Abstract
Gamma oscillations are a robust component of sensory responses but are also part of the background spontaneous activity of the brain. To determine whether the properties of gamma oscillations in cortex are specific to their mechanism of generation, we compared in mouse visual cortex in vivo the laminar geometry and single-neuron rhythmicity of oscillations produced during sensory representation with those occurring spontaneously in the absence of stimulation. In mouse visual cortex under anesthesia (isoflurane and xylazine), visual stimulation triggered oscillations mainly between 20 and 50 Hz, which, because of their similar functional significance to gamma oscillations in higher mammals, we define here as gamma range. Sensory representation in visual cortex specifically increased gamma oscillation amplitude in the supragranular (L2/3) and granular (L4) layers and strongly entrained putative excitatory and inhibitory neurons in infragranular layers, while spontaneous gamma oscillations were distributed evenly through the cortical depth and primarily entrained putative inhibitory neurons in the infragranular (L5/6) cortical layers. The difference in laminar distribution of gamma oscillations during the two different conditions may result from differences in the source of excitatory input to the cortex. In addition, modulation of superficial gamma oscillation amplitude did not result in a corresponding change in deep-layer oscillations, suggesting that superficial and deep layers of cortex may utilize independent but related networks for gamma generation. These results demonstrate that stimulus-driven gamma oscillations engage cortical circuitry in a manner distinct from spontaneous oscillations and suggest multiple networks for the generation of gamma oscillations in cortex.
Keywords: gamma oscillation, visual cortex, cortical column, spontaneous activity, GABAA receptor
oscillatory activity in the gamma frequency band is a ubiquitous rhythm throughout the brain that has been implicated in multiple aspects of information processing. Gamma oscillations have been associated with sensory representation (Eckhorn et al. 1993; Gray et al. 1989; Ribary et al. 1991), sensorimotor integration (Sanes and Donoghue 1993; Schoffelen et al. 2011), and cognitive processes including memory (Gruber et al. 2004; Herrmann et al. 2004; Sederberg et al. 2003), attention (Debener et al. 2003; Fries et al. 2001), and perception (Keil et al. 1999; Ribary 2005). The association of gamma-band rhythmic activity with information processing is found across a wide range of species from insects (Laurent 1996) and rodents (Nase et al. 2003; Sukov and Barth 1998) to nonhuman primates (Eckhorn et al. 1993) and humans (Ribary et al. 1991).
In addition to its association with information processing, gamma-frequency activity is a robust component of the ongoing background activity of the brain. Spontaneous gamma oscillations occur during REM and slow-wave sleep, under various anesthetics, and in the awake, restful state (Contreras et al. 1996; Destexhe et al. 1999; MacDonald et al. 1996; Steriade 2006; Steriade et al. 1996a; Steriade and Amzica 1996). Given that cortical activity during the spontaneous and sensory representation brain states clearly generates different functional and perceptual outcomes, it seems likely that the networks participating in these states may show different spatial and temporal properties. However, a number of studies have reported that spontaneous and sensory-driven cortical activities have a similar geometry and may share core networks of neurons (Arieli et al. 1996; Kenet et al. 2003; Luczak and MacLean 2012; MacLean et al. 2005; Tsodyks et al. 1999). These results have been interpreted to suggest that spontaneous activity consists of samples from all of the different possible patterns of sensory-driven cortical activity (Luczak et al. 2009). However, recent work looking at cortical network activity during different brain states has identified key state-dependent differences related to laminar structure that are consistent with the functional anatomy of cortex (Maier et al. 2011; Sakata and Harris 2012). In the auditory cortex, stimulus-driven population activity originates in the thalamorecipient layers 4 and 6 and spreads quickly across columns, while spontaneous activity initiates in deep layers and propagates upward slowly. Results such as these suggest that the functional spread of activity through the cortical laminae may differ with brain state.
Like the similarities observed in the broadband local field potential (LFP), the gamma-frequency component of cortical activity has been reported to have a similar magnitude and frequency between spontaneous and sensory-driven cortical activity (Lakatos et al. 2005). However, the modulation of the laminar properties of gamma-frequency activity with respect to brain state has been incompletely described. Characterizing the modulation of the spatiotemporal distribution of gamma oscillations in the vertical axis of the cortex would help identify the components of gamma-frequency spatiotemporal geometry that are specific to the representation of sensory information.
