Abstract
To further evaluate the parameters whereby intracerebral administration of recombinant α-synuclein (αS) induces pathological phenotypes in mice, we conducted a series of studies where αS fibrils were injected into the brains of M83 (A53T) and M47 (E46K) αS transgenic (Tg) mice, and non-transgenic (nTg) mice. Using multiple markers to assess αS inclusion formation, we find that injected fibrillar human αS induced widespread cerebral αS inclusion formation in the M83 Tg mice, but in both nTg and M47 Tg mice, induced αS inclusion pathology is largely restricted to the site of injection. Furthermore, mouse αS fibrils injected into nTg mice brains also resulted in inclusion pathology restricted to the site of injection with no evidence for spread. We find no compelling evidence for extensive spread of αS pathology within white matter tracts, and we attribute previous reports of white matter tract spreading to cross-reactivity of the αS pSer129/81A antibody with phosphorylated neurofilament subunit L (NFL). These studies suggest that with the exception of the M83 mice which appear to be uniquely susceptible to induction of inclusion pathology by exogenous forms of αS there are significant barriers in mice to widespread induction of αS pathology following intracerebral administration of amyloidogenic αS.
Keywords: amyloid, Parkinson’s disease, pathology, α-synuclein, transgenic mice
Introduction
α-Synucleinopathies, a spectrum of neurodegenerative disorders, most notably Parkinson’s disease (PD), are characterized by the presence of intracellular α-synuclein (αS) inclusions. These inclusions are formed from the amyloidogenic aggregation of the normally soluble presynaptic protein αS [13,37,93]. Mature αS inclusions are also labeled with other markers of intracellular protein aggregates including ubiquitin and p62 [58,65]. A direct causal role for αS in neurodegeneration is supported by missense mutations or increased copy number of the αS gene (SNCA) in patients with familial PD [5,25,52,57,61,75,76,85,101].
α-Synucleinopathies are progressive neurodegenerative disorders and multiple experimental and pathologic studies have been interpreted as supportive of a “prion-like” spread mechanism where amyloidogenic αS serves as a template to drive the conversion of soluble, natively unfolded αS to a conformationally-altered, aggregated form which transmits from cell-to-cell [40,48,56,62,63,66,67,70,74,77,83,90,91]. For example, in post-mortem studies of PD patients who had received therapeutic striatal transplants of fetal dopaminergic neurons, neurons subsequently developed αS pathology [56,62,63], and a similar phenomenon has also been reported in wild type mouse neurons transplanted into an αS Tg mouse model [41]. However, other mechanisms could also explain this phenomenon (reviewed in [9]). In recent studies, it has also been shown that intracerebral injections of extracts from brains of humans with αS pathology or amyloidogenic fibrillar forms of human αS can induce pathology in M83 (A53T) αS transgenic (Tg) mice [67,70,91]. In addition, amyloidogenic murine αS was reported to induce widespread pathology and neurodegeneration within 1 month in non-transgenic (nTg)/native mice [66]. In cell culture studies, it has been reported that the addition of preformed exogenous αS amyloid seeds induces intracellular αS aggregates in both αS overexpressing and naïve primary mouse neuronal cultures [19,40,90], with evidence in these studies for both strain like conformational templating, passaging, and possibly cell-to cell transmission [83].
Despite the growing evidence for prion like induction of αS pathology both in culture and in vivo [40,48,56,62,63,66,67,74,77,83,90,91], alternative and perhaps synergistic mechanisms of pathology induction have not been excluded. Indeed, we have observed that non-amyloidogenic form of αS (human Δ71–82 αS) that does not form amyloid in vitro or seed αS inclusion formation in culture can induce inclusion formation in vivo [82]. Moreover, the extent of pathology induction in vivo and the time course of induction that have been reported are also quite variable. Given the potential importance of these induced models for mechanistic and therapeutic studies, we conducted a series of in vivo studies in which different forms and types of αS fibrils were injected into the brains of M83 (A53T) and M47 (E46K) αS transgenic (Tg) mice, and non-transgenic (nTg) mice. At post-injection times similar to what has been previously reported, we demonstrate that fibrillar human αS induced widespread cerebral αS inclusion formation only in the M83 Tg mice, but not in nTg and M47 Tg mice. In both nTg and M47 mice αS pathology is induced but it is largely restricted to the site of injection and in the nTg mice actually diminishes over time, confirming that there are significant barriers to widespread induction of pathology except in the M83 mice. Finally, we find that the αS pSer129/81A antibody used in many of the studies to report widespread pathology induction in vivo (and also white matter pathology in human samples) shows significant cross reactivity with phosphorylated neurofilament subunit L (NFL). Use of this antibody to track αS pathology can result in a marked overestimation of pathology induction, and thus raises additional questions about the mechanisms of pathology induction that have been reported to occur through white matter tracts.
Results
Pathological changes following intrahippocampal injection of preformed αS fibrils in M83 Tg mice
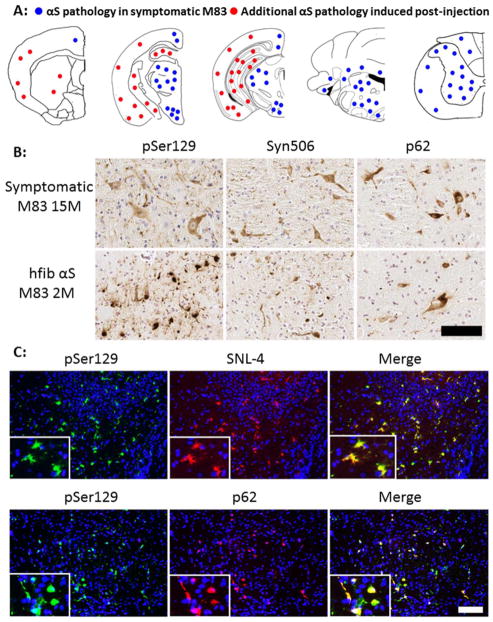
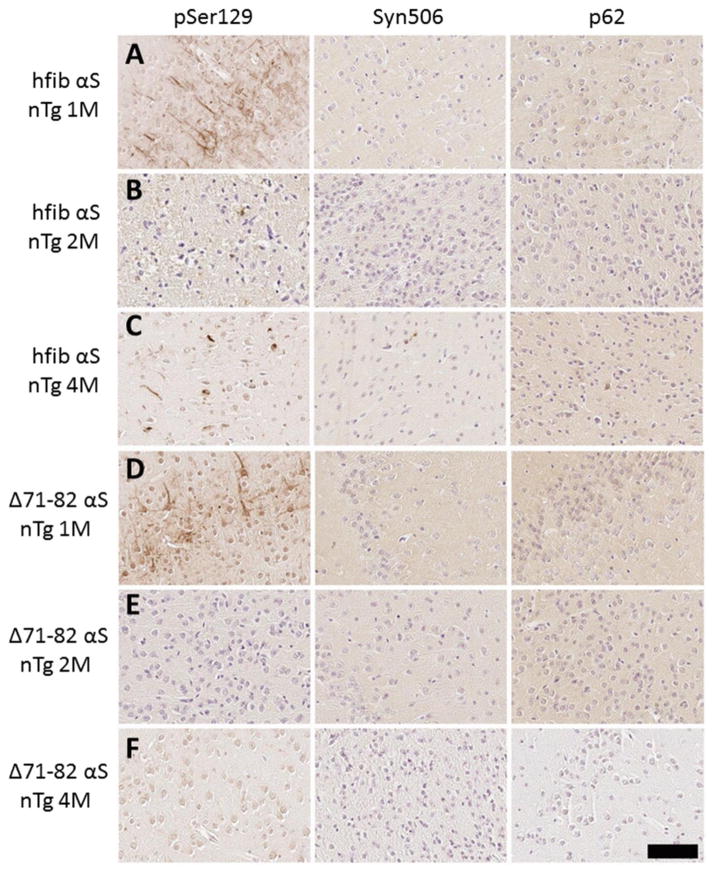
Recent studies have shown robust induction of widespread CNS αS pathology using M83 Tg mice challenged with adult brain injection of human fibrillar (hfib) αS [67]. M83 Tg mice intrinsically develop a severe motor phenotype associated with flame-like αS inclusions. On the current genetic background, the motor phenotype develops over a wide range of time from 7–15 months. Inclusion pathology occurs throughout the neuroaxis, but largely spares the hippocampus, striatum, and the cortex [33]. To limit confounds from intrinsic pathology formation, intrahippocampal injection of 21–140 hfib αS was performed in young 2 month-old M83 Tg mice, which were analyzed 2 months later, a time when no intrinsic pathology is observed (Table 1). We injected N-terminally truncated protein 21–140 αS, as we and others previously have shown that it can seed αS inclusions in cultured cells as efficiently as full-length αS [68,83,95,96], and it enables monitoring of the aggregation of endogenous αS with N-terminal specific antibodies such as SNL-4 and Syn506 [21,34,92]. 21–140 hfib αS intrahippocampal injection resulted in widespread αS inclusion formation that was found in all the regions where intrinsic αS pathology would form. Abundant inclusions were also noted in areas where inclusion formation does not intrinsically occur in these mice including the hippocampus, where the protein was injected, and the striatum and cortex, though the striatal/cortical inclusions were less frequent (Fig. 1; Supp. Figs. 1–3). Intrahippocampal injection of PBS resulted in no αS pathology formation. At the site of injection, Δ71–82 αS, which has a deletion in the middle of the hydrophobic region of αS that lacks the ability to form or seed αS amyloid in vitro and in cultured cells [35,68,83,98], resulted in sparse altered pSer129/81A staining of perikaryal cell bodies and structures that resembles dystrophic synaptic terminals or that could be Jucker bodies (Supp. Fig. 4; data not shown). Similar to the αS inclusions seen in symptomatic M83 Tg mice (Fig. 1B), those seen post-injection of 21–140 hfib αS are predominantly flame-like, filling the somatodendritic compartment, and are robustly recognized by the αS antibody Syn506 and by an antibody to p62, which is specific for inclusion formation [58] (Fig. 1; Supp. Figs. 1–3).
Table 1.
