Abstract
Objective
Define the demographics, natural history, and clinical management of patients with inclusion body myositis (IBM).
Background
Few studies of the demographics, natural history, and clinical management of IBM have been performed in a large patient population.
Methods
A cross-sectional, self-reporting survey was conducted.
Results
The mean age of the 916 participants was 70.4 years, the male-to-female ratio was 2:1, and the majority reported difficulty with ambulation and activities of daily living. The earliest symptoms included impaired use and weakness of arms and legs. The mean time from first symptoms to diagnosis was 4.7 years. Half reported that IBM was their initial diagnosis. A composite functional index negatively associated with age, disease duration, and positively associated with participation in exercise.
Conclusion
These data are valuable for informing patients how IBM manifestations are expected to impair daily living and indicate that self-reporting could be used to establish outcome measures in clinical trials.
Keywords: Inclusion body myositis, survey, myopathy, demographics, natural history
INTRODUCTION
Inclusion body myositis (IBM) is both an autoimmune and degenerative disorder of skeletal muscle of unknown etiology 1–3. Patients with IBM experience slowly progressive muscle weakness that can occasionally be asymmetric. Muscles most frequently affected include finger and wrist flexors, quadriceps, and the muscles of the lower leg. This muscle involvement is a consistent pattern that is evident clinically and can be also visualized through imaging 4. The disease manifests as slowly progressive hand and finger grip impairment and difficulty with ambulation. The etiology and pathogenesis of IBM are understood poorly, but aging, genetics, and environment may each play a role 5,6. IBM is typically sporadic in nature (often termed sIBM) and most often occurs after age 45 7. The prevalence of IBM in North America has not been reported. However, limited studies in the USA estimate the prevalence per million in Connecticut to 10.7 and 71 in a single county in Minnesota 8. A national survey in Australia reported that the disease affects 14.9 per million individuals, and this prevalence increases to 51.3 per million when the estimate is restricted to people over age 50 9.
The literature on the demographics and clinical history of patients with IBM comprises a very limited number of studies, each of which involves a very small sample of patients. These studies include a cross-sectional study of 64 patients 10; a long-term observational study of 136 patients at 2 separate centers 11; a study of 51 patients, in which 23 participated in a one-year follow-up 12; and separate clinical studies of 18 13 patients and 15 patients 14;and a study focused on demographic features of 73 patients in Japan 15. In addition the Myositis Association of America (renamed The Myositis Association) compiled a report of their survey that included 364 adult patients with sporadic IBM. Collectively, these studies offer a limited and highly localized portrait of the general and clinical characteristics of sporadic IBM, including the mean age of patients, age of onset, period between symptoms and diagnosis, which muscle groups are most affected, and daily living tasks that are difficult. As a means to consolidate findings from these studies into a valuable metric, the Muscle Study Group (MSG) 16 developed an IBM functional rating scale (IBMFRS). This was used to evaluate 30 IBM patients in a placebo-controlled trial. The scale is based on 10 questions related to tasks associated with daily living.
The rarity of such studies and their limited number of included patients emphasize the difficulty of performing studies in IBM. Moreover, given the rarity of the disease, surveys of considerable size are not possible in single centers. Accordingly, we undertook a self-reporting survey of IBM patients in North America in order to collect both clinical and demographic data from a relatively large cohort of subjects. There were 4 aims of this study. The first was to describe the demographics of the IBM patient population; the second was to describe the nature of IBM disability by characterizing the trajectory of disease symptoms, its impact on activities of daily living, and its effect on quality of life; the third was to ascertain the means of diagnosis; and the fourth was to determine whether the natural history of disease varies between population groups. The survey included questions related to demographics, diagnosis, clinical care, and daily living queries that build upon the IBMFRS.
