Abstract
Objective
Human papillomavirus (HPV) causes almost all cervical cancer in women and contributes to vaginal, anal, oropharyngeal, and penile cancer morbidity and mortality. Although vaccines effective in preventing up to nine types of HPV are available, vaccination rates are low nationally. We assessed HPV vaccination coverage by age, sex, and county using Washington State Immunization Information System data.
Methods
We calculated on-time dose coverage by county and statewide among adolescents aged 11–12 years and assessed coverage by age 18 years. We calculated missed opportunities as the number of visits at which doses of other adolescent vaccines were administered without administration of the first dose of HPV vaccine (HPV1).
Results
In 2013, HPV vaccination coverage estimates with one, two, and three doses (HPV1-3) for adolescents aged 11–12 years were 48.5%, 32.4%, and 18.3% among girls and 31.2%, 17.1%, and 8.1% among boys. The three-dose HPV vaccine coverage estimate increased to 40.1% among girls by age 18 but was unchanged for boys. Coverage estimates varied by age, sex, and county. One-third of eligible unvaccinated girls and two of five eligible boys aged 11–17 years had at least one missed opportunity to receive HPV1.
Conclusion
Despite a recommendation to vaccinate adolescents aged 11–12 years, HPV vaccination is often delayed and coverage levels among all age groups are below national target levels. Improved understanding of the variability of HPV vaccination coverage rates by age, sex, and county can inform targeted interventions statewide.
Each year in the United States, human papillomavirus (HPV) causes an estimated 26,000 new cancers, with about 65% of these cancers occurring in women and 35% occurring in men.1 More than 150 HPV types exist, about 40 of which are infectious in humans and are characterized as high or low risk by their ability to cause cancer.1 High-risk HPV causes cancers of the cervix, vagina, vulva, penis, anus, and oropharynx.1,2 Low-risk HPV causes anogenital warts and recurrent respiratory papillomatosis.1
In 2006, the U.S. Food and Drug Administration (FDA) licensed the first vaccine to prevent HPV infections in females aged 9–26 years.3 In 2009, approval was extended to males and a second vaccine was approved for females; in 2014, a 9-valent vaccine was approved for use in both sexes.4–6 All vaccines are administered in a series of three injections. The Advisory Committee on Immunization Practices (ACIP) recommended routine HPV vaccination for girls in January 2007 and for boys in October 2011 for adolescents aged 11–12 years.7,8 Catch-up vaccinations are recommended for females aged 13–26 years, males aged 13–21 years, or high-risk males aged 13–26 years, including men who have sex with men aged 13–26 years. Any approved vaccines can be used for routine vaccination. On-time vaccination is important to optimize protection before exposure; one study found up to one-third of females initiating HPV vaccination already had evidence of sexual experience and associated morbidity.9
HPV vaccination coverage is low nationally.10 The 2007–2013 National Immunization Survey–Teen (NIS-Teen) found that coverage with ≥1 dose of HPV vaccine increased from 25.1% in 2007 to 57.3% in 2013 in adolescent girls aged 13–17 years.10 About one-third (37.8%) of adolescent girls received all three recommended doses in 2013. For adolescent boys, coverage with ≥1 dose was approximately 35% and coverage with ≥3 doses was 14% in 2013. The Centers for Disease Control and Prevention (CDC) named slow uptake of HPV vaccine as one of the top five health threats for 2014.11 Documented reasons for low coverage rates include difficulty completing the three-dose series, lack of parental education, lack of strong recommendations from health-care providers, and missed opportunities for vaccination.10,12 Prior studies found that geographic location, race/ethnicity, health-care coverage, and provider specialty affected coverage rates.13–18
In 2014, a CDC document for state and local immunization programs detailed action areas for improving HPV vaccination rates.19 One action area was using state data for program decision making, stating, “If data on adolescent vaccination from your state immunization registry are robust, assess vaccination coverage levels and evaluate the frequency of missed opportunities.”19 The Washington State Department of Health launched its immunization registry in 1993 and expanded it in 2012 to include adolescents and adults as the Washington State Immunization Information System (WAIIS). The WAIIS links to Washington State's vital records system, allowing automatic input of electronic records of each year's birth cohort, regardless of vaccinations. Birth certificates are the definitive source for patient date of birth. Provider records are the best source for immunization information and updated demographic data.20 The WAIIS is currently estimated to cover 98% of children younger than 6 years of age and 84% of adolescents aged 11–17 years, and is used by all public providers and 81% of private providers in Washington State.21,22 Historical immunization records can be added to the system at any time. We assessed vaccination data in the WAIIS for completeness from 2006–2010 and validated data for research purposes in a separate study.23
Based on prior studies, we hypothesized that vaccination coverage rates vary by age, sex, and geographic location in Washington State. Our objectives were twofold: (1) estimate vaccination coverage levels for the state overall and stratified by county and (2) assess the frequency of missed opportunities for the first dose of HPV vaccine (HPV1). We hypothesized that HPV vaccine initiation and completion of ≥3 doses (HPV3) vary by completion of childhood vaccines, as well as characteristics such as county size or median income. This analysis can serve as an example for other states interested in assessing their HPV immunization registry data.
