Abstract
Post-human genome revelation observes the emergence of ‘Nutigenomics’ as one of the exciting scientific advancement influencing mankind around the world. Food or more precisely 'nutrition' has the major impact in defining the cause-response interaction between nutrient (diet) and human health. In addition to substantial understanding of nutrition-human-health interaction, bases of 'nutrigenomic' development foster on advent in transcriptomics, genomics, proteomics and metabolomics as well as insight into food as health supplement. Interaction of selected nutrient with associated genes in specific organ or tissue necessary to comprehend that how individual's genetic makeup (DNA transcribed into mRNA and then to proteins) respond to particular nutrient. It provided new opportunities to incorporate natural bioactive compounds into food for specific group of people with similar genotype. As inception of diabetes associated with change in gene expression of, not limited to, protein kinase B, insulin receptor, duodenal homeobox and glucokinase, thus, targeting such proteins by modifying or improving the nutritional availability or uptake may help to devise novel food, supplements, or nutraceuticals. In this article, various aspects of R&D in nutrigenomics are reviewed to ascertain its impact on human health, especially with life-style associated diseases.
Keywords: Cardiovascular, Diabetes, Ethics, Inflammation, Nutrient, Nutrigenomics, Protein.
1. INTRODUCTION
Health is a symbol of quality life and prosperity of any civilization, which inevitably associated with socio-economic status and living environment of local inhabitants across continents. World Health Organization in its constitution define health ‘as a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity and fundamental rights of every human being without distinction of race, religion, political belief, socio-economic condition’ [1]. Health signifies the human’s functional or metabolic ability of self-acclimatization under adverse conditions. Recent past has witnessed huge shift in the pattern of diseases owing to transition in urbanization, food habit, living life style and environment. The studies suggested a marked increase in non-communicable diseases especially in fast evolving countries [2]. Inherited character, genetic make-up and availability of nutritious food is not only factors symbolic to health status, but it is much more complex interaction between individual’s genome (entire set of genes present) and environmental factors faced over lifetime. In addition, nutritional level, concentration of bioactive and their ability to influence health status are some of the crucial factors, which need to be addressed in studies aiming food-nutrient-health interaction. Thus, the science involved in development of dietary supplement, nutraceuticals or functional food, especially for disease prevention is highly complex.
Alteration in functioning of particular gene (s) or their protein product (s) during progression of a particular disease is actually influenced by subsequent modification in nutritional composition of food or diet [3]. The phenomenon of interaction between nutrients - genes - diseases is a highly complex. However, the scientific pursuit starting from isolation of DNA (1869), elucidation of its structure (1953) and finally revelation of human genome (2003) help to resolve many such complexities. The realization of these scientific breakthroughs was only possible due to parallel technological advancements, especially in the area of ‘Omics’ (transcriptomics, epigenomics, proteomics & metabolomics) and ‘Bioinformatics’ [4]. Post human genome era led to surfacing of ‘Nutrigenomics’ a nuptials between nutrition and genomics. It brings together the science of nutrition, genomics, computational biology & bioinformatics, and molecular medicine to tackle these chronic diseases (Fig. 1). It enables nutritionist to understand potential of food fortification or supplementation of diet with particular nutrient and its molecular effect in human health and reduce the risk of life style related disease. It render help in development of designed food for individuals with specific genetic backgrounds and also enable policymakers to strategize the development of safer and more effective dietary interventions. Challenges are enormous, but manageable, as most of the metabolic risk factors are associated with diet nutrition. In addition, a comprehensive approach to diagnostic and treatment is required and post genomic era with advancement in omic technologies provided excellent opportunity to the researcher in the form of ‘Nutrigenomics’ to tackle these colossal destroyers of human health.
Fig. (1).
Interdisciplinary approaches in nutrigenomics.
1.1. Life Style Associated Metabolic Diseases
Unhealthy living practices are the major factor associated with present life style. It is culminating into high mortality rate diseases, especially non-communicable chronic diseases (NCDs) that are responsible for most of the deaths in past decade [5]. Life style associated diseases are a group of diseases resulted from exposure of humankind over longer period to unhealthy diet, lifestyle and living environment. These diseases share almost similar risk factors, owing to, slow in progression, non-infectious and non-transmissible e.g. cardiovascular, nutrition-induced cancers, diabetes, chronic bronchitis, renal failure, hypertension etc. [6, 7].
Primary motive in selection of life style associated diseases in present review is entirely based on their impact in human health. WHO report suggested a rapid change in disease profile for past few decades from communicable diseases to non-communicable diseases irrespective of region, ethnicity and economy [8]. About 60 % deaths worldwide resulted from life style associated chronic diseases, double than infectious diseases [9]. In India too, the noncommunicable diseases were responsible for 53 % deaths, out of which 24 % causalities alone contributed by cardiovascular disease (CVDs) [5]. Generally, these diseases have specific metabolic risk factors associated cellular mechanism that results mainly in mitochondrial alterations, oxidative stress and inflammation etc. like epidemiological characteristics. These responses to changed environment contribute significantly in the inception and progression of lifestyle related diseases [10]. Primarily, it is the unhealthy diet that led to increase in metabolic risk factors of bloods like pressure, glucose, lipids etc. [11]. Diseases associated with modern life style also alter human body inflammation process. It is a self-limiting and controlled process executed by innate immune system (IIS), required to restrict incursion of foreign material and limit further damage to the human body [12]. Inflammation process is controlled by eicosanoids, a metabolite from fatty acids (arachidonic, eicosapentaenoic and docosahexaenoic acid). Inflammation reported to be negatively influenced by unhealthy diet and social environmental stress faced by humankind in present. Diets having high saturated fatty acids and antinutrients (lectines, saponins), low dietary fibre and vitamins (D & K), and imbalanced antioxidants considered responsible for poor IIS activity [13]. Scientific investigations revealed the genes (plasminogen activator inhibitor-1 associated with obesity) in animal model accountable for such alteration [14]. The life style and environmental transitions not only impacting the human physiology and metabolic processes, but also altering intestinal microbiome leading to such health complications [15]. An earlier notion that links these diseases with developed nations was shattered, as social, economical, and environmental factors are more favorable for their spread in low and middle-income countries [16]. If such transition continuous, it will further escalate the disease profiles, especially in developing and under developed nations.
2. FOOD, NUTRITION AND HEALTH INTERACTION
‘Food’ is a substance of plant, microbial or animal origin containing essential nutrients such as carbohydrates, fats, proteins, vitamins, or minerals essential for normal functioning of human body. Owing to their nutritional properties, food also has human-health benefits. World-over ingredients of certain foods or plants are in medical practices since time immemorial in prevention or treatment of human diseases e.g. Charaka samhita, a traditional Indian medication system heavily based on foods and herbs having medicinal properties [17]. In recent years, food has become the central dogma for nutritionist in establishing interaction between life style associated metabolic diseases. Reconsideration of ‘food as medicine’ by general public, scientific developments in health sector and demand for disease-combating diet open up new area for intervention in health-related issues, which allow individuals with improved and healthier [18].
The type and composition of food are the basis for availability and amount of energy, macro- and micro-nutrients and growth promoting factors in a diet. It provides the basis of relationships between food, health and disease. Dietary nutrition plays significant role in onset of the major chronic diseases like diabetes, cancer, atherosclerosis, cardiovascular etc. being faced by human generation at present[19, 20]. Nutritional deficiency, found to associate with specific disease conditions, e.g. lack of vitamin C were associated with scurvy, niacin (vitamin B3) with pellagra, etc [21]. Evidences are available to suggest that besides deficiency of a particular element, presence of various other compounds in diet in adequate nutrient also plays major role in management and disease protection of human health [22]. Many of the CVDs are thought to be triggered by free radicals and inflammation processes. Metabolite associated studies suggest that many natural compounds such as combination of β-carotene and vitamin A have the potential to target multiple pathways in this disease [23]. As a whole, food intake, nature and amount of nutrient available, individual makeup and local environment are the key factors to be associated in the progression of the common life style related diseases [24, 25]. Nutrients can have variable effects including up- and/or down-regulation of gene(s) and changes in protein expression levels in the fed and fasted states [26]. Epigenetic changes such as DNA methylation and histone modification relevant to adult chronic diseases are also possible [27]. Therefore, multidisciplinary efforts will be necessary to understand the rationale behind the association between food as medicine and human health.
3. INCEPTION OF NUTRIGENOMICS
World has made substantial progress in terms of food availability, since Universal Declaration of Human Rights (1948), where ‘right to food’ is recognized for first time as a core element for adequate standard of living [28]. Past decades witnessed opening up of international trade that contributed towards the availability of food around the world irrespective of region, religion and resource. International agencies like Codex Alimentarius Commission (CAC) have established international standards, which are applicable and acceptable globally and provide guidance for improving food safety and nutrition [29]. However, the importance of ‘nutritional food as a right’ received attention of policy maker’s world-over in late 1990s, with completion of human genome project. Also, World Food Summit in 1996 emphasized on linkage between food, nutrition and health. Over recent years, easy availability of processed foods, increased income and availability of diversified food owing to diminishing trade barrier has impacted significantly the health and nutritional status of populations, especially in developing and under developed countries [30]. Increased and diversified food intake (e.g. animal and partially hydrogenated fats consumption) and sedentary life style led to reduced energy expenditure, which ultimately increased the life style-related diseases[31]. The post genomic era has witnessed scientific revolutionin understanding the molecular mechanism of the interaction between food, nutrition and genes influencing human health. A drastic shift was observed in the approach from drug as medicine to food as medicine for management of nutrition related diseases that led to development of “Nutritional genomics or Nutrigenomics”. The historical milestones contributed immensely in emergence of this new scientific subject are also tabulated (Table 1).
Table (1).
Significant milestones in nutrigenomics.
| Year | Milestone (s) |
|---|---|
| 1859 | Publication of Charles Darwin's theory on origin of species and their evolution |
| 1865 | Gregor Mendel describes that heredity is transmitted in discrete units |
| 1869 | Friedrich Miescher was the first to identify DNA as a distinct molecule i.e. “nuclein” |
| 1879 | Walter Flemming observed mitosis and described chromosome behavior |
| 1902 | Archibald Garrod observed orderly Inheritance of alkaptonuria disease according to Mendelian rules. |
| 1903 | Walter Sutton connect Mendel's laws of heredity and given chromosome theory of heredity |
| 1909 | Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity |
| 1943 | Erwin Schrödinger proposed gene as the information carrier |
| 1944 | Oswald Avery, Colin MacLeod, and Maclyn McCarty identified DNA as the “transforming principle” and showed that it can transform the properties of cells |
| 1944 | Barbara McClintock observed that genes can "jump" or be transposed from one position to another on chromosomes and revealed that the genome was much more dynamic |
| 1952 | Alfred Hershey and Martha Chase experiments proved that genes are made of DNA |
| 1953 | Francis Crick and James Watson described the double helix structure of DNA |
| 1955 | Joe Hin Tjio defined 46 as the exact number of human chromosomes |
| 1956 | V.M. Ingram discovered that cause of disease (sickle-cell anemia) is a specific chemical alteration in a hemoglobin protein |
| 1966 | Marshall Nirenberg, Har Khorana and Severo Ochoa and their colleagues elucidated the genetic code Genetic Code Cracked Khorana and Nirenberg, along with Robert Holley interpretation of the genetic code and its function in protein synthesis |
| 1968 | Restriction nucleases enzymes described that revolutionized ability of DNA manipulation. |
| 1983 | First genetic disease (Huntington) mapped using DNA polymorphisms |
| 1990 | Launch of human genome project by U.S. Department of Energy (DOE), National Institutes of Health (NIH) and internation groups |
| Ethical, Legal and Social Implications (ELSI) programs founded | |
| 1994 | DOE initiated microbial genome program |
| 1997 | Galileo laboratories became the first Nutrigenomics company |
| 1999 | Nancy-Fogg Johnson and Alex Merolli used ‘Nutrogenomics’ term publically |
| 1991 | Food and Drug Administration (FDA) approved Gleevec, an oral medication to treat chronic myeloid leukemia (CML) |
| 2003 | Establishment of National Centre of Excellence in Nutritional Genomics in US to explore link between diet, genes and diseases |
| Human genome project completed and published | |
| 2004 | The European Nutrigenomics organization (NuGO) born |
| 2011 | Personalised nutrition: An integrated analysis of opportunities and challenges (Food processing) |
“Nutrigenomics is the science of how bioactive chemicals in foods and supplements alter the molecular expression and/or structure of an individual’s genetic makeup” [32].