METHODS
Survey implementation
We developed the questionnaire (Table S1, available online), with guidance from experts in the fields of rheumatology, neurology, economics, operations research, patient registry design, and health care management policy. The survey was implemented in both electronic and paper formats. The electronic version was prepared using the online design, distribution, and analysis software of Qualtrics, a market research and enterprise feedback organization (www.qualtrics.com). A sample of persons with IBM was assembled with the help of 2 patient organizations. The Myositis Association (TMA) is an international organization of roughly 9,000 patients living with inflammatory muscle diseases, including IBM (www.myositis.org). TMA sent an electronic message on our behalf to its approximately 1,400 IBM members with known email addresses. A reminder email was also sent to these persons 2 weeks later. Individuals were asked to voluntarily complete the survey anonymously via the Qualtrics online website. Persons who felt uncomfortable or unable to complete the survey online were provided with information to obtain a hard-copy version. TMA mailed a paper copy of the cover letter, consent form, and survey to approximately 1,500 U.S. and Canadian IBM patients in its database who lacked an email address. The Muscular Dystrophy Association (MDA) is a nonprofit health agency dedicated to finding treatments and cures for neuromuscular diseases, including IBM (www.mda.org). The MDA posted a brief article in Quest, its online newsletter, (quest.mda.org) describing the survey and providing a link to the Qualtrics website. MDA also e-mailed the article to its registered IBM patients.
The original survey did not include a sufficient query to accurately determine the time between the appearance of symptoms and the date of first diagnosis. We therefore created a supplementary survey (Table S1, available online) that asked 2 short questions about symptom onset and time of diagnosis. We also asked again for year and place of birth so that we could match these supplemental survey responses with those of the original survey without asking respondents to reveal their protected identity. The request to patients to participate in this supplementary survey was emailed to patients in the TMA database, and an article about the supplemental survey also appeared in the MDA Quest newsletter.
Inclusion and exclusion criteria
We included all the records submitted (via paper or electronically) by patients confirming a diagnosis of IBM. A few participants (n=10) responded indirectly via a caregiver (a spouse or child), and several participants (n=29) asked (and received) permission to respond on behalf of a deceased loved one; these records were included. Exclusion was only applied in instances of duplication. Duplicate records were identified first by examining records for matching year and place of birth. Records that matched in these 2 categories were then examined for matching in 6 additional categories: gender, ethnicity, height, education level, annual income, and marital status. Duplicates that matched in all 8 categories were eliminated.
Ethics
All patients provided informed consent to participate in the survey. The survey instrument, which included the questionnaire, accompanying consent forms, and data management protocols, was approved by the Human Research Protection Program at Yale School of Medicine. No identifiers or any other information that could be used to identify or locate survey respondents were collected. At no time did either TMA or MDA share their mailing lists with the investigators. Completion and submission of surveys was completely voluntary and anonymous.
Statistical analyses
Column statistics were calculated with Microsoft Excel. The MEANS procedure for calculating latency was performed with Statistical Analysis System (SAS) software. A composite index of function was constructed as the sum of response scores (0–6) over 10 categories of disability measured in the survey (cutting food and handling utensils, dressing, fine motor tasks, handwriting, hygiene, sit to stand, swallowing, turning in bed/adjusting covers, walking, and climbing stairs). A higher composite index reflects greater ability. To explore the relationships between age, time since onset of disease, and exercise on overall functional status, fixed effects models were fitted to the composite index. The models include combinations of the following variables: age, gender, time since diagnosis (in years), the type of the exercise in which a respondent engages, and the hours spent on that exercise in a week. To evaluate different aspects of the relationship with exercise, we estimated 3 separate fixed effects models that include indicators for either: any participation in exercise, type of exercise, or hours of exercise per week. These models also include age and gender as control variables. An algorithm was applied to deal with missing data if a respondent did not answer a question and to deal with multiple variables. All computations were performed using Statistical Analysis System 9.3 (SAS). Least squares (LS) means for each exercise category (defined by type of exercise and hours per week), 95% confidence intervals (CIs) for pairwise differences in LS means, and P-values for these pairwise comparisons were generated. Normal probability plots and residual plots were used to assess the assumptions of the above model.