METHODS
Data sources
We obtained data on February 4, 2014, by extracting WAIIS records for children with birthdates from July 1, 1988, through June 30, 2001. Variables included unique identifiers, vaccines administered, administration dates, age at administration, sex, race/ethnicity, county, and ZIP code. We imported data into SAS® version 9.324 and cleaned the data to include only records marked as active, with residence in Washington State, and with a valid county or ZIP code included in the address. Records in the WAIIS are inactivated according to the American Immunization Registry Association's best practice document: if a record matches a death certificate, if an out-of-state address is listed, or if certain criteria are met pertaining to lack of health-care provider, date of last immunization, age, or validity of mailing address. We assessed all doses of HPV vaccine for administration at appropriate ages and intervals (per ACIP guidelines), and we discounted any dose given before licensure, before the earliest recommended age, or too soon after a previous dose. Only records with nonmissing data on sex were included in the analysis. More than 85% of records lacked information on race or ethnicity, precluding further use of these variables.
Coverage level estimation
We assessed vaccination coverage in three ways. First, we compared state coverage-level estimates using the 2013 WAIIS data with coverage estimates from NIS-Teen. We replicated previous methods, using WAIIS data from all adolescents born from January 11, 1995, through February 13, 2001.25 We assessed coverage levels for ≥1, ≥2, and ≥3 doses of HPV vaccine in girls and boys aged 13–17 years. We estimated the denominators for these proportions in two ways. First, we used 2013 census estimates from the Washington State Office of Financial Management (OFM) to estimate total populations by sex for adolescents born within the appropriate dates. Second, we used WAIIS to determine the number of adolescents born from January 11, 1995, through February 13, 2001. We compared these two WAIIS coverage estimates with state estimates from NIS-Teen.
Second, we assessed coverage with ≥1, ≥2, and ≥3 doses of HPV vaccine for 13- and 18-year-olds and graphed data for 2006–2013 for both sexes. The numerator for each calculation was the number of adolescents turning 13 or 18 years of age in the year of assessment who received doses of vaccine given on time (i.e., before the 13th birthday) or before the 18th birthday. Denominators for coverage calculations were census estimates for the total 13- and 18-year-old populations by sex for the years 2006–2013.
For the third assessment, we stratified coverage levels among 13- and 18-year-olds for 2013 by county and mapped them using ArcGIS.26 We assessed HPV vaccine series initiation and completion (three doses of vaccine with minimum spacing intervals per ACIP guidelines) by sex and age for 2013 and calculated 95% confidence intervals (CIs) for all estimates. The numerator for coverage estimate proportions was the number of adolescents with ≥1 dose or ≥3 doses of HPV vaccine administered before their 13th or 18th birthday recorded in the WAIIS. The denominator was based on 2013 census estimates for total 13- and 18-year-olds by sex and county. OFM provided estimates for the total population of 18-year-olds by sex in each county. An estimate for the total 13-year-old population in each county was not available; to estimate this number, the population of 10- to 14-year-olds was divided by five; we assumed equal distribution among the ages. Kittitas, Walla Walla, Whatcom, and Whitman counties had census estimates for 18-year-olds that were much higher than expected, most likely due to the presence of universities in counties with small populations. We used the number of 17-year-olds in 2012 as a proxy denominator for these counties.
We created choropleth maps for 2013 by county and sex for each of the following: on-time series initiation, on-time series completion, series initiation by age 18 years, and series completion by age 18 years. To further assess changes in coverage levels by county, we graphed initiation or completion of HPV vaccination by age 13 and age 18 for the years 2006–2013 to visualize trends for boys and girls.
