Abstract
The maturation of HIV-1 viral particles is essential for viral infectivity. During maturation, many copies of the capsid protein (CA) self-assemble into a capsid shell to enclose the viral RNA. The mechanistic details of the initiation and early stages of capsid assembly remain to be delineated. We present coarse-grained simulations of capsid assembly under various conditions, considering not only capsid lattice self-assembly but also the potential disassembly of capsid upon delivery to the cytoplasm of a target cell. The effects of CA concentration, molecular crowding, and the conformational variability of CA are described, with results indicating that capsid nucleation and growth is a multi-stage process requiring well-defined metastable intermediates. Generation of the mature capsid lattice is sensitive to local conditions, with relatively subtle changes in CA concentration and molecular crowding influencing self-assembly and the ensemble of structural morphologies.
 Significant morphological changes occur during the conversion of the immature HIV virion into a mature infectious form. Here the authors use coarse-grained molecular dynamics simulations to model HIV-1 capsid self-assembly and disassembly events that suggests several metastable capsid intermediates sensitive to local conditions.
Significant morphological changes occur during the conversion of the immature HIV virion into a mature infectious form. Here the authors use coarse-grained molecular dynamics simulations to model HIV-1 capsid self-assembly and disassembly events that suggests several metastable capsid intermediates sensitive to local conditions.
Significant morphological changes convert an ‘immature' virus particle (virion) of HIV-1 into the mature and infectious form1,2,3. During maturation, enzymatic cleavage of Gag polypeptide4 releases capsid protein (CA) to self-assemble into a conical lattice structure enclosing the viral RNA (the capsid, Fig. 1). Failure to generate a suitable capsid precludes infectivity5,6,7,8,9 and so the details of capsid generation are of significant interest as a therapeutic target.
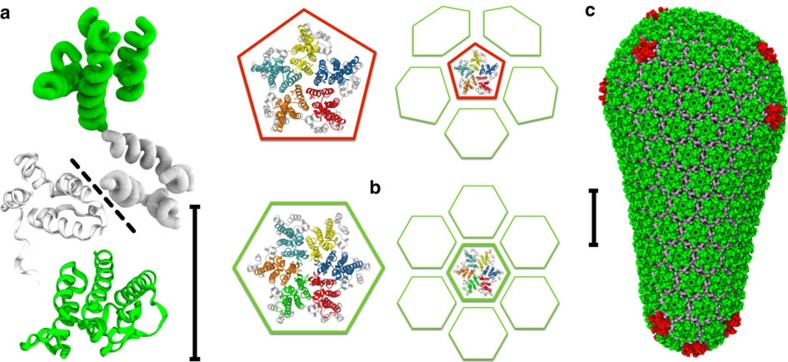
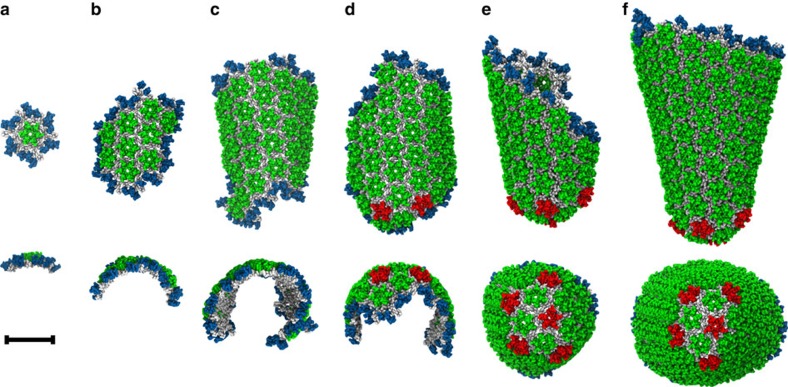
Figure 1. The fullerene cone model of the HIV-1 capsid is assembled from ‘mature-style' capsid lattice with hexameric (green) and pentameric (red) building blocks.
(a) CA dimer structure with both CG (tube) and all-atom (ribbon) monomer representations (NTDs green and CTDs grey). CTD dimer interface marked by dashed line; scale bar, 5 nm. (b) Quasi-equivalent pentamer and hexamer capsid inclusions (NTDs distinguished by colour, all CTDs grey), with schematic of adjacent packing in capsid lattice. (c) Mature capsid structure after Pornillos et al.21. NTDs of hexamer-associated CA shown in green, with NTDs of pentamer-associated CA shown in red (all CTDs grey); scale bar, 20 nm.
HIV-1 capsid protein is mainly dimeric under physiological conditions, with CA monomers connected by a well-characterized interface between C-terminal domains (CTDs)10. Given suitable conditions in vitro, CA can spontaneously self-assemble into a wide variety of structures11,12,13,14,15,16,17,18, but the prototypical mature virion contains a single conical capsid with a complex of viral RNA and nucleocapsid protein (NC) condensed within the broader terminus. In agreement with fullerene cone models14, pentameric and hexameric CA oligomers were identified as the basic components of mature-style CA lattice, with CA N-terminal domains (NTDs) arranged into quasi-equivalent rings15,19,20,21, and with pentamers believed to be located in regions of higher lattice curvature21,22. Although the NTD and CTD structures of CA are well conserved, inter-domain flexibility allows CA in solution to dynamically transition between conformations that are compatible and incompatible with the mature lattice23.
Controlled study of HIV-1 capsid assembly is complicated by the inherent variability of virions: non-trivial differences in average radius (≈630±50 Å) and Gag content (≈2,400±700 molecules) are reported in vivo24,25,26, and these values bound a significant potential range of CA concentrations. The large numbers of Gag, in addition to viral RNA and sundry molecules sequestered from the host cell27,28, ensure the virion interior is a highly crowded environment with internal organic mass densities as high as ≈300 mg ml−1 (ref. 24). Molecular-crowding effects are known to influence protein behaviours29 and yet, remarkably, virion maturation consistently produces similar capsid morphologies. The relatively fast maturation process30,31 makes in vivo study of capsid self-assembly pathways difficult. Nonetheless, Woodward et al.32 show capsid formation proceeding via a hook-shaped precursor to the broad end of the capsid, in contrast with models of capsid assembly that begin with the narrow end of the cone33.
As the average virion contains approximately twice the CA present in a mature capsid, a capsid exists in equilibrium with a relatively high concentration of solution-state CA. Viral infection requires the transfer of virion contents into a target cell, producing a rapid dilution of this CA solution. Rapid dilution destabilizes the CA lattice in vitro under physiological salt concentrations12, but the potential significance of this effect on capsid stability is unclear.
Computer simulations of CA self-assembly offer a valuable complement to other experimental techniques. Atomic-resolution molecular dynamics (MD) simulations of pre-assembled capsids have been reported for HIV-1 (ref. 22), but the long-time stability of such MD models has not been demonstrated and their great computational expense prevents such models being used to examine capsid self-assembly. Coarse-grained (CG) models using simpler molecular representations34,35 provide an appealing alternative; for example, extensive CG simulations of icosahedral capsid self-assembly have been reported36,37,38,39,40, but the small, well-defined end products are in striking contrast to the structural pleomorphism observed with HIV-1 CA. Non-equilibrium CG simulations featuring irreversible subunit binding can generate capsid-relevant morphologies41,42, but may struggle to represent any dynamic reorganization that might occur at the leading edges of lattice growth. The CG self-assembly of CA dimers was explored using Monte Carlo43 and MD simulations34 on two-dimensional or quasi-two-dimensional surfaces, and recent work extended Monte Carlo simulations to three-dimensional (3D) systems44 albeit using rigid models that cannot capture the innate NTD/CTD flexibility of the capsid protein.
