Abstract
INTRODUCTION
Posterior interosseous nerve (PIN) syndrome is a rare compression neuropathy. Electrodiagnostic studies (EDX) combined with neuromuscular ultrasound (US) enable precise lesion localization and may improve patient outcome.
METHODS
In 4 patients with finger extension weakness, US was used to accurately localize concentric EMG needle placement in PIN muscles and to visualize the lesion site.
RESULTS
EMG with US guidance showed decreased recruitment with abnormal configuration in PIN muscles. Active denervation was not always observed. US scanning demonstrated larger PIN diameter in the affected arm. All patients had surgical intervention to confirm EDX and US findings with improved outcome on follow-up.
CONCLUSION
These cases demonstrate the benefits of augmenting EDX with US by guiding accurate electrode localization and providing diagnostic information about lesion location.
Keywords: Ultrasound, Posterior Interosseous Nerve Syndrome, Electrodiagnosis, Surgery, Outcome
Introduction
Posterior interosseous nerve (PIN) syndrome is a rare compression neuropathy of the deep branch of the radial nerve in the region of the supinator muscle. Annual incidence is estimated to be less than 0.7% (1). Typical patients with PIN syndrome present with motor symptoms, such as finger or thumb extension weakness. Furthermore, patients have tenderness during palpation at the lateral epicondyle and with resisted supination and pronation of the forearm (2). There are 5 possible PIN compression sites, the most common located at the Arcade of Frohse (3). Other sites include fibrous bands of tissue anterior to the radiocapitellar joint between the brachialis and the brachioradialis muscles, recurrent radial vessels at the level of the radial neck also known as “leash of Henry,” the medial proximal edge of the extensor radialis brevis (ECRB), and the distal edge of the supinator muscle (3). Etiologies include compression from lipomas, synovial overgrowth, ganglia, trauma, post-traumatic fibrosis from fractures/dislocations, and microtrauma from repetitive pronation/supination movements (4). With chronic compression, there may be extensor compartment muscle atrophy.
Since the ECRB overlays the extensor digitorum (ED), and the brachioradialis overlays the supinator, blind EMG needle electrode localization is difficult. Inaccurate electrode placement can compromise PIN diagnosis and delay appropriate care. Several cadaver studies have evaluated the accuracy of non- image-guided EMG needle placement in the hands of experienced electromyographers, and accuracy has ranged from 0% to 100% depending on the muscles examined (5–8). With neuromuscular ultrasound (US) guidance, accuracy rates can be enhanced consistently, and reported improvement is up to 96% (7). Though expert electromyographers can identify muscles by landmarks and palpation, certain situations may hinder muscle identification, and ultrasound could be used as a complementary tool. Examples would include large body habitus, altered anatomy, deep muscles, muscles examined rarely, and locations next to vital structures (9).
Here we describe 4 patients where the diagnosis of PIN syndrome was enhanced by combining electrodiagnostic studies (EDX) and US findings. US was used to guide needle electrode placement and to provide important diagnostic structural information complementary to often-subtle EDX findings.
Methods
For all four patients, after completion of the history and exam, standard sensory and motor conduction studies were completed (10). High resolution US using an 8–13 MHZ linear array transducer (GE logic, Little Chalfont, United Kingdom) was used to guide needle electrode localization in the extensor forearm to examine the ECRB and distal PIN muscles including the ED, extensor indicis (EI), extensor carpi ulnaris (ECU), and supinator. Muscles were confirmed using passive and active movements in addition to known geometry of the muscle. Out of plane (transverse) views were preferred to guide the needle electrode into targeted muscles. Table 1 summarizes the pertinent findings for all 4 cases.
Table 1.
