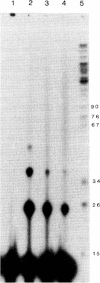
Abstract
Experimentally determined changes in free energy (delta G(o)) for thymine-thymine interactions occurring in oligo(dT).poly(dA) are dependent on the method used for preparation of the double-stranded template. A rapid laser cross-linking technique was used to examine the equilibrium between oligomers of (dT) bound to either poly(dA) or poly(rA). The single-pulse (4-6 nsec) ultraviolet laser excitation of these polynucleotides causes pyrimidine dimer formation between contiguous oligo(dT) molecules, resulting in a "ligation" of the oligomers. Analysis of the resulting data using standard binding isotherms allowed determination of the degree of cooperativity existing between oligomers. Using the cooperativity, delta G(o), delta H(o), and delta S(o) are calculated, thereby providing thermodynamic parameters for this interaction. The measured cooperativity of oligo(dT) molecule interactions allows direct calculation of the number of 3' ends available as nicked structures or the number of 3' ends associated with gaps for oligo(dT).poly(dA) when used as a substrate for DNA synthesis.
Full text
PDF
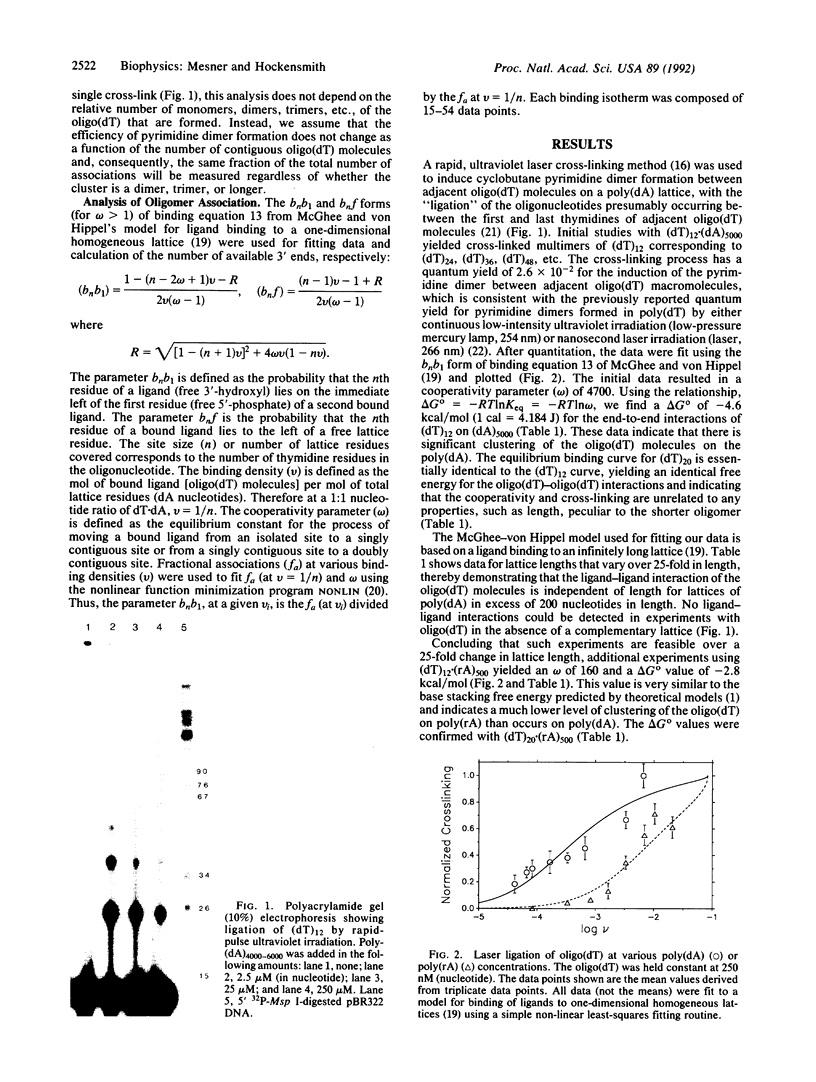

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breslauer K. J., Frank R., Blöcker H., Marky L. A. Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3746–3750. doi: 10.1073/pnas.83.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani G. R., Bollum F. J. Oligodeoxythymidylate: polydeoxyadenylate and oligodeoxyadenylate: polydeoxythymidylate interactions. Biochemistry. 1969 Oct;8(10):3928–3936. doi: 10.1021/bi00838a008. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Bohn E. W., Planck S. R., Wilson S. H. Mouse DNA polymerase alpha. Subunit structure and identification of a species with associated exonuclease. J Biol Chem. 1979 Nov 25;254(22):11678–11687. [PubMed] [Google Scholar]
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcourt S. G., Blake R. D. Stacking energies in DNA. J Biol Chem. 1991 Aug 15;266(23):15160–15169. [PubMed] [Google Scholar]
- Detera S. D., Wilson S. H. Studies on the mechanism of Escherichia coli DNA polymerase I large fragment. Chain termination and modulation by polynucleotides. J Biol Chem. 1982 Aug 25;257(16):9770–9780. [PubMed] [Google Scholar]
- Edmondson S. P. Polynucleotide base-pair orientation in solution: linear dichroism and molecular mechanical studies of poly[d(A)]-poly[d(T)]. Biopolymers. 1987 Nov;26(11):1941–1956. doi: 10.1002/bip.360261108. [DOI] [PubMed] [Google Scholar]
- Fay P. J., Johanson K. O., McHenry C. S., Bambara R. A. Size classes of products synthesized processively by DNA polymerase III and DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1981 Jan 25;256(2):976–983. [PubMed] [Google Scholar]
- Griffith J., Huberman J. A., Kornberg A. Electron microscopy of DNA polymerase bound to DNA. J Mol Biol. 1971 Jan 28;55(2):209–214. doi: 10.1016/0022-2836(71)90192-6. [DOI] [PubMed] [Google Scholar]
