Abstract
Vegetable oil utilization is determined by its fatty acid composition. In soybean and other grain crops, during the seed development oil accumulation is important trait for value in food or industrial applications. Seed development is relatively short and sensitive to unfavorable abiotic conditions. These stresses can lead to a numerous undesirable qualitative as well as quantitative changes in fatty acid production. Fatty acid manipulation which targets a higher content of a specific single fatty acid for food or industrial application has gained more attention. Despite several successes in modifying the ratio of endogenous fatty acids in most domesticated oilseed crops, numerous obstacles in FA manipulation of seed maturation are yet to be overcome. Remarkably, connections with plant hormones have not been well studied despite their critical roles in the regulation and promotion of a plethora of processes in plant growth and development. While activities of phytohormones during the reproductive phase have been partially clarified in seed physiology, the biological role of plant hormones in oil accumulation during seed development has not been investigated. In this review seed development and numerous effects of abiotic stresses are discussed. After describing fatty acid and phytohormone metabolism and their interactions, we postulate that the endogenous plant hormones play important roles in fatty acid production in soybean seeds.
Keywords: Soybean, Seed development, Cytokinin, Abscisic acid, Fatty acid.
1. INTRODUCTION
Plant oils have become an important and integral part of our economy. They are important as feedstock, for food uses and for an abundance of industrial applications such as biodiesel fuel, lubricants, engine oils, polyesters, pesticides or inks. Vegetable oils have been identified as a potential replacement for fossil oils based on the strikingly similar chemical structure of their constituents. This has provided a strong case for plant oils as valuable potential replacements for the non-renewable oil in essentially any application [1]. However, current and future energy demands rely on fossil oil and difficulties associated with plant oil production limit the potential for such a transition. Restrictions, including available farmland and crop productivity, make such a transition virtually impossible. In contrast, the prospects for the use of plant oils in the material and chemical industry are much more promising. The material and chemical production industry only accounts for 10% of all fossil oil use. However, the value of the industry is equal to that of all the remaining fossil oil usage worldwide [2]. The material and chemical industry could capture much of this added value, by sourcing plant macromolecules which can be optimized for end use and thereby minimize the downstream processing costs [1].
Soybean (Glycine max, Gm) is one of the most important oilseed crops, contributing to 59% of all the world oilseed production in 2014 [3] and is one of the world’s most widely used and healthy edible oils. In addition to this, the industrial products and uses for soybean oil are becoming increasingly popular and diverse. Soybean oil utilization is determined by fatty acid (FA) composition. In the chemical industry, each specific type of FA is important to a particular but limited production use. This limitation can be overcome through a process that allows for the isolation of specific FA from the oil mixture, but this comes with a significant extra cost [1, 4].
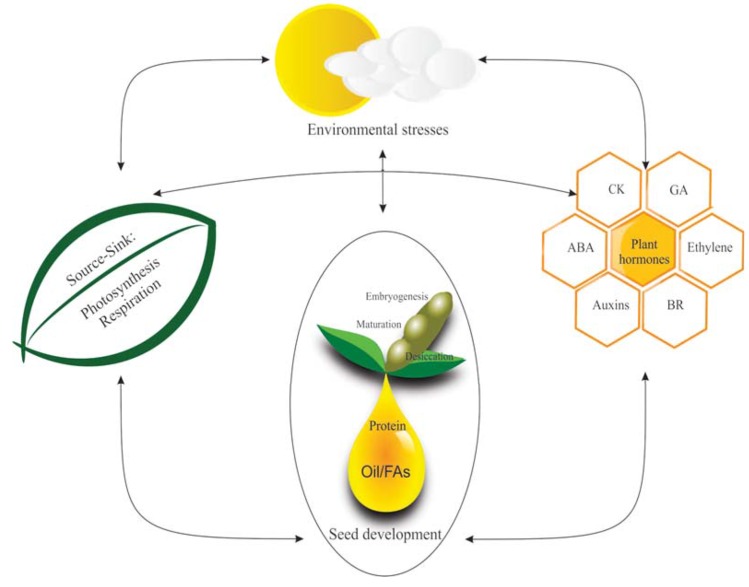
In soybean as well as other oil-grain crops, oil production and accumulation take place during seed development and maturation [5]. Generally, seed development is highly regulated and follows an ordered progression through three phases of embryonic development: morphogenesis, cell enlargement/reserve accumulation, and desiccation/developmental arrest. This progression, from the beginning of flowering stage through the embryogenesis, seed maturation, desiccation and germination, has undergone a lot of research to elucidate many different important regulatory factors. Much evidence points towards the participation of both hormonal and non-hormonal developmental controls, some of which act synergistically [6]. While the role of many of these hormones in seed development have been reported, their long term effects on the accumulation in dried matter composition, especially FAs or oil, during seed maturation have yet to be investigated thoroughly. In this review seed development, FA, phytohormone metabolism and their interactions (Fig. 1) will be considered in order to highlight the connections between plant hormone dynamics and FA production during soybean reproductive development.
Fig. (1).
Seed development and the regulation network.
2. SOYBEAN SEED REPRODUCTIVE DEVELOPMENT
In order to best describe the reproductive phases (R) and seed development of soybean, authors introduced a development scale ranging from R1-R8 based on morphological descriptions of these phases known as growth stages (GS) (Table 1) [7]. Previous studies, which consider compositional changes during seed development, have utilized sampling methods based on a system defined as Day After Flowering (DAF) [8, 9]. Unfortunately, the DAF sampling method introduces variability towards the rate of seed development caused by environmental and genotypic influences. Furthermore, Bewley and Black [10] found that similar studies which define seed development rates could not be compared to one another because, although the pattern of seed development was consistent, the timing was not. The advantage to utilizing growth stages is that they serve as precise and objective descriptions of plant development and are applicable to determinate and indeterminate soybean cultivars in all environments. For these reasons, the use of GS in describing soybean development has increased and continues to gain acceptance [7, 11, 12].
Table 1.
Reproductive stages and development.
| R1 | Beginning bloom: One flower at any node. |
| R2 | Full bloom: Open flower at one of the two uppermost nodes. |
| R3 | Beginning pod: Pod 0.5cm (1/4 inch) long at one of the four uppermost nodes. |
| R4 | Full pod: Pod 2 cm (3/4 inch) long at one of the four uppermost nodes. |
| R5 | Beginning seed: Beans beginning to develop at one the four uppermost nodes. |
| R6 | Full seed: Pod containing a green seed that fills the pod cavity at one of the four uppermost nodes. |
| R7 | Beginning maturity: One pod anywhere with its mature color. |
| R8 | Full maturity: 95% of the pods have reached their mature color. |
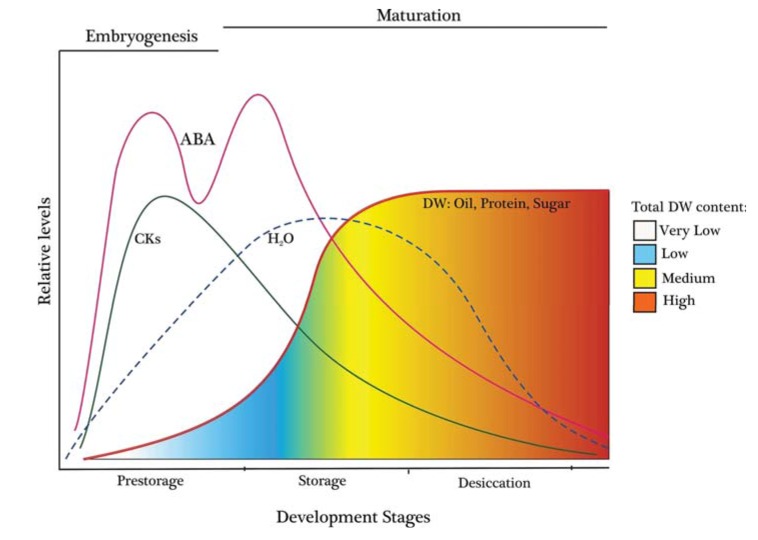
On the other hand, according to embryo differentiation a general staging system has been described that divides seed development into three parts: cell division or prestorage phase, maturation or storage phase, and desiccation phase (Fig. 2). For crop seeds, maturation or seed filling duration is of special interest. During this stage, a regulatory network initiates the accumulation of storage products, on multiple levels. These reserves accumulate during seed maturation to nourish the young plant during germination and supply a high nutrient and chemical value. This has led to many investigations into maturation during seed development with a view to producing better grain crops. Much work has been done in legumes and in particular on Arabidopsis, which strongly implicates metabolite and hormone responsive pathways as key contributors [6, 13, 14].
Fig. (2).
A generalized graph showing the relative levels of water, dry weight (DW), and hormones during the stages of seed development (adapted from [6]).
2.1. Soybean Prestorage Stage
Prestorage or morphogenesis begins with the fertilization of the first flower, follows on to the completion of embryogenesis, and ends once pod development has been achieved. Prestorage includes GS R1-R4. Moreover, the zygote undergoes extensive cell divisions, and resembles the globular heart stage. This cell differentiation subsequently results in the tissue types required to form the root-shoot axis [15] and large cotyledon where oil, protein and starch reserves are localized during seed maturation. In the early stage of embryogenesis, the embryo is supported by a temporary organ called a suspensor, which provides a connection for the embryo to the surrounding nutrient-providing tissues. Measurements of endogenous hormone concentrations during morphogenesis have shown that cytokinins (CKs), abscisic acid (ABA), gibberellin (GA) and indole-3-acetic acid (IAA) are all transiently high and significantly active [6, 16, 17]. Tissue culture studies involving Phaseolus (common bean) have shown that the addition of exogenous GA can substitute for a detached suspensor in promoting embryonic growth, suggesting that the suspensor may normally provide GAs as well as nutrients to the developing embryo. Similarly, other studies involving a focus on either exogenous hormone addition, genetic responses or exudates from tissue culture all suggest that the roles of GAs and CKs are primarily nutritive. IAA has shown to play a major role in establishing the embryonic body-plan via effects on apical-basal polarity/pattern formation and vascular development [18, 19]. ABA can act to prevent seed abortion and promote embryo growth during the early embryogenesis [20, 21]. Despite the low levels of ABA generally detected during early embryogenesis, the ABA biosynthetic pathway is apparently active at this stage. In agreement, high ABA levels have been found in the pedicel/placento-chalazal complex of maize kernels [22]. CKs have been implicated in a number of processes including support of suspensor function, significant promotion of embryonic growth to reduce seed abortion, and enhancement of grain filling and seed yield via the promotion of cell division, especially within the cotyledons [23, 24].
In dicots such as soybean, prestorage cell division is critical as it dictates the total number of cells that will exist within, and in doing so lays down the ground work for cell enlargement during maturation. Moreover, once the number of embryonic cells has been defined by the key contributors, the seed cotyledon will enlarge and accumulate the important constituents based upon the available number of cells, assimilate supply, and regulatory signals. Accumulation of oils/FAs and proteins occurs throughout cell enlargement and is central to cotyledon development. Inside the cells of cotyledons, oil is stored in small discrete oil bodies in the form of triacylglycerols (TAGs) [25]. It is believed that the more intracellular volume is available, the more space oil bodies can occupy. However, this limited available intracellular space must be shared between both protein bodies and TAGs. Thus, it is well-known that the production of TAGs and protein bodies is inversely correlated [26].
2.2. Seed Maturation and Desiccation Stage
Following the first phase, the reserve accumulation is the next critical period in soybean seed production. Soybean seed value is determined in this phase as lipid bodies and proteins are synthesized and stored throughout development stage of R5 until the end of R6. This is one of the last two phases of embryonic development and is sometimes collectively referred to as “maturation”. At that time, seeds acquire the ability to survive desiccation and become ready to initiate growth of the next generation, independent of the maternal plant. Seed maturation begins when developing embryos cease growth by cell division; this coincides with an increase in seed ABA, a hormone which induces expression of a cyclin-dependent kinase inhibitor (ICK1) that could lead to cell cycle arrest at the G1/S transition [6]. As demonstrated in the Arabidopsis seed model, ABA, classically associated with seed maturation, is produced first in maternal tissues and later in the embryo [27]. Maternal ABA, synthesized in the seed coat and translocated to the embryo, promotes its growth and prevents abortion [21]. A major increase in ABA levels occurs during the maturation phase corresponds to the positive regulation of a number of genes for seed reserves [28, 29].
The middle stage of seed development is a period of massive reserve accumulation and cell enlargement as cells fill with protein and lipid bodies [30, 31]. Multiple seed mass and composition studies on “Williams 79” soybean seeds by Dornbos and McDonald [32] demonstrated that stages R5 and R7 corresponded to seed filling initiation and physiological maturity, respectively. Between those phases, water content (% fresh weight - FW) declines steadily although the total amount of water per embryo is still increasing. The most abundant hormone at this stage is ABA, which reaches peak levels during the period of maximal seed weight gain.
In the late-developmental stage, ABA induces dormancy and inhibits germination in the matured seeds by up-regulating its own levels and down-regulating GA synthesis [33-36]. During the final phase of seed development, embryos become desiccation tolerant, lose water, and become relatively metabolically inactive. A decrease in the ABA level during the desiccation phase is also expected to result from decreased ABA synthesis [29]. After harvesting at the full maturity stage, R8, the dry weight of a soybean seed consists of the following elements: oil (20%), protein (40%), carbohydrates (30%), crude fiber (5%) and ash (5%) [7]. Typically, soybean oil consists of approximately: 13% palmitic (C16:0), 4% stearic (C18:0), 20% oleic (C18:1), 55% linoleic (C18:2) and 8% linolenic (C18:3) acid at 13% moisture [4, 37]. In addition to these five major Fas, a numerous minor FAs, which may also have commercial value could be found in soybean oil such as myristic acid (C14:0), arachidic acid (C20:0), behenic acid (C22:0) or erucic acid (C22:1) [38].
Despite that seed development is a highly regulated process, dry matter accumulation at seed filling is affected by both genetic and environmental factors which lead to changes of oil and protein concentrations of crops. In soybean, the FA accumulation during seed maturation takes place in a short period about 4 to 6 weeks as opposed to those of other oil plants such as olive, oil palm or avocado. Crops are sensitive to stressful conditions during their short seed filling period, and this makes them susceptible to incurring permanent changes in oil content and FA profile as well as crop quality and productivity [39].
2.3. Effect of Environmental Stresses on Seed Development
Soybean is an important source of dietary proteins and lipids for humans and animals. Both of these compounds make up about 60% of the seed total dry weight. This part of the review addresses mainly the influence of major abiotic environmental stresses on total oil concentration and changes in oil composition of soybean and compares those to other oil crops. However, it is well known that oil content is inversely related to protein in soybean [26], and thus, the effects of stresses on protein content will be also discussed.
Abiotic stress factors such as drought, heat, soil salinity, tropospheric ozone and excess UV radiation are already causing significant agricultural yield losses and will become even more prevalent in the coming decades due to the effects of global climate change [40-42]. The growing impact of the climate change is increasing the frequency of adverse environment conditions and has driven worldwide attempts to adapt agricultural production to such stresses [43, 44]. Consequences of exposure to abiotic stresses include various physiological changes in crop plants, such as: alterations in the photosynthetic gas exchange and assimilate translocation [45], altered water uptake and evapotranspiration, effects on nutrient uptake and translocation [46], antioxidant reactions [47], programmed cell death [48], and altered gene expression and enzyme activity [49, 50]. These exposures are likely to have numerous effects on the chemical composition of crops and, consequently, the quality of agricultural products.
Oil and protein concentrations of crops are sensitive to both genetic and environmental factors. The major stress factors that have been investigated are: drought, salinity, ozone and heat. The observed effects are variable and depend on the stress type, crop species, and experimental conditions, but some typical patterns can be characterized. A decrease in the lipid concentration has been reported in almost every study involving crops grown under unfavorable conditions. By contrast, these stresses usually stimulate higher protein concentration in the harvested fraction of crops, with only a few studies showing no effect or lower protein concentration [39].
2.3.1. Effect of Abiotic Stresses On Oil Content and Fatty Acid Composition
The FA profile of soybean oil is a fundamental quality attribute. Genotype is the main determinant of FA composition, but environmental factors such as climate conditions have been linked to variations in oil quality and yield. Remarkable variability of effects was observed, depending on the stress type, crop species and experimental conditions; however, some general patterns can be discerned. Notably, the majority of the studies reported decreases in the lipid concentration when crops were grown under stressful conditions [51, 39]. Liu et al. [52] and Feng et al. [53] indicated UV-B radiation decreased total biomass and seed yield per plant. These losses were mainly attributed to the change of pod number per plant and seed size. In a report on seed development gene expression, Fatihi et al. [54] indicated that a reduced seed size is primarily associated with reduced TAG content in the embryos of Arabidopsis.
