulated by genetic engineering. As mentioned previously stearidonic acids give similar health benefits as EPA [13]. Some plants such as Borago officinalis can produce SDA in their seeds [34, 35]. However, these plants are not part of human diet and agronomically not amenable to cultivation. Oilseeds can therefore be engineered to produce this nutritionally important fatty acid by transferring ∆6-desaturase gene to produce SDA. The ∆6-desaturase can use both linoleic and α-linolenic acid as a substrate and convert them into γ-linolenic acid (GLA) and stearidonic acid (SDA) respectively (see Fig. 2). When ∆6-desaturase from Borago officinalis was expressed constitutively in tobacco, it led to production of both 13.2% GLA and 9.6% SDA in leaves and up to 27% GLA in stem [34, 35]. When a borage ∆6-desaturase gene was expressed along with an Arabidopsis ∆15-desaturase gene in soybean seed under seed-specific β-conglycinin promoter, SDA content of as high as 29% was observed. The overall omega-3 fatty acid profile was increased to 60% in the transgenic seeds [36]. When ∆6-desaturase gene from M. alpina was expressed in low α-linolenic canola along with ∆12-desaturase gene, it resulted in production of up to 40% w/w GLA in seeds [37]. Expression of ∆6-desaturase (PiD6), from an oleaginous fungus, Pythium irregulare gene in Brassica juncea under seed-specific napin promoter resulted in production of GLA up to 40% of the total seed fatty acids [38]. Similarly, when the Δ6-desaturase gene from Saprolegnia diclina was expressed in cultivars of safflower producing high levels of LA, the transgenic plants produced >70% (v/v) of GLA whereas when the Δ6-desaturase gene from M. alpina was expressed, 50% GLA levels were achieved. The difference in level of accumulation was due to differences in Δ6-desaturase activity [39]. However, the effectiveness of SDA content in plant oils when compared to EPA/DHA containing oils as a nutritional supplement is less and depends upon human capacity to convert LA/ALA into VLC-PUFA [33, 40]. Thus the focus now has to shift to directly engineer plants to produce and accumulate EPA/DHA as an alternative source.
8. TRANSGENIC PRODUCTION OF ARA/EPA/DHA
Considering the growing list of health benefits of omega-3 fatty acids and limited fish oil source, there is a need to develop alternative plant-based source for production of these FAs. As described earlier, (Fig. 2) depicts major pathways for production of nutritionally important FAs. A preliminary demonstration of ETA biosynthesis involved use of alternative pathway using a combination of genes for three enzymes viz. ∆9-elongase, ∆8-desaturase and ∆5-desaturase under a constitutive promoter. It resulted in production of low levels of ETA and ARA but showed that it is possible to engineer plants for VLC-PUFA [41]. Other preliminary attempts to produce EPA and DHA involved use of enzymes of ∆6-desaturase pathway from Phaeodactylum tricornutum (∆5- and ∆6-desaturase) and ∆6-elongase from Physcomitrella patens [42]. This study demonstrated that accumulation of intermediates and low levels of EPA or ARA in transgenic plants was due to low levels of acyl-CoA precursor pool of FAs, which are used as a substrate and is elongated by ∆6-elongase (see Fig. 2). Thus this step is apparently a rate limiting. By coexpressing genes for ∆9-elongase from Isochrysis galbana and ∆8- and ∆5-desaturase from Pavlova salina in Arabidopsis seeds resulted in accumulation up to 20% ARA and 2% EPA in storage oil and ~10% ARA in B. napus [43]. Petrie et al. [44] engineered the pathway for DHA production in A. thaliana that resulted in accumulation of up to 15% DHA, almost similar (18%) to fish oil. The ∆6-desaturase pathway to produce DHA from oleic acid involved coexpression of genes for ∆12-desaturase (Lachancea kluyveri), ∆15-desaturase (Pichia pastoris), along with ∆6-desaturase (Micromonas pusilla), ∆5- and ∆4-desaturase (Pavlova salina) and ∆6- and ∆5-elongase (Pyramimonas cordata) (see Fig. 2). A greater success was also achieved using acyl-CoA-dependent ∆6-desaturase from Ostreococcus tauri. Ruiz-Lopez et al. [45, 46] reported a 10-fold increase in EPA/DHA production. Petrie et al. [47] also engineered Camelina sativa to produce up to 15% of DHA in seed oil with a high w3/w6 ratio, which is more than the quantity found in bulk fish oil. The EPA and DHA were incorporated at sn-1,3 position of TAG. Betancore et al. [48] generated seeds of transgenic C. sativa that produces up to 20% of EPA. The oil from such transgenic plants can replace fish meal in aquaculture without affecting the nutritional quality of farmed fishes. This, therefore, has potential to decrease the load on marine life by serving as an alternate source of omega fatty acids. Attempts have also been made to produce EPA and ETA in seeds of C. sativa using alternative pathway [16]. Transgenic seeds accumulate EPA and ETA up to 26.4% confirming that it is possible to produce these PUFAs at significant levels.
9. PRODUCTION OF COMMON FATTY ACIDS
Besides omega-3, there are other fatty acids with potential health benefits. FAs such as stearic acid and monounsaturated oleic acid can substitute palm oil and partially hydrogenated oils in applications such as baking. Oleic acid has increased shelf life and higher oxidative stability than linoleic acid due to presence of one less double bond. Thus, it is desirable to develop vegetable oils with high level of monounsaturated fatty acids such as oleic acid and low level of linoleic acid. Oleic acid is produced by dehydrogenation of stearic acid by action of enzyme stearoyl-CoA 9-desaturase (SAD). The fad2 gene codes for enzyme oleate desaturase (∆12-desaturase), which converts oleic acid into linoleic acid. High oleic acid lines have been developed in maize, canola, and soybean. All of these lines have mutations in the fad2 gene [49-51]. Seed-specific silencing of fad2 gene resulted in increasing levels of oleic acid in Arabidopsis and Brassica napus [52-54]. The oleic acid content was increased along with reduction of linolenic acid. When fad2 gene is also silenced in soybean, along with fatB gene that codes for a thioesterase, oleic acid is increased up to 85% from 75% in plants in which only fad2 alone is silenced [55]. By down regulating both fad2 and fae1 genes, Peng et al. [56] were able to increase oleic acid content to 75%, in addition to reduction of PUFA to 10% and complete elimination of erucic acid. Similarly the saturated fatty acid levels can be manipulated in seed oils so that it becomes nutritionally superior replacement in baking.