- Hockensmith J. W., Kubasek W. L., Vorachek W. R., Evertsz E. M., von Hippel P. H. Laser cross-linking of protein-nucleic acid complexes. Methods Enzymol. 1991;208:211–236. doi: 10.1016/0076-6879(91)08015-a. [DOI] [PubMed] [Google Scholar]
- Hockensmith J. W., Kubasek W. L., Vorachek W. R., von Hippel P. H. Laser cross-linking of nucleic acids to proteins. Methodology and first applications to the phage T4 DNA replication system. J Biol Chem. 1986 Mar 15;261(8):3512–3518. [PubMed] [Google Scholar]
- Kawasaki K., Enomoto T., Suzuki M., Seki M., Hanaoka F., Yamada M. Detection and characterization of a novel factor that stimulates DNA polymerase alpha. Biochemistry. 1986 May 20;25(10):3044–3050. doi: 10.1021/bi00358a046. [DOI] [PubMed] [Google Scholar]
- Leng M., Felsenfeld G. A study of polyadenylic acid at neutral pH. J Mol Biol. 1966 Feb;15(2):455–466. doi: 10.1016/s0022-2836(66)80121-3. [DOI] [PubMed] [Google Scholar]
- Leroy J. L., Charretier E., Kochoyan M., Guéron M. Evidence from base-pair kinetics for two types of adenine tract structures in solution: their relation to DNA curvature. Biochemistry. 1988 Dec 13;27(25):8894–8898. doi: 10.1021/bi00425a004. [DOI] [PubMed] [Google Scholar]
- Lewis R. J., Hanawalt P. C. Ligation of oligonucleotides by pyrimidine dimers--a missing 'link' in the origin of life? Nature. 1982 Jul 22;298(5872):393–396. doi: 10.1038/298393a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Enzymic joining of polynucleotides. 3. The polydeoxyadenylate-polydeoxythymidylate homopolymer pair. J Mol Biol. 1968 Sep 14;36(2):261–274. doi: 10.1016/0022-2836(68)90380-x. [DOI] [PubMed] [Google Scholar]
- Patapoff T. W., Thomas G. A., Wang Y., Peticolas W. L. Polarized Raman scattering from oriented single microcrystals of d(A5T5)2 and d(pTpT). Biopolymers. 1988 Mar;27(3):493–507. doi: 10.1002/bip.360270310. [DOI] [PubMed] [Google Scholar]
- Peticolas W. L., Wang Y., Thomas G. A. Some rules for predicting the base-sequence dependence of DNA conformation. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2579–2583. doi: 10.1073/pnas.85.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A. A HYBRID HELIX CONTAINING BOTH DEOXYRIBOSE AND RIBOSE POLYNUCLEOTIDES AND ITS RELATION TO THE TRANSFER OF INFORMATION BETWEEN THE NUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1044–1053. doi: 10.1073/pnas.46.8.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- Swillens S., Cochaux P., Lecocq R. A pitfall in the computer-aided quantitation of autoradiograms. Trends Biochem Sci. 1989 Nov;14(11):440–441. doi: 10.1016/0968-0004(89)90097-2. [DOI] [PubMed] [Google Scholar]
- Tan C. K., Castillo C., So A. G., Downey K. M. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. J Biol Chem. 1986 Sep 15;261(26):12310–12316. [PubMed] [Google Scholar]
- Vesnaver G., Breslauer K. J. The contribution of DNA single-stranded order to the thermodynamics of duplex formation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3569–3573. doi: 10.1073/pnas.88.9.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]