In case of the drought stressed crops, almost all studies reported a decrease in the lipid concentration of the harvested products compared to that of the sufficiently watered plants [39]. Few studies failed to detect significant decreases in oil concentration due to drought, and only one study reported an increase in oil concentration, in olive fruits [51, 55]. It is important to note that, in seeds of annual crops, such as soybean and sunflower, oil accumulates at a high rate during a short period of time (between 30 and 45 days). On the other hand, in olive fruit – similar to those of oil palm and avocado – oil accumulates principally in the mesocarp at low rate, over a long period (100 to 140 days). Thus, it is possible that greater opportunities for recovery to normal values after a high-temperature event might exist in olives [51].
A similar trend towards declining oil concentration was seen under salinity and heat stress, for which only a few studies reported increases or no effects on lipid concentrations [39]. In contrast, ozone stress seemed to be an exception, as the available studies reported either no effect, or even an increase in lipid concentration [39].
Temperature effects on seed growth [56] are well documented in annual crops, including oil-seed species. Seed oil concentration decreased in response to high temperatures during the period of oil synthesis [57]. Processes indirectly linked to oil synthesis such as photosynthesis or respiration could also be simultaneously modulating the oil concentration. Photosynthesis of both leaves and fruit are likely negatively affected by exposure to high temperatures. Increases in leaf temperature above 32oC in growth chambers resulted in a decline in photosynthetic rate [58]. The high temperature stress decreases the duration of seed filling period via accelerated leaf senescence, and consequently oil accumulation is stopped before fulfilling seed oil capacity, when the seed is ready for desiccation.
The environmental stresses not only change the oil contents of oil crops but also affect oil composition [39]. A general trend indicated an increase in the saturation level of the oil fraction due to various abiotic stresses has been reported [39]. The proportion of polyunsaturated FAs (PUFA) in soybean oil dropped considerably under heat stress [59]. The same pattern was observed under drought stress in the oil fractions of sunflower [60], groundnut [61] and sage [62]; and under salt stress in sunflower [63], olive [64], cotton [65], sage [66], and coriander [67]. These decreases in PUFA (especially linoleic acid, C18:2) were consistently accompanied by increases in the proportion of oleic acid (C18:1) [63, 65, 66].
FA composition varies depending on the timing of the high temperature event. For example, in sunflower [57], when high temperature was applied during the final portion of oil accumulation phase, the proportion of C18:1 increased while that of C18:2 decreased. In soybean, as well as in sunflower, lower latitudes leading to the increase of temperature have been associated with high C18:1 oils [65, 66]. A high C18:1 concentration in sunflower was shown to be associated with increased temperature also when heat was applied to the plants as an experimental factor [68]. In addition, it has been demonstrated that the differences in night temperatures are better indicator of the changes in FA composition than daily average temperatures in annual oil-seed crops (sunflower: [69, 70]; soybean: [71]).
The observed changes in FA composition are believed to be a result of the activity of enzymes involved in lipid synthesis and conversion [60, 72]. FA synthesis in oil seeds starts its early steps in the plastids and then C18:1 as the main product of plastidal lipid synthesis is exported to the cytosol. The enzyme activity in which oleate desaturase (OD) moderates the cytosolic desaturation of C18:1 to form PUFA (i.e. C18:2) is believed as an explanation for shifts in C18:1/C18:2 ratio in several crops under various types of stress, including: salinity, drought, and heat [63, 73]. A numerous studies have demonstrated the temperature dependence of this enzyme [74, 75]. In sunflower, the highest OD activity was observed at 20oC and its activity dropped considerably at higher temperatures. In contrast in safflower OD was more heat stable and maintained its full activity up to 30oC. Two factors including (i) the heat stability of the enzyme, and (ii) the effects of temperature on the internal oxygen concentration of seeds, which is a key regulator of OD activity [75] have been proposed in order to explain this temperature-dependent decline in enzyme activity. Besides enzymatic desaturation of FAs, transport of plastidal FAs to the cytosol is potentially affected by environmental stresses [60].
2.3.2. Effect of Abiotic Stresses On Protein
It is generally considered that the common stress factors including drought, heat and tropospheric ozone result in an increased protein concentration in wheat grains and soybean seeds [39]. It has been previously suggested in many reports that the common negative effect of stress on dry matter accumulation, content of oil, starch and other residuals, can ultimately lead to the relatively higher protein concentration. Studies found that in most cases the nitrogen harvest index, which represents the proportion of nitrogen contained in the grain relative to the total above-ground nitrogen content, was less affected under abiotic stresses than dry matter harvest index, which results in increase of nitrogen concentration in the harvested fractions of crops [76,77].
Pleijel et al. [78] reported a negative linear correlation between grain yield and grain protein concentration in wheat exposed to different concentrations of ozone and carbon dioxide across different countries. Using meta-analysis, Rotundo and Westgate [79] analyzed the environmental effects on metabolite contents and concentrations in soybean seeds. They concluded that the increase in protein level did not necessarily result from a stimulation of protein synthesis, but rather from a concentration effect due to reduced biomass production under stress. Similarly, Singh et al. [80] grew two soybean varieties under elevated ozone to evaluate yield reduction. They reported that the yield reduction can be mainly attributed to the reduction in weight of the seeds. Zhang et al. [81] reported that ozone causes the reduction of soybean seed yield via decreased photosynthetic rates and this effect is greater in the seed filling stage than the flowering stage. Additionally, an increase in protein concentration was consistently associated with a decrease in grain yield of wheat when water deficit occurred throughout the growing season [82].
Another common response of plants to environmental stress is accelerated leaf senescence accompanied by shortened grain development period [83, 84]. In order to compensate for the decrease in seed filling duration and reduced nitrogen uptake from soil under stress conditions, remobilization of nitrogen from vegetative tissues, and amino acids derived from the protein degradation is enabled [85]. As a result of this remobilization, protein synthesis is less affected by stress factors than other components [79]. The adverse growing conditions that promote leaf senescence during grain filling tend to favor protein deposition over starch accumulation in the grain, because the production and translocation of carbohydrates to the grain is more sensitive to environmental stresses than protein accumulation [86, 87]. In a number of studies, grain starch concentration under stress conditions was reduced because of a shortened duration of starch accumulation [88,89], or an inhibition of key enzymes involved in starch synthesis [89].
In fact, the synthesis of all the major seed components (protein, oil and residuals) is inhibited during water or heat stress, but the accumulation of individual components is not obstructed to the same extent [79]. The negative impact was more pronounced on oil and residuals than on protein, which resulted in a significant increase in protein concentration. Therefore the changes in seed protein concentration could be explained largely by a simple concentration effect [39].
In summary, environmental stress effects on seed development include: increased flower abortion, shortened embryogenesis and seed filling duration, accelerated leaf senescence and reduced photosynthetic rate. Shortened embryogenesis could lead to a limited number of cells in cotyledons where the nutrients can accumulate. Cotyledon cell number, along with the cell volume, is one of two major components determining seed size. On the other hand, if the seed filling duration is shortened, there would be less time for protein, oil and other compounds accumulation. This would lead to the reduction in cell volume, and subsequently, to a smaller seed size. Contributing in the loss of grain yield, the effects of stresses on the increase of flower abortion could cause the reduction of number of pods per plant and number of seed per plant and lead to a sink limitation. Finally, stress responses via acceleration of leaf senescence and reduction of photosynthesis or respiration rates could result in shortened seed maturation and may lead to a limited source for the supply of assimilates to the developing seed.
3. FATTY ACID ACCUMULATION AND COMPOSITION IN SEED DEVELOPMENT
3.1. Fatty Acid Biosynthesis
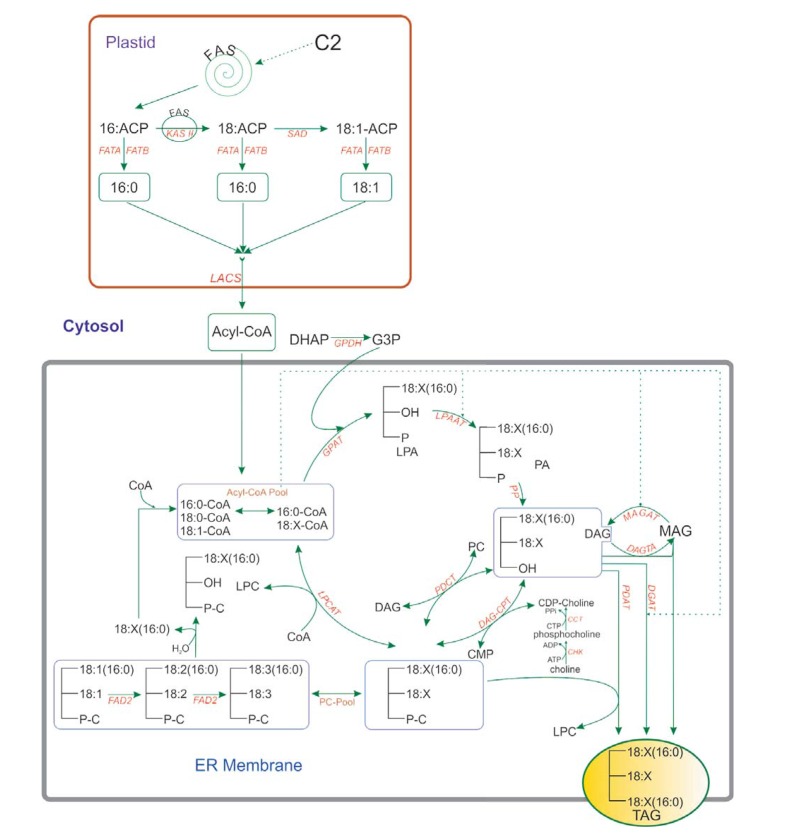
The biosynthesis of seed storage oils containing the five major FAs occurs primarily in two subcellular compartments. FA biosynthesis occurs in the plastid of cells and involves the cyclic condensation of two-carbon units in which acetyl coenzyme A (acetyl-CoA) is the precursor. When conjugated to the acyl carrier protein (ACP), the FA chain is referred to as acyl-ACP. The first committed step in the pathway is the synthesis of malonyl-CoA from acetyl-CoA and CO2 by the enzyme acetyl-CoA carboxylase [90,91]. In the following step, some 16:0-ACP is released from the FA synthase machinery, but most molecules that are elongated to 18:0-ACP are efficiently converted to 18:1-ACP by a desaturase enzyme (Fig. 3).
Fig. (3).
Fatty acid and triacylglycerol synthesis of the five common FAs in in soybean (adapted from [91] and [224]). Abbreviations: ABCAT, ABC acyl transporter; ACBP, acyl-CoA binding protein; ACP, acyl carrier protein; FAS, fatty acid synthase; FATA (B), fatty acyl thioesterase A (B); KAS, ketoacyl-ACP synthase; LACS, long-chain acyl-CoA synthetase; SAD, stearoyl-ACP desaturase. CCT, cholinephosphate cytidylyltransferase; CHK, choline kinase; DAG, diacylglycerol; DAG-CPT, diacylglycerol cholinephosphotransferase; DAGTA, diacylglycerol transacylase; DGAT, acyl-CoA: diacylglycerol acyltransferase; DHAP, dihydroxyacetone phosphate; FAD2, oleate desaturase; FAD3, linoleate desaturase; G3P, glycerol-3-phosphate; GPAT, glycerol-3-phosphate acyltransferase; GPDH, glycerol-3-phosphate dehydrogenase; LPA,2-lysophosphatidic acid; LPAAT, 2-lysophosphatidic acid acyltransferase; LPC, 2-lysophosphatidylcholine; LPCAT, 2-lysophosphatidylcholine acyltransferase; MAG, monoacylglycerol; MAGAT, monoacylglycerol acyltransferase; PA, phosphatidic acid; PDAT, phospholipid:diacylglycerol acyltransferase; PDCT, phosphatidylcholine:diacylglycerol cholinephosphotransferase; PLA2, phospholipase A2; PP, phosphatidate phosphatase.
(Fig. 3) depicts the biosynthesis of the five common FAs present in the oil of annual oil crops and the main enzyme steps involved. The first three FAs (C16:0, C18:0 and C18:1) are produced by de novo synthesis and desaturation in the plastids [91]. Elongation and desaturation are carried out while the FAs are attached to an acyl carrier protein (ACP). After removal of the ACP group by acyl-ACP thioesterases (FatA or FatB), the FAs are exported from the plastid and incorporated into the cytosolic acyl-CoA by the action of an acyl-CoA synthetase (ACS). 18:1 is then acylated onto the membrane lipid phosphatidylcholine (PC), mainly by the action of the lysophosphatidylcholine acyltransferase (LPCAT) [91]. Further desaturations of the 18:1 to 18:2 and 18:3 are catalysed by FA desaturase 2 (FAD2) and FAD3 while the acyl substrates are acylated to PC. Storage TAGs are synthesized by the Kennedy pathway (Fig. 2) in developing seeds [91]. The enzymes involved are probably located in the endoplasmic reticulum (ER) and act by the sequential acylation of the sn-1, -2 and -3 positions of glycerol-3-phosphate, with the removal of the phosphate group occurring before the final acylation step. The distribution of acyl groups on the glycerol backbone is often non-random because of the substrate selectivity of the acyltransferases for different FAs [91]. In detail, TAGs can be formed through three sequential acyl-CoA-dependent acylations of the glycerol backbone beginning with sn-glycerol-3-phosphate. The acylation of sn-glycerol-3-phosphate is catalyzed by acyl-CoA:sn-glycerol-3-phosphate acyltransferase (GPAT). The second acylation is catalyzed by acyl-CoA:lyso-phosphatidic acid acyltransferase (LPAAT). After removal of the phosphate group to generate sn-1,2-diacylglycerol (sn-1,2-DAG), the final acyl-CoA-dependent acylation is catalyzed by acyl-CoA:diacylglycerol acyltransferase (DGAT) to form TAG [90,91].
3.2. Storage Lipid Accumulation
During soybean seed development, the rate of lipid biosynthesis per seed increases markedly, resulting in a mature seed containing 20 to 25% lipid on a seed dry weight basis at 13% moisture. During the seed filling period, the rate of FA accumulation per seed increases by 10 to 20 fold. Most of the lipid in soybean seeds is stored in the cotyledons in the form of triglycerides packaged in specialized structures, often referred to as oil bodies or spherosomes [92]. These organelles consist of a TAG core surrounded by a phospholipid monolayer decorated with a number of different proteins. The most abundant of these are the oleosins, but others such as caleosins and steroleosins are also present [93]. Oleosins contain a hydrophic oil body–binding domain flanked by two amphipathic domains. Mutant analysis has confirmed that oleosins determine the size of oil bodies and, thereby, facilitate mobilization of the TAG storage reserves during seed germination by maximizing the surface-to-volume ratio of the oil bodies [94,95]. Caleosins also appear to play a role in TAG mobilization during germination, possibly by facilitating interactions with vacuoles [96]. Steroleosins, in addition to an oil body–anchoring domain, possess a sterol-binding dehydrogenase that might play a role in signal transduction [97].
Soybean oil consists predominantly (96 – 98%) of TAGs with the FAs distributed among different molecular species [38,98]. The physical and chemical properties of TAG generally reflect that expected from the FA composition including chain length, degree of unsaturation and branching of the chain, and FA distribution in TAG molecules, for instances TAG melting behavior or oxidation stability [99, 100]. TAG rich in long-chain (from C16) and saturated acids are high melting, and those rich in polyunsaturated acid are lower melting. Owing to the distribution of FAs in TAG molecules as well as the difference in TAG composition, the melting behavior could be more complicatedly to determine. Because of the high proportion of polyunsaturated FAs, soybean oil is considerably low oxidative stability that limits the uses of soybean oil in food products and industrial applications [101]. Although enhancing the oxidative stability of oils for food applications has been addressed historically through partial hydrogenation, this approach to the enhancement of oxidative stability will have a negative impact on the cold flow properties of biodiesel [101]. However, a very good compromise can be reached by developing an oil high in the mono-unsaturated fatty acids, such as C18:1 or C16:1 and low in both saturated and polyunsaturated FAs, thereby simultaneously improving oxidative stability whilst augmenting cold flow [99, 101].