In this work, we consider the nucleation and growth of CG HIV-1 capsid protein in 3D systems under various conditions relevant to the viral lifecycle. In particular, we examine the effects of CA concentration and molecular crowding on mature CA lattice generation, the influence of dynamic transitions between populations of CA that are compatible and incompatible with mature lattice formation, and the effects of rapid CA dilution on previously self-assembled CA lattice structures. While comparisons to fully assembled HIV-1 capsid structures from experimental data are difficult (our system sizes are restricted by computational expense), and hence caution is warranted when considering large-scale structural assemblies, the early stages of nucleation and growth for capsid protein lattice are accessible in our simulations. In total, we present the results of 51 separate CG simulations and examine general trends across the data sets.
Results
The effects of CA concentration and molecular crowding
As noted, there is a dynamic population of CA conformations dictated by the flexible linker between the NTD and CTD. For our simulations, the equilibrium NTD-CTD conformation was based on the curved hexagonal lattice within CA tubes (Supplementary Fig. 1). Coarse-grained molecular dynamics (CGMD) simulations were performed (see Methods and Supplementary Fig. 1A–C) using CA concentrations [CA] of 1, 2, 3 and 4 mM (with [CA] in a typical virion estimated as ≈3.8 mM)24. For each [CA] studied, inert CG crowding agent densities ρCR from 0 to 200 mg ml−1 were present with increments of 50 mg ml−1 to study the effects of molecular crowding on CG self-assembly12. A single simulation was performed for each data point for 2 × 108 MD time steps, with results summarized in Table 1 (full data in Supplementary Fig. 2).
Table 1. CG self-assembly for varying [CA] and inert crowding levels.
For [CA]=1 mM, no stable self-assembly of mature-style capsid lattice occurred for any level of crowding studied. Instead, populations of metastable ‘trimer-of-dimers' structures34 rapidly formed (Fig. 2a,b), with average trimer populations increasing with ρCR. A similar trend was observed for [CA]=2 mM until 200 mg ml−1 of crowder was present, at which point the self-assembly behaviour changed dramatically. Rather than generating a steady-state population of trimers, as was apparently the case for ρCR=150 mg ml−1, a new self-assembly regime emerged: the steady production of mature-style CA lattice with quasi-equivalent pentamers and hexamers. Similar effects were observed for [CA] of 3 and 4 mM, with metastable trimers generated below the onset of CA lattice growth at 100<ρCR≤150 mg ml−1 (for [CA]=3 mM) and 50<ρCR≤100 mg ml−1 ([CA]=4 mM). The transition from producing only trimers to the nucleation of mature-style lattice was thus a function of both [CA] and ρCR, with higher [CA] requiring lower ρCR to induce lattice growth. Differences of ≤50 mg ml−1 in ρCR therefore led to markedly different behaviours for [CA]≥2 mM.
Figure 2. Dimers of the CA protein are the units of self-assembly of HIV-1 capsid-like CG structures.
(a) Simulation snapshot of a population of metastable trimer-of-dimers. CA NTDs are blue, with CTDs grey (non-aggregated CA shown transparent for clarity). (b) Detail of a CG trimer-of-dimers, where ‘edges' are CA dimers, with two NTDs per triangle ‘vertex'. NTDs coloured by dimer for clarity, scale bar 10 nm. Final simulation snapshots for ρCR=200 mg ml−1 and [CA]=2 mM (c), 3 mM (d) and 4 mM (e) are presented with NTDs coloured by monomer presence in a trimer (blue), pentamer (red) or hexamer (green). All CTDs grey, scale bar 20 nm. (f) Multiple aggregated capsid structures as revealed by electron cryotomography, with capsid structures highlighted in orange (g). Scale bar, 20 nm; f and g adapted from ref. 45.
Where simulations generated only steady-state trimer populations, the steady state was rapidly attained (<5 × 106 MD time steps). For simulations producing mature-style CA lattice, the appearance of stable pentameric inclusions lags the production of stable hexamers (see Supplementary Fig. 2). In every simulation producing mature-style lattice, nucleation and growth of multiple lattice regions occurred. For example, with [CA]=2 mM and ρCR=200 mg ml−1, two independent lattice regions were generated: an incomplete cone fragment, and a small array of hexamers (Fig. 2c). For [CA]=3 mM and ρCR=200 mg ml−1, several independent lattice regions appeared, resulting in a pill-shaped structure with a closed lattice surface and the fusion of an incomplete pill shape with a cylinder fragment (Fig. 2d). For [CA]=4 mM and ρCR=200 mg ml−1, multiple lattice regions fused into a semi-amorphous assembly (Fig. 2e), reminiscent of multiple capsid structure aggregations observed in electron cryotomograms (Fig. 2f,g)45. The CG model is thus capable of producing a wide variety of curvatures, as required by the continuous curvatures observed in pleomorphic HIV-1 capsids.
Given CA molecular mass≈25 kDa, [CA] of 2, 3, and 4 mM correspond to CA mass densities of ≈50, ≈75 and ≈100 mg ml−1. Onset of mature-style lattice growth therefore occurred for total mass densities of between 200 and 250 mg ml−1 (for [CA]=2 mM), 175 and 225 mg ml−1 ([CA]=3 mM), and 150 and 200 mg ml−1 ([CA]=4 mM). Total non-lipid mass density of HIV-1 virions was estimated as ≈200–300 mg ml−1 (ref. 24), and so the CG model self-assembly initiated at mass density levels comparable to the virion.
These results indicate sensitivity to both [CA] and local molecular crowding for CG self-assembly. Well-regulated nucleation and growth pathways are therefore critical for capsid production, given significant natural variability in HIV-1 virions24,25,26,27,28.
Effects of CA concentration with constant molecular crowding
Inert molecular-crowding effects can significantly alter CG self-assembly. However, CA is also a self-crowding agent as it occupies volume in the simulations. Direct comparison between self-assembly behaviours of different [CA] with the same quantity of inert crowder is therefore non-trivial.
To examine the effects of CA concentration on CG self-assembly under constant molecular crowding, the initial CA content of identical systems (4 mM CA, ρCR=200 mg ml−1) is partitioned into fixed ‘active' ([CA]+) and ‘inactive' ([CA]−) populations. After the initial partitioning, CA dimers remain either ‘active' or ‘inactive' for the duration of the simulation. This partitioning is motivated by the experimental results of Deshmukh et al.23 suggesting a relatively small population of ‘assembly-competent' CA dimers in solution. Inactive CA is identical to active CA in all aspects in our CG model except for the removal of attractive interactions, and thus both types of CA occlude equivalent volume. By fixing [CA]+ between 250 μM and 4 mM (with increments of 250 μM), CG self-assembly is studied as a function of CA concentration alone while maintaining fixed, virion-relevant crowding levels. The formation of trimer-of-dimers, hexamer, and pentamer structures are recorded over 2 × 108 CGMD time steps (one simulation per CA concentration), with selected results summarized in Fig. 3 (full data in Table 2 and Supplementary Fig. 3).
