SUMMARY
BACKGROUND
The Xpert® MTB/RIF assay can diagnose tuberculosis (TB) rapidly and with great accuracy. The effect of Xpert placement at point of care (POC) vs. at an off-site laboratory on patient management remains unknown.
DESIGN
At a primary care clinic in Johannesburg, South Africa, we compared TB diagnosis and treatment initiation among 1861 individuals evaluated for pulmonary TB using Xpert performed either at POC or offsite.
RESULTS
When Xpert was performed at POC, a higher proportion of Xpert-positive individuals started treatment (95% vs. 87%, P = 0.047) and time to treatment initiation was shorter (median 0 vs. 5 days, P < 0.001). In contrast, among Xpert-negative TB cases, a higher proportion (87% vs. 72%, P =0.001) started treatment when the sample was sent to the laboratory, with a shorter time to treatment (median 9 vs. 13 days, P = 0.056). While the overall proportion of presumed TB patients starting treatment was independent of Xpert placement, the proportion started based on a bacteriologically confirmed diagnosis was higher when Xpert was performed at POC (73% vs. 58%, P = 0.006).
CONCLUSIONS
Placement of Xpert at POC resulted in more Xpert-positive patients receiving treatment, but did not increase the total number of presumed TB patients starting treatment. When samples were sent to a laboratory for Xpert testing, empiric decision making increased.
Keywords: tuberculosis, diagnostics, South Africa
IMPROVING THE AVAILABILITY, accuracy and speed of tuberculosis (TB) diagnosis has the potential to reduce transmission and hasten the decline of incidence rates, particularly in high-burden settings.1,2 The Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) assay has improved diagnostic accuracy compared to smear microscopy,3 and can deliver results in 2 h at point of care (POC).4 POC placement of Xpert has been demonstrated to be feasible, facilitating same-day initiation of anti-tuberculosis treatment.5–7
The South African Department of Health (DOH), together with the National Health Laboratory Service (NHLS), has been a global leader in rolling out the Xpert assay and replacing smear microscopy with Xpert as the initial diagnostic for all individuals with presumptive TB.8,9 Based on logistical and financial considerations, Xpert instruments were placed in NHLS laboratories performing smear microscopy rather than at POC in clinics. The transportation of samples to laboratories creates a diagnosis–treatment gap due to the delay in the availability of results, which increases the risk of loss to follow-up during the diagnostic process. This may undermine the huge potential of this new technology to improve patient outcomes.10
Two randomised controlled trials have compared patient outcomes when using Xpert vs. smear microscopy, either sending all samples to a laboratory (XPert for TB: Evaluating a New Diagnostic Trial, or EXTEND TB) or performing both Xpert and smear microscopy at POC (TB diagnosis in smear-negative and HIV-infected persons in high-burden countries, or the TB NEAT study).7,11 While these studies assessed the incremental benefit of Xpert vs. smear microscopy, they could not answer the important question of the extent to which Xpert placement impacts patient management. We therefore compared TB diagnosis and treatment initiation in individuals with presumed pulmonary TB who were assessed by Xpert placed at POC or at an offsite laboratory. We hypothesised that Xpert at POC would result in a greater proportion of individuals starting treatment compared to laboratory placement.
METHODS
Participants in this prospective cohort study received care at the Witkoppen Health and Welfare Centre, a primary care clinic in northern Johannesburg. All clinic clients were offered provider-initiated human immunodeficiency virus (HIV) counselling and testing, as well as screening for TB symptoms. Patients were evaluated for pulmonary TB if they were HIV-infected and presented with cough, fever, unintentional weight loss or night sweats of any duration, or were non-HIV-infected and presented with prolonged (≥2 weeks) cough, fever, night sweats or unintentional weight loss. Clients were referred to a nearby hospital for chest X-ray upon clinician request.
From July 2011 to July 2012, Xpert testing was performed in the clinic at the POC using two four-module instruments operated by HIV counsellors with high school education or lower. Two spontaneous sputum samples were collected: one was processed using POC Xpert, the other was sent to an NHLS laboratory in Johannesburg for fluorescent sputum smear microscopy and liquid culture. Xpert assays with invalid results were repeated whenever possible using a new sputum sample. In August 2012, POC Xpert was stopped, and all samples were transported to the NHLS laboratory for offsite Xpert testing, according to the new Department of Health algorithm.12 According to NHLS guidelines, a sample for Xpert testing was rejected if submitted within 60 days of a previous sample with a successful Xpert result. Xpert results from the NHLS laboratory could be accessed online in real time by clinic staff.