3.3. Manipulation of the Levels of the Five Common Fatty Acids Using Genetic Techniques
All the genes involved in biosynthesis of the five common FAs are known, and a number of acyltransferase genes that are putative candidates for the reactions leading to the synthesis of TAG with these five FAs have been cloned. Most domesticated oilseed crops have been successfully modified through either breeding or genetic engineering approaches to optimize the ratio of endogenous FAs in the storage oil for specific end uses [102]. For example, suppression of the oleate D12-desaturase gene (which normally converts C18:1 to C18:2) in soybean, sunflower, cotton and canola has resulted in the production of oils with a high C18:1 content, which have greater oxidative stability and improved performance in high-temperature cooking applications. Oils with a high C18:1 content are also desired by the chemical industry, as C18:1 can be used in a variety of applications including detergents, soaps, lubricants, cosmetics and emulsifying agents, and as a source of C9 monomers for plastics [103]. Buhr et al. [104] described the development of transgenic soybean events in which the expression of FAD2-1 and FatB was simultaneously down-regulated in a seed-specific fashion, thereby generating soybean oil with a reduced content of C16:0 (< 5%) and significantly increased C18:1 (> 85%). Recently, Pham et al. [4,37] have found mutant alleles of FAD2-1A and FAD2-1B in their soybean plant introduction (PI) collections. The non-transgenic soybean lines carrying both homozygous mutant FAD2-1A alleles and mutant FAD2-1B alleles have an average of 82-86% C18:1 content, as opposed to 20% in conventional soybean. They also have low levels of C18:2 and C18:3 [4, 37]. On the other hand, up-regulating the ER oleoyl and linoleoyl desaturases by over-expressing these genes in oil seeds has led to substantial increases in PUFAs in the oil. A striking example of this is the overexpression of a fungal bifunctional Δ12 and Δ15 desaturase in soybean, resulting in somatic embryo oils with over 70% of C18:3, compared to 19% in the wild type [105].
The amount of the saturated C16:0 in the oil is mainly controlled by the balance of activities between two key plastidic enzymes, namely ketoacyl-ACP synthase (KAS) II and the FatB thioesterase. By overexpression of a palmitoyl specific FatB, an increase in C16:0 in rape seed from 6% to 34% was achieved [106]. In cottonseed, an increase of C16:0 from 27% to 77% was obtained by RNAi silencing of KASII [107]. The level of stearic acid is controlled by the activity of the stearoyl-ACP desaturase in combination with the activity of the acyl-ACP thioesterases acting on 18:0-ACP. Down-regulating the stearoyl-ACP desaturase or the silencing of the gene encoding for stearoyl-ACP desaturase led to an increase of C18:0 in rape seed and turnip rape oil from 2% to 40% [108, 109].
FA profiles appeared in membrane lipids and different lipid storage forms are controlled by soluble and membrane-bound enzyme activities which are mutually expressed by means of the cooperation between plastids and the extraplastidial compartments [102]. The two common FAs (C16:0 and C18:1) derived from lipid biosynthesis are necessary for membrane lipids. In addition, it should be noted that the biosynthesis of TAGs and membrane lipids share DAG as a common precursor. As a result, corresponding FA changes as engineered in the oil will occur in the membrane lipids of the seed and might affect overall membrane functioning. A complete absence of PUFAs might have deleterious effects in the seed and the manipulation of C16:0 and C18:0 levels in the oil. This will cause similar changes in the extraplastidic membrane lipids. Due to the high melting point of these FAs, the membrane lipids will crystallize and cause collapse of cell metabolism if the levels are too high [1]. In addition, all the genes involved in biosynthesis of the five common FAs are known and have been cloned; however, there is a very limited knowledge about the involvement of these genes in TAG synthesis in vivo [91]. Consequently, besides continuing to improve these limitations, a focus should be to find more efficient approaches for the optimization of FAs and TAG compositions accompanying increases in crop yields. Most soybean oils are processed either for nutritive uses or industrial purposes. Industrial processing requires different compositions of FA in soybean oil. It means that some of these FA are more desirable than others. The alteration of FA profiles in soybean to improve oil quality is an important and evolving theme in soybean research to meet nutritional needs and industrial criteria in the modern market [4, 110]. The oil profile modification approach has led to great interest into regulatory activity during seed maturation and seed development, as a whole. Potential regulators of embryo maturation have been identified by determining which conditions or metabolites are present in seeds at these stages and testing their functional significance by physiological and genetic assays.
All of the known hormone groups participate in some aspects of seed development. Early embryos require auxin transport and signaling to mediate pattern formation, whereas GAs and CK are most important for nutritive effects and general growth promotion at that stage. ABA is important for inducing the transition from growth by cell division to cell enlargement during maturation. Seed maturation is subject to overlapping control mechanisms, only some of which are ABA-dependent. Many of the mediators of hormonal signaling during seed development are transcription factors showing complex patterns of cross-regulation, with evidence for both hierarchical and combinatorial interactions [6]. Many of these transcription factors are members of families with partially redundant, and sometimes antagonistic function. In fact, the oil accumulation of soybean or other oil crops takes place during seed maturation which is highly regulated by means of the endogenous plant hormones. It seems an oversight that lipid and FA production has been looked at separately from plant hormone metabolism in seeds, even though their activities take place during the same periods. Thus, the potential interaction between lipid production and plant hormone activity during the seed maturation is an area with great scientific and industrial potential. For example, while the activities of plant hormones during the reproductive phase have been partially clarified in seed physiology, one should ask, what is the biological role of plant hormones in oil accumulation during the seed maturation? Remarkably, plant hormones have critical roles in the regulation and promotion of a plethora of processes in plant growth and development; therefore, we postulate that the endogenous plant hormones play an important role in FA-TAG composition and their accumulation in soybean seeds.
4. PHYTOHORMONE METABOLISM
All of the known hormone groups participate in some aspects of seed development. Early embryos require auxin transport and signaling to mediate pattern formation of the embryo, whereas GAs along with CKs are the most important for nutritive effects and general growth promotion at that stage. CKs are also in charge of cell division promotion during the early embryogenesis. In the latter stages of seed development, ABA and sucrose trigger seed maturation [111] to start dry matter accumulation in the seed. Later ABA interacts with GAs to start seed desiccation and induce seed dormancy [6]. ABA also enhances the seed storage of protein [35], while CKs and brassinosteroids (BRs) participate in the promotion of the seed filling process [112, 113]. In this review, FA production during seed development and its interaction with phytohormone metabolism are the main focus. In all these important hormones, CKs and ABA extensively involve in seed development. Therefore, we will mainly discuss these two hormones in the following parts in order to postulate a potential biological role of endogenous hormones in oil accumulation during the seed maturation.
4.1. Cytokinins
More than a half century ago, Skoog and his collaborators purified and crystallized a compound that strongly stimulated cell proliferation from autoclaved herring sperm DNA extracts [114,115]. This growth regulating substance is known as Kinetin. Originally the name CK was a generic name for kinetin-like substances which promote cell division and exerts other growth regulatory effects [114, 115]. The compounds we now refer to as CKs, have been established as a prominent part of seed development research. The first naturally occurring CK was isolated from immature maize endosperm and termed Zeatin (trans-zeatin, tZ) and it was implicated as a key factor in seed development [116]. Subsequently, high CK biosynthetic activity was found to take place not only in the roots, but also shoot meristems, and developing seeds [117]. Over the years, many types and forms of CKs have been described and they have been isolated from an array or organisms including: plants, fungi, bacteria, and algae [118]. These phytohormones have been shown to play a crucial role in the beginning of a plant life cycle by regulating the proliferation and differentiation of plant cells [119], but they are also involved in many aspects of growth including: the balancing of nutrient source and sink relationships [120, 121], control of shoot/root investment [122], transduction of nutritional signals [123], decrease of flower and seed abortion and enhancement of seed filling [124], increased crop productivity [113, 124], and delayed senescence [119].
4.1.1. The Role of Cytokinins in Seed Development Processes
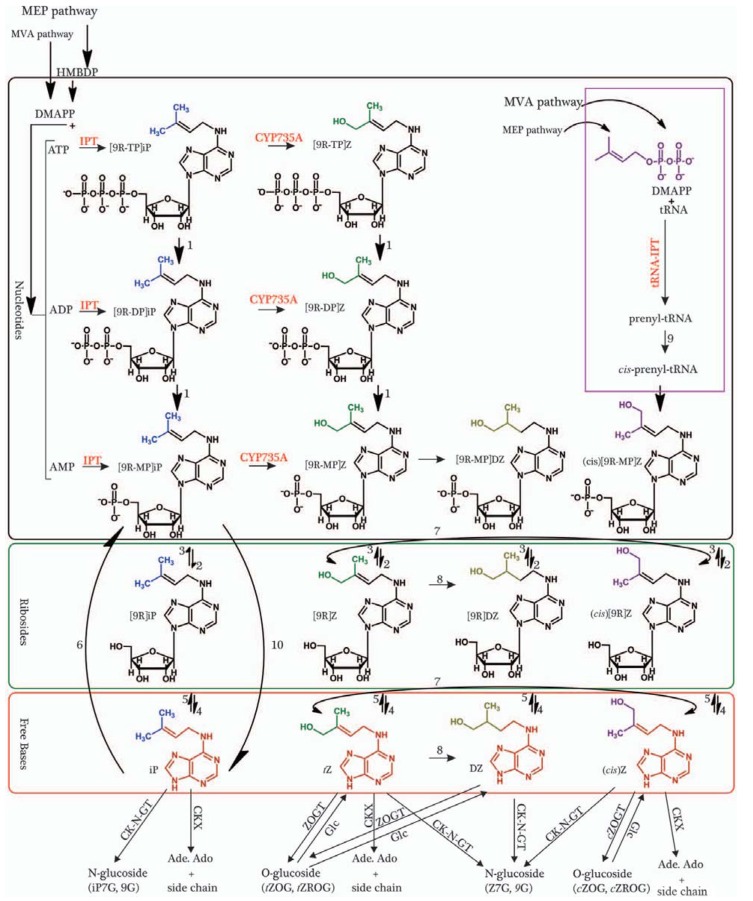
The synthesis of CKs is strongly influenced by the isopentenyltransferase (IPT) gene family. The IPT catalytic enzyme (Fig. 4) initiates the synthesis of CKs and plays a major role in controlling CK levels in plant tissues [125-127]. The IPT gene was first characterized in Agrobacterium tumefaciens, a crown gall-forming bacterium [128]. In the subsequent studies, IPT genes were identified in many different plants such as: Arabidopsis [129, 130], petunia [131], maize, and hops [132]. In soybean, the first three IPT genes discovered, were GmIPT1, GmIPT2, GmIPT3 [133, 134]. Their discovery was enabled by the sequencing of the whole soybean genome [135]. To date, 14 putative IPT genes numbered from GmIPT1 to GmIPT14 have been discovered [136]. Since the discovery of the CK biosynthesis IPT enzyme, a reverse role catalyst, CK oxidase/dehydrogenase (CKX), was shown to cause the irreversible degradation of CKs [137, 138]. These gene families appear to mechanistically control CK levels throughout much of the plant. CK levels in different tissues are regulated by CK oxidase/dehydrogenase (CKX) enzymes, which preferentially cleave isoprenoid CKs, (aromatic CKs are degraded with lower reaction rates) [139]. The enzymes are encoded by a multigene family of CKX genes that catalyze the irreversible degradation of the CKs in a single enzymatic step by oxidative side chain cleavage [138]. This gene family has been characterized in the model plant Arabidopsis thaliana [120], and some cereals such as: rice [113], maize [140, 141], wheat and barley [142]. After the entire soybean genome sequence was introduced [135], Tran and his group [136] have identified 17 putative CKX genes in soybean. The distinct expression profiles of the various genes and the subcellular localizations of these gene products suggest specialized functions adapted specifically to certain organs [143]. The proteins encoded by the CKX family can largely differ in their biochemical properties, including their particular substrate affinity for different CKs [139]. Generally the biologically activity of CKs is controlled by a balance of synthesis, catabolism, inactivation of conjugations [125, 144], CK signaling [145], and CK activation [146].
Fig. (4).
Proposed cytokinin biosynthesis and metabolic pathways in Arabidopsis with numbers representing enzymes responsible for the illustrated conversions: (1) phosphatase, (2) 5’-ribonucleotide phosphohydrolase, (3) adenosine kinase, (4) adenosine nucleosidase, (5) purine nucleoside phosphorylase, (6) adenine phosphoribosyltransferase, (7) cis-trans isomerase, (8) zeatin reductase, (9) cis-hydroxylase, (10) LOG phosphoribohydrolase (Modified from [125, 126, 146, 225]). Abbreviations: HMBDP: (E)-4-hydroxy- 3-methyl-but-2-enyl diphosphate; DMAPP: Dimethylallyl pyrophosphate; MEP: Methylerythritol phosphate pathway; MVA: the mevalonate pathway; CYP735A: CK transhydroxylase; iP: Isopentenyladenine; Z: trans Zeatin; cZ: cis Zeatin; DZ: Dihydrozeatin; [9R] iP: Isopentenyladenosine; [9R]Z: trans Zeatin riboside; [9R]Z: cis Zeatin riboside, (cis; [9R]DZ: Dihydrozeatin riboside; [9R-NT]iP: Isopentenyladenosine monophosphate; [9R-NT]Z: trans Zeatin riboside monophosphate; [9R-NT] Z: cis Zeatin riboside monophosphate, (cis); [9R-NT]DZ: Dihydrozeatin riboside monophosphate; Ado: Adenosine; Ade: Adenine; AMP: Adenosine monophosphate; ADP: Adenosine diphosphates; ATP: Adenosine triphosphates.
CK metabolites and CKX activity have been studied extensively during rice development, with the ultimate goal of manipulating yield [113]. Within the early stages of embryonic development of cereals, there is a narrow period during which CK content rises and, after which, it rapidly declines.
The decline in CK level is accompanied by an increase in CKX activity. Consequently seed filling, eventual seed size and yield are enhanced by both maintaining a higher level of CKs by lowering the level of CKX activity [24, 113, 124].
Disruption of CKX3 and CKX5 genes in Arabidopsis resulted in higher CK levels, which subsequently led to: larger inflorescences and floral meristems, increased size of the WUSCHEL expression domain, supernumerary ovules and increased seed yield of the ckx3/ckx5 double mutant [24, 124]. This suggested that more beneficial phenotypes resulted from an overabundance of CKs which brought about increased seed yield and decreased abortion rates.
In many instances, agriculturally important traits are regulated by quantitative trait loci (QTL) or traits controlled by multiple genes. Research involving isolation and deletion of the OsCKX2 gene from a QTL in different cultivars of rice (Oryza sativa, Os), allowed for altered expression of CK oxidase activity. A null mutation or partial expression of OsCKX2 enhanced the size of inflorescence meristems and increased the number of reproductive organs, resulting in greater overall grain yield [113]. A similar effect on plant productivity was obtained in barley (Hordeum vulgare, Hv) with the silencing of the HvCKX1 gene [24]. This decreased the CKX enzyme activity and led to higher plant yield. Additionally, these traits were associated with a greater root mass. These cases underline, once more, the importance of CKs in developmental processes. Likewise, the addition of Zeatin had a positive effect on the expression of genes that encode cell wall invertases and hexose transporters [147, 148]. Increased activity of sugar-associated proteins is essential for the mobilization and flow of the nutrients to the storage organs [149]. Further studies into this phenomenon showed that two out of the three studied rice cell wall invertases are strongly expressed in the aleurone layer and chalazal vasculature during the seed filling stage [150]. Thus, the reduced ability HvCKX in the regulatory aleurone layer of the filling grains [151] could lead to an increased concentration of CKs with a direct enhancement on the activity of the cell wall invertases and hexose transporters. The increased flow of sucrose through the aleurone layer then results in higher starch accumulation in the endosperm [24].