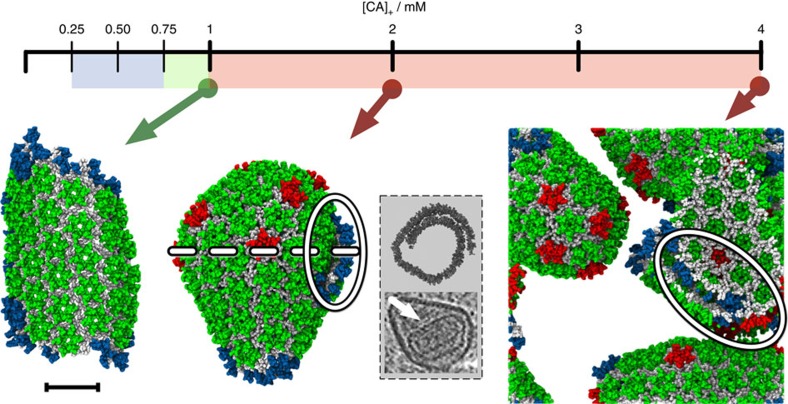
Figure 3. Only a narrow range (indicated in green) of CA concentrations results in the nucleation and growth of a single-lattice region.
CG self-assembly as a function of active CA concentration [CA]+ under fixed crowding conditions. Colour in the concentration bar indicates formation of only trimer-of-dimers structures (blue) and the nucleation and growth of single (green) or multiple (red) lattice regions. Example final structures are shown for [CA]+=1, 2 and 4 mM (arrows). CA colour scheme as in Fig. 2, lamellar regions highlighted by ovals. Final structure for [CA]+=2 mM formed via two lattice regions fusing. Grey panel shows cross-sectional slices (not to scale) to illustrate lamellar lattice in structure for [CA]+=2 mM (top, cross-sectional plane indicated by dashed white line) and an example lamellar CA lattice inside a virion from electron cryotomography (bottom, CA lattice indicated by white arrow). Final structures for [CA]+=4 mM wrap around periodic boundaries. Scale bar, 20 nm.
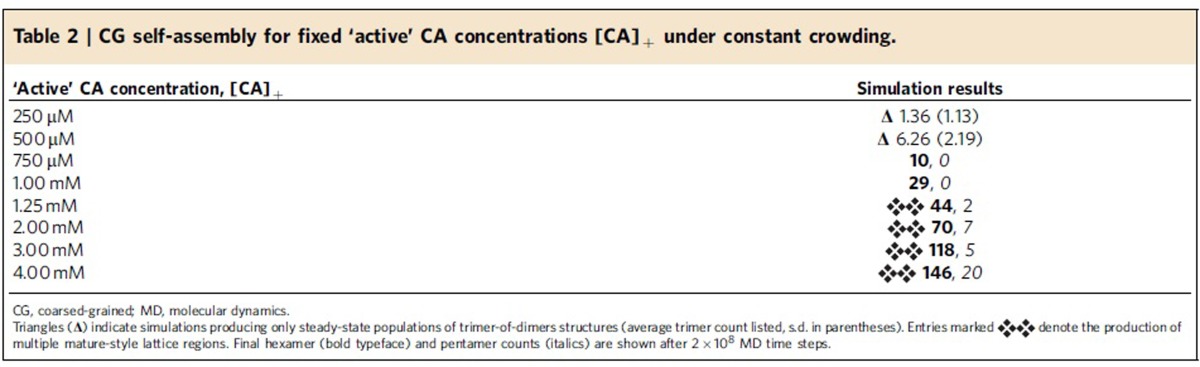
Table 2. CG self-assembly for fixed ‘active' CA concentrations [CA]+ under constant crowding.
For 250≤[CA]+≤500 μM, no generation of mature-style CA lattice occurs, but metastable trimer-of-dimer populations emerge. Elevating [CA]+ to 750 μM produced an apparent initial steady-state trimer population before a single region of mature-style lattice nucleated and grew after ≈4 × 107 MD time steps. With [CA]+=1 mM, the nucleation and growth of a single-lattice region began after ≈1 × 107 MD time steps. For both [CA]+=750 μM and [CA]+=1 mM, the lattice region adopted a cylindrical conformation as growth proceeded; although pentameric inclusions regularly formed at the growing edge of the lattice, the inclusions were transient and thus the lattice was essentially hexameric. For all [CA]+≥1.25 mM nucleation and growth of multiple lattice structures occurred, with regions of high local curvature around the pentamers. Also evident at higher [CA]+ were lamellar regions of CG lattice (Fig. 3) reminiscent of structures observed in electron cryotomograms. Lamellar and off-pathway capsid structures are indicated to be significantly more common in virion systems lacking RNA/NC complex46, as is the case in these CG simulations.
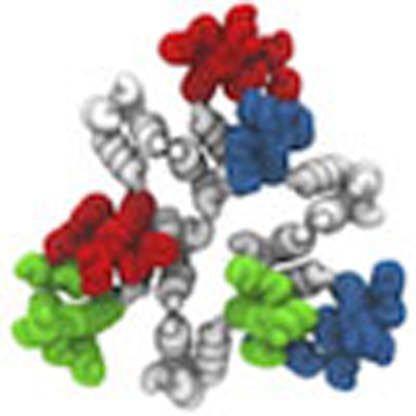
The relatively controlled nucleation and growth for lower [CA]+ reveals specific details of CG lattice nucleation. Visual inspection of simulation trajectories indicates lattice nucleation occurs by the addition of CA dimers onto existing trimer-of-dimers structures, producing trimers with shared edges (Fig. 4) and leading to a central hexamer stabilized by a peripheral trimer ‘skirt' from which lattice growth proceeds. Metastable trimers therefore appear to seed the nucleation and growth of CG lattice34, rather than independent trimers aggregating. Transient structures along this pathway are observed in simulations that do not produce mature lattice, but these intermediates are unstable and dissociate before nucleation of lattice growth.
Figure 4. Putative assembly from 12 CA dimers that nucleates CG capsid lattice growth.
(a) Reversible addition of CA dimers (CA monomers depicted as black triangles, CTD/CTD interface as a dashed line) onto existing aggregates produces trimers with shared edges, eventually generating a central hexamer stabilized by trimer ‘skirt'. (b) Front and side view of example structure from CG simulation, with mild innate curvature visible. Hexamer-associated NTDs are green, NTDs in trimer skirt blue, CTDs grey. Scale bar, 20 nm.
Examination of the solution-state [CA]+ reveals that lattice growth continues below the level of solution-state [CA]+ required for initial nucleation (for example, Fig. 5a), in agreement with standard nucleation and growth theories47. As a region of mature lattice grows, there is also a reduction in the number of separate trimers in solution (for example, Fig. 5b). Progressive reduction of not only the ‘available' capsid protein but also the number of trimers in solution (the potential nucleating seeds) can thus provide an elegant feedback mechanism to deter multiple capsid formation.
Figure 5. Example CG simulation data under fixed molecular crowding.
