Abstract
Background
Previous studies have implicated the important and active role of vitamin D in the immune system.
Objectives
The aim of this study was to evaluate serum levels of 25-hydroxyvitamin D in children with burn injuries.
Materials and Methods
In this cross-sectional study, 118 patients with various degrees of burn injuries were enrolled. A checklist consisting of demographic data, total body surface area (TBSA) affected by burn, degree of burn, serum level of 25(OH)D, total protein, albumin, electrolytes, and parathyroid hormone was recorded for each patient.
Results
Sixty-eight (57.6%) males and 50 (42.4%) females with a mean age of 4.04 years (SD = 3.04) were evaluated. The mean level of 25(OH)D was 14.58 ng/mL (SD = 6.94). Levels of 25(OH)D in four (3.39%) cases were higher than 30 ng/mL, while 95 (81.35%) cases had levels lower than 20 ng/mL, and 19 (16.10%) cases had levels of 21 - 30 ng/mL. The level of 25(OH)D was below recommended levels in 96.61% of cases, while 81.34% had vitamin D deficiency and 16.1% had insufficiency. We found a significant correlation between vitamin 25(OH)D and total protein, albumin, and total and ionized calcium (P < 0.001). There was also a significant negative correlation between 25(OH)D and TBSA affected by burn (P = 0.001).
Conclusions
The levels of 25(OH)D in children suffering from severe burns were low. Supplementation might be useful in patients with very low levels of serum vitamin D.
Keywords: Pediatric, Burn, Serum 25-Hydroxyvitamin D
1. Background
Burns and their adverse effects are major causes of mortality and morbidity around the world (1). Burn injuries affect 30% - 40% of children and adolescents, and are more prevalent in these age groups. Approximately 85% of burns are thermal (most commonly from hot water). Thermal burns are the most common type of burn injury in children under 4 years of age and are often associated with destructive consequences. Such injuries are largely preventable, and the implementation of preventive and safety protocols would prevent the occurrence of most of these events (2, 3).
Vitamin D, a fat-soluble vitamin, is an essential element in establishment of bone structures through the metabolism of calcium and phosphorus. Vitamin D receptors have been found in many body tissues, and vitamin D takes part in the regulation of immune system cell proliferation and differentiation (4). Vitamin D deficiency results in weakening of the immune system and may be involved in the pathogenesis of certain types of cancer, as well as in diabetes and infections. Recent studies have indicated that several non-skeletal diseases are also associated with vitamin D deficiency (4, 5).
Previous studies have evaluated the serum levels of 25-hydroxyvitamin D (25(OH)D) in children and adults with burn injuries (6-8). However, there are some controversial issues regarding the measurement of vitamin D metabolites in these patients. For instance, there are questions regarding which metabolite should be measured, and whether it is beneficial to begin the administration of vitamin D supplementation after burn injuries.
2. Objectives
This study aimed to measure serum levels of 25(OH)D in burned children, and to discuss the controversial issues regarding the administration of supplemental vitamin D in these patients.
3. Materials and Methods
This cross-sectional study was carried out in the pediatric ward of a burn hospital (Shahid Motahari burn hospital) in Tehran, Iran, during 2012 - 2014. One hundred eighteen burned patients were enrolled in this study after informed consent was obtained from their parents. Blood samples were sent to the laboratory during the first week after the acute burn injury. The testing kits used were from Roche (Germany), and the unit of measurement was ng/mL (9). Serum 25(OH)D, total protein, albumin, phosphorus, total calcium, ionized calcium, and PTH were measured. A checklist consisting of age, sex, degree of burn, and total body surface area (TBSA) affected by burn was also completed for each patient.
Frequency, mean, and standard deviation (SD) were calculated for numeric variables. The chi-square test was used for comparing qualitative variables. Student’s t-test was used for comparing the means of vitamin D levels in the different groups. All statistical analyses were performed using PASW 16.0 (SPSS Inc. Chicago, IL, USA).
This study was approved by the ethics committee of Iran University of Medical Sciences and was therefore performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki and its later amendments.