RESULTS
Participation and general demographics
We received a total of 973 responses (795 online; 178 paper), of which 57 were considered duplicates. The online survey response rate was 795/1400 (56.7%) based on the original TMA solicitation through email. Additional online responses were received following the announcement in the TMA newsletter that could not be attributed exclusively to the email solicitation. The response rate for the mailed paper surveys was 178/1500 (11.8%). Of the collective 916 unique respondents 613 (66.9%) were men, yielding a male-female ratio of 2:1 (Table 1). The mean age of the subjects was 70.4 (SD, 10.2) years (Table 1). The age range was 32 – 100 years. The majority (65.0%) of the subjects were between ages 60–80 years. Only 3 (0.3%) of the 916 respondents were less than 40 years of age. Additional demographics that were collected included race, living arrangements, employment, height, and weight (Table S2, available online). The race of the responding majority was white (95.5%), and their ethnicity was non-Hispanic/Latino (97.3%). Many of the respondents were retired (75.3%). Most respondents still lived at home (91.7%), and a high fraction lived with a spouse (75.6%). The likelihood of residing in an assisted living facility was higher for those respondents living alone (10.0%) than for those living with a spouse (1.5%). The weight and height distributions of the respondents were unremarkable. However, the body mass index (BMI) of a considerable fraction (43%), was greater than 27.
Table 1.
Age and gender of the survey participants
| Demographic Category | Respondents (n) | Responding Yes (%) |
|---|---|---|
| Age | 916 | |
| < 40 | 3 | 0.33 |
| 40–49 | 27 | 2.9 |
| 50–59 | 157 | 17.1 |
| 60–69 | 303 | 33.1 |
| 70–79 | 292 | 31.9 |
| ≥ 80 | 134 | 14.6 |
| Gender | 916 | |
| Men | 613 | 66.9 |
| Women | 303 | 33.1 |
Ambulation and daily living
Daily living was evaluated through questions we developed using the IBMFRS disability scale 16 as a guide. While the validity of the IBMFRS for self-administration has yet to be established, such an assessment is beyond both the exploratory scope of the current study and our use of the IBMFRS in guiding the development of our survey. Our questions included rating scales of difficulty in daily tasks including handwriting, eating, fine motor tasks, dressing, standing, walking, and climbing stairs (Table S3, available online). Among these assessments, those that were notably influenced by the disease were standing from sitting and walking. Many subjects reported that rising from the sitting position requires use of arms (44.5%) or requires assistance from a device or person (34.2%). Similarly, the majority of subjects reported difficulty with walking as only 5.7% qualified their ability as normal. Difficulty included unsteadiness while walking (17.0%), dependence on an assistive device (24.6%), and dependence on a wheelchair (26.6%). Disability was apparent in stair climbing assessment. The majority of subjects (56.8%) reported the inability to climb stairs, while a further 17.4% depend on hand rails or additional support (17.3%). Two-thirds (66.2%) reported the inability to walk more than a block. Nearly half of the subjects (46.6%) reported participation in some form of exercise.
A majority of subjects reported that IBM produced either extreme (41.2%) or considerable (32.6%) interference with work both in and out of the home, while very few (2.0%) indicated that it had no influence. Similarly, most of the subjects reported that IBM influenced their energy levels some (23.7%), all (32.0%), or most (37.7%) of the time. Slightly more than half (55.3%) operated a motor vehicle alone, and the majority traveled away from home. More than half indicated that the disease influenced their mood toward depression. Only a third of the respondents (36.7%) reported that eating or swallowing was unaffected, while nearly half reported difficulty swallowing (44.0%), and 12.0% reported that they choke frequently. Tasks that require motor skills, including handwriting, handling utensils, dressing, hygiene, adjusting in bed, and fine motor skills were all influenced in many subjects and were rarely reported as normal. In spite of these difficulties, 58.4% of the subjects reported that they did not employ assistance in their activities of daily living.
Diagnosis
Subjects were asked several questions related to their diagnosis and clinical care (Table S4, available online). Regarding the symptom(s) that compelled them to first seek medical care, the majority cited weakness (69.9%) as the chief ailment followed by difficulty in climbing stairs (59.6%), falls (56.8%), and impaired use of arms and legs (53.4%). Fewer reported fatigue (32.0%) and trouble swallowing (23.0%). The time between the observation of these initial symptoms and diagnosis was greater than 2 years in nearly half of the subjects (45.9%). Other respondents reported shorter intervals: between 1–2 years in 22.8%; 6–12 months in 14.3%, and less than 6 months in the remaining 15.8% of the subjects. The mean latency between disease onset and establishment of the diagnosis was 4.7 years for the subset of patients (n=280) that completed the second follow-up survey. This value compares favorably with estimates (5.7 years) from other series that report this metric 13,17,18,14. Approximately half of the subjects (50.9%) reported their initial diagnosis as IBM. The other half was diagnosed initially with polymyositis (18.8%) or arthritis (4.3%), and the remainder did not know or were diagnosed with another disease that was not disclosed. This initial diagnosis was provided to the majority of the subjects (69.3%) by a neurologist. Both rheumatologists (14.4%) and primary care providers (10.7%) provided the initial diagnosis in a noticeably smaller subset. Neurologists provided the first diagnosis of IBM in 78.3% of the subjects. Nearly all (90.8%) reported that a muscle biopsy was associated with the diagnosis of IBM. Finally, almost all (97.7%) had no knowledge of IBM remissions through their own experience or that of other patients.