These strong lines of evidence leave no doubt that CKs play a critical role in the development of reproductive organs and seed yields [136]. Therefore, to gain greater insight into the CK metabolism in reproductive organs, authors [136] have analysed the expression of CK metabolic genes in flowers, full pods and R5 seeds. Their results shed a lot of light into the specific involvement of the 14 putative GmIPT and 17 GmCKX genes in the functional reproductive process. Findings indicate that GmIPT02 is ubiquitously expressed in all three reproductive tissues examined while five other GmIPTs (GmIPT03, 05, 08, 10 and 12) were not detected, implicating they played no role. GmIPT02 was the major transcript in flowers, GmIPT01 and 02 were expressed in the greatest abundance in full pods, while GmIPT01, 02 and 11 were the most abundant transcripts in R5 seeds. The variation in the GmIPT transcript levels in flowers, pods and R5 seeds suggested that each of these organs require different GmIPT genes, or combinations thereof, for CK biosynthesis. GmIPT02 and GmIPT11 may play more important roles in flowers and R5 seeds, respectively, while GmIPT01 and 02 appear to be equally important in full pods, based on their transcription levels. The expression levels of GmCKX genes were also determined in flowers, pods and R5 seeds. In flowers, five GmCKX mRNAs were dominant, including GmCKX04, 07, 08, 12 and 16. In contrast to the situation in flowers, GmCKX08 transcript was in highest abundance in pods. In R5 seeds, GmCKX08 was still the most highly abundant transcript, while the second most abundant was GmCKX16. Meanwhile, the expression of 9 of 17 GmCKXs had either very low levels detected or were not detected in the three reproductive tissues. GmCKX13 was highly expressed in various vegetative tissues. These data suggested that GmCKX08 is perhaps the key regulator of CK levels in reproductive organs. On the other hand, in flower tissues, the concerted actions of at least five GmCKXs are required for maintaining CK homeostasis [136]. These results are quite interesting and consistent with observed morphological patterns.
4.1.2. CK and Drought Tolerance
It is well known that CKs control many aspects of plant growth, development, and the responses of plants to abiotic and biotic stresses [121]. They prevent senescence [152] which is important considering that rapid, premature leaf senescence and death often occurs when water is limited during the grain-filling period. Genotypes possessing a stay-green phenotype and more photosynthetically active traits which lead to stress tolerance, have been shown to contain elevated levels of CKs in xylem sap [153]. A significant amount of evidence indicates that appropriate manipulation of CK levels may enhance tolerance to drought stress [154-156]. On one end of the CK spectrum, a reduction of CK levels by the transgenic overexpression of CKX genes in roots promotes primary root elongation and root branching, resulting in an increase in root biomass for improvement of drought tolerance [136, 157]. At the other end, drought stress has been shown to accelerate leaf senescence, which is associated with a decrease in CK content and suppression of CK signaling [119, 158, 159]. But an overproduction of CKs during plant maturation, just prior to the onset of senescence, significantly increased drought tolerance with minimal yield loss due to the delay of drought-induced senescence associated with this pre-programmed increase in CK levels [156, 160, 161].
Analogously, transgenic cassava plants over-expressing CKs show significant drought tolerance as a result of stay-green capacity under stress [162]. The CK biosynthesis gene IPT, has now been over-expressed in several plant species under different promoters, and the resulting transgenic plants have demonstrated improved stress tolerance [163], including field analyses [121, 161, 164]. The localized expression in tobacco (Nicotiana tabacum) of a promoter-less IPT, enhanced the local sink strength and quickly mobilized nutrients to the tissues with elevated CKs [165]. Changes in source – sink relationships were also observed in CK–deficient tobacco shoots and roots [166]. Elevated CK levels enhanced the survival of plants under water-stress conditions [156]. The overexpression of IPT under the control of senescence associated receptor kinase (SARK; a maturation and stress-induced promoter) improved the drought tolerance of both eudicots [156, 161] and monocots [154]. During the reproductive stages (pre- and post-anthesis) and after a water-stress episode, the transgenic PSARK::IPT rice plants displayed higher grain yield than the wild type [154]. The transgenic PSARK::IPT rice exhibited a differential expression of the genes encoding enzymes associated with hormone synthesis and hormone-regulated pathways. These results suggested that the modification of source/sink relationships was induced by the changes in hormone homeostasis in the transgenic plants, resulting in higher grain yields under stress conditions [154]. Plant hormones affect, either directly or indirectly, these pathways and appear to be acting antagonistically or synergistically when responding to environmental stress [164].
Under drought stress, total plant CK content decreases, and this reduction in CKs stimulates the plants to over-produce ABA [167], inducing stomata closure and inhibiting photosynthesis [168]. This CK – ABA relationship has been reviewed in many models. Such findings suggest, stress-induced CK synthesis, driven by a stress-induced promoter, protected against the deleterious effects of water deficit on the photosynthetic apparatus, allowing higher photosynthetic rates and higher yields after water deficit in tobacco [160] and cotton (Gossypium hirsutum) plants grown in the greenhouse [169] and peanut (Arachis hypogaea) plants grown under field conditions [161].
4.1.3. Cytokinin and the Modification of FA Profile in Seeds
Seeds are a rich source of CKs. The first natural occurring CK was detected within seeds, and the highest levels of CKs documented to date have been found in seeds [23]. This, in itself spurs efforts to investigate the role of these hormones and their biochemical pathways during seed development and maturation. In soybean and other legumes, maturation is a critical time when the ultimate market value is established via nutrients, FAs/oils and other dry matter accumulations. Almost a half century ago, research began to investigate the possibility of a link between hormone regulation and FAs/oils synthesis in plant tissues, It was in the 1970s, when several reports [170-172] stated that the synthesis of lipids in leaves was promoted by kinetin. In 1974 Ulrich and Roland`s study was conducted that found kinetin inhibited the degradation of FAs in spinach chloroplasts. Furthermore, treatment of spinach leaves with kinetin and zeatin for a period of four weeks led to an increase in C18:3 and a decrease of C16:0 [172]. A similar study was conducted on soybean callus resulting in congruent findings [171].
To the best of our knowledge, and surprisingly, the potential link between CKs and FAs has not been strongly pursued. More recently the investigation has been rekindled. For example, the incentive for enhanced seed filling [124] and increased crop productivity [113, 124] was the impetus for a CK manipulation through the use of an IPT gene coupled with over-expression of the KASHIII enzyme. This resulted in modification of the FA profile of tobacco plants [173] which raised the interest, once again, about the role of hormones in FA/oil synthesis.
These above studies have shown there is a real possibility in manipulating FA biosynthesis in soybean using the application of exogenous or manipulation of endogenous plant hormones and we speculate that CK plays a crucial role in FA composition.
4.2. ABA Metabolism in Seed Development
ABA was discovered by three independent research groups [174]. It was called as “abscisin” as well as “dormin” and later was relabelled “abscisic acid” and the abbreviation “ABA” was agreed at the Sixth International Congress on the Plant Growth Substances in 1967 [175]. ABA belongs to a class of metabolites known as isoprenoids, also called terpenoids [35]. ABA occurs in all higher plants, ferns, mosses, liverworts, and algae. As the result of the universal distribution in the plant kingdom and many physiological studies, ABA has been classified as a plant hormone [174]. ABA regulates many physiological processes including: germination, growth, flowering, seed maturation, senescence, ABA is also implicated in adaptation to various environmental stresses such as: water deficiency, low temperature, and freezing [35, 176]. Exogenous ABA shows unique activities in plants, which reflect its physiological role as a hormone.
Seeds are rich in ABA which may be imported from the leaves or in situ [177]. During seed development, ABA has a triple role in the entire embryo growth, as deduced from the physiological analysis of ABA-deficient mutants. In early embryogenesis, ABA can act as a promoter to prevent seed abortion and promote embryo growth [18, 30]. ABA is very active and classically associated with seed maturation in order to control seed filling and promote the synthesis of seed proteins. ABA stimulates dormancy and inhibits germination in the matured seeds by positively regulating its own levels and down-regulating GA synthesis [35, 36]. In the early stages of embryogenesis, ABA content is quite high. After the first stage (R1), its level goes down from R2 to R4 before finally increasing and reaching its peak at R5 in 10 tested soybean varieties (K Coates, N Emery unpublished data). ABA is associated with seed maturation and control of seed filling. In addition to protein synthesis promotion, there is significant evidence derived from exogenous ABA treatments which suggest that ABA also plays an important role in FA accumulation during seed maturation.
Studies with microspore-derived embryos of rapeseed have shown that oil synthesis was stimulated by 50% with the addition of ABA to the incubation medium. The exogenous ABA induced expression of oleosin, and FA elongase genes, and increased the accumulation of TAGs and monounsaturated FAs including eicosenoic acid (C20:1) and erucic acid (C22:1) [178]. Furthermore, preliminary studies with Arabidopsis mutants, which are both ABA synthesis-deficient and ABA-insensitive, have shown that these mutants accumulate considerably less lipids than the wild type [179, 180]. In vitro model systems, such as cell-suspension cultures of carrot and somatic embryos of carrot [181] or white spruce [182] have shown that the deposition of storage lipids is favored by the presence of ABA and/or by high osmoticum. The application of exogenous ABA or high osmoticum has been found to promote the accumulation of seed specific storage lipids containing C20:1 and C22:1 in developing zygotic embryos of rape cultured in vitro [183]. Moreover, cultured embryos accumulated significantly less C18:1 and had higher levels of C18:3 than embryos that remained on the plant, regardless of exogenous ABA levels [183]. This shows a crucial function of exogenous ABA in enhancing FA biosynthesis in Arabidopsis, white spruce or rape. Recently ABA was shown to have a significant effect on the increase of lipid production of a microalga known as Chlamydomonas reinhardtii [184]. All these earlier promising results further inspire the effort to clarify the role of endogenous ABA in FA biosynthesis in soybean seeds.
4.2.1. Sucrose-ABA Interaction: A Trigger of Seed Maturation
The transition of embryos into the maturation phase is triggered by both sugars and ABA and their interactions. Sugar responses are linked to those of ABA [13, 111]. Alternatively, ABA could enhance the ability to respond to sugar signals [185]. Transcript levels of ABA biosynthesis genes in Arabidopsis leaves are raised by low concentrations of glucose, indicating a nutritional control on ABA biosynthesis [186]. In addition, certain stress conditions can play a role. Sucrose has a dual function as transport and nutrient sugar and as a signal molecule triggering storage-associated processes [187, 188]. Several lines of evidence suggest that sucrose can induce storage-associated gene expression. Before maturation, young embryos contain moderately low levels of sucrose. At maturation actively elongating and starch-accumulating cells contain the highest sucrose concentrations, which are correlated with transcripts of storage-associated enzymes [189, 190]. Sucrose acts at the transcriptional level causing up-regulation of enzymes like sucrose synthase and adenosine diphosphoglucose pyrophosphorylase (ADPG-pyrophosphorylase) [215]. The genes for the enzymes sucrose-phosphate synthase (SPS) and phosphoenolpyruvate carboxylase (PEPC) are repressed and induced, respectively, in response to sucrose [216]. In vitro sucrose feeding disrupts the meristematic state, induces cell expansion and endopolyploidization in young explanted cotyledons [191-193], and promotes cotyledonary storage activity at the transcript level [194, 195]. In legume seeds a switch of the principal sugars from hexoses to sucrose initiates embryo maturation. The switch could result in nutrient stress and/or energy limitation, which may stimulate ABA synthesis [196]. The data indicate that impaired storage metabolism in seeds and tubers is due to decreased sucrose levels rather than to hexose accumulation. Together, these findings suggest that sucrose, together with ABA, signals the transition of embryo into the storage mode. Consequently, stimulation of lipid synthesis could be achieved by increased sucrose unloading which stimulates acetyl-CoA carboxylase (ACC), a starting enzyme in FA biosynthesis pathway [197].
4.2.2. ABA Metabolism in Drought Stress
Exposure to environmental stresses, such as drought, causes adverse effects on the growth of plants and the production of crops. It leads to the decrease of seed filling/maturation duration and, consequently, FA accumulation is reduced. ABA has long been recognized as a plant hormone that is up-regulated in response to the soil water deficit around roots. Drought osmotically stresses the organisms, and this causes dehydration and inhibition of water uptake in plants. ABA accumulates under osmotic stress conditions and plays an important role in the stress response and tolerance of plants [6, 198]. It rapidly limits transpiration within hours or even minutes [199] through its well-known ability to induce stomatal closure by guard cell turgor and reduced leaf/canopy expansion to maintain water temporary in plants as a first response to the drought condition.
ABA has a reported involvement in another process by which a plant could escape drought stress. Adaptive ‘deeper’ root growth, and other aspects of architectural modification, under drought [200, 201] are activated by means of ABA signals. In addition, ABA-induced increases in hydraulic conductivity via improved aquaporin functioning and/or osmotic regulation will tend to oppose stomatal closure in the long run, or indeed may fully overcome it [202, 203], potentially leading to sustained growth under stress. However, if the plant could not successfully overcome the water deficit, ABA would trigger early leaf senescence which leads to a significant yield loss in soybean and other grain crops. Hence, manipulation of ABA signal via advance agricultural practices and genetic improvement breeding programs are become promising approaches to overcome drought or other abiotic stresses, and consequently improve crop yields with increased/sustained oil accumulation under stresses.
Advances in ABA signaling science have already allowed refined development of crop management techniques for reducing irrigation input, exemplified by the form of deficit irrigation known as partial root-zone drying (PRD), which is practiced in parts of Southern Europe and China. The partial water deficit induced ABA signals from the dry root area and lead to a reduction in transpiration rate. As discussed above, ABA started to enhance the water “hunting” process with roots in plants to sustain yields under reduced irrigation. Other potential management techniques involving altered ABA signaling could include the use of relatively acidic foliar sprays to reduce stomatal ABA sensitivity [36] or soil/seed inoculation with ABA producing or suppressing bacteria, and the search for these is fully active [204].
In another approach, breeding for changes in ABA biology (increased concentration or perception) that increase field performance and yield in crop species has been attracting a lot attention [205, 206]. The control of the expression of ABA signaling factors may improve tolerance to environmental stresses. Several groups reported the transgenic plant overexpressing AREB/ABFs showed improved drought tolerance and yields under drought conditions [206-208]. Engineering the constitutive over-expression of LeNCED1, an enzyme that catalyses a rate-limiting reaction in the synthesis of ABA in tomato, resulted in increased ABA accumulation and drought tolerance [205]. Drought-inducible over-expression of the gene ABA3/LOS5 (integral to ABA biosynthesis) in field-grown rice resulted in significantly increased yields [209]. Using numerous drought-inducible promoters (from Arabidopsis) for increased plant ABA, also resulted in enhanced yield [210]. However, this approach remains a great challenge, because of the necessity for avoiding issues such as: inadvertent/concomitant reductions in carbon gain upon stomatal closure, ABA-induced reductions in seed germination, and ABA-induced pollen sterility and abortion [211]. In addition to the complexities arising from opposing direct and indirect effects of ABA itself on stomatal aperture and shoot growth rates, other problems with breeding for manipulation of ABA biology can potentially arise through interactions between ABA and other hormones or signaling molecules, such as ethylene and CKs. In fact, the interactions of ABA signals with other hormone signaling pathways were reviewed in Arabidopsis [28]. Other plant hormones such as CKs auxin, and ethylene, may alter the effectiveness of ABA to modify its target cells directly, or indirectly by altering its biosynthesis [36].
4.3. Nutrient Source and Sink Relationships in Seed Development and the Roles of Plant Hormones
Limitations to crop yields are frequently attributed to two factors: 1) the source of assimilates or 2) the sinks which are the sites of assimilate utilization. These divisions recognize the two major processes involved in the determination of seed yield: the assimilate supply in the leaves or roots and utilization of those assimilates by the developing seeds.
Source – sink relationships also play an important role during embryogenesis [23, 117, 212-214]. In this process, a developed embryo with a constant number of cells has been set and this number will not change during embryo or seed maturation. It marks a sink limitation in seed filling since dry matter can only accumulate into the existing number of cells in a seed and at certain seed growth rates (SGR). Under ideal conditions, the nutrient source saturates cell demand and the sink sets a maximum potential number of cells in the embryo. In this case, the storage capacity is maximized, and the seed filling depend on the SGR which now becomes the major sink limitation. The SGR would vary among different soybean genotypes and when optimized a maximum grain yield could be achieved. The SGR is significantly impacted by cell signals such as hormones. For example ABA accompanied with sucrose trigger seed maturation processes [111] to start dry matter accumulation in seed and seed desiccation. Later ABA interacts with GAs to put the seed into dormancy [6]. ABA also enhances the seed storage of protein [35], while CKs and brassinosteroids (BRs) participate in the promotion of the seed filling process [112, 113].