Demographic and clinical data on consecutive adults (age ≥15 years) who underwent Xpert testing for pulmonary TB were collected from reviews of medical charts, electronic records and clinic registers over two 6-month study periods: December 2011–May 2012, when Xpert was performed at POC (POC Xpert period), and August 2012–January 2013, when samples were sent to a laboratory for Xpert testing (laboratory Xpert period).
The main outcome of interest was initiation of anti-tuberculosis treatment in the 3 months following initial sputum collection. Those not initiating treatment by 3 months were considered lost to follow-up. Cases who started treatment based on a positive Xpert, smear microscopy or culture were classified as bacteriologically confirmed,13 while those who started treatment in the absence of, or prior to, any positive bacteriological test were classified as empiric treatment. Xpert-negative TB was defined as an individual testing negative on Xpert but diagnosed with TB based on smear, culture, X-ray or clinical presentation. Time to anti-tuberculosis treatment initiation was defined as the time between collection of the initial sputum sample for Xpert testing and treatment initiation. Participants with drug resistance detected by any method were excluded from the time-to-treatment analysis. Laboratory turnaround times for Xpert were calculated from receipt of the sample at the laboratory until test results were authorised for release. Samples without a valid Xpert result included those with an invalid result, those that leaked in transit and those rejected by the laboratory as a duplicate request if they were submitted as a repeat after an error result or leaked sample.
We constructed Kaplan-Meier curves to estimate time to treatment initiation, and compared the basis of treatment by study period using a proportions test. We used a Cox proportional hazards model to compare time to treatment initiation between study groups among Xpert-positive individuals. All statistical analyses were conducted using Stata, v 12 (Stata Corp, College Station, TX).
The study was approved by the institutional review boards of the University of North Carolina, Chapel Hill and the University of the Witwatersrand in Johannesburg, South Africa. All participants provided written consent for research use of routinely collected clinical data.
RESULTS
Study population
A total of 1861 adults were assessed using Xpert as the initial diagnostic for presumptive pulmonary TB. About equal proportions were evaluated by POC Xpert (n =870, 47%) and laboratory Xpert (n =991, 53%) (Table 1). Most presented with cough (84%), were female (59%) and HIV-infected (72%). Compared to those in the POC Xpert group, individuals assessed by laboratory Xpert were more likely to be co-infected with HIV (76% vs. 67%, P < 0.001) and be on antiretroviral treatment (ART) (45% vs. 42%, P =0.027). The proportion with a CD4 count below the ART initiation threshold of 350 cells/mm3 was similar for both groups (59% POC Xpert and 60% laboratory Xpert, P = 0.712).
Table 1.
Baseline characteristics of 1861 individuals evaluated for TB by Xpert placed at POC or offsite at a laboratory
| POC Xpert (n = 870) n (%) |
Offsite Xpert (n = 991) n (%) |
P value | |
|---|---|---|---|
| Age category, years | 0.036 | ||
| <30 | 239 (27) | 221 (22) | |
| 30–45 | 416 (48) | 505 (51) | |
| >45 | 215 (25) | 265 (27) | |
| Female sex | 506 (58) | 597 (60) | 0.129 |
| HIV | <0.001 | ||
| Infected | 580 (67) | 756 (76) | |
| Non-infected | 279 (31) | 231 (23) | |
| Unknown | 11 (1) | 4 (<1) | |
| On ART | 241 (42) | 369 (48) | 0.027 |
| CD4 count, cells/mm3, mean (95%CI) | 294 (274–314) | 319 (302–321) | 0.065 |
| CD4 count, cells/mm3, median [IQR] | 251 [107–429] | 294 [149–454] | 0.013 |
| CD4 category, cells/mm3 | 0.001 | ||
| <200 | 222 (38) | 240 (32) | |
| 201–350 | 120 (21) | 210 (28) | |
| >350 | 182 (31) | 281 (37) | |
| Missing CD4 count | 56 (10) | 25 (3) | |
| Presenting with TB symptoms | |||
| Cough | 750 (85) | 816 (82) | 0.083 |
| Fever | 192 (22) | 219 (22) | 0.988 |
| Night sweats | 295 (34) | 293 (30) | 0.065 |
| Weight loss | 220 (25) | 309 (31) | 0.004 |
POC = point of care; HIV = human immunodeficiency virus; ART =antiretroviral therapy; CI = confidence interval; IQR = interquartile range; TB = tuberculosis.