10. GENETIC ENGINEERING FOR FATTY ACIDS OF INDUSTRIAL IMPORTANCE
In the past, plant breeders have made selection and developed high oil-yielding varieties largely for food-based applications. Besides their use for edible purpose, the utilization of vegetable oil is now expanding from nutrition point of view to industrial-based applications to biofuels. It is now possible to modify plant metabolism especifically fatty acid synthesis, accumulation and composition. In addition, many fatty acids of unique importance as mentioned earlier, which either come from non-plant sources or are produced by repertoire of plants which are not amenable for commercial cultivation. Thus genetic engineering provides an opportunity to transfer genes coding for production of novel fatty acids of industrial as well as nutritional importance to oilseed crops and produce application-based designer oils.
11. PRODUCTION OF HYDROXY FATTY ACIDS IN NON-NATIVE PLANTS
Unusual fatty acids like hydroxy fatty acids are of immense importance as they serve as a feedstock for various industrial applications. Ricinoleic acid is a type of hydroxy fatty acid that accumulates in seeds of castor bean. Ricinoleic acid is produced by action of enzyme ∆12-hydroxylase (FAH-12) present in endoplasmic reticulum (ER), and is stored as triacylglycerols (TAG) [57]. The ∆12-hydroxylase enzyme transfers hydroxyl group to the delta-12 position of oleic acid. Castor seeds contain up to 80-90% ricinoleic acid. When ∆12-hydroxylase gene was expressed in Arabidopsis plant, the transgenic plants accumulated HFAs ~17% of the seed oil [58]. Similar results were observed in Brassica napus [59] and Camelina [60]. This accumulation is much less as compared to the castor plants indicating that there are other factors involved in accumulation of this unusual fatty acid. In fact, plants producing more than 20% HFA have reduced oil content and seed viability, indicating that this unusual fatty acid has a role in physiology of the seed [61]. Thus, expressing only gene for FAH-12 is insufficient to produce ricinoleic acid for industrial purpose as other genes play a role in hydroxy fatty acid synthesis and accumulation as well.
Mechanisms involved in synthesis and storage of hydroxy fatty acids are being studied using Arabidopsis and castor bean as model plants. Several genes have been characterized that are involved in fatty acid accumulation. These studies have led to an understanding that lack of corresponding acyltransferases that catalyze synthesis of TAG or enzymes involved in editing functions, may be the reason behind it. As mentioned earlier, TAG is the major storage form of the oilseeds. The DGAT enzyme catalyzes acylation of DAG and thus influences fatty acid accumulation by being involved in TAG synthesis. Over expressing these enzymes in seed-specific manner increases the quantity of oil content and seed weight [62]. Coexpression of genes for DGAT2, and ∆12-hydroxylase from castor bean, increases the accumulation of HFA to 30% compared to 17% in transgenic plants expressing only the ∆12-hydroxylase gene. Interestingly, oil content of seeds is also comparable to that of the control plants [61]. Bates et al. [63] reported that a high level of HFA reduces fatty acid synthesis by a post-translational mechanism affecting plastidial acetyl–CoA carboxylase (ACCase) activity. In transgenic plants for ∆12-hydroxylase gene alone, inefficient incorporation of ricinoleic acid into TAG causes inhibition of ACCase activity. This bottleneck was alleviated by coexpressing gene for HFA-specific DGAT2 that efficiently and specifically incorporates ricinoleic acid into TAG and in turn restores seed oil content. Similarly in tung tree that produces an unusual fatty acid; α-eleostearic acid, DGAT2 preferentially incorporates eleostearic acid into TAG [64]. Thus low levels of accumulation of unusual fatty acids in transgenic plants could be the result of lack of their incorporation into TAG.
Ricinoleic acid is normally produced in phospholipids in ER, and needs to be removed from phospholipids and transferred to TAGs for storage during seed development. This can occur by two known mechanisms: one mechanism involves an enzyme phospholipid:diacylglycerol acyltransferase (PDAT) which transfers an acyl group from phospholipid pool to DAG. This DAG is then used for TAG synthesis as well. In castor bean, there are three PDAT enzymes reported of which PDAT1-2 is predominantly expressed in seeds. This when coexpressed in conjunction with ∆12-hydroxylase improves incorporation of hydroxy fatty acid into TAG in seeds of transgenic Arabidopsis plants. Moreover, elevated levels of HFA in these transgenic plants did not affect the seed physiology. It was also found that no further increase was seen in transgenic plants expressing three genes for FAH12, PDAT1-2, DGAT2 [65, 66]. Besides acyltransferases, castor bean electron donor cytochrome b5 (RcCb5) and NADH;cytochrome b5 reductase (RcCBR1) were found to be important in HFA accumulation in transgenic plants [67]. However, when RcCBR1 and RcCb5 were coexpressed in FAH12 trangenic plants, no increase in HFA levels was seen [68]. A study by van Erp et al. [69] showed that by reducing competition from endogenous acyltransferases (AtDGAT1), the levels of HFA can be further increased in transgenic plants expressing FAH12 and RcDGAT2. Another enzyme that seems to play a role in hydroxy fatty acid accumulation is phosphocholine diacylglycerol choline phosphotransferase (PDCT). This enzyme controls flux of interconversion of PC and DAG which is then incorporated into TAG for storage [70].
It has also been proposed that low levels of unusual fatty acid accumulation in transgenic plants is due to futile cycle of β-oxidation as analyzed in transgenic plants expressing California bay lauroyl–acyl carrier protein thioesterase [71]. Thus multiple factors seem to influence fatty acid synthesis and accumulation.