Factors correlated with function and disability
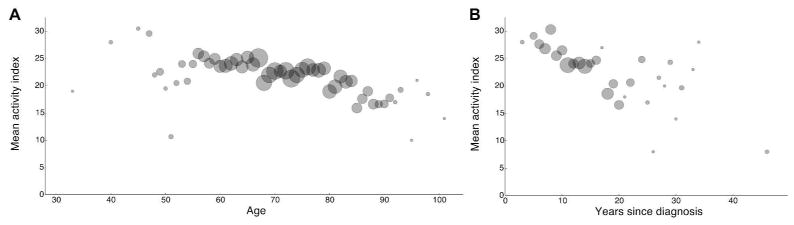
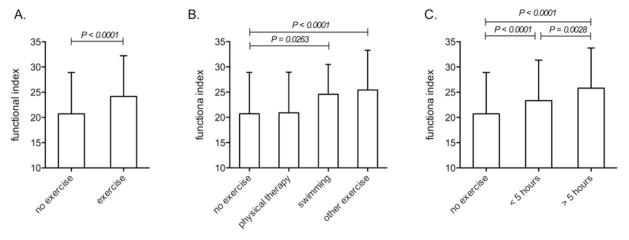
A functional index was used to determine whether disability was associated with demographics and daily living. There was an association between age and functional ability (P < 0.0001), indicating that older respondents experienced greater disability (Figure 1A). Similarly, for every year since diagnosis, there was an average decrease of 0.38 in the overall functional index (P < 0.0001), highlighting disease progression and impairment (Figure 1B). We also found associations between exercise and degree of function (Figure 2) by comparing the functional index of subjects who exercised (controlling for age and gender) to that of subjects who did not. We observed that participation in exercise was significantly associated (P < 0.0001) with increased functional ability (Figure 2A). Subjects who participated in swimming or other unspecified forms of exercise reported greater functional ability (Figure 2B) than respondents who did not exercise (P=0.0263 for swimming and P < 0.0001 for unspecified forms). Participation in physical therapy was not associated significantly (P=0.5042) with increased functional ability (Figure 2B). The amount of time spent exercising was associated significantly with increased functional ability. Respondents who spent either up to 5 hours or more than 5 hours per week had significantly higher functional index scores (P < 0.0001 for both categories) compared to respondents who did not exercise (Figure 2C). Moreover, respondents who spent more than 5 hours per week participating in exercise had greater functional ability (P=0.0028) than those whose exercise was limited to 5 hours or less (Figure 2C).
Figure 1.
Age and disease duration associate with disability in patients with IBM. A functional index, calculated from 10 scores reporting ambulation and daily living activities, negatively associated with both the age of respondents and the number of years since diagnosis. The mean of the functional activity index was plotted against the age (years) of respondents (A) or disease duration (B) indicated by the number of years since the diagnosis of IBM. The size of the data points in each graph represents the relative number of respondents in each age group.
Figure 2.
Participation in exercise associates with better function in patients with IBM. A functional index, calculated from scores reporting ambulation and daily living activities, was compared among survey respondents. Three fixed effects models were estimated that include age and gender, as well as indicators for either: any participation in exercise (A), or type of exercise (swimming, physical therapy, or other unspecified forms of exercise) (B), or hours of exercise per week (C). Using these indicators, comparisons were made relative to no exercise (A–C), and between types of exercise (B) or hours of exercise (C). Statistical differences are indicated when significant.
