Abstract
Aims:
To evaluate the outcome of nonendoscopic endonasal dacryocystorhinostomy (NEN-DCR) in patients with nasolacrimal duct obstruction (NLDO) in India.
Methods:
Retrospective case series of NEN-DCR between July 2012 and October 2014. All patients had follow-up >3 months. Success was defined anatomically as patency on irrigation and functionally as relief from epiphora.
Statistical Analysis Used:
Fischer's exact test and Chi-square test.
Results:
A total of 122 patients (134 eyes; 81 female; mean age 37 ± 18 years) were included. Indications were primary acquired NLDO in 92 (68%) eyes of adults (>18 years), NLDO in children (<18 years) in 22 eyes (16%), acute dacryocystitis in 13 eyes, failed prior DCR in six eyes, and secondary acquired NLDO in one eye. Mean duration of surgery was 36 min (range: 16–92). At a median follow-up of 6 months (range: 3–15), 86% eyes had functional success and 85% had anatomical success. Revision NEN-DCR was successful in 13/16 eyes. All patients with acute dacryocystitis were completely symptom-free at final visit. In children, (17/22) 77% achieved functional success after primary NEN-DCR which improved to 100% after one revision. Tube-related epiphora and granuloma in ten eyes resolved after removal.
Conclusion:
NEN-DCR gives good outcome in primary NLDO and is also effective in those with acute dacryocystitis and in children with NLDO. The technique obviates the need for an endoscope and has an acceptable safety profile and thus may be particularly suited for the developing nations.
Keywords: Dacryocystorhinostomy, endonasal, nonendoscopic
Caldwell and Toti deserve the credit for describing the endonasal and external approaches to dacryocystorhinostomy (DCR).[1,2] More than a century after it was first described external (Ext)-DCR has undergone several minor modifications to gain wide acceptance among ophthalmologists as the treatment of choice for nasolacrimal duct obstruction (NLDO). Unfortunately, endonasal DCR fell out of favor in the first half of the 20th century owing to difficulties encountered in the visualization of the nasal cavity with the then available instrumentation. Interest in endonasal DCR saw a resurgence around 1970 with the availability of rigid and subsequently fiber-optic imaging systems. Many ophthalmologists prefer external over endonasal DCR because they are not familiar with the nasal anatomy. In 1990 Massaro et al. described the ingenious technique of transilluminating the lacrimal sac with a vitrectomy light pipe in cadavers and rekindled interest in endonasal DCR.[3] In the last two decades, the available technology and surgical technique of endonasal DCR have evolved. The main advantages of endonasal DCR include the absence of a visible scar, minimal postoperative morbidity, faster recovery, and success rates (>90%) comparable to that of Ext-DCR.[4,5,6,7,8] We report the experience of a single surgeon in 134 eyes after nonlaser, non-endoscopic endonasal (NEN)-DCR at a Tertiary Eye Care Center in India.
Methods
This was a retrospective, noncomparative interventional study of all consecutive patients who underwent NEN-DCR between July 2012 and October 2014. The study adheres to the Declaration of Helsinki 1975 and was initiated after getting approval from the Institute Ethics Committee. In all patients, regurgitation of mucopurulent material on pressure over the lacrimal sac was assessed to diagnose NLDO. In those who had no regurgitation on pressure, a diagnostic irrigation and probing were performed. All patients were counseled and offered to choose between endonasal and Ext-DCR. This series includes those who opted for endonasal DCR surgery. Those children who had persistent congenital NLDO after one or more failed attempts of probing were also included in this series. All patients were asked about a history of nasal blockage and bleeding. A basic nasal examination was done in the clinic to rule out gross pathology involving the nose. All surgeries were performed by a single surgeon after formal training in the technique.