This work investigates the columnar circuitry and excitatory and inhibitory network contribution during stimulus-driven and spontaneous gamma activity in mice in vivo. The well-documented modulation of gamma activity by visual stimuli, the robust gamma oscillations in the mouse primary visual cortex (V1) (Nase et al. 2003), and the characterization of its single-unit response properties (Niell and Stryker 2008) make the mouse V1 ideally suited for our study of the characteristics of gamma-frequency oscillations. In higher mammals, visually driven oscillatory activity occurs in the frequency range from 30 to 80 Hz, ascribed to the gamma frequency. In mouse visual cortex, visual stimulation triggered oscillations mainly between 20 and 50 Hz, which, because of their functional significance similar to gamma oscillations in higher mammals, we define here as gamma range.
We show that in the V1 of the anesthetized mouse in vivo visually driven and ongoing spontaneous gamma oscillations have similar magnitudes and peak frequencies but differ in their distribution through the depth of the cerebral cortex. Sensory representation in visual cortex specifically increases gamma oscillation amplitude in the superficial layers and primarily entrains putative excitatory neurons. In contrast, spontaneous gamma oscillations are distributed evenly through the cortical depth and strongly entrain putative inhibitory neurons in the deep layers. The laminar distribution of gamma oscillations during different functional states may result from differences in the source of excitatory input to the cortex. In addition, modulation of superficial gamma oscillation amplitude without a corresponding change in deep-layer oscillations suggests that superficial and deep layers of cortex may utilize independent, but related, networks for gamma generation. These results imply that the laminar geometry of gamma-frequency activity in V1 depends on the functional context in which they are generated and suggest that the laminar spatiotemporal structure of gamma activity may be a specific component of the sensory response.
MATERIALS AND METHODS
Surgery.
All animal experiments were performed in accordance with the guidelines of the National Institutes of Health and protocols reviewed and approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Adult C57/B6 mice (12–24 wk) were sedated with an initial dose of xylazine (13 mg/kg) and anesthetized with brief exposure to a high concentration of isoflurane (5%). Anesthesia was maintained with light isoflurane (0.1–0.7%) and the administration of booster doses of xylazine as needed, typically every 2 h. Anesthetic level was monitored by toe pinch, respiration, and pupil dilation. This anesthesia protocol was chosen because it allowed for the stable, long-term maintenance of the animal at a relatively light anesthesia state. Under very deep anesthesia, such as the state achieved with the use of 1–1.5% isoflurane without xylazine, the visually evoked responses were eliminated. Body temperature was maintained at 36–37°C throughout the experiment with a heating pad (FHC). Mice were positioned in a stereotaxic apparatus (David Kopf Instruments) and rotated 60° so that one eye was directed toward a LCD monitor. Skin incisions were infused with lidocaine, and the eye was covered with ointment (Lacri-Lube) to prevent drying. During the recording session, ointment was removed and eye condition was carefully monitored. A craniotomy (∼1.5 × 1.5 mm) was made above the V1 contralateral to the eye facing the monitor. The dura was removed to allow the insertion of either a silicon multielectrode probe (NeuroNexus Technologies, Ann Arbor, MI) or multiple tetrodes (Thomas Recording, Giessen, Germany).
Electrophysiology.
LFP recordings were obtained from multielectrode probes (NeuroNexus Technologies) with 16 channels arranged in a vertical configuration, with either 50- or 100-μm spacing between probes (model a1x16-3mm50-177 or a1x16-3mm100-177), inserted normal to the surface of the cortex. In some experiments, two probes were inserted with ∼1-mm space between the probes. LFP signals were filtered from 0.1 to 3,000 Hz online (n = 18 animals, 21 probes). For single-unit recording, simultaneous extracellular and LFP recordings were obtained from multiple tetrodes inserted via a microcontroller (Thomas Recording). The extracellular signal was filtered from 600 to 9,000 Hz, and spiking events were detected online by voltage threshold crossing (n = 14 animals, 47 tetrodes, 215 cells). A 1.5-ms waveform sample was acquired around the time of threshold crossing and was analyzed off-line. All signals were recorded with the Cheetah 32-channel acquisition system at 30 kHz (Neuralynx).