Missed opportunities for vaccination
We calculated missed opportunities as the number of visits at which any doses of tetanus, diphtheria, acellular pertussis; seasonal influenza; or meningococcal conjugate vaccines were administered without administration of HPV1 in eligible adolescents. Dates of vaccine administration were de-duplicated to account for multiple doses received at one visit. We assessed the number of missed opportunities in adolescents aged 11–17 years who had not received HPV1, stratified by sex. We considered the date of vaccine licensing (June 8, 2006, for girls and October 16, 2009, for boys) as the first date for a potential missed opportunity, and we calculated missed opportunities through 2013.
Predictors for vaccination
We created univariable logistic regression models for both sexes at age 13 and age 18, modeling series initiation or series completion as outcomes. We used the county's median household income as a proxy for socioeconomic status and created income quartiles using the range of incomes by county (i.e., <$42,500, $42,500–<$46,857, $46,857–<$54,500, and ≥$54,500). Similarly, we modeled urban and rural settings by county population size quartiles (i.e., <20,000, 20,000–<60,000, 60,000–<120,000, and ≥120,000). We developed a dichotomous variable to identify adolescents who completed their primary childhood immunization series (two doses of measles, mumps, rubella; four doses of polio-containing vaccine; and five doses of diphtheria and tetanus toxoids-containing vaccine, each with appropriate intervals) before age 7 years. We developed similar dichotomous variables for adolescents who received at least one of each type of childhood vaccine or adolescents who completed at least one childhood vaccine dose. We assessed odds ratios (ORs) for significant associations with series initiation and completion at a=0.01.
RESULTS
Study population
The final dataset for analysis contained records for 1,391,069 individuals, including 697,296 males and 693,773 females ever eligible to receive HPV vaccine.
Vaccination coverage
Census-denominator estimates were similar to NIS-Teen Washington State estimates for girls with one (HPV1) or two (HPV2) doses of HPV vaccine, –0.1 and –4.0 percentage-point differences, respectively. The WAIIS/census estimate for girls with three doses of HPV vaccine was more than 10 percentage points below the NIS-Teen estimate, the only estimate outside of the 95% CIs for NIS-Teen estimates. The WAIIS/census estimates for boys were above the NIS-Teen Washington State estimates for one or two vaccine doses (+4.6 and +3.4 percentage points, respectively) and below the NIS-Teen estimates for three doses (–1.0 percentage point) (Table 1).
Table 1.
Comparison of human papillomavirus vaccine coverage estimates among adolescents aged 13–17 years, by sex and number of doses, using NIS-Teen and WAIIS study data, Washington State, 2013a
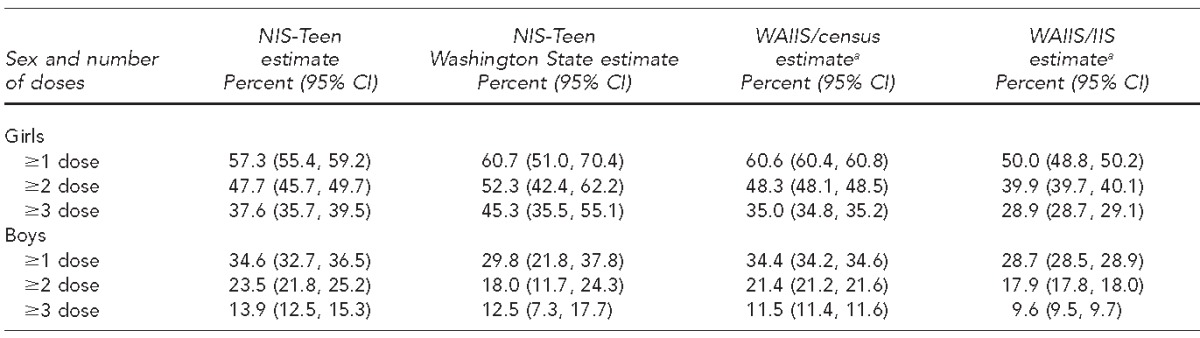
Data sources: NIS-Teen (Elam-Evans LD, Yankey D, Jeyarajah J, Singleton JA, Curtis CR, MacNeil J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2013. MMWR Morb Mortal Wkly Rep 2014;63[29]:625-33.). WAIIS: Unpublished data. Washington State Department of Health, Washington Immunization Information System, accessed February 4, 2014. Census: Washington State Office of Financial Management. Census 2010 data [cited 2014 Feb 4]. Available from: http://www.ofm.wa.gov/pop/census2010/data.asp
NIS = National Immunization Survey
WAIIS = Washington State Immunization Information System
CI = confidence interval
The WAIIS-denominator estimates were lower than the census-denominator estimates because of larger population estimates from WAIIS than from OFM. Because of the closer similarity between the NIS-Teen and census-denominator estimates than between the NIS-Teen and WAIIS-denominator estimates, the census-denominator estimates were considered a more accurate representation of our population and were used for subsequent analyses.
