Abstract
Approximately one quarter of individuals with an autism spectrum disorder (ASD) display self-injurious behavior (SIB) ranging from head banging to self-directed biting and punching. Sometimes, these behaviors are extreme and unresponsive to pharmacological and behavioral therapies. We have found electroconvulsive therapy (ECT) can produce life-changing results, with more than 90% suppression of SIB frequency. However, these patients typically require frequent maintenance ECT (mECT), as often as every 5 days, to sustain the improvement gained during the acute course. Long-term consequences of such frequent mECT started as early as childhood in some cases are unknown. Accordingly, there is a need for alternative forms of chronic stimulation for these patients. To explore the feasibility of deep brain stimulation (DBS) for intractable SIB seen in some patients with an ASD, we utilized two genetically distinct mouse models demonstrating excessive self-grooming, namely the Viaat-Mecp2−/y and Shank3B−/− lines, and administered high-frequency stimulation (HFS) via implanted electrodes at the subthalamic nucleus (STN-HFS). We found that STN-HFS significantly suppressed excessive self-grooming in both genetic lines. Suppression occurs both acutely when stimulation is switched on, and persists for several days after HFS is stopped. This effect was not explained by a change in locomotor activity, which was unaffected by STN-HFS. Likewise, social interaction deficits were not corrected by STN-HFS. Our data show STN-HFS suppresses excessive self-grooming in two autism-like mouse models, raising the possibility DBS might be used to treat intractable SIB associated with ASDs. Further studies are required to explore the circuitry engaged by STN-HFS, as well as other potential stimulation sites. Such studies might also yield clues about pathways, which could be modulated by non-invasive stimulatory techniques.
INTRODUCTION
Restricted, repetitive, and stereotyped behavior (RRSB) is a key feature of autism spectrum disorders (ASDs; American Psychiatric Association, 2013). RRSBs have great potential for harm when manifested as self-injurious behavior (SIB), which afflicts approximately one quarter of individuals with an ASD, ranging from head banging to self-directed biting and punching (Minshawi et al, 2014; Bartak and Rutter, 1976; Richards et al, 2012; Shattuck et al, 2007). A small subgroup of these patients demonstrates extremely high-frequency, high-intensity staccato-like repetitive SIB that has devastating consequences for the patient and their family (Wachtel and Dhossche, 2013). Such behaviors are not associated with secondary gain and, at times, resemble a stereotyped movement disorder.
Stereotyped movements are a recognized symptom of catatonia (American Psychiatric Association, 2013). A small number of patients with an ASD not only demonstrate involuntary, mechanical, and repetitive SIB, but also more classical akinetic motor signs, characteristic of catatonic states associated with mood disorders or psychosis. In fact, in recent years large population-based studies have estimated that 12–18% of autistic adolescents and young adults manifest some symptoms of catatonia (Billstedt et al, 2005; Ghaziuddin et al, 2012; Wing and Shah, 2000). Consistent with the sensitivity of catatonia to electroconvulsive therapy (ECT; Fink and Taylor, 2006), we and others have found ECT can produce life-changing results in ASD patients with extreme SIB that is unresponsive to traditional pharmacological and behavioral therapies (Wachtel and Dhossche, 2013; Consoli et al, 2013; Haq and Ghaziuddin, 2014). At our clinic, we have observed a >90% reduction in frequency of SIB in ASD patients with the most severe forms of self-injury (Wachtel et al, 2008, 2009, 2010, 2014, 2015).
Although these patients respond extraordinarily well to ECT, they remain dependent on it to sustain the improvement gained during the acute ECT course. Such maintenance ECT (mECT) regimes can be as frequent as one treatment every 5 days (Wachtel and Dhossche, 2013; Haq and Ghaziuddin, 2014; Wachtel et al, 2011, 2012). The long-term effects of such frequent ECT started as early as childhood in some cases are unknown. Although cognitive testing up to two years following the commencement of ECT has not shown deleterious effects (Wachtel et al, 2011, 2012), there are no data available on longer term outcomes. Furthermore, ~27% of children with ASD and moderate intellectual disability have comorbid epilepsy (Berg and Plioplys, 2012), and there are additional complexities and potential complications associated with delivering ECT to epileptics who take anticonvulsant medications that interfere with ECT (Lunde et al, 2006). These concerns underscore the need for the development of alternative therapies that mimic the beneficial effects of ECT without seizure induction or stimulation of brain regions uninvolved in the pathophysiology of SIB.
Neuromodulation by direct deep brain stimulation (DBS) has the potential to achieve anatomical specificity with minimal off-target effects, such as the cognitive side effects associated with ECT. DBS therapy is delivered by implanted electrodes and repeated anesthesia is not required, unlike for mECT. The risk of perioperative complications including infection, hemorrhage, and seizure is low (Cheng et al, 2015), however, off-target stimulation can result in serious neuropsychiatric symptoms (Morishita et al, 2010). An additional drawback of DBS is the need to periodically replace the battery of the implanted pulse generator. These potential limitations of DBS would need to be weighed against the unknown effects of frequent mECT starting at a young age. Of note, implanted DBS electrodes do not preclude ECT (Ducharme et al, 2011; Vila-Rodriguez et al, 2014), which is an important consideration given DBS candidate patients would likely be receiving mECT. Also, while brain and skull growth would preclude the accurate and consistent targeting of electrode leads in children and adolescents with ASD, severe cases of SIB typically persist into adulthood when DBS implantation surgery could become a viable alternative to mECT. Another justification for this study is that uncovering potential DBS target sites using mouse models may provide further insight into the neural pathways that might regulate SIB, and could be amenable to focal non-invasive brain stimulation therapies. Along these lines, Sokhadze et al (2014) recently reported low-frequency repetitive transcranial magnetic stimulation (rTMS) over the dorsolateral prefrontal cortex, which is inhibitory, suppressed stereotypic behavior in children with an ASD.