Summary of intracerebral injections of mice.
| Authentic αS pathologya | |||||||
|---|---|---|---|---|---|---|---|
| Strain | Number of mice | Injection site | Inoculum | Time post-injection | Induced pSer129/81A staining | Detection by multiple markers | Spread of αS pathology outside of injection site |
| M83 (A53T αS) | 4 | HC | 2μL 2mg/mL 21–140WT hfib αS | 2 months | yes | yes | yes |
| M83 (A53T αS) | 4 | HC | 2μL 2mg/mL Δ71–82 αS | 2 months | yes, but limitedb | no | no |
| M83 (A53T αS) | 4 | HC | 2μL PBS | 2 months | no | no | no |
| M47 (E46K αS) | 6 | HC | 2μL 2mg/mL 21–140 WT hfib αS | 4 months | yes | yes | minimalb |
| M47 (E46K αS) | 6 | HC | 2μL 2mg/mL E46K FL hfib αS | 4 months | yes | yes | minimalb |
| M47 (E46K αS) | 5 | HC | 2μL 2mg/mL A53T FL hfib αS | 4 months | yes | yes | minimalb |
| C57BL6/C3H | 4 | HC | 2μL 2mg/mL 21–140WT hfib αS | 1 month | yes | yes | no |
| C57BL6/C3H | 4 | HC | 2μL 2mg/mL 21–140WT hfib αS | 2 months | yes | yes | no |
| C57BL6/C3H | 4 | HC | 2μL 2mg/mL 21–140WT hfib αS | 4 months | yes | yes | no |
| C57BL6/C3H | 5 | HC | 2μL 2mg/mL FL WT mfib αS | 2 months | yes | yes | no |
| C57BL6/C3H | 4 | CTX | 2μL 2mg/mL 21–140WT hfib αS | 1 month | yes | yes, sparse | no |
| C57BL6/C3H | 4 | CTX | 2μL 2mg/mL 21–140WT hfib αS | 2 months | yes | yes, sparse | no |
| C57BL6/C3H | 4 | CTX | 2μL 2mg/mL 21–140WT hfib αS | 4 months | yes | yes, sparse | no |
| C57BL6/C3H | 4 | CTX | 2μL 2mg/mL Δ71–82 αS | 1 month | yes | no | no |
| C57BL6/C3H | 4 | CTX | 2μL 2mg/mL Δ71–82 αS | 2 months | no | no | no |
| C57BL6/C3H | 4 | CTX | 2μL 2mg/mL Δ71–82 αS | 4 months | no | no | no |
| C57BL6/C3H | 4 | CTX | 2μL PBS | 1 month | no | no | no |
| C57BL6/C3H | 4 | CTX | 2μL PBS | 2 months | no | no | no |
| C57BL6/C3H | 4 | CTX | 2μL PBS | 4 months | no | no | no |
αS pathology confirmed with multiple αS antibodies and aggregate markers.
See text.
Fig 1. Induction of αS pathology at 2 months post intrahippocampal injection of 21–140 hfib αS in M83 Tg mice.
(A) Schematic map showing rostral-caudal distribution of αS pathology in M83 Tg mice following hippocampal injection of 21–140 hfib αS. In these mice there was robust induction of αS pathology at the site of injection, but also throughout the CNS. Red dots indicate locations of intrinsic αS pathology observed when M83 Tg mice become symptomatic with aging [33] and blue dots indicate locations of additional αS pathology uniquely induced by hippocampus injection with 21–140 hfib αS. Therefore, in M83 Tg mice injected with 21–140 hfib αS, αS neuronal inclusions were observed in the location indicated by both red and blue dots. Similar density and distribution of αS pathology was seen bilaterally. This distribution of αS inclusions was assessed with both pSer129/81A and Syn506 antibodies. (B) IHC of brainstem tissue sections from a symptomatic 15 month-old M83 Tg mouse and a M83 Tg mouse at 2 months post intrahippocampal injection of 21–140 hfib αS showing pSer129/81A+ inclusions that fill the cell bodies and extend out into the processes along with the presence of dystrophic neurites. These αS inclusions are also readily detected by staining with Syn506 and p62 antibodies. Tissue sections were counterstained with hematoxylin. (C) Double immunofluorescence analysis of the hippocampal region of a M83 Tg mouse at 2 months post intrahippocampal injection of 21–140 hfib αS for pSer129 (green) and SNL-4 (upper panels) or p62 (lower panels) shows that all pSer129/81A+ perikaryal inclusions are also SNL-4+ and that the majority of pSer129/81A+ inclusions are also p62+. Cell nuclei were counter stained with DAPI. Scale bar = 100 μm and 25 μm (inset).
Although our findings are consistent with those of Luk and colleagues in the ability of intracerebral injection of exogenous αS fibrils to rapidly induce αS inclusion pathology M83 Tg mice, using multiple αS antibodies including Syn506, we were not able to observe robust evidence of inclusion pathology within brain white matter tracts as previously reported [67]. The vast majority of the white matter staining by pSer129/81A antibody was not reactive with other αS antibodies (Supp. Fig. 5). This difference is significant as it was used to support the concept of axonal spread of inclusion pathology from the site of injection [67]. Only sparse αS inclusions that could be labeled with other αS antibodies were observed in the brain white matter tracts of hfib αS injected M83 Tg mice. pSer129/81A readily labeled αS inclusions as shown by double-labeling; however, it did not stain areas abundant in normal αS such as the neuropil areas of the cerebellum (Supp. Fig. 5), consistent with previous findings that αS is only modestly phosphorylated at Ser129 under normal conditions but hyperphosphorylated in aggregates [4,27,94]. However, the intense pSer129/81A white matter tract staining was still present throughout the CNS in SNCA−/− mice as shown by immunofluorescence microscopy and IHC (Supp. Figs. 5–6). This finding is consistent with our recent studies that also revealed robust pSer129/81A staining of cultured neuronal processes from SNCA−/− mice [83], indicating that this staining is due to cross-reactivity of the pSer129/81A antibody with a non-αS target.
Analyses of intrahippocampal injection of preformed αS fibrils in nTg mice
To further study the pathological changes resulting from cerebral challenge of exogenous preformed αS fibrils, we performed similar intrahippocampal injection of 21–140 hfib αS in nTg mice (Table 1). This treatment resulted in the accumulation of neuronal pSer129 immunostaining localized to the site of injection with very limited pathology in the cortex (Fig. 2A). Intrahippocampal injections also resulted in the formation of some αS inclusions that could be detected with Syn506 and p62 antibodies (Fig. 2B). pSer129/81A, Syn506, and p62 immunoreactive perikaryal and neuritic inclusions in the hippocampus of nTg mice challenged with 21–140 hfib αS persisted for up to 4 months, although with decreasing abundance (Fig. 2B). During this time period there was no evidence of spread beyond the initial sites of pathology.
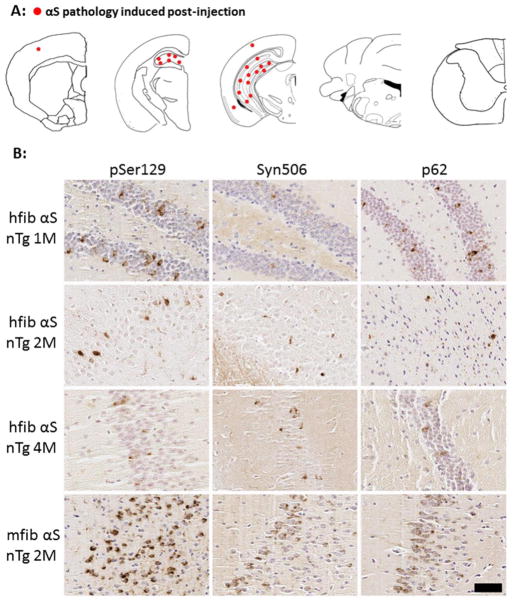
Fig 2. Induction of αS inclusions in nTg mice after intrahippocampal injection of 21–140 hfib αS or full-length mfib αS.
(A) Schematic map showing rostral-caudal distribution of αS in nTg mice at 4 months post-injection. Similar density and distribution of αS pathology was seen bilaterally. Inclusions were mainly localized around the site of injection (hippocampus) with sparse pathology seen in the cortex for both human and mouse derived αS amyloidogenic fibrils. (B) Representative images of IHC in the hippocampal region surrounding the site of injection showing decreasing pSer129/81A pathology at 1 month, 2 months, and 4 months post-injection of hfib αS. Some inclusions reactive with Syn506, a mouse monoclonal antibody that conformationally detects αS aggregates, and p62, a rabbit polyclonal antibody that nonspecifically recognizes protein aggregates, were also observed. Similar staining also shows αS inclusion formation resulting from the hippocampal injection of mfib αS at 2 months post-injection. Some of these αS inclusions are also detected by Syn506 and p62. Tissue sections were counterstained with hematoxylin. Scale bar = 50 μm.
The limited level of αS pathology induced after intrahippocampal injection of 21–140 hfib αS prompted us to examine whether there is a cross-species barrier inhibiting inclusion formation. We performed intrahippocampal injection of full-length mouse fibrillar (mfib) αS. At 2 months post-injection of mfib αS, there were αS inclusions readily detected with pSer129/81A, Syn506, and p62 antibodies at the site of injection, and the amount of pathology was more robust than for 21–140 hfib αS (Fig. 2B); however, there was no difference in the distribution pattern of pathology. The more robust induction of local pathology by mfib αS could simply be due to the fact that mouse αS intrinsically have the A53T substitution that promotes αS aggregation and seeding [12,36,79]. Therefore, our findings are consistent with those recently reporting that mfib αS can induce the formation of some αS inclusions in nTg mice [67,69], but in our studies αS pathology was highly localized to the site of injection.
Analyses of intrahippocampal injection of preformed αS fibrils in M47 Tg mice
Since we observed rapid and widespread induction of αS pathology in M83 Tg mice, but not in nTg mice, we next examined induction of αS inclusion formation following stereotactic brain injection of fib αS in a Tg mouse model expressing human αS with the E46K missense mutation (line M47, Table 1). These mice typically develop Lewy body-like αS inclusions with a distribution similar to M83 Tg mice but not before 15 months of age [23]. At 4 months post hippocampal injection of hfib αS, αS inclusions were predominantly found at the site of injection in the hippocampus, with modest amounts in the cortex, midbrain, and brainstem (Fig. 3A). The rounded, perinuclear and neuritic inclusions similar to those seen in symptomatic M47 Tg mice were also detectable by Syn506 and p62 (Fig. 3B–C). We further investigated whether αS fibrils with pathogenic mutants such as A53T or E46K could be more potent at inducing αS pathology in M47 Tg mice. The hippocampal injection of fibrils comprised of E46K αS in M47 Tg mice also recapitulates the condition where the same protein is injected as is expressed, alleviating concerns of cross-species differences. The location and formation of αS pathology in M47 Tg mice injected with A53T or E46K αS fibs was similar to the mice injected with the 21–140 hfib αS (Fig. 4).