(a) ‘Available' assembly-competent CA in solution for initial [CA]+=0.5 mM (resulting in no nucleation and growth of mature lattice) and initial [CA]+=1.0 mM (producing nucleation and growth of a single-lattice region, see the main text and Table 2). Lattice growth continues below the level of available [CA]+ required for nucleation on the same timescale. (b) Number of separate lattice regions containing key structural motifs for [CA]+=1 mM. The number of trimers in solution (blue curve) reduces significantly as a single region of lattice grows.
These results indicate a marked sensitivity of CG self-assembly processes to CA concentration under constant molecular crowding. Increases of as little as 250 μM in [CA]+ can drive the system from steady-state populations of metastable trimer structures ([CA]+<750 μM) into the nucleation and growth of single (750 μM≤[CA]+≤1 mM) or multiple lattice regions ([CA]+≥1.25 mM) over the timescales examined. Greater quantities of [CA]+ may encourage pentamer formation and/or stability over the course of the simulations: for [CA]+≤1 mM, no stable pentamers were detected (Table 2).
The effects of NTD/CTD conformational freedom
The NTD and CTD structures of CA are well conserved, but a flexible linker region allows significant conformational freedom in solution. Deshmukh et al.23 estimate that the solution-state CA with NTD/CTD arrangements compatible with mature lattice might be as low as ≈5%. Furthermore, this population is inherently dynamic, with correlation times for inter-domain motions estimated as between 2 and 5 ns (ref. 23). The CGMD simulations with fixed populations of ‘active' ([CA]+) and ‘inactive' ([CA]−) CA effectively describe a limiting case of this behaviour: [CA]+ corresponds to a population of CA whose instantaneous structure is compatible with mature lattice (and hence may directly self-assemble) and the fixed populations of [CA]+ and [CA]− correspond to infinitely long correlation times for inter-domain motions (that is, specific proteins remain in either assembly-competent or -incompetent conformations for the entire simulation).
The effects of interconversion between structural populations are now considered. Rather than initial fixing of [CA]+ and [CA]− populations, all ‘free' CA in solution (that is, CA which is not currently aggregated) is periodically identified and then randomly assigned to [CA]+ or [CA]− populations to maintain a specific target ratio of [CA]+ and [CA]− in solution. The interval between random population assignments is thus a simplified proxy for time correlations in CA structural conformation: once assigned to either the [CA]+ or [CA]− populations, a CA dimer remains ‘assembly-competent' or ‘assembly-incompetent' at least until the next random population assignment. Aggregated CA is not included in this process to avoid perturbation of existing aggregates, and to reflect CA conformational stability in mature CA lattice. Specific CA can disaggregate and, in the absence of re-aggregation, is subject to the next random population switch. This approach allows significant control over the quantity of assembly-competent CA in solution to mimic the coexistence of assembly-competent and -incompetent populations.
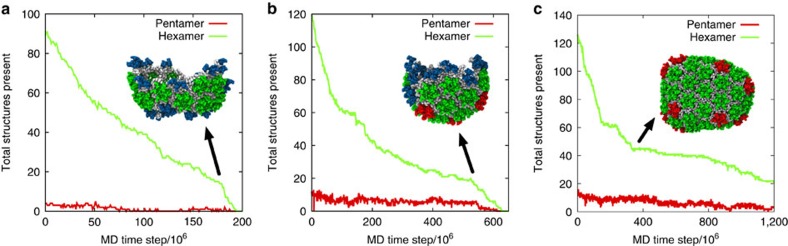
For an identical baseline system (4 mM total CA, 200 mg ml−1 crowder), target ‘assembly-competent' proportions [CA]% of 2.5, 5, 10, 25 and 50% of solution-state CA were studied (one simulation per [CA]% value). Random population switching was performed with intervals of 1 × 104, 1 × 105 and 5 × 105 CGMD time steps, corresponding to 0.1, 1 and 5 ns of CG time respectively, but caution should be exercised in any direct comparison of CG timescales to those suggested by NMR experiments (see note regarding CG timescales in Supplementary Note 4). Simulations were performed for 2 × 108 MD time steps unless explicitly noted, with results summarized in Table 3 and Fig. 6 (full data in Supplementary Fig. 4).
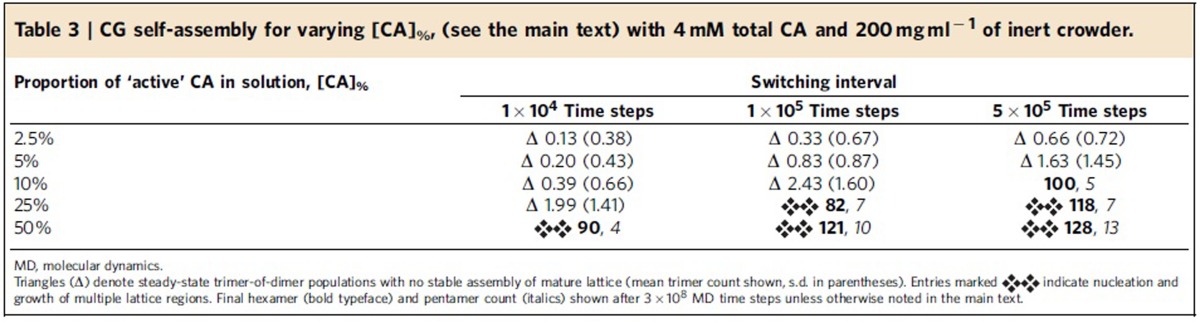
Table 3. CG self-assembly for varying [CA]%, (see the main text) with 4 mM total CA and 200 mg ml−1 of inert crowder.
Figure 6. Steps in the assembly of the HIV-1 capsid by polymerization of CA dimers for [CA]%=10% and conformational switching interval of 5 × 105 time steps (see the main text).
Simulation snapshots at 120 × 106 (a), 240 × 106 (b), 440 × 106 (c), 460 × 106 (d), 600 × 106 (e) and 1,700 × 106 MD time steps (f) are shown with views perpendicular and parallel to the major structural axis. Colour scheme as in Fig. 2. Scale bar, 20 nm.
Apparent from Table 3 is the influence of conformational switching intervals on CG self-assembly. For the most rapid switching rate (interval=1 × 104 time steps), no self-assembly of mature-style CA lattice emerges until some 50% of the total CA in solution is considered to be assembly-competent: below this level, only metastable trimer-of-dimer structures appear. With a slower switching rate (interval=1 × 105 time steps), the transition from steady-state trimers into mature-style lattice occurs for 10%<[CA]%≤25%. For the slowest switching rate studied (5 × 105 time steps), the transition occurs at 5%<[CA]%≤10%. Where simulations produced only metastable trimers, faster switching rates reduced the average trimer population for identical [CA]% (with an additional 1 × 108 MD time steps performed to improve statistical estimates). These results suggest that NTD/CTD correlation times affect CG self-assembly, with faster switching between assembly-competent and -incompetent forms suppressing lattice formation for otherwise identical [CA]%.