To evaluate the possible use of DBS to treat intractable SIB in ASD, we initially tested the efficacy of high-frequency stimulation (HFS) delivered by implanted electrodes in an autism-like mouse model in which the methyl-CpG binding (Mecp2) gene is conditionally deleted in neurons that release the inhibitory neurotransmitter γ-aminobutyric acid (GABA; Viaat-Mecp2−/y; Chao et al, 2010). These mice display GABAergic dysfunction including reduced miniature inhibitory postsynaptic currents, transcription of genes encoding glutamic acid decarboxylase and GABA immunoreactivity. We also tested the efficacy of HFS in a genetically distinct autism-like mouse model featuring constitutive deletion of the PDZ domain of the Shank3 gene (Shank3B−/−; Peca et al, 2011). These mice exhibit reduced levels of glutamate receptor subunits in the striatum and an associated decrease in frequency of miniature excitatory postsynaptic currents. Importantly, both transgenic mouse models also show excessive self-grooming with development of skin lesions as well as social deficits.
MATERIALS AND METHODS
Animals
Experiments complied with NIH guidelines for animal care and were approved by the Johns Hopkins University Institutional Animal Care and Use Committee. Mice were maintained on a 12-h light/dark cycle and were given access to food and water ad libitum. We used two established, genetically distinct, transgenic animal models of autism that feature excessive self-grooming causing injury, namely the Viaat-Mecp2−/y (Chao et al, 2010) and Shank3B−/− (Peca et al, 2011) mice. Both lines also exhibit deficits in social interaction. Viaat-Mecp2−/y mice were obtained by breeding Viaat-cre FVB males to Mecp2loxp/loxp 129SvJ females. Shank3B−/− mice were bred from heterozygotes and maintained on a 50 : 50 mixed background of C57BL6 and 129SvJ. All original breeders were obtained from Jackson Laboratory. For all experiments, we compared 10- to 15-week-old male knockout mice of each strain to age-matched wild-type siblings.
Electroconvulsive Seizure
At around 10 weeks of age, animals were administered a single electroconvulsive seizure (ECS; Ugo Basile, Collegeville, PA, USA) using bilateral earclip electrodes (100 Hz, 40-mA, pulse width 0.9 ms, stimulus duration 1 s), as previously described (Reti and Baraban, 2000). Following the generalized tonic–clonic seizure, mice were allowed to recover for 5 min before being placed back in their home cage. Sham-treated mice were handled identically to those receiving ECS except for administration of the electrical current.
Stereotactic Surgery
Mice were bilaterally implanted with monopolar stimulation electrodes (Supplementary Figure S1A and B). Animals were placed into a sealed chamber to induce anesthesia with a mixture of 3% isoflurane in oxygen delivered at 2 l/min. Mice were then placed in a stereotaxic apparatus and maintained on 1% isoflurane to retain sedation throughout the procedure. Antiseptic iodine–povidone was applied to the scalp prior to exposing the skull. Ethanol was then used to clean the skull and remove any particulates from the surgical site. Ensheathed in 30-gauge needle tubing, monopolar Teflon-insulated platinum iridium stimulation microwires of 75 μm diameter (cat#777000; A-M Systems, WA, USA) were targeted to the subthalamic nucleus (STN; AP −1.8; ML ±1.6; DV −4.4; Franklin and Paxinos, 1996) and secured to the skull with cyanoacrylate glue and dental cement. Control mice were implanted in the visual association area V2 of the visual cortex (V2; AP −3.88; ML±2.5; DV −1.25; Franklin and Paxinos, 1996). Extracranial wires and a skull ground wire were connected to pedestal connection sockets (cat#4-1571552-2; Digi-Key, MN, USA) and attached to the skull with an additional layer of dental cement. The scalp was then sutured while ensuring that the connection sockets remained exposed. Animals recovered on a heating pad. We waited a minimum of two weeks post-surgery before commencing experimental testing. Single housing was required to prevent mice from damaging the HFS headstage implant.
High Frequency Stimulation
Tethering wires were constructed from a one-meter bundle of 10 34-gauge wires. The use of long, thin wiring allowed mice to have uninhibited free movement without the need for a commutator, while bundling rectified the high impedance of individual 34-gauge wires. Tethering wires were hung from a one-meter height above the animal, and given enough slack for rotational and translational movements. Animals were habituated to electrical cable tethering for 3 days prior to initiating stimulation procedures. Animals receiving active HFS were administered constant-current square waves (130 Hz, 60 μs, 30 μA; S88, Grass Telefactor, Warwick, RI, USA) at each electrode. These parameters were chosen for their adherence with safety standards for current density, the absence of motor abnormalities upon stimulation, and their similarity to typical DBS settings used in humans (Montgomery, 2010). Because current amplitude is directly proportional to the square of the distance between the farthest activated neuron and the electrode tip, and based upon empirical values of comparable tissue resistivity (Tehovnik, 1996), we estimate the maximum radius of tissue activation surrounding our stimulation electrodes is approximately 0.093–0.332 mm. According to these estimates, our electrode placements potentially induce activation of the internal capsule fibers that form the rostral border of the STN. Hence, as with all studies using HFS delivered by implanted electrodes, we take caution in attributing any therapeutic benefit to the stimulation of a single neuronal element. Sham-treated mice were handled in the same manner but received no stimulation. In preliminary studies, we confirmed that our stimulation parameters produced neuronal stimulation in the STN by monitoring Arc expression (Supplementary Figure S1C). For all HFS experiments, stimulation was administered for a 3-hour period on a given day, with a total maximum of three consecutive days of stimulation.
Behavioral Testing
For all behavioral testing, mice were transported in their home cage to the testing room where they habituated for 1 h prior to being placed in the testing arena. All testing was conducted between 1200 hours and 1600 hours to avoid circadian cycle variability. For all HFS experiments, mice were tethered to stimulation equipment at the onset of this habituation period, but did not receive any stimulation until behavioral testing began. For HFS paradigms in which HFS was continued after initial behavioral testing was completed, mice were placed back in their home cages for the remaining duration of stimulation. Videos of behavioral responses were captured and then scored in duplicate using ethnographic software, JWatcher (Blumstein et al, 2012). Scoring was performed blind to genotype and treatment.
Self-Grooming and Locomotion Activity
As described by Silverman et al (2010), animals were placed into empty plexiglass cages that were approximately the same dimensions as their home cages. After a 10-min habituation period, mice were videotaped and scored for duration of self-grooming behaviors (syntactic grooming and scratching behavior) during the subsequent 10-min period (Supplementary Data, video). Simultaneously, animals were monitored for locomotor activity (ambulation and rearing) between grooming bouts.