Pharmacology.
To specifically manipulate the gamma oscillations in superficial layers, in a subset of experiments superficial gamma activity was disrupted through pharmacological manipulation of the GABAergic system with picrotoxin. Because gamma oscillation production depends on inhibitory function (Cardin et al. 2009; Sohal et al. 2009; Takada et al. 2014), the application of the GABA antagonist picrotoxin increases overall depolarization but decreases the magnitude of gamma oscillations. In these experiments, a small dose (75 μl) of picrotoxin (0.75 μM; Sigma) was applied to the surface of the cortex, and the excess was immediately removed. Recordings were started 5 min after the application of picrotoxin and lasted for ∼20 min. This dose was selected as the lowest possible dilution that still produced an increase in the magnitude of the evoked response in the LFP. The very dilute dose of picrotoxin in combination with the short duration of recordings allowed us to isolate the effects of picrotoxin in the superficial layers of cortex. The penetration of picrotoxin into the cortex was monitored indirectly by changes in the size of the evoked response in the LFP. Under these conditions, only the evoked responses in the superficial layers of cortex were affected.
Visual stimuli.
Visual stimuli were generated with a ViSaGe stimulation generation system (Cambridge Research Systems, Cambridge, UK) and the accompanying MATLAB toolbox. Stimuli were displayed on a gamma-corrected 19-in. LCD monitor configured at 75 Hz refresh rate and positioned 30 cm away from the mouse's eye to occupy ∼70° of visual space. Full-screen drifting gratings were presented for 1 s with long (2–5 s) interstimulus intervals to allow for a sufficient return to baseline between trials. Stimulus parameters (95% contrast, 0.08–0.1 cycles/°, 3 Hz) were chosen to maximally drive the visual response in both single units (Drager 1975; Niell and Stryker 2008) and LFPs.
Analysis.
Analysis was carried out with custom routines in IGOR (WaveMetrics) except where specified. Neural signals were filtered at 0.1–300 Hz for LFP recordings and at 20–50 Hz to isolate gamma-frequency oscillations. LFPs from the 16-channel probes were used to calculate the current source density (CSD) of the cortical stimulus-evoked responses according to the methods of Swadlow and colleagues (Swadlow et al. 2002). Briefly, the one-dimensional CSD was derived from the second spatial derivative of the LFP data as described by Freeman and Nicholson (1975):
where Φ is the LFP, z is the vertical coordinate depth of the probe, and Δz is the interrecording site distance (50 or 100 μm in the present study). Upper and lower boundaries for CSD calculation were obtained by extrapolating recordings from the first and last recording sites. Cortical layers were identified by the appearance of current sinks and sources, relating to extracellular current flow following sensory input. The earliest current sink appears in L4, followed by sinks in L2/3 and L5/6. The latency and amplitude of the LFP-evoked response were quantified by finding the local peak (which is negative since the excitatory input causes a negative wave in the LFP) following stimulus onset. Values reported in text correspond to those found in L4, based on CSD calculations, unless otherwise specified. Power spectra were generated by fast Fourier transform at a single-trial level for 1-s epochs either immediately before (baseline condition) or after (stimulus condition) the onset of the drifting grating. Baseline and stimulus spectra were averaged across trials for each electrode and then divided (stimulus/baseline) to give a ratio measurement of the fold increase above baseline. Peak frequency, amplitude, and width measurements were calculated from the ratio spectra (see Fig. 2A for an example). Latency to onset of gamma oscillations was calculated from LFP traces filtered for the gamma range (20–50 Hz) and was measured as the time from stimulus onset to the first positive peak of the gamma-filtered trace that crossed a significance threshold [2.5 standard deviations (SD) above the mean]. Coherence measurements were computed with multitapered methods and the Chronux package (http://chronux.org/) (Mitra and Bokil 2007) in MATLAB (MathWorks). Wave-triggered averages(WTAs) were calculated by averaging LFPs on the peaks of gamma oscillations that occurred during the presentation of the drifting grating and were above the significance threshold (2.5 SD above the mean). Representative LFPs from L2/3, L4, and L5/6 were averaged on the peaks of gamma from the L4 LFP. Phase shift was quantified by comparing the time of the center peak in the WTA of LFPs from L2/3, L4, and L5/6.