Diagnostic Findings Summarized
| Case | Exam | EMG PIN Muscles | US findings | Surgical findings |
|---|---|---|---|---|
| 1 | Finger extensor weakness |
|
|
PIN compression within a fibrous Arcade of Frohse. |
| 2 | Finger and thumb extensor weakness Dorsal forearm atrophy |
|
|
Significant compression at the Arcade of Frohse. |
| 3 | Finger and wrist extensor weakness |
|
|
Significant compression at the Arcade of Frohse and an enlarged blue colored PIN. |
| 4 | Finger and thumb extension weakness |
|
|
Significant compression at the Arcade of Frohse and nerve diameter “twice the size of normal” |
abbreviations: EMG (electromyography), US (ultrasound), PIN (posterior interosseous nerve)
The PIN was evaluated with US using an algorithm that follows the published methods of Djurdjevic et al (4) and Won et al (11). Side-to-side PIN comparisons with measurement of PIN cross sectional area (CSA) were made, since all patients had unilateral symptoms:
-
1)
The forearm was placed in a supine semi-flexed position.
-
2)
The common radial nerve was identified on a transverse scan at the dorsolateral aspect in the mid-part of the humerus where it runs close to the bone.
-
3)
From here the course was followed distally to the bifurcation into the superficial sensory and deep (PIN) branches.
-
4)
The PIN, identified by its hypoechoic echotexture, was then followed to the level of the supinator overlying the radius, identified by cortical shadowing.
-
5)
The PIN was then followed as it entered underneath the fascia covering the supinator muscle (Arcade of Frohse). In this view, the brachioradialis is seen superior to the arcade.
-
5)
Power doppler sonography was used to exclude blood vessels in this region and to confirm that the hypoechoic nerve was not a blood vessel.
-
6)
The cross sectional area (CSA) of the PIN was measured in the proximal portion of the Arcade of Frohse.
-
7.)
The surrounding soft tissues were examined to identify possible PIN compression sites by secondary causes.
Results
Patient 1 is a 69-year-old right handed man with right thumb and finger extension weakness that was progressive over 1 year. EDX demonstrated normal superficial radial sensory nerve action potentials. Radial motor conduction studies recording over the ED demonstrated symmetric amplitudes, but increased distal latency on the right. Concentric needle EMG using US guidance for PIN muscles demonstrated active denervation with decreased recruitment and rapid firing rates of polyphasic motor unit potentials (MUPs) in all PIN-innervated muscles tested. The CSA of the PIN on the right was 5.0 mm2 vs. 4.0 mm2 on the left side. Furthermore, the Arcade of Frohse was thickened with hyperechoic echotexture compared to the left side. Surgical exploration a few months later revealed a degenerative fibrous leash at the Arcade of Frohse leading to compression at the proximal edge of the supinator muscle. Upon telephone follow up, the patient endorsed improvement of hand strength.
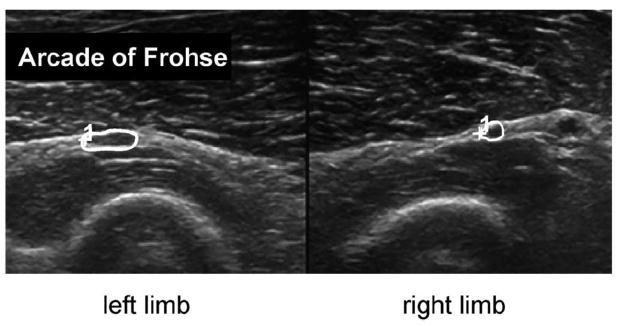
Patient 2 is a 57-year-old man with left finger and thumb extensor weakness and dorsal forearm atrophy. Superficial radial sensory nerve conduction studies were normal, but affected radial compound muscle action potential amplitude was one-fourth the size of the unaffected side. Concentric needle EMG in combination with US guidance demonstrated large mean amplitude MUPs that had decreased recruitment and rapid firing rate in ED, ECU, and EI. No active denervation was noted. US scanning demonstrated a hyperechoic Arcade of Frohse and a 6.6 mm2 versus 3.1 mm2 CSA of PIN on the affected side (Figure 1). In addition, the shape of the affected PIN was more oblong instead of round. The operative report described “very significant amount of compression” at a fibrous Arcade of Frohse. Chart review revealed that patient had improved strength at his follow up appointment.