When a target active proportion [CA]% of 10% was combined with a switching interval of 5 × 105 time steps, a cone-shaped structure reminiscent of a mature capsid was produced (Fig. 6). A small region of hexameric lattice initially formed, which curled into a semi-cylinder under growth. Stable pentamer incorporation appeared alongside higher local curvatures at one end of the structure, generating the narrow end of a cone. At this point lattice growth essentially stopped because of insufficient assembly-competent CA in solution, as the system contained only 50% of the CA content of a typical virion24,25,26. Although transient pentameric inclusions regularly formed at the exposed lattice edges, all permanent capsomer additions were hexameric after this point. It is interesting to note that pentamers were occasionally embedded slightly behind the growing edge of the lattice, but were replaced by hexamers in a process of local remodelling. The CG self-assembly can thus demonstrate a degree of ‘error correction', provided the growing lattice edge does not advance too far beyond the pentamers. A repeat simulation was performed using a different initial momentum distribution with quite similar results (Supplementary Fig. 5), but it should not be inferred that these specific conditions are unique in producing this outcome.
Stability and uncoating of self-assembled CG structures
Capsid assembly is critical for HIV-1 infectivity, but capsid destruction is also crucial to the viral lifecycle. Successful infection is temporally sensitive to capsid uncoating after cell entry, with both premature and delayed uncoating detrimental to viral replication6,48. Assuming a virion radius ∼630 Å (ref. 24), the transfer of virion contents into a cell of radius≈10 μm (ref. 49) produces rapid dilution of the CA solution surrounding the capsid. To examine the effects of rapid dilution, simulations using previously self-assembled lattice structures were performed with [CA]%=0 to approximate the negligible background CA concentration after transferring virion contents into a cell. Importantly, this approach preserves the level of molecular crowding and conformational switching rate under which the lattice structures originally formed, allowing investigation of rapid dilution on several different structures that are otherwise stable under identical conditions. This avoids the use of a single model structure, whose natural stability may differ subtly under different test conditions even in the absence of rapid dilution. The simulations therefore probe the effects of rapid dilution specifically, representing a cytoplasmic environment that is otherwise identical to the notional virion in which the structures were generated.
Maximally assembled systems (that is, using [CA]%=50%) for the three conformational switching rates described previously were used as starting configurations for rapid dilution, with [CA]% now set to 0% in each case. Figure 7 presents the resultant time series of structural data from one rapid dilution simulation per starting conformation, with CA lattice dissolution evident in all systems. Conformational switching intervals of 1 × 104 time steps (Fig. 7a) and 1 × 105 time steps (Fig. 7b) show a distinct and rapid destruction of any remaining lattice at ≈175 × 106 and ≈550 × 106 CGMD time steps, respectively, indicating lattice dissolution is not a simple function of time (lattice structures at these points shown in Fig. 7, inset). Interestingly, the 5 × 105 time step switching interval showed a long-lived plateau between ≈350 × 106 and ≈800 × 106 MD time steps corresponding to reorganization of the remaining lattice into a pill-like structure with closed surface (Fig. 7c). This enhanced stability is because of the absence of exposed lattice edges: CA in edge-free lattice requires significant reorganization of surrounding lattice components to escape, but can dissolve from exposed edges directly.
Figure 7. Disassembly of self-assembled CG CA lattice under simulated rapid dilution with constant molecular crowding.
Conformational switching intervals of 1 × 104 time steps (a), 1 × 105 time steps (b) and 5 × 105 time steps (c) are shown. Simulation snapshots (inset) correspond to the systems at ≈175 × 106 (a), ≈550 × 106 (b) and ≈350 × 106 (c) CG MD time steps. Colour scheme as in Fig. 2.
These results demonstrate the instability of CG capsid lattice under rapid dilution, even under conditions otherwise identical to those in which the lattice assembled. Furthermore, the results suggest that CA lattice structures with no exposed edges may be significantly more stable upon transfer into a cell.
Discussion
Our CG model is capable of self-assembly into structures that reproduce native protein/protein interfaces in the mature HIV-1 capsid lattice HIV-1 capsid lattice, including subtle effects such as quasi-equivalent NTD packing in pentameric and hexameric rings. While care should be taken in assuming any CG model to be a direct representation of a real, fully atomistically resolved system, the reproduction of specific experimental phenomena suggests that our CG model can capture the early and intermediate stages of nucleation and growth of the capsid lattice. Even so, we note that the computational expense still limits the system sizes considered in this work, preventing the generation of full capsid structures after the depletion of the CA concentration. Our study demonstrates that CG self-assembly is sensitive to CA concentration, molecular crowding, and NTD/CTD conformational correlation times. A single CG CA protein model generates surprisingly varied morphologies, encompassing the wide range of local curvatures required in the viral capsid15. Evident in the results is a reversible multi-stage process triggering mature lattice self-assembly50, where metastable trimer-of-dimers structures rapidly emerge to act as potential seeds for the nucleation of mature lattice growth (Fig. 4). The steady-state population of trimers increases as a function of both concentration and molecular crowding, until suitable conditions produce mature-style capsid lattice over the simulation lengths examined. Lattice growth progressively reduces both the solution-state CA and the population of independent trimers to deter the nucleation of additional lattice regions (for example, Fig. 5). The transition between self-assembly regimes appears to be quite stable, in which relatively small changes in the molecular environment are capable of producing different outcomes, in agreement with, for example, previous studies of self-assembly for icosahedral capsid models37,38,39,40,51,52.
The general effects of molecular crowding in our simulations are in agreement with those observed using other computational approaches for self-assembly. For example, high levels of crowding can reduce nucleation lag times to effectively zero (see, for example, Supplementary Figs 2–4), changing the self-assembly process from nucleation-limited to non-nucleation-limited. Smith et al.39 also noted such effects in discrete event simulations of icosahedral capsid self-assembly: multiple parallel nucleation and growth events depleted the available capsid components, arresting complete capsid assembly and producing off-pathway growth. Similar kinetic traps and multiple nucleation were also observed by, for example, Johnston and co-workers in Monte Carlo investigations of icosahedral capsid assembly37,38,39,40,51,52.
In the absence of RNA, CA is typically observed to assemble into tubes of variable diameter in vitro10,12,13,15,16,17,18, albeit with occasional cone-shaped structures12 and other non-tubular morphologies13,17. These experiments typically feature CA concentrations significantly below that expected in a virion, which likely assists in producing regular, purely hexameric cylinders via orderly nucleation and growth.
Sigmoidal ‘assembly curve' characteristics have been observed in experimentally for capsid assembly in, for example, HIV-1 (ref. 12) and HPV-11 (ref. 53). Our results follow this general prediction, with assembly lag periods that are very short or effectively zero with suitable CA concentration and crowding (see, for example, Supplementary Note 2). We note that simulations lacking mature-style lattice production do not necessarily indicate that CG lattice growth cannot occur under those conditions, but rather that nucleation and growth were not detected over the simulation timescales examined. As the main focus of this study is the nucleation and growth of capsid lattice, converged simulations (that is, those attaining some final equilibrium) do not offer significantly more information regarding these aspects. Nonetheless, examination of structural data as a function of time (for example, Supplementary Figs 2–4) indicates that many simulations are approaching the plateau of a sigmoidal growth curve, with significant additional lattice growth unlikely.
Trimer-of-dimers structures have been reported previously in computer simulations34,43, and have also been assumed as the fundamental building block in mathematical models of capsid cylinder growth54. Our results suggest that trimer structures are metastable in isolation, but addition of CA dimers onto these templates (Fig. 4) provides the basic nucleation pathway for capsid assembly rather than the aggregation of independent trimer-of-dimer structures.