4. Results
One hundred eighteen burned children with a mean age of 4.04 years (SD = 3.04) were evaluated in this study, including 68 (57.6%) males and 50 (42.4%) females (Figure 1). The demographic data are shown in Table 1, and the laboratory data are shown in Table 2. There were no significant differences between these factors in boys versus girls (P > 0.05). The frequencies of patients in accordance with burn degree and mean 25(OH)D levels are depicted in Table 3.
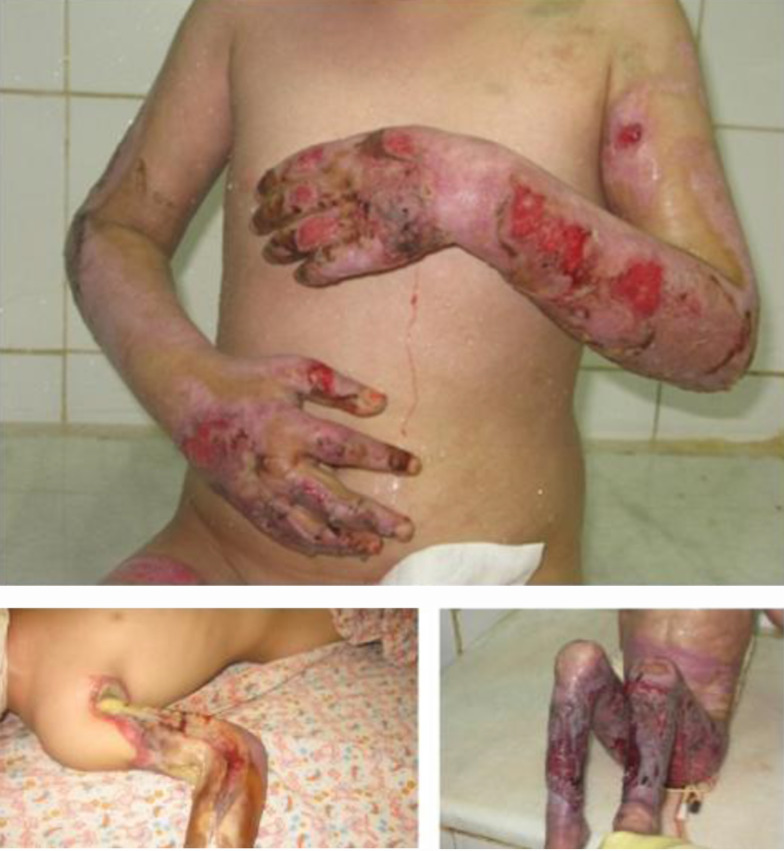
Figure 1. Skin Damage in Burn Patients is the Major Risk Factor for Losing Vitamin D.
Table 1. Demographics and Burn Classifications.
| Variables | Values |
|---|---|
| Gender a | |
| Female | 42.2 |
| Male | 57.6 |
| Ethnicity a | |
| White b | 82.3 |
| Non-white c | 17.7 |
| Classification of burn a | |
| Scald | 80 |
| Flame | 10 |
| Contact | 10 |
| Age, y d | 4.04 ± 3.04 |
a Values unit is %.
b White = Caucasian.
c Non-white = brunette.
d Data are presented as mean ± SD.
Table 2. Laboratory Data.
| Gender a | Data b |
|---|---|
| Male | |
| Phosphorus | 3.37 ± 0.71 |
| Alb | 3.33 ± 0.69 |
| Total Ca | 7.65 ± 0.59 |
| Ionized Ca | 1.29 ± 0.06 |
| PTH | 11.09 ± 5.75 |
| Vit D | 15.37 ± 7.07 |
| Female | |
| Phosphorus | 3.20 ± 0.56 |
| Alb | 3.23 ± 0.61 |
| Total Ca | 7.63 ± 0.46 |
| Ionized Ca | 1.29 ± 0.07 |
| PTH | 10.93 ± 7.60 |
| Vit D | 13.50 ± 6.73 |
a There was no statistically significant difference based on gender (P = 0.6).
b Data are presented as mean ± SD.