The duration of seed maturation and dry matter accumulation is another important parameter in seed development that is affected by hormones. It shows a strong relationship with SGR and the availability of photosynthates. In an ideal growth condition, the assimilate supply – from photosynthesis in leaves and in seeds could reach the optimum level and this will depend on the genotype. This highest assimilate level become a limitation if the assimilate demand exceeds what the plant could supply. For instance when a huge number of seeds is produced in a variety, its seed size is likely small because of the source limitation. The highest yielding soybean varieties would have a medium seed size, a large number of seeds, long lasting photosynthetically active foliage and a maximum SGR operating during the longest possible seed filling period.
Focusing on sources and sinks provides a simple two-component model system; unfortunately, this type of analysis does not always clearly identify the yield-limiting processes [214]. Modification of source activity of soybean during flowering and pod set usually results in a corresponding change in pod and seed number, indicating a source limitation. Increasing canopy photosynthesis by increasing levels of atmospheric CO2 [215] or irradiance [216] increases pod and seed number, while reducing photosynthesis by shading [217, 218] or defoliation [219] decreases pod and seed number. The effects of source-sink dynamics during seed filling, after pod and seed number are fixed, are more complex. Depodding to increase assimilate supply to the remaining seeds usually increases seed size (weight per seed), but does not always change individual SGR’s [214]. Reducing assimilate supply by defoliation reduced seed size, but the effect of shade was more variable [213, 214]. Source limitations during seed filling seem to be relatively common based on changes in seed size, but SGR is not as responsive to changes in source activity and can be more sink limited. Recent analysis of changes in starch levels in soybean leaves during seed filling supports this contention by suggesting that intermittent sink limitations can occur [214].
The response of the sink (the seed) to source-sink alterations during seed filling depends upon the effects of the assimilate levels in the seed and the ability of the seed to respond to a change in assimilate supply [220]. The response could be manifested in changes in SGR or seed fill duration, with interaction of those two factors ultimately determining final seed size. The response of seed fill duration to assimilate availability is not well known [214]. However, studies with in vitro culture systems demonstrated that SGR exhibits a classic saturation response to increased sucrose concentrations [221, 222]. Unfortunately, the effect of gross manipulations of source-sink ratios in planta on seed sucrose levels are rarely reported, which makes it difficult to extrapolate in vitro results to those of whole plants [223]. The results suggest that soybean SGR is generally sink limited if photosynthesis increases during seed filling, but source limited if photosynthesis is reduced [224, 225].
CK regulation and seed size: CK helps to accelerate cell divisions and interacts with GA mainly for nutritional responses during embryogenesis. It means CK signals (together with genotype dependence) play a big part in determining how many cells will be produced during embryogenesis or, on the other hand, to enhance the embryogenesis to match its developmental potential for a larger seed size. CK is also well known to help augment source activity via enhancing and regulating photosynthesis while concomitantly strengthening the sink during embryogenesis [117, 125-127].
Previous studies report that seed size and oil body accumulation have a positive correlation [66]. The larger seeds become, the more oil-bodies accumulate so that a higher oil content is obtained with large seed varieties. Most of the lipid in soybean seeds is stored in the cotyledons in the form of triglycerides packaged in specialized structures, often referred to as oil bodies or spherosomes [25]. Cytological studies of soybean embryogenesis have shown that cell division in the seed is completed at an early stage of development (R4) while the embryo is still quite small [25]. The major increase in seed size occurs from the beginning of R5 to the end of R6 and is brought about through enlargement of preexisting cells [32]. During this period of cell enlargement, the majority of the oil, protein, and carbohydrate synthesis and storage in the seed occurs. Thus, the enlarging seed must simultaneously partition its photosynthate among three major reserves.
In summary, plant hormone signals have significant roles in all source – sink regulations that lead to set patterns of seed maturation during seed development. If plant hormone signals could be optimized, seed development could be matched to its development potential to achieve better seeds. It is not a simple model of source – sink relationships and plant hormone regulation; but, rather it is a complex network of these limitations. With a deep knowledge in seed development regulation as well as storage accumulation, an optimization for every step throughout a whole plant development cycle will be a target for breeder to get a better grain crop.
SUMMARY
Soybean is one of the most important oilseed crops and facets of its seed development determine its value. The progression, from the beginning of flowering stage through the embryogenesis until seed maturation and desiccation as well as its regulators, hormonal and non-hormonal factors decide the crop quality and productivity. Environmental stresses have always had the big impact on seed development generally and seed filling particularly, which leads to significant changes at the harvest stage. As mentioned above, seed development, FA accumulation and their hormonal and non-hormonal regulations have been revealed separately, but there is a gap between our understanding of their mechanisms and the possible improvements and manipulations of these processes. In order to fully control seed development in planta from which can stabilize and enhance the crop production of desire products, a deeper understanding in optimization and customization of seed development related processes is crucial.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Carlsson A.S., Yilmaz J.L., Green A.G., Stymne S., Hofvander P. Replacing fossil oil with fresh oil - with what and for what? Eur. J. Lipid Sci. Technol. 2011;113(7):812–831. doi: 10.1002/ejlt.201100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall J. Who Needs Oil? New Sci. 2007;195:28–31. doi: 10.1016/S0262-4079(07)61712-6. [DOI] [Google Scholar]
- 3.SoyStats. International: World Oilseed Production 2014. http://soystats.com/international-world-oilseed-production/ [(accessed May 19, 2015].
- 4.Pham A-T., Lee J-D., Shannon J.G., Bilyeu K.D. Mutant alleles of FAD2-1A and FAD2-1B combine to produce soybeans with the high oleic acid seed oil trait. BMC Plant Biol. 2010;10:195. doi: 10.1186/1471-2229-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fehr W.R., Caviness C.E., Burmood D.T., Pennington J.S. Stage of Development Descriptions for Soybeans, Glycine Max (L.) Merrill. Crop Sci. 1971;11:929–931. doi: 10.2135/cropsci1971.0011183X001100060051x. [DOI] [Google Scholar]
- 6.Finkelstein R.R. The Role of Hormones during Seed Development and Germination. Plant Horm; 2010. pp. 549–573. [DOI] [Google Scholar]
- 7.Fehr W.R., Caviness C.E. Stages of Soybean Development. Iowa: Iowa State University of Science and Technology Ames; 1977. [Google Scholar]
- 8.Bils R.F., Howell R.W. Biochemical and Cytological Changes in Developing Soybean Cotyledons1. Crop Sci. 1963;3:304–308. doi: 10.2135/cropsci1963.0011183X000300040008x. [DOI] [Google Scholar]
- 9.Rubel A., Rinne R.W., Canvin D.T. Protein, Oil, and Fatty Acid in Developing Soybean Seeds. Crop Sci. 1972;12:739–741. doi: 10.2135/cropsci1972.0011183X001200060006x. [DOI] [Google Scholar]
- 10.Bewley J.D., Black M. Physiology and Biochemistry of Seeds in Relation to Germination. 1982. [DOI] [Google Scholar]
- 11.Fraser J., Egli D.B., Leggett J.E. Pod and Seed Development in Soybean Cultivars with Differences in Seed Size. Agron. J. 1982;74:81–85. doi: 10.2134/agronj1982.00021962007400010022x. [DOI] [Google Scholar]
- 12.Spaeth S.C., Sinclair T.R. Soybean Seed Growth II. Individual Seed Mass and Component Compensation. Agron. J. 1984;76:128–133. doi: 10.2134/agronj1984.00021962007600010031x. [DOI] [Google Scholar]
- 13.Gibson S.I. Sugar and phytohormone response pathways: navigating a signalling network. J. Exp. Bot. 2004;55(395):253–264. doi: 10.1093/jxb/erh048. [DOI] [PubMed] [Google Scholar]
- 14.Wobus U., Weber H. Seed maturation: genetic programmes and control signals. Curr. Opin. Plant Biol. 1999;2(1):33–38. doi: 10.1016/S1369-5266(99)80007-7. [DOI] [PubMed] [Google Scholar]
- 15.Berger F. Endosperm: the crossroad of seed development. Curr. Opin. Plant Biol. 2003;6(1):42–50. doi: 10.1016/S1369526602000043. [DOI] [PubMed] [Google Scholar]
- 16.Audran C., Liotenberg S., Gonneau M., North H., Frey A., Tap-Waksman K., Vartanian N., Marion-Poll A. Localisation and Expression of Zeaxanthin Epoxidase mRNA in Arabidopsis in Response to Drought Stress and during Seed Development. Funct. Plant Biol. 2001;28:1161–1173. doi: 10.1071/PP00134. [DOI] [Google Scholar]
- 17.Bewley J.D., Bradford K., Hilhorst H. Seeds: Physiology of Development, Germination and Dormancy. Springer; 2012. [Google Scholar]
- 18.Souter M., Lindsey K. Polarity and signalling in plant embryogenesis. J. Exp. Bot. 2000;51(347):971–983. doi: 10.1093/jexbot/51.347.971. [DOI] [PubMed] [Google Scholar]
- 19.Vogler H., Kuhlemeier C. Simple hormones but complex signalling. Curr. Opin. Plant Biol. 2003;6(1):51–56. doi: 10.1016/S1369-5266(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 20.Cheng W-H., Endo A., Zhou L., Penney J., Chen H.C., Arroyo A., Leon P., Nambara E., Asami T., Seo M., Koshiba T., Sheen J. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell. 2002;14(11):2723–2743. doi: 10.1105/tpc.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frey A., Godin B., Bonnet M., Sotta B., Marion-Poll A. Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia. Planta. 2004;218(6):958–964. doi: 10.1007/s00425-003-1180-7. [DOI] [PubMed] [Google Scholar]
- 22.Jones R.J., Brenner M.L. Distribution of Abscisic Acid in Maize Kernel during Grain Filling. Plant Physiol. 1987;83(4):905–909. doi: 10.1104/pp.83.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery R.J., Ma Q., Atkins C.A. The forms and sources of cytokinins in developing white lupine seeds and fruits. Plant Physiol. 2000;123(4):1593–1604. doi: 10.1104/pp.123.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zalewski W., Galuszka P., Gasparis S., Orczyk W., Nadolska-Orczyk A. Silencing of the HvCKX1 gene decreases the cytokinin oxidase/dehydrogenase level in barley and leads to higher plant productivity. J. Exp. Bot. 2010;61(6):1839–1851. doi: 10.1093/jxb/erq052. [DOI] [PubMed] [Google Scholar]
- 25.Ohlrogge J.B., Kuo T-M. Control of Lipid Synthesis during Soybean Seed Development: Enzymic and Immunochemical Assay of Acyl Carrier Protein. Plant Physiol. 1984;74(3):622–625. doi: 10.1104/pp.74.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung J., Babka H.L., Graef G.L., Staswick P.E., Lee D.J., Cregan P.B., Shoemaker R.C., Specht J.E. The Seed Protein, Oil, and Yield QTL on Soybean Linkage Group I. Crop Sci. 2003;43:1053. doi: 10.2135/cropsci2003.1053. [DOI] [Google Scholar]
- 27.Karssen C.M., Brinkhorst-van der Swan D.L., Breekland A.E., Koornneef M. Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta. 1983;157(2):158–165. doi: 10.1007/BF00393650. [DOI] [PubMed] [Google Scholar]
- 28.Finkelstein R.R., Gampala S.S., Rock C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14(Suppl.):S15–S45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Audran C., Borel C., Frey A., Sotta B., Meyer C., Simonneau T., Marion-Poll A. Expression studies of the zeaxanthin epoxidase gene in nicotiana plumbaginifolia. Plant Physiol. 1998;118(3):1021–1028. doi: 10.1104/pp.118.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg R.B., De Paiva G., Yadegari R. Plant Embryogenesis: Zygote to Seed. Sci. York Then Washington; 1994. p. 605. [DOI] [PubMed] [Google Scholar]
- 31.Harada J.J. Cellular and molecular biology of plant seed development. Springer; 1997. Seed Maturation and Control of Germination. pp. 545–592. [DOI] [Google Scholar]
- 32.Dornbos D.L., McDonald M.B. Mass and Composition of Developing Soybean Seeds at Five Reproductive Growth Stages. Crop Sci. 1986;26:624–630. doi: 10.2135/cropsci1986.0011183X002600030042x. [DOI] [Google Scholar]
- 33.Gazzarrini S., Tsuchiya Y., Lumba S., Okamoto M., McCourt P. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev. Cell. 2004;7(3):373–385. doi: 10.1016/j.devcel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Nambara E., Hayama R., Tsuchiya Y., Nishimura M., Kawaide H., Kamiya Y., Naito S. The role of ABI3 and FUS3 loci in Arabidopsis thaliana on phase transition from late embryo development to germination. Dev. Biol. 2000;220(2):412–423. doi: 10.1006/dbio.2000.9632. [DOI] [PubMed] [Google Scholar]
- 35.Nambara E., Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson S., Davies W.J. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ. 2010;33(4):510–525. doi: 10.1111/j.1365-3040.2009.02052.x. [DOI] [PubMed] [Google Scholar]
- 37.Pham A-T., Lee J-D., Shannon J.G., Bilyeu K.D. A novel FAD2-1 A allele in a soybean plant introduction offers an alternate means to produce soybean seed oil with 85% oleic acid content. Theor. Appl. Genet. 2011;123(5):793–802. doi: 10.1007/s00122-011-1627-3. [DOI] [PubMed] [Google Scholar]
- 38.Jokic´ S., Sudar R., Svilovic´ S., Vidovic´ S., Bilic´ M., Velic´ D., Jurkovic´ V. Fatty Acid Composition of Oil Obtained from Soybeans by Extraction with Supercritical Carbon Dioxide. Czech J. Food Sci. 2013;31:116–125. [Google Scholar]
- 39.Wang Y., Frei M. Stressed Food – The Impact of Abiotic Environmental Stresses on Crop Quality. Agric. Ecosyst. Environ. 2011;141:271–286. doi: 10.1016/j.agee.2011.03.017. [DOI] [Google Scholar]
- 40.Ashmore M., Toet S., Emberson L. Ozone -- a significant threat to future world food production? New Phytol. 2006;170(2):201–204. doi: 10.1111/j.1469-8137.2006.01709.x. [DOI] [PubMed] [Google Scholar]
- 41.Feng Z., Kobayashi K. Assessing the Impacts of Current and Future Concentrations of Surface Ozone on Crop Yield with Meta-Analysis. Atmos. Environ. 2009;43:1510–1519. doi: 10.1016/j.atmosenv.2008.11.033. [DOI] [Google Scholar]