Nutrigenomics illustrate the influence of nutrients on gene/protein expression that are regulated at specific time under particular environment and also generate response resulted from simultaneous functioning of gene/protein networks in regulation of related human disease or disorder. The underlying criterion for any nutrigenomic studies are based on the fact that food nutrients present in a diet alter and regulate specific genes, particular diet in specific population (individual genetic makeup) may be contributory factor for occurrence or progression of specific diseases and nutritional modification can be used to manage or mitigate the selected disease [32].
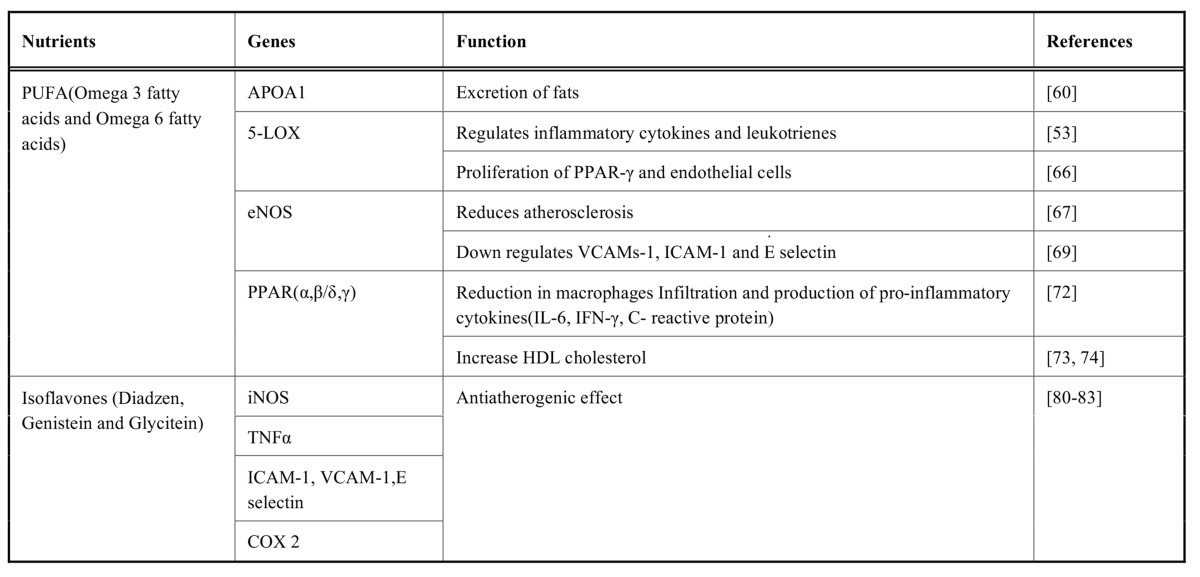
4. NUTRIENT-GENE INTERACTION IN LIFE-STYLE ASSOCIATE DISEASES
4.1. Nutrient Interaction in Diabetes
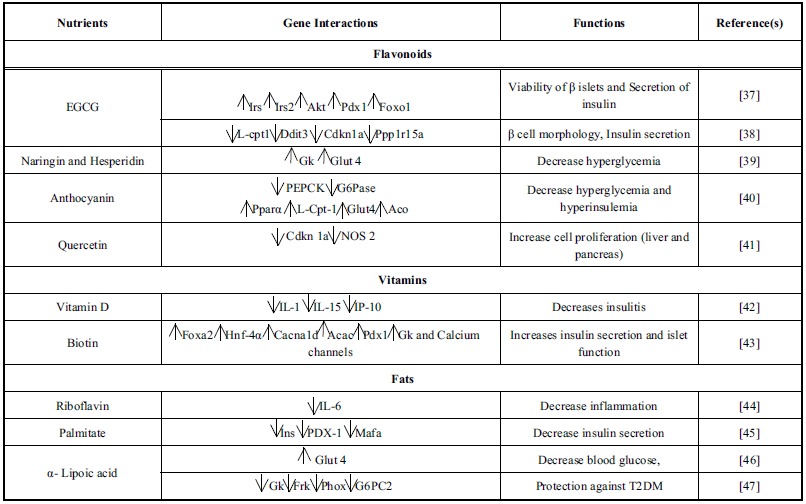
Diabetes mellitus (DM) is a complicated lifestyle associated metabolic disease designated with alteration in insulin secretion as well as function, or both resulting in hyperglycemia. A long-term complication resulted in failure and dysfunction of multiple organs such as heart, kidneys, nerves and blood vasculature [33]. Globally, the prevalence of diabetes is 8.3%, affecting 387 Million world population and expected further increase upto 592 million by 2035 [34]. In general, the diabetic cases are categorized into two type’s i.e. type 1 (T1DM) and type 2 (T2DM). T1DM is an autoimmune disease results from destruction of pancreatic β islets by T lymphocytes infiltration and consequent loss of β cells alongwith deficiency in insulin secretion. At the onset of disease about 70% of cells are damaged [35]. However, T2DM is the most prevalent type of complex disease, which accounts for > 90% diabetic cases [33]. A number of factors like insulin deficiency, insulin resistance, β cell dysfunction, impaired insulin signaling and oxidative stress associated with this disease that leads to complications like neuropathy, retinopathy, nephropathy, macro- and micro vascular conditions [36]. DM is more commonly among peoples between 40 and 59 years of age [34]. Several factors such as genes & diet, metabolic profile, environmental changes and their interactions plays crucial role in the disease progression (Table 2) [37-47]. ‘Omics’ approaches have been extensively used in exploring the impact of the dietary ingredients upon gene functioning. These approaches enable us to find new targets like genes, proteins and their interactions with nutrients [48]. The nutrient gene interaction studies are important to understand the associated factors, in the etiopathogenesis of metabolic syndromes. These factors modulate the gene expression directly or by activating various signalling molecules of complex metabolic pathways [49].
Table (2).
Interaction between nutrient, gene and their functions in diabetes.
|
4.1.1. Dietary Flavonoids Interaction
In vitro as well as in vivo studies revealed the beneficial effect of dietary flavonoids in DM. These secondary metabolites showed improved insulin signalling and its secretion, carbohydrate metabolism, and glucose uptake in various insulin-sensitive tissues [50]. Moreover, clinical studies have revealed that anthocyanin rich food, particularly from apples, blueberries and pears related with a lower risk of DM [51]. Generally, human diet containing vegetables, fruits, herbs, cocoa, tea, soya and other plant food products found to be rich in flavonoids. Scientific evidences showed that these metabolites exert beneficial effect in glucose homeostasis and regulates carbohydrate digestion through various insulin signaling pathways required in the management of DM [50, 51].
Epigallocatechin gallate (EGCG) is a flavan-3-ols that improves the viability of β cells and insulin secretion by activating insulin receptor proteins (IRS2) and AMP-activated protein kinase (AMK) signaling pathways in glucotoxic conditions [52]. EGCG aid in the downstream signaling of protein kinase B (Akt), insulin receptor (Ir), insulin receptor substrate-2 (Irs2), pancreatic duodenal homeobox1 (Pdx1), and the forkhead box protein O1 (Foxo1) [37]. In addition, it improves morphology of β cell and insulin secretion in db/db mice model. It was resulted from reduction in the expression level of carnitine palmitoyltransferase 1 (L-Cpt-1) (a mitochondrial fatty acid transporter), which in response reduces the level of fatty acids in blood circulation. EGCG supplementation also found to reduce the expression level of DNA-damage-inducible transcript-3 (Ddit-3), an endoplasmic reticulum stress marker and its downstream signaling targets like Cdkn1a as well as protein phosphatase 1, regulatory subunit 15A (Ppp1r15a). Reduced expression of these markers enhances pancreatic function and lowers the insulin resistance in diabetic mouse model [38].
In vivo studies employing naringin and hesperidin flavanones also showed antihyperglycemic properties in C57BL/Ks-db/db mice model. Increased expression of glucose transporter type 4 (Glut 4) and glucokinase (Gk) was observed in adipocytes that activates peroxisome proliferator-activated receptors (PAPRs) [39]. Anthocyanin exerts antidiabetic effect by improving insulin resistance and hyperglycemia in T2DM. It found to downregulate expression of gluconeogenic enzymes (PEPCK and G6Pase), while upregulated the PAPR-α, Glut4, L-Cpt-1 and aconitase (Aco) expression in the liver tissue [40]. Quercetin is one of the most abundant flavonol distributed in plant kingdom. It also found to downregulate expression of Cdkn 1a (regulator of cell cycle) and nitric oxide synthase inducible (NOS 2) genes in liver and pancreas of the streptozotocin induced diabetic mice models [41]. These studies suggest that flavonoids present in diet have significant antidiabetic potential as evident from gene expression in liver, pancreas and skeletal muscles. Thus, these metabolites possibly has regulatory role in the nutrient-induced responses such as release of insulin from β islets, sensitivity in insulin responding tissues, proliferation as well as regeneration of β islets and their survival.
4.1.2. Vitamin Interaction
Vitamins are the class of nutrient that exerts protective effect by acting as antioxidants. These antioxidant properties are directly related with decreased lipid peroxidation, lowered DNA damage or inhibition of various in vitro malignancies and other degenerative diseases [53]. Under in vivo experiments vitamin D able to preserve β cell function during the insulitis by down regulating the expression levels of inflammatory mediators like interleukins (IL-1, IL-15), and interferon -γ-inducible protein 10 (IP-10) in non-obese diabetic (NOD) mice model during the insulitis [42]. Biotin (vitamin H) an activator of carboxylase enzymes found to modify the expression of various genes at both transcriptional as well as the posttranscriptional levels. Biotin supplementation significantly intensifies glucose induced insulin secretion and functioning of β islets in mice models. Expression level of insulin, alpha 1D subunit (Cacna1d), forkhead box A2 (Foxa2), acetyl-CoA carboxylase (Acac), hepatocyte nuclear factor 4α (Hnf-4α), Pdx1, Gk and calcium channel also increased by biotin [43, 33]. Riboflavin, a cofactor of FAD and FMN significantly down regulated the cytokine-induced increased expression of IL-6 mRNA in NIT-1 insulinoma cells as well as in cultured islets [44].
4.1.3. Dietary Fat Interaction
Evidences show that dietary fats, a major macro energy nutrient, effectively maintain the balance between lipogenesis and fatty acid oxidation in human body. Certain dietary fatty acids also affect cell metabolism and gene expression in array of metabolic disorders. However, high fat diet intake is associated with high risk of T2DM [54]. Hagman et al.; 2005 found that elevated level of palmitate inhibits the glucose induced insulin gene expression. It reduces the binding of transcription factors i.e. pancreas-duodenal homeobox-1 (Pdx-1) and mammalian homologue of avian (MafA) to the insulin promoters resulting in the decreased secretion of insulin from rat islets [45].
α- lipoic acid is known to improve glucose metabolism in T2DM. In animal models, it decreases the blood glucose level and enhances the insulin stimulated glucose uptake into muscles through Glut 4 receptors [46]. In addition, the lipoic acid prevents the increased NAD(P)H oxidase subunit- p22 (phox) and glucose-6-phosphatase (G6pc2), Gk, fructokinase (Frk) gene expression in high fructose diet fed diabetic rats [47].
4.2. Nutrients-gene Interaction in Cardio Vascular Diseases
Cardio Vascular Diseases (CVD) is characterized by formation of intimal lesions due to lipid deposition, inflammatory response, fibrosis and cell death in the blood vessels [55]. CVD is the major cause of mortality, according to WHO report [56]. Global estimations revealed that, it is responsible for 17.5 million deaths in 2012 and representing 31% of all global deaths [57]. Nutrition plays important role in the management and prevention of CVDs [58]. Genes responsible for the metabolism and biosynthesis of lipids includes Arachidonate 5-lipoxygenase (ALOX5), fatty acid synthase (FASN), apolipoprotein E (APOE), lipoprotein lipase (LPL), peroxisome proliferator activated receptors (PPARs) etc, which can be modulated in a protective manner by shift to a healthy diet [59].