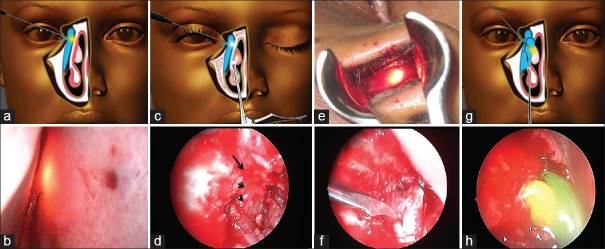
The technique of NEN-DCR was similar to that described by Dolman [Fig. 1a and b].[4] Surgery was performed under local anesthesia (LA) or general anesthesia (GA) based on the preference of the patient and a comprehensive systemic examination. After punctal dilation with a Nettleship dilator, a 23-gauge vitrectomy light pipe was gently introduced through the upper canaliculus until a hard stop was felt [Fig. 2a and b]. A nasal speculum with 5 cm long blades and a guard was introduced into the nasal cavity. A myringotomy sickle knife was used to incise the lateral nasal mucosa showing the transillumination effect. The incision for the mucosal flap was begun 8 mm above the insertion of the middle turbinate and then carried out vertically or in a curvilinear fashion down to the bone. A freer periosteal elevator was used to elevate the incised nasal mucosa and expose the frontal process of the maxilla. The posteriorly-hinged nasal mucosal flap was excised with Weil-Blakesley forceps. With the use of a 3 mm forward-biting straight Kerrison rongeurs, the thick bone of the frontal process of the maxilla was sequentially removed, and the osteotomy was gradually enlarged [Fig. 2c and d]. An ostium was considered to be of an optimum size and position if it allowed easy passage of a horizontally directed light pipe from the lower canaliculus into the lacrimal sac. Finally, the medial wall of the lacrimal sac was incised with a myringotomy sickle knife to create a marsupialized sac [Fig. 2e–g]. Irrigation through the lower canaliculus confirmed patency of the drainage system [Fig. 2h]. Mitomycin C (MMC) 0.04% was applied for 3 min as per discretion of the surgeon. Bi-canalicular silicone tubes were introduced and secured in all patients.
Figure 1.
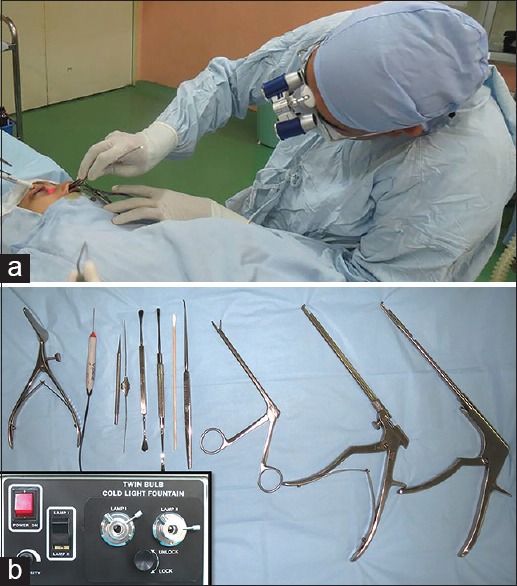
(a) Surgeon's position for performing nonendoscopic endonasal dacryocystorhinostomy; (b) instrumentation for nonendoscopic endonasal dacryocystorhinostomy
Figure 2.
(a) Transillumination of the lacrimal sac with the vitrectomy light pipe touching the medial wall of the nasal cavity; (b) transnasal view of the glow in the medial wall of the nasal cavity; (c) bony ostium being made with the Kerrison rongeur; (d) the lateral nasal wall shows bony ostium (small arrow) with the pale lacrimal sac mucosa showing through the ostium (arrowhead), and the nasal mucosal edge above it (large arrow); (e) transillumination of lacrimal sac after bony osteotomy; (f and g) Incision on the lacrimal sac wall with a myringotomy sickle knife; (h) free flow of viscoelastic substance stained with fluorescein through the ostium at the conclusion of surgery
The patients were seen on the 1st postoperative day and at 2 weeks, 6 weeks, 3 months, and 6 months after surgery. At each visit, irrigation of the lacrimal passage was done to assess anatomical outcome and the patient was specifically asked about epiphora to assess functional outcome. Success was defined anatomically as patency on irrigation and functionally as relief from epiphora. Tubes were usually removed after 6–8 weeks of surgery and/or earlier if there was a spontaneous extrusion.