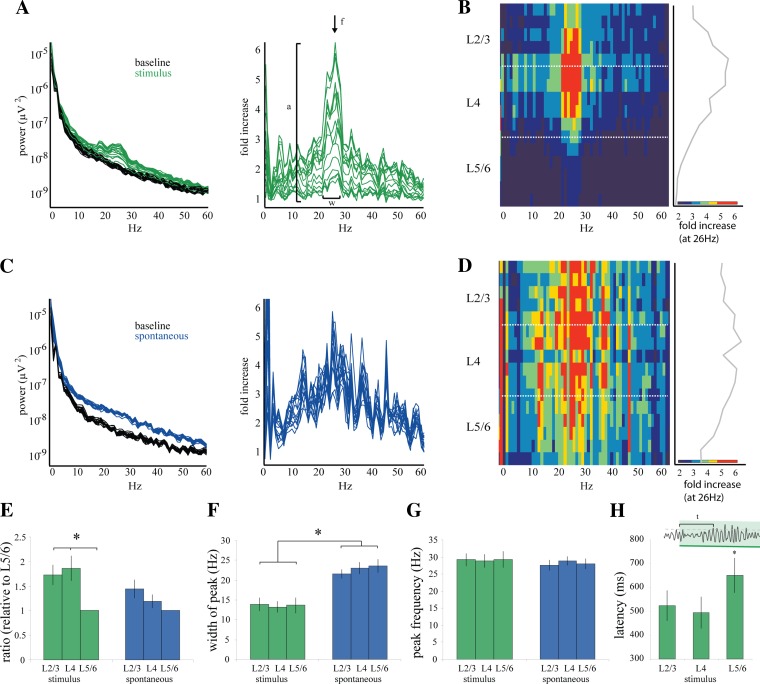
Fig. 2.
Laminar characteristics of stimulus-driven and spontaneous gamma activity. A, left: power spectra from each of the 16 channels for 1 experiment. Power spectra are calculated for the 1-s window during the presentation of the stimulus (green) and for the baseline period 1 s immediately before the stimulus onset (black). Spectra are calculated for each single trial and then averaged across all trials. Right: for each channel, the stimulus spectrum (green) is divided by the baseline spectrum (black) to reveal the frequencies with the greatest increase above baseline during the presentation of the stimulus. The largest difference is within the gamma frequency band (20–30 Hz). B: values of the stimulus-to-baseline ratio (A, right) plotted with respect to cortical depth. Left: fold increase over baseline is represented by pseudocolor, with red indicating the largest increase (∼6-fold increase). Right: values for the frequency with the largest increase (26 ± 1 Hz, designated by arrow in A, right) plotted with respect to depth. Width of the gamma peak is 11.3 ± 1.8 Hz. C, left: spontaneous bouts of gamma are identified manually and confirmed by threshold crossing (2.5 SD + mean). Power spectra are calculated in the same manner as in A for the 1-s window during the spontaneous bout (blue) and the 1-s baseline period immediately preceding each bout (black). Right: the spectra during the spontaneous bout are then divided by the corresponding baseline spectra for each channel to reveal a ratio measurement. D: ratio measurements calculated in C plotted with respect to depth. Pseudocolor scale indicates increase over baseline and is the same as in B. Maximum increase (∼6-fold) occurs at frequency 25.2 ± 1 Hz, and width of the gamma peak is 18.5 ± 1.4 Hz. E: ratio measurements shown in A and C for stimulus and spontaneous gamma, respectively, were quantified (stimulus n = 18 probes, 13 animals; spontaneous n = 9 probes, 7 animals). Increase over baseline was quantified as the maximum amplitude for each channel, indicated by “a” in A, right. To compare between stimulus and spontaneous, these values were normalized to the value in L5/6. *Increase in visually driven gamma was significantly higher in L2/3 (1.83-fold greater, P = 0.022) and L4 (1.86-fold greater, P = 0.012) than in L5/6 only during stimulus driven gamma. F: width of the gamma peak includes those frequencies in which the ratio measurement is greater than baseline spectral noise, demonstrated by “w” in A, right. Width of the gamma peak was similar throughout the layers of cortex but encompassed a wider range of frequencies during spontaneous gamma (23.2 ± 1.9 Hz) than stimulus (13.4 ± 2.7 Hz, *P = 0.02). G: peak frequency was designated as the frequency with the largest increase over baseline, demonstrated by “f” in A, right. Peak frequency was constant through the layers of cortex and was the same during stimulus-driven (29.5 ± 3.4 Hz) and spontaneous (29.1 ± 1.8 Hz) bouts of gamma. H: latency to the first bout of gamma was only calculated for stimulus-driven gamma; since this measurement is made from stimulus onset it cannot be calculated for spontaneous gamma. Latency was determined by finding the first gamma peak to cross the significance threshold after the onset of the stimulus. The population average showed no difference in onset latency between L2/3 (524 ± 246 ms) and L4 (495 ± 252 ms), but the latency in L5/6 was significantly longer than in L4 (652 ± 279 ms, *P = 0.04).