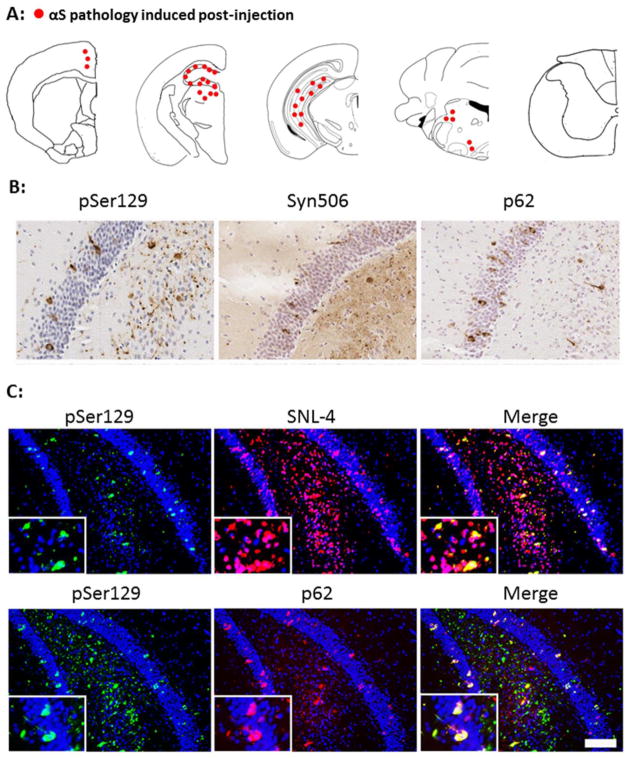
Fig 3. Characterization and distribution of αS inclusion pathology at 4 months post intrahippocampal injection of 21–140 hfib αS in M47 Tg mice.
(A) Diagram shows rostral/caudal distribution of αS inclusion pathology in M47 Tg mice. Tissue sections were stained with antibodies pSer129/81A and Syn506 to detect αS inclusion pathology. Intrahippocampal injections resulted in the induction of αS pathology at the site of injection with some additional pathology in the midbrain and brainstem. Similar density and distribution of αS pathology was seen bilaterally. (B) Brain tissue sections with representative regions of the hippocampus in injected M47 Tg mice show rounded, perinuclear inclusions and neuritic pathology stained with pSer129/81A. Inclusions were also recognized by Syn506, a mouse monoclonal antibody that conformationally recognizes αS inclusions, and p62, a rabbit polyclonal antibody that non-specifically recognizes protein aggregates. (C) Double immunofluorescence analysis of the hippocampal region stained for pSer129/81A (green) and SNL-4 (red; upper panels) or p62 (red; lower panels) shows that most hyperphosphorylated αS inclusions are SNL-4+ and that a large portion are also p62+ in M47 Tg mice injected with 21–140 hfib αS. There is also significant p62 immunoreactivity that does not co-localize with pSer129 indicating the formation of more than one type of protein aggregate. Tissue sections were counterstained with hematoxylin (B) and DAPI (C). Scale bar = 50 μm (B), and 100 μm and 25 μm (C; insets).
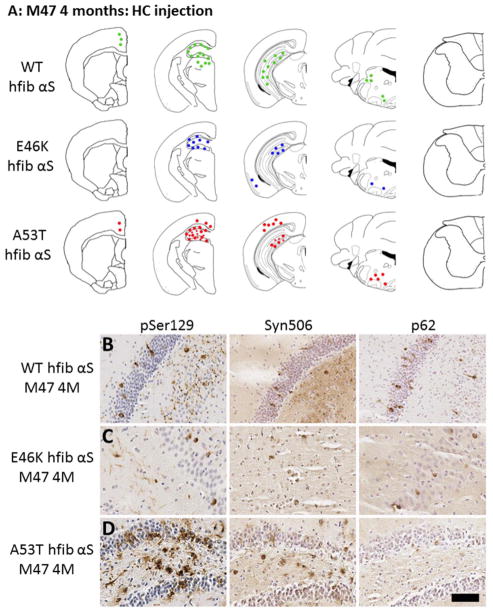
Fig 4. Induction of αS induction pathology in M47 Tg mice following hippocampal injection of wild-type, E46K, or A53T hfib αS.
(A) Schematic summary of αS pathology distribution at 4 months post intrahippocampal injection of wild-type 21–140 (WT), or full-length E46K or A53T hfib αS in M47 Tg mice. Diagrams show rostral/caudal distribution of αS pathology in M47 Tg mice. Tissue sections were stained with pSer129 and Syn506 to detect αS pathology. (B–D) Immunohistochemistry showing similar αS pathology induced by intrahippocampal injection of WT, E46K, or A53T hfib αS in M47 Tg mice at 4 months post-injection. Brain tissue sections with representative regions of the hippocampus surrounding the injection site in M47 Tg mice injected with WT (B), E46K (C), or A53T (D) hfib αS show rounded, perinuclear inclusions and neuritic pathology regardless of the type of inoculum detected with pSer129/81A antibody. Inclusions were also recognized by Syn506, a mouse monoclonal antibody that conformationally recognizes αS inclusions, and p62, a rabbit polyclonal antibody that non-specifically recognizes protein aggregates. Tissue sections were counterstained with hematoxylin. Scale bar = 50 μm.
Analyses of intracortical injection of preformed αS fibrils in nTg mice
Since we were unable to induce widespread αS inclusion pathology by the hippocampal injection of exogenous αS fibrils in nTg mice as previously reported [66], we explored the possibility that the site of injection (cortical vs hippocampal) may have contributed to this difference although Luk and colleagues have already reported that similar induction of αS should result from the injection in various brain regions including cortex, striatum, and hippocampus [66]. We performed cortical injections of 21–140 hfib αS or Δ71–82 αS in nTg mice with analyses conducted at 1, 2, and 4 months post-injection (Table 1). At 1 month post-injection, we observed a robust increase in pSer129/81A perikaryal, neuronal immunoreactivity that radiates throughout the cortex from the site of injection following both types of injections (Fig 5. and Supp. Figs. 7–8). The intense induced pSer129/81A cortical staining was not observed in PBS injected nTg mice (Fig. 5 and Supp. Figs. 7–8).
Fig 5. Immunohistochemical analysis of αS immunoreactivity in nTg mice after intracerebral injection with 21–140 hfib αS or Δ71–82 αS.
At one month post-injection of both 21–140 hfib αS (A) and Δ71–82 αS (D) robust staining with pSer129/81A was seen at the site of injection in the cortex; however, the increased pSer129 immunoreactivity was not detected by other markers of αS pathology, Syn506 or p62. At 2 and 4 months post-injection of 21–140 hfib αS (B, C) and Δ71–82 αS (E, F) almost all the intense but diffuse perikaryal pSer129 immunoreactivity had subsided. At 4 months post injection of 21–140 hfib αS (C), scattered dense cortical pSer129 reactive inclusions were present in the cortex and rarely in the hippocampus, but most of these were not detected with either Syn506 or p62 antibodies. Tissue sections were counterstained with hematoxylin. Scale bar = 50 μm.
In nTg mice injected with 21–140 hfib αS, only some scattered more compacted pSer129/81A immunoreactive perikaryal or neuritic profiles persisted up to 4 months post-injection, but the initial induced cortical pSer129/81A immunoreactivity that radiated from the injection had subsided (Fig. 5). Similarly at 2 and 4 months post-injection of Δ71–82 αS, the initially induced perikaryal pSer129/81A staining was no longer detected (Fig. 5). Staining of these perikaryal neuronal cell bodies as seen with the anti-pSer129/81A antibody at 1 month post injection was not observed with a panel of other αS antibodies (Syn506, Syn303, Syn505, Syn514, SNL-1, SNL-4, and HuA) or p62 (Fig. 5, Supp. Fig. 9, and data not shown) even after systematically evaluating various antigen retrieval methods [21,34,92]. In comparison, bona fide mature αS inclusions in symptomatic M83 A53T αS Tg mice were readily detected with multiple αS antibodies (Fig. 1, Supp. Figs. 2, 9, and data not shown).
Identification of the major axonal pSer129/81A cross-reactive protein as NFL phosphorylated at Ser473
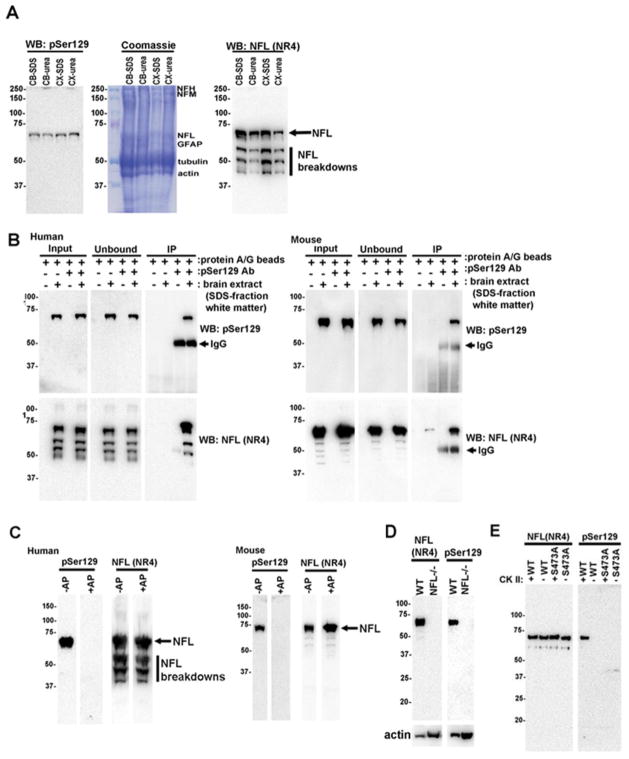
The antibody pSer129/81A has been used in many seminal reports to track αS pathology [40,66,67,90]. Previous findings [83,94] and the data shown here (Fig. 5 and Supp. Figs 5–6, 7–8) indicate that this antibody cross-reacts with a non-αS pSer129 target with increased immunoreactivity following exogenous αS treatment. Therefore we conducted studies to identify this non-αS pSer129 target. We first performed biochemical fractionations from CNS areas enriched in grey matter (cortex) and white matter (spinal cord and brain stem) from nTg and SNCA−/− mice. Western blot analysis of these fractions demonstrated that αS is predominantly present in the HS fractions, and the presence of modestly pSer129 phosphorylated αS in the HS-soluble fraction of only nTg mice (Supp. Fig. 9). This is consistent with previous characterizations of normal αS protein demonstrating that it is largely soluble and only modestly phosphorylated at Ser129 [4,18,27,94,99]. As previously reported, endogenous αS is also more highly expressed in the cortex than the spinal cord [32,33]. Importantly, this analysis demonstrates that pSer129/81A also strongly reacts with a ~70 kDa non-αS target that is still present in SNCA−/− mouse brain and enriched in the white matter. Biochemically, this protein is enriched in the SDS-urea fraction, but is also present in lesser amounts as a soluble form in the HS-fraction (Supp. Fig. 9). This finding is also consistent with our previous characterization, where we showed this antibody can cross-react with a detergent insoluble ~70 kDa non-αS target enriched in white matter brain tissue, like cerebellar white matter [94], and we hypothesized that the reactivity with this protein was largely responsible for the intense pSer129/81A staining observed in neuronal processes still present in SNCA−/− mice [83].