4.2.1. Polyunsaturated Fatty Acids (PUFA)
4.2.1.1. Apolipoprotein A
Apolipoprotein (APOA1) is a 28.1 kDa protein, an important component of high density lipoprotein (HDL) responsible for excretion from body [60]. This gene is used as a biomarker for prediction of CVDs like myocardial infarction [61]. Therefore, clearance of HDL is important from plasma by intake of low fat content diet. PUFA plays a crucial role in modulating expression of factors playing important role in metabolism of carbohydrates and lipids. Studies (Table 3) have shown that intake of these essential fatty acids reduced the LDL- cholesterol level in patients [62].
Table (3).
Interaction between nutrient, gene and their functions in CVD.
4.2.1.2. Lipoxygenase
Lipoxygenase or arachidonate 5-lipoxigenase (5-LOX) gene is an important enzyme that regulates synthesis of leukotrienes and inflammatory cytokines and chemokines. [63]. Its expression level is enhanced in case of atherosclerotic lesions, thereby mobilizing more inflammatory cells. Studies on APOE-/- 5-LO-/- and LDLR-/- 5-LO-/- mice models revealed the role of 5-LOX as a candidate gene in the progression of atherosclerosis [64]. Omega-3 PUFA diet is shown to nullify release of leukotrienes production by affecting eicosanoid biosynthesis [65]. In vivo studies revealed that omega -3 fatty acids provided protection to mice model of retinopathy owing to oxidation of 4-hydroxy-docosahexaenoic acid (4-HDHA), which inhibits angiogenesis by directly acting on PPAR-γ and endothelial cell proliferation [66] (Table 3).
4.2.1.3. Endothelial Nitric Oxide Synthase (eNOS)
Endothelial nitric oxide synthase functions in generating nitric oxide (NO) from L- arginine in blood vessels. NO diffusion to vascular smooth cells dilutes blood cells by stimulating guanylyl cyclase receptor and increasing concentration of cGMP. It also acts as an inhibitor of leukotrienes and platelet aggregation, which help in reduction of risk of adhesion of white blood cells and atherosclerosis. It was reported that increased eNOS expression associated with production of H2O2 and caused endothelial dysfunction, which leads to CVD [67].
L-arginine found to have beneficial effect in hypercholesterolemia in animals as well as humans [68]. n-3 PUFAs improve eNOS expression leading to vasorelaxation, and also lowers circulating markers like E-selectin, inter-cellular (ICAM-1) and vascular cell adhesion molecule-1 (VCAMs-11) [69] (Table 3).
4.2.1.4. Peroxisome Proliferator Activated Receptors (PPARs)
PPARs belong to nuclear receptor proteins, playing important role in cellular metabolism and development. It consists of 3 isoforms, PPAR-α, PPAR-β/δ, and PPAR-γ with variation in tissue distribution [70]. PPARs can be activated by long chain fatty acids or thiazolidinedione’s (PPAR-γ) ligand. FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) investigation revealed that PPAR-α activating drugs showed reduction in CVD along with myocardial infarction [71]. The possible mechanism under is reduction possibly by infiltration of macrophages at the site of plaque formation and production of pro-inflammatory mediators (IL-6, IFN-γ, C-reactive proteins etc.) [72]. PPAR-β/δ suggested to activate fatty acid oxidation in heart and muscle. It also increases HDL cholesterol, thereby decreasing fat deposits and help in management of CVD [73, 74]. PPAR-γ agonists showed beneficial effect by activating PPAR-γ, resulting in decline of matrix metalloproteinase 9, CD 40, plasma IL-6, IL-8 etc[75]. PUFA regulates gene expression either through generation of alternative ligand or by altering membrane fluidity [76]. The discussed results are clearly suggestive that depending upon individual makeup or genotype, dietary intervention can be used to treat or manage CVD (Table 3).
4.2.2. Cholesterol and Expression of VCAM-1
Vascular cell adhesion molecule-1 (VCAM-1) play significant role in endothelium and leukocytes interaction. Atherosclerosis onset binds monocytes and T lymphocytes by VCAM-1, which leads to plaque formation [77]. However, oxidized lipids required for VCAM-1 expression are induced through inflammatory pathway that involves pro-inflammatory cytokines such as tumor necrosis factor- α (TNF-α) and interleukin-1 (IL-1) [78] (Table 3).
4.2.3. Isoflavones Interventions in the Progression of CVD
Isoflavones constitute a subclass of the flavonoids having antioxidant properties. Diadzen, genistein and glycitein forms of the isoflavones reported to modulate expression of pro-inflammatory cytokines, including improvement in vascular reactivity and inhibition of platelet aggregation [79]. These compounds regulates the expression of various biomarkers like inducible nitric oxide synthase (iNOS), inflammatory cytokines (e.g. TNF-α), cell adhesion molecules (e.g. VCAM-1, ICAM-1, E-selectin) and cyclooxygenase-2 [80, 83] (Table 4).
Table (4).
Interaction between nutrient, gene and their functions in inflammation.
|
4.3. Diet and Inflammation
Nutritional composition of diet systems are known to affect the inflammatory process as a result of immune response modulation either by alteration of gene expression or interfering into the signaling cascade. Inflammation is a biological response of human body against any invasion or injuries, which may be acute (self-healing, short term) or chronic (required medication, long term). Later inflammation type can last for years and in due course lead to several diseases like some cancers, intestinal allergies, atherosclerosis etc. In a recent study, researchers have found that nutrients and certain food items influence inflammation [84]. Inflammatory process is regulated by immune system, in which several immunological mediators plays significant role [85].
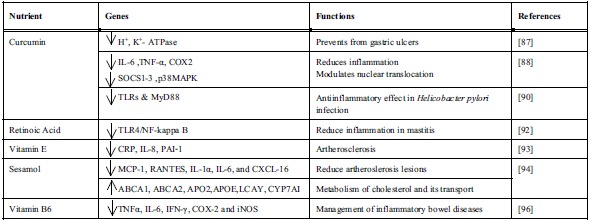
Curcuma longa (turmeric) well known golden spice has long been in use as Ayurvedic medicine against inflammatory conditions. It contains number of bioactive compounds, however, ‘curcumin’ is a major metabolites that accounts for numerous pharmacological activities i.e. antioxidant, antimicrobial and strong antiinflammatory properties. Evidences are available that advocate curcumin as a highly pleiotropic molecule, capable of interacting with molecular targets of inflammation. It was reported to be a highly potential therapeutic agent not only for inflammatory conditions, but also in certain types of cancers [86]. In rat animal model, curcumin was shown to have a preventive and therapeutic effect in gastric ulcer disease by acting as a strong inhibitor of H+, K+-ATPase activity. It downregulated the acetylation of histone H3, thereby inhibiting the transcription and expression of H+, K+-ATPase gene [87]. Curcumin strongly inhibits the expression of LPS-induced inflammatory mediators along with IL-6, TNF-α and COX-2 mRNAs in macrophages via modulating the expression of cytokine signaling-1(SOCS-1 and 3) and p38 MAPKsuppressors. Further amendment of nuclear translocation prevents the inhibition of LPS-induced SOCS-1 and -3 expressions and activation of p38 MAPK kinase [88]. Recent in vitro, in vivo and human clinical investigation on curcumin (diferyloylmethane) orally bioavailability and safety revealed that it inhibits TNF-α action and its production [89]. Curcumin also showed promising role in the prevention of Helicobacter pylori induced inflammation. H. pylori infection activates a sequence of gastric alterations with induction of gastric mucosa inflammation, which some time may evolves to gastric cancer. Curcumin exerted a significant antiinflammatory effect and even attenuate inflammation process by amending the expression of toll-like receptors (TLRs) and myeloid differentiation primary response gene 88 (MyD88) [90]. It is a promising example of nutritional approach in the prevention of H. pylori induced inflammation. Vitamin A supplementation proved beneficial against number of inflammatory conditions including various skin related disorders and various precancerous as well as cancerous states [91]. Retinoid, a derivative of vitamin A, is known to inhibit neoplasm and improve immune system. Owing to retinoic acid treatment, a significant increase in expressions of toll-like receptor 4 (TLR4) gene with decreased level of IL-1b β in mammary gland and NF-kappa B DNA binding activity was observed in lipopolysaccharide (LPS) induced mastitis rat model and mammary epithelial primary cell cultures [92]. It was deduced from the results that retinoic acid due to suppression of TLR4/NF-kappa B signaling attenuated the LPS induced inflammatory response.
Several evidences are available exhibiting inflammation’s role in CVDs or atherogenesis. Vitamin E and its isoforms have potent antioxidant and antiinflammatory properties. Tocopherol (α‐T) supplementation found responsible for decreasing C‐reactive protein (CRP), IL‐8 and plasminogen activator inhibitor-1 (PAI‐1) levels in human and animal subjects. Isoform γ‐T was effective in decreasing reactive nitrogen species and also appear to have antiinflammatory properties [93]. In vivo studies using experimental mice demonstrated sesame oil (sesamol as an active ingredient) application as an alternative treatment to atherosclerosis. Sesame oil diet significantly reduced atherosclerotic conditions, plasma cholesterol, LDL cholesterol and triglyceride levels in LDLR-/- mice models. Significant reduction of plasma inflammatory cytokines such as IL-6, IL-1α, regulated on activation, normal T cell expressed and secreted (RANTES), monocyte chemo attractant protein-1 (MCP-1), and chemokine (C-X-C motif) ligand 16 (CXCL-16) were, suggestive of its antiinflammatory properties. Gene array studies also revealed that sesame oil was able to induce ATP-binding cassette transporter (ABCA1 and ABCA2), lecithin- cholesterol acyltransferase (LCAT), APOE, and cholesterol 7α‐hydroxylase (CYP7A1) genes, which are involved in cholesterol metabolism and reverse cholesterol transport [94]. Although Vitamin B6 deficiency is not very common, however in certain population it can increases the risk of inflammation-related diseases [95]. Pyridoxal-5-phosphate (PLP), a bioactive derivative of vitamin B6 serves as a cofactor to numerous biochemical reactions. Plasma concentrations of PLP found inversely related to C-reactive proteins, which is a inflammation markers, and low PLP associated with inflammatory conditions like inflammatory bowel disease (IBD). Low as well as high PLP plasma concentration found significantly associated with suppression of colon specific molecular inflammation (TNFα, IL-6, IFN-γ, COX-2 and iNOS expression) and histological markers. Therefore, vitamin B6 supplementation suggested to be an additional means of IBD management [96] (Table 4).
5. DIETARY INTERVENTIONS IN MANAGEMENT OF LIFE-STYLE ASSOCIATE METABOLIC DISEASES
In recent time, there is more interest in nutrigenomics research to understand the effects of nutritious food and medication on metabolic disorders associated with lifestyle transition. Dietary interventions are supposed to play essential role in maintenance of individual’s well being and transforming immunity. In this section, an effort has been made to recapitulate the beneficial effects of food supplements in prevention or management of life-style associated diseases.