12. GENETIC ENGINEERING OF PLANTS FOR PRODUCTION OF ERUCIC ACID
The major source of erucic acid is HEAR which produces a maximum of up to 50% in its seed oil. This fatty acid is not incorporated at the sn-2 position of the TAG. It was found that the B. napus LPAAT that is involved in DAG synthesis via the Kennedy pathway, lacks specificity for erucoyl-CoA. In order to further increase the erucic acid, genetic engineering of rapeseed using LPAAT enzyme having specificity for erucoyl-CoA as an acyl substrate was performed [72]. A gene coding for LPAAT was isolated from Limnanthes species and expressed in rapeseed [73]. Erucic acid was incorporated into sn-2 position and trierucin was obtained. However, the total erucic acid content did not increase. The other strategy used for erucic acid production revolves around elongation of oleic acid into erucic acid. When fae1 gene, which is involved in fatty acid elongation from oleic acid to erucic acid, was overexpressed under seed specific promoter, an increase in erucic acid content was observed in transgenic plants [74]. When fae1 was overexpressed along with LPAAT gene from Limnanthes douglasii in HEAR lines, which was further combined with plants carrying mutant alleles for PUFA i.e. linoleic and linolenic acid, plants producing up to 72% erucic acid and reduced PUFA were obtained [75]. Similar results were obtained with up to 73% upon genetic modification of crambe which is another example where genetic engineering has advanced production of fatty acids [76]. Jadhav et al. [77] used cosuppresion and antisense method to down regulate fad2 in B. carinata, and obtained increased erucic acid content in transgenic plants.
Besides HFA and erucic acid, genetic engineering has been used to modify plant oils for production of other industrially important fatty acids as well. For example, by expressing an engineered plastidial ∆9-16:0-ACP desaturase from Doxantha unguis-cati, down-regulation of ketoacyl-ACP synthase II 16:0 elongase, and coexpression of fungal 16:0-ACP desaturases led to production of up to ~71% w-7 fatty acids in Arabidopsis. This level is comparable to that of Doxantha seeds [78]. Similarly, lauric acid (12:0) is a type of saturated, medium chain fatty acid that is widely used for production of soaps and detergents. Major sources of this FA are coconut and palm tree. As an alternative, vegetable oils rich in lauric acid have been obtained by expressing a heterologous thioesterase gene under seed-specific napin promoter. This enzyme which has a high specificity for lauroyl-ACP, releases the lauric acid into the lipid pool. This gene when expressed in canola yielded up to 50% lauric acid [79]. An additional increase of 5% in the lauric acid content was achieved by expressing a gene for lysophosphatidic acid acyltransferase (LPAAT) [80]. (Table 1) summarizes the attempts made for transgenic production of FAs of nutritional and industrial importance.
Table (1).
Genetic engineering of plants for production of FAs of nutritional and industrial importance.
| Fatty Acids Specific Utility |
Major Natural Source | Target Crop | Gene Source | Gene | Remarks | Reference |
|---|---|---|---|---|---|---|
| DHA/EPA/ARA | Fish, Algae |
Tobacco Linseed Arabidopsis A. thaliana Camelina sativa Camelina sativa |
Phaeodactylum tricornutum Physcomitrella patens Isochrysis galbana Pavlova salina Lachancea kluyveri Pichia pastoris Micromonas pusilla Pavlova salina Pyramimonas cordata Ostreococcus tauri Thraustochytrium sp. Phytophthora infestans Phytophthora sojae Emiliania huxleyi P. patens O. tauri |
∆5- and ∆6-desaturase ∆6-elongase ∆9-elongase ∆8- and ∆5-desaturases ∆12-desaturase ∆15-desaturase ∆6-desaturase ∆5- and ∆4-desaturases ∆6- and ∆5-elongases ∆6-desaturase ∆5-desaturase w 3-desaturase ∆12-desaturase ∆4-desaturase ∆ 6-elongase ∆ 5-elongase |
5% including ARA and EPA >20% ARA and 2% EPA in seeds 15% DHA in seed Two iterations 1) up to 31% EPA levels 2) up to 12% EPA and 14% DHA in seed 20% DHA in seed |
[42] [43] [44, 47] [45] [48] |
| GLA | Borago officinalis | Tobacco Brassica juncea low α-linolenic canola Safflower (producing high levels of LA) |
Borago officinalis Pythium irregular Mortierella alpina Saprolegnia diclina M. alpina |
∆6-desaturase ∆6-desaturase ∆6-desaturase gene And ∆12-desaturase ∆6-desaturase ∆6-desaturase |
13.2% GLA in leaves 27% GLA in stem 40% GLA in seeds 40% GLA in seeds >70% GLA 50% GLA |
[34, 35] [37] [38] [39] |
| SDA | Seafood B. officinalis Black current |
Tobacco Soybean |
Borago officinalis B. officinalis Arabidopsis |
∆6-desaturase ∆6-desaturase and ∆15-desaturase gene |
9.6% SDA in stem 29% SDA in seed |
[34, 35] [36] |
| Hydroxy fatty acid | Castor, Lesquerella | Castor | Arabidopsis | Oleate-12-hydroxylase Oleate-12-hydroxylase And DGAT2 Oleate-12-hydroxylase and PDAT1-2 |
17% HFA 30% HFA 27 % HFA |
[58] [61] [65] |
| Erucic acid | Brassica Crambe |
Brassica napus (HEAR) B. napus Crambe abyssinica |
B. napus B. napus Limnanthes douglasii B. napus Limnanthes douglasii C. abyssinica |
Fatty acid elongase (Bn-FAE1.1) BnFAE1, LdLPAAT BnFAE1, LdLPAAT and CaFAD2-RNAi |
60% erucic acid 72% erucic acid 73.9% erucic acid |
[74] [75] [76] |
| Stearic acid | Palm kernel | B. napus | B. rapa | Stearoyl-ACP D-9 desaturase (antisense) | 2%- 40% stearic acid |
[81] |
| Lauric acid | Coconut Palm kernel |
B. napus B. napus |
Umbellularia californica Umbellularia californica Cocos nucifera |
Acyl-ACP thioesterase Acyl-ACP thioesterase (UcFatB1) and (CnLPAT) |
Upto 50% lauric acid Further 5% increase |
[79] [80] |
| Capric acid | Coconut | B. napus | Cuphea hookeriana | Acyl-ACP thioesterase (ChFatB2) |