- 42.Wassmann R., Jagadish S.V., Heuer S., Ismail A., Redona E., Serraj R., Singh R.K., Howell G., Pathak H., Sumfleth K. Chapter 2 Climate Change Affecting Rice Production: The Physiological and Agronomic Basis for Possible Adaptation Strategies. Adv. Agron. 2009;101:59–122. doi: 10.1016/S0065-2113(08)00802-X. [DOI] [Google Scholar]
- 43.Lobell D.B., Burke M.B., Tebaldi C., Mastrandrea M.D., Falcon W.P., Naylor R.L. Prioritizing climate change adaptation needs for food security in 2030. Science. 2008;319(5863):607–610. doi: 10.1126/science.1152339. [DOI] [PubMed] [Google Scholar]
- 44.Wassmann R., Jagadish S.V., Sumfleth K., Pathak H., Howell G., Ismail A., Serraj R., Redona E., Singh R.K., Heuer S. Chapter 3 Regional Vulnerability of Climate Change Impacts on Asian Rice Production and Scope for Adaptation. Adv. Agron. 2009;102:91–133. doi: 10.1016/S0065-2113(09)01003-7. [DOI] [Google Scholar]
- 45.Morgan P.B., Bernacchi C.J., Ort D.R., Long S.P. An in vivo analysis of the effect of season-long open-air elevation of ozone to anticipated 2050 levels on photosynthesis in soybean. Plant Physiol. 2004;135(4):2348–2357. doi: 10.1104/pp.104.043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sánchez-Rodríguez E., del Mar Rubio-Wilhelmi M., Cervilla L.M., Blasco B., Rios J.J., Leyva R., Romero L., Ruiz J.M. Study of the Ionome and Uptake Fluxes in Cherry Tomato Plants under Moderate Water Stress Conditions. Plant Soil. 2010;335:339–347. doi: 10.1007/s11104-010-0422-2. [DOI] [Google Scholar]
- 47.Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 48.Kangasjärvi J., Jaspers P., Kollist H. Signalling and Cell Death in Ozone-Exposed Plants. Plant Cell Environ. 2005;28:1021–1036. doi: 10.1111/j.1365-3040.2005.01325.x. [DOI] [Google Scholar]
- 49.Yamakawa H., Hirose T., Kuroda M., Yamaguchi T. Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiol. 2007;144(1):258–277. doi: 10.1104/pp.107.098665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frei M., Tanaka J.P., Chen C.P., Wissuwa M. Mechanisms of ozone tolerance in rice: characterization of two QTLs affecting leaf bronzing by gene expression profiling and biochemical analyses. J. Exp. Bot. 2010;61(5):1405–1417. doi: 10.1093/jxb/erq007. [DOI] [PubMed] [Google Scholar]
- 51.Palese A.M., Nuzzo V., Favati F., Pietrafesa A., Celano G., Xiloyannis C. Effects of Water Deficit on the Vegetative Response, Yield and Oil Quality of Olive Trees (Olea Europaea L., Cv Coratina) Grown under Intensive Cultivation. Sci. Hortic. (Amsterdam) 2010;125:222–229. doi: 10.1016/j.scienta.2010.03.025. [DOI] [Google Scholar]
- 52.Liu B., Liu X., Li Y-S., Herbert S.J. Effects of Enhanced UV-B Radiation on Seed Growth Characteristics and Yield Components in Soybean. F. Crop. Res. 2013;154:158–163. doi: 10.1016/j.fcr.2013.08.006. [DOI] [Google Scholar]
- 53.Huyuan F., Lizhe A., Shuyan C., Xunling W., Guodong C. The Interactive Effects of Enhanced UV-B Irradiation and Water Deficit on Physiological Properties of Spring Wheat Seedling. Acta Ecol. Sin. 2002;22:1564–1568. [Google Scholar]
- 54.Fatihi A., Zbierzak A.M., Dörmann P. Alterations in seed development gene expression affect size and oil content of Arabidopsis seeds. Plant Physiol. 2013;163(2):973–985. doi: 10.1104/pp.113.226761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baghalian K., Haghiry A., Naghavi M.R., Mohammadi A. Effect of Saline Irrigation Water on Agronomical and Phytochemical Characters of Chamomile (Matricaria Recutita L.). Sci. Hortic. (Amsterdam) 2008;116:437–441. doi: 10.1016/j.scienta.2008.02.014. [DOI] [Google Scholar]
- 56.Wardlaw I.F., Blumenthal C., Larroque O., Wrigley C.W. Contrasting Effects of Chronic Heat Stress and Heat Shock on Kernel Weight and Flour Quality in Wheat. Funct. Plant Biol. 2002;29:25. doi: 10.1071/PP00147. [DOI] [PubMed] [Google Scholar]
- 57.Rondanini D., Savin R., Hall A.J. Dynamics of Fruit Growth and Oil Quality of Sunflower (Helianthus Annuus L.) Exposed to Brief Intervals of High Temperature during Grain Filling. F. Crop. Res. 2003;83:79–90. doi: 10.1016/S0378-4290(03)00064-9. [DOI] [Google Scholar]
- 58.Bongi G., Long S.P. Light-Dependent Damage to Photosynthesis in Olive Leaves during Chilling and High Temperature Stress. Plant Cell Environ. 1987;10:241–249. [Google Scholar]
- 59.Dornbos D.L., Mullen R.E. Soybean Seed Protein and Oil Contents and Fatty Acid Composition Adjustments by Drought and Temperature. J. Am. Oil Chem. Soc. 1992;69:228–231. doi: 10.1007/BF02635891. [DOI] [Google Scholar]
- 60.Flagella Z., Rotunno T., Tarantino E., Di Caterina R., De Caro A. Changes in Seed Yield and Oil Fatty Acid Composition of High Oleic Sunflower (Helianthus Annuus L.) Hybrids in Relation to the Sowing Date and the Water Regime. Eur. J. Agron. 2002;17:221–230. doi: 10.1016/S1161-0301(02)00012-6. [DOI] [Google Scholar]
- 61.Dwivedi S.L., Nigam S.N., Rao R.C., Singh U., Rao K.V. Effect of Drought on Oil, Fatty Acids and Protein Contents of Groundnut (Arachis Hypogaea L.) Seeds. F. Crop. Res. 1996;48:125–133. doi: 10.1016/S0378-4290(96)01027-1. [DOI] [Google Scholar]
- 62.Bettaieb I., Zakhama N., Wannes W.A., Kchouk M.E., Marzouk B. Water Deficit Effects on Salvia Officinalis Fatty Acids and Essential Oils Composition. Sci. Hortic. (Amsterdam) 2009;120:271–275. doi: 10.1016/j.scienta.2008.10.016. [DOI] [Google Scholar]
- 63.Di Caterina R., Giuliani M.M., Rotunno T., De Caro A., Flagella Z. Influence of Salt Stress on Seed Yield and Oil Quality of Two Sunflower Hybrids. Ann. Appl. Biol. 2007;151:145–154. doi: 10.1111/j.1744-7348.2007.00165.x. [DOI] [Google Scholar]
- 64.Chartzoulakis K.S. Salinity and Olive: Growth, Salt Tolerance, Photosynthesis and Yield. Agric. Water Manage. 2005;78:108–121. doi: 10.1016/j.agwat.2005.04.025. [DOI] [Google Scholar]
- 65.Ahmad S., Anwar F., Hussain A.I., Ashraf M., Awan A.R. Does Soil Salinity Affect Yield and Composition of Cottonseed Oil? J. Am. Oil Chem. Soc. 2007;84:845–851. doi: 10.1007/s11746-007-1115-8. [DOI] [Google Scholar]
- 66.Ben Taarit M., Msaada K., Hosni K., Marzouk B. Changes in Fatty Acid and Essential Oil Composition of Sage (Salvia Officinalis L.) Leaves under NaCl Stress. Food Chem. 2010;119:951–956. doi: 10.1016/j.foodchem.2009.07.055. [DOI] [Google Scholar]
- 67.Neffati M., Marzouk B. Changes in Essential Oil and Fatty Acid Composition in Coriander (Coriandrum Sativum L.) Leaves under Saline Conditions. Ind. Crops Prod. 2008;28:137–142. doi: 10.1016/j.indcrop.2008.02.005. [DOI] [Google Scholar]
- 68.Maestri D.M., Labuckas D.O., Meriles J.M., Lamarque A.L., Zygadlo J.A., Guzmán C.A. Seed Composition of Soybean Cultivars Evaluated in Different Environmental Regions. J. Sci. Food Agric. 1998;77:494–498. doi: 10.1002/(SICI)1097-0010(199808)77:4<494::AID-JSFA69>3.0.CO;2-B. [DOI] [Google Scholar]
- 69.Izquierdo N.G., Aguirrezábal L.A., Andrade F.H., Cantarero M.G. Modeling the Response of Fatty Acid Composition to Temperature in a Traditional Sunflower Hybrid. Agron. J. 2006;98:451. doi: 10.2134/agronj2005.0083. [DOI] [Google Scholar]
- 70.Pereyra-Irujo G.A., Aguirrezábal L.A. Sunflower Yield and Oil Quality Interactions and Variability: Analysis through a Simple Simulation Model. Agric. For. Meteorol. 2007;143:252–265. doi: 10.1016/j.agrformet.2007.01.001. [DOI] [Google Scholar]
- 71.Gibson L.R., Mullen R.E. Soybean Seed Quality Reductions by High Day and Night Temperature. Crop Sci. 1996;36:1615. doi: 10.2135/cropsci1996.0011183X003600060034x. [DOI] [Google Scholar]
- 72.Bouchereau A., Clossais-Besnard N., Bensaoud A., Leport L., Renard M. Water Stress Effects on Rapeseed Quality. Eur. J. Agron. 1996;5:19–30. doi: 10.1016/S1161-0301(96)02005-9. [DOI] [Google Scholar]
- 73.Hernández M.L., Padilla M.N., Mancha M., Martínez-Rivas J.M. Expression analysis identifies FAD2-2 as the olive oleate desaturase gene mainly responsible for the linoleic acid content in virgin olive oil. J. Agric. Food Chem. 2009;57(14):6199–6206. doi: 10.1021/jf900678z. [DOI] [PubMed] [Google Scholar]
- 74.Esteban A.B., Sicardo M.D., Mancha M., Martínez-Rivas J.M. Growth temperature control of the linoleic acid content in safflower (Carthamus tinctorius) seed oil. J. Agric. Food Chem. 2004;52(2):332–336. doi: 10.1021/jf030581m. [DOI] [PubMed] [Google Scholar]
- 75.Rolletschek H., Borisjuk L., Sánchez-García A., Gotor C., Romero L.C., Martínez-Rivas J.M., Mancha M. Temperature-dependent endogenous oxygen concentration regulates microsomal oleate desaturase in developing sunflower seeds. J. Exp. Bot. 2007;58(12):3171–3181. doi: 10.1093/jxb/erm154. [DOI] [PubMed] [Google Scholar]
- 76.Gooding M.J., Ellis R.H., Shewry P.R., Schofield J.D. Effects of Restricted Water Availability and Increased Temperature on the Grain Filling, Drying and Quality of Winter Wheat. J. Cereal Sci. 2003;37:295–309. doi: 10.1006/jcrs.2002.0501. [DOI] [Google Scholar]
- 77.Weightman R.M., Millar S., Alava J., John Foulkes M., Fish L., Snape J.W. Effects of Drought and the Presence of the 1BL/1RS Translocation on Grain Vitreosity, Hardness and Protein Content in Winter Wheat. J. Cereal Sci. 2008;47:457–468. doi: 10.1016/j.jcs.2007.05.011. [DOI] [Google Scholar]
- 78.Pleijel H. Grain Protein Accumulation in Relation to Grain Yield of Spring Wheat (Triticum Aestivum L.) Grown in Open-Top Chambers with Different Concentrations of Ozone, Carbon Dioxide and Water Availability. Agric. Ecosyst. Environ. 1999;72:265–270. doi: 10.1016/S0167-8809(98)00185-6. [DOI] [Google Scholar]
- 79.Rotundo J.L., Westgate M.E. Meta-Analysis of Environmental Effects on Soybean Seed Composition. F. Crop. Res. 2009;110:147–156. doi: 10.1016/j.fcr.2008.07.012. [DOI] [Google Scholar]
- 80.Singh E., Tiwari S., Agrawal M. Variability in Antioxidant and Metabolite Levels, Growth and Yield of Two Soybean Varieties: An Assessment of Anticipated Yield Losses under Projected Elevation of Ozone. Agric. Ecosyst. Environ. 2010;135:168–177. doi: 10.1016/j.agee.2009.09.004. [DOI] [Google Scholar]
- 81.Zhang W., Wang G., Liu X., Feng Z. Effects of elevated O3 exposure on seed yield, N concentration and photosynthesis of nine soybean cultivars (Glycine max (L.) Merr.) in Northeast China. Plant Sci. 2014;226:172–181. doi: 10.1016/j.plantsci.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 82.Flagella Z., Giuliani M.M., Giuzio L., Volpi C., Masci S. Influence of Water Deficit on Durum Wheat Storage Protein Composition and Technological Quality. Eur. J. Agron. 2010;33:197–207. doi: 10.1016/j.eja.2010.05.006. [DOI] [Google Scholar]
- 83.Barnabás B., Jäger K., Fehér A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008;31(1):11–38. doi: 10.1111/j.1365-3040.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 84.Booker F., Muntifering R., McGrath M., Burkey K., Decoteau D., Fiscus E., Manning W., Krupa S., Chappelka A., Grantz D. The ozone component of global change: potential effects on agricultural and horticultural plant yield, product quality and interactions with invasive species. J. Integr. Plant Biol. 2009;51(4):337–351. doi: 10.1111/j.1744-7909.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- 85.Triboi E., Triboi-Blondel A-M. Productivity and Grain or Seed Composition: A New Approach to an Old Problem—invited Paper. Eur. J. Agron. 2002;16:163–186. doi: 10.1016/S1161-0301(01)00146-0. [DOI] [Google Scholar]
- 86.Fernandez-Figares I., Marinetto J., Royo C., Ramos J.M., Garcia del Moral L.F. Amino-Acid Composition and Protein and Carbohydrate Accumulation in the Grain of Triticale Grown under Terminal Water Stress Simulated by a Senescing Agent. J. Cereal Sci. 2000;32:249–258. doi: 10.1006/jcrs.2000.0329. [DOI] [Google Scholar]
- 87.Rharrabti Y., Royo C., Villegas D., Aparicio N., García del Moral L. Durum Wheat Quality in Mediterranean Environments. F. Crop. Res. 2003;80:123–131. doi: 10.1016/S0378-4290(02)00176-4. [DOI] [Google Scholar]
- 88.Guedira M., Paulsen G.M. Accumulation of Starch in Wheat Grain under Different Shoot/root Temperatures during Maturation. Funct. Plant Biol. 2002;29:495. doi: 10.1071/PP01006. [DOI] [PubMed] [Google Scholar]
- 89.Hurkman W., McCue K., Altenbach S. Expression of Genes for Starch Biosynthesis Is Regulated by High Temperature in Developing Wheat Endosperm. Plant Sci. 2003 doi: 10.1016/S0168-9452(03)00076-1. [DOI] [Google Scholar]
- 90.Chapman K.D., Ohlrogge J.B. Compartmentation of triacylglycerol accumulation in plants. J. Biol. Chem. 2012;287(4):2288–2294. doi: 10.1074/jbc.R111.290072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li-Beisson Y., Shorrosh B., Beisson F., Andersson M.X., Arondel V., Bates P.D., Baud S., Bird D., Debono A., Durrett T.P., Franke R.B., Graham I.A., Katayama K., Kelly A.A., Larson T., Markham J.E., Miquel M., Molina I., Nishida I., Rowland O., Samuels L., Schmid K.M., Wada H., Welti R., Xu C., Zallot R., Ohlrogge J. Acyl-lipid metabolism. Arabidopsis Book. 2013;11:e0161. doi: 10.1199/tab.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Napier J.A., Stobart A.K., Shewry P.R. The structure and biogenesis of plant oil bodies: the role of the ER membrane and the oleosin class of proteins. Plant Mol. Biol. 1996;31(5):945–956. doi: 10.1007/BF00040714. [DOI] [PubMed] [Google Scholar]
- 93.Jolivet P., Roux E., D’Andrea S., Davanture M., Negroni L., Zivy M., Chardot T. Protein composition of oil bodies in Arabidopsis thaliana ecotype WS. Plant Physiol. Biochem. 2004;42(6):501–509. doi: 10.1016/j.plaphy.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Siloto R.M., Findlay K., Lopez-Villalobos A., Yeung E.C., Nykiforuk C.L., Moloney M.M. The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell. 2006;18(8):1961–1974. doi: 10.1105/tpc.106.041269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimada T.L., Shimada T., Takahashi H., Fukao Y., Hara-Nishimura I. A novel role for oleosins in freezing tolerance of oilseeds in Arabidopsis thaliana. Plant J. 2008;55(5):798–809. doi: 10.1111/j.1365-313X.2008.03553.x. [DOI] [PubMed] [Google Scholar]
- 96.Poxleitner M., Rogers S.W., Lacey Samuels A., Browse J., Rogers J.C. A role for caleosin in degradation of oil-body storage lipid during seed germination. Plant J. 2006;47(6):917–933. doi: 10.1111/j.1365-313X.2006.02845.x. [DOI] [PubMed] [Google Scholar]
- 97.Lin L-J., Tai S.S., Peng C-C., Tzen J.T. Steroleosin, a sterol-binding dehydrogenase in seed oil bodies. Plant Physiol. 2002;128(4):1200–1211. doi: 10.1104/pp.010982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reske J., Siebrecht J., Hazebroek J. Triacylglycerol Composition and Structure in Genetically Modified Sunflower and Soybean Oils. J. Am. Oil Chem. Soc. 1997;74:989–998. doi: 10.1007/s11746-997-0016-1. [DOI] [Google Scholar]