The regular appearance of transient pentamers at the growing edge of CG lattice was observed, suggesting a natural aspect of lattice growth. If growth is slow, local remodelling can remove the pentamers provided the expanding lattice edge does not advance too far. For more rapid assembly (for example, higher crowding or CA concentration) the lattice advances too quickly for reliable remodelling, producing high local curvature via stably embedded pentamers. This process can explain why relatively low concentrations of CA typically produce cylindrical structures in vitro10,12,15,16,17,18,19,20, and why increased CA concentration reduces cylinder length16: early pentamer incorporation redirects lattice growth, suppressing the formation of longer cylinders. Inherent instability in lattice edges suggest that CG models allowing dissociation more accurately reflect capsid assembly41,42,50.
Switching between active and inactive CA populations in solution, akin to the conformational dynamism recorded in vitro23, has pronounced effects on CG lattice self-assembly. Faster random mixing of the populations suppresses CG lattice production (Table 3), with a low instantaneous proportion of assembly-competent CA limiting uncontrolled nucleation and growth. Longer intervals between population switching might allow CA that dissociates from a lattice edge to remain assembly-competent long enough to reassociate directly, and thus maintain the overall lattice structure, but more investigation is required to elucidate this mechanism. In any case, dynamic conformational switching can ensure that growth is not prevented by the exhaustion of an otherwise limited quantity of assembly-competent CA. This suggests that CG models incorporating this phenomenon may better represent a key aspect of HIV-1 capsid assembly.
It is interesting to consider HIV-1 virion maturation in light of these results. Although the prototypical mature virion contains a single capsid, multiple capsid structures are sometimes observed9,31,32,33,55, both with and without RNA/NC encapsulation32, suggesting a delicate balance in the virion. Multiple capsids appear correlated with larger virions33,55, consistent with sensitivity of CG assembly to local conditions: lattice growth reduces CA in solution, so larger virions (with larger numbers CA for the same overall CA concentration) could maintain CA levels above the critical nucleating value after an initial nucleation, increasing the likelihood of subsequent nucleation events. This is compatible with observations that multiple cores are often comparable in size55, suggestive of separate nucleation events in quick succession. The sensitivity of CG self-assembly to CA concentration and local molecular crowding, in combination with natural virion variability, may complicate analyses that assume multiple cores are unrelated to CA concentration55.
Our CG simulations produced virion-relevant morphologies such as the extended cone-shaped structure shown in Fig. 6. While the narrow end of this cone formed first, electron cryotomography indicates capsid growth typically proceeds from a small region of CA lattice adsorbed to the RNA/NC complex, generating the broad end of the capsid first (although capsid growth can also occur without RNA/NC)32. The presence of a RNA/NC complex would obstruct high local curvature (such as in the narrow end of a cone), selecting against the initial formation of a narrow end. Our simulations therefore do not fully recapitulate the virion environment, but instead explore principles relevant to capsid self-assembly. Nonetheless, the results are in general agreement with models of capsid generation where a small sheet of CA lattice curls under growth, producing a cup shaped structure from which assembly proceeds32.
Our results suggest the importance of relatively slow, controlled growth of the HIV-1 capsid, allowing significant relaxation of both the local and global structure to produce a metastable capsid despite weak interactions between CA dimers11,12. This phenomena suggests caution is appropriate when fitting atomic-resolution models into relatively low-resolution capsid data: for example, CTD/CTD dimer interfaces present in the atomistic MD simulations of HIV-1 viral capsids by Zhao et al.49 can display significant shearing and distortions from the expected structures10,23 (Supplementary Fig. 6). It is possible that such deformations occur naturally in a viral capsid, but further experimental support is needed to clarify this situation.
The stability of CG lattice structures was sensitive to CA concentration in the supporting solution. Rapid dilution under otherwise identical conditions destabilized the capsid lattice, indicating the metastability of CA structures in agreement with experimental observations12. Similar effects have also been observed in simulations of icosahedral capsids56, albeit these simulations did not examine this effect under constant crowding conditions. Sealed, edge-free CA lattices appear more resistant to this process (Fig. 7c).
Cellular responses to HIV-1 include the weak binding of TRIM5α protein to capsids, forming a hexameric super-lattice on the capsid exterior57. TRIM5α binding promotes rapid capsid uncoating48,58,59,60,61,62,63, and while TRIM5α is digested by the proteasome64, capsid protein does not appear to be destroyed in this manner58,59,63. TRIM5α restriction is less efficient under proteasomal inhibition65,66,67, and we speculate this somewhat counterintuitive data can be explained by TRIM5α inducing local faults in the capsid lattice: slight mismatches in CA and TRIM5α lattice spacing induces additional stress in the metastable CA lattice68, with local tears providing an exposed edge from which CA can dissolve. Proteasomal degradation then ensures that a TRIM5α ‘scaffold' cannot exert any stabilizing influence on the overall capsid superstructure, enabling fragmentation of the capsid to further accelerate uncoating. Partial support for this hypothesis is offered by TRIM5α inducing only mild disruption of CA cylinders in vitro61, but as these studies used above-physiological salt concentrations with no rapid dilution (conditions in which the CA lattice may be over-stable)12 complete breakdown of CA lattice was not observed. Experimental data suggests HIV-1 capsids often feature exposed lattice edges, with an estimated 25% of cytoplasmic capsids featuring seams or holes large enough to allow the escape of green fluorescent protein (GFP)42, and so TRIM5α could help to accelerate natural lattice dissipation under rapid dilution. For capsid structures lacking exposed lattice edges, however, TRIM5α would provide a powerful accelerator of uncoating. This process would offer a relatively simple description of the general effects of TRIM restriction on HIV-a capsids, but we note that the natural TRIM-restriction processes could be significantly more complicated.
Methods
Model details
Full-model details provided in Supplementary Note 1. Briefly, CG CA is modelled directly from experimental hexamer (PDB 3H4E) and pentamer (3P0A) data20,21, and also heavily influenced by work from the same authors (B.K.G-P. and M.Y.) with cylindrical CA assemblies in vitro14,15,16,19,20. CA is represented as a dimer, the prevalent species in solution10,23, which is required for CA lattice assembly11. The NTD and CTD of a CA monomer are represented as independent stiff elastic network models (ENMs) comprising the Cα atoms from CA-α helices, which are well conserved across experimental structures34. An additional weaker ENM connects the NTD and CTD in each monomer to provide limited inter-domain structural flexibility. The CTD/CTD dimer interface is represented by a stiff ENM based on PDB structure 2KOD (ref. 10) relevant for the mature lattice23. CG beads have excluded volume radii to deter unphysical overlaps as indicated by experimental structural data. Important CA protein/protein interfaces required for self-assembly9,10 are represented by specific attractive interactions, with the locations of energy minima parameterized using experimental structures20,21 and attractive strengths chosen to allow CA self-assembly under the experimentally relevant mass densities and conditions we study here (Supplementary Note 1). These attractive interactions can be dynamically enabled and disabled, allowing the generation of a fixed solution-state ratio of assembly-competent and assembly-incompetent populations as indicated by NMR data23. Inert crowding agent is based on the excluded volume and relative mass of Ficoll 70 (ref. 12). The combination of internal NTD/CTD flexibility in CA monomers, ‘switching' capability, and freedom of motion in a 3D simulation domain with explicit crowding molecules provides an advanced and relatively detailed molecular model with which to study CA self-assembly.