We found that the ~70 kDa pSer129/81A reactive protein was soluble in either 4M urea or 2% SDS and that it was similarly abundant in white matter from human cortex or cerebellum (Fig. 6A). Compared to total protein Coomassie stained gel, we found that the pSer129/81A reactive ~70 kDa band aligned with the low-molecular-mass neurofilament subunit (NFL) (Fig. 6A). To ascertain that pSer129/81A could be reacting with NFL, we performed immunoprecipitation (IP) assays with pSer129/81A and showed by immunoblotting that pSer129/81A antibody could immunoprecipitate NFL from both human and mouse white matter (Fig. 6B). We then performed dephosphorylation studies to show that the reactivity of the pSer129/81A antibody with the ~70 kDa protein band was completely dependent on phosphorylation (Fig. 6C). To confirm that the ~70 kDa protein recognized by pSer129/81A antibody was NFL, we analyzed extracts from NFL−/− mice and showed that it was completely absent in tissue from NFL−/− mice (Fig. 6D). To further confirm that pSer129/81A was reacting with phosphorylated NFL we purified recombinant NFL from bacteria and phosphorylated the protein with casein kinase II in vitro. We found that the pSer129/81A antibody only reacted with recombinant NFL phosphorylated in vitro (Fig. 6E). The major in vivo phosphorylation site in NFL is Ser473 (normally modified at ~73%), which corresponds to Ser472 in bovine NFL [88,100]. Like the Ser129 phosphorylation site in αS, this site in NFL is also an excellent substrate for casein kinase II [45,72,94] and analysis of the sequence revealed homology between each site: DEPPpSEGEAE in NFL versus AYEMPpSEEGYQ in αS (the sequence used to make the pSer129/81A antibody)[94]. Furthermore, the amino acid residues surrounding Ser473 in NFL are conserved across all species [30,49,50,88,100]. Therefore, we generated recombinant NFL with the Ser473Ala mutation and showed that this mutation ablated the reactivity of pSer129/81A with NFL incubated with casein kinase II (Fig. 6E). Collectively, these studies show that the ~70 kDa protein recognized by pSer129/81A antibody is NFL phosphorylated at Ser473.
Fig 6. The ~70 kDa protein recognized by pSer129/81A antibody is NFL phosphorylated at Ser473.
(A) Immunoblotting with pSer129/81A and anti-NFL antibody NR4 or Coomassie R250 stained 8% polylacrylamide gels of 2% SDS or 4M urea fractions from the cortical (CX) or cerebellar (CB) white matter of human brain. (B) pSer129/81A antibody immunoprecipitates NFL from human cortical white matter or mouse spinal cord/brain stem. Immunoprecipitation was performed as described in Material and Methods. Input, unbound, and immunoprecipitated fractions were analyzed by immunoblotting with pSer129/81A and anti-NFL antibody NR4. (C) In situ alkaline phosphatase treatment showing that the pSer129/81A-reactive ~70 kDa protein is a phospho-protein in both human and mouse brain. Nitrocellulose membranes with SDS fractions from human cortical white matter or murine SDS/urea spinal cord and brain stem were incubated without (−) or with (+) alkaline phosphatase (AP) as described in Material and Methods and immunoblotted with pSer129 or anti-NFL NR4 antibodies. (D) Immunoblotting of SDS/urea fractions from nTg/wild-type (WT) and NFL−/− mice with pSer129/81A or anti-NFL NR4 antibodies showing that the ~70 kDa protein band is NFL. (E) In vitro casein kinase II phosphorylation of recombinant wild-type (WT) and Ser473Ala NFL followed by immunoblotting with either anti-NFL antibody NR4 or pSer129/81A antibody demonstrating that pSer129/81A antibody recognized NFL phosphorylated at Ser473. GFAP, glial fibrillary acid protein; NFL, low molecular-mass neurofilament subunit; NFM, mid-sized molecular-mass NF subunit; NFH, heavy molecular-mass NF subunit.
Analysis of pSer129/81A detection of phosphorylated NFL in white matter tracts of nervous tissue
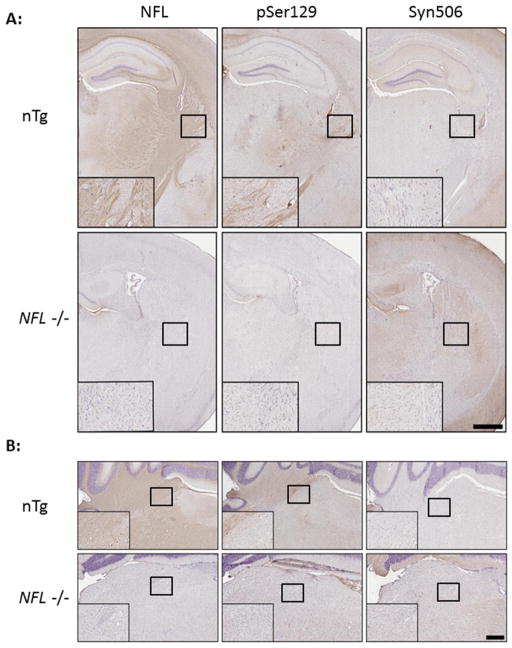
Since it has been previously suggested that αS pathology spreads from the site of intracerebral injection of fibrillar αS via white matter tracts based on pSer129/81A staining [67], we investigated the extent to which pSer129/81A detects phosphorylated NFL in the white matter tracts of nTg mice. In untreated nTg mice, IHC can readily detect NFL in the white matter tracts of the corpus callosum, hippocampus, striatum, brain stem, and cerebellum (Fig. 7). IHC staining with pSer129/81A shows a similar neuroanatomical staining pattern of white matter tracts as NFL antibody staining. Conversely, IHC with anti-αS antibody Syn506 does not stain white matter tracts since αS is normally a presynaptic protein [31,46,47](Fig. 7). Immunofluorescent analysis also demonstrated that pSer129/81A immunoreactivity co-localizes with phosphorylated NFL in the majority of white matter tracts and also in a subset of cell bodies (Supp. Fig. 10). For comparison, we stained nervous tissue from NFL−/− mice and show that the loss of the NFL staining pattern is accompanied by a similar loss of white matter pSer129/81A staining, but the pSer129/81A and NFL staining of white matter tracts are unaltered in SNCA−/− mice (Supp. Fig 10). This data indicates that pSer129/81A can readily detect phosphorylated NFL in white matter tracts and some cell bodies.
Fig 7. Immunocytochemical pSer129/81A staining of the white matter tracts in nTg/native mice is absent in NFL−/− mice.
(A) White matter tracts of the corpus callosum and internal capsule in nTg mice are detected by staining for NFL. Areas within those tracts are also detected with pSer129/81A, but not by αS marker Syn506, indicating that pSer129/81A is detecting a non-αS target in the tracts. In NFL−/− mice, there is no staining for NFL or pSer129/81A in these white matter tracts. (B) Similarly, white matter tracts in the cerebellum of nTg mice, which are detected by NFL, also stain with pSer129/81A and not Syn506. These are also unstained for NFL and pSer129/81A in NFL−/− mice. Tissue sections were counterstained with hematoxylin. Scale bar = 150 μm and 50 μm (inset) for A and B.
To further investigate if pSer129/81A could react with inclusions comprised of neurofilaments (NFs), we then stained the nervous tissue from NFH-LacZ Tg mice, which develop intracellular NF inclusions throughout the brain and brain stem [24,89]. We found that pSer129/81A strongly detects the Lewy body-like perikaryal NF inclusions and these inclusions do not contain αS, as they are not detected by the αS antibody SNL-4 (Supp. Fig 11). The NF inclusions in NFH-LacZ Tg mice were also not stained with more than 10 other αS antibodies [21,34].
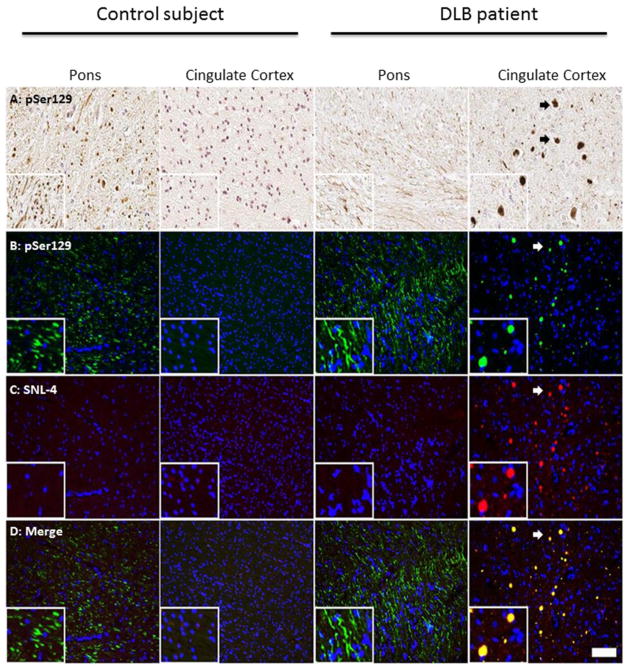
Expanding upon our findings in murine nervous tissue, we next tested the specificity of pSer129/81A staining in human nervous tissue and in murine primary mixed neuronal cultures. In nervous tissue from a control human subject, we found that pSer129/81A also stains phosphorylated NFL in the white matter tracts in the absence of accumulated endogenous αS (Supp. Fig. 12). Similar to staining in mouse CNS tissue, anti-pSer129/81A can robustly cross-react with the non-αS target in white matter tracts in human nervous system tissue that can be easy misinterpreted as αS pathology (Fig. 8). Having identified phosphorylated NFL as the major non-αS target recognized by antibody pSer129/81A, we performed double-label immunofluorescence analysis of the tissue sections from nTg mice that were cortically injected with αS and demonstrated robust pSer129/81A increased immunoreactivity that could not be detected with other non-phospho-specific αS antibodies. These studies revealed that the majority of the robust induced pSer129/81A immunoreactivity co-localized with NFL staining (Supp. Fig 13). These data indicates that most of this induced pSer129/81A immunoreactivity was likely due to hyperphosphorylation of NFL and/or accumulation of NFL.