- 99.Refaat A.A. Correlation between the Chemical Structure of Biodiesel and Its Physical Properties. Int. J. Environ. Sci. Technol. 2009;6:677–694. doi: 10.1007/BF03326109. [DOI] [Google Scholar]
- 100.Gunstone F. Vegetable Oils in Food Technology: Composition. Properties and Uses; 2011. [DOI] [Google Scholar]
- 101.Graef G., LaVallee B.J., Tenopir P., Tat M., Schweiger B., Kinney A.J., Van Gerpen J.H., Clemente T.E. A high-oleic-acid and low-palmitic-acid soybean: agronomic performance and evaluation as a feedstock for biodiesel. Plant Biotechnol. J. 2009;7(5):411–421. doi: 10.1111/j.1467-7652.2009.00408.x. [DOI] [PubMed] [Google Scholar]
- 102.Drexler H., Spiekermann P., Meyer A., Domergue F., Zank T., Sperling P., Abbadi A., Heinz E. Metabolic engineering of fatty acids for breeding of new oilseed crops: strategies, problems and first results. J. Plant Physiol. 2003;160(7):779–802. doi: 10.1078/0176-1617-01025. [DOI] [PubMed] [Google Scholar]
- 103.Metzger J.O., Bornscheuer U. Lipids as renewable resources: current state of chemical and biotechnological conversion and diversification. Appl. Microbiol. Biotechnol. 2006;71(1):13–22. doi: 10.1007/s00253-006-0335-4. [DOI] [PubMed] [Google Scholar]
- 104.Buhr T., Sato S., Ebrahim F., Xing A., Zhou Y., Mathiesen M., Schweiger B., Kinney A., Staswick P., Tom Clemente Ribozyme termination of RNA transcripts down-regulate seed fatty acid genes in transgenic soybean. Plant J. 2002;30(2):155–163. doi: 10.1046/j.1365-313X.2002.01283.x. [DOI] [PubMed] [Google Scholar]
- 105.Damude H.G., Zhang H., Farrall L., Ripp K.G., Tomb J-F., Hollerbach D., Yadav N.S. Identification of bifunctional delta12/ω3 fatty acid desaturases for improving the ratio of ω3 to ω6 fatty acids in microbes and plants. Proc. Natl. Acad. Sci. USA. 2006;103(25):9446–9451. doi: 10.1073/pnas.0511079103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jones A., Davies H.M., Voelker T.A. Palmitoyl-acyl carrier protein (ACP) thioesterase and the evolutionary origin of plant acyl-ACP thioesterases. Plant Cell. 1995;7(3):359–371. doi: 10.1105/tpc.7.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Q., Singh S., Green A. Genetic Manipulation of Fatty Acid Composition in Cottonseed Oil.; 18th Int. Symp. Plant Lipids; 2008. [Google Scholar]
- 108.Knutzon D.S., Thompson G.A., Radke S.E., Johnson W.B., Knauf V.C., Kridl J.C. Modification of Brassica seed oil by antisense expression of a stearoyl-acyl carrier protein desaturase gene. Proc. Natl. Acad. Sci. USA. 1992;89(7):2624–2628. doi: 10.1073/pnas.89.7.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Q., Singh S.P., Green A.G. High-stearic and High-oleic cottonseed oils produced by hairpin RNA-mediated post-transcriptional gene silencing. Plant Physiol. 2002;129(4):1732–1743. doi: 10.1104/pp.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Narine S.S., Yue J., Kong X. Production of Polyols from Canola Oil and Their Chemical Identification and Physical Properties. J. Am. Oil Chem. Soc. 2007;84:173–179. doi: 10.1007/s11746-006-1021-5. [DOI] [Google Scholar]
- 111.Brocard-Gifford I.M., Lynch T.J., Finkelstein R.R. Regulatory networks in seeds integrating developmental, abscisic acid, sugar, and light signaling. Plant Physiol. 2003;131(1):78–92. doi: 10.1104/pp.011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vriet C., Russinova E., Reuzeau C. Boosting crop yields with plant steroids. Plant Cell. 2012;24(3):842–857. doi: 10.1105/tpc.111.094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ashikari M., Sakakibara H., Lin S., Yamamoto T., Takashi T., Nishimura A., Angeles E.R., Qian Q., Kitano H., Matsuoka M. Cytokinin oxidase regulates rice grain production. Science. 2005;309(5735):741–745. doi: 10.1126/science.1113373. [DOI] [PubMed] [Google Scholar]
- 114.Miller C.O., Skoog F., Von Saltza M.H., Strong F.M. Kinetin, a Cell Division Factor from Deoxyribonucleic Acid 1. J. Am. Chem. Soc. 1955;77:1392–1392. doi: 10.1021/ja01610a105. [DOI] [Google Scholar]
- 115.Amasino R. 1955: kinetin arrives: the 50th anniversary of a new plant hormone. Plant Physiol. 2005;138(3):1177–1184. doi: 10.1104/pp.104.900160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Letham D.S. Zeatin, a Factor Inducing Cell Division Isolated from. Life Sci. 1963;8:569–573. doi: 10.1016/0024-3205(63)90108-5. [DOI] [PubMed] [Google Scholar]
- 117.Emery N., Atkins C., Basra A.S. Cytokinins and Seed Development. Handb. seed Sci. Technol; 2006. pp. 63–93. [Google Scholar]
- 118.Spíchal L. Cytokinins - Recent News and Views of Evolutionally Old Molecules. Funct. Plant Biol. 2012;39:267. doi: 10.1071/FP11276. [DOI] [PubMed] [Google Scholar]
- 119.Gan S., Amasino R.M. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270(5244):1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- 120.Werner T., Motyka V., Strnad M., Schmülling T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA. 2001;98(18):10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reguera M., Peleg Z., Abdel-Tawab Y.M., Tumimbang E.B., Delatorre C.A., Blumwald E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol. 2013;163(4):1609–1622. doi: 10.1104/pp.113.227702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Werner T., Motyka V., Laucou V., Smets R., Van Onckelen H., Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15(11):2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sakakibara H. Cytokinin biosynthesis and regulation. Vitam. Horm. 2005;72:271–287. doi: 10.1016/S0083-6729(05)72008-2. [DOI] [PubMed] [Google Scholar]
- 124.Bartrina I., Otto E., Strnad M., Werner T., Schmülling T. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell. 2011;23(1):69–80. doi: 10.1105/tpc.110.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- 126.Farrow S.C., Emery R.N. Concurrent profiling of indole-3-acetic acid, abscisic acid, and cytokinins and structurally related purines by high-performance-liquid-chromatography tandem electrospray mass spectrometry. Plant Methods. 2012;8(1):42. doi: 10.1186/1746-4811-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frébort I., Kowalska M., Hluska T., Frébortová J., Galuszka P. Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 2011;62(8):2431–2452. doi: 10.1093/jxb/err004. [DOI] [PubMed] [Google Scholar]
- 128.Barry G.F., Rogers S.G., Fraley R.T., Brand L. Identification of a cloned cytokinin biosynthetic gene. Proc. Natl. Acad. Sci. USA. 1984;81(15):4776–4780. doi: 10.1073/pnas.81.15.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kakimoto T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant Cell Physiol. 2001;42(7):677–685. doi: 10.1093/pcp/pce112. [DOI] [PubMed] [Google Scholar]
- 130.Takei K., Sakakibara H., Sugiyama T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J. Biol. Chem. 2001;276(28):26405–26410. doi: 10.1074/jbc.M102130200. [DOI] [PubMed] [Google Scholar]
- 131.Zubko E., Adams C.J., Macháèková I., Malbeck J., Scollan C., Meyer P. Activation tagging identifies a gene from Petunia hybrida responsible for the production of active cytokinins in plants. Plant J. 2002;29(6):797–808. doi: 10.1046/j.1365-313X.2002.01256.x. [DOI] [PubMed] [Google Scholar]
- 132.Sakano Y., Okada Y., Matsunaga A., Suwama T., Kaneko T., Ito K., Noguchi H., Abe I. Molecular cloning, expression, and characterization of adenylate isopentenyltransferase from hop (Humulus lupulus L.). Phytochemistry. 2004;65(17):2439–2446. doi: 10.1016/j.phytochem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 133.Ye C., Wu S., Kong F., Zhou C., Yang Q., Sun Y., Wang B. Identification and Characterization of an Isopentenyltransferase (IPT) Gene in Soybean (Glycine Max L.). Plant Sci. 2006;170:542–550. doi: 10.1016/j.plantsci.2005.10.008. [DOI] [Google Scholar]
- 134.Brugiere N. Soybean Isopentenyl Transferase Genes and Methods of Use. 2009 Patent US7893236 B2. [Google Scholar]
- 135.Schmutz J., Cannon S.B., Schlueter J., Ma J., Mitros T., Nelson W., Hyten D.L., Song Q., Thelen J.J., Cheng J., Xu D., Hellsten U., May G.D., Yu Y., Sakurai T., Umezawa T., Bhattacharyya M.K., Sandhu D., Valliyodan B., Lindquist E., Peto M., Grant D., Shu S., Goodstein D., Barry K., Futrell-Griggs M., Abernathy B., Du J., Tian Z., Zhu L., Gill N., Joshi T., Libault M., Sethuraman A., Zhang X.C., Shinozaki K., Nguyen H.T., Wing R.A., Cregan P., Specht J., Grimwood J., Rokhsar D., Stacey G., Shoemaker R.C., Jackson S.A. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278):178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- 136.Le D.T., Nishiyama R., Watanabe Y., Vankova R., Tanaka M., Seki M., Ham H., Yamaguchi-Shinozaki K., Shinozaki K., Tran L.S. Identification and expression analysis of cytokinin metabolic genes in soybean under normal and drought conditions in relation to cytokinin levels. PLoS One. 2012;7(8):e42411. doi: 10.1371/journal.pone.0042411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Galuszka P., Frébort I., Šebela M., Sauer P., Jacobsen S., Peč P. Cytokinin oxidase or dehydrogenase? Mechanism of cytokinin degradation in cereals. Eur. J. Biochem. 2001;268(2):450–461. doi: 10.1046/j.1432-1033.2001.01910.x. [DOI] [PubMed] [Google Scholar]
- 138.Gajdosová S., Spíchal L., Kamínek M., Hoyerová K., Novák O., Dobrev P.I., Galuszka P., Klíma P., Gaudinová A., Zizková E., Hanus J., Dancák M., Trávnícek B., Pesek B., Krupicka M., Vanková R., Strnad M., Motyka V. Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J. Exp. Bot. 2011;62(8):2827–2840. doi: 10.1093/jxb/erq457. [DOI] [PubMed] [Google Scholar]
- 139.Galuszka P., Popelková H. Biochemical Characterization of Cytokinin Oxidases/dehydrogenases from Arabidopsis Thaliana Expressed in Nicotiana Tabacum L. J. Plant Growth Regul. 2007;26(3):255–267. doi: 10.1007/s00344-007-9008-5. [DOI] [Google Scholar]
- 140.Houba-Hérin N., Pethe C., d’Alayer J., Laloue M. Cytokinin oxidase from Zea mays: purification, cDNA cloning and expression in moss protoplasts. Plant J. 1999;17(6):615–626. doi: 10.1046/j.1365-313X.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 141.Vyroubalová S., Václavíková K., Turecková V., Novák O., Smehilová M., Hluska T., Ohnoutková L., Frébort I., Galuszka P. Characterization of new maize genes putatively involved in cytokinin metabolism and their expression during osmotic stress in relation to cytokinin levels. Plant Physiol. 2009;151(1):433–447. doi: 10.1104/pp.109.142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Galuszka P., Frébortová J., Werner T., Yamada M., Strnad M., Schmülling T., Frébort I. Cytokinin oxidase/dehydrogenase genes in barley and wheat: cloning and heterologous expression. Eur. J. Biochem. 2004;271(20):3990–4002. doi: 10.1111/j.1432-1033.2004.04334.x. [DOI] [PubMed] [Google Scholar]
- 143.Werner T., Schmülling T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 2009;12(5):527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 144.Mok D.W., Mok M.C. Cytokinin Metabolism and Action. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:89–118. doi: 10.1146/annurev.arplant.52.1.89. [DOI] [PubMed] [Google Scholar]
- 145.Ferreira F.J., Kieber J.J. Cytokinin signaling. Curr. Opin. Plant Biol. 2005;8(5):518–525. doi: 10.1016/j.pbi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 146.Kurakawa T., Ueda N., Maekawa M., Kobayashi K., Kojima M., Nagato Y., Sakakibara H., Kyozuka J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445(7128):652–655. doi: 10.1038/nature05504. [DOI] [PubMed] [Google Scholar]
- 147.Ehness R., Roitsch T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J. 1997;11(3):539–548. doi: 10.1046/j.1365-313X.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- 148.Godt D.E., Roitsch T. Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol. 1997;115(1):273–282. doi: 10.1104/pp.115.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roitsch T., González M-C. Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 2004;9(12):606–613. doi: 10.1016/j.tplants.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 150.Wang E., Wang J., Zhu X., Hao W., Wang L., Li Q., Zhang L., He W., Lu B., Lin H., Ma H., Zhang G., He Z. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 2008;40(11):1370–1374. doi: 10.1038/ng.220. [DOI] [PubMed] [Google Scholar]
- 151.Mashiguchi K., Urakami E., Hasegawa M., Sanmiya K., Matsumoto I., Yamaguchi I., Asami T., Suzuki Y. Defense-related signaling by interaction of arabinogalactan proteins and beta-glucosyl Yariv reagent inhibits gibberellin signaling in barley aleurone cells. Plant Cell Physiol. 2008;49(2):178–190. doi: 10.1093/pcp/pcm175. [DOI] [PubMed] [Google Scholar]
- 152.Davies P.J. The Plant Hormones: Their Nature, Occurrence, and Functions. Plant Horm; 2010. pp. 1–15. [DOI] [Google Scholar]
- 153.Borrell A.K., Hammer G.L., Douglas A.C. Does Maintaining Green Leaf Area in Sorghum Improve Yield under Drought? I. Leaf Growth and Senescence. Crop Sci. 2000;40:1026. doi: 10.2135/cropsci2000.4041026x. [DOI] [Google Scholar]
- 154.Peleg Z., Apse M., Blumwald E. Engineering Salinity and Water-Stress Tolerance in Crop Plants: Getting Closer to the Field. Adv. Bot. Res. 2011;••• doi: 10.1016/B978-0-12-387692-8.00012-6. [DOI] [Google Scholar]
- 155.Ha S., Vankova R., Yamaguchi-Shinozaki K., Shinozaki K., Tran L-S. Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012;17(3):172–179. doi: 10.1016/j.tplants.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 156.Rivero R.M., Kojima M., Gepstein A., Sakakibara H., Mittler R., Gepstein S., Blumwald E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Natl. Acad. Sci. USA. 2007;104(49):19631–19636. doi: 10.1073/pnas.0709453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Werner T., Nehnevajova E., Köllmer I., Novák O., Strnad M., Krämer U., Schmülling T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell. 2010;22(12):3905–3920. doi: 10.1105/tpc.109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim H.J., Ryu H., Hong S.H., Woo H.R., Lim P.O., Lee I.C., Sheen J., Nam H.G., Hwang I. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006;103(3):814–819. doi: 10.1073/pnas.0505150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Riefler M., Novak O., Strnad M., Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18(1):40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rivero R.M., Shulaev V., Blumwald E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol. 2009;150(3):1530–1540. doi: 10.1104/pp.109.139378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qin H., Gu Q., Zhang J., Sun L., Kuppu S., Zhang Y., Burow M., Payton P., Blumwald E., Zhang H. Regulated expression of an isopentenyltransferase gene (IPT) in peanut significantly improves drought tolerance and increases yield under field conditions. Plant Cell Physiol. 2011;52(11):1904–1914. doi: 10.1093/pcp/pcr125. [DOI] [PubMed] [Google Scholar]