Social Interaction
To test social interaction in animals tethered to stimulation equipment, we constructed a modified, open top three-chamber test apparatus (393 × 285 × 194 mm) with removable Plexiglass partitions and gates (Chao et al, 2010; Peca et al, 2011). The partitions equally divided the apparatus into left, middle, and right chambers, and the gates of the partitions could be removed to allow the test mouse to roam freely throughout each chamber. The left and right chambers each contained a cylindrical cage used to contain a stranger mouse during testing procedures. The chambers and partitions were wiped with ethanol and paper towels between trials. No bedding was used.
The three-chamber test consisted of two, 10-min phases, habituation, and social interaction. During habituation, the animal was kept within the middle chamber, free to view the uninhabited left and right chambers. To test for social interaction, a stranger mouse was then placed in the cylindrical cage in one side chamber, while the cage in the other side chamber remained empty. The position of the stranger mouse was alternated between trials between left and right chambers. We scored both time spent in each chamber and in direct interaction with the stranger mouse (sniffing and rearing).
Accuracy of Electrode Placement
We checked the accuracy of electrode placement in all mice. Animals were killed by perfusion fixation and serial sections were made through the STN and stained with cresyl violet. Only animals in which electrode tips were located within a 0.1-mm radius of the STN border bilaterally were included in statistical analyses of behavioral paradigms (Supplementary Figure S1D).
Statistical Analysis
All statistical analyses were conducted using IBM SPSS software. Unless otherwise specified, the repeated measures two-way ANOVA was used to evaluate the regulation of behavioral paradigms by genotype, treatment, and/or time. Post hoc analyses were performed using LSD test criteria. Specifically, these pairwise comparisons were made to interpret whether overall effects were due to differences between knockouts receiving either sham or active stimulation, and if so, when these differences occurred. Effects were considered significant at P-values <0.05.
RESULTS
ECS Suppresses Excessive Self-Grooming in Viaat-mecp2−/y Mice
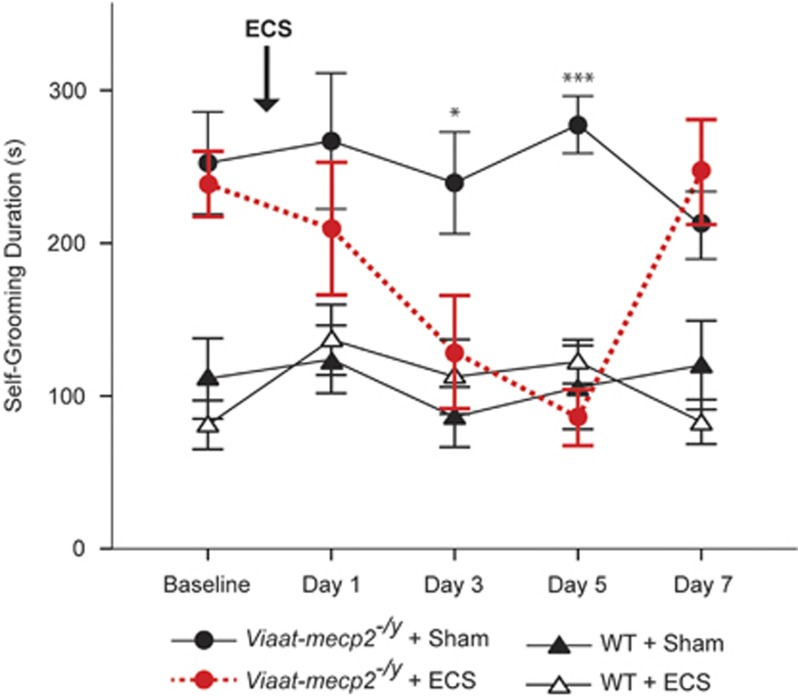
To determine whether the Viaat-mecp2−/y mouse model responds to ECS, we first checked the response of excessive self-grooming by these mice to a single ECS for up to a week after treatment. Mimicking the effect observed clinically, ECS selectively suppressed excessive self-grooming in Viaat-mecp2−/y mice (Figure 1). Importantly, ECS did not completely abolish self-grooming behavior, instead normalizing it to levels comparable to wild-type mice (genotype × treatment × time interaction effect: F(3,84)=4.30, P=0.007). Over the course of the week following ECS administration, excessive self-grooming in Viaat-mecp2−/y mice declined progressively, reaching significant suppression between days three (post-hoc test: P=0.018) and five (post hoc test: P<0.0001). As expected, the effects of ECS were transient, with rates of excessive self-grooming in ECS-treated Viaat-mecp2−/y mice returning to baseline by day eight after treatment.
Figure 1.
Electroconvulsive seizure (ECS) suppresses excessive self-grooming in Viaat-mecp2−/y mice. Time-course analysis of self-grooming behavior following a single ECS treatment showed significant reductions in Viaat-mecp2−/y mice, but not in wild-type controls (n=8–10). Over the course of the week following treatment, excessive self-grooming was significantly reduced on days 3 and 5 in Viaat-mecp2−/y mice receiving ECS compared with those receiving sham treatment (*P<0.05, **P<0.005, ***P<0.0005).