Simulation details
All simulations were performed with our UCG-MD code69. The simulation domain was an 800 Å cube (≈50% of typical virion volume due to computational expense, with max. 616 CA dimers present at [CA]=4 mM) with periodic boundaries. Temperature of 300 K was maintained by Langevin dynamics with relaxation period 100 ps−1 to minimize influence of thermostat, integration time step 10 fs. Visualizations of simulation data were created using VMD70, with CG CA depicted as tubes connecting CG particles in each notional α helix to emphasize the local secondary structure of the CG model.
Data availability
All relevant data are available from the authors on request.
Additional information
How to cite this article: Grime, J. M. A. et al. Coarse-grained simulation reveals key features of HIV-1 capsid self-assembly. Nat. Commun. 7:11568 doi: 10.1038/ncomms11568 (2016).
Supplementary Material
Supplementary Figures 1-6, Supplementary Tables 1-4, Supplementary Notes 1-6 and Supplementary References
Acknowledgments
This research was supported by National Institutes of Health grants P50 GM082545 (G.A.V. and M.Y.) and R01 GM066087 (M.Y.). The computations in this work are part of the Blue Waters sustained-petascale computing project, which is supported by the National Science Foundation (awards OCI-0725070 and ACI-1238993) and the state of Illinois. Blue Waters is a joint effort of the University of Illinois at Urbana-Champaign and its National Center for Supercomputing Applications. This work is also part of the ‘Ultra-Coarse-Grained Simulations of Biomolecular Processes at the Petascale' Petascale Computing Resource Allocation (PRAC) support by the National Science Foundation (award number OCI-1440027).
Footnotes
Author contributions J.M.A.G., J.F.D. and G.A.V. conceived the study, designed the simulations and wrote the manuscript. J.M.A.G. performed the simulations. Additional experimental data were provided by C.L.W. Data analysis was performed by J.M.A.G., J.F.D., B.K.G.-P., G.J.J., M.J.Y. and G.A.V.
References
- Briggs J. A. & Krausslich H. G. The molecular architecture of HIV. J. Mol. Biol. 410, 491–500 (2011). [DOI] [PubMed] [Google Scholar]
- Ganser-Pornillos B. K., Yeager M. & Sundquist W. I. The structural biology of HIV assembly. Curr. Opin. Struct. Biol. 18, 203–217 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist W. I. & Krausslich H. G. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2, a006924 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit S. C. et al. The p2 domain of human immunodeficiency virus type 1 Gag regulates sequential proteolytic processing and is required to produce fully infectious virions. J. Virol. 68, 8017–8027 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman T., Bukovsky A., Ohagen A., Hoglund S. & Gottlinger H. G. Functional domains of the capsid protein of human immunodeficiency virus type 1. J. Virol. 68, 8180–8187 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshey B. M., von Schwedler U., Sundquist W. I. & Aiken C. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J. Virol. 76, 5667–5677 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammano F., Ohagen A., Hoglund S. & Gottlinger H. G. Role of the major homology region of human immunodeficiency virus type 1 in virion morphogenesis. J. Virol. 68, 4927–4936 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S. et al. Human immunodeficiency virus type 1N-terminal capsid mutants that exhibit aberrant core morphology and are blocked in initiation of reverse transcription in infected cells. J. Virol. 75, 9357–9366 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwedler U. K., Stray K. M., Garrus J. E. & Sundquist W. I. Functional surfaces of the human immunodeficiency virus type 1 capsid protein. J. Virol. 77, 5439–5450 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byeon I. J. et al. Structural convergence between Cryo-EM and NMR reveals intersubunit interactions critical for HIV-1 capsid function. Cell 139, 780–790 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra R. et al. Association equilibrium of the HIV-1 capsid protein in a crowded medium reveals that hexamerization during capsid assembly requires a functional C-domain dimerization interface. Biophys. J. 104, 884–893 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Alamo M., Rivas G. & Mateu M. G. Effect of macromolecular crowding agents on human immunodeficiency virus type 1 capsid protein assembly in vitro. J. Virol. 79, 14271–14281 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich L. S., Agresta B. E. & Carter C. A. Assembly of recombinant human immunodeficiency virus type 1 capsid protein in vitro. J. Virol. 66, 4874–4883 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganser B. K., Li S., Klishko V. Y., Finch J. T. & Sundquist W. I. Assembly and analysis of conical models for the HIV-1 core. Science 283, 80–83 (1999). [DOI] [PubMed] [Google Scholar]
- Ganser-Pornillos B. K., Cheng A. & Yeager M. Structure of full-length HIV-1 CA: a model for the mature capsid lattice. Cell 131, 70–79 (2007). [DOI] [PubMed] [Google Scholar]
- Ganser-Pornillos B. K., von Schwedler U. K., Stray K. M., Aiken C. & Sundquist W. I. Assembly properties of the human immunodeficiency virus type 1 CA protein. J. Virol. 78, 2545–2552 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattinger M. et al. In vitro assembly properties of wild-type and cyclophilin-binding defective human immunodeficiency virus capsid proteins in the presence and absence of cyclophilin A. Virology 257, 247–260 (1999). [DOI] [PubMed] [Google Scholar]
- Li S., Hill C. P., Sundquist W. I. & Finch J. T. Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature 407, 409–413 (2000). [DOI] [PubMed] [Google Scholar]
- Pornillos O., Ganser-Pornillos B. K., Banumathi S., Hua Y. & Yeager M. Disulfide bond stabilization of the hexameric capsomer of human immunodeficiency virus. J. Mol. Biol. 401, 985–995 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornillos O. et al. X-ray structures of the hexameric building block of the HIV capsid. Cell 137, 1282–1292 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornillos O., Ganser-Pornillos B. K. & Yeager M. Atomic-level modelling of the HIV capsid. Nature 469, 424–427 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G. et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 497, 643–646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh L. et al. Structure and dynamics of full-length HIV-1 capsid protein in solution. J. Am. Chem. Soc. 135, 16133–16147 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson L. A. et al. Three-dimensional analysis of budding sites and released virus suggests a revised model for HIV-1 morphogenesis. Cell Host Microbe 4, 592–599 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson L. A. et al. Cryo electron tomography of native HIV-1 budding sites. PLoS Pathog. 6, e1001173 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. R. et al. Electron cryotomography of immature HIV-1 virions reveals the structure of the CA and SP1 Gag shells. EMBO J. 26, 2218–2226 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott D. E. Cellular proteins detected in HIV-1. Rev. Med. Virol. 18, 159–175 (2008). [DOI] [PubMed] [Google Scholar]
- Ott D. E. et al. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J. Virol. 70, 7734–7743 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A. P. Influence of macromolecular crowding upon the stability and state of association of proteins: predictions and observations. J. Pharm. Sci. 94, 1668–1675 (2005). [DOI] [PubMed] [Google Scholar]