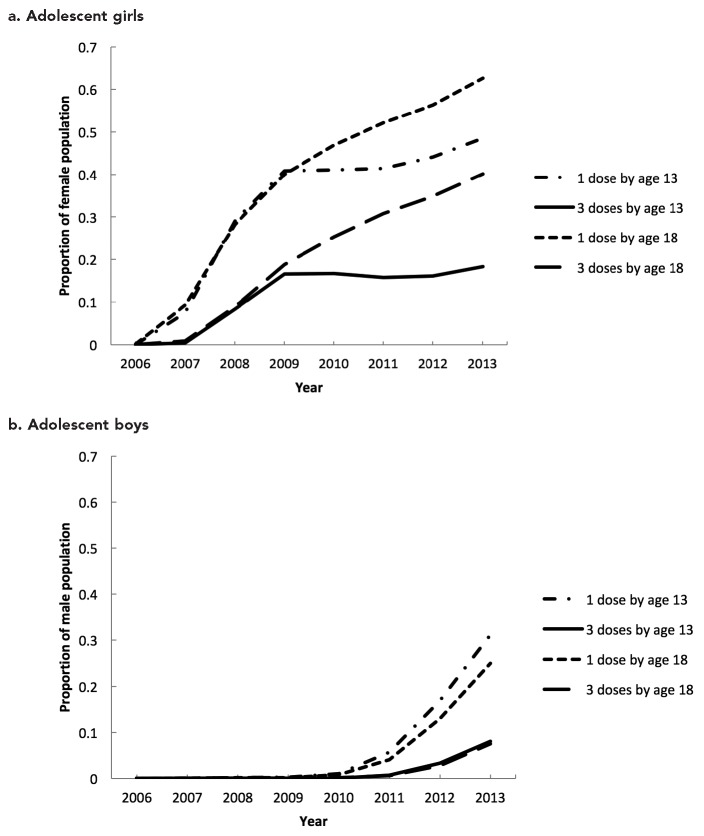
The proportion of adolescents receiving HPV vaccine in Washington increased over time (Figure 1a–b). From 2006 through 2009, vaccine uptake in girls for on-time and catch-up doses increased rapidly. During 2009–2010, on-time vaccination rates leveled off, and this stagnation persisted. On-time HPV vaccination coverage for girls decreased with each sequential dose; in 2013, coverage was 48.5% for HPV1, 32.4% for HPV2, and 18.3% for HPV3. The proportion of the population vaccinated by 18 years of age continued to increase over time, but coverage again decreased with each sequential dose. In 2013, HPV vaccination coverage in girls by age 18 years was 62.6% for HPV1, 52.0% for HPV2, and 40.1% for HPV3 (Figure 1a).
Figure 1.
Total proportion of (a) adolescent girls and (b) adolescent boys vaccinated with ≥1 or ≥3 doses of human papillomavirus vaccine by age 13 or age 18 based on doses recorded in the WAIIS and census population estimates, Washington State, 2006–2013a
aData sources: Unpublished data. Washington State Department of Health, Washington Immunization Information System, accessed February 4, 2014. Washington State Office of Financial Management. Census 2010 data [cited 2014 Feb 4]. Available from: http://www.ofm.wa.gov/pop/census2010/data.asp
WAIIS = Washington State Immunization Information System
Vaccination uptake in boys for on-time and catch-up doses increased during 2010 through 2013. However, overall vaccination coverage in boys was low. In 2013, on-time coverage was 31.2% for HPV1, 17.1% for HPV2, and 8.1% for HPV3. By age 18 years, coverage in boys in 2013 was 25.0% for HPV1, 14.5% for HPV2, and 7.5% for HPV3 (Figure 1b).