Single units were first identified with an automated clustering algorithm based on the mixture-of-Gaussians model (Harris et al. 2000). Clusters were refined manually on the basis of waveform shape and interspike interval (SpikeSort3D, Neuralynx). Quality of separation was determined based on the Mahalanobis distance and L ratio (Schmitzer-Torbert et al. 2005) with the MClust package (MATLAB). Units were identified as visually responsive if they maintained an average firing rate of 0.05 Hz during at least 20 presentations of the stimulus.
All 215 units were then classified as regular (RS) or fast (FS) spiking on the basis of properties of their average waveforms, at the electrode site with largest amplitude. Three parameters were used for discrimination: the height of the positive peak relative to the subsequent negative trough, the time from peak to trough, and the slope of the waveform 0.5 ms after the initial peak. These parameters have been found optimal for separation of neurons in all layers of the mouse V1 (Niell and Stryker 2008).
To determine whether single units were entrained to the gamma oscillations in the LFP recorded on the same tetrode, we created perievent histograms (PEHs) using the peaks of the gamma oscillations that crossed a significance threshold as time stamps. To ascertain whether the modulation of these events was significant, we computed a rhythmicity index (RI) for each cell. The RI was calculated by averaging the spike counts of the difference between the three center peaks and troughs and dividing this by the average of the entire histogram, which normalizes the measurement for spike rate (Popescu et al. 2009). This calculation was repeated 1,000 times with shuffled spike times, and the RI of the cell was normalized with respect to the resulting histogram of spike-shuffled rhythmicity values. A normalized rhythmicity value of 0.95 or greater, corresponding to a RI > 95% of the shuffled values, was considered significant. All rhythmicity values reported in the text and figures are normalized. Another measure of spike-to-LFP relationship, the spike field coherence, was computed with multitapered methods and the Chronux package (http://chronux.org/) (Mitra and Bokil 2007) in MATLAB (MathWorks).
To confirm that the spike waveforms of rhythmic cells were not contributing to the gamma power measured on the corresponding LFP, we removed 4 ms of the LFP waveform centered on each spike time stamp. This 4-ms snippet was replaced with the average value of the point on the LFP waveform immediately on either side of the missing snippet. This segment was median filtered to remove any abrupt transitions in LFP values, and then the gamma oscillation power was recalculated for the LFP.
Statistical analysis was performed with the Mann-Whitney U-test or the Wilcoxon signed-rank test where appropriate. Variability is reported as the SD unless specified to be the standard error (SE). All error bars in figures represent SE.
RESULTS
Spontaneous and visually driven gamma-frequency oscillations occur robustly through the depth of V1.