Fig 8. pSer129/81A antibody can intensely detect white matter tracts that is not due to αS in human nervous tissue sections.
IHC (A) and immunofluorescence (B–D) analyses of fixed CNS tissue from a control subject or a dementia with LBs (DLB) patient stained with pSer129/81A and SNL-4. White matter tracts in the pons of the control subject and DLB patient were detected with pSer129/81A by both IHC (A) and IF (B) staining; however, this reactivity was not detectable when staining for αS with SNL-4 (C). pSer129/81A staining in the gray matter area of the cingulate cortex of a DLB patient depicting cortical LBs (indicated by arrows) detected by both IHC (A) and immunofluorescence analysis (B) that was also detectable with SNL-4 (C, D). Cell nuclei were counter stained with hematoxylin (A) and DAPI (B–D). Scale bar = 100 μm and 25 μm (insets).
We and others have used primary neuronal cultures as in vitro modeling systems for αS pathology [83,90]. We have previously shown in mixed neuronal cultures from untreated nTg and SNCA−/− mice that pSer129/81A antibody demonstrated robust cross-reactivity with an unknown non-αS target in the cell bodies and processes [83]. We show that in these cultures the pSer129/81A antibody also can almost completely co-localize with the NFL profile indicating that the majority of the pSer129 staining in native neuronal cultures is due to reactivity with Ser473 phosphorylated αS (Supp. Fig 14).
Discussion
Our studies demonstrate that direct intracerebral injection of fib αS can reproducibly and rapidly induce widespread αS inclusion pathology in M83 αS Tg mice but not in nTg or M47 αS Tg mice. Although these studies confirm the primary observation reported in several recent studies [66,67,69] that αS pathology can be induced by exogenous challenge with fibrillar forms of αS, they indicate that except in a line of mice that is most intrinsically vulnerable to αS pathology, induction of αS pathology in focal areas does not invariably lead to rapid widespread dissemination of the pathology within the brain. This observation is important as it suggests that there are biological barriers, which can regulate the rate and extent of αS pathology induction. These data may be viewed as controversial, but we believe that a reappraisal of previous data that relied heavily on pSer129/81A antibody and even other pSer129 antibody staining to track pathology induction is essential. We have now shown that pSer129/81A antibody recognizes pSer473 in NFL as a cross-reactive epitope. Indeed, our data suggests that the phospho-NFL cross-reactivity likely accounts for the majority of staining within white matter tracts and neuronal processes that has been used to support the hypothesis that αS pathology spreads in vivo via axonal connections [67]. Furthermore, we show that in nTg mice cortical injection of amyloidogenic (fibrillar) and non-amyloidogenic (Δ71–82) forms of αS can induce robust, but transient pSer129/81A staining that is predominantly due to NFL phosphorylation and not αS aggregation. Although it could be argued that the time frame (up to 4 months) for our studies was not sufficiently long to result in “prion-like” spread of αS pathology in nTg and M47 Tg mice, the 4 month time point is longer than most time points that were reportedly demonstrated to result in widespread αS pathology in nTg mice [66]. Furthermore, we find that while hfib αS induces some focal αS pathology at the injection site, the pathology actually diminishes over time rather than progressively spreads.
Though data from numerous laboratories, including our own, suggested that αS, like almost all amyloids, can behave like a prion in some experimental conditions [40,66–68,77,83,91,95], several findings in this study and previous studies suggest that it is premature to conclude that the spread of pathology observed in vivo is attributable, or at least solely attributable, to prion-like mechanisms. The observation that soluble Δ71–82 αS, which is non-amyloidogenic in vitro and in cell culture studies, can induces αS pathology in vivo, albeit less efficiently than fib αS [82], is challenging to reconcile with a prion-like only replication hypothesis. It is possible that additional factors in vivo can convert the soluble non amyloidogenic Δ71–82 αS into a form capable of conformationally templating endogenous αS. However, it is also possible that other mechanism such as the pro-inflammatory properties of α-synuclein, direct toxicity, or disrupted proteostasis could induce αS pathology [3,7,10,14,15,17,22,26,51,53–55,60,64,78,80,86,87,102]. In addition, our data and that of others, rather than being indicative of “spread” could be attributable to initial diffusion of the injected αS within the brain, which then induces pathology at sites distal to the site of injection. A gradient of injected αS away from the injection site, possibly along normal anatomical connections, could lead to the progressive appearance of αS pathology. In this scenario there could be progressive “seeding” but no true spread except for distribution of the exogenous seeds. Finally, we would like to point out that in no study in which an αS aggregate containing brain homogenate has been used to induce robust αS pathology in mice, has an αS depleted control homogenate been shown to not induce αS pathology. In a recent report by Recasens and colleagues, it was shown that the nigral injection of LB-enriched extracts from PD brains could result in progressive dopaminergic neuronal demise in mice and monkeys and this activity could be immune-depleted with αS antibodies, but only subtle accumulations of αS and not formation of authentic αS pathological inclusions was reported [77]. These findings are in fact in line with an alternative hypothesis that exogenous αS may lead to progression of disease due to neuronal toxicity as has been reported in many studies [15,17,22,51,64].
As noted above, our data support the concept that biological barriers exist that regulate the induction of αS pathology in vivo by exogenous αS. This extends observations made in various lines of unmanipulated αS Tg mice that clearly demonstrate a differential intrinsic susceptibility to inclusion formation and neurodegenerative phenotypes between the various models expressing human WT and PD-linked mutant forms of αS, despite similar levels of expression [23]. There are several non-exclusive hypotheses that could explain both the differential intrinsic susceptibility of these various αS Tg models and the differential vulnerability observed here following exogenous αS Tg challenge. One hypothesis would be that αS can assume different conformations and that these different conformers or “strains” may i) initially aggregate with different kinetics and ii) propagate through conformational templating with differing kinetics, cell-to-cell transmissibility, and mutability. αS conformers most efficient at conformational templating and transmissibility would thus be most likely to induce pathology both intrinsically and in seeding paradigms. There is growing evidence that ultrastructural differences in αS polymers, which can in some cases be attributed to PD-linked mutations in αS and could be referred to as conformers, may have distinct biological activities [8,11,36,39,43,73,83]. There is more limited evidence that distinct conformers might be associated with different levels of toxicity and capacity to induce inclusion formation in vivo. Indeed, it may be speculated that this may account for the heterogeneity of clinical disease associated with α-synucleinopathy in humans. Perhaps the most provocative findings in this regard are that homogenates from brains of humans with multiple systems atrophy are capable of seeding pathology in M83 Tg mice, but again this was only shown in this primed animals and no immune-depletion of brain extracts were reported [91]. Nevertheless, in humans there is no evidence that an α-synucleinopathy would begin through an amyloid seeded mechanism; it more likely begins through an intrinsic disruption of mechanisms that maintain normal proteostasis. In any case, it will be very challenging to dissociate subsequent prion like spreading from progressive disruptions of proteostasis in vivo, as the two may be inexorably linked. The second hypothesis is that the M83 Tg mice are uniquely primed to develop αS pathology, and that exogenous αS results in a stress signal that simply accelerated the development of the intrinsic phenotype. Indeed, exogenous stressors such as lipopolysaccharide can trigger αS pathology in the M83 Tg mouse line [28,29], and there is growing evidence that exogenous amyloidogenic proteins like αS, especially when aggregated, act as danger associated molecular patterns to activate innate immunity [38,81]. Alternatively, exogenous αS that is internalized by cells may also create a proteotoxic stress that leads to a more generalized disruption of proteostasis. Notably, strain like conformational templating in which distinct morphology of inclusions is induced by different mutant fibrils could be used in future studies to distinguish between some of these mechanisms. One would predict that if prion-like mechanisms predominate then, as we have observed in primary cell culture systems [83], the exogenous αS strains will dictate the conformation and morphology of the αS pathology.
The lengthy time course of these in vivo studies and the numerous experimental variables including but not limited to different conditions of αS protein purification, fibril preparation, and different inoculation paradigms, could clearly contribute to differential results obtained by various groups. We have taken great care in these studies to insure that the fibrillar and non-fibrillar αS we use in these studies are well characterized. For the fibrillar and soluble αS we insure they are capable of efficiently inducing or do not induce pathology, respectively, in our primary cell culture systems. As opposed to PrP derived prions, which once conformationally-templated, are highly immutable in terms of their properties, αS conformers like many other amyloids can be heterogeneous. We believe that in the future it will be essential for researchers to exchange αS preparations between laboratories to establish how much “strain-like” differences in αS conformers contribute to the differential results reported to date. One key observation in the prion field is that different strains of prions can require different incubation times; thus, at this time we cannot exclude that injection of exogenous αS in M47 and nTg may lead to more extensive delayed induction of αS inclusion pathology [2,69,82].
pSer129 immunostaining with antibody 81A and other similar antibodies not only has been used as a major method to assess αS pathology in recent mouse studies of “seeding”; it has also been used to assess αS pathology in human studies [6,42,44,59,66,67,69,71,84,91]. Anti-pSer129 reactivity is a sensitive marker of αS pathology, because αS is normally only marginally phosphorylated while it is hyperphosphorylated in inclusions [4,27,94]. However, we attribute the pSer129/81A immunoreactivity that we have observed in brain sections from control patients that mimics LB pathology to cross-reactivity with NFL. Indeed, this antibody can readily label the NF inclusions in NFH-LacZ Tg mice. Thus, the conclusions drawn from any study using this antibody as a sole marker of αS pathology must be viewed cautiously until the pathology is confirmed with other αS antibodies. Although other pSer129 antibodies are available commercially and some have been used to document peripheral αS inclusion pathology [6,42,44,59,71,84], it is likely that some preparations of rabbit polyclonal pSer129 can also react with NFL phosphorylated at Ser473 due to the homology between both epitopes, but each preparation of pSer129 αS antibodies will need to be specifically tested. We have tried to test the specificity of several pSer129 antibodies from several manufacturers and have discovered that some of the antibodies such as EP1536Y are either no longer available due to lack of specificity or are of low quality giving very low signal (i.e. not detecting αS) or reacting with non-phosphorylated αS. We have only specifically shown cross-reactivity with the pSer129/81A antibody, which is the most commonly used antibody for seminal reports [19,40,66,67,90]. We do not know if pSer129 antibody 1175 that has been used in one study to report delayed induction of αS pathology following cerebral injection of αS fibrils cross-reacts with phosphorylated NFL [69], but this antibody was not biochemically characterized for specificity, and Masuda-Suzukake and colleagues did not document αS inclusion formation with any other αS antibodies [69].