Table 3. Frequency of Patients for Burn Degree and Mean Vitamin 25(OH)D Level.
| Burn Degree | Number of patients | Vitamin D level, ng/mL a |
|---|---|---|
| IIA+IIB | 45 | 15.46 ± 5.95 |
| IIB+III-IV | 29 | 12.76 ± 8.06 |
| IIA | 2 | 21.25 ± 10.53 |
| IIB | 27 | 16.19 ± 6.47 |
| III-IV | 14 | 11.51 ± 7.19 |
| I+IIA+IIB | 1 | 13.50 ± 0 |
a Data are presented as mean ± SD.
The lowest 25(OH)D level was 1.1 ng/mL and the highest was 36.9 ng/mL. Serum levels of 25(OH)D were < 13.9 ng/mL in half of the patients (n = 59) and < 18.2 ng/mL in 75% of the patients (n = 89). Only four (3.39%) patients had 25(OH)D levels of > 30 ng/mL. In addition, the serum level of 25(OH)D was < 20 ng/mL in 96 (81.35%) patients, indicating vitamin D deficiency. The 25(OH)D levels were 21 - 30 ng/mL (insufficient) in 19 (10.16%) patients.
The mean level of 25(OH)D was 15.37 ng/mL (SD = 7.07) in males and 13.50 ng/mL (SD = 6.73) in females, a difference that was not statistically significant (P = 0.14).
According to the TBSA involved in the burn injury, the patients were divided into four groups: A: 1% - 30% of TBSA, B: 31% - 40% of TBSA, C: 41% - 50% of TBSA, and D: > 50% of TBSA. Fifty-nine (50%), 34 (28.8%), 17 (14.4%), and 8 (6.8%) patients were in groups A, B, C, and D, respectively.
The mean levels of 25(OH)D were 16.49 ng/mL (SD = 7.49), 13.91 ng/mL (SD = 5.61), 10.53 ng/mL (SD = 4.63), and 11.92 ng/mL (SD = 8.20) in groups A, B, C, and D, respectively. We found a statistically significant difference between serum 25(OH)D levels in groups A and C (P = 0.01). There was no statistically significant difference in a comparison of serum 25(OH)D levels between the other groups (P > 0.05).
The mean levels of serum protein, albumin, and total calcium in group A were statistically higher than in the other groups. Details are presented in Table 4.
Table 4. Mean Levels of Protein, Albumin, and Total Calcium in Different Burn-Percent Groups.
| Burn-Percent | Mean ± SD |
|---|---|
| Protein | |
| 1 - 30 | 5.95 ± 0.86 |
| 31 - 40 | 5.23 ± 0.63 |
| 41 - 50 | 4.84 ± 0.81 |
| > 50 | 4.62 ± 0.42 |
| Albumin | |
| 1 - 30 | 3.65 ± 0.60 |
| 31 - 40 | 3.07 ± 0.41 |
| 41 - 50 | 2.80 ± 0.58 |
| > 50 | 2.61 ± 0.55 |
| Total calcium | |
| 1 - 30 | 7.88 ± 0.45 |
| 31 - 40 | 7.41 ± 0.51 |
| 41 - 50 | 7.45 ± 0.51 |
| > 50 | 7.30 ± 0.50 |
There was a significant positive correlation between serum levels of 25(OH)D and protein (r = 0.35), albumin (r = 0.42), total calcium (r = 0.40), and ionized calcium (r = 0.39, P < 0.001), showing that 25(OH)D levels will decrease by reducing these factors. We also found a significant negative correlation between serum levels of 25(OH)D and TBSA affected by burn, in that 25(OH)D will decrease with an increasing TBSA affected by burn (r = −0.29, P = 0.001).
5. Discussion
The normal mean level of 25(OH)D is considered to be 30 ng/mL, but the minimum bodily requirement for 25(OH)D is still controversial (10). In the present study, the mean 25(OH)D level was 14.58 ng/mL (SD = 6.96), and 114 (96.61%) of the patients had levels of < 30 ng/mL.
The main site for endogenous synthesis of 25(OH)D is the skin, which provides the majority of vitamin D precursor, with a small amount being absorbed through dietary intake (11). In burn patients, this source is impaired; however, other factors also contribute to 25(OH)D deficiency in these patients, including electrolyte disturbances, malabsorption, reduced albumin levels, post-burn hypermetabolism, and immobilization (12).