11 and 27 Mol% | [82] |
| Palmitoleic acid | Doxantha unguis-cati | Arabidopsis | Castor Aspergillus nidulans Stagonospora nodorum Arabidopsis |
Plastidial ∆9-16:0-ACP desaturase (variant), ∆9-16:0-ACP desaturases and ketoacyl-ACP synthase II 16:0 elongase (downregulation). | 71% w-7 fatty acids | [78] |
13. INCREASE IN OIL CONTENT
Other area of interest is to increase oil production. Several biochemical pathways involved in oil biosynthesis in plants have been characterized elucidating key steps and rate limiting enzymes [1]. As mentioned earlier several bottlenecks have been identified in fatty acid biosynthesis and their flux is under coordinated regulation between various pathways as well as carbohydrate metabolism. For example targeting acetyl-coenzyme A carboxylase (ACCase) to plastids results in an increase of ~5% oil content in rapeseed [83]. Expression of genes for DGAT, DGAT2A, DGAT1-2 in Arabidopsis, soybean, and maize increases overall oil content [62, 84, 85]. Besides known genes involved in fatty acid metabolism, other genes that may play a role in increasing storage reserves could also serve as important tools to boost the oil content. The gene encoding transcription factor, LEC1 is a potential candidate for controlling both quality and quantity of oil [86]. Another transcription factor in B. napus, WRINKLED1, seems to control metabolic processes that influences fatty acid accumulation. Ectopic expression of WRINKLED1 under CAMV 35S promoter results in increase in TAG content in seedlings [87]. Several comparative transcriptomics studies are now identifying genes involved at rate limiting steps [88]. Chandrasekaran et al. [89] studied the role of abcissic acid (ABA) signaling during seed filling and storage of oil in castor bean seeds using transcriptomic studies. The focus now is not only seed specific metabolic engineering for fatty acid but its use for production of TAG in leaf tissue as well [90]. Petris et al. [91] have reported an alternative pathway for production of TAG in plants. This pathway involves enzyme GPAT4 or GPAT6 that synthesizes sn-2-monoacylglycerol (MAG) using dicarboxylic and w-hydroxyl acyl-CoA fatty acids. This MAG is converted to DAG by MGAT acyltransferases or even to TAG by action of a mouse bifunctional enzyme M/DGAT1 acyltransferases. This pathway that bypasses the Kennedy pathway may prove useful for increasing levels of TAG in transgenic plants.
CONCLUSION
Significant advancements have been made in understanding the biochemistry of plant lipid metabolism and several bottlenecks have been documented. Factors that may influence novel FA accumulation include substrate availability or substrate specificity of enzymes, their incorporation into TAG or negative post-translational regulatory controls within and between pathways. These bottlenecks may be bypassed by use of knowledge of alternative pathways or identifying enzymes from other sources that can overcome these bottlenecks. Use of engineered enzymes may also provide better resources for transgenic production. The other major constraint is where, when and how the transgenes and endogenous genes are integrated to give best results and avoid problems such as biosynthetic intermediates or futile cycles. Plant biotechnology has now opened new vistas with several promising demonstration of successful genetic manipulation using multiple genes for nutritionally important fatty acids. A fair progress has also been made regarding industrially important fatty acids with several reports of success. Use of master regulators (transcription factors) can be further explored to increase seed oil content. The future global demands of oilseed production will be influenced from industrial or nutritional perspectives. Designer oilseeds will help achieve the targets not earlier possible.
ACKNOWLEDGEMENTS
Authors would like to thank Mr Kaushik Dubey for critical reading of the reference section of the manuscript. This work was supported financially by DBT Bio-CARe/02/763/ 2011-12.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Bates P.D., Stymne S., Ohlrogge J. Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 2013;16(3):358–364. doi: 10.1016/j.pbi.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Thelen J.J., Ohlrogge J.B. Metabolic engineering of fatty acid biosynthesis in plants. Metab. Eng. 2002;4(1):12–21. doi: 10.1006/mben.2001.0204. [DOI] [PubMed] [Google Scholar]
- 3.Graham I.A., Larson T., Napier J.A. Rational metabolic engineering of transgenic plants for biosynthesis of omega-3 polyunsaturates. Curr. Opin. Biotechnol. 2007;18(2):142–147. doi: 10.1016/j.copbio.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Rogalski M., Carrer H. Engineering plastid fatty acid biosynthesis to improve food quality and biofuel production in higher plants. Plant Biotechnol. J. 2011;9(5):554–564. doi: 10.1111/j.1467-7652.2011.00621.x. [DOI] [PubMed] [Google Scholar]
- 5.Harwood J.L., Guschina I.A. The versatility of algae and their lipid metabolism. Biochimie. 2009;91(6):679–684. doi: 10.1016/j.biochi.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Cahoon E.B., Shockey J.M., Dietrich C.R., Gidda S.K., Mullen R.T., Dyer J.M. Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: solving bottlenecks in fatty acid flux. Curr. Opin. Plant Biol. 2007;10(3):236–244. doi: 10.1016/j.pbi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Damude H.G., Kinney A.J. Engineering oilseed plants for a sustainable, land-based source of long chain polyunsaturated fatty acids. Lipids. 2007;42(3):179–185. doi: 10.1007/s11745-007-3049-1. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-López N., Sayanova O., Napier J.A., Haslam R.P. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J. Exp. Bot. 2012;63(7):2397–2410. doi: 10.1093/jxb/err454. [DOI] [PubMed] [Google Scholar]