Persistent Suppression of Excessive Self-Grooming in Viaat-mecp2−/y Mice After STN-HFS
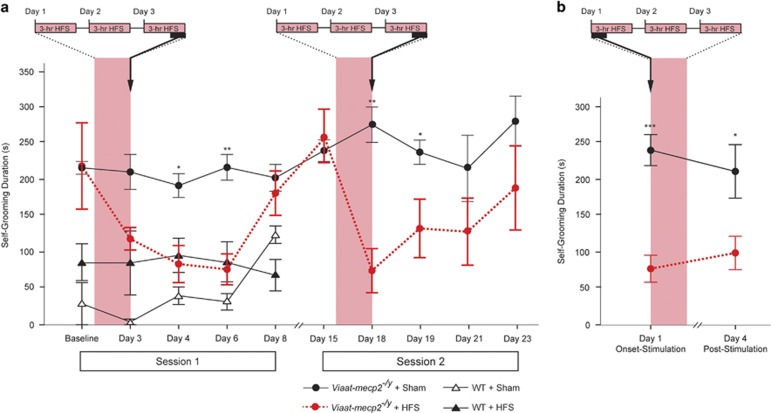
In order to test whether HFS could exert similar effects in these mice, we targeted the STN, as STN-HFS is effective in suppressing repetitive stereotyped behaviors in monkeys (Baup et al, 2008) and humans suffering with severe obsessive compulsive disorder (OCD; Luigjes et al, 2013). Self-grooming in Viaat-mecp2−/y mice was initially evaluated following three consecutive days of 3-h STN-HFS sessions. This cumulative 9-h stimulation paradigm was chosen as a practical model of chronic stimulation. Analysis of self-grooming in animals before and after 3 days of either sham or active STN-HFS revealed that behavioral suppression was exclusively seen in the Viaat-mecp2−/y mice receiving electrical stimulation (Figure 2a, session 1; genotype × treatment × time interaction effect: F(4,52)=3.70, P=0.01). The behavioral responses paralleled those seen with ECS treatment, as rates of self-grooming progressively and significantly reduced up to three days after active stimulation had ceased (post hoc test: day 4, P=0.01; day 6, P=0.001). As with ECS, self-grooming normalized to self-grooming levels of wild-type controls during this period, and soon returned to pre-stimulation levels 5 days post-HFS. Suppression of self-grooming by STN-HFS was not accounted for by depressed locomotor activity (Supplementary Figure S2). Two weeks following the first session of STN-HFS, we confirmed that self-grooming in Viaat-mecp2−/y mice had returned to baseline levels. Because the excessive self-grooming was comparable on the fifth day and the second week following our initial stimulation paradigm, we concluded that the suppressive effects of HFS only lasted for 5 days. We then checked the efficacy of a second round of STN-HFS in these mice, which showed they retained their initial sensitivity to stimulation, with excessive grooming progressively decreasing to levels comparable to wild-type mice (Figure 2a, session 2; genotype × treatment × time interaction effect: F(4,32)=4.04, P=0.009). Re-stimulation generated a shorter latency to grooming suppression and relapse compared with the first session of STN-HFS, with significant reductions in grooming being present on the last day of stimulation and the following day (post hoc test: day 18, P=0.001; day 19, P=0.043), suggesting the mice might have become sensitized to STN-HFS. Further studies are needed to characterize the extent of any such sensitization response, including the interval between stimulation courses and the mechanisms that mediate it. The combination of persistent suppression of self-grooming after cessation of HFS and the presence of sensitization after restimulation in the Viaat-mecp2−/y mice raises the possibility that chronic active stimulation, the norm for DBS in the clinical setting, may be unnecessary to maintain suppression of excessive self-grooming in these mice long-term.
Figure 2.
STN-HFS suppresses excessive self-grooming in Viaat-mecp2−/y mice. (a) Session 1: Timecourse analysis of self-grooming behavioral responses after nine cumulative hours of STN-HFS showed SIB suppression in Viaat-mecp2−/y mice, but not wild-type control mice (n=3–6). Specifically, Viaat-mecp2−/y mice exhibited significant reductions in excessive self-grooming on days 4 and 6 when comparing STN-HFS to sham treatment. Session 2: Analysis of Viaat-mecp2−/y self-grooming behavior response to a second round of STN-HFS mimicked the first session results (n=5). Compared with animals receiving sham treatment, significant grooming suppression lasted from the completion of the stimulation paradigm on day 18 until the following day 19. (b) Within the first 10 min of delivering STN-HFS, self-grooming suppression in Viaat-mecp2−/y mice (n=5–6) was comparable to the effects seen after administering the full, 9-h course of chronic stimulation (*P<0.05, **P<0.005, ***P<0.0005). HFS, high-frequency stimulation; STN-HFS, HFS via implanted electrodes at the subthalamic nucleus.
STN-HFS Acutely Suppresses Excessive Self-Grooming in Viaat-mecp2−/y Mice
While STN-HFS suppressed self-grooming after a total of 9 h of stimulation, it remained unclear whether stimulation efficacy was immediate or progressive. Therefore, a new cohort of Viaat-mecp2−/y mice were administered the same 3-day stimulation paradigm, but monitored during the first 10 min of stimulation, and after the cumulative 9 h of stimulation (Figure 2b). Indeed, self-injurious grooming was significantly reduced in Viaat-mecp2−/y mice from the onset of stimulation compared with sham treated mice (main treatment effect: F(1,9)=16.99, P=0.003; post hoc tests: day 1, P=0.0003; day 4, P=0.037). Moreover, the level of suppression at stimulation onset was comparable to that seen after animals had received the full, 9-h course of STN-HFS. This finding raised the possibility that STN-HFS could potentially suppress intractable SIB in ASD patients both immediately with stimulation onset and persistently after cessation of stimulation.
STN-HFS Does Not Reduce Heightened Social Interaction in Viaat-mecp2−/y Mice
Although the social interactions of these mice may have been affected by being housed alone after implantation of HFS electrodes, we nonetheless checked the effect of STN-HFS on the increased social interaction the Viaat-mecp2−/y mice demonstrate (Berg and Plioplys, 2012). We first confirmed that Viaat-mecp2−/y mice with implanted electrodes demonstrate increased social interaction compared with wild-type control mice (Supplementary Figure S3A–D). When STN-HFS was administered to Viaat-mecp2−/y mice, we did not detect any significant decrease in the social interaction of the Viaat-mecp2−/y mice compared with animals given sham stimulation (Supplementary Figure S3E and F).