Figure 1.
US of patient 2 shows side-to-side comparisons of the PIN (circled). Note PIN enlargement on the affected left side, within a thickened Arcade of Frohse. Left image is the left arm, and right image is the right arm.
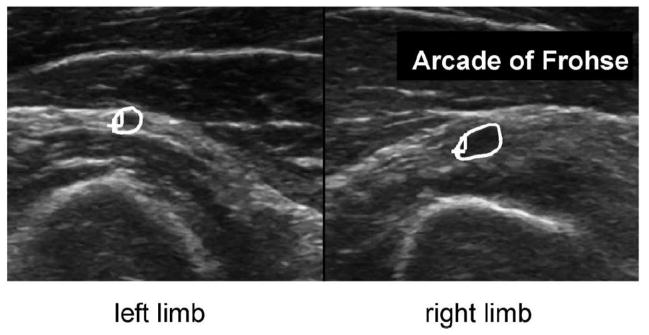
Patient 3 is a 48-year-old man who presented with a 2-year history of weakness in his right arm and hand. On physical exam he had right wrist and finger extension weakness. Nerve conduction studies demonstrated normal superficial radial sensory action potential and compound muscle action potential amplitude and normal latencies. EMG data were significant for large amplitude MUPs with decreased recruitment and rapid firing rate in the right ED, ECU, and EI. No active denervation was appreciated. Ultrasound images demonstrated an enlarged PIN within the right Arcade of Frohse (5.6 mm2 on the right vs 2.8 mm2 CSA on the left, Figure 2). The operative report noted “very significant compression” at the Arcade of Frohse with the nerve distal to the compression appearing “bluish” in color compared to proximal to the compression. Chart review confirmed that patient had improved hand strength on follow up.
Figure 2.
US side-to-side comparisons of the PIN (circled) in patient 3. Note PIN enlargement on the affected right side within a thickened Arcade of Frohse. Left image is the left arm, and right image is the right arm.
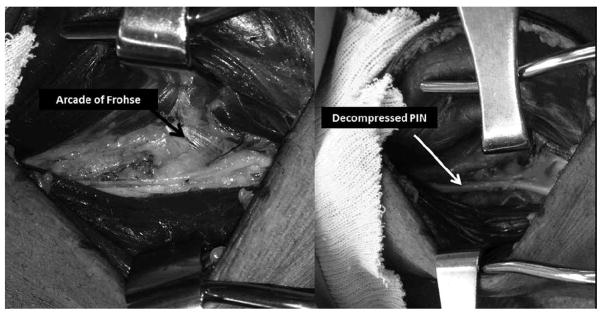
Patient 4 is a 58 year-old man who presented with a 1-month history of increasing left hand weakness with some numbness and tingling in the forearm. On physical exam he was unable to extend his fingers and thumb. Nerve conduction studies demonstrated normal superficial radial sensory action potential and radial compound motor action potential amplitudes. EMG data were significant for decreased recruitment and rapid firing in the ECU with polyphasic MUPs. In the more distal PIN-innervated muscles there were fibrillation potentials (3+) and positive sharp waves (3+) in the ED, extensor pollicis brevis (EPB), and EI. He had polyphasic MUPs in the EI and no MUPs with attempted activation of the ED and EPB. US showed a very swollen PIN just prior to and entering the Arcade of Frohse compared to the right side (8.0 mm2 circumference on the affected side vs. 1.9 mm2 on the unaffected side). The operative report noted that during dissection the PIN was under “a great deal of pressure” at the Arcade of Frohse and was “twice the size” of normal just proximal to the site of compression (Figure 3). Upon follow up, the patient had less pain and some improvement of strength, though the exam demonstrated ongoing extensor weakness.
Figure 3.
Two intraoperative pictures of patient 4 demonstrate the PIN before and after decompression. On the left, a tendinous appearing Arcade of Frohse is shown (arrow). The PIN was enlarged to approximately twice its normal diameter proximal to the site of compression. The picture on the right depicts the decompressed PIN (white arrow) after release of the Arcade of Frohse.