Results
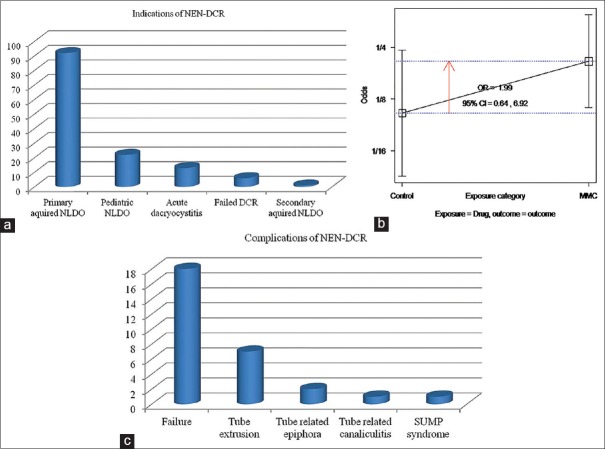
NEN-DCR was performed in 122 patients (134 eyes) in the study period. Surgery was performed under GA in 82/134 (61.1%) and LA in 52/134 (38.8%) patients. The mean age of the group was 37 ± 18 years (range: 3–71 years). A large majority 81 (66%) were female and procedure was performed in 69 (51%) right eyes. The indications for NEN-DCR were primary acquired NLDO in 92 (68.6%) eyes of adults, NLDO in children (<18 years) in 22 eyes (16.4%), acute dacryocystitis in 13 (10%) eyes, failed prior DCR in six eyes, and secondary acquired NLDO in one eye [Fig. 3a]. At a median follow-up of 6 months (range: 3–15) an overall functional success (relief from epiphora) was achieved in 116/134 (86.5%) eyes and anatomical success (patency on irrigation) in 93/109 (85.3%) eyes after primary NEN-DCR.
Figure 3.
(a) Bar diagram showing indications of nonendoscopic endonasal dacryocystorhinostomy in our study (N-134); (b) odds ratio plot showing no association between mitomycin C use and surgical success rate; (c) bar diagram showing complications of nonendoscopic endonasal dacryocystorhinostomy in our study (N-134)
Eighteen eyes had persistent epiphora after primary surgery. Revision NEN-DCR was performed in 16 eyes at a median interval of 142 days after primary surgery. In 10/16 (62.5%) eyes at revision, the ostium was obstructed by dense granulation tissue which was excised and MMC 0.04% applied for 3 min followed by bi-canalicular intubation. Overall, after revision surgery, 13/16 (81%) eyes were relieved of epiphora. Finally, functional success was achieved in 129/134 (96.2%) eyes and anatomical success in 106/109 (97.2%) eyes at a median follow-up of 7 months (range: 3–15 months).
Of the three patients who failed revision, one was found to have a residual bone at the ostium which was removed followed by MMC and silicone tubes. He complained of persistent epiphora 6 months after revision and opted out of any further intervention. Remaining two patients had a canalicular obstruction. In one, a conjunctivodacryocystorhinostomy was done which failed subsequently and the other patient declined further intervention. Among patients who had successful revision was a 43-year-old female who presented with an inflamed mucocele before primary surgery. Six months later she complained of recurrent swelling in the lacrimal sac region and foul smelling discharge in her mouth when the pressure was applied on the swollen lacrimal sac. As there was patency on irrigation she was diagnosed to have “sump syndrome.” Revision surgery showed a superiorly placed ostium with granulation tissue seen inferiorly. This was removed followed by MMC application and intubation. Six months later she was symptom-free.
The mean duration of surgery was 36 min (range: 16–92 min). All patients with acute dacryocystitis were completely symptom-free at the final visit. In children, 17/22 (77.2%) achieved functional success after primary NEN-DCR, which improved to 100% after revision. MMC 0.04% was used in seventy patients and not used in 64 patients. Comparison of outcome with and without MMC application [Fig. 3b] did not show a statistically significant difference (odds ratio: 1.99, 95% confidence intervals: 0.64–6.92, P = 0.21 using Fischer's exact test).
A univariate analysis for risk factors predictive of failure included age, gender, presence of regurgitation on pressure, indication for NEN-DCR, and right/left eye. None was found to be statistically significant by Pearson Chi-square test. Complications included failure after the primary procedure in 18/134 (13.4%) eyes, tube-related complications in ten (7%) eyes (tube extrusion in seven, tube-related epiphora in two, and tube-related canaliculitis in one), and sump syndrome in one patient [Fig. 3c]. Most tube-related complications resolved after removal of the tube.
Discussion
NEN-DCR retains the benefits of an endonasal approach and can be done without using expensive video-endoscope or laser systems. Our experience in 134 eyes at Tertiary Eye Care Center in India showed NEN-DCR had a good outcome and acceptable safety profile.