Klein et al. showed that vitamin D deficiency occurs in children suffering from burn injuries. Moreover, they found that the conversion of 7-dehydrocholesterol into previtamin D3 was impaired in biopsies from both the scar tissue and adjacent healthy skin. This finding indicates that the burden of the inability of the skin to synthesize vitamin D goes beyond the TBSA and involves a larger area (6).
Serum albumin is the main protein that binds to D1,25. Serum albumin concentrations are reduced following thermal damage, and can remain low for a long period of time (13). A significant correlation was observed between the 25(OH)D levels and albumin in the present study, which is in concordance with this finding.
It has been shown that many growth factors and cytokines play active roles in inflammatory and immunological responses after injury. These factors are also effective in bone remodeling and pathological destruction. The associations of 25(OH)D metabolism with these new factors and cytokines have recently been studied, but their impact on 25(OH)D levels after burn injury is unclear (14). Bone metabolism is a complex and multifactorial process that is regulated by systemic hormones. Since the consumption of corticosteroids is associated with bone loss and bone fractures, increasing endogenous glucocorticoid production, which occurs after a burn, can be associated with vitamin D abnormalities (6, 15).
Several studies have looked at supplementation for vitamin D-deficient patients. A study by Gottschlich et al. used ergocalciferol in children younger than three years at a dose of 800 IU daily, and for patients older than three years, they used 1600 IU daily for two weeks. They doubled the supplemental dose every two weeks, up to a maximum dose of ten times the recommended dietary allowance (RDA), or 4000 IU daily, if there was no improvement in the serum levels of vitamin D in these patients. It was found that patients receiving vitamin D supplementation had consistently lower levels of 25(OH)D and D1,25. It was presumed that there might be a malabsorptive defect or an inability to hydroxylate vitamin D (16). In another study, Klein et al. assessed supplementation with ergocalciferol in eight burned children suffering from vitamin D deficiency (D2). Vitamin D2 400 IU (10 ug) was administered daily for six months in order to raise serum levels of 25(OH)D back to normal. The 25(OH)D levels were raised in these patients, but not enough to compensate for the deficiency; the increase was also not statistically meaningful in comparison to the control group, who had not received the supplementation (17). Both of these studies may have been limited by their choice of vitamin D supplementation, as ergocalciferol is less effective than cholecalciferol in raising serum levels of vitamin D.
On the other hand, several additional studies have indicated the benefits of vitamin D supplementation in burn patients. Mayes et al. followed 39 burn patients for fractures after discharge while giving them vitamin D2 and D3 supplementation for one year. The results showed that vitamin D3 may have been beneficial in reducing the fracture risk in these patients (18). In a randomized clinical trial by Rousseau et al. on 15 burn patients followed for one year, the patients were divided into two groups. One group received intramuscular injection of cholecalciferol (200000 IU) every three months with oral daily calcium, and the other group received normal saline every three months with oral daily lactose as a placebo. The results indicated that calcidiol (25(OH)D) levels increased significantly in the experimental group that received vitamin D supplementation and calcium. However, there was no significant change in bone health in either of the groups. Quadriceps strength did improve in the case group (19). Table 5 shows the results of some previous studies.
Table 5. Summary of Previous Studies.
Although there is controversy with regard to administering vitamin D supplementation to patients after acute burn injuries, some studies strongly suggest that vitamin D is beneficial in these patients. We also began administering vitamin D to our burn patients as a part of their treatment protocol; however, the follow-up is still ongoing, and the results are not yet ready to be published. Whatever the results indicate, it is obvious that patients with acute burn injuries will need essential vitamins and minerals more than normal individuals will. Therefore, we recommend administering vitamins and minerals to this group of patients, beyond the usual and common RDA.
5.1. Conclusions
Based on the results of this study, we can conclude that 25-hydroxyvitamin D levels are low in children after acute burns, and these patients should be given vitamin D supplementation at 2 - 3 times the physiologic range (800 - 1200 IU/day). The present study also showed a higher frequency of vitamin D deficiency compared with other studies; vitamin D levels were lower than recommended levels in 96.61% of patients, while 81.35% had deficiencies and 16.10% had insufficiencies.
Acknowledgments
Authors wish to thank all the staff and nurses working at the Shahid Motahari burn hospital for their cooperation in this project.