5.1. Functional Foods
Health and nutrition are at forefront of scientific community owing to their interactive revelation with diseases, which paved the way towards development of health promoting food prototype (s). According to ‘The Institute of Medicine's Food and Nutrition Board’ - Functional food is any food or food component that may provide a health benefit beyond the traditional nutrients it contains. Plant derived functional foods such as flavonoids, omega-3-fatty acids, dietary fibres, antioxidant vitamins, proteins, amino acids and prebiotics etc. found useful in managing diseases related to lifestyle e.g. congestive heart failure, arrhythmias, hypertension, angina and hyperlipidemias [97]. The information pertaining to various plant derived functional foods with their possible role in prevention and treatment of CVDs and diabetes is tabulated (Table 5) for precise documentation [98-11]. Curcumin, a dietary flavonoid, exhibited antiadipogenic role in human and murine pre-adipocytes [112]. Ciardi and his coworkers found that adipocytes incubated with curcumin showed a dose-dependent decrease in leptin and lipopolysaccharide induced IL-6 secretion [113]. Curcumin was found to reduce hyperglycemia and improved insulin sensitivity in T2DM induced male Sprague Dawley rats, along with concurrently reduction in the level of TNF-α [114]. Oral administration of Trigonella foenum graecum seed extract to streptozotocin-induced diabetic rats showed low blood glucose level, total cholesterol, triglycerides, while increase in HDL cholesterol in a dose-dependent manner [115]. The hypoglycemic property of Trigonella foenum graecum (fenugreek) seed powder is mainly exerted by its bioactive components such as 4- hydroxyisoleucine (amino acid) and steroid saponin trigonelline. A clinical trial consists of T2DM infested 24 adults was conducted using hot water soaked fenugreek seeds for eight weeks [116]. The results revealed significant decrease in the fasting blood sugar, triglycerides and very low density lipoproteins (VLDLs). Another nine week human clinical trial, which included 56 healthy men and women, was conducted to evaluate the effect of soy protein (25 g/d) with varied isoflavones concentration. Authors observed dose-dependent reduction in total and low density lipoprotein cholesterol levels, but did not showed any effect on the concentration of HDL and triglycerides [117]. In addition to natural health-promoting functional ingredients of plant origin, various bioactive components in animal products also have positive role in management of lifestyle related disorders. Likewise omega-3 fatty acids (docosahexaenoic and eicosapentaenoic acid) derived from fish oil play vital role in number of ailments, primarily cancer and CVD [118, 119]. A conjugated fatty acid i.e. linoleic acid (mixture of isomers of linoleic acid) isolated from grilled beef was found to possess anticarcinogenic activity [120]. Conjugated linoleic acid (CLA) supplements also reported to possess strong antiobesitic and hypolipidemic effects. Linoleic acid treatment found to raise body protein concentration, while concomitantly decreased the body fat [121, 122]. In vivo studies suggest that CLA supplementation enhances lipolysis in adipocytes, whereas reduced the fat deposition [123]. Probiotics are well known health promoting fermented food products. Reports are available that demonstrated hypocholesterolemic effect of Lactobacillus acidophilus by inhibiting 3-hydroxy-3-methylglutaryl CoA reductase activity, a key enzyme of cholesterol biosynthesis [124, 125]. Few clinical studies showing the effect of different functional food components on life style associated diseases are tabulated (Table6) [126-135].
Table (5).
Potential of functional foods in life style associated diseases.
| S. No | Functional Foods | Active Component | Potential Mechanism of Action | References |
|---|---|---|---|---|
| 1 | Green Tea | Catechins | Prevent both obesity and obesity-induced T2DM | [98] |
| 2 | Flaxseed | Lignans | LDL, total cholesterol and lipoprotein were reduced | [99] |
| 3 | Fenugreek | Saponins | Lowers lipid peroxidation and increase the antioxidant level. | [100] |
| 4. | Banaba leaves extract | Corosolic acid, ellagitannins | Antihyperglycemic(decrease fasting as well as postprandial blood glucose levels in humans) and antiobesity effect | [101] |
| 5 | Soy proteins | Phytoestrogens, Genistein and Daidzein | Lower blood cholesterol, reduces lipid peroxidation, antioxidant potential, |
[102] |
| 6. | Grapes and its products | Anthocyanidin, Flavan-3-ols, Flavonols | Shows hypotensive, hypolipidaemic and antiatherosclerotic effects |
[103] |
| 7 | Dark chocolate | Flavanols | Decrease blood pressure, decreases serum LDL cholesterol, improves flow-mediated dilation | [104] |
| 9. | Red wine, Berries, Peers, Apples | Proanthocyanidins | Antioxidant effects and inhibition of LDL oxidation, inhibition of the inflammatory response in atherosclerosis, improvement of endothelial function | [105] |
| 10. | Fruits and vegetables | Dietary fibre | Reduces fasting blood glucose and also affects glycosylated hemoglobin | [106] |
| 11. | Stevia rebaudina | Stevioside | Reduces postprandial blood glucose levels in Type 2 Diabetes Mellitus patients | [107] |
| 12. | Curcuma longa | Curcumin | Possess antidiabetic activity | [108, 109] |
| 13. | Allii cepae Bulbus | Allyl propyl disulfide and S-methyl cysteine sulfoxide | Antidiabetic and antihyperlipidemic effect | [110, 111] |
Table (6).
Clinical trials showing effect of functional food components on life style associated diseases.
| Extract/Compound | Study | Period | Experiment Design | Total Dosing | Effects | Year | Reference |
|---|---|---|---|---|---|---|---|
| Apple juice | Healthy men and women | 6 weeks | Unblinded, randomized, crossover design | 375 ml of apple juice or 340 g of whole apple | Decrease in plasma low-density lipoprotein oxidation | 2000 | [126] |
| Omega-3 fatty acids [DHA and EPA] | Dyslipidemic subjects (n=38) | 7 weeks | Parallel, double-blind trial | 3 gm EPA/d (n = 12), 3 gm DHA/d (n = 12) |
Increases systemic arterial compliance, Lowers total plasma and VLDL triacylglycerol | 2002 | [127] |
| Cocoa (Flavanol) | CAD (n =40) | 6-week | Randomized double-blind placebo-controlled | Flavanol-rich chocolate bar (flavanols 444 mg/day) or placebos total flavanols, 19.6 mg/day | Brachial artery FMD (flow-mediated dilation) and SAC (systemic arterial compliance) were assessed | 2006 | [128] |
| Black tea | Patients with mild serum cholesterol or triglycerites elevation (n=21) | 28 days | Randomized controlled parallel design | 5 cups per day of black tea | Improve vasodilator function in conduit arteries. | 2002 | [129] |
| Soy protein (isoflavones) | Healthy volunteers (n= 156) | 9 weeks | Double blind randomized parallel trial | 3, 27, 37, or 62mg isoflavones | Lower total and LDL cholesterol level | 1999 | [117] |
| Trigonella foenum graecum seeds | Type 2 diabetic patients (n=25) | 2 months | Double blind placebo controlled | 1 gm/day extract of fenugreek seeds | Increase in HDL cholesterol and decrease in serum triglycerides | 2001 | [130] |
| Hazelnut | Hypercholesterolemic men (n=15) | 8-week | - | MUFA-rich hazelnut (40 g/day) | Reduction in VLDL cholesterol, triacylglycerol, apolipoprotein B and increase in HDL, Total/HDL and LDL/HDL cholesterol ratios | 2007 | [131] |
| Cinnamon | Type 2 diabetic patients (n=60) | 40 days | - | Cinnamon dose 1, 3, 6 g/day | Decrease in serum glucose, triglyceride, LDL and total cholesterol in T2D diabetic people | 2003 | [132] |
| L-arginine | Patients with moderate and severe heart failure (n=15) | 6 weeks | Double-blind, placebo-controlled study | 6 to 12.6 g/d | Increase peripheral blood flow in patients with moderate to severe heart failure | 1996 | [133] |
| Stevioside | Type 2 diabetic patients (n=12) | - | Paired cross-over study | Meal supplemented with 1 g of stevioside or 1 g of maize starch (control) | Reduces postprandial blood glucose levels in T2D diabetic patients | 2004 | [134] |
| Probiotics | Type 2 diabetic patients (n=120) | 26 weeks | Randomized, double-blind, placebo-controlled trial | 2 g freeze-dried powder of the probiotics | Improves gut microbiota in T2DM, and also alters the levels of systemic endotoxin in systemic inflammatory condition | 2013 | [135] |
5.2. Nutraceuticals
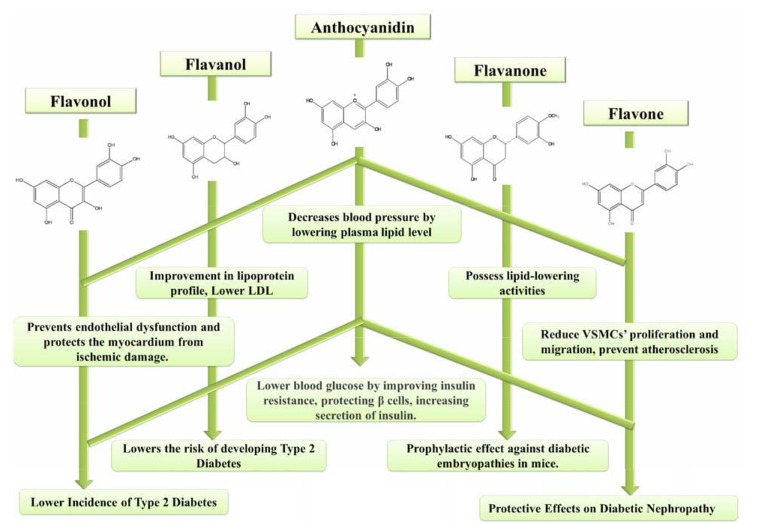
Past few decades has witnessed tremendous improvement in lifestyle of human kind, however, with some new friends in the form of `lifestyle associated metabolic diseases. Erroneous food habits suspected to be prime accused in nutritional debacle that led to present state of health. Nutraceutical, term was coined by Stephen DeFelice in 1989, who described it as “any substance that is a food or a part of food provides medical or health benefits, including the prevention and treatment of disease”[136]. The major cluster of nutraceuticals includes dietary phytochemicals such as antioxidant flavonoids, phytoestrogens, glycosinolates, dietary fibre and carotenoids. Dietary phytochemicals are naturally occurring bioactive plant derived compounds e.g. anthocyanins in blueberries, flavanols in green tea, lycopene in tomatoes, and resveratrol in grape seeds that have a positive effect on human health. Flavonoids exhibited inverse relationship between its dietary ingestion and risk of both diabetes as well as CVDs [137, 138]. The possible mechanism of dietary flavonoids in prevention and/or treatment of CVDs and diabetes is depicted in figure (Fig. 2). Green tea flavanol, epigallocatechin-3-O-gallate (EGCG), lowered the VCAM-1 and ICAM1 genes expression by inhibiting the protein and mRNA in human umbilical vein endothelial cells (HUVEC) induced by angiotensin II in concentration dependent manner (10 to 50 µM) [139]. Later, a significant relation was found with consumption of flavan-3-ols in lowering risk of coronary heart disease mortality [140]. Dihydrochalcone, phloridzin also demonstrated to have role in management of postprandial hyperglycemia. Dihydrochalcone fed at different oral doses (5, 10, 20 and 40 mg/kg body weight) reported to reduce blood glucose levels and improve dyslipidemia in streptozotocin-induced diabetic rat [141]. In another study, results showed that quercetin (0.08%) supplemented diet provided for 6 weeks to T2DM mice raised HDL-cholesterol, while lowered the total cholesterol. Dietary quercetin supplementation also reported to lower the increased activitiy of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) antioxidant enzymes in liver tissue [142]. Besides reducing liver fat accumulation in mice fed on western diet, quercetin supplementation also report to improve hyperglycemia, hyperinsulinemia and dyslipidemia by reducing the expression of PPAR-α [143]. In different study, addition of naringenin-7-O-glucoside in dose dependent manner (10, 20 and 40 μM) showed significant improvement in proliferation of rat cardiac H9C2 cell line [144]. The proliferation of cells attributed to increased expression of caspase-3 and caspase-9 mRNA along with elevated HO-1 mRNA expression. Polyphenols present in lemon such as flavanone showed protective effects against high-fat diet-induced obesity in mice model. Diet of obese mice supplemented with lemon polyphenols (eriocitrin, hesperidin and naringin etc.) found to increase the expression level of PPARs, and acyl-CoA oxidase, and also showed reduction in body weight [145]. Results revealed that the supplemented diet improved insulin resistance in experimental animal models by adjusting serum insulin and glucose level. Oral administration of citrus polyphenol hesperidin (500 mg/d) for three weeks to volunteers (n=24) with metabolic syndrome in a randomized, placebo-controlled, double-blind, crossover trial found to improve endothelial dysfunction, while reduced the activities of circulating inflammatory biomarkers such as C-reactive protein, serum amyloid A protein and soluble E-selectin [146]. It was concluded from these results that citrus polyphenol ingestion had vasculoprotective actions, which might highlight its positive cardiovascular effects. Oral administration of hesperidin and naringin (50 mg/kg body weight) for 30 days to high fat fed streptozotocin induced T2DM rats showed antihyperglycemic and antidyslipidemic effects [147]. The experimental animal showed improved level of glucose and serum enzymes (aspartate aminotransferase, lactate dehydrogenase and creatine kinase), while serum insulin and muscle glycogen content were decreased. Hence, in addition to nutrient supply, nutraceutical also possess potential to manage and/or prevent nutrition-linked diseases.