Turnaround time for Xpert results
The majority of POC Xpert results (84%) were available on the same working day (by 4 pm), while the remainder (16%) were run overnight, with results available the following day. When performed at the laboratory, the majority (58%) of Xpert results were available online by the end of the day following sputum collection, and 96% of results were available within 2 days.
Xpert results, TB diagnosis and treatment initiation
The proportion of those evaluated for TB who tested positive on initial Xpert was similar in the two groups, with 11% having a positive result in both the POC Xpert (100/870) and laboratory Xpert groups (104/991). The proportion of individuals with rifampicin resistance detected using Xpert was relatively low, but was similar in the two groups (POC, 3%; laboratory Xpert, 5%; P = 0.467).
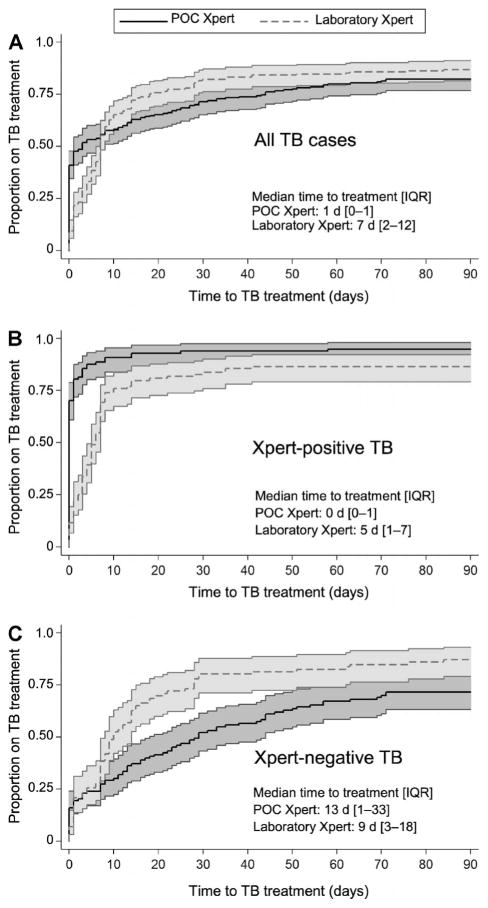
The impact of Xpert placement on the cumulative proportion of all TB cases started on anti-tuberculosis treatment, regardless of diagnostic basis, varied by time since initial evaluation (Figure, A). By 5 days following evaluation, 54% (95% confidence interval [CI] 47–60) of all TB cases were on treatment in the POC Xpert group compared to 38% (95%CI 32–46) in the laboratory Xpert group. However, by 15 days, more laboratory Xpert TB cases were on treatment (73%, 95%CI 67–79) than POC Xpert TB cases (63%, 95%CI 56–69). By 90 days, the gap between the two groups had narrowed (POC Xpert 82%, 95%CI 77–87; laboratory Xpert 87%, 95%CI 82–91). The median time to treatment in the POC Xpert group was 1 day (interquartile range [IQR] 0–14), while that of the laboratory Xpert group was 7 days (IQR 2–12, P < 0.001). More patients were started on treatment on the basis of bacteriological confirmation when Xpert was at POC (72%, 95%CI 65–79) vs. offsite at the laboratory (58%, 95%CI 48–63, P = 0.002).
Figure.
Estimated time to anti-tuberculosis treatment by Xpert status and Xpert placement. A) All patients with a TB diagnosis, regardless of its basis; B) all patients who were Xpert-positive; and C) all patients who were diagnosed with Xpert-negative TB. TB=tuberculosis; IQR=interquartile range; POC=point of care.