- Konnyu B. et al. Gag-Pol processing during HIV-1 virion maturation: a systems biology approach. PLoS Comput. Biol. 9, e1003103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei S. et al. Induced maturation of human immunodeficiency virus. J. Virol. 88, 13722–13731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward C. L., Cheng S. N. & Jensen G. J. Electron cryotomography studies of maturing HIV-1 particles reveal the assembly pathway of the viral core. J. Virol. 89, 1267–1277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs J. A. et al. The mechanism of HIV-1 core assembly: insights from three-dimensional reconstructions of authentic virions. Structure 14, 15–20 (2006). [DOI] [PubMed] [Google Scholar]
- Grime J. M. & Voth G. A. Early stages of the HIV-1 capsid protein lattice formation. Biophys. J. 103, 1774–1783 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders M. G. & Voth G. A. Coarse-graining methods for computational biology. Annu. Rev. Biophys 42, 73–93 (2013). [DOI] [PubMed] [Google Scholar]
- Hagan M. F. Modeling viral capsid assembly. Adv. Chem. Phys. 155, 1–68 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I. G., Louis A. A. & Doye J. P. K. Modelling the self-assembly of virus capsids. J. Phys. Cond. Matter 22, 104101 (2010). [DOI] [PubMed] [Google Scholar]
- Nguyen H. D., Reddy V. S. & Brooks C. L. Invariant polymorphism in virus capsid assembly. J. Am. Chem. Soc. 131, 2606–2614 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Xie L., Lee B. & Schwartz R. Applying molecular crowding models to simulations of virus capsid assembly in vitro. Biophys. J. 106, 310–320 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber A. W., Doye J. P. K., Louis A. A. & Lewis A. C. F. Monodisperse self-assembly in a model with protein-like interactions. J. Chem. Phys. 131, 175102 (2009). [DOI] [PubMed] [Google Scholar]
- Levandovsky A. & Zandi R. Nonequilibirum assembly, retroviruses, and conical structures. Phys. Rev. Lett. 102, 198102 (2009). [DOI] [PubMed] [Google Scholar]
- Yu Z. et al. Unclosed HIV-1 capsids suggest a curled sheet model of assembly. J. Mol. Biol. 425, 112–123 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. & Tycko R. Simulated self-assembly of the HIV-1 capsid: protein shape and native contacts are sufficient for two-dimensional lattice formation. Biophys. J. 100, 3035–3044 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X., Jeon J., Weber J., Zhu F. & Chen B. Mechanism of polymorphism and curvature of HIV capsid assemblies probed by 3D simulations with a novel coarse grain model. Biochim. Biophys. Acta 1850, 2353–2367 (2015). [DOI] [PubMed] [Google Scholar]
- Benjamin J., Ganser-Pornillos B. K., Tivol W. F., Sundquist W. I. & Jensen G. J. Three-dimensional structure of HIV-1 virus-like particles by electron cryotomography. J. Mol. Biol. 346, 577–588 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei S. et al. RNA and nucleocapsid are dispensable for mature HIV-1 capsid assembly. J. Virol. 89, 9739–9747 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes R. J. & Evans D. F. Fundamentals of Interfacial Engineering Wiley-VCH (1997). [Google Scholar]
- Yufenyuy E. L. & Aiken C. The NTD-CTD intersubunit interface plays a critical role in assembly and stabilization of the HIV-1 capsid. Retrovirology 10, 29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. et al. Intracellular water specific MR of microbead-adherent cells: the HeLa cell intracellular water exchange lifetime. NMR Biomed. 21, 159–164 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D. C. Role of reversibility in viral capsid growth: a paradigm for self-assembly. Phys. Rev. Lett. 101, 186101 (2008). [DOI] [PubMed] [Google Scholar]
- Nguyen H. D., Reddy V. S. & Brooks C. L. Deciphering the kinetic mechanism of spontaneous self-assembly of icosahedral capsids. Nano Lett. 7, 338–344 (2007). [DOI] [PubMed] [Google Scholar]
- Wilber A. W. et al. Reversible self-assembly of patchy particles into monodisperse icosahedral clusters. J. Chem. Phys. 127, 085106 (2007). [DOI] [PubMed] [Google Scholar]
- Casini G. L., Graham D., Heine D., Garcea R. L. & Wu D. T. In vitro papillomavirus capsid assembly analyzed by light scattering. Virology 325, 320–327 (2004). [DOI] [PubMed] [Google Scholar]
- Tsiang M. et al. A trimer of dimers is the basic building block for human immunodeficiency virus-1 capsid assembly. Biochemistry 51, 4416–4428 (2012). [DOI] [PubMed] [Google Scholar]
- Briggs J. A., Wilk T., Welker R., Krausslich H. G. & Fuller S. D. Structural organization of authentic, mature HIV-1 virions and cores. EMBO J. 22, 1707–1715 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan M. F. & Chandler D. Dynamic pathways for viral capsid assembly. Biophys. J. 91, 42–54 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganser-Pornillos B. K. et al. Hexagonal assembly of a restricting TRIM5alpha protein. Proc. Natl Acad. Sci. USA 108, 534–539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F. et al. Comparative requirements for the restriction of retrovirus infection by TRIM5alpha and TRIMCyp. Virology 369, 400–410 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F. et al. Modulation of retroviral restriction and proteasome inhibitor-resistant turnover by changes in the TRIM5alpha B-box 2 domain. J. Virol. 81, 10362–10378 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutluay S. B., Perez-Caballero D. & Bieniasz P. D. Fates of retroviral core components during unrestricted and TRIM5-restricted infection. PLoS Pathog. 9, e1003214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier C. R. et al. Biochemical characterization of a recombinant TRIM5alpha protein that restricts human immunodeficiency virus type 1 replication. J. Virol. 82, 11682–11694 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama E. E. & Shioda T. Anti-retroviral activity of TRIM5 alpha. Rev. Med. Virol. 20, 77–92 (2010). [DOI] [PubMed] [Google Scholar]
- Stremlau M. et al. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl Acad. Sci. USA 103, 5514–5519 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F. et al. Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology 349, 300–315 (2006). [DOI] [PubMed] [Google Scholar]
- Javanbakht H., Diaz-Griffero F., Stremlau M., Si Z. & Sodroski J. The contribution of RING and B-box 2 domains to retroviral restriction mediated by monkey TRIM5alpha. J. Biol. Chem. 280, 26933–26940 (2005). [DOI] [PubMed] [Google Scholar]
- Lienlaf M. et al. Contribution of E3-ubiquitin ligase activity to HIV-1 restriction by TRIM5alpha(rh): structure of the RING domain of TRIM5alpha. J. Virol. 85, 8725–8737 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M. et al. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427, 848–853 (2004). [DOI] [PubMed] [Google Scholar]
- Zhao G. et al. Rhesus TRIM5alpha disrupts the HIV-1 capsid at the inter-hexamer interfaces. PLoS Pathog. 7, e1002009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime J. M. A. & Voth G. A. Highly scalable and memory efficient ultra-coarse-grained molecular dynamics simulations. J. Chem. Theory Comput. 10, 423–431 (2014). [DOI] [PubMed] [Google Scholar]
- Humphrey W., Dalke A. & Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 27-38 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-6, Supplementary Tables 1-4, Supplementary Notes 1-6 and Supplementary References
Data Availability Statement
All relevant data are available from the authors on request.