Fig. (2).
Atheroprotective and antidiabetic activity of dietary flavonoids.
5.3. Dietary Supplements (DS)
Dietary supplements are the products intended to supplement human diet and not anticipated to prevent any disease. DS are available in the form of pills, capsules, tablets and used to meet requirement of specific nutrient, fitness, optimum physical health or improve overall well-being health [148]. Generally the DS contain biologically active crude or purified primary or secondary metabolites. However, DS can be categorized depending upon constituent or health application e.g. vitamin supplements, dietary fiber supplement and mineral supplements, and supplements for weight-loss, colds, ageing population, brain, bone health, immune system, heart health, gastrointestinal health etc. [149]. A clinical study conducted on around 40,000 male volunteers 40-75 years aged suggested that vitamin E helps in reduction of coronary heart disease (CHD) risk. Results also revealed that dietary supplementation of ≥ 60 vitamin E IU/d lowers the CHD damage by 36% than men those consumed ≤ 7.5 IU/d [150]. These findings get supported by another study, which involved 87,245 female nurses. The data showed that vitamin E supplementation for ≥ 2 years of the subjects resulted in reduction of coronary disease damage up to 41% [151]. In addition, oral ingestion of arginine (a nonessential amino acid) showed protective effect against cardiovascular diseases. In a clinical study, 15 subjects were given arginine hydrochloride supplementation at varied concentration (6 to 12.6 g/d) for 6 weeks and results showed significantly higher blood flow (from 5.1±2.8 to 6.6±3.4 mL/min/dL) in forearm [152]. Obesity is one of the leading health issues in present scenario and it is directly related to other health disorders such as T2DM, hypertension and heart diseases. Secondary metabolites like flavonoids, hydroxycitric acid, conjugated linoleic acid, pyruvate etc. are found as an active ingredient in weight-loss supplements to improve fat and carbohydrate metabolism [153]. However, these compounds are also linked to diabetes. High-fat diet supplemented with luteolin (0.002 and 0.01%) given to male C57BL/6 mice for 12 week, not only able to suppress the induced body weight gain, but also improved glucose intolerance and insulin sensitivity [154]. A pilot study carried out on 13 T1DM subjects showed that daily omega-3-fatty acid diet supplementation (5.4 gm of eicosapentaenoic and 2.3 g of docosahexaenoic acid) for 4 week, decreased the level of blood glucose, triglycerides and HDL cholesterol [155]. Also, cocoa polyphenols reported to possess protective effect in management of cardiovascular and inflammatory diseases. Meta analysis revealed that cocoa supplements given to 173 hypertensive subjects for 2 weeks significantly reduced blood pressure [156]. In another study, subjects with T2DM given cocoa phenolic rich chocolate, showed elevated level of HDL cholesterol, and decreased total cholesterol and HDL ratio [157]. The data reported on EGCG supplementation diet fed to pathologically altered islets revealed prevention of T2DM and instead showed that modified diet increased the size and number of islets in db/db mice [158]. The study performed to evaluate the effect of probiotics (dose of 200 mL @ 4 × 108 CFU/100 mL concentration symbiotic cultures of Lactobacillus acidophilus and Bifidobacterium bifidum) in T2DM patient’s demonstrated decrease in total cholesterol and triglycerides as well as HDL cholesterol level [159]. A randomized, double-blind, controlled clinical study was conducted to evaluate the effect of probiotic yogurt (given Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12; 300 g/d for 6 weeks) in T2DM patients. The information generated revealed that supplementation resulted in decreased levels of fasting glucose and HbA1c, though antioxidant activity, erythrocyte SOD and GSH-Px activities found to increase [160]. In vivo study involving male albino Wistar rat, fed on high fructose diet supplemented with ‘dahi’(milk based food), showed improvement in blood glucose level, dyslipidemia, oxidative damage and insulin secretion. Thus, advocating ‘dahi’ (milk fermented by mixed cultures of L. acidophilus, L. casei and L. lactis) as probiotic supplement in management of T2DM disease [161].
The above discussed observations from various epidemiological studies clearly supports the notion that functional food/nutraceutical/dietary supplements can be a part of a dietary interventions based on nutrigenomics in management of life style related metabolic human health complications, especially CVDs, diabetes and inflammation.
6. ETHICAL AND REGULATORY ISSUES
Similar to pharmacogenomics, nutrigenomics also expected to demonstrate proof of concept prior to become a reality. A diverse opinion regarding the disease claims exists owing to predictive nature of nutritional genomics studies [162-164]. Hence, reservations concerning outcomes or products of nutrigenomic are obvious with respect to their acceptability across region, ethnicity, age-group, gender etc. [165]. The regulatory guidelines about offering of nutrigenomic products and services by public or private means or through health practitioner are also an issue of intense debate [166]. Health benefits of food constituents, dietary supplements, functional food or even nutraceuticals depends upon individual’s genetic variation and life style environment against particular disease [167]. In present case also, pharmacogenomics model can be look upto, where human volunteers are being employed to answer pertinent scientific queries thorough long term clinical trials [168]. Now, it is well established that primary and/or secondary metabolites present in food have profound effects on individual’s genetic profile, thereby influencing the phenotype [165]. The ethical guidelines that are established for conventional nutritional research can be followed in nutrigenomics, as it also involve human model for collection of sample or information from such model [169]. However, the twist in nutrigenomics lies with the type of data collected and information generated i.e. diet-gene interaction, genotypic characterization, reusability of stored data in biobanks, and sharing of such information across boundaries [170]. Hence, a different set of socio-ethical guidelines and regulatory system may be needed to assess the nutrigenomics products. In response, NuGO was established by European Union (EU) with an aim to mobilize nutrigenomics studies and since 2010 looking for bigger role globally. It is an association of various institution engaged in nutritional genomics R&D activities across Europe. NuGO evolved from an EU Sixth Framework Network of Excellence with a primary objective of formulating guidelines and procedures for conducting nutrigenomics studies [169]. The ethical guidelines were devised after deliberations among individual researchers, expert groups and organizations and summarized into four components i.e. revelation of experiments to the volunteers, and their prior consent for participation; protection of participants against bigotry and misinterpretation of genotype data or information; preservation and maintenance of collected biological samples in repository with respect to genetic information and personal data of volunteers; and finally access or exchange of participant’s samples or data for future R&D developments.
In addition, University of Montreal, Canada based OMICS-ETHICS Research Group also looking into ethical issues (such as ethnicity, age, regional specificity, gender, exaggerated claims etc.) of studies including nutrigenomics, which apply ‘OMICS’ and related technologies. The emphasize of this group is to establish evidence -based system that enable to validate socio- ethical issues in relation to omic technologies and study their feasibility to devise suitable guidelines for conducting R&D in the field of nutritional genomics [171].
CONCLUSION
Transition in dietary system & eating habits, availability of diversified food, improved living environment & work standard, easy lifestyle, increased economic status are some examples of present life style. No doubt that this shift has improved present human living standard by many-fold, but not without compromising their health status, as evident from staggering human mortality rate of past decade owned by non-communicable diseases. Fortunately, past few decades has also witnessed the revelation of human genome along with high-through put tools, which equipped nutritional and medical science researchers to countenance the posed challenges. Nutrigenomics has not only raised expectation of unhealthy population, but it also becomes popular among healthy people to utilize generated information for maintenance of present well being and devise future health plan through diet modification. The world over efforts resulted in number of in vitro, in vivo and clinical studies, which raised expectation of general public of healthy living through dietary intervention. However, researchers need to maintain high standard of integrity and address these expectation through a validated regulatory mechanism, supported by ethical perseverance.
ACKNOWLEDGEMENTs
The authors wish to thank the Director, CSIR-IHBT Palampur for continuous encouragement and support. SK wishes to thank Supriya Sharma for her support during part of manuscript writing. All authors contributed substantially for the manuscript development.
CONFLICT OF INTEREST
Authors acknowledge Council of Scientific and Industrial Research, New Delhi for financial support through projects (BSC 0105 & MLP 0039). Shiv Kumar acknowledges RJNF-UGC for Junior Research Fellowship. The authors declare that there is no conflict of interest.
REFERENCES
- 1.Constitution of the World Health Organization Basic Documents; Official Document No. 240 (Washington). The Constitution of WHO was adopted at the International Health Conference held in 1946 in New York, where it was signed by the representatives of sixty-one states (hereafter coted as WHO Constitution); 1991. [Google Scholar]
- 2.Neeha V.S., Kinth P. Nutrigenomics research: a review. J Food Sci Technol. 2013;50(3):415–428. doi: 10.1007/s13197-012-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Diet, nutrition and the prevention of chronic diseases. Report of a WHO Study Group. (WHO Technical Report Series, No. 797), Geneva. 1990. [PubMed] [Google Scholar]
- 4.Ovesná J., Slabý O., Toussaint O., Kodícek M., Marsík P., Pouchová V., Vanĕk T. High throughput ‘omics’ approaches to assess the effects of phytochemicals in human health studies. Br. J. Nutr. 2008;99 E(Suppl. 1):ES127–ES134. doi: 10.1017/S0007114508965818. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Non-communicable Diseases Country Profile. Geneva: 2011. [Google Scholar]
- 6.Fatma A.M. Lifestyle diseases: an economic burden on the health services. UN Chronicle- Achieving global health. Vol. XLVII No. 2. 2010 [Google Scholar]
- 7.Shetty P.S. Nutrition transition in India. Public Health Nutr. 2002;5(1A):175–182. doi: 10.1079/PHN2001291. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Preventing Chronic Diseases: a vital investment. Geneva: 2005. [Google Scholar]
- 9.World Health Organization . Interventions on diet and physical activity. What works? Geneva, 2008. [PubMed] [Google Scholar]
- 10.Ebrahim S., Pearce N., Smeeth L., Casas J.P., Jaffar S., Piot P. Tackling non-communicable diseases in low- and middle-income countries: is the evidence from high-income countries all we need? PLoS Med. 2013;10(1):e1001377. doi: 10.1371/journal.pmed.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khatibzadeh S., Micha M., Afshin A., Rao M., Yakoob M.Y., Mozaffarian D. Major dietary risk factors for chronic diseases: a systematic review of the current evidence for causal effects and effect Sizes. Circulation. 2012;125:AP060. [Google Scholar]
- 12.Bosma-den Boer M.M., van Wetten M.L., Pruimboom L. Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutr. Metab. (Lond) 2012;9(1):32. doi: 10.1186/1743-7075-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordain L., Eaton S.B., Sebastian A., Mann N., Lindeberg S., Watkins B.A., O’Keefe J.H., Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 2005;81(2):341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzawa Y. [Life style-related disease]. Nippon Rinsho. 2001;59(1):188–194. [PubMed] [Google Scholar]
- 15.West C.E., Renz H., Jenmalm M.C., Kozyrskyj A.L., Allen K.J., Vuillermin P., Prescott S.L., in-FLAME Microbiome Interest Group The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J. Allergy Clin. Immunol. 2015;135(1):3–13. doi: 10.1016/j.jaci.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Nugent R. Chronic diseases in developing countries: health and economic burdens. Ann. N. Y. Acad. Sci. 2008;1136:70–79. doi: 10.1196/annals.1425.027. [DOI] [PubMed] [Google Scholar]
- 17.Paliyath G., Bakovic M., Shetty K. Functional Foods, Nutraceuticals, and, Degenerative Disease Prevention. UK: Wiley Blackwell; 2011. [DOI] [Google Scholar]
- 18.Ohlhorst S.D., Russell R., Bier D., Klurfeld D.M., Li Z., Mein J.R., Milner J., Ross A.C., Stover P., Konopka E. Nutrition research to affect food and a healthy life span. J. Nutr. 2013;143(8):1349–1354. doi: 10.3945/jn.113.180638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lillycrop K.A., Burdge G.C. Epigenetic mechanisms linking early nutrition to long term health. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26(5):667–676. doi: 10.1016/j.beem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Virmani A., Binienda Z., Ali S., Gaetani F. Links between nutrition, drug abuse, and the metabolic syndrome. Ann. N. Y. Acad. Sci. 2006;1074:303–314. doi: 10.1196/annals.1369.027. [DOI] [PubMed] [Google Scholar]
- 21.Jukes T.H. The prevention and conquest of scurvy, beri-beri, and pellagra. Prev. Med. 1989;18(6):877–883. doi: 10.1016/0091-7435(89)90023-6. [DOI] [PubMed] [Google Scholar]
- 22.Boeing H., Bechthold A., Bub A., Ellinger S., Haller D., Kroke A., Leschik-Bonnet E., Müller M.J., Oberritter H., Schulze M., Stehle P., Watzl B. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012;51(6):637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omenn G.S., Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Glass A., Keogh J.P., Meyskens F.L., Valanis B., Williams J.H., Barnhart S., Hammar S. Effects of a combination of β carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 24.Virmani A., Pinto L., Binienda Z., Ali S. Food, nutrigenomics, and neurodegeneration--neuroprotection by what you eat! Mol. Neurobiol. 2013;48(2):353–362. doi: 10.1007/s12035-013-8498-3. [DOI] [PubMed] [Google Scholar]
- 25.Roche H.M. Nutrigenomics – new approaches for human nutrition research. J. Sci. Food Agric. 2006;86:1156–1163. doi: 10.1002/jsfa.2484. [DOI] [Google Scholar]
- 26.Kersten S., Mandard S., Tan N.S., Escher P., Metzger D., Chambon P., Gonzalez F.J., Desvergne B., Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 2000;275(37):28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 27.Waterland R.A., Jirtle R.L. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20(1):63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Frankenberger T.R., Maxwell S. Food Security – Concepts, indicators, measurements – A technical review. New York: UNICEF/IFAD; 1992. [Google Scholar]
- 29.Ramsingh B. Codex Alimentarius Commission. 2012. [DOI] [Google Scholar]
- 30.World Health Organization . Diet, nutrition and the prevention of chronic diseases. Report of a Joint WHO/FAO Expert Consultation, Geneva. 2003. [Google Scholar]
- 31.Popkin B.M. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006;84(2):289–298. doi: 10.1093/ajcn/84.1.289. [DOI] [PubMed] [Google Scholar]
- 32.Kaput J., Rodriguez R.L. Nutritional genomics: the next frontier in the postgenomic era. Physiol. Genomics. 2004;16(2):166–177. doi: 10.1152/physiolgenomics.00107.2003. [DOI] [PubMed] [Google Scholar]
- 33.Diagnosis and Classification of Diabetes Mellitus, American Diabetes Association Diabetes Care. 2010;33:62–69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Diabetes Federation IDF Diabetes Atlas, 6th ed. http://www.idf.org/sites/default/files/EN 6E Atlas Full 0.pdf . 2013. [Accessed January 20, 2015].