STN-HFS Suppresses Excessive Self-Grooming in Shank3B−/− Mice
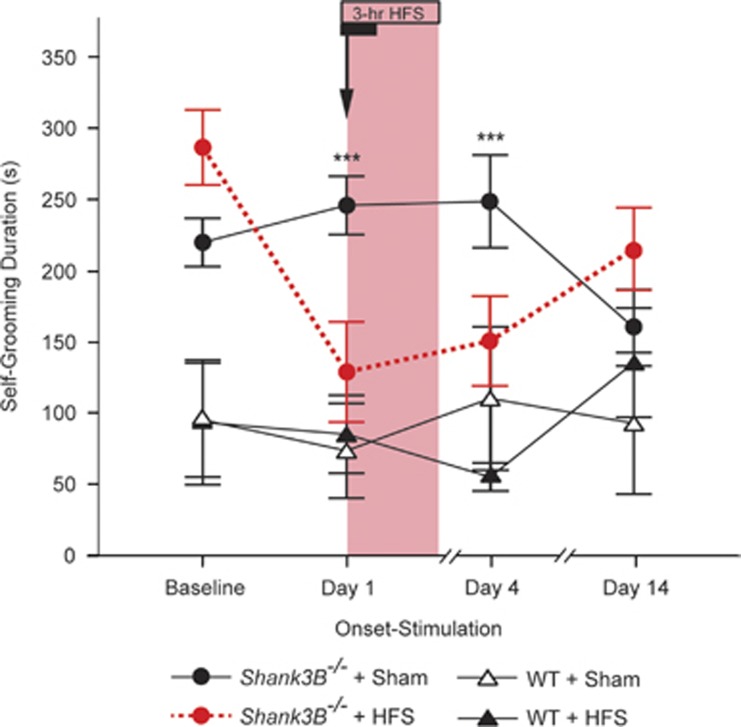
To check whether the effect of STN-HFS generalizes to other autism-like mouse models that exhibit excessive self-grooming, we chose a second transgenic model, the Shank3B−/− mouse. Because the response in Viaat-mecp2−/y mice was both immediate and persistent beyond the cumulative nine-hour stimulation paradigm, we sought to confirm that the Shank3B−/− mice exhibit a similar pattern of response. However, to check whether the required stimulus duration to produce a persistent suppression of excessive self-grooming after cessation of stimulation might be shorter than 9 h over 3 days, we evaluated the effect of a single 3-h session of STN-HFS. We found that STN-HFS triggered both immediate and persistent suppression of excessive self-grooming in the Shank3B−/− mice (Figure 3; genotype × treatment × time interaction effect: F(2,48)=8.96, P=0.0005). Within the first 10 min of stimulation, Shank3B−/− mice exhibited markedly less self-injurious grooming compared with sham-treated animals (post hoc test: P=0.002). Of note, the suppressive effect of STN-HFS on excessive self-grooming persisted 4 days later after just 3 hs of stimulation on day 1 (post hoc test: P=0.002). After a wash-out period of 14 days, self-grooming levels in the Shank3B−/− mice had returned to baseline. As with the Viaat-mecp2−/y line, suppression of self-grooming by STN-HFS in the Shank3B−/− mice was not accounted for by depressed locomotor activity (Supplementary Figure S2).
Figure 3.
STN-HFS suppresses excessive self-grooming in Shank3B−/− mice. Shank3B−/− mice, but not their wild-type controls, exhibit both immediate and persistent self-grooming suppression in response to 3-h of STN-HFS (n=6–8). Compared with sham treatment, marked reduction in Shank3B−/− excessive self-grooming was achieved within the first 10 min of STN-HFS, and persisted up to 4 days without any additional stimulation beyond the 3-h STN-HFS session that continued after day-1 behavioral testing (*P<0.05, **P<0.005, ***P<0.0005).
V2-HFS Fails to Suppress Excessive Self-Grooming in Shank3B−/− Mice
To evaluate the specificity of the STN as a therapeutic target for HFS, we monitored self-grooming behavior in response to stimulation in a control brain region, namely the visual association area V2 of the visual cortex (V2-HFS). Applying the prior stimulation and monitoring paradigms used to test STN-HFS in Shank3B−/− mice, we found that HFS in V2 did not impact excessive self-grooming either acutely or 4 days later (Supplementary Figure S4).
DISCUSSION
The goal of this translational study is the development of an alternate form of chronic stimulation to ECT that could suppress intractable SIB in ASD patients. To this end, we initially showed that the excessive self-grooming of Viaat-Mecp2−/y mice is suppressed by ECS, mimicking the effect we see in patients, and demonstrating that this mouse is a heuristic model for our clinical observations. We then showed that STN-HFS significantly suppressed excessive self-grooming in both the Viaat-Mecp2−/y line and in the genetically distinct Shank3B−/− mouse model. Unexpectedly, we found that suppression of excessive self-grooming by STN-HFS not only occurs acutely when stimulation is switched on, but is also persistent for several days after HFS is turned off. After excessive self-grooming returns to elevated baseline levels following cessation of STN-HFS, the stimulation retains its efficacy once it is reapplied to Viaat-Mecp2−/y mice. The effect of STN-HFS on excessive self-grooming is also selective. Locomotor activity is not affected by STN-HFS indicating the suppression in excessive self-grooming is not due to hypoactivity. Also, the heightened social interaction of Viaat-Mecp2−/y mice does not appear to be corrected by STN-HFS. Finally, HFS in a control brain region V2 had no effect on excessive self-grooming in Shank3B−/− mice, indicating the therapeutic effect of STN-HFS is not due to non-specific stimulation of the brain. As far as we are aware this is the first study reporting the use of HFS to suppress excessive self-grooming in autism-like mouse models.
It is not clear how ECT works to suppress excessive self-grooming in both Viaat-Mecp2−/y mice and ASD patients exhibiting intractable SIB. In ASD patients who exhibit both catatonia and SIB, we have found that benzodiazepines, which are allosteric modifiers of the GABAA receptor and considered a first-line intervention for catatonia, may also suppress SIB when effective for catatonia (Wachtel et al, 2008, 2010). Also, the effect of ECT on catatonia appears to be mediated by enhancing GABAergic function and ECT increases cortical GABA concentration and increases cortical inhibition post-seizure (Reti, 2015; Sanacora et al, 2003). Taken together, these data suggest that the effect of ECT on excessive self-grooming in Viaat-Mecp2−/y mice and SIB in ASD patients could be mediated by the enhancing effect of ECT on GABAergic tone. However, it is unclear how the effect of ECT on GABAergic function actually suppresses SIB. Decreased inhibitory tone from the output nuclei of the basal ganglia, namely the substantia nigra reticulata (SNr) and globus pallidus interna (GPi), to the thalamus, appears to have a role in the pathophysiology of SIB in patients with ASD. For example, injections into SNr of muscimol, a GABA receptor agonist, trigger dose-dependent increases in self-biting behavior (Baumeister and Frye, 1984), and diminished GPi volume has been shown to correlate with the frequency of RRSBs in ASD (Estes et al, 2011). Therefore, one possibility based on expression studies of GABA in rodents after ECS (Ferraro et al, 1990) is that ECT enhances the inhibitory tone of the SNr and GPi, which may mute abnormal motor thalamic spike patterns associated with stereotypy (Lewis and Kim, 2009). Consistent with this hypothesis, Viaat-Mecp2−/y mice have reduced GABA in the striatum (Chao et al, 2010) which could contribute to their stereotypies and which might be restored by ECS. Finally, Hagen et al (2015) recently reported response of another autism-like mouse model demonstrating excessive self-grooming, BTBR mice, to ECS, although they required multiple ECS sessions to suppress self-grooming. One possible explanation for why the Viaat-Mecp2−/y mice were sensitive to just one ECS in our hands is that we used a pulse width of 0.9 ms compared with a pulse width of 0.1 ms used by Hagen and colleagues. The ECS pulse width has a key role in determining which cells are recruited into a seizure (O'Donovan et al, 2012; Peterchev et al, 2010), as well as therapeutic outcome (Sackeim et al, 2008).