Discussion
We found that EDX in combination with US helped to accurately diagnose PIN. Precise needle electrode localization with US allowed accurate MUP analysis, which was the only EMG abnormality in 2 patients. US demonstrated structural abnormalities that correlated with EMG findings and helped to precisely locate the site of the lesion. The collective findings of EDX and US led to a clear diagnosis that guided surgical intervention and resulted in symptom improvement in all patients. The operative findings correlated with the diagnostic studies and likely led to improved outcomes.
Our literature search revealed a few PIN case reports and small studies that demonstrated correlation among symptoms, ultrasound, MRI, EDX, and operative findings (12–16). Only a case report of a single patient correlated US, EDX, and operative data, but it did not report outcome data or a description of the methodology used (12). Our case series provides more detailed methodology, description, and comparison of findings with the unaffected arm.
These findings are consistent with the limited literature regarding PIN syndrome and the diagnostic utility of US as an adjunct to EDX. One of the largest US studies of PIN retrospectively compared US findings in 13 patients with clinical and electrophysiological evidence of PIN syndrome with normative data from healthy volunteers (4). They concluded that high resolution US is useful for diagnosing PIN syndrome based on enlargement of the anteroposterior nerve diameter at the Arcade of Frohse compared to the unaffected side. In that study, anteroposterior diameter and not CSA was used to measure the PIN. Normative data can be highly variable in small diameter nerves such as the PIN, (11, 17) and may not be as clinically relevant as side-to-side comparisons. In patient 1, both sides would have fallen outside the range of normal reference values for the PIN (2.03 ± 0.46 mm2 on right side and 1.95 ± 0.39 mm2 on left side) (16). Importantly, the Arcade of Frohse in patient 1 was hyperechoic compared to the unaffected side, which provided additional important diagnostic information regarding lesion location. Furthermore, another study demonstrated low intra-participant side-to-side variability, which suggests that the unaffected contralateral side can be used as an internal control in patients with unilateral neuropathy (17).
In conclusion, US aided precise needle electrode localization of PIN-innervated muscles during EDX studies and allowed visualization of the course of the nerve, including the site of entrapment. In all 4 patients, surgical intervention was expedited due to our findings, and patients had improvement of symptoms on follow up. We believe that the combination of EDX and US in PIN can improve diagnosis and patient outcomes.
Abbreviations
- EDX
electrodiagnostic studies
- EMG
electromyography
- ECRB
extensor carpi radialis brevis
- EDC
extensor digitorum
- ECU
extensor carpi ulnaris
- EI
extensor indicis
- EPB
extensor pollicis brevis
- MUPs
motor unit potentials
- PIN
posterior interosseous nerve syndrome
- US
ultrasound
Footnotes
The authors of this manuscript have no conflicts of interest or financial disclosures to report.
References
- 1.Bevelaqua AC, Hayter C, Feinberg J, Rodeo S. Posterior Interosseous Neuropathy: Electrodiagnostic Evaluation. Hospital of Special Surgery Journal. 2012;8:184–189. doi: 10.1007/s11420-011-9238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheeless Clifford., III Posterior Interosseous Nerve Compression Syndrome. [Accessed 2015 April 25];Wheeless’ Textbook of Orthopedics Online. Updated 2013. Available at http://www.wheelessonline.com/ortho/posterior_interosseous_nerve_compression_syndrome.