Among Xpert-positive patients, a higher cumulative proportion tested using POC Xpert (95%, 95%CI 89–98) than those who underwent laboratory-based Xpert (87%, 95%CI 79–92, P = 0.047) started treatment by 90 days following initial evaluation (Figure, B). Among Xpert-positives who started anti-tuberculosis treatment, the median time to treatment with POC Xpert was 0 days (IQR 0–1), while with laboratory Xpert it was 5 days (IQR 1–7, P <0.001). The proportion of Xpert-positive patients starting treatment on the same day as TB testing was also higher among those assessed using POC Xpert (68/97, 70% vs. 12/99, 12%; P < 0.001). Among those who tested positive on laboratory Xpert, 23% (23/99) were started on treatment empirically before the Xpert result was available to the clinician. Among Xpert-positives, instrument placement was the only variable associated with time to treatment initiation in the multivariate Cox proportional hazards model (adjusted hazard ratio 0.45, 95%CI 0.33–0.61, P < 0.001, after accounting for age, sex and HIV status, Table 2), suggesting that Xpert placement at POC resulted in a faster time to treatment initiation, independent of patient characteristics, which differed according to study period.
Table 2.
Factors associated with time to initiation of anti-tuberculosis treatment among Xpert-positive individuals
| Univariate
|
Multivariate
|
|||
|---|---|---|---|---|
| HR (95%CI) | P value | aHR (95%CI) | P value | |
| Xpert placement | ||||
| POC | Reference | |||
| Laboratory | 0.45 (0.33–0.61) | <0.001 | 0.45 (0.33–0.61) | <0.001 |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 1.0 (0.75–1.3) | 0.995 | 1.0 (0.78–1.4) | 0.761 |
| Age category, years | ||||
| <30 | Reference | Reference | ||
| 30–45 | 0.97 (0.70–1.3) | 0.863 | 1.1 (0.78–1.5) | 0.604 |
| >45 | 0.79 (0.49–1.2) | 0.306 | 0.85 (0.53–1.4) | 0.490 |
| HIV | ||||
| Negative | Reference | Reference | ||
| Positive | 0.77 (0.56–1.1) | 0.144 | 0.77 (0.54–1.1) | 0.140 |
| Unknown | 0.32 (0.04–2.4) | 0.265 | 0.42 (0.06–3.1) | 0.401 |
| ART | ||||
| Not on ART | Reference | |||
| On ART | 1.2 (0.72–1.9) | 0.539 | ||
| CD4 category,* cells/mm3 | ||||
| <200 | Reference | |||
| 201–350 | 1.2 (0.73–1.9) | 0.493 | ||
| >350 | 0.92 (0.52–1.6) | 0.774 | ||
Excludes 14 HIV-infected individuals with missing CD4 count.
HR = hazard ratio; CI = confidence interval; aHR = adjusted HR; POC = point of care; HIV = human immunodeficiency virus; ART =antiretroviral therapy.
Among Xpert-negative patients, the cumulative proportion started on treatment based on clinical examination, smear, culture or chest X-ray was similar in the two groups: 11% (95%CI 9–13) in the POC Xpert and 8% (95%CI 7–11) in the laboratory Xpert groups (P = 0.173). More Xpert-negative TB cases were initiated on treatment during the laboratory Xpert period (87%, 95%CI 79–93) than in the POC Xpert period (72%, 95%CI 63–80, P =0.001) (Figure, C). Among Xpert-negative TB cases starting anti-tuberculosis treatment, the median time to treatment was 13 days (IQR 1–33) in the POC Xpert group and 9 (IQR 3–18) in the laboratory Xpert group (P = 0.056).
Xpert errors and diagnostic follow-up
The proportion of assays with invalid or error results was similar in both groups: 4% at POC and 3% at offsite laboratory (P = 0.238). In addition, 33 (3%) samples leaked during transportation to the laboratory and 10 samples were rejected by the laboratory because a previous sample had been processed within the past 60 days (Table 3). The overall proportion of sputum samples without a valid Xpert result was therefore higher at the laboratory than at POC (8% vs. 4%, P < 0.001).
Table 3.