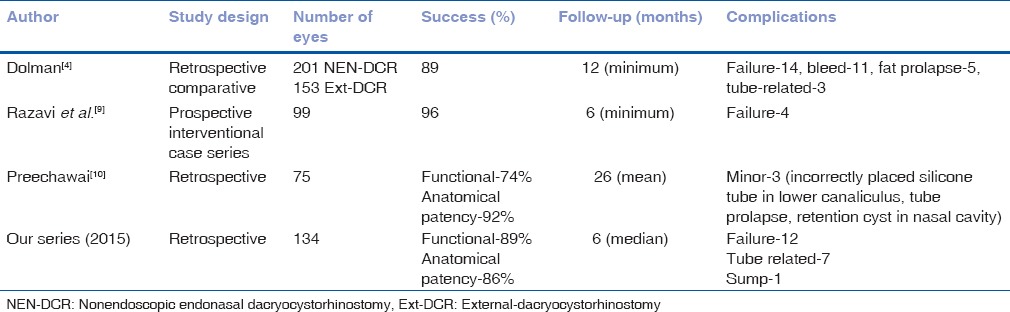
In a large comparative series of 354 patients reported by Dolman in 2003, comparable success was achieved in 89.1% of NEN-DCRs versus 90.2% in traditional Ext-DCR.[4] Further, 90% of patients who underwent revision NEN-DCR achieved success and complete relief from symptoms in the above series of patients.[4] In 2009 Razavi et al. reported a combined symptomatic relief and anatomic patency in 96% patients in a series of 99 NEN-DCRs performed in 95 patients.[9] Our success after primary NEN-DCR was 86% (116/134) and this improved to 96% (129/134) after one revision in 16 patients. This outcome was comparable to that reported by Dolman (89%) and Razavi et al. (96%) [Table 1].[4,9]
Table 1.
Summary of nonendoscopic endonasal dacryocystorhinostomy studies done till date
While a majority of NEN-DCR was performed under GA around 40% of patients underwent the procedure under LA. This is interesting as most groups prefer performing endonasal DCR under GA.[9,10] While patient preferences for local or GA may vary, the fact that a significant number of NEN DCR was performed under LA makes the technique suited for developing regions.
Ophthalmologists often prefer external over endonasal DCR as many ophthalmologists are not familiar with the nasal anatomy. This is primarily responsible for a longer learning curve in the endonasal approach. Preechawai studied the learning curve of NEN-DCR.[10] Seventy-five NEN-DCRs were performed by the author who had no prior training in nasal endoscopy and by residents under his supervision. The functional success rate in their study was 74.7% and anatomical patency was 92%.[10] Onerci et al. observed that success of endoscopic DCR could range from 94% in the hands of experienced surgeons to 58% in inexperienced hands.[11] The above studies may point toward a steeper learning curve for NEN-DCR compared to the gradual learning in endoscopic DCR.[10,11] The simpler instrumentation and lacrimal sac transillumination in NEN-DCR may be responsible for easier learning of the technique.
Acute dacryocystitis is a suppurative inflammation of lacrimal sac. Conventional management of acute dacryocystitis included conservative treatment followed by Ext-DCR after quiescence of several weeks.[12,13] Ext-DCR is not preferred in acute dacryocystitis due to the potential risks of exacerbation and spread of infection, associated surgical difficulty and unpredictable scarring.[14,15,16] In addition, the disadvantages of conservative management include prolonged and recurrent infection, cutaneous scar or fistula formation, and risks of failure of subsequent lacrimal surgery because of scarring or granuloma formation in the sac.[16,17,18,19,20] Acute dacryocystitis is an indication for endonasal DCR. In our series, all patients with acute dacryocystitis had a good outcome. Other authors like Razavi et al. have reported similar results.[9]
In children, the lacrimal sac is usually anterior to the middle turbinate because the agger nasi cells are not well pneumatized, and the ascending process of the maxilla is minimally differentiated. These characteristics of the pediatric nasal anatomy facilitate access to the lacrimal sac. However, introducing the instruments to the nasal cavity and performing the surgery are more difficult due to the narrow nasal passages and vestibules. The few studies available in the literature for endonasal endoscopic DCR in the pediatric population have reported a success rate ranging from 76% to 94.4%.[21,22,23,24,25] To the best of our knowledge, no previous studies have reported the outcome of NEN-DCR in children. Our primary outcome in children (77%) was comparable and this significantly improved after one revision. Thus, NEN-DCR appears to be a safe alternative for the management of refractory NLDO in children.