- 3.Dang AC, Rodner CM. Unusual Compression Neuropathies of the Forearm, Part I: Radial Nerve. The Journal of Hand Surgery. 2009;34:1906–1914. doi: 10.1016/j.jhsa.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Djurdjevic T, Loizides A, Löscher W, Gruber H, Plaikner M, Peer S. High resolution ultrasound in posterior interosseous nerve syndrome. Muscle Nerve. 2014;49:35–39. doi: 10.1002/mus.23867. [DOI] [PubMed] [Google Scholar]
- 5.Chiodo A, Goodmurphy C, Haig A. Cadaveric study of methods for subscapularis muscle needle insertion. Am J Phys Med Rehabil. 2005;84:662–665. doi: 10.1097/01.phm.0000171174.96352.d9. [DOI] [PubMed] [Google Scholar]
- 6.Chiodo A, Goodmurphy C, Haig A. Cadaver evaluation of EMG needle insertion techniques used to target muscles of the thorax. Spine. 2006;31:E241–E243. doi: 10.1097/01.brs.0000214941.46628.1c. [DOI] [PubMed] [Google Scholar]
- 7.Haig AJ, Goodmurphy CW, Harris AR, Ruiz AP, Eternad J. The accuracy of needle placement in lower-limb muscles: a blinded study. Arch Phys Med Rehabil. 2003;84:877–882. doi: 10.1016/s0003-9993(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 8.Boon AJ, Oney-Marlow TM, Murthy NS, Harper CM, McNamara TR, Smith J. Accuracy of Electromyography Needle Placement in Cadavers: Non-Guided vs. Ultrasound Guided Muscle Nerve. 2011;44:45–49. doi: 10.1002/mus.22008. [DOI] [PubMed] [Google Scholar]
- 9.Boon, Andrea Ultrasonography and Electrodiagnosis: Are They Complementary Techniques? PM R. 2013;5:S100–S106. doi: 10.1016/j.pmrj.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Dumitru D, Amato A, Zwats M. Electrodiagnostic Medicine. Philadelphia: Hanley and Belfus, Inc; 2002. pp. 194–208. [Google Scholar]
- 11.Won SJ, Kim BJ, Park KS, Yoon JS, Choi H. Reference values for nerve ultrasonography in the upper extremity. Muscle Nerve. 2013;47:864–71. doi: 10.1002/mus.23691. [DOI] [PubMed] [Google Scholar]
- 12.Joy V, Therimadasamy A, Cheun CY, Wilder-Smith E. Diagnostic utility of ultrasound in posterior interosseous nerve syndrome. Arch Neurol. 2009;66:902–903. doi: 10.1001/archneurol.2009.109. [DOI] [PubMed] [Google Scholar]
- 13.Chien AJ, Jamadar DA, Jacobson JA, Hayes CW, Louis DS. Sonography and MR imaging of posterior interosseous nerve syndrome with surgical correlation. Am J Roentgenol. 2003;181:219–221. doi: 10.2214/ajr.181.1.1810219. [DOI] [PubMed] [Google Scholar]
- 14.Kinni V, Craig J, van Holsbeeck M, Ditmars D. Entrapment of the posterior interosseous nerve at the arcade of Frohse with sonographic, magnetic resonance imaging, and intraoperative confirmation. J Ultrasound Med. 2009;28:807–812. doi: 10.7863/jum.2009.28.6.807. [DOI] [PubMed] [Google Scholar]
- 15.Bodner G, Harpf C, Meirer R, Gardetto A, Kovacs P, Gruber H. Ultrasonographic appearance of supinator syndrome. J Ultrasound Med. 2002;21:1289–1293. doi: 10.7863/jum.2002.21.11.1289. [DOI] [PubMed] [Google Scholar]
- 16.Boehm J, Scheidl E, Bereczki D, Schelle T, Arányi Z. High-resolution ultrasonography of peripheral nerves: measurements on 14 nerve segments in 56 healthy subjects and reliability assessments. Ultraschall Med. 2014;35:459–67. doi: 10.1055/s-0033-1356385. [DOI] [PubMed] [Google Scholar]
- 17.Tagliafico A, Martinoli C. Reliability of Side-to-Side Sonographic Cross- sectional Area Measurements of Upper Extremity Nerves in Healthy Volunteers. J Ultrasound Med. 2013;32:457–462. doi: 10.7863/jum.2013.32.3.457. [DOI] [PubMed] [Google Scholar]