Diagnostic follow-up of invalid or laboratory errors on initial Xpert by Xpert placement
| POC Xpert n/N (%) |
Laboratory Xpert n/N (%) |
P value | |
|---|---|---|---|
| Total errors | 32/870 (4) | 77/991 (8) | <0.001 |
| Error on initial Xpert | |||
| Invalid | 32/32 (100) | 34/77 (44) | |
| Leaked | — | 33/77 (43) | |
| Rejected* | — | 10/77 (13) | |
| Repeated with Xpert | 32/32 (100) | 33/77 (43) | <0.001 |
| Result on Xpert repeat | |||
| Positive | 6/32 (19) | 3/33 (9) | |
| Negative | 26/32 (81) | 16/33 (48) | |
| Invalid | — | 2/33 (6) | |
| Leaked | — | 1/33 (3) | |
| Rejected | — | 11/33 (33) | |
| Repeated with Xpert, smear or culture | 32/32 (100) | 41/77 (53) | <0.001 |
A sample that was incorrectly rejected as having been submitted within 60 days of a sample with a valid Xpert result (e.g., the sample was rejected although it was a repeat after an error result).
POC = point of care.
DISCUSSION
In this prospective cohort study, we found that although Xpert placement at POC did not increase the overall proportion of presumed TB patients initiating treatment, POC Xpert did improve patient management. Almost all (95%) of those testing positive on POC Xpert started anti-tuberculosis treatment, mostly on the day of presentation, while 13% of those with a positive result in the laboratory Xpert group failed to initiate treatment, resulting in an 8% decrease in the proportion of cases confirmed by Xpert initiating anti-tuberculosis treatment. Empiric treatment was less common in the POC Xpert period than in the laboratory Xpert period. POC placement of the Xpert apparatus also altered the management of Xpert-negative TB cases, with a lower proportion started on treatment and a longer time to treatment initiation than in the case of laboratory placement of Xpert.
Our findings are in line with two recent studies that failed to demonstrate significant impact on patients of the use of Xpert when compared to smear microscopy for the initial diagnostic.7,11 In a trial where patients were randomised to same-day Xpert at POC vs. same-day smear microscopy at POC (TB-NEAT study), no effect was observed on morbidity, mortality or the proportion of patients started on treatment.7 However, similar to our findings, Xpert improved patient management, with a higher proportion of culture-confirmed TB cases initiating treatment in the Xpert arm (91% vs. 84%) and a lower proportion initiating empiric treatment in the Xpert arm (17% vs. 26%, P < 0.001). In a cluster randomised trial of laboratory-based Xpert vs. laboratory-based smear microscopy (EXTEND-TB study), the use of Xpert also did not increase the proportion of presumed TB patients started on treatment, and no effect on mortality was observed.11 Taken together, these results support the hypothesis proposed by Theron et al. that high rates of empiric treatment may reduce the potential positive effect of more accurate or immediate TB diagnosis on patient outcomes in high-burden settings.14 In our study, without access to same-day Xpert results, clinicians frequently initiated empiric treatment, with 23% of patients in the laboratory group initiating treatment empirically, before the positive Xpert result became available. The population impact of Xpert at POC will likely depend heavily on changes in patient management that accompany its implementation.
It has been demonstrated that the diagnostic accuracy of Xpert when carried out at POC and that of Xpert performed at a laboratory were comparable. We found that the proportion of Xpert assays with an invalid result was likewise independent of Xpert placement, confirming that the assay can be performed in the clinic setting by low-skilled health workers.5–7 Placement of Xpert at a laboratory created problems related to the transportation of samples (3% leaked), and fewer invalid results or errors being repeated.
Despite the strengths of a large sample size and implementation under real-world conditions, several limitations should be considered when interpreting these findings. The operational nature of our study design did not incorporate rigorously controlled diagnostic processes and resulted in the absence of culture results in all participants. Allocation to Xpert at POC or laboratory occurred by study period and was not randomised, possibly introducing bias by factors other than Xpert placement that may have changed over time. The study was set at a single primary care clinic, possibly limiting its generalisability due to clinical practices that were particular to this setting. For example, the daily presence of a physician and access to X-ray may not be representative of primary care settings in many high TB burden settings. The laboratory characteristics may also not have been representative, given the excellent infrastructure, including roadways and telecommunications, which allowed twice-daily transportation of samples to the laboratory and the electronic relay of results. These clinic and laboratory characteristics may have resulted in an underestimation of the effect of Xpert placement at POC. Placement of Xpert at POC in settings with fewer resources, lower density of laboratories or in rural settings, where patients travel long distances to access care and where sample transportation and communication infrastructure is less reliable, may result in a greater impact.