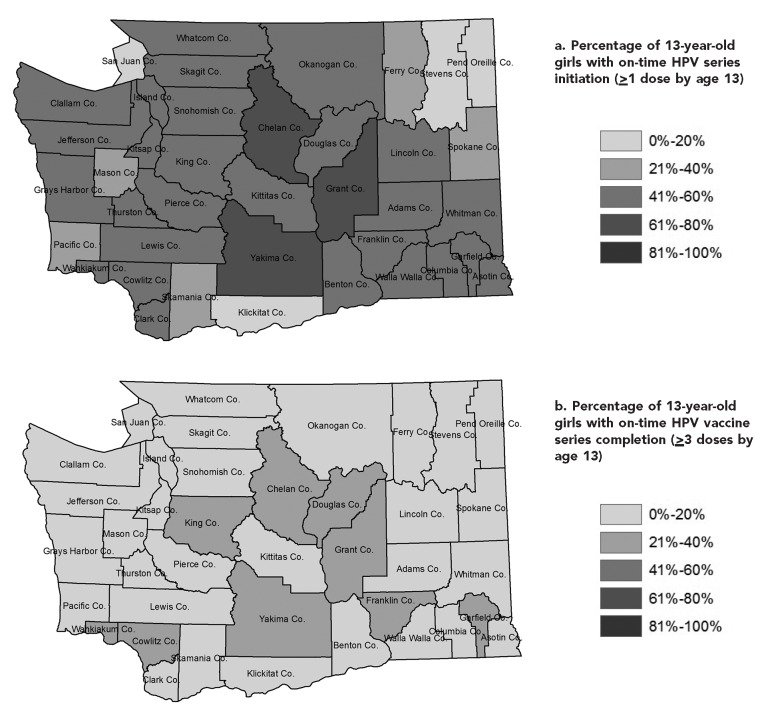
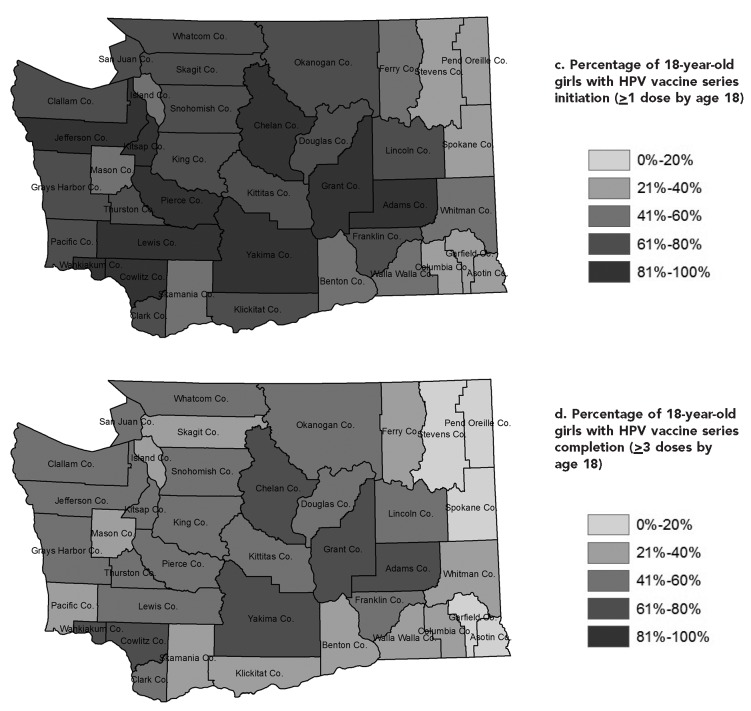
HPV vaccination coverage varied by age, sex, and county. Central Washington counties had higher vaccination coverage and peripheral counties had lower coverage. The patterns for on-time vaccine series initiation and completion were similar in both sexes, although no county had more than 50% series initiation or 20% series completion (Figures 2a–d, male data not shown). Series initiation or completion by age 18 years was also low in boys across the state. Trends in vaccination coverage over time varied by county, and several counties did not show the same recent statewide plateauing in on-time doses.
Figure 2.