- 9.Kitessa S.M., Abeywardena M., Wijesundera C., Nichols P.D. DHA-containing oilseed: a timely solution for the sustainability issues surrounding fish oil sources of the health-benefitting long-chain omega-3 oils. Nutrients. 2014;6(5):2035–2058. doi: 10.3390/nu6052035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damude H.G., Kinney A.J. Enhancing plant seed oils for human nutrition. Plant Physiol. 2008;147(3):962–968. doi: 10.1104/pp.108.121681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napier J.A., Graham I.A. Tailoring plant lipid composition: designer oilseeds come of age. Curr. Opin. Plant Biol. 2010;13(3):330–337. doi: 10.1016/j.pbi.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Lemke S.L., Vicini J.L., Su H., Goldstein D.A., Nemeth M.A., Krul E.S., Harris W.S. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: randomized, double-blind clinical study of efficacy and safety. Am. J. Clin. Nutr. 2010;92(4):766–775. doi: 10.3945/ajcn.2009.29072. [DOI] [PubMed] [Google Scholar]
- 13.Harris W.S. Stearidonic acid-enhanced soybean oil: a plant-based source of (n-3) fatty acids for foods. J. Nutr. 2012;142(3):600S–604S. doi: 10.3945/jn.111.146613. [DOI] [PubMed] [Google Scholar]
- 14.Casey J.M., Banz W.J., Krul E.S., Butteiger D.N., Goldstein D.A., Davis J.E. Effect of stearidonic acid-enriched soybean oil on fatty acid profile and metabolic parameters in lean and obese Zucker rats. Lipids Health Dis. 2013;12:147–163. doi: 10.1186/1476-511X-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haslam R.P., Ruiz-Lopez N., Eastmond P., Moloney M., Sayanova O., Napier J.A. The modification of plant oil composition via metabolic engineering--better nutrition by design. Plant Biotechnol. J. 2013;11(2):157–168. doi: 10.1111/pbi.12012. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Lopez N., Usher S., Sayanova O.V., Napier J.A., Haslam R.P. Modifying the lipid content and composition of plant seeds: engineering the production of LC-PUFA. Appl. Microbiol. Biotechnol. 2015;99(1):143–154. doi: 10.1007/s00253-014-6217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kris-Etherton P.M., Harris W.S., Appel L.J., American Heart Association. Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 18.Raheja B.S., Sadikot S.M., Phatak R.B., Rao M.B. Significance of the N-6/N-3 ratio for insulin action in diabetes. Ann. N. Y. Acad. Sci. 1993;683:258–271. doi: 10.1111/j.1749-6632.1993.tb35715.x. [DOI] [PubMed] [Google Scholar]
- 19.Swanson D., Block R., Mousa S.A. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv. Nutr. 2012;3(1):1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molfino A., Gioia G., Rossi Fanelli F., Muscaritoli M. The role for dietary omega-3 fatty acids supplementation in older adults. Nutrients. 2014;6(10):4058–4073. doi: 10.3390/nu6104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F., Bhat K., Doucette M., Zhou S., Gu Y., Law B., Liu X., Wong E.T., Kang J.X., Hsieh T.C., Qian S.Y., Wu E. Docosahexaenoic acid (DHA) sensitizes brain tumor cells to etoposide-induced apoptosis. Curr. Mol. Med. 2011;11(6):503–511. doi: 10.2174/156652411796268740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simopoulos A.P. Human requirement for N-3 polyunsaturated fatty acids. Poult. Sci. 2000;79(7):961–970. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y., Qian S.Y. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed. J. 2014;37(3):112–119. doi: 10.4103/2319-4170.131378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emken E.A., Adlof R.O., Gulley R.M. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta. 1994;1213(3):277–288. doi: 10.1016/0005-2760(94)00054-9. [DOI] [PubMed] [Google Scholar]
- 25.Riediger N.D., Azordegan N., Harris-Janz S., Ma D.W., Suh M., Moghadasian M.H. ‘Designer oils’ low in n-6:n-3 fatty acid ratio beneficially modifies cardiovascular risks in mice. Eur. J. Nutr. 2009;48(5):307–314. doi: 10.1007/s00394-009-0015-0. [DOI] [PubMed] [Google Scholar]
- 26.Wendell S.G., Baffi C., Holguin F. Fatty acids, inflammation, and asthma. J. Allergy Clin. Immunol. 2014;133(5):1255–1264. doi: 10.1016/j.jaci.2013.12.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord J.M., Roberts L.M., Robertus J.D. Ricin: structure, mode of action, and some current applications. FASEB J. 1994;8(2):201–208. [PubMed] [Google Scholar]
- 28.Sakuradani E., Ando A., Ogawa J., Shimizu S. Improved production of various polyunsaturated fatty acids through filamentous fungus Mortierella alpina breeding. Appl. Microbiol. Biotechnol. 2009;84(1):1–10. doi: 10.1007/s00253-009-2076-7. [DOI] [PubMed] [Google Scholar]
- 29.Liang Y., Maeda Y., Sunaga Y., Muto M., Matsumoto M., Yoshino T., Tanaka T. Biosynthesis of polyunsaturated fatty acids in the oleaginous marine diatom Fistulifera sp. strain JPCC DA0580. Mar. Drugs. 2013;11(12):5008–5023. doi: 10.3390/md11125008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H.Y., Lu Y., Zheng J.W., Yang W.D., Liu J.S. Biochemical and genetic engineering of diatoms for polyunsaturated fatty acid biosynthesis. Mar. Drugs. 2014;12(1):153–166. doi: 10.3390/md12010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamilton M.L., Haslam R.P., Napier J.A., Sayanova O. Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metab. Eng. 2014;22:3–9. doi: 10.1016/j.ymben.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie D., Jackson E.N., Zhu Q. Sustainable source of omega-3 eicosapentaenoic acid from metabolically engineered Yarrowia lipolytica: from fundamental research to commercial production. Appl. Microbiol. Biotechnol. 2015;99(4):1599–1610. doi: 10.1007/s00253-014-6318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James M.J., Ursin V.M., Cleland L.G. Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am. J. Clin. Nutr. 2003;77(5):1140–1145. doi: 10.1093/ajcn/77.5.1140. [DOI] [PubMed] [Google Scholar]