A large meta-analysis of 850 patients reported by Xue et al. in 2014 failed to show a significant beneficial effect of MMC application in primary endonasal DCR. However, MMC was shown to significantly lower the failure rates in primary Ext-DCR and revision endonasal DCR.[26] This discrepancy in the beneficial role of MMC can only be explained by the varied concentration and duration of MMC application in the patient groups included in the meta-analysis. While our result aligns with the current body of evidence, the role of MMC in primary NEN-DCR will only be known with randomized controlled trials with adequate sample size.
NEN-DCR is a relatively safe procedure with few serious complications reported.[4,9,10] Unlike Ext-DCR, the average intraoperative bleeding is minimal in NEN-DCR.[9] More serious but rare complications reported are orbital fat prolapse, medial rectus incarceration, orbital and subcutaneous emphysema, conjunctival fistula formation, and retrobulbar hemorrhage.[9,10] None of these major complications were seen in our series. Tube-related complications include punctal erosion, granuloma formation, and spontaneous extrusion.[9] However, most of these resolve after removal of the tube as was seen in our series.
Conclusion
NEN-DCR has a good outcome in primary NLDO, acute dacryocystitis, and children with NLDO. Transillumination of the lacrimal sac makes learning easier even for a novice surgeon. It can be performed without expensive instrumentation and therefore may be particularly suited for the developing nations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Caldwell GW. Two new operations for obstruction of the nasal duct, with preservation of the canaliculi and an incidental description of a new lacrimal probe. N Y Med J. 1893;57:581–2. [Google Scholar]
- 2.Toti A. Nuovo metodo conservatore di cura radicale delle suppurazioni croniche del sacco lacrimale (dacriocistorinostomia) Clin Mod Fir. 1904;10:385–7. [Google Scholar]
- 3.Massaro BM, Gonnering RS, Harris GJ. Endonasal laser dacryocystorhinostomy. A new approach to nasolacrimal duct obstruction. Arch Ophthalmol. 1990;108:1172–6. doi: 10.1001/archopht.1990.01070100128048. [DOI] [PubMed] [Google Scholar]
- 4.Dolman PJ. Comparison of external dacryocystorhinostomy with nonlaser endonasal dacryocystorhinostomy. Ophthalmology. 2003;110:78–84. doi: 10.1016/s0161-6420(02)01452-5. [DOI] [PubMed] [Google Scholar]
- 5.Tsirbas A, Wormald PJ. Endonasal dacryocystorhinostomy with mucosal flaps. Am J Ophthalmol. 2003;135:76–83. doi: 10.1016/s0002-9394(02)01830-5. [DOI] [PubMed] [Google Scholar]
- 6.Hartikainen J, Grenman R, Puukka P, Seppä H. Prospective randomized comparison of external dacryocystorhinostomy and endonasal laser dacryocystorhinostomy. Ophthalmology. 1998;105:1106–13. doi: 10.1016/S0161-6420(98)96015-8. [DOI] [PubMed] [Google Scholar]
- 7.Tarbet KJ, Custer PL. External dacryocystorhinostomy. Surgical success, patient satisfaction, and economic cost. Ophthalmology. 1995;102:1065–70. doi: 10.1016/s0161-6420(95)30910-4. [DOI] [PubMed] [Google Scholar]
- 8.Tsirbas A, Davis G, Wormald PJ. Mechanical endonasal dacryocystorhinostomy versus external dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2004;20:50–6. doi: 10.1097/01.IOP.0000103006.49679.23. [DOI] [PubMed] [Google Scholar]
- 9.Razavi ME, Eslampoor A, Noorollahian M, O'Donnell A, Beigi B. Non-endoscopic endonasal dacryocystorhinostomy – Technique, indications, and results. Orbit. 2009;28:1–6. doi: 10.1080/01676830802414772. [DOI] [PubMed] [Google Scholar]
- 10.Preechawai P. Results of nonendoscopic endonasal dacryocystorhinostomy. Clin Ophthalmol. 2012;6:1297–301. doi: 10.2147/OPTH.S33030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onerci M, Orhan M, Ogretmenoglu O, Irkeç M. Long-term results and reasons for failure of intranasal endoscopic dacryocystorhinostomy. Acta Otolaryngol. 2000;120:319–22. doi: 10.1080/000164800750001170. [DOI] [PubMed] [Google Scholar]