DISCUSSION
Overall, our data set agreed very well with the existing literature on IBM. We obtained a male:female ratio of 2:1 which is in agreement with a number of other studies19,18,12,11,15. However, other observational studies of IBM report ratios that are more extreme (6:1) 14 and also closer to equal distribution between the genders (1.25:1) 13. IBM is reported rarely in patients less than age 40 years 14,11; our data reflected this, in that only 0.3% of the respondents were less than age 40 years. The rate of functional decline over time in IBM patients varies based on the age of onset. Those in whom the disease onset occurs between 40–59 years progress more slowly than those whose onset occurs between 60–79 years of age 20. Given that IBM develops slowly and starts insidiously, by the time medical help is pursued there is already significant muscle weakness, atrophy, and advanced degeneration even in clinically strong muscles. Our data reflect this, in that a large fraction (nearly 70%) of respondents reported muscle weakness as the reason for seeking medical advice. Only half of the subjects in our study reported IBM as their initial diagnosis, indicating that misdiagnosis of IBM is common. Other studies that queried this also reveal that rates of initial misdiagnosis can reach as high as 85% 18. Neurologists, confirming another survey, most often perform evaluations and provide a diagnosis of IBM 18. These findings highlight that patients suspected of having myositis should be directed to clinicians who specialize in inflammatory myopathies. Overall, our study provided data that were in alignment with a number of prior clinical studies, indicating that self-reporting may be valuable for acquiring outcome measures in clinical studies of IBM.
A few interesting patterns emerged from our post-hoc analysis of associations. We observed that approximately three-fourths of the respondents lived at home with a spouse. Respondents living alone were much more likely to reside in an assisted living facility than respondents who lived with a spouse. These data taken together may reflect that spousal help is an important contributing factor in keeping those with the disease in their homes. We also focused our inquiry of associations with the degree of disability. Given the progressive nature of the disease it was not unexpected that individuals with longer disease duration experienced less ability in ambulation and tasks accompanying daily living. The same was true of age. Older subjects also experienced increased disability compared to their younger counterparts. Interestingly, participation in exercise and the duration of participation were strongly associated with increased ambulation and performance of daily living tasks. Exercise provides a number of beneficial effects in polymyositis and dermatomyositis 21. Although studies in IBM are few, it appears to prevent loss of muscle strength/deterioration 22,23. While the associations we found do not imply causality, we cannot discern whether exercise slows disability or if persons with less severe disability are more likely to exercise, they warrant further exploration.
Although this study has provided a wealth of information, the limitations must be considered. The origin of this study was founded in the objective to build a national registry of IBM patients and the associated demographics and clinical data. Any study, such as ours, in which a convenience sample is used, includes inherent bias. Self-reporting allows a single study to cover a huge geographic area and therefore a very large cohort of subjects; further research is needed, however, to confirm the validity of our question set for self-administration. Among the obvious limitations are that a clinical evaluation was not performed, thus the possibility that some respondents have not been properly diagnosed remains. However, our data set matches other surveys of these patients, and more than 90% reported undergoing muscle biopsy during diagnosis. We are aware that surveys conducted in the context of patient care will capture a wide diversity of patients. Our online survey would exclude those unfamiliar with the Internet. We sought to address this limitation by including a standard mail version of the survey. Additional comorbidities were not queried which may have contributed to conditions that we ascribed to IBM. Overall, our data derived from a number of queries was in agreement with several other studies, suggesting the veracity of our data set in light of these limitations. Finally, we did not include a question that would allow us to distinguish between hereditary inclusion body myopathy and sporadic IBM. Hereditary inclusion body myopathy encompasses a heterogeneous group of vacuolar myopathies that can be either autosomal recessive or dominant 24; they are rare, as a very small number of cases have been reported 24–26.
Although the 2 diseases share a number of pathologic features, hereditary inclusion body myopathy is not associated with an intramuscular lymphocyte infiltrate, thus it is termed a myopathy rather than a myositis, and the disease spares the quadriceps. Additionally, unlike sporadic IBM, which mostly affects individuals over age 50 years, the clinical onset of hereditary inclusion body myopathy usually occurs in individuals aged between 20–40 27. Given the age of onset of our subjects, that none of the respondents reported knowing any family members with IBM, and that this disease is extraordinary rare, we reason that our data include very few, if any cases of hereditary inclusion body myopathy.