- 34.Sayanova O., Smith M.A., Lapinskas P., Stobart A.K., Dobson G., Christie W.W., Shewry P.R., Napier J.A. Expression of a borage desaturase cDNA containing an N-terminal cytochrome b5 domain results in the accumulation of high levels of delta6-desaturated fatty acids in transgenic tobacco. Proc. Natl. Acad. Sci. USA. 1997;94(8):4211–4216. doi: 10.1073/pnas.94.8.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayanova O.V., Beaudoin F., Michaelson L.V., Shewry P.R., Napier J.A. Identification of primula fatty acid delta 6-desaturases with n-3 substrate preferences. FEBS Lett. 2003;542(1-3):100–104. doi: 10.1016/s0014-5793(03)00358-2. [DOI] [PubMed] [Google Scholar]
- 36.Eckert H., La Vallee B., Schweiger B.J., Kinney A.J., Cahoon E.B., Clemente T. Co-expression of the borage Delta 6 desaturase and the Arabidopsis Delta 15 desaturase results in high accumulation of stearidonic acid in the seeds of transgenic soybean. Planta. 2006;224(5):1050–1057. doi: 10.1007/s00425-006-0291-3. [DOI] [PubMed] [Google Scholar]
- 37.Liu J.W., DeMichele S., Bergana M., Bobik E.J., Hastilow C., Chuang L.T., Mukerji P., Uang Y.S. Characterization of oil exhibiting high γ-linolenic acid from a genetically transformed canola strain. J. Am. Oil Chem. Soc. 2001;78:489–493. [Google Scholar]
- 38.Hong H., Datla N., Reed D.W., Covello P.S., MacKenzie S.L., Qiu X. High-level production of γ-linolenic acid in Brassica juncea using a delta6 desaturase from Pythium irregulare. Plant Physiol. 2002;129(1):354–362. doi: 10.1104/pp.001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nykiforuk C.L., Shewmaker C., Harry I., Yurchenko O.P., Zhang M., Reed C., Oinam G.S., Zaplachinski S., Fidantsef A., Boothe J.G., Moloney M.M. High level accumulation of gamma linolenic acid (C18:3Δ6.9,12 cis) in transgenic safflower (Carthamus tinctorius) seeds. Transgenic Res. 2012;21(2):367–381. doi: 10.1007/s11248-011-9543-5. [DOI] [PubMed] [Google Scholar]
- 40.Clemente T.E., Cahoon E.B. Soybean oil: genetic approaches for modification of functionality and total content. Plant Physiol. 2009;151(3):1030–1040. doi: 10.1104/pp.109.146282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi B., Fraser T., Mugford S., Dobson G., Sayanova O., Butler J., Napier J.A., Stobart A.K., Lazarus C.M. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat. Biotechnol. 2004;22(6):739–745. doi: 10.1038/nbt972. [DOI] [PubMed] [Google Scholar]
- 42.Abbadi A., Domergue F., Bauer J., Napier J.A., Welti R., Zähringer U., Cirpus P., Heinz E. Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell. 2004;16(10):2734–2748. doi: 10.1105/tpc.104.026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrie J.R., Shrestha P., Belide S., Mansour M.P., Liu Q., Horne J., Nichols P.D., Singh S.P. Transgenic production of arachidonic acid in oilseeds. Transgenic Res. 2012;21(1):139–147. doi: 10.1007/s11248-011-9517-7. [DOI] [PubMed] [Google Scholar]
- 44.Petrie J.R., Shrestha P., Zhou X.R., Mansour M.P., Liu Q., Belide S., Nichols P.D., Singh S.P. Metabolic engineering plant seeds with fish oil-like levels of DHA. PLoS One. 2012;7(11):e49165. doi: 10.1371/journal.pone.0049165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz-Lopez N., Haslam R.P., Napier J.A., Sayanova O. Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J. 2014;77(2):198–208. doi: 10.1111/tpj.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz-Lopez N., Haslam R.P., Usher S.L., Napier J.A., Sayanova O. Reconstitution of EPA and DHA biosynthesis in arabidopsis: iterative metabolic engineering for the synthesis of n-3 LC-PUFAs in transgenic plants. Metab. Eng. 2013;17:30–41. doi: 10.1016/j.ymben.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrie J.R., Shrestha P., Belide S., Kennedy Y., Lester G., Liu Q., Divi U.K., Mulder R.J., Mansour M.P., Nichols P.D., Singh S.P. Metabolic engineering Camelina sativa with fish oil-like levels of DHA. PLoS One. 2014;9(1):e85061. doi: 10.1371/journal.pone.0085061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Betancor M.B., Sprague M., Usher S., Sayanova O., Campbell P.J., Napier J.A., Tocher D.R. A nutritionally-enhanced oil from transgenic Camelina sativa effectively replaces fish oil as a source of eicosapentaenoic acid for fish. Sci. Rep. 2015;5:8104. doi: 10.1038/srep08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu X., Sullivan-Gilbert M., Gupta M., Thompson S.A. Mapping of the loci controlling oleic and linolenic acid contents and development of fad2 and fad3 allele-specific markers in canola (Brassica napus L.). Theor. Appl. Genet. 2006;113(3):497–507. doi: 10.1007/s00122-006-0315-1. [DOI] [PubMed] [Google Scholar]
- 50.Beló A., Zheng P., Luck S., Shen B., Meyer D.J., Li B., Tingey S., Rafalski A. Whole genome scan detects an allelic variant of fad2 associated with increased oleic acid levels in maize. Mol. Genet. Genomics. 2008;279(1):1–10. doi: 10.1007/s00438-007-0289-y. [DOI] [PubMed] [Google Scholar]
- 51.Pham A.T., Lee J.D., Shannon J.G., Bilyeu K.D. Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biol. 2010;10:195. doi: 10.1186/1471-2229-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoutjesdijk P.A., Hurlestone C., Singh S.P., Green A.G. High-oleic acid Australian Brassica napus and B. juncea varieties produced by co-suppression of endogenous Delta12-desaturases. Biochem. Soc. Trans. 2000;28(6):938–940. [PubMed] [Google Scholar]