(a) Percentage of 13-year-old girls with on-time human papillomavirus (HPV) vaccine series initiation (≥1 dose by age 13) and (b) completion (≥3 doses by age 13) and (c) 18-year-olds with on-time HPV vaccine series initiation (≥1 dose by age 18) and (d) completion (≥3 doses by age 18), by county, Washington State, 2013a
aData sources: Unpublished data. Washington State Department of Health, Washington Immunization Information System, accessed February 4, 2014. Washington State Office of Financial Management. Census 2010 data [cited 2014 Feb 4]. Available from: http://www.ofm.wa.gov/pop/census2010/data.asp
Missed opportunities for vaccination
The proportion of adolescent visits that were missed opportunities for HPV1 administration was 32.9% for girls and 38.7% for boys (Table 2). Unvaccinated girls aged 11–17 years eligible to receive HPV1 had 1–12 missed opportunities. Unvaccinated boys aged 11–17 years eligible to receive HPV1 had 1–9 missed opportunities. Both groups averaged 1.7 missed opportunities.
Table 2.
Adolescents aged 11–17 years not vaccinated against human papillomavirus (HPV) and with missed opportunitiesa for first dose of HPV vaccine, Washington State, 2013b
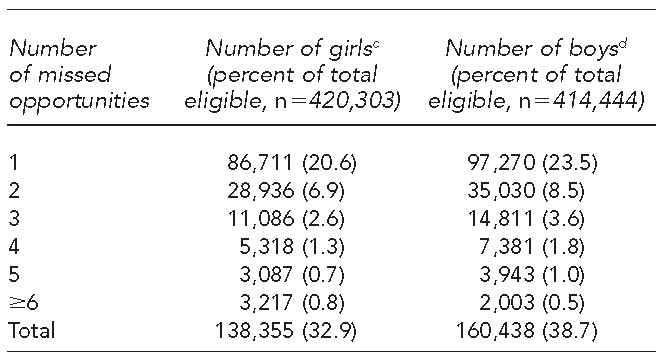
A missed opportunity is defined as a health-care visit for an eligible adolescent at which another adolescent vaccine was administered (e.g., tetanus, diphtheria, acellular pertussis; seasonal influenza; or meningococcal conjugate vaccine) without corresponding administration of the HPV vaccine.
Data source: Unpublished data. Washington State Department of Health, Washington Immunization Information System, accessed February 4, 2014.
cFor girls, the vaccine licensing date of June 8, 2006, was used as the first date at which a missed opportunity could occur.
dFor boys, the vaccine licensing date of October 16, 2009, was used as the first date at which a missed opportunity could occur.
Logistic regression modeling
Univariable logistic regression modeling showed an association between HPV vaccine series initiation by age 13 years and having a single childhood vaccine dose in both sexes (girls: OR=2.1, 95% CI 2.0, 2.3, p<0.001; boys: OR=2.0, 95% CI 1.9, 2.1, p<0.001), having one dose of each type (girls: OR=2.7, 95% CI 2.6, 2.8, p<0.001; boys: OR=2.5, 95% CI 2.3, 2.6, p<0.001), or having all childhood doses complete (girls: OR=2.1, 95% CI 2.0, 2.1, p<0.001; boys: OR=1.8, 95% CI 1.7, 1.9, p<0.001). Likewise, HPV vaccine series initiation by age 18 years and series completion by age 13 or 18 years were significantly associated with all childhood vaccination variables at p<0.001. We found no clear dose-response pattern by median county income or county population size, although some income quartiles and population-size quartiles were associated with vaccination.
DISCUSSION
The results of our analysis support previous findings that HPV vaccination coverage in Washington State is similar to national averages. Although coverage increased statewide upon introduction, on-time vaccination plateaued over time. Missed opportunities occurred in at least one-third of unvaccinated adolescents, indicating that low vaccination rates are not solely the result of a lack of health-care visits or objections to vaccination in general, as noted elsewhere.10 HPV vaccination coverage in Washington State varied by age, sex, and county of residence. Some counties had vaccination rates that were much higher than state averages, and other counties continued to see growth in on-time dose uptake instead of the plateauing on-time coverage seen statewide. Central Washington counties had higher vaccination coverage and peripheral counties had lower coverage, which paralleled generalized childhood vaccination coverage patterns in the state. Vaccination uptake in boys was delayed compared with vaccination uptake in girls, which was expected because of the delayed FDA approval and ACIP recommendation for use of HPV vaccines in boys.