- 35.Soltani N., Qiu H., Aleksic M., Glinka Y., Zhao F., Liu R., Li Y., Zhang N., Chakrabarti R., Ng T., Jin T., Zhang H., Lu W.Y., Feng Z.P., Prud’homme G.J., Wang Q. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA. 2011;108(28):11692–11697. doi: 10.1073/pnas.1102715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002;23(5):599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 37.Cai E.P., Lin J.K. Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J. Agric. Food Chem. 2009;57(20):9817–9827. doi: 10.1021/jf902618v. [DOI] [PubMed] [Google Scholar]
- 38.Ortsäter H., Grankvist N., Wolfram S., Kuehn N., Sjöholm A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr. Metab. (Lond) 2012;9:11–13. doi: 10.1186/1743-7075-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung U.J., Lee M.K., Park Y.B., Kang M.A., Choi M.S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006;38(7):1134–1145. doi: 10.1016/j.biocel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Takikawa M., Inoue S., Horio F., Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010;140(3):527–533. doi: 10.3945/jn.109.118216. [DOI] [PubMed] [Google Scholar]
- 41.Kobori M., Masumoto S., Akimoto Y., Takahashi Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol. Nutr. Food Res. 2009;53(7):859–868. doi: 10.1002/mnfr.200800310. [DOI] [PubMed] [Google Scholar]
- 42.Gysemans C.A., Cardozo A.K., Callewaert H., Giulietti A., Hulshagen L., Bouillon R., Eizirik D.L., Mathieu C. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146(4):1956–1964. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 43.Lazo de la Vega-Monroy M.L., Larrieta E., German M.S., Baez-Saldana A., Fernandez-Mejia C. Effects of biotin supplementation in the diet on insulin secretion, islet gene expression, glucose homeostasis and beta-cell proportion. J. Nutr. Biochem. 2013;24(1):169–177. doi: 10.1016/j.jnutbio.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Cobianchi L., Fornoni A., Pileggi A., Molano R.D., Sanabria N.Y., Gonzalez-Quintana J., Bocca N., Marzorati S., Zahr E., Hogan A.R., Ricordi C., Inverardi L. Riboflavin inhibits IL-6 expression and p38 activation in islet cells. Cell Transplant. 2008;17(5):559–566. doi: 10.3727/096368908785096060. [DOI] [PubMed] [Google Scholar]
- 45.Hagman D.K., Hays L.B., Parazzoli S.D., Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J. Biol. Chem. 2005;280(37):32413–32418. doi: 10.1074/jbc.M506000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konrad D., Somwar R., Sweeney G., Yaworsky K., Hayashi M., Ramlal T., Klip A. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes. 2001;50(6):1464–1471. doi: 10.2337/diabetes.50.6.1464. [DOI] [PubMed] [Google Scholar]
- 47.Castro M.C., Francini F., Gagliardino J.J., Massa M.L. Lipoic acid prevents fructose-induced changes in liver carbohydrate metabolism: Role of oxidative stress. Biochim. Biophys. Acta. 2014;1840:1145–1151. doi: 10.1016/j.bbagen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Ovesna J., Slaby O., Toussaint O., Kodícek M. Marsík. P.; Pouchova, V.; Vanek, T. High throughput ‘omics’ approaches to assess the effects of phytochemicals in human health studies. Br. J. Nutr. 2008;99:127–134. doi: 10.1017/S0007114508965818. [DOI] [PubMed] [Google Scholar]
- 49.Mutch D.M., Wahli W., Williamson G. Nutrigenomics and nutrigenetics: the emerging faces of nutrition. FASEB J. 2005;19(12):1602–1616. doi: 10.1096/fj.05-3911rev. [DOI] [PubMed] [Google Scholar]
- 50.Babu P.V., Liu D., Gilbert E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013;24(11):1777–1789. doi: 10.1016/j.jnutbio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wedick N.M., Pan A., Cassidy A., Rimm E.B., Sampson L., Rosner B., Willett W., Hu F.B., Sun Q., van Dam R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012;95(4):925–933. doi: 10.3945/ajcn.111.028894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin C.L., Lin J.K. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human hepG2 hepatoma cells. Mol. Nutr. Food Res. 2008;52(8):930–939. doi: 10.1002/mnfr.200700437. [DOI] [PubMed] [Google Scholar]
- 53.Sies H., Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995;62(6) Suppl.:1315S–1321S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- 54.Misra A., Singhal N., Khurana L. Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: role of dietary fats and oils. J. Am. Coll. Nutr. 2010;29(3) Suppl.:289S–301S. doi: 10.1080/07315724.2010.10719844. [DOI] [PubMed] [Google Scholar]
- 55.Mangge H., Becker K., Fuchs D., Gostner J.M. Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. 2014;6(6):462–477. doi: 10.4330/wjc.v6.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pagidipati N.J., Gaziano T.A. Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation. 2013;127(6):749–756. doi: 10.1161/CIRCULATIONAHA.112.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization. cardiovascular disease Fact sheet No.317, Updated January, 2015. http://www.who.int/mediacentre/factsheets/fs317/en/ 2015. [accessed on Feburary, 2015].
- 58.Bazzano L.A., Serdula M.K., Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr. Atheroscler. Rep. 2003;5(6):492–499. doi: 10.1007/s11883-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 59.Dimitriou M.E., Dedoussis G.V. Gene–diet interactions in cardiovascular disease. Curr. Nutr. Rep. 2012;1:153–160. doi: 10.1007/s13668-012-0020-4. [DOI] [Google Scholar]
- 60.Iacoviello L., Santimone I., Latella M.C., de Gaetano G., Donati M.B. Nutrigenomics: a case for the common soil between cardiovascular disease and cancer. Genes Nutr. 2008;3(1):19–24. doi: 10.1007/s12263-008-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McQueen M.J., Hawken S., Wang X., Ounpuu S., Sniderman A., Probstfield J., Steyn K., Sanderson J.E., Hasani M., Volkova E., Kazmi K., Yusuf S., INTERHEART study investigators Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372(9634):224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 62.Mata P., Lopez-Miranda J., Pocovi M., Alonso R., Lahoz C., Marin C., Garces C., Cenarro A., Perez-Jimenez F., de Oya M., Ordovas J.M. Human apolipoprotein A-I gene promoter mutation influences plasma low density lipoprotein cholesterol response to dietary fat saturation. Atherosclerosis. 1998;137(2):367–376. doi: 10.1016/S0021-9150(97)00265-7. [DOI] [PubMed] [Google Scholar]
- 63.Funk C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 64.Poeckel D., Funk C.D. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc. Res. 2010;86(2):243–253. doi: 10.1093/cvr/cvq016. [DOI] [PubMed] [Google Scholar]
- 65.Ferretti A., Nelson G.J., Schmidt P.C., Kelley D.S., Bartolini G., Flanagan V.P. Increased dietary arachidonic acid enhances the synthesis of vasoactive eicosanoids in humans. Lipids. 1997;32(4):435–439. doi: 10.1007/s11745-997-0057-5. [DOI] [PubMed] [Google Scholar]
- 66.Sapieha P., Stahl A., Chen J., Seaward M.R., Willett K.L., Krah N.M., Dennison R.J., Connor K.M., Aderman C.M., Liclican E., Carughi A., Perelman D., Kanaoka Y., Sangiovanni J.P., Gronert K., Smith L.E. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids. Sci. Transl. Med. 2011;3(69):69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Förstermann U., Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 2011;164(2):213–223. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Förstermann U., Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 69.Mozaffarian D., Wu J.H. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 70.Mukherjee R., Jow L., Noonan D., McDonnell D.P. Human and rat peroxisome proliferator activated receptors (PPARs) demonstrate similar tissue distribution but different responsiveness to PPAR activators. J. Steroid Biochem. Mol. Biol. 1994;51(3-4):157–166. doi: 10.1016/0960-0760(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 71.FIELD Study Investigators The need for a large-scale trial of fibrate therapy in diabetes: the rationale and design of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Cardiovasc. Diabetol. 2004;•••:3. doi: 10.1186/1475-2840-3-9. [ISRCTN64783481]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilde A.J., Fruchart J.C., Staels B. Peroxisome Proliferator-Activated Receptors at the Crossroads of Obesity, Diabetes, and Cardiovascular Disease. Journ. Annu. Diabetol. Hotel Dieu. 2007;48:24–32. [PubMed] [Google Scholar]
- 73.Luquet S., Lopez-Soriano J., Holst D., Fredenrich A., Melki J., Rassoulzadegan M., Grimaldi P.A. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17(15):2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- 74.Leibowitz M.D., Fiévet C., Hennuyer N., Peinado-Onsurbe J., Duez H., Bergera J., Cullinan C.A., Sparrow C.P., Baffic J., Berger G.D., Santini C., Marquis R.W., Tolman R.L., Smith R.G., Moller D.E., Auwerx J. Activation of PPARdelta alters lipid metabolism in db/db mice. FEBS Lett. 2000;473(3):333–336. doi: 10.1016/S0014-5793(00)01554-4. [DOI] [PubMed] [Google Scholar]
- 75.Wilding J.P. PPAR agonists for the treatment of cardiovascular disease in patients with diabetes. Diabetes Obes. Metab. 2012;14(11):973–982. doi: 10.1111/j.1463-1326.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 76.Tai E.S., Demissie S., Cupples L.A., Corella D., Wilson P.W., Schaefer E.J., Ordovas J.M. Association between the PPARA L162V polymorphism and plasma lipid levels: the Framingham Offspring Study. Arterioscler. Thromb. Vasc. Biol. 2002;22(5):805–810. doi: 10.1161/01.ATV.0000012302.11991.42. [DOI] [PubMed] [Google Scholar]
- 77.Huo Y., Ley K. Adhesion molecules and atherogenesis. Acta Physiol. Scand. 2001;173(1):35–43. doi: 10.1046/j.1365-201X.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 78.Libby P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006;83(2):456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 79.Rimbach G., Boesch-Saadatmandi C., Frank J., Fuchs D., Wenzel U., Daniel H., Hall W.L., Weinberg P.D. Dietary isoflavones in the prevention of cardiovascular disease--a molecular perspective. Food Chem. Toxicol. 2008;46(4):1308–1319. doi: 10.1016/j.fct.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 80.Gottstein N., Ewins B.A., Eccleston C., Hubbard G.P., Kavanagh I.C., Minihane A.M., Weinberg P.D., Rimbach G. Effect of genistein and daidzein on platelet aggregation and monocyte and endothelial function. Br. J. Nutr. 2003;89(5):607–616. doi: 10.1079/BJN2003820. [DOI] [PubMed] [Google Scholar]