The results are of value for 2 main purposes. The first is that these data will be valuable for informing patients of the manifestations of the disease. These data may help clinicians explain to their IBM patients their prognoses and specifically what they can expect to experience in their daily lives based on the experiences of a large group of their peers. Second, further value may be realized by overcoming the absence of standardized outcome measures able to capture meaningful changes connected with disability and quality of life. We suggest that self-reporting surveys of patients with IBM are accurate in terms of assessing demographics and, more importantly, evaluating disability. Thus, such instruments may be considered for use as outcome measures for clinical trials, as this approach could reduce considerably both clinic visits and, by extension, the costs of clinical trials.
Supplementary Material
Acknowledgments
The authors are grateful to The Myositis Association (TMA) and the Muscular Dystrophy Association (MDA) for providing assistance in distributing the survey and to the Cowles Foundation for Research in Economics at Yale University. KCO is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R03AR061529. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- BMI
body mass index
- CI
confidence interval
- IBM
inclusion body myositis
- IBMFRS
IBM functional rating scale
- LS
least squares
- MSG
Muscle Study Group
- SAS
Statistical Analysis System
- TMA
The Myositis Association
References
- 1.Greenberg SA. Pathogenesis and therapy of inclusion body myositis. Curr Opin Neurol. 2012;25(5):630–639. doi: 10.1097/WCO.0b013e328357f211. [DOI] [PubMed] [Google Scholar]
- 2.Amato AA, Barohn RJ. Inclusion body myositis: old and new concepts. J Neurol Neurosurg Psychiatry. 2009;80(11):1186–1193. doi: 10.1136/jnnp.2009.173823. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt J, Dalakas MC. Inclusion body myositis: from immunopathology and degenerative mechanisms to treatment perspectives. Expert Rev Clin Immunol. 2013;9(11):1125–1133. doi: 10.1586/1744666X.2013.842467. [DOI] [PubMed] [Google Scholar]
- 4.Cox FM, Reijnierse M, van Rijswijk CS, Wintzen AR, Verschuuren JJ, Badrising UA. Magnetic resonance imaging of skeletal muscles in sporadic inclusion body myositis. Rheumatology (Oxford) 2011;50(6):1153–1161. doi: 10.1093/rheumatology/ker001. [DOI] [PubMed] [Google Scholar]
- 5.Askanas V, Engel WK, Nogalska A. Pathogenic considerations in sporadic inclusion-body myositis, a degenerative muscle disease associated with aging and abnormalities of myoproteostasis. J Neuropathol Exp Neurol. 2012;71(8):680–693. doi: 10.1097/NEN.0b013e31826183c8. [DOI] [PubMed] [Google Scholar]
- 6.Hohlfeld R, Engel AG, Goebels N, Behrens L. Cellular immune mechanisms in inflammatory myopathies. Curr Opin Rheumatol. 1997;9(6):520–526. doi: 10.1097/00002281-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Rose MR. 188th ENMC International Workshop: Inclusion Body Myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Disord. 2013;23(12):1044–1055. doi: 10.1016/j.nmd.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Wilson FC, Ytterberg SR, St Sauver JL, Reed AM. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol. 2008;35(3):445–447. [PubMed] [Google Scholar]
- 9.Needham M, Corbett A, Day T, Christiansen F, Fabian V, Mastaglia FL. Prevalence of sporadic inclusion body myositis and factors contributing to delayed diagnosis. J Clin Neurosci. 2008;15(12):1350–1353. doi: 10.1016/j.jocn.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Badrising UA, Maat-Schieman ML, van Houwelingen JC, van Doorn PA, van Duinen SG, van Engelen BG, et al. Inclusion body myositis. Clinical features and clinical course of the disease in 64 patients. J Neurol. 2005;252(12):1448–1454. doi: 10.1007/s00415-005-0884-y. [DOI] [PubMed] [Google Scholar]
- 11.Benveniste O, Guiguet M, Freebody J, Dubourg O, Squier W, Maisonobe T, et al. Long-term observational study of sporadic inclusion body myositis. Brain. 2011;134(Pt 11):3176–3184. doi: 10.1093/brain/awr213. [DOI] [PubMed] [Google Scholar]