In summary, in an urban clinic with good infrastructure, POC placement of Xpert increased the health care worker’s ability to rapidly start Xpert-positive patients on treatment and avoid loss to follow-up, but did not influence the overall proportion of presumed TB patients started on treatment and the time to treatment initiation. When samples were transported to a laboratory, empiric anti-tuberculosis treatment was an important form of treatment decision making, resulting in greater initiation of treatment in Xpert-negatives. The impact of the introduction of new diagnostic tests for TB may depend largely on the diagnostic practices and quality of the health care system. In settings with intermittent sample transportation, cumbersome communication of test results, low patient access to care or in which empiric treatment is infrequent, the placement of Xpert at POC can be expected to have a greater impact on patient outcomes. Economic evaluations and modelling studies that take these complexities into account are needed to guide policies on Xpert placement in high-burden countries to maximise patient impact.
Acknowledgments
The authors sincerely thank the patients and clinical staff at the Witkoppen Health and Welfare Centre, Johannesburg, South Africa, as well as V Modise for assistance in collecting patient data.
This work was supported by the National Institute for Allergy and Infectious Diseases at the National Institutes of Health, Bethesda, MD, USA (grant number UM1 AI069463), and the United States Agency for International Development, Washington DC, USA (grant number AID-674-A-12-00033).
Footnotes
Conflicts of interest: none declared.
References
- 1.Lin HH, Dowdy D, Dye C, Murray M, Cohen T. The impact of new tuberculosis diagnostics on transmission: why context matters. Bull World Health Organ. 2012;90:739–747A. doi: 10.2471/BLT.11.101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun AY, Pai M, Salje H, Satyanarayana S, Deo S, Dowdy DW. Modeling the impact of alternative strategies for rapid molecular diagnosis of tuberculosis in southeast Asia. Am J Epidemiol. 2013;178:1740–1749. doi: 10.1093/aje/kwt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steingart KR, Sohn H, Schiller I, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;1:CD009593. doi: 10.1002/14651858.CD009593.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6:1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clouse K, Page-Shipp L, Dansey H, et al. Implementation of Xpert MTB/RIF for routine point-of-care diagnosis of tuberculosis at the primary care level. S Afr Med J. 2012;102:805–807. doi: 10.7196/samj.5851. [DOI] [PubMed] [Google Scholar]
- 6.Hanrahan CF, Selibas K, Deery CB, et al. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PLOS ONE. 2013;8:e65421. doi: 10.1371/journal.pone.0065421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383:424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 8.National Health Laboratory Service. NPP Implementation of GeneXpert for TB testing. Johannesburg, South Africa: NHLS; 2013. [Accessed March 2015]. http://www.nhls.ac.za/?page=genexpert_for_tb_testing&id=69. [Google Scholar]
- 9.World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach. Geneva, Switzerland: WHO; 2013. [Accessed March 2015]. 2003 revision. http://www.who.int/3by5/publications/documents/arv_guidelines/en/ [Google Scholar]
- 10.Lawn SD, Kerkhoff AD, Wood R. Location of Xpert® MTB/RIF in centralised laboratories in South Africa undermines potential impact. Int J Tuberc Lung Dis. 2012;16:701. doi: 10.5588/ijtld.12.0131. author reply 2. [DOI] [PubMed] [Google Scholar]
- 11.Churchyard G. Effect of Xpert MTB/RIF on early mortality in adults with suspected TB: a pragmatic randomized trial. Conference on Retroviruses and Opportunistic Infections (CROI); Boston, MA, USA. 3–6 March 2014; [Oral Abstract 95] [Google Scholar]
- 12.National Health Laboratory Services. GeneXpert testing algorithm. Johannesburg, South Africa: NHLS; 2013. [Accessed March 2015]. http://www.nhls.ac.za/assets/files/GeneXpert%20poster.pdf. [Google Scholar]
- 13.World Health Organization. Definitions and reporting framework for tuberculosis. Geneva, Switzerland: WHO; 2013. 2013 revision. WHO/HTM/TB/2013.2. [Google Scholar]
- 14.Theron G, Peter J, Dowdy D, Langley I, Squire SB, Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014;14:527–532. doi: 10.1016/S1473-3099(13)70360-8. [DOI] [PubMed] [Google Scholar]