In conclusion, as we have hypothesized in a recent review, the “spread” of αS and other pathologies is likely to be complex and not mediated by a single mechanism [38]. “Prion-like” seeding, induction of a toxic environment, and intrinsic disruptions of proteostasis may all synergistically contribute to the induction and spread of αS inclusion pathology as well as the proteinopathies that underlie other neurodegenerative CNS diseases. Additional studies will be needed to more conclusively determine the relative contribution of these various mechanisms to induction and propagation of αS pathology. Resolving the mechanism(s) of disease progression is important for biological, experimental, medical, and social reasons, but it also has direct therapeutic implications. Indeed, if we can identify the barriers to induction and spread of αS, then we may be able to harness these barriers to therapeutically intervene in PD and other α-synucleinopathies.
Materials and Methods
Expression and purification of recombinant αS proteins
The pRK172 cDNA constructs expressing human αS with amino acid Δ71–82 deleted (Δ71–82), N-terminal truncated wild-type 21–140 human αS (with a Met codon added before amino acid 21), and full-length murine αS or full-length human αS with the A53T or E46K mutations were previously described [35,95,97,98]. Recombinant αS proteins were expressed in E. coli BL21 (DE3) and purified to homogeneity as previously described [35,39].
Fibril preparation of recombinant αS for mouse brain injection
21–140 human αS, murine αS, and A53T or E46K human αS proteins were assembled into filaments by incubation at 37°C at 5 mg/ml in sterile phosphate buffered saline (PBS, Invitrogen) with continuous shaking at 1050 rpm (Thermomixer R, Eppendorf, Westbury, NY) for 2 days, and then transferred to a new tube for testing and sonication. αS amyloid fibril assembly was monitored as previously described with K114 fluorometry [16,98]. αS fibrils were diluted to a concentration of 1–2 mg/mL in sterile PBS and treated by mild water bath sonication for 2 hours that fragments the typical elongate αS fibrils into smaller polymers and fibrils (see Supp. Fig 15). These fibrils were tested for robust induction of intracellular amyloid inclusion formation as previously described [83,95,96].
Mice husbandry and stereotactic injections
All procedures were performed according to the NIH Guide for the Care and Use of Experimental Animals and were approved by the University of Florida Institutional Animal Care and Use Committee or the Canadian Council on Animal Care and approved by the Faculty of Medicine Animal Care Committee at the University of Toronto, Canada. M47 and M83 Tg mice expressing human αS with the E46K mutation or the A53T mutation, respectively, were previously described [23,33]. SNCA−/− mice [1] were obtained from The Jackson Laboratory (Bar Harbor, MA). NFH-LacZ Tg mice that accumulate widespread brain NF inclusions were previously described [24,89]. NFL null mice [103] were a kind gift from Dr. Jean-Pierre Julien (Laval University, Canada) and maintained on a C57BL/6J background. nTg, SNCA−/−, and M83 and M47 Tg mice at 2 months of age were bilaterally stereotaxically injected with 2 μL of 1mg/mL αS proteins or PBS in the hippocampus (coordinates from Bregma: A/P −1.7, L +/−1.6, D/V −2.0) or the cortex (coordinates from Bregma: A/P −1.7, L +/−1.6, D/V −1.0) as indicated in the text (Table 1). The inoculum was injected at a rate of 0.2 μL per min (total volume of 2 μL per hemisphere) with the needle in place for 15 min at each site. Mice n≥4 for all cohorts, and without gender bias.
Antibodies
pSer129, also known as clone 81A, is a mouse monoclonal antibody that reacts with αS phosphorylated at Ser129 [94]. SNL-4 and SNL-1 are rabbit polyclonal antibodies raised against synthetic peptides corresponding to amino acids 2–12 and 104–119 of αS, respectively [34]. Syn506 is an anti-αS mouse monoclonal antibody that recognizes the N-terminus of αS [21,92]. Anti-p62 (SQSTM1; Proteintech; Chicago, IL) is a rabbit polyclonal antibody. Mouse anti-actin (clone C4) monoclonal antibody reacts with all forms of vertebrate actin (Millipore, Billerica, MA). NR4 is a mouse monoclonal antibody specific for NFL (Sigma-Aldrich). Chicken and rabbit polyclonal antibodies for NFL were generously provided by Dr. Gerry Shaw (Encor Biotechnology Inc.). Rabbit anti-NFL monoclonal antibody C28E10 was purchased from Cell Signaling Technology.
Immunohistochemical analysis
Mice were sacrificed with CO2 euthanization and perfused with PBS/heparin, followed by perfusion with 70% ethanol/150mM NaCl. The brain and spinal cord were then removed and fixed for at least 24 hours. Tissues were dehydrated and infiltrated with paraffin as previously described [20]. The tissues were cut into 7μm sections. Immunostaining of the sections was performed using previously described methods [20] using avidin-biotin complex (ABC) system (Vectastain ABC Elite Kit, Vector Laboratories, Burlingame, CA) and immunocomplexes were visualized with the chromogen 3,3′-diaminobenzidine. Sections were counterstained with hematoxylin. All slides were scanned using an Aperio ScanScope CS (40× magnification; Aperio Technologies Inc., Vista, CA) and images of representative areas of αS pathology were taken using the ImageScopeTM software (40× magnification; Aperio Technologies Inc.).
Double-labeling immunofluorescence analysis of mouse brain tissue
Paraffin-embedded tissue sections were deparaffinized and hydrated through a series of graded ethanol solutions followed by 0.1M Tris, pH 7.6. The sections were blocked with 5% dry milk/0.1M Tris, pH 7.6 and were incubated simultaneously with combinations of primary antibodies diluted in 5% dry milk/0.1M Tris, pH 7.6. After extensive washing, sections were incubated with secondary antibodies conjugated to Alexa 594 or Alexa 488 (Invitrogen, Eugene, OR). Sections were post-fixed with formalin, incubated with Sudan Black, and stained with 4′,6-diamidino-2-phenylindole (DAPI)(Invitrogen, Eugene, OR). The sections were coverslipped with Fluoromount-G (SouthernBiotech, Birmingham, AL) and visualized using an Olympus BX51 microscope mounted with a DP71 Olympus digital camera to capture images.
Biochemical fractionation and immunoblotting analysis of mouse brain tissue
Cortex and brainstem/spinal cord from 4 month-old nTg, SNCA−/− and NFL−/− mice were biochemically fractionated as previously described [33]. Briefly, tissue samples were dissected, weighed, and homogenized in 3mL/g of high-salt (HS) buffer (50mM Tris, pH 7.5, 750mM NaCl, 20mM NaF, 5mM EDTA) with protease inhibitors and sedimented at 100,000 x g for 20 min. Pellets were re-extracted in HS buffer, followed by subsequent extractions in HS/1% Triton, HS/1M sucrose, RIPA buffer (50mM Tris, pH 8.0, 150mM NaCl, 5mM EDTA, 1% NP40, 0.5% sodium deoxycholate, and 0.1% SDS with the protease inhibitors), and 2%SDS/4M urea. SDS/urea fractions were then sonicated. 2% SDS was added to all the fractions, which were heated to 100°C for 10 minutes, except for those fractions containing urea. For some experiments the RIPA homogenate was separated in half and the subsequent pellets were re-suspended in 4M urea or 2% SDS by sonication. Protein concentrations were quantified using the bicinchoninic acid (BCA) assay and bovine serum albumin as a standard (Pierce Biotechnology, Rockford, IL).
SDS-PAGE and immunoblotting analysis
Protein was resolved by SDS-PAGE on 7% (w/v), 8% or 13% polyacrylamide gels, as indicated, followed by electrophoretic transfer onto nitrocellulose membranes. Some gels were directly stained with Coomassie R-250 or transferred by electrophoresis onto nitrocellulose membranes. For Western blotting, membranes were blocked in Tris buffered saline (TBS) with 5% dry milk, and incubated with primary antibodies in TBS/5% dry milk or TBS/5% BSA for pSer129/81A overnight. Following washes, membranes were incubated with a goat anti-mouse antibody conjugated to horseradish peroxidase (HRP) or a goat anti-rabbit antibody conjugated to HRP. Protein bands were detected using chemiluminescent reagent (NEN, Boston, MA) and a FluorChem E and M Imager (Proteinsimple, San Jose, California).
In situ nitrocellulose protein dephosphorylation
For dephosphorylation of protein directly on the membrane, following transfer from SDS-polyacrylamide gel, the membrane was blocked with 5% BSA in TBS overnight. The membrane was then rinsed in phosphatase buffer (50mM Tris, pH 9.2, 0.5mM MgCl2) and incubated in phosphatase buffer with or without 250U/mL bovine intestinal mucosa alkaline phosphatase (Sigma-Aldrich) overnight. The membranes were rinsed with TBS and blocked for 1 hour with 5% skimmed milk in TBS and processed for immunoblotting as described above.
Immunoprecipitation
pSer129/81A antibody was pre-absorbed with protein A/G agarose beads (Santa Cruz Biotechnology, Inc) and exchanged in CSK buffer (50mM Tris, 150mM NaCl, pH 7.4, 20mM NaF, 1mM EDTA, 1% Triton X-100). The SDS fraction from human cortical white matter was diluted in 20 volumes of CSK buffer with protease inhibitors and incubated with the pSer129/81A-protein A/G agarose beads overnight at 4°C with rotation. After extensive washing with CSK buffer, the immuno-precipitated complex was eluted with SDS sample buffer and heating to 100°C for 5 minutes.
Expression, purification, and phosphorylation of murine NFL from bacteria
Recombinant NFL was expressed in Escherichia coli BL21 (DE3) using mouse NFL cDNA cloned into the pET-23d expression vector (Novagen, Inc. Madison, WI). The same construct with the Ser473Ala mutation was generated using the QuickChange Site Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and specific oligonucleotides.