- 162.Zhang P., Wang W.Q., Zhang G.L., Kaminek M., Dobrev P., Xu J., Gruissem W. Senescence-inducible expression of isopentenyl transferase extends leaf life, increases drought stress resistance and alters cytokinin metabolism in cassava. J. Integr. Plant Biol. 2010;52(7):653–669. doi: 10.1111/j.1744-7909.2010.00956.x. [DOI] [PubMed] [Google Scholar]
- 163.Peleg Z., Blumwald E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011;14(3):290–295. doi: 10.1016/j.pbi.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 164.Wilkinson S., Kudoyarova G.R., Veselov D.S., Arkhipova T.N., Davies W.J. Plant hormone interactions: innovative targets for crop breeding and management. J. Exp. Bot. 2012;63(9):3499–3509. doi: 10.1093/jxb/ers148. [DOI] [PubMed] [Google Scholar]
- 165.Guivarc’h A., Rembur J., Goetz M., Roitsch T., Noin M., Schmülling T., Chriqui D. Local expression of the ipt gene in transgenic tobacco (Nicotiana tabacum L. cv. SR1) axillary buds establishes a role for cytokinins in tuberization and sink formation. J. Exp. Bot. 2002;53(369):621–629. doi: 10.1093/jexbot/53.369.621. [DOI] [PubMed] [Google Scholar]
- 166.Werner T., Holst K., Pörs Y., Guivarc’h A., Mustroph A., Chriqui D., Grimm B., Schmülling T. Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J. Exp. Bot. 2008;59(10):2659–2672. doi: 10.1093/jxb/ern134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Davies W.J., Zhang J. Root Signals and the Regulation of Growth and Development of Plants in Drying Soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991;42:55–76. doi: 10.1146/annurev.pp.42.060191.000415. [DOI] [Google Scholar]
- 168.Rivero R.M., Gimeno J., Van Deynze A., Walia H., Blumwald E. Enhanced cytokinin synthesis in tobacco plants expressing PSARK:IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol. 2010;51(11):1929–1941. doi: 10.1093/pcp/pcq143. [DOI] [PubMed] [Google Scholar]
- 169.Kuppu S., Mishra N., Hu R., Sun L., Zhu X., Shen G., Blumwald E., Payton P., Zhang H. Water-deficit inducible expression of a cytokinin biosynthetic gene IPT improves drought tolerance in cotton. PLoS One. 2013;8(5):e64190. doi: 10.1371/journal.pone.0064190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kull U., Büxenstein R. Effect of Cytokinins on the Lipid Fatty Acids of Leaves. Phytochemistry. 1974;13:39–44. doi: 10.1016/S0031-9422(00)91264-0. [DOI] [Google Scholar]
- 171.Stearns E.M., Jr, Morton W.T. Effects of Growth Regulators on Fatty Acids of Soybean Suspension Cultures. Phytochemistry. 1975;14:619–622. doi: 10.1016/0031-9422(75)83004-4. [DOI] [Google Scholar]
- 172.Kull U., Kühn B., Schweizer J., Weiser H. Short-Term Effects of Cytokinins on the Lipid Fatty Acids of Green Leaves. Plant Cell Physiol. 1978;19:801–810. [Google Scholar]
- 173.Dunne A., Maple-Grødem J., Gargano D., Haslam R.P., Napier J.A., Chua N.H., Russell R., Møller S.G. Modifying fatty acid profiles through a new cytokinin-based plastid transformation system. Plant J. 2014;80(6):1131–1138. doi: 10.1111/tpj.12684. [DOI] [PubMed] [Google Scholar]
- 174.Addicott F. Abscisic Acid. 1983. p. 607. [Google Scholar]
- 175.Addicott F.T., Lyon J.L., Ohkuma K., Thiessen W.E., Carns H.R., Smith O.E., Cornforth J.W., Milborrow B.V., Ryback G., Wareing P.F. Abscisic acid: a new name for abscisin II (dormin). Science. 1968;159(3822):1493–1493. doi: 10.1126/science.159.3822.1493. [DOI] [PubMed] [Google Scholar]
- 176.Hetherington A., Quatrano R. Mechanisms of Action of Abscisic Acid at the Cellular Level. New Phytol. 1991;119(1) doi: 10.1111/j.1469-8137.1991.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 177.Hein M.B., Brenner M.L., Brun W.A. Concentrations of Abscisic Acid and Indole-3-Acetic Acid in Soybean Seeds during Development. Plant Physiol. 1984;76(4):951–954. doi: 10.1104/pp.76.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Holbrook L.A., Magus J.R., Taylor D.C. Abscisic Acid Induction of Elongase Activity, Biosynthesis and Accumulation of Very Long Chain Monounsaturated Fatty Acids and Oil Body Proteins in Microspore-Derived Embryos of Brassica napus L. Cv Reston. Plant Sci. 1992;84:99–115. doi: 10.1016/0168-9452(92)90213-6. [DOI] [Google Scholar]
- 179.De Bruijn S.M., Ooms J.J.J., Basra A.S., van Lammeren A.A.M., Vreugdenhil D. Influence of Abscisic Acid on Storage of Lipids and Carbohydrates in Developing Arabidopsis Seeds. 1993:103–108. [Google Scholar]
- 180.De Bruijn S.M., Ooms J.J., Karssen C.M., Vreugdenhil D. Effects of Abscisic Acid on Reserve Deposition in Developing Arabidopsis Seeds. Acta Bot. Neerl. 1997;46:263–277. [Google Scholar]
- 181.Ross J.H., Murphy D.J. Differential Accumulation of Oleosins, Starch, Storage Proteins and Triacylglycerols in Embryos and Cell Cultures of Daucus Carota. L. Plant Sci. 1993;88:1–11. doi: 10.1016/0168-9452(93)90103-7. [DOI] [Google Scholar]
- 182.Attree S.M., Pomeroy M.K., Fowke L.C. Manipulation of conditions for the culture of somatic embryos of white spruce for improved triacylglycerol biosynthesis and desiccation tolerance. Planta. 1992;187(3):395–404. doi: 10.1007/BF00195664. [DOI] [PubMed] [Google Scholar]
- 183.Finkelstein R., Somerville C. Abscisic Acid or High Osmoticum Promote Accumulation of Long-Chain Fatty Acids in Developing Embryos of Brassica Napus. Plant Sci. 1989;61:213–217. doi: 10.1016/0168-9452(89)90227-6. [DOI] [Google Scholar]
- 184.Park W-K., Yoo G., Moon M., Kim C.W., Choi Y-E., Yang J-W. Phytohormone supplementation significantly increases growth of Chlamydomonas reinhardtii cultivated for biodiesel production. Appl. Biochem. Biotechnol. 2013;171(5):1128–1142. doi: 10.1007/s12010-013-0386-9. [DOI] [PubMed] [Google Scholar]
- 185.Rook F., Corke F., Card R., Munz G., Smith C., Bevan M.W. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J. 2001;26(4):421–433. doi: 10.1046/j.1365-313X.2001.2641043.x. [DOI] [PubMed] [Google Scholar]
- 186.León P., Sheen J. Sugar and hormone connections. Trends Plant Sci. 2003;8(3):110–116. doi: 10.1016/S1360-1385(03)00011-6. [DOI] [PubMed] [Google Scholar]
- 187.Koch K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004;7(3):235–246. doi: 10.1016/j.pbi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 188.Smeekens S. Sugar-Induced Signal Transduction in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- 189.Borisjuk L., Walenta S., Rolletschek H., Mueller-Klieser W., Wobus U., Weber H. Spatial analysis of plant metabolism: sucrose imaging within Vicia faba cotyledons reveals specific developmental patterns. Plant J. 2002;29(4):521–530. doi: 10.1046/j.1365-313x.2002.01222.x. [DOI] [PubMed] [Google Scholar]
- 190.Heim U., Weber H., Bäumlein H., Wobus U. A sucrose-synthase gene of Vicia faba L.: expression pattern in developing seeds in relation to starch synthesis and metabolic regulation. Planta. 1993;191(3):394–401. doi: 10.1007/BF00195698. [DOI] [PubMed] [Google Scholar]
- 191.Weber H., Heim U., Golombek S., Borisjuk L., Wobus U. Assimilate Uptake and the Regulation of Seed Development. Seed Sci. Res. 1998;8:331–346. doi: 10.1017/S0960258500004268. [DOI] [Google Scholar]
- 192.Golombek S., Heim U., Horstmann C., Wobus U., Weber H. Phosphoenolpyruvate carboxylase in developing seeds of Vicia faba L.: gene expression and metabolic regulation. Planta. 1999;208(1):66–72. doi: 10.1007/s004250050535. [DOI] [PubMed] [Google Scholar]
- 193.Weber H., Borisjuk L., Wobus U. Controlling Seed Development and Seed Size in Vicia Faba: A Role for Seed Coat‐associated Invertases and Carbohydrate State. Plant J. 1996;10:823–834. doi: 10.1046/j.1365-313X.1996.10050823.x. [DOI] [Google Scholar]
- 194.Ambrose M.J., Wang T.L., Cook S.K., Hedley C.L. An Analysis of Seed Development in Pisum Sativum IV. Cotyledon Cell Populations in Vivo and in Vitro. J. Exp. Bot. 1987;38:1909–1920. doi: 10.1093/jxb/38.11.1909. [DOI] [Google Scholar]
- 195.Corke F.M., Hedley C.L., Wang T.L. An Analysis of Seed Development inPisum Sativum L. Protoplasma. 1990;155:127–135. doi: 10.1007/BF01322622. [DOI] [Google Scholar]
- 196.Geigenberger P. Regulation of sucrose to starch conversion in growing potato tubers. J. Exp. Bot. 2003;54(382):457–465. doi: 10.1093/jxb/erg074. [DOI] [PubMed] [Google Scholar]
- 197.Yazdi-Samadi B., Rinne R., Seif R. Components of Developing Soybean Seeds: Oil, Protein, Starch, Organic Acids and Amino Acids. Agron. J. 1976;69(3) [Google Scholar]
- 198.Yamaguchi-Shinozaki K., Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 199.Sobeih W.Y., Dodd I.C., Bacon M.A., Grierson D., Davies W.J. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J. Exp. Bot. 2004;55(407):2353–2363. doi: 10.1093/jxb/erh204. [DOI] [PubMed] [Google Scholar]
- 200.Spollen W.G., LeNoble M.E., Samuels T.D., Bernstein N., Sharp R.E. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol. 2000;122(3):967–976. doi: 10.1104/pp.122.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Giuliani S., Sanguineti M.C., Tuberosa R., Bellotti M., Salvi S., Landi P. Root-ABA1, a major constitutive QTL, affects maize root architecture and leaf ABA concentration at different water regimes. J. Exp. Bot. 2005;56(422):3061–3070. doi: 10.1093/jxb/eri303. [DOI] [PubMed] [Google Scholar]
- 202.Tardieu F., Parent B., Simonneau T. Control of leaf growth by abscisic acid: hydraulic or non-hydraulic processes? Plant Cell Environ. 2010;33(4):636–647. doi: 10.1111/j.1365-3040.2009.02091.x. [DOI] [PubMed] [Google Scholar]
- 203.Travaglia C., Reinoso H., Cohen A., Luna C., Tommasino E., Castillo C., Bottini R. Exogenous ABA Increases Yield in Field-Grown Wheat with Moderate Water Restriction. J. Plant Growth Regul. 2010;29:366–374. doi: 10.1007/s00344-010-9147-y. [DOI] [Google Scholar]
- 204.Prudent M., Salon C., Souleimanov A., Emery R.J., Smith D.L. Soybean Is Less Impacted by Water Stress Using Bradyrhizobium Japonicum and Thuricin-17 from Bacillus Thuringiensis. Agron. Sustain. Dev. 2014;35:749–757. doi: 10.1007/s13593-014-0256-z. [DOI] [Google Scholar]
- 205.Thompson A.J., Andrews J., Mulholland B.J., McKee J.M., Hilton H.W., Horridge J.S., Farquhar G.D., Smeeton R.C., Smillie I.R., Black C.R., Taylor I.B. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol. 2007;143(4):1905–1917. doi: 10.1104/pp.106.093559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nakashima K., Yamaguchi-Shinozaki K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013;32(7):959–970. doi: 10.1007/s00299-013-1418-1. [DOI] [PubMed] [Google Scholar]
- 207.Fujita Y., Fujita M., Shinozaki K., Yamaguchi-Shinozaki K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011;124(4):509–525. doi: 10.1007/s10265-011-0412-3. [DOI] [PubMed] [Google Scholar]
- 208.Kim J-S., Mizoi J., Yoshida T., Fujita Y., Nakajima J., Ohori T., Todaka D., Nakashima K., Hirayama T., Shinozaki K., Yamaguchi-Shinozaki K. An ABRE promoter sequence is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible genes in Arabidopsis. Plant Cell Physiol. 2011;52(12):2136–2146. doi: 10.1093/pcp/pcr143. [DOI] [PubMed] [Google Scholar]
- 209.Xiao B-Z., Chen X., Xiang C-B., Tang N., Zhang Q-F., Xiong L-Z. Evaluation of seven function-known candidate genes for their effects on improving drought resistance of transgenic rice under field conditions. Mol. Plant. 2009;2(1):73–83. doi: 10.1093/mp/ssn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang Y., Ying J., Kuzma M., Chalifoux M., Sample A., McArthur C., Uchacz T., Sarvas C., Wan J., Dennis D.T., McCourt P., Huang Y. Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J. 2005;43(3):413–424. doi: 10.1111/j.1365-313X.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 211.Ji X., Dong B., Shiran B., Talbot M.J., Edlington J.E., Hughes T., White R.G., Gubler F., Dolferus R. Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol. 2011;156(2):647–662. doi: 10.1104/pp.111.176164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Munier-Jolain N.G., Munier-Jolain N.M., Roche R., Ney B., Duthion C. Seed Growth Rate in Grain Legumes I. Effect of Photoassimilate Availability on Seed Growth Rate. J. Exp. Bot. 1998;49:1963–1969. doi: 10.1093/jxb/49.329.1963. [DOI] [Google Scholar]
- 213.Egli D.B., Guffy R.D., Meckel L.W., Leggett J.E. The Effect of Source-Sink Alterations on Soybean Seed Growth. Ann. Bot. (Lond.) 1985;55:395–402. [Google Scholar]
- 214.Egli D. Source-Sink Relationships, Seed Sucrose Levels and Seed Growth Rates in Soybean. Ann. Bot. (Lond.) 2001;88:235–242. doi: 10.1006/anbo.2001.1449. [DOI] [Google Scholar]
- 215.Hardman L.L., Brun W.A. Effect of Atmospheric Carbon Dioxide Enrichment at Different Developmental Stages on Growth and Yield Components of Soybeans1. Crop Sci. 1971;11:886. doi: 10.2135/cropsci1971.0011183X001100060037x. [DOI] [Google Scholar]
- 216.Schou J.B., Jeffers D.L., Streeter J.G. Effects of Reflectors, Black Boards, or Shades Applied at Different Stages of Plant Development on Yield of Soybeans1. Crop Sci. 1978;18:29. doi: 10.2135/cropsci1978.0011183X001800010009x. [DOI] [Google Scholar]
- 217.Egli D.B., Zhen-wen Y. Crop Growth Rate and Seeds per Unit Area in Soybean. Crop Sci. 1991;31:439. doi: 10.2135/cropsci1991.0011183X003100020043x. [DOI] [Google Scholar]
- 218.Andrade F.H., Ferreiro M.A. Reproductive Growth of Maize, Sunflower and Soybean at Different Source Levels during Grain Filling. F. Crop. Res. 1996;48:155–165. doi: 10.1016/S0378-4290(96)01017-9. [DOI] [Google Scholar]
- 219.Board J.E., Tan Q. Assimilatory Capacity Effects on Soybean Yield Components and Pod Number. Crop Sci. 1995;35:846. doi: 10.2135/cropsci1995.0011183X003500030035x. [DOI] [Google Scholar]
- 220.Jenner C., Ugalde T., Aspinall D. The Physiology of Starch and Protein Deposition in the Endosperm of Wheat. Aust. J. Plant Physiol. 1991;18:211. doi: 10.1071/PP9910211. [DOI] [Google Scholar]
- 221.Thompson J.F., Madison J.T., Muenster A.M. In Vitro Culture of Immature Cotyledons of Soya Bean (Glycine Max L. Merr.). Ann. Bot. (Lond.) 1977;41:29–39. [Google Scholar]
- 222.Egli D.B., Ramseur E.L., Zhen-wen Y., Sullivan C.H. Source-Sink Alterations Affect the Number of Cells in Soybean Cotyledons. Crop Sci. 1989;29:732. doi: 10.2135/cropsci1989.0011183X002900030039x. [DOI] [Google Scholar]
- 223.Jenner C. Effects of Shading or Removing Spikelets in Wheat: Testing Assumptions. Aust. J. Plant Physiol. 1980;7:113. doi: 10.1071/PP9800113. [DOI] [Google Scholar]
- 224.Ohlrogge J.B., Jaworski J.G. Regulation of Fatty Acid Synthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997;48:109–136. doi: 10.1146/annurev.arplant.48.1.109. [DOI] [PubMed] [Google Scholar]
- 225.Frébort I., Kowalska M., Hluska T., Frébortová J., Galuszka P. Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 2011;62(8):2431–2452. doi: 10.1093/jxb/err004. [DOI] [PubMed] [Google Scholar]