- 12.Ali MJ, Joshi SD, Naik MN, Honavar SG. Clinical profile and management outcome of acute dacryocystitis: Two decades of experience in a tertiary eye care center. Semin Ophthalmol. 2015;30:118–23. doi: 10.3109/08820538.2013.833269. [DOI] [PubMed] [Google Scholar]
- 13.Cahill KV, Burns JA. Management of acute dacryocystitis in adults. Ophthal Plast Reconstr Surg. 1993;9:38–41. doi: 10.1097/00002341-199303000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Wu W, Yan W, MacCallum JK, Tu Y, Jiang AC, Yang Y, et al. Primary treatment of acute dacryocystitis by endoscopic dacryocystorhinostomy with silicone intubation guided by a soft probe. Ophthalmology. 2009;116:116–22. doi: 10.1016/j.ophtha.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Lee TS, Woog JJ. Endonasal dacryocystorhinostomy in the primary treatment of acute dacryocystitis with abscess formation. Ophthal Plast Reconstr Surg. 2001;17:180–3. doi: 10.1097/00002341-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Morgan S, Austin M, Whittet H. The treatment of acute dacryocystitis using laser assisted endonasal dacryocystorhinostomy. Br J Ophthalmol. 2004;88:139–41. doi: 10.1136/bjo.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duggal P, Mahindroo NK, Chauhan A. Primary endoscopic dacryocystorhinostomy as treatment for acute dacryocystitis with abscess formation. Am J Otolaryngol. 2008;29:177–9. doi: 10.1016/j.amjoto.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Madge SN, Chan W, Malhotra R, Ghabrial R, Floreani S, Wormald PJ, et al. Endoscopic dacryocystorhinostomy in acute dacryocystitis: A multicenter case series. Orbit. 2011;30:1–6. doi: 10.3109/01676830.2010.535952. [DOI] [PubMed] [Google Scholar]
- 19.Naik SM, Appaji MK, Ravishankara S, Mushannavar AS, Naik SS. Endonasal DCR with silicon tube stents: A better management for acute lacrimal abscesses. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl 2):343–9. doi: 10.1007/s12070-012-0507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamal S, Ali MJ, Pujari A, Naik MN. Primary powered endoscopic dacryocystorhinostomy in the setting of acute dacryocystitis and lacrimal abscess. Ophthal Plast Reconstr Surg. 2015;31:293–5. doi: 10.1097/IOP.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 21.Dolmetsch AM, Gallon MA, Holds JB. Nonlaser endoscopic endonasal dacryocystorhinostomy with adjunctive mitomycin C in children. Ophthal Plast Reconstr Surg. 2008;24:390–3. doi: 10.1097/IOP.0b013e3181831f56. [DOI] [PubMed] [Google Scholar]
- 22.Jones DT, Fajardo NF, Petersen RA, VanderVeen DK. Pediatric endoscopic dacryocystorhinostomy failures: Who and why? Laryngoscope. 2007;117:323–7. doi: 10.1097/01.mlg.0000250266.39362.1b. [DOI] [PubMed] [Google Scholar]
- 23.Komínek P, Cervenka S. Pediatric endonasal dacryocystorhinostomy: A report of 34 cases. Laryngoscope. 2005;115:1800–3. doi: 10.1097/01.mlg.0000175678.73264.88. [DOI] [PubMed] [Google Scholar]
- 24.Komínek P, Cervenka S, Matousek P, Pniak T, Zeleník K. Primary pediatric endonasal dacryocystorhinostomy – A review of 58 procedures. Int J Pediatr Otorhinolaryngol. 2010;74:661–4. doi: 10.1016/j.ijporl.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Celenk F, Mumbuc S, Durucu C, Karatas ZA, Aytaç I, Baysal E, et al. Pediatric endonasal endoscopic dacryocystorhinostomy. Int J Pediatr Otorhinolaryngol. 2013;77:1259–62. doi: 10.1016/j.ijporl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Xue K, Mellington FE, Norris JH. Meta-analysis of the adjunctive use of mitomycin C in primary and revision, external and endonasal dacryocystorhinostomy. Orbit. 2014;33:239–44. doi: 10.3109/01676830.2013.871297. [DOI] [PubMed] [Google Scholar]