There is little data to guide placement of DBS electrodes for suppressing severe SIB in patients with ASD. We are unaware of other preclinical studies evaluating HFS for suppressing excessive self-grooming in autism-like mouse models, and know of only three case reports utilizing DBS to treat SIB exhibited by ASD patients who have not responded to traditional interventions. Sturm et al (2013) report the case of a 13-year old with an ASD and intractable SIB and aggression whose behaviors were ameliorated by DBS at the basolateral amygdala. Stocco and Baizabal-Carvallo (2014) report two similar cases: one patient responded to GPi-DBS and the other had a transient response to stimulation at the GPi and anterior limb of the internal capsule (ALIC). We targeted the STN based on both the similarity of SIB to other conditions that have been successfully treated with STN-DBS and its proposed role in regulating repetitive behaviors. STN-HFS has been shown to be effective in suppressing the repetitive stereotyped behaviors induced in two monkeys by bicuculline injection of the globus pallidus externa (Baup et al, 2008). Also, patients with OCD demonstrate repetitive, ritualistic behaviors that resemble the stereotypies seen in ASD patients with SIB, and have likewise been successfully treated with STN-DBS (Luigjes et al, 2013).
To the extent that the SNr and GPi mediate the excessive self-grooming in our autism-like mouse models, we chose to stimulate the glutamatergic STN which directly innervates both these nuclei. Multiple lines of evidence point to the STN as a key node which integrates ‘no-go' signals and conveys this information to the basal ganglia output nuclei to suppress development of aberrant behavior such as stereotypy (Heida et al, 2008; Scheel-Kruger et al, 1981). Indeed, while the direct effects of STN-HFS on the STN itself are unclear, studies utilizing electrophysiologic measures and neuronal activation markers consistently demonstrate that STN-HFS activates downstream SNr and GPi neural populations (Chiken and Nambu, 2014). Moreover, not only STN-DBS, but also GPi-DBS suppresses other conditions with phenotypes similar to the SIB seen in some ASD patients, such as Lesch–Nyhan syndrome (Cif et al, 2007; Taira et al, 2003), Tourette's syndrome (Cannon et al, 2012) and OCD (Nair et al, 2014). Taken together, these studies converge on a central idea that the basal ganglia is a behavioral filter, which can be shifted toward conservative or liberal restriction based on sufficient activation or inhibition of key ‘no-go' nuclei such as the STN, GPi, and SNr (Bergman et al, 2015). Finally, it is also possible that STN-HFS could be effective in our study because of stimulation of fibers of passage in the neighboring internal capsule and zona incerta. Interestingly, DBS target preferences for OCD have shifted away from the STN to the ALIC because of growing evidence of greater improvement when electrodes are placed in the ALIC (Prosée and Denys, 2015).
STN-HFS immediately suppressed excessive self-grooming in our mouse models, similar to the effect observed for Parkinsonian tremor (Groiss et al, 2009). It was also effective at suppressing SIB for up to 4 days after cessation of stimulation that was as brief as 3 h. Such a long-lasting effect after stimulation resembles the impact of a single ECS and is reminiscent of effects after brief optogenetic stimulation (Ahmari et al, 2013). It is unclear how STN-HFS triggers both acute suppression at stimulation onset, and suppression of SIB that persists beyond the stimulus, although long-lasting neuronal plasticity is a key feature of all brain stimulation interventions (Baraban, 2015). Noting also that we found HFS restimulation to be effective, our data raise the possibility that if STN-DBS were used for ASD patients with intractable SIB, one could consider on-demand stimulation and real-time feedback to slow the development of tolerance if an electrophysiological signal for the stereotyped self-injury could be identified (Reti and Chang, 2015). Habituation to the therapeutic effects of DBS has been described for both essential tremor and Parkinson's disease, the cause of which is unknown, but on-demand stimulation in response to local field potentials appears to lengthen the time DBS is effective and increase battery life (Kronenbuerger et al, 2006; Little et al, 2013).
We have explored the feasibility of DBS for intractable SIB in ASD patients, by evaluating the effect of HFS in suppressing excessive self-grooming in autism-like mouse models. However, focal brain stimulation engages neural elements that form part of distinct, parallel-organized, functional basal ganglia-thalamocortical circuits (Reti and Chang, 2015). Accordingly, in future studies it will be important to evaluate the efficacy of HFS at other sites, including cortical regions that project to the STN and GPi such as the orbitofrontal cortex and supplementary motor area, which have also been implicated in mediating intractable SIB seen in ASD patients (Muehlmann and Lewis, 2012). Especially in light of the recent case report by Sturm et al (2013), future studies in autism-like mouse models should also assess the impact of HFS at the amygdala noting that the amygdala regulates both aggression and social processing. Finally, translational studies using optogenetic techniques could also help optimize targeting in patients. Such studies might provide clues about brain circuitry, which could potentially be harnessed by less invasive techniques such as epidural cortical stimulation as well as non-invasive, nonconvulsive neuromodulatory techniques such as rTMS and transcranial direct current stimulation.
FUNDING AND DISCLOSURE
This work was supported by an Explorer Award from the Simons Foundation Autism Research Initiative (IMR) and by the Dana Foundation Program in Clinical Neuroscience Research (IMR). IMR has received research support from NIH, Department of Defense, Simons Foundation Autism Research Initiative, Dana Foundation, HDRF, Neuronetics Inc. and Brainsway Inc. JMB and GYF have received research support from NIH. The remaining authors declare no conflict of interest.