Our goal was to characterize the spatiotemporal distribution of gamma oscillations through the depth of the mouse V1 and compare visually driven oscillations with those occurring during spontaneous background activity, i.e., in the absence of visual stimulus. The response in visual cortex was dominated by oscillatory activity between 20 and 50 Hz, which we defined as our gamma range because of these oscillations' functional identity with gamma oscillations in higher mammals (30–80 Hz). We recorded LFPs with a multisite probe (NeuroNexus Technologies) with 16 evenly spaced recording sites (50- or 100-μm interelectrode distance) inserted normal to the cortical surface and spanning the cortical depth. In a separate cohort, we recorded single cells from layers 2–6 with five to seven independently movable tetrodes (Thomas Recording). Mice were anesthetized with a mix of isoflurane and xylazine in order to obtain a stable low-amplitude baseline pattern recorded in the LFPs (Fig. 1A). The visual responses described here were readily abolished or became highly variable under deep anesthesia, as indicated by large-amplitude slow oscillations in the background activity, and all recording sessions of this nature were discarded. We obtained recordings from 21 probes from 18 animals, from which we selected 17 probes from 13 animals for analysis on the basis of stability and amplitude of visual responses and the absence of slow oscillations in the baseline. Among these, we further selected nine probes from seven animals for the analysis of baseline activity because of the presence of long periods of stable nonstimulated activity.
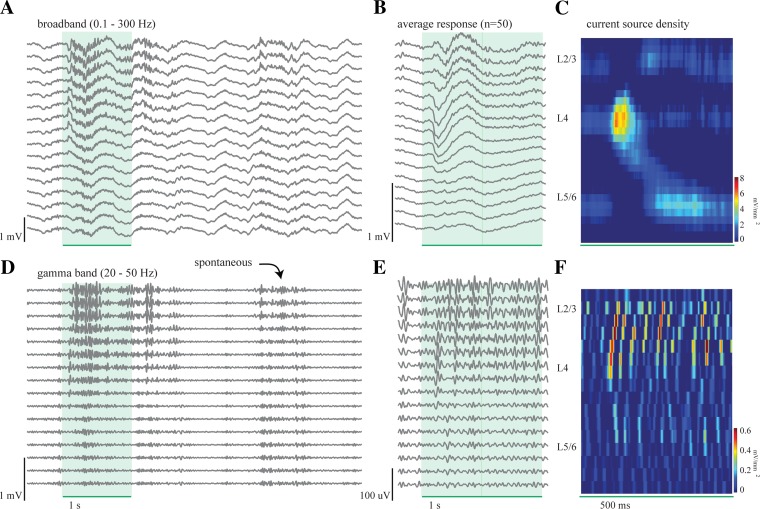
Fig. 1.
Local field potential (LFP) activity during the presentation of a drifting grating. A: 16 LFPs, filtered at 0.1–300 Hz, showing the evoked response to a single presentation of a full-screen vertical drifting grating (95% contrast, 3 Hz, 0.08 cycles/°) for 1 s, as indicated by green rectangle. An evoked response and corresponding fast frequency activity following stimulus onset are clearly visible during this single trial. B: average evoked response for 50 presentations of the drifting grating. The maximum amplitude of the evoked response occurs 90 ms after stimulus onset within L4. C: current source density (CSD) analysis of the average response in B shows a large initial sink in L4 at 90 ms followed by sinks in L2/3 (182 ms) and L5/6 (201 ms). D: same data as presented in A but filtered for the gamma frequency range, 20–50 Hz. A stimulus-driven increase in gamma activity occurs during the presentation of the drifting grating, and a spontaneous bout of gamma activity occurs several seconds later. E: average evoked response as in B filtered for gamma frequency range. Because gamma activity is not time-locked to stimulus onset, these averages only represent a small portion of the total gamma activity, as reflected by the smaller amplitude of the averages compared with the single-trial activity in D. F: CSD analysis of the average evoked gamma. Note difference in y-axis scale. Unlike the CSD analysis of the broadband LFP (C), there is not one initial sink in L4 followed by distinct sinks in the other layers but instead nonsignificant, multiple sinks and sources distributed over space and time.