- 81.Comalada M., Ballester I., Bailón E., Sierra S., Xaus J., Gálvez J., de Medina F.S., Zarzuelo A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochem. Pharmacol. 2006;72(8):1010–1021. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 82.Rimbach G., Weinberg P.D., de Pascual-Teresa S., Alonso M.G., Ewins B.A., Turner R., Minihane A.M., Botting N., Fairley B., Matsugo S., Uchida Y., Cassidy A. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim. Biophys. Acta. 2004;1670:229–237. doi: 10.1016/j.bbagen.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 83.Hermenegildo C., Oviedo P.J., García-Pérez M.A., Tarín J.J., Cano A. Effects of phytoestrogens genistein and daidzein on prostacyclin production by human endothelial cells. J. Pharmacol. Exp. Ther. 2005;315(2):722–728. doi: 10.1124/jpet.105.090456. [DOI] [PubMed] [Google Scholar]
- 84.Shivappa N., Hébert J.R., Rietzschel E.R., De Buyzere M.L., Langlois M., Debruyne E., Marcos A., Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015;113(4):665–671. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rankin J.A. Biological mediators of acute inflammation. AACN Clin. Issues. 2004;15(1):3–17. doi: 10.1097/00044067-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 86.Parsad S., Aggarwal B. 2011. [Google Scholar]
- 87.He P., Zhou R., Hu G., Liu Z., Jin Y., Yang G., Li M., Lin Q. Curcumin-induced histone acetylation inhibition improves stress-induced gastric ulcer disease in rats. Mol. Med. Rep. 2015;11(3):1911–1916. doi: 10.3892/mmr.2014.2958. [DOI] [PubMed] [Google Scholar]
- 88.Guimarães M.R., Leite F.R., Spolidorio L.C., Kirkwood K.L., Rossa C., Jr Curcumin abrogates LPS-induced pro-inflammatory cytokines in RAW 264.7 macrophages. Evidence for novel mechanisms involving SOCS-1, -3 and p38 MAPK. Arch. Oral Biol. 2013;58(10):1309–1317. doi: 10.1016/j.archoralbio.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aggarwal B.B., Gupta S.C., Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013;169(8):1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Santos A.M., Lopes T., Oleastro M., Gato I.V., Floch P., Benejat L., Chaves P., Pereira T., Seixas E., Machado J., Guerreiro A.S. Curcumin inhibits gastric inflammation induced by Helicobacter pylori infection in a mouse model. Nutrients. 2015;7(1):306–320. doi: 10.3390/nu7010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reifen R. Vitamin A as an anti-inflammatory agent. Proc. Nutr. Soc. 2002;61(3):397–400. doi: 10.1079/PNS2002172. [DOI] [PubMed] [Google Scholar]
- 92.Gu B., Miao J., Fa Y., Lu J., Zou S. Retinoic acid attenuates lipopolysaccharide-induced inflammatory responses by suppressing TLR4/NF-kappaB expression in rat mammary tissue. Int. Immunopharmacol. 2010;10(7):799–805. doi: 10.1016/j.intimp.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 93.Singh U., Devaraj S., Vitamin E. Vitamin E: inflammation and atherosclerosis. Vitam. Horm. 2007;76:519–549. doi: 10.1016/S0083-6729(07)76020-X. [DOI] [PubMed] [Google Scholar]
- 94.Narasimhulu C.A., Selvarajan K., Litvinov D., Parthasarathy S. Anti-atherosclerotic and anti-inflammatory actions of sesame oil. J. Med. Food. 2015;18(1):11–20. doi: 10.1089/jmf.2014.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lotto V., Choi S.W., Friso S. Vitamin B6: a challenging link between nutrition and inflammation in CVD. Br. J. Nutr. 2011;106(2):183–195. doi: 10.1017/S0007114511000407. [DOI] [PubMed] [Google Scholar]
- 96.Selhub J., Byun A., Liu Z., Mason J.B., Bronson R.T., Crott J.W. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J. Nutr. Biochem. 2013;24(12):2138–2143. doi: 10.1016/j.jnutbio.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramaa C.S., Shirode A.R., Mundada A.S., Kadam V.J. Nutraceuticals--an emerging era in the treatment and prevention of cardiovascular diseases. Curr. Pharm. Biotechnol. 2006;7(1):15–23. doi: 10.2174/138920106775789647. [DOI] [PubMed] [Google Scholar]
- 98.Bae K.C., Park J.H., Na A.Y., Kim S.J., Ahn S., Kim S.P., Oh B.C., Cho H.C., Kim Y.W., Song D-K. Effect of green tea extract/poly-γ-glutamic acid complex in obese Type 2 Diabetic Mice. Diabetes Metab. J. 2013;37(3):196–206. doi: 10.4093/dmj.2013.37.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peterson J., Dwyer J., Adlercreutz H., Scalbert A., Jacques P., McCullough M.L. Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 2010;68(10):571–603. doi: 10.1111/j.1753-4887.2010.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ravikumar P., Anuradha C.V. Effect of fenugreek seeds on blood lipid peroxidation and antioxidants in diabetic rats. Phytother. Res. 1999;13(3):197–201. doi: 10.1002/(SICI)1099-1573(199905)13:3<197::AID-PTR413>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 101.Miura T., Takagi S., Ishida T. Management of diabetes and its complications with Banaba (Lagerstroemia speciosa L.) and corosolic acid. eCAM. 2012;2012 doi: 10.1155/2012/87149. Article ID 871495, 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiseman H., O’Reilly J.D., Adlercreutz H., Mallet A.I., Bowey E.A., Rowland I.R., Sanders T.A. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am. J. Clin. Nutr. 2000;72(2):395–400. doi: 10.1093/ajcn/72.2.395. [DOI] [PubMed] [Google Scholar]
- 103.Pérez-Jiménez J., Saura-Calixto F. Grape products and cardiovascular disease risk factors. Nutr. Res. Rev. 2008;21(2):158–173. doi: 10.1017/S0954422408125124. [DOI] [PubMed] [Google Scholar]
- 104.Grassi D., Necozione S., Lippi C., Croce G., Valeri L., Pasqualetti P., Desideri G., Blumberg J.B., Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46(2):398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 105.Rasmussen S.E., Frederiksen H., Struntze Krogholm K., Poulsen L. Dietary proanthocyanidins: occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005;49(2):159–174. doi: 10.1002/mnfr.200400082. [DOI] [PubMed] [Google Scholar]
- 106.Post R.E., Mainous A.G., III, King D.E., Simpson K.N. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J. Am. Board Fam. Med. 2012;25(1):16–23. doi: 10.3122/jabfm.2012.01.110148. [DOI] [PubMed] [Google Scholar]
- 107.Gregersen S., Jeppesen P.B., Holst J.J., Hermansen K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism. 2004;53(1):73–76. doi: 10.1016/j.metabol.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 108.Ali Hussain H.E., Hussain A. Hypoglycemic, hypolipidemic and antioxidant properties of combination ofCurcumin fromCurcuma longa, Linn, and partially purified product fromAbroma augusta, Linn. in streptozotocin induced diabetes. Indian J. Clin. Biochem. 2002;17(2):33–43. doi: 10.1007/BF02867969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tank R., Sharma N., Sharma I., Dixit V.P. Anti-diabetic activity of C.longa in alloxan induced diabetic rats. Indian drugs. 1989;27:587–589. [Google Scholar]
- 110.Kumari K., Mathew B.C., Augusti K.T. Antidiabetic and hypolipidemic effects of S-methyl cysteine sulfoxide isolated from Allium cepa Linn. Indian J. Biochem. Biophys. 1995;32(1):49–54. [PubMed] [Google Scholar]
- 111.Augusti K.I., Roy V.C., Semple M. Effect of allyl propyl disulphide isolated from onion (Allium cepa L.) on glucose tolerance of alloxan diabetic rabbits. Experientia. 1974;30(10):1119–1120. doi: 10.1007/BF01923642. [DOI] [PubMed] [Google Scholar]
- 112.Kim C.Y., Le T.T., Chen C., Cheng J.X., Kim K.H. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. J. Nutr. Biochem. 2011;22(10):910–920. doi: 10.1016/j.jnutbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 113.Ciardi C., Jenny M., Tschoner A., Ueberall F., Patsch J., Pedrini M., Ebenbichler C., Fuchs D. Food additives such as sodium sulphite, sodium benzoate and curcumin inhibit leptin release in lipopolysaccharide-treated murine adipocytes in vitro. Br. J. Nutr. 2012;107(6):826–833. doi: 10.1017/S0007114511003680. [DOI] [PubMed] [Google Scholar]
- 114.El-Moselhy M.A., Taye A., Sharkawi S.S., El-Sisi S.F., Ahmed A.F. The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-α and free fatty acids. Food Chem. Toxicol. 2011;49(5):1129–1140. doi: 10.1016/j.fct.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 115.Xue W-L., Li X-S., Zhang J., Liu Y.H., Wang Z.L., Zhang R.J. Effect of Trigonella foenum-graecum (fenugreek) extract on blood glucose, blood lipid and hemorheological properties in streptozotocin-induced diabetic rats. Asia Pac. J. Clin. Nutr. 2007;16(Suppl. 1):422–426. [PubMed] [Google Scholar]
- 116.Kassaian N., Azadbakht L., Forghani B., Amini M. Effect of fenugreek seeds on blood glucose and lipid profiles in type 2 diabetic patients. Int. J. Vitam. Nutr. Res. 2009;79(1):34–39. doi: 10.1024/0300-9831.79.1.34. [DOI] [PubMed] [Google Scholar]
- 117.Crouse J.R., III, Morgan T., Terry J.G., Ellis J., Vitolins M., Burke G.L. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch. Intern. Med. 1999;159(17):2070–2076. doi: 10.1001/archinte.159.17.2070. [DOI] [PubMed] [Google Scholar]
- 118.Vrablík M., Prusíková M., Snejdrlová M., Zlatohlávek L. Omega-3 fatty acids and cardiovascular disease risk: do we understand the relationship? Physiol. Res. 2009;58(Suppl. 1):S19–S26. doi: 10.33549/physiolres.931860. [DOI] [PubMed] [Google Scholar]
- 119.Laviano A., Rianda S., Molfino A., Rossi Fanelli F. Omega-3 fatty acids in cancer. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16(2):156–161. doi: 10.1097/MCO.0b013e32835d2d99. [DOI] [PubMed] [Google Scholar]
- 120.Kelley N.S., Hubbard N.E., Erickson K.L. Conjugated linoleic acid isomers and cancer. J. Nutr. 2007;137(12):2599–2607. doi: 10.1093/jn/137.12.2599. [DOI] [PubMed] [Google Scholar]
- 121.Syvertsen C., Halse J., Høivik H.O., Gaullier J.M., Nurminiemi M., Kristiansen K., Einerhand A., O’Shea M., Gudmundsen O. The effect of 6 months supplementation with conjugated linoleic acid on insulin resistance in overweight and obese. Int. J. Obes. 2007;31(7):1148–1154. doi: 10.1038/sj.ijo.0803482. [DOI] [PubMed] [Google Scholar]
- 122.Akahoshi A., Koba K., Ichinose F., Kaneko M., Shimoda A., Nonaka K., Yamasaki M., Iwata T., Yamauchi Y., Tsutsumi K., Sugano M. Dietary protein modulates the effect of CLA on lipid metabolism in rats. Lipids. 2004;39(1):25–30. doi: 10.1007/s11745-004-1197-3. [DOI] [PubMed] [Google Scholar]
- 123.Azain M.J. Role of fatty acids in adipocyte growth and development. J. Anim. Sci. 2004;82(3):916–924. doi: 10.2527/2004.823916x. [DOI] [PubMed] [Google Scholar]
- 124.Fuller R. In: Chapman & Hall. Probiotics R.F., editor. N.Y.: 1992. pp. 1–8. [Google Scholar]