Acknowledgments
We thank Dr Wayne Frankel at Jackson Laboratory for providing Viaat-cre mice, and Dr Hsiao-Tuan Chao for advice on genotyping and maintaining the Viaat-mecp2−/y colony.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K et al (2013). Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science 340: 1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th edn. American Psychiatric Press: Washington, DC, USA. [Google Scholar]
- Baraban JM (2015). Viewing brain stimulation from a plasticity perspective. In: Reti IM (ed). Brain Stimulation: Methodologies and Interventions, 1st edn. Wiley Blackwell: NJ, USA, pp 45–56. [Google Scholar]
- Bartak L, Rutter M (1976). Differences between mentally retarded and normally intelligent autistic children. J Autism Child Schizophr 6: 109–120. [DOI] [PubMed] [Google Scholar]
- Baumeister AA, Frye GD (1984). Self-injurious behavior in rats produced by intranigral microinjection of GABA agonists. Pharmacol Biochem Behav 21: 89–95. [DOI] [PubMed] [Google Scholar]
- Baup N, Grabli D, Karachi C, Mounayar S, Francois C, Yelnik J et al (2008). High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates. J Neurosci 28: 8785–8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Plioplys S (2012). Epilepsy and autism: is there a special relationship? Epilepsy Behav 23: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Katabi S, Slovik M, Deffains M, Arkadir D, Israel Z et al (2015). Motor pathways, basal ganglia physiology and pathophysiology. In: Reti IM (ed). Brain Stimulation: Methodologies and Interventions, 1st edn. Wiley Blackwell: NJ, USA, pp 29–44. [Google Scholar]
- Billstedt E, Gillberg IC, Gillberg C (2005). Autism after adolescence: population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. J Autism Dev Disord 35: 351–360. [DOI] [PubMed] [Google Scholar]
- Blumstein D, Evans C, Daniel J (2012). JWatcher. Available at: http://www.jwatcher.ucla.edu.
- Cannon E, Silburn P, Coyne T, O'Maley K, Crawford JD, Sachdev PS (2012). Deep brain stimulation of anteromedial globus pallidus interna for severe Tourette's syndrome. Am J Psychiatry 169: 860–866. [DOI] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J et al (2010). Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JJ, Anderson WS, Lenz FA (2015). Neurological indications for deep brain stimulation. In: Reti IM (ed). Brain Stimulation: Methodologies and Interventions, 1st edn. Wiley Blackwell: NJ, USA, pp 271–288. [Google Scholar]
- Chiken S, Nambu A (2014). Disrupting neuronal transmission: mechanism of DBS? Front Syst Neurosci 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cif L, Biolsi B, Gavarini S, Saux A, Robles SG, Tancu C et al (2007). Antero-ventral internal pallidum stimulation improves behavioral disorders in Lesch-Nyhan disease. Mov Disord 22: 2126–2129. [DOI] [PubMed] [Google Scholar]
- Consoli A, Cohen J, Bodeau N, Guinchat V, Wachtel L, Cohen D (2013). Electroconvulsive therapy in adolescents with intellectual disability and severe self-injurious behavior and aggression: a retrospective study. Eur Child Adolesc Psychiatry 22: 55–62. [DOI] [PubMed] [Google Scholar]
- Ducharme S, Flaherty AW, Seiner SJ, Dougherty DD, Morales OG (2011). Temporary interruption of deep brain stimulation for Parkinson's disease during outpatient electroconvulsive therapy for major depression: a novel treatment strategy. J Neuropsychiatry Clin Neurosci 23: 194–197. [DOI] [PubMed] [Google Scholar]
- Estes A, Shaw DW, Sparks BF, Friedman S, Giedd JN, Dawson G et al (2011). Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Res 4: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Hare TA (1990). Repeated electroconvulsive shock selectively alters gamma-aminobutyric acid levels in the rat brain: effect of electrode placement. Convuls Ther 6: 199–208. [PubMed] [Google Scholar]
- Fink M, Taylor MA (2006) Catatonia: A Clinician's Guide to Diagnosis and Treatment. Cambridge University Press: Cambridge, UK. [Google Scholar]
- Franklin BJ, Paxinos GT (1996) The Mouse Brain in Stereotaxic Coordinates. Academic Press: NY, USA. [Google Scholar]
- Ghaziuddin N, Dhossche D, Marcotte K (2012). Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand 125: 33–38. [DOI] [PubMed] [Google Scholar]
- Groiss SJ, Wojtecki L, Sudmeyer M, Schnitzler A (2009). Deep brain stimulation in Parkinson's disease. Ther Adv Neurol Disord 2: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen E, Shprung D, Minakova E, Washington J, Kumar U, Shin D et al (2015). Autism-like behavior in BTBR mice is improved by electroconvulsive therapy. Neurotherapeutics 12: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq AU, Ghaziuddin N (2014). Maintenance electroconvulsive therapy for aggression and self-injurious behavior in two adolescents with autism and catatonia. J Neuropsychiatry Clin Neurosci 26: 64–72. [DOI] [PubMed] [Google Scholar]
- Heida T, Marani E, Usunoff KG (2008). The subthalamic nucleus part II: modelling and simulation of activity. Adv Anat Embryol Cell Biol 199: 1–85, vii. [PubMed] [Google Scholar]
- Kronenbuerger M, Fromm C, Block F, Coenen VA, Rohde I, Rohde V et al (2006). On-demand deep brain stimulation for essential tremor: a report on four cases. Mov Disord 21: 401–405. [DOI] [PubMed] [Google Scholar]
- Lewis M, Kim SJ (2009). The pathophysiology of restricted repetitive behavior. J Neurodev Disord 1: 114–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M et al (2013). Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 74: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigjes J, de Kwaasteniet BP, de Koning PP, Oudijn MS, van den Munckhof P, Schuurman PR et al (2013). Surgery for psychiatric disorders. World Neurosurg 80: S31.e17–e28. [DOI] [PubMed] [Google Scholar]
- Lunde ME, Lee EK, Rasmussen KG (2006). Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav 9: 355–359. [DOI] [PubMed] [Google Scholar]
- Minshawi NF, Hurwitz S, Fodstad JC, Biebl S, Morriss DH, McDougle CJ (2014). The association between self-injurious behaviors and autism spectrum disorders. Psychol Res Behav Manag 7: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery EB (2010) Deep Brain Stimulation Programming: Principles and Practice. Oxford University Press: Oxford, UK. [Google Scholar]