Following transformation, bacteria were grown to an OD600 of 0.6 and the expression of the recombinant protein was induced with 1mM isopropyl-β-D-thiogalactopyranoside for 2 hours. NFL was purified using inclusion bodies procedure. Cells were pelleted, re-suspended into lysis buffer (25% sucrose, 50mM Tris, pH 8.0, 1mM EDTA, 2mg/mL lysozyme, and a cocktail of protease inhibitors) and incubated on ice for 30 minutes. Ten mM MgCl2, 10μg/mL DNase1 and 1μg/mL RNaseA were added to the homogenate, which was incubated on ice for another 30 minutes. Ten mL of detergent buffer (0.2M NaCl, 1% deoxycholic acid, 1% IGEPAL CA630, 20mM Tris, pH 8.0, 2mM EDTA) per mL of lysis buffer were added and, after vigorous mixing, the insoluble material was sedimented at 5,000 × g for 30 minutes. The supernatant was discarded and the pellet was repeatedly washed with 1mM EDTA/0.5% Triton to generate a highly compact pellet, which was solubilized in 8M urea, 10mM Tris, pH 8.0, 1mM EDTA. Protein concentration was determined using Bradford assay (Thermo-Fisher) and bovine serum albumin as the standard.
Recombinant NFL (1mg/mL) was dialyzed overnight in 20mM Tris pH 7.5, 50mM KCl. Kinase reaction was performed by adding 10mM MgCl2, 200μM ATP and 10000U casein kinase II/mL (control reaction had no kinase added). Reactions were incubated at 30°C for 1 hour. Sample buffer was added to the reactions and incubated at 100°C for 10 minutes.
Mixed neuronal-glial primary cultures
Primary cultures were prepared from P0 C3HBL/6 mouse brains (Harlan Labs). Cerebral cortices were dissected from P0 mouse brains and dissociated in 2mg/mL papain (Worthington) and 50 μg/mL DNAase I (Sigma) in sterile Hank’s Balanced Salt Solution (HBSS, Life Technologies) at 37°C for 20 minutes. They were then washed three times in sterile HBSS to inactivate the papain and switched to 5% fetal bovine serum (HyClone) in Neurobasal-A growth media (Gibco), which includes 0.5mM L-glutamine (Gibco), 0.5mM GlutaMax (Life Technologies), 0.01% antibiotic-antimycotic (Gibco), and 0.02% SM1 supplement (Stemcell). The tissue mixture was then triturated three times using a 5 mL pipette followed by a Pasteur pipette, and strained through a 70 μm cell strainer. The cell mixture was then centrifuged at 200 × g for 3 minutes, and re-suspended in fresh Neurobasal-A media. They were then plated onto poly-D lysine coated chamber slides (Life Technologies) or dishes at around 100,000–200,000 cells/cm2. Cells were maintained in the Neurobasal-A growth media without fetal bovine serum at 37°C in a humidified 5% CO2 chamber.
Negative Staining Electron Microscopy
αS filaments were absorbed to 300 mesh carbon coated copper grids and stained with 1% uranyl acetate as described previously [36]. Images were captured with a JEOL 1010 transmission electron microscope (Peabody, MA) mounted with a Hamamatsu digital camera (Bridgewater, MA) using AMT software (Danvers, MA).
Supplementary Material
Acknowledgments
This work was supported by the Ellison Medical Foundation Senior Scholar Award to TEG and funding from the University of Florida. We thank the University of Florida NeuroMedicine Human Tissue Brain Bank, which is supported by a gift from Eve and Joe Wilder, for the patient brain tissues. JR holds a Tier 2 Canada Research Chair and is funded by the Canadian Institutes of Health Research.
Footnotes
Author Contribution
ANS designed the study, performed the injections, analyzed the data, and drafted the manuscript. MB collected the samples and analyzed the data. MAT and ABM performed the injections and analyzed the data. NHM, NLR, and CCD analyzed the data. JR participated in the design of the study. TEG and BIG participated in the design and coordination of the study, analyzed the data, and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25 (1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 2.Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8 (7):552–561. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011;69 (4):337–342. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281 (40):29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 5.Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, Aasly JO, Rajput A, Rajput AH, Jon SA, Farrer MJ. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov Disord. 2013;28:811–813. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 6.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG Arizona Parkinson’s Disease C. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119 (6):689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beraud D, Twomey M, Bloom B, Mittereder A, Ton V, Neitzke K, Chasovskikh S, Mhyre TR, Maguire-Zeiss KA. alpha-Synuclein Alters Toll-Like Receptor Expression. Front Neurosci. 2011;5:80. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K, Olieric V, Bockmann A, Meier BH, Melki R. Structural and functional characterization of two alpha-synuclein strains. Nature Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: the enigma of Parkinson’s disease pathology spread. Nat Rev Neurosci. 2008;9 (10):741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- 10.Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, de BM. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS One. 2013;8 (1):e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comellas G, Lemkau LR, Nieuwkoop AJ, Kloepper KD, Ladror DT, Ebisu R, Woods WS, Lipton AS, George JM, Rienstra CM. Structured regions of alpha-synuclein fibrils include the early-onset Parkinson’s disease mutation sites. J Mol Biol. 2011;411 (4):881–895. doi: 10.1016/j.jmb.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4 (11):1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 13.Cookson MR. The biochemistry of Parkinson’s disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 14.Couch Y, Alvarez-Erviti L, Sibson NR, Wood MJ, Anthony DC. The acute inflammatory response to intranigral alpha-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. J Neuroinflammation. 2011;8:166. doi: 10.1186/1742-2094-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, Bertoncini CW, Wood NW, Knowles TP, Dobson CM, Klenerman D. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell. 2012;149 (5):1048–1059. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crystal AS, Giasson BI, Crowe A, Kung MP, Zhuang ZP, Trojanowski JQ, Lee VM. A comparison of amyloid fibrillogenesis using the novel fluorescent compound K114. J Neurochem. 2003;86 (6):1359–1368. doi: 10.1046/j.1471-4159.2003.01949.x. [DOI] [PubMed] [Google Scholar]
- 17.Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27 (34):9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273 (16):9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 19.Dryanovski DI, Guzman JN, Xie Z, Galteri DJ, Volpicelli-Daley LA, Lee VM, Miller RJ, Schumacker PT, Surmeier DJ. Calcium entry and alpha-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J Neurosci. 2013;33 (24):10154–10164. doi: 10.1523/JNEUROSCI.5311-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duda JE, Giasson BI, Gur TL, Montine TJ, Robertson D, Biaggioni I, Hurtig HI, Stern MB, Gollomp SM, Grossman M, Lee VMY, Trojanowski JQ. Immunohistochemical and biochemical studies demonstrate a distinct profile of alpha-synuclein permutations in multiple system atrophy. J Neuropathol Exp Neurol. 2000;59 (9):830–841. doi: 10.1093/jnen/59.9.830. [DOI] [PubMed] [Google Scholar]
- 21.Duda JE, Giasson BI, Mabon ME, Lee VMY, Trojanoswki JQ. Novel antibodies to oxidized α-synuclein reveal abundant neuritic pathology in Lewy body disease. Ann Neurol. 2002;52:205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 22.El-Agnaf OM, Jakes R, Curran MD, Middleton D, Ingenito R, Bianchi E, Pessi A, Neill D, Wallace A. Aggregates from mutant and wild-type alpha-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of beta-sheet and amyloid-like filaments. FEBS letters. 1998;440 (1–2):71–75. doi: 10.1016/s0014-5793(98)01418-5. [DOI] [PubMed] [Google Scholar]
- 23.Emmer KL, Waxman EA, Covy JP, Giasson BI. E46K human alpha-synuclein transgenic mice develop Lewy-like and tau pathology associated with age-dependent, detrimental motor impairment. J Biol Chem. 2011;286 (40):35104–35118. doi: 10.1074/jbc.M111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eyer J, Peterson A. Neurofilament-deficient axons and perikaryal aggregates in viable transgenic mice expressing a neurofilament-beta-galactosidase fusion protein. Neuron. 1994;12 (2):389–405. doi: 10.1016/0896-6273(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 25.Farrer M, Gwinn-Hardy K, Hutton M, Hardy J. The genetics of disorders with synuclein pathology and parkinsonism. Hum Mol Genet. 1999;8 (10):1901–1905. doi: 10.1093/hmg/8.10.1901. [DOI] [PubMed] [Google Scholar]
- 26.Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2013;61 (3):349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. α-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 28.Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28 (30):7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS. Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect. 2011;119 (6):807–814. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geisler N, Plessmann U, Weber K. The complete amino acid sequence of the major mammalian neurofilament protein (NF-L) FEBS letters. 1985;182 (2):475–478. doi: 10.1016/0014-5793(85)80357-4. [DOI] [PubMed] [Google Scholar]
- 31.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15 (2):361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 32.Giasson BI, Duda JE, Forman MS, Lee VMY, Trojanoswki JQ. Prominent perikaryal expression of α- and β-synuclein in neurons of dorsal root ganglion and in medullary neurons. Exp Neurol. 2001;172:354–362. doi: 10.1006/exnr.2001.7805. [DOI] [PubMed] [Google Scholar]
- 33.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34 (4):521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 34.Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, Lee VMY. A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson’s disease. J Neurosci Res. 2000;59 (4):528–533. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Giasson BI, Murray IV, Trojanowski JQ, Lee VMY. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 36.Giasson BI, Uryu K, Trojanowski JQ, Lee VMY. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274 (12):7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 37.Goedert M. Familial Parkinson’s disease. The awakening of alpha-synuclein. Nature. 1997;388 (6639):232–233. doi: 10.1038/40767. [DOI] [PubMed] [Google Scholar]
- 38.Golde TE, Borchelt DR, Giasson BI, Lewis J. Thinking laterally about neurodegenerative proteinopathies. J Clin Invest. 2013;123:1847–1855. doi: 10.1172/JCI66029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha-synuclein increases amyloid fibril formation. JBiolChem. 2005;280 (9):7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 40.Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B, Riddle DM, Kwong LK, Xu Y, Trojanowski JQ, Lee VM. Distinct alpha-Synuclein Strains Differentially Promote Tau Inclusions in Neurons. Cell. 2013;154 (1):103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121 (2):715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, Broughton E, Hagan H, Carroll C. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2013 doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- 43.Hoyer W, Antony T, Cherny D, Heim G, Jovin TM, Subramaniam V. Dependence of alpha-synuclein aggregate morphology on solution conditions. J Mol Biol. 2002;322 (2):383–393. doi: 10.1016/s0022-2836(02)00775-1. [DOI] [PubMed] [Google Scholar]
- 44.Ikemura M, Saito Y, Sengoku R, Sakiyama Y, Hatsuta H, Kanemaru K, Sawabe M, Arai T, Ito G, Iwatsubo T, Fukayama M, Murayama S. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol. 2008;67 (10):945–953. doi: 10.1097/NEN.0b013e318186de48. [DOI] [PubMed] [Google Scholar]