We found no clear dose-response patterns for county-level variables and HPV vaccination coverage. Further research is needed to understand predictors of higher vaccination rates and the plateauing of on-time dose coverage. This detailed picture of variation in vaccination can help direct research, programs, and interventions to improve HPV vaccination rates statewide in a targeted and efficient way.
Strengths and limitations
Strengths of this study included the wide-reaching coverage of WAIIS data, which included data collected more than a decade before introduction of HPV vaccines. The large number of records allowed for stratification of HPV vaccination coverage by three variables: age, sex, and county of residence. Previously, the only data available for Washington State were from the NIS-Teen study, which is based on a sample of adolescents that is insufficient to stratify by county and groups adolescents in one age category. Information from this study identified areas for improvement; in each county in Washington State, we assessed on-time vaccination coverage, coverage by sex, three-dose series completion, and change in vaccination coverage over time. Additionally, we assessed the number and proportion of vaccination visits that were missed opportunities for HPV1 administration. These metrics will allow tracking of coverage improvements over time and reductions in missed opportunities in Washington State.
The data were also subject to several limitations. Information in the WAIIS is estimated to cover 84% of adolescents aged 11–17 years in Washington State; however, the WAIIS denominator overestimated the population. Because of difficulties with denominator estimation using WAIIS data, we used the census denominator instead. Using the census denominator forced the assumption that adolescents migrating into the state, whose doses may not be included in the WAIIS registry, have a similar vaccination history as those migrating out of the state, whose records should have been marked as inactive. Additionally, an estimated 16% of adolescents aged 11–17 years are not covered by the registry, which likely led to an underestimation of vaccination coverage. Demographic information was also missing from a large number of WAIIS records.
The definition used for a missed opportunity most likely led to an underestimation of missed opportunities for administration of HPV vaccine. A missed opportunity was defined as a health-care visit for an eligible adolescent during which another adolescent vaccine was administered without corresponding administration of HPV1. This definition was necessary because other health-care visits were unknown. Some adolescents likely had visits at which no vaccines were administered; accounting for these visits would lead to a more accurate estimation of missed opportunities. Additionally, this study assessed missed opportunities only among the study population of 11- to 17-year-olds from vaccine licensing through 2013; missed opportunities likely varied over time, with more missed opportunities immediately following vaccine licensing and decreasing over time.
Lastly, the results from the univariable logistic regression analysis were difficult to interpret. Our large population size made almost all associations significant, but it is unclear if any associations were meaningful. A history of childhood vaccination may be an important predictor for adolescent vaccination, but this association may be confounded by whether or not a child's health-care provider enters information on doses into the WAIIS. Significant associations, but no clear dose-response patterns, were seen for some county income quartiles and county population-size quartiles; however, it is unclear if these associations are meaningful. These county-level variables do not represent individual-level data, which may obscure any true effects of socioeconomic status or urban vs. rural setting. This modeling should be repeated using individual-level data, which were not available for this study, to further assess these relationships.
CONCLUSION
HPV vaccination coverage in Washington State was similar to national averages, but we found significant variation at the county level. Information on varying vaccination coverage by age, sex, and county should be used to target programs and interventions to improve HPV vaccination rates statewide. Further research is needed to understand predictors of plateauing on-time vaccination rates and geographical variation in vaccination coverage.
Footnotes
This study was supported in part by an appointment to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists and funded by the Centers for Disease Control and Prevention cooperative agreement #1U38OT000143-02. The Washington State Institutional Review Board deemed this project exempt from review.
REFERENCES
- 1.Dunne EF, Markowitz LE, Saraiya M, Stokley S, Middleman A, Unger ER, et al. CDC Grand Rounds: reducing the burden of HPV-associated cancer and disease. MMWR Morb Mortal Wkly Rep. 2014;63(4):69–72. [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Simard EP, Dorell C, Noone A-M, Markowitz LE, Kohler B, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Drug Administration (US) June 8, 2006, approval -letter—human papillomavirus quadrivalent (types 6, 11, 16, 18) vaccine, recombinant [cited 2014 Aug 7] Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm111283.htm.
- 4.Food and Drug Administration (US) October 16, 2009, approval letter—Gardasil [cited 2014 Aug 7] Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186991.htm.