- 12.Cortese A, Machado P, Morrow J, Dewar L, Hiscock A, Miller A, et al. Longitudinal observational study of sporadic inclusion body myositis: implications for clinical trials. Neuromuscul Disord. 2013;23(5):404–412. doi: 10.1016/j.nmd.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Lindberg C, Persson LI, Bjorkander J, Oldfors A. Inclusion body myositis: clinical, morphological, physiological and laboratory findings in 18 cases. Acta Neurol Scand. 1994;89(2):123–131. doi: 10.1111/j.1600-0404.1994.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 14.Amato AA, Gronseth GS, Jackson CE, Wolfe GI, Katz JS, Bryan WW, et al. Inclusion body myositis: clinical and pathological boundaries. Ann Neurol. 1996;40(4):581–586. doi: 10.1002/ana.410400407. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi H, Koike H, Matsuo K, Tanaka F, Noda T, Fujikake A, et al. Demographic features of Japanese patients with sporadic inclusion body myositis: a single-center referral experience. Intern Med. 2013;52(3):333–337. doi: 10.2169/internalmedicine.52.8910. [DOI] [PubMed] [Google Scholar]
- 16.Jackson CE, Barohn RJ, Gronseth G, Pandya S, Herbelin L. Inclusion body myositis functional rating scale: a reliable and valid measure of disease severity. Muscle Nerve. 2008;37(4):473–476. doi: 10.1002/mus.20958. [DOI] [PubMed] [Google Scholar]
- 17.Lotz BP, Engel AG, Nishino H, Stevens JC, Litchy WJ. Inclusion body myositis. Observations in 40 patients. Brain. 1989;112( Pt 3):727–747. doi: 10.1093/brain/112.3.727. [DOI] [PubMed] [Google Scholar]
- 18.Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine. 2001;80(5):320–327. doi: 10.1097/00005792-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Chahin N, Engel AG. Correlation of muscle biopsy, clinical course, and outcome in PM and sporadic IBM. Neurology. 2008;70(6):418–424. doi: 10.1212/01.wnl.0000277527.69388.fe. [DOI] [PubMed] [Google Scholar]
- 20.Peng A, Koffman BM, Malley JD, Dalakas MC. Disease progression in sporadic inclusion body myositis: observations in 78 patients. Neurology. 2000;55(2):296–298. doi: 10.1212/wnl.55.2.296. [DOI] [PubMed] [Google Scholar]
- 21.Alemo Munters L, Alexanderson H, Crofford LJ, Lundberg IE. New insights into the benefits of exercise for muscle health in patients with idiopathic inflammatory myositis. Curr Rheumatol Rep. 2014;16(7):429. doi: 10.1007/s11926-014-0429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnardottir S, Alexanderson H, Lundberg IE, Borg K. Sporadic inclusion body myositis: pilot study on the effects of a home exercise program on muscle function, histopathology and inflammatory reaction. J Rehabil Med. 2003;35(1):31–35. doi: 10.1080/16501970306110. [DOI] [PubMed] [Google Scholar]
- 23.Alexanderson H, Lundberg IE. Exercise as a therapeutic modality in patients with idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2012;24(2):201–207. doi: 10.1097/BOR.0b013e32834f19f5. [DOI] [PubMed] [Google Scholar]
- 24.Askanas V, Engel WK. Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: current concepts of diagnosis and pathogenesis. Curr Opin Rheumatol. 1998;10(6):530–542. doi: 10.1097/00002281-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Sivakumar K, Semino-Mora C, Dalakas MC. An inflammatory, familial, inclusion body myositis with autoimmune features and a phenotype identical to sporadic inclusion body myositis. Studies in three families Brain. 1997;120( Pt 4):653–661. doi: 10.1093/brain/120.4.653. [DOI] [PubMed] [Google Scholar]
- 26.Sivakumar K, Dalakas MC. The spectrum of familial inclusion body myopathies in 13 families and a description of a quadriceps-sparing phenotype in non-Iranian Jews. Neurology. 1996;47(4):977–984. doi: 10.1212/wnl.47.4.977. [DOI] [PubMed] [Google Scholar]
- 27.Simmons Z, Towfighi J. Sporadic inclusion body myositis and hereditary inclusion body myopathy. J Clin Neuromuscul Dis. 2002;3(3):122–132. doi: 10.1097/00131402-200203000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.