- Morishita T, Foote KD, Burdick AP, Katayama Y, Yamamoto T, Frucht SJ et al (2010). Identification and management of deep brain stimulation intra- and postoperative urgencies and emergencies. Parkinsonism Relat Disord 16: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlmann AM, Lewis MH (2012). Abnormal repetitive behaviours: shared phenomenology and pathophysiology. J Intellect Disabil Res 56: 427–440. [DOI] [PubMed] [Google Scholar]
- Nair G, Evans A, Bear RE, Velakoulis D, Bittar RG (2014). The anteromedial GPi as a new target for deep brain stimulation in obsessive compulsive disorder. J Clin Neurosci 21: 815–821. [DOI] [PubMed] [Google Scholar]
- O'Donovan S, Kennedy M, Guinan B, O'Mara S, McLoughlin DM (2012). A comparison of brief pulse and ultrabrief pulse electroconvulsive stimulation on rodent brain and behaviour. Prog Neuropsychopharmacol Biol Psychiatry 37: 147–152. [DOI] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN et al (2011). Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterchev AV, Rosa MA, Deng ZD, Prudic J, Lisanby SH (2010). Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT 26: 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosée R, Denys D (2015). Psychiatric indications for deep brain stimulation. In: Reti IM (ed). Brain Stimulation: Methodologies and Interventions, 1st edn. Wiley Blackwell: NJ, USA, pp 289–304. [Google Scholar]
- Reti IM (2015). How does electroconvulsive therapy work? In: Reti IM (ed). Brain Stimulation: Methodologies and Interventions, 1st edn. Wiley Blackwell: NJ, USA, pp 107–122. [Google Scholar]
- Reti IM, Baraban JM (2000). Sustained increase in Narp protein expression following repeated electroconvulsive seizure. Neuropsychopharmacology 23: 439–443. [DOI] [PubMed] [Google Scholar]
- Reti IM, Chang AD (2015). Introduction to brain stimulation. In: Reti IM (ed). Brain Stimulation: Methodologies and Interventions, 1st edn. Wiley Blackwell: NJ, USA, pp 1–12. [Google Scholar]
- Richards C, Oliver C, Nelson L, Moss J (2012). Self-injurious behaviour in individuals with autism spectrum disorder and intellectual disability. J Intellect Disabil Res 56: 476–489. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N et al (2008). Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul 1: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB et al (2003). Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 160: 577–579. [DOI] [PubMed] [Google Scholar]
- Scheel-Kruger J, Magelund G, Olianas M (1981). The role of GABA in the basal ganglia and limbic system for behaviour. Adv Biochem Psychopharmacol 29: 23–36. [PubMed] [Google Scholar]
- Shattuck PT, Seltzer MM, Greenberg JS, Orsmond GI, Bolt D, Kring S et al (2007). Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 37: 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN (2010). Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11: 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze EM, El-Baz AS, Sears LL, Opris I, Casanova MF (2014). rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front Syst Neurosci 8: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco A, Baizabal-Carvallo JF (2014). Deep brain stimulation for severe secondary stereotypies. Parkinsonism Relat Disord 20: 1035–1036. [DOI] [PubMed] [Google Scholar]
- Sturm V, Fricke O, Buhrle CP, Lenartz D, Maarouf M, Treuer H et al (2013). DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci 6: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira T, Kobayashi T, Hori T (2003). Disappearance of self-mutilating behavior in a patient with lesch-nyhan syndrome after bilateral chronic stimulation of the globus pallidus internus. Case report. J Neurosurg 98: 414–416. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ (1996). Electrical stimulation of neural tissue to evoke behavioral responses. J Neurosci Methods 65: 1–17. [DOI] [PubMed] [Google Scholar]
- Vila-Rodriguez F, McGirr A, Tham J, Hadjipavlou G, Honey CR (2014). Electroconvulsive therapy in patients with deep brain stimulators. J ECT 30: e16–e18. [DOI] [PubMed] [Google Scholar]
- Wachtel L, Commins E, Park M, Rolider N, Stephens R, Reti I (2015). Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand 132: 319–320. [DOI] [PubMed] [Google Scholar]
- Wachtel LE, Contrucci-Kuhn SA, Griffin M, Thompson A, Dhossche DM, Reti IM (2009). ECT for self-injury in an autistic boy. Eur Child Adolesc Psychiatry 18: 458–463. [DOI] [PubMed] [Google Scholar]
- Wachtel LE, Dhossche D (2013). ECT for self-injurious behavior. In: Ghaziuddin N, Walter G (eds). Electroconvulsive Therapy for Children and Adolescents. Oxford University Press: Oxford, UK, pp 247–280. [Google Scholar]
- Wachtel LE, Dhossche DM, Reti IM, Hughes-Wheatland R (2012). Stability of intellectual functioning during maintenance electroconvulsive therapy. Pediatr Neurol 47: 219–221. [DOI] [PubMed] [Google Scholar]
- Wachtel LE, Griffin M, Reti IM (2010). Electroconvulsive therapy in a man with autism experiencing severe depression, catatonia, and self-injury. J ECT 26: 70–73. [DOI] [PubMed] [Google Scholar]
- Wachtel LE, Kahng S, Dhossche DM, Cascella N, Reti IM (2008). ECT for catatonia in an autistic girl. Am J Psychiatry 165: 329–333. [DOI] [PubMed] [Google Scholar]
- Wachtel LE, Reti IM, Dhossche DM, Slomine BS, Sanz J (2011). Stability of neuropsychological testing during two years of maintenance electroconvulsive therapy in an autistic man. Prog Neuropsychopharmacol Biol Psychiatry 35: 301–302. [DOI] [PubMed] [Google Scholar]
- Wachtel LE, Reti IM, Ying H (2014). Stability of intraocular pressure after retinal reattachment surgery during electroconvulsive therapy for intractable self-injury in a 12-year-old autistic boy. J ECT 30: 73–76. [DOI] [PubMed] [Google Scholar]
- Wing L, Shah A (2000). Catatonia in autistic spectrum disorders. Br J Psychiatry 176: 357–362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.