- 53.Belide S., Petrie J.R., Shrestha P., Singh S.P. Modification of seed oil composition in Arabidopsis by artificial microRNA-mediated gene silencing. Front. Plant Sci. 2012;3:168. doi: 10.3389/fpls.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baoming T., Dandan S., Yuli L., Haiyan S., Hua L., Xin Z., Bonan W., Zhenqiang P. Analysis of the RNAi targeting FAD2 gene on oleic acid composition in transgenic plants of Brassica napus. Afr. J. Microbiol. Res. 2011;5:817–822. [Google Scholar]
- 55.Buhr T., Sato S., Ebrahim F., Xing A., Zhou Y., Mathiesen M., Schweiger B., Kinney A., Staswick P., Clemente T., Tom Clemente Ribozyme termination of RNA transcripts down-regulate seed fatty acid genes in transgenic soybean. Plant J. 2002;30(2):155–163. doi: 10.1046/j.1365-313x.2002.01283.x. [DOI] [PubMed] [Google Scholar]
- 56.Peng Q., Hu Y., Wei R., Zhang Y., Guan C., Ruan Y., Liu C. Simultaneous silencing of FAD2 and FAE1 genes affects both oleic acid and erucic acid contents in Brassica napus seeds. Plant Cell Rep. 2010;29(4):317–325. doi: 10.1007/s00299-010-0823-y. [DOI] [PubMed] [Google Scholar]
- 57.van de Loo F.J., Broun P., Turner S., Somerville C. An oleate 12-hydroxylase from Ricinus communis L. is a fatty acyl desaturase homolog. Proc. Natl. Acad. Sci. USA. 1995;92(15):6743–6747. doi: 10.1073/pnas.92.15.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Broun P., Somerville C. Accumulation of ricinoleic, lesquerolic, and densipolic acids in seeds of transgenic Arabidopsis plants that express a fatty acyl hydroxylase cDNA from castor bean. Plant Physiol. 1997;113(3):933–942. doi: 10.1104/pp.113.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broun P., Boddupalli S., Somerville C. A bifunctional oleate 12-hydroxylase: desaturase from Lesquerella fendleri. Plant J. 1998;13(2):201–210. doi: 10.1046/j.1365-313x.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- 60.Lu C., Kang J. Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep. 2008;27(2):273–278. doi: 10.1007/s00299-007-0454-0. [DOI] [PubMed] [Google Scholar]
- 61.Burgal J., Shockey J., Lu C., Dyer J., Larson T., Graham I., Browse J. Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol. J. 2008;6(8):819–831. doi: 10.1111/j.1467-7652.2008.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jako C., Kumar A., Wei Y., Zou J., Barton D.L., Giblin E.M., Covello P.S., Taylor D.C. Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol. 2001;126(2):861–874. doi: 10.1104/pp.126.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bates P.D., Johnson S.R., Cao X., Li J., Nam J.W., Jaworski J.G., Ohlrogge J.B., Browse J. Fatty acid synthesis is inhibited by inefficient utilization of unusual fatty acids for glycerolipid assembly. Proc. Natl. Acad. Sci. USA. 2014;111(3):1204–1209. doi: 10.1073/pnas.1318511111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shockey J.M., Gidda S.K., Chapital D.C., Kuan J.C., Dhanoa P.K., Bland J.M., Rothstein S.J., Mullen R.T., Dyer J.M. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell. 2006;18(9):2294–2313. doi: 10.1105/tpc.106.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Erp H., Bates P.D., Burgal J., Shockey J., Browse J. Castor phospholipid:diacylglycerol acyltransferase facilitates efficient metabolism of hydroxy fatty acids in transgenic Arabidopsis. Plant Physiol. 2011;155(2):683–693. doi: 10.1104/pp.110.167239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H.U., Lee K.R., Go Y.S., Jung J.H., Suh M.C., Kim J.B. Endoplasmic reticulum-located PDAT1-2 from castor bean enhances hydroxy fatty acid accumulation in transgenic plants. Plant Cell Physiol. 2011;52(6):983–993. doi: 10.1093/pcp/pcr051. [DOI] [PubMed] [Google Scholar]
- 67.Kumar R., Wallis J.G., Skidmore C., Browse J. A mutation in Arabidopsis cytochrome b5 reductase identified by high-throughput screening differentially affects hydroxylation and desaturation. Plant J. 2006;48(6):920–932. doi: 10.1111/j.1365-313X.2006.02925.x. [DOI] [PubMed] [Google Scholar]
- 68.Wayne L.L., Browse J. Homologous electron transport components fail to increase fatty acid hydroxylation in transgenic Arabidopsis thaliana. F1000 Res. 2013;2:203. doi: 10.12688/f1000research.2-203.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Erp H., Shockey J., Zhang M., Adhikari N.D., Browse J. Reducing isozyme competition increases target fatty acid accumulation in seed triacylglycerols of transgenic Arabidopsis. Plant Physiol. 2015;168(1):36–46. doi: 10.1104/pp.114.254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu Z., Ren Z., Lu C. The phosphatidylcholine diacylglycerol cholinephosphotransferase is required for efficient hydroxy fatty acid accumulation in transgenic Arabidopsis. Plant Physiol. 2012;158(4):1944–1954. doi: 10.1104/pp.111.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eccleston V.S., Ohlrogge J.B. Expression of lauroyl-acyl carrier protein thioesterase in brassica napus seeds induces pathways for both fatty acid oxidation and biosynthesis and implies a set point for triacylglycerol accumulation. Plant Cell. 1998;10(4):613–622. doi: 10.1105/tpc.10.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bernerth R., Frentzen M. Utilization of erucoyl-CoA by acyltransferases from developing seeds of Brassica napus (L.) involved in triacylglycerol biosynthesis. Plant Sci. 1990;67:21–29. [Google Scholar]