References
- 1.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19(2):403–34. doi: 10.1128/cmr.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toon MH, Maybauer DM, Arceneaux LL, Fraser JF, Meyer W, Runge A, et al. Children with burn injuries--assessment of trauma, neglect, violence and abuse. J Inj Violence Res. 2011;3(2):98–110. doi: 10.5249/jivr.v3i2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris HA. Vitamin D: a hormone for all seasons--how much is enough? Clin Biochem Rev. 2005;26(1):21–32. [PMC free article] [PubMed] [Google Scholar]
- 4.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–6. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangin M, Sinha R, Fincher K. Inflammation and vitamin D: the infection connection. Inflamm Res. 2014;63(10):803–19. doi: 10.1007/s00011-014-0755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein GL. Burn-induced bone loss: importance, mechanisms, and management. J Burns Wounds. 2006;5:e30905. [PMC free article] [PubMed] [Google Scholar]
- 7.Klein GL, Langman CB, Herndon DN. Vitamin D depletion following burn injury in children: a possible factor in post-burn osteopenia. J Trauma. 2002;52(2):346–50. doi: 10.1097/00005373-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Klein GL. Burns: where has all the calcium (and vitamin D) gone? Adv Nutr. 2011;2(6):457–62. doi: 10.3945/an.111.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cap Today. Roche Diagnostics, cobas e411 (Immunoassay 2015). Available from: http://www.captodayonline.com/productguides/instruments/automated-immunoassay-analyzers-july-2015/roche-diagnostics-cobas-e411-immunoassay-analyzers-july-2015.html.
- 10.Sim JJ, Lac PT, Liu IL, Meguerditchian SO, Kumar VA, Kujubu DA, et al. Vitamin D deficiency and anemia: a cross-sectional study. Ann Hematol. 2010;89(5):447–52. doi: 10.1007/s00277-009-0850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringe JD, Kipshoven C. Vitamin D-insufficiency: An estimate of the situation in Germany. Dermatoendocrinol. 2012;4(1):72–80. doi: 10.4161/derm.19829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez NA, Jeschke MG, Williams FN, Kamolz LP, Herndon DN. Nutrition in burns: Galveston contributions. JPEN J Parenter Enteral Nutr. 2011;35(6):704–14. doi: 10.1177/0148607111417446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Guisado J, de Haro-Padilla JM, Rioja LF, Derosier LC, de la Torre JI. Serum albumin levels in burn people are associated to the total body surface burned and the length of hospital stay but not to the initiation of the oral/enteral nutrition. Int J Burns Trauma. 2013;3(3):159–63. [PMC free article] [PubMed] [Google Scholar]
- 14.Schwacha MG. Gammadelta T-cells: potential regulators of the post-burn inflammatory response. Burns. 2009;35(3):318–26. doi: 10.1016/j.burns.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper MS. Glucocorticoid-induced osteoporosis: how best to avoid fractures. Ther Adv Chronic Dis. 2010;1(1):17–23. doi: 10.1177/2040622310368737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschlich MM, Mayes T, Khoury J, Warden GD. Hypovitaminosis D in acutely injured pediatric burn patients. J Am Diet Assoc. 2004;104(6):931–41. doi: 10.1016/j.jada.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Klein GL, Herndon DN, Chen TC, Kulp G, Holick MF. Standard multivitamin supplementation does not improve vitamin D insufficiency after burns. J Bone Miner Metab. 2009;27(4):502–6. doi: 10.1007/s00774-009-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayes T, Gottschlich MM, Khoury J, Kagan RJ. Investigation of Bone Health Subsequent to Vitamin D Supplementation in Children Following Burn Injury. Nutr Clin Pract. 2015;30(6):830–7. doi: 10.1177/0884533615587720. [DOI] [PubMed] [Google Scholar]
- 19.Rousseau AF, Foidart-Desalle M, Ledoux D, Remy C, Croisier JL, Damas P, et al. Effects of cholecalciferol supplementation and optimized calcium intakes on vitamin D status, muscle strength and bone health: a one-year pilot randomized controlled trial in adults with severe burns. Burns. 2015;41(2):317–25. doi: 10.1016/j.burns.2014.07.005. [DOI] [PubMed] [Google Scholar]