- 45.Ishii A, Nonaka T, Taniguchi S, Saito T, Arai T, Mann D, Iwatsubo T, Hisanaga S, Goedert M, Hasegawa M. Casein kinase 2 is the major enzyme in brain that phosphorylates Ser129 of human alpha-synuclein: Implication for alpha-synucleinopathies. FEBS letters. 2007;581 (24):4711–4717. doi: 10.1016/j.febslet.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 46.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14 (2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 47.Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345 (1):27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 48.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501 (7465):45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Julien JP, Grosveld F, Yazdanbaksh K, Flavell D, Meijer D, Mushynski W. The structure of a human neurofilament gene (NF-L): a unique exon-intron organization in the intermediate filament gene family. Biochim Biophys Acta. 1987;909 (1):10–20. doi: 10.1016/0167-4781(87)90041-8. [DOI] [PubMed] [Google Scholar]
- 50.Julien JPMD, Flavel D, Hurst J, Grosveld F. Cloning and developmental expression of the murine neurofilament gene family. Mol Brain Res. 1986;1:243–250. doi: 10.1016/0169-328x(86)90030-6. [DOI] [PubMed] [Google Scholar]
- 51.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300 (5618):486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 52.Kiely AP, Asi YT, Kara E, Limousin P, Ling H, Lewis P, Proukakis C, Quinn N, Lees AJ, Hardy J, Revesz T, Houlden H, Holton JL. alpha-Synucleinopathy associated with G51D SNCA mutation: a link between Parkinson’s disease and multiple system atrophy? Acta Neuropathol. 2013;125:753–769. doi: 10.1007/s00401-013-1096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong LS, Masliah E, Hwang D, Lee HJ, Lee SJ. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, McGeer PL. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006;20 (12):2000–2008. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- 55.Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29 (5):739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14 (5):504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 57.Kruger R, Muller T, Riess O. Involvement of alpha-synuclein in Parkinson’s disease and other neurodegenerative disorders. J Neural Transm. 2000;107 (1):31–40. doi: 10.1007/s007020050002. [DOI] [PubMed] [Google Scholar]
- 58.Kuusisto E, Parkkinen L, Alafuzoff I. Morphogenesis of Lewy bodies: dissimilar incorporation of alpha-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol. 2003;62 (12):1241–1253. doi: 10.1093/jnen/62.12.1241. [DOI] [PubMed] [Google Scholar]
- 59.Lebouvier T, Chaumette T, Damier P, Coron E, Touchefeu Y, Vrignaud S, Naveilhan P, Galmiche JP, Bruley des Varannes S, Derkinderen P, Neunlist M. Pathological lesions in colonic biopsies during Parkinson’s disease. Gut. 2008;57 (12):1741–1743. doi: 10.1136/gut.2008.162503. [DOI] [PubMed] [Google Scholar]
- 60.Lee SB, Park SM, Ahn KJ, Chung KC, Paik SR, Kim J. Identification of the amino acid sequence motif of alpha-synuclein responsible for macrophage activation. Biochem Biophys Res Commun. 2009;381 (1):39–43. doi: 10.1016/j.bbrc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Lesage S, Anheim M, Letournel F, Bousset L, Honore A, Rozas N, Pieri L, Madiona K, Durr A, Melki R, Verny C, Brice A. G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. AnnNeurol. 2013 doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 62.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14 (5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 63.Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, Brundin P. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord. 2010;25 (8):1091–1096. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- 64.Liu CW, Giasson BI, Lewis KA, Lee VM, Demartino GN, Thomas PJ. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J Biol Chem. 2005;280 (24):22670–22678. doi: 10.1074/jbc.M501508200. [DOI] [PubMed] [Google Scholar]
- 65.Lowe J, Blanchard A, Morrell K, Lennox G, Reynolds L, Billett M, Landon M, Mayer RJ. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson’s disease, Pick’s disease, and Alzheimer’s disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and mallory bodies in alcoholic liver disease. J Pathol. 1988;155 (1):9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- 66.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338 (6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012;209 (5):975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl AcadSci USA. 2009;106 (47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136 (Pt 4):1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mougenot AL, Nicot S, Bencsik A, Morignat E, Verchere J, Lakhdar L, Legastelois S, Baron T. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 2012;33 (9):2225–2228. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 71.Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, Shill HA, Caviness JN, Samanta JE, Beach TG Arizona Parkinson’s Disease C. Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2013;72 (2):119–129. doi: 10.1097/NEN.0b013e3182801cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamura Y, Hashimoto R, Kashiwagi Y, Wada Y, Sakoda S, Miyamae Y, Kudo T, Takeda M. Casein kinase II is responsible for phosphorylation of NF-L at Ser-473. FEBS letters. 1999;455 (1–2):83–86. doi: 10.1016/s0014-5793(99)00832-7. [DOI] [PubMed] [Google Scholar]
- 73.Ono K, Ikeda T, Takasaki J, Yamada M. Familial Parkinson disease mutations influence alpha-synuclein assembly. Neurobiol Dis. 2011;43 (3):715–724. doi: 10.1016/j.nbd.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 74.Polymenidou M, Cleveland DW. Prion-like spread of protein aggregates in neurodegeneration. J Exp Med. 2012;209 (5):889–893. doi: 10.1084/jem.20120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276 (5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 76.Proukakis C, Dudzik CG, Brier T, Mackay DS, Cooper JM, Millhauser GL, Houlden H, Schapira AH. A novel alpha-synuclein missense mutation in Parkinson disease. Neurology. 2013;80 (11):1062–1064. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez A, Fernagut PO, Blesa J, Parent A, Perier C, Farinas I, Obeso JA, Bezard E, Vila M. Lewy body extracts from parkinson’s disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 2013 doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 78.Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, Ciborowski P, Cerny R, Gelman B, Thomas MP, Mosley RL, Gendelman HE. Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. J Neurochem. 2008;104 (6):1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- 79.Rochet JC, Conway KA, Lansbury PT. Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse alpha-synuclein. Biochemistry. 2000;39 (35):10619–10626. doi: 10.1021/bi001315u. [DOI] [PubMed] [Google Scholar]
- 80.Roodveldt C, Christodoulou J, Dobson CM. Immunological features of alpha-synuclein in Parkinson’s disease. J Cell Mol Med. 2008;12 (5B):1820–1829. doi: 10.1111/j.1582-4934.2008.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28 (10):429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Sacino AN, Brooks M, McGarvey NH, McKinney AB, Thomas MA, Levites Y, Ran Y, Golde TE, Giasson BI. Induction of CNS alpha-synuclein pathology by fibrillar and non-amyloidogenic recombinant alpha-synuclein. Acta Neuropathol Commun. 2013;1 (1):38. doi: 10.1186/2051-5960-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sacino AN, Thomas MA, Ceballos-Diaz C, Cruz PE, Rosario AM, Lewis J, Giasson BI, Golde TE. Conformational templating of alpha-synuclein aggregates in neuronal-glial cultures. Mol Neurodegen. 2013;8:17. doi: 10.1186/1750-1326-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sengoku R, Saito Y, Ikemura M, Hatsuta H, Sakiyama Y, Kanemaru K, Arai T, Sawabe M, Tanaka N, Mochizuki H, Inoue K, Murayama S. Incidence and extent of Lewy body-related alpha-synucleinopathy in aging human olfactory bulb. J Neuropathol Exp Neurol. 2008;67 (11):1072–1083. doi: 10.1097/NEN.0b013e31818b4126. [DOI] [PubMed] [Google Scholar]
- 85.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302 (5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 86.Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29 (11):1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37 (3):510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trimpin S, Mixon AE, Stapels MD, Kim MY, Spencer PS, Deinzer ML. Identification of endogenous phosphorylation sites of bovine medium and low molecular weight neurofilament proteins by tandem mass spectrometry. Biochemistry. 2004;43 (7):2091–2105. doi: 10.1021/bi030196q. [DOI] [PubMed] [Google Scholar]
- 89.Tu PH, Robinson KA, de Snoo F, Eyer J, Peterson A, Lee VM, Trojanowski JQ. Selective degeneration fo Purkinje cells with Lewy body-like inclusions in aged NFHLACZ transgenic mice. J Neurosci. 1997;17 (3):1064–1074. doi: 10.1523/JNEUROSCI.17-03-01064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72 (1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, Dearmond SJ, Prusiner SB. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl AcadSci USA. 2013;110 (48):19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waxman EA, Duda JE, Giasson BI. Characterization of antibodies that selectively detect alpha-synuclein in pathological inclusions. Acta Neuropathol. 2008;116 (1):37–46. doi: 10.1007/s00401-008-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Waxman EA, Giasson BI. Molecular mechanisms of alpha-synuclein neurodegeneration. Biochim Biophys Acta. 2008;1792:616–624. doi: 10.1016/j.bbadis.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waxman EA, Giasson BI. Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. J Neuropathol Exp Neurol. 2008;67 (5):402–416. doi: 10.1097/NEN.0b013e3186fc995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waxman EA, Giasson BI. A novel, high-efficiency cellular model of fibrillar alpha-synuclein inclusions and the examination of mutations that inhibit amyloid formation. J Neurochem. 2010;113 (2):374–388. doi: 10.1111/j.1471-4159.2010.06592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Waxman EA, Giasson BI. Characterization of kinases involved in the phosphorylation of aggregated alpha-synuclein. J Neurosci Res. 2011;89 (2):231–247. doi: 10.1002/jnr.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Waxman EA, Giasson BI. Induction of intracellular tau aggregation is promoted by alpha-synuclein seeds and provides novel insights into the hyperphosphorylation of tau. J Neurosci. 2011;31 (21):7604–7618. doi: 10.1523/JNEUROSCI.0297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Waxman EA, Mazzulli JR, Giasson BI. Characterization of hydrophobic residue requirements for alpha-synuclein fibrillization. Biochemistry. 2009;48 (40):9427–9436. doi: 10.1021/bi900539p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35 (43):13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 100.Xu ZS, Liu WS, Willard M. Identification of serine 473 as a major phosphorylation site in the neurofilament polypeptide NF-L. J Neurosci. 1990;10(6):1838–1846. doi: 10.1523/JNEUROSCI.10-06-01838.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez TE, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55 (2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 102.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19 (6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 103.Zhu Q, Couillard-Despres S, Julien JP. Delayed maturation of regenerating myelinated axons in mice lacking neurofilaments. Exp Neurol. 1997;148 (1):299–316. doi: 10.1006/exnr.1997.6654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.