- 73.Lassner M.W., Levering C.K., Davies H.M., Knutzon D.S. Lysophosphatidic acid acyltransferase from meadowfoam mediates insertion of erucic acid at the sn-2 position of triacylglycerol in transgenic rapeseed oil. Plant Physiol. 1995;109(4):1389–1394. doi: 10.1104/pp.109.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han J., Lühs W., Sonntag K., Zähringer U., Borchardt D.S., Wolter F.P., Heinz E., Frentzen M. Functional characterization of beta-ketoacyl-CoA synthase genes from Brassica napus L. Plant Mol. Biol. 2001;46(2):229–239. doi: 10.1023/a:1010665121980. [DOI] [PubMed] [Google Scholar]
- 75.Nath U.K., Wilmer J.A., Wallington E.J., Becker H.C., Möllers C. Increasing erucic acid content through combination of endogenous low polyunsaturated fatty acids alleles with Ld-LPAAT + Bn-fae1 transgenes in rapeseed (Brassica napus L.). Theor. Appl. Genet. 2009;118(4):765–773. doi: 10.1007/s00122-008-0936-7. [DOI] [PubMed] [Google Scholar]
- 76.Li X., van Loo E.N., Gruber J., Fan J., Guan R., Frentzen M., Stymne S., Zhu L.H. Development of ultra-high erucic acid oil in the industrial oil crop Crambe abyssinica. Plant Biotechnol. J. 2012;10(7):862–870. doi: 10.1111/j.1467-7652.2012.00709.x. [DOI] [PubMed] [Google Scholar]
- 77.Jadhav A., Katavic V., Marillia E.F., Michael Giblin E., Barton D.L., Kumar A., Sonntag C., Babic V., Keller W.A., Taylor D.C. Increased levels of erucic acid in Brassica carinata by co-suppression and antisense repression of the endogenous FAD2 gene. Metab. Eng. 2005;7(3):215–220. doi: 10.1016/j.ymben.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen H.T., Mishra G., Whittle E., Pidkowich M.S., Bevan S.A., Merlo A.O., Walsh T.A., Shanklin J. Metabolic engineering of seeds can achieve levels of ω-7 fatty acids comparable with the highest levels found in natural plant sources. Plant Physiol. 2010;154(4):1897–1904. doi: 10.1104/pp.110.165340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Voelker T.A., Hayes T.R., Cranmer A.C., Davies H.M. Genetic engineering of a quantitative trait: metabolic and genetic parameters influencing the accumulation of laurate in rapeseed. Plant J. 1996;9:229–241. [Google Scholar]
- 80.Knutzon D.S., Hayes T.R., Wyrick A., Xiong H., Voelker T.A., Voelker T.A., Maelor Davies H Lysophosphatidic acid acyltransferase from coconut endosperm mediates the insertion of laurate at the sn-2 position of triacylglycerols in lauric rapeseed oil and can increase total laurate levels. Plant Physiol. 1999;120(3):739–746. doi: 10.1104/pp.120.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knutzon D.S., Thompson G.A., Radke S.E., Johnson W.B., Knauf V.C., Kridl J.C. Modification of Brassica seed oil by antisense expression of a stearoyl-acyl carrier protein desaturase gene. Proc. Natl. Acad. Sci. USA. 1992;89(7):2624–2628. doi: 10.1073/pnas.89.7.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dehesh K., Jones A., Knutzon D.S., Voelker T.A. Production of high levels of 8:0 and 10:0 fatty acids in transgenic canola by overexpression of Ch FatB2, a thioesterase cDNA from Cuphea hookeriana. Plant J. 1996;9(2):167–172. doi: 10.1046/j.1365-313x.1996.09020167.x. [DOI] [PubMed] [Google Scholar]
- 83.Roesler K., Shintani D., Savage L., Boddupalli S., Ohlrogge J. Targeting of the Arabidopsis homomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds. Plant Physiol. 1997;113(1):75–81. doi: 10.1104/pp.113.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lardizabal K., Effertz R., Levering C., Mai J., Pedroso M.C., Jury T., Aasen E., Gruys K., Bennett K. Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol. 2008;148(1):89–96. doi: 10.1104/pp.108.123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng P., Allen W.B., Roesler K., Williams M.E., Zhang S., Li J., Glassman K., Ranch J., Nubel D., Solawetz W., Bhattramakki D., Llaca V., Deschamps S., Zhong G.Y., Tarczynski M.C., Shen B. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat. Genet. 2008;40(3):367–372. doi: 10.1038/ng.85. [DOI] [PubMed] [Google Scholar]
- 86.Mu J., Tan H., Zheng Q., Fu F., Liang Y., Zhang J., Yang X., Wang T., Chong K., Wang X-J., Zuo J. LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol. 2008;148(2):1042–1054. doi: 10.1104/pp.108.126342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cernac A., Benning C. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J. 2004;40(4):575–585. doi: 10.1111/j.1365-313X.2004.02235.x. [DOI] [PubMed] [Google Scholar]
- 88.Ke T., Yu J., Dong C., Mao H., Hua W., Liu S. ocsESTdb: a database of oil crop seed EST sequences for comparative analysis and investigation of a global metabolic network and oil accumulation metabolism. BMC Plant Biol. 2015;15:19. doi: 10.1186/s12870-014-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chandrasekaran U., Xu W., Liu A. Transcriptome profiling identifies ABA mediated regulatory changes towards storage filling in developing seeds of castor bean (Ricinus communis L.). Cell Biosci. 2014;4:33. doi: 10.1186/2045-3701-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vanhercke T., El Tahchy A., Liu Q., Zhou X.R., Shrestha P., Divi U.K., Ral J-P., Mansour M.P., Nichols P.D., James C.N., Horn P.J., Chapman K.D., Beaudoin F., Ruiz-López N., Larkin P.J., de Feyter R.C., Singh S.P., Petrie J.R. Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol. J. 2014;12(2):231–239. doi: 10.1111/pbi.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petrie J.R., Vanhercke T., Shrestha P., El Tahchy A., White A., Zhou X.R., Liu Q., Mansour M.P., Nichols P.D., Singh S.P. Recruiting a new substrate for triacylglycerol synthesis in plants: the monoacylglycerol acyltransferase pathway. PLoS One. 2012;7(4):e35214. doi: 10.1371/journal.pone.0035214. [DOI] [PMC free article] [PubMed] [Google Scholar]