- 5.Food and Drug Administration (US) October 16, 2009, approval letter—Cervarix [cited 2014 Aug 7] Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186959.htm.
- 6.Food and Drug Administration (US) December 10, 2014, approval letter—Gardasil 9 [cited 2014 Aug 7] Available from: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm426520.htm.
- 7.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2007;56:1–24. (early release) [PubMed] [Google Scholar]
- 8.Dunne ER, Markowitz LE, Chesson H, Curtis CR, Saraiya M, Gee J, et al. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–8. [PubMed] [Google Scholar]
- 9.Hofstetter AM, Stockwell MS, Al-Husayni N, Ompad D, Natarajan K, Rosenthal SL, et al. HPV vaccination: are we initiating too late? Vaccine. 2014;32:1939–45. doi: 10.1016/j.vaccine.2014.01.084. [DOI] [PubMed] [Google Scholar]
- 10.Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, et al. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–4. [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (US) CDC looks back at 2013 health challenges, ahead to 2014 health worries; press release 2013 Dec 16 [cited 2015 Aug 7] Available from: http://www.cdc.gov/media/releases/2013/p1216-eoy2013.html.
- 12.Brewer NT, Gottileb SL, Reiter PL, McRee AL, Liddon N, Markowitz L, et al. Longitudinal predictors of HPV vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011;38:197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher H, Trotter CL, Audrey S, MacDonald-Wallis K, Hickman M. Inequalities in the uptake of human papillomavirus vaccination: a systematic review and meta-analysis. Int J Epidemiol. 2013;42:896–908. doi: 10.1093/ije/dyt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins RB, Brogly SB, Adams WG, Freund KM. Correlates of human papillomavirus vaccination rates in low-income, minority adolescents: a multicenter study. J Womens Health. 2012;21:813–20. doi: 10.1089/jwh.2011.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiter PL, McRee AL, Gottlieb SL, Brewer NT. Correlates of receiving recommended adolescent vaccines among adolescent females in North Carolina. Human Vaccin. 2011;7:67–73. doi: 10.4161/hv.7.1.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey A, Cohn L, Dalton V, Ruffin M. Patient and clinic factors associated with adolescent human papillomavirus vaccine utilization within a university-based health system. Vaccine. 2010;28:989–95. doi: 10.1016/j.vaccine.2009.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiro JA, Tsui J, Bauer HM, Yamada E, Kobrin S, Breen N. Human papillomavirus vaccine use among adolescent girls and young adult women: an analysis of the 2007 California Health Interview Survey. J Womens Health (Larchmt) 2012;21:656–65. doi: 10.1089/jwh.2011.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laz TH, Rahman M, Berenson AB. An update on human papillomavirus vaccine uptake among 11–17 year old girls in the United States: National Health Interview Survey, 2010. Vaccine. 2012;30:3534–40. doi: 10.1016/j.vaccine.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (US) Atlanta: CDC; 2014. May, How state and local immunization programs can help improve HPV vaccination rates to reduce future cancers. [Google Scholar]
- 20.Hills RA, Revere D, Altamore R, Abernethy NF, Lober WB. Timeliness and data element completeness of immunization data in Washington State in 2010: a comparison of data exchange methods. AMIA Annu Symp Proc. 2012;2012:340–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (US) 2012 IISAR data participation rates [cited 2014 Aug 8] Available from: http://www.cdc.gov/vaccines/programs/iis/annual-report-IISAR/2012-data.html.
- 22.Centers for Disease Control and Prevention (US) 2009 IISAR data participation rates [cited 2014 Aug 8] Available from: http://www.cdc.gov/vaccines/programs/iis/annual-report-IISAR/2009-data.html.
- 23.Jackson ML, Henrikson NB, Grossman DC. Evaluating Washington State's immunization information system as a research tool. Acad Pediatr. 2014;14:71–6. doi: 10.1016/j.acap.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 24.SAS Institute, Inc. Cary (NC): SAS Institute, Inc.; 2013. SAS®: Version 9.3. [Google Scholar]
- 25.Elam-Evans LD, Yankey D, Jeyarajah J, Singleton JA, Curtis CR, MacNeil J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(29):625–33. [PMC free article] [PubMed] [Google Scholar]
- 26.ESRI. Redlands (CA): ESRI; 2010. ArcMap: Version 10. [Google Scholar]