The presentation of high-contrast, full-screen drifting gratings evoked a large response in the LFP signal, which was evident at the single-trial level (Fig. 1) both in the broadband (0.1–300 Hz; Fig. 1A)- and gamma frequency (20–50 Hz; Fig. 1D)-filtered data. The stimulus-locked average of the visual response in the broadband LFP (Fig. 1B) consisted of a negative deflection with a latency to peak of 90 ms after stimulus onset, as quantified by the local minimum of the LFP trace within L4. The amplitude and latency of the stimulus-locked response varied systematically with cortical depth, which we quantified with CSD analysis (Freeman and Nicholson 1975; Swadlow et al. 2002). Because the sinks generated by sensory inputs have the shortest latency in L4, we were able to use the CSD analysis to reliably identify channels located in L4. We included the channels that were 300 μm superficial to the upper boundary of L4 in our analysis of L2/3 and those that were 400 μm deeper than the lower boundary of L4 in our analysis of L5/6. In this example, the CSD revealed an initial large sink in L4 with a latency to peak of 90 ms followed 92 and 111 ms later by sinks in L2/3 and L5/6, respectively (Fig. 1C). For the population, the latency to peak of the evoked response in L4 was 95.7 ± 15.5 ms (population measures are means ± SD, unless otherwise noted) and the latency to peak of the CSD response for L4 was 103 ± 16 ms, followed by the peaks in L2/3 and L5/6 at additional latencies of 69 ± 66 ms and 78 ± 73 ms, respectively. These latencies are longer than others have previously reported for rodent V1 (Heynen and Bear 2001; Niell and Stryker 2008) and auditory cortex (Szymanski et al. 2009). However, this may be a function of the drifting grating stimuli, which produce a more gradual onset than a flashed or contrast-reversing grating. Additionally, differences in peak latency may be anesthesia dependent.
Visual stimulation also triggered a robust increase in gamma-frequency activity (Fig. 1D). Unlike the LFP-evoked response, which is time-locked to the stimulus, sensory-driven gamma showed a variable trial-to-trial latency from stimulus onset and therefore was greatly reduced in magnitude by the stimulus-locked averaging (Fig. 1E; note the change in vertical scale). Indeed, the CSD analysis of the averaged gamma revealed multiple sinks and sources, whose amplitudes were not above background noise and therefore showed no laminar arrangement (Fig. 1F; note change in z-scale). For this reason, our quantification of visually driven gamma-frequency activity was performed at the single-trial level and not on the stimulus-locked average.
In addition to visually driven gamma oscillations, the spontaneous background activity (not related to the visual stimulus) showed bouts of gamma activity with amplitude and duration similar to those triggered by the drifting gratings. Spontaneous gamma bouts occurred robustly and regularly throughout the depth of V1 in all recording sessions (Fig. 1D). The presence of spontaneous gamma oscillations agrees with previous observations indicating that gamma oscillations are a robust component of the background activity of the brain (Steriade et al. 1996b).
Sensory-driven and spontaneous gamma frequency oscillations have different spatiotemporal geometries.
We compared the characteristics of visually driven and spontaneous gamma oscillations through the vertical depth of the cortex. Because visually driven gamma oscillations are not time-locked to stimulus onset, our quantification is based on single-trial data. Single-trial analysis is immune to the reduction in amplitude of gamma oscillations that inevitably results from averaging responses with varying latency. We calculated, from the raw unfiltered LFP in each trial, the power spectrum from a 1-s window after stimulus onset (response) and from a 1-s window immediately preceding stimulus onset (baseline). We then averaged the single-trial broadband spectra for the response and baseline (Fig. 2A, left) and calculated the ratio between the two for all 16 channels (Fig. 2A, right). Thus the ratio spectra quantify the fold increase in power, across all frequencies, triggered by the visual response with respect to the spontaneous activity immediately preceding stimulus onset. The ratio spectra for the example illustrated in Fig. 2A showed an increase in power over baseline limited to a relatively narrow band of frequencies (width = 11.3 ± 1.8 Hz; Fig. 2A, right, “w”) surrounding a sharp peak at 26 ± 1 Hz (Fig. 2A, right, “f”), at which the maximum increase of 6.4-fold was observed for the electrode at the top of L4 (Fig. 2A, right, “a”). This specific increase in power, centered in the gamma band, was not uniform through cortical depth but was much stronger in granular (L4) and supragranular (L2/3) layers. This was illustrated by representing the ratio spectra as a two-dimensional plot in which magnitude was color coded (Fig. 2B, left). The asymmetry between layers was clear when plotting the fold increase of the peak frequency (26 Hz) vs. cortical depth (Fig. 2B, right), which shows a 2.8-fold difference between L4 and L5/6 and a maximum increase of 6.4-fold at top of L4.