- 125.Mital B.K., Garg S.K. Anticarcinogenic, hypocholesterolemic, and antagonistic activities of Lactobacillus acidophilus. Crit. Rev. Microbiol. 1995;21(3):175–214. doi: 10.3109/10408419509113540. [DOI] [PubMed] [Google Scholar]
- 126.Hyson D., Studebaker-Hallman D., Davis P.A., Gershwin M.E. Apple juice consumption reduces plasma low-density lipoprotein oxidation in healthy men and women. J. Med. Food. 2000;3(4):159–166. doi: 10.1089/jmf.2000.3.159. [DOI] [PubMed] [Google Scholar]
- 127.Nestel P., Shige H., Pomeroy S., Cehun M., Abbey M., Raederstorff D. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am. J. Clin. Nutr. 2002;76(2):326–330. doi: 10.1093/ajcn/76.2.326. [DOI] [PubMed] [Google Scholar]
- 128.Farouque H.M., Leung M., Hope S.A., Baldi M., Schechter C., Cameron J.D., Meredith I.T. Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: a randomized double-blind placebo-controlled study. Clin. Sci. 2006;111(1):71–80. doi: 10.1042/CS20060048. [DOI] [PubMed] [Google Scholar]
- 129.Hodgson J.M., Puddey I.B., Burke V., Watts G.F., Beilin L.J. Regular ingestion of black tea improves brachial artery vasodilator function. Clin. Sci. 2002;102(2):195–201. doi: 10.1042/cs1020195. [DOI] [PubMed] [Google Scholar]
- 130.Gupta A., Gupta R., Lal B. Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. J. Assoc. Physicians India. 2001;49:1057–1061. [PubMed] [Google Scholar]
- 131.Mercanlıgil S.M., Arslan P., Alasalvar C., Okut E., Akgül E., Pınar A., Geyik P.O., Tokgözoğlu L., Shahidi F. Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. Eur. J. Clin. Nutr. 2007;61(2):212–220. doi: 10.1038/sj.ejcn.1602518. [DOI] [PubMed] [Google Scholar]
- 132.Khan A., Safdar M., Ali Khan M.M., Khattak K.N., Anderson R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 133.Rector T.S., Bank A.J., Mullen K.A., Tschumperlin L.K., Sih R., Pillai K., Kubo S.H. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation. 1996;93(12):2135–2141. doi: 10.1161/01.CIR.93.12.2135. [DOI] [PubMed] [Google Scholar]
- 134.Gregersen S., Jeppesen P.B., Holst J.J., Hermansen K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism. 2004;53(1):73–76. doi: 10.1016/j.metabol.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 135.Alokail M.S., Sabico S., Al-Saleh Y., Al-Daghri N.M., Alkharfy K.M., Vanhoutte P.M., McTernan P.G. Effects of probiotics in patients with diabetes mellitus type 2: study protocol for a randomized, double-blind, placebo-controlled trial. Trials. 2013;14:195. doi: 10.1186/1745-6215-14-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stephen D.F. The nutraceutical revolution, its impact on food industry. Trends Food Sci. Technol. 1995;6:59–61. doi: 10.1016/S0924-2244(00)88944-X. [DOI] [Google Scholar]
- 137.Mulvihill E.E., Huff M.W. Antiatherogenic properties of flavonoids: implications for cardiovascular health. Can. J. Cardiol. 2010;26(Suppl. A):17A–21A. doi: 10.1016/S0828-282X(10)71056-4. [DOI] [PubMed] [Google Scholar]
- 138.Nicolle E., Souard F., Faure P., Boumendjel A. Flavonoids as promising lead compounds in type 2 diabetes mellitus: molecules of interest and structure-activity relationship. Curr. Med. Chem. 2011;18(17):2661–2672. doi: 10.2174/092986711795933777. [DOI] [PubMed] [Google Scholar]
- 139.Chae Y.J., Kim C.H., Ha T.S., Hescheler J., Ahn H.Y., Sachinidis A. Epigallocatechin-3-O-gallate inhibits the angiotensin II-induced adhesion molecule expression in human umbilical vein endothelial cell via inhibition of MAPK pathways. Cell. Physiol. Biochem. 2007;20(6):859–866. doi: 10.1159/000110446. [DOI] [PubMed] [Google Scholar]
- 140.Arts I.C., Hollman P.C., Feskens E.J., Bueno de Mesquita H.B., Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am. J. Clin. Nutr. 2001;74(2):227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 141.Najafian M., Jahromi M.Z., Nowroznejhad M.J., Khajeaian P., Kargar M.M., Sadeghi M., Arasteh A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2012;39(5):5299–5306. doi: 10.1007/s11033-011-1328-7. [DOI] [PubMed] [Google Scholar]
- 142.Jeong S.M., Kang M-J., Choi H-N., Kim J-H., Kim J-I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012;6(3):201–207. doi: 10.4162/nrp.2012.6.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kobori M., Masumoto S., Akimoto Y., Oike H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol. Nutr. Food Res. 2011;55(4):530–540. doi: 10.1002/mnfr.201000392. [DOI] [PubMed] [Google Scholar]
- 144.Han X., Ren D., Fan P., Shen T., Lou H. Protective effects of naringenin-7-O-glucoside on doxorubicin-induced apoptosis in H9C2 cells. Eur. J. Pharmacol. 2008;581(1-2):47–53. doi: 10.1016/j.ejphar.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 145.Fukuchi Y., Hiramitsu M., Okada M., Hayashi S., Nabeno Y., Osawa T., Naito M. Lemon polyphenols suppress diet-induced obesity by upregulation of mRNA levels of the enzymes involved in beta-oxidation in mouse white adipose tissue. J. Clin. Biochem. Nutr. 2008;43(3):201–209. doi: 10.3164/jcbn.2008066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rizza S., Muniyappa R., Iantorno M., Kim J.A., Chen H., Pullikotil P., Senese N., Tesauro M., Lauro D., Cardillo C., Quon M.J. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011;96(5):E782–E792. doi: 10.1210/jc.2010-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahmed O.M., Mahmoud A.M., Abdel-Moneim A., Ashour M.B. Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol. Croat. 2012;41:53–67. [Google Scholar]
- 148.Food And Drug Administration CFR - Code of Federal Regulations Title 21. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/ cfcfr/CFRSearch.cfm?fr=101.36 . [accessed Feburary 18, 2015].
- 149.Supplements: who needs them? A behind the headlines report - NHS Choices. http://www.nhs.uk/news/2011/05May/Documents/BtH_supplements.pdf . 2011. [accessed March 2015].
- 150.Rimm E.B., Stampfer M.J., Ascherio A., Giovannucci E., Colditz G.A., Willett W.C. Vitamin E consumption and the risk of coronary heart disease in men. N. Engl. J. Med. 1993;328(20):1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 151.Stampfer M.J., Hennekens C.H., Manson J.E., Colditz G.A., Rosner B., Willett W.C. Vitamin E consumption and the risk of coronary disease in women. N. Engl. J. Med. 1993;328(20):1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 152.Rector T.S., Bank A.J., Mullen K.A., Tschumperlin L.K., Sih R., Pillai K., Kubo S.H. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation. 1996;93(12):2135–2141. doi: 10.1161/01.CIR.93.12.2135. [DOI] [PubMed] [Google Scholar]
- 153.Saper R.B., Eisenberg D.M., Phillips R.S. Common dietary supplements for weight loss. Am. Fam. Physician. 2004;70(9):1731–1738. [PubMed] [Google Scholar]
- 154.Xu N., Zhang L., Dong J., Zhang X., Chen Y.G., Bao B., Liu J. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol. Nutr. Food Res. 2014;58(6):1258–1268. doi: 10.1002/mnfr.201300830. [DOI] [PubMed] [Google Scholar]
- 155.Landgraf-Leurs M.M., Drummer C., Fröschl H., Steinhuber R., Von Schacky C., Landgraf R. Pilot study on omega-3 fatty acids in type I diabetes mellitus. Diabetes. 1990;39(3):369–375. doi: 10.2337/diab.39.3.369. [DOI] [PubMed] [Google Scholar]
- 156.Andujar I., Recio M.C., Giner R.M., Rios J.L. 2012. [Google Scholar]
- 157.Mellor D.D., Sathyapalan T., Kilpatrick E.S., Beckett S., Atkin S.L. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in Type 2 diabetes patients. Diabet. Med. 2010;27(11):1318–1321. doi: 10.1111/j.1464-5491.2010.03108.x. [DOI] [PubMed] [Google Scholar]
- 158.Ortsater H., Grankvist N., Wolfram S., Kuehn N., Sjoholm A. Diet supplementation with green tea extract epigallocatechingallate prevents progression to glucose intolerance in db/db mice. Nutr. Metab. 2012;14:9–11. doi: 10.1186/1743-7075-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moroti C., Souza Magri L.F., de Rezende Costa M., Cavallini D.C., Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;11:29. doi: 10.1186/1476-511X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ejtahed H.S., Mohtadi-Nia J., Homayouni-Rad A., Niafar M., Asghari-Jafarabadi M., Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28(5):539–543. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 161.Yadav H., Jain S., Sinha P.R. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23(1):62–68. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 162.Arab L. Individualized nutritional recommendations: do we have the measurements needed to assess risk and make dietary recommendations? Proc. Nutr. Soc. 2004;63(1):167–172. doi: 10.1079/PNS2003325. [DOI] [PubMed] [Google Scholar]
- 163.Kaput J., Rodriguez R.L. Nutritional genomics: the next frontier in the postgenomic era. Physiol. Genomics. 2004;16(2):166–177. doi: 10.1152/physiolgenomics.00107.2003. [DOI] [PubMed] [Google Scholar]
- 164.Cooper R.S., Psaty B.M. Genomics and medicine: distraction, incremental progress, or the dawn of a new age? Ann. Intern. Med. 2003;138(7):576–580. doi: 10.7326/0003-4819-138-7-200304010-00014. [DOI] [PubMed] [Google Scholar]
- 165.Müller M., Kersten S. Nutrigenomics: goals and strategies. Nat. Rev. Genet. 2003;4(4):315–322. doi: 10.1038/nrg1047. [DOI] [PubMed] [Google Scholar]
- 166.David Castle D., Cline C., Daar A.S., Tsamis C., Singer P.A. In: Nutritional Genomics: Discovering the Path to Personalized Nutrition, J. Kaput. Raymond L.R., editor. USA: John Wiley & Sons, Inc.; 2006. pp. 419–434. [DOI] [Google Scholar]
- 167.David Castle D., Cline C., Daar A.S., Tsamis C., Singer P.A. Science, Society and the Supermarket: The Opportunities and Challenges of Nutrigenomics. USA: Wiley; 2006. [DOI] [Google Scholar]
- 168.Horton R. Vioxx, the implosion of Merck, and aftershocks at the FDA. Lancet. 2004;364(9450):1995–1996. doi: 10.1016/S0140-6736(04)17523-5. [DOI] [PubMed] [Google Scholar]
- 169.Bergmann M.M., Bodzioch M., Bonet M.L., Defoort C., Lietz G., Mathers J.C. Bioethics in human nutrigenomics research: European Nutrigenomics Organisation workshop report. Br. J. Nutr. 2006;95(5):1024–1027. doi: 10.1079/BJN20061758. [DOI] [PubMed] [Google Scholar]
- 170.Mathers J.C. Chairman’s introduction: what can we expect to learn from genomics? Proc. Nutr. Soc. 2004;63(1):1–4. doi: 10.1079/PNS2003322. [DOI] [PubMed] [Google Scholar]
- 171.Godard B., Graham J., Hurlimann T., Lafreniere D., Menuz V., Robitaille J., Stenne R., Vohl M. Scientific and Ethical Challenges in Nutrigenomics & Nutrigenetics Research- A Survey of Researchers’ Perceptions, Omics-Ethics Research Group. Canada: University of Montreal; 2013. [Google Scholar]