Spontaneous gamma oscillations had distinct spectral and spatiotemporal properties compared with visually driven gamma. We detected spontaneous gamma bouts by the crossing of a significance threshold of 2.5 SD above the mean applied to the gamma band-filtered data (see materials and methods). We then used those time stamps to calculate the power spectrum from the raw, unfiltered data of 1 s after each detection time and their corresponding 1 s preceding baselines. We averaged these single trial spectra (Fig. 2C, left) and calculated the ratio spectra (spontaneous bout/baseline) for all 16 channels (Fig. 2C, right). As illustrated by the example in Fig. 2C, spontaneous bouts were similar to sensory-triggered bouts in their amplitude and peak frequency (maximum 6.2-fold increase at the peak frequency of 25.2 ± 1 Hz). In contrast, spontaneous oscillatory bouts included a much wider spectral increase, spanning beyond the gamma-range (between 10 and 60 Hz; example of Fig. 2C, right: width = 18.5 ± 1.4 Hz), and were relatively uniform in magnitude over the cortical layers. The more uniform distribution of spontaneous gamma bouts through cortical depth is illustrated by the pseudocolor plot of the ratio spectra and by the laminar profile of the peak frequency of 26 Hz in Fig. 2D. Thus, in contrast with those driven by visual stimuli, spontaneous gamma oscillations were much wider spectrally and lacked a distinct laminar structure.
To generate population measures, we averaged the responses of the channels located in the supragranular (L2/3), granular (L4), and infragranular (L5/6) laminae, according to the L4 location indicated by the CSD analysis. Because of the variability in spectral power magnitude across experiments, we normalized the ratio spectra of L2/3 and L4 to that of L5/6 for each experiment (Fig. 2E). The population data showed that the increase in visually driven gamma was significantly higher in L2/3 (1.83-fold greater, P = 0.022) and L4 (1.86-fold greater, P = 0.012) than L5/6. In contrast, the increase in gamma power during spontaneous bouts did not show significant differences between layers, despite a tendency to a larger increase in L2/3 (Fig. 2E).
The width (in Hz) of the increased power in the ratio spectra was measured as the distance between points rising 1 SD above spectral noise (as in Fig. 2A, right, “w”). The width did not change as a function of depth in either the spontaneous or the stimulus-driven condition and thus was averaged across all channels for each experiment. The width was significantly larger for spontaneous (23.2 ± 1.9 Hz) than for visually driven (13.4 ± 2.7 Hz) bouts (Fig. 2F), and while the visually driven increase generally fell between 20 and 50 Hz, spontaneous oscillations increased over a broader range from 10 to 60 Hz. Consistent with the example in Fig. 2, the population peak frequency in the ratio spectra did not change over cortical depth and was averaged across all channels. The average population peak frequency was not different between spontaneous (29.5 ± 3.4 Hz) and visually driven (29.1 ± 1.8 Hz) activity (Fig. 2G). We quantified the latency to onset of visually driven gamma oscillations as the time from onset of the drifting grating presentation to the first threshold-detected peak on the gamma band-filtered LFP. The population average showed a small but not significant difference in onset latency between L2/3 (524 ± 246 ms) and L4 (495 ± 252 ms), but the latency in L5/6 was significantly longer than in L4 (652 ± 279 ms, P = 0.04). In summary, sensory-driven and spontaneous gamma activity showed peaks with similar frequency and magnitude but different bandwidth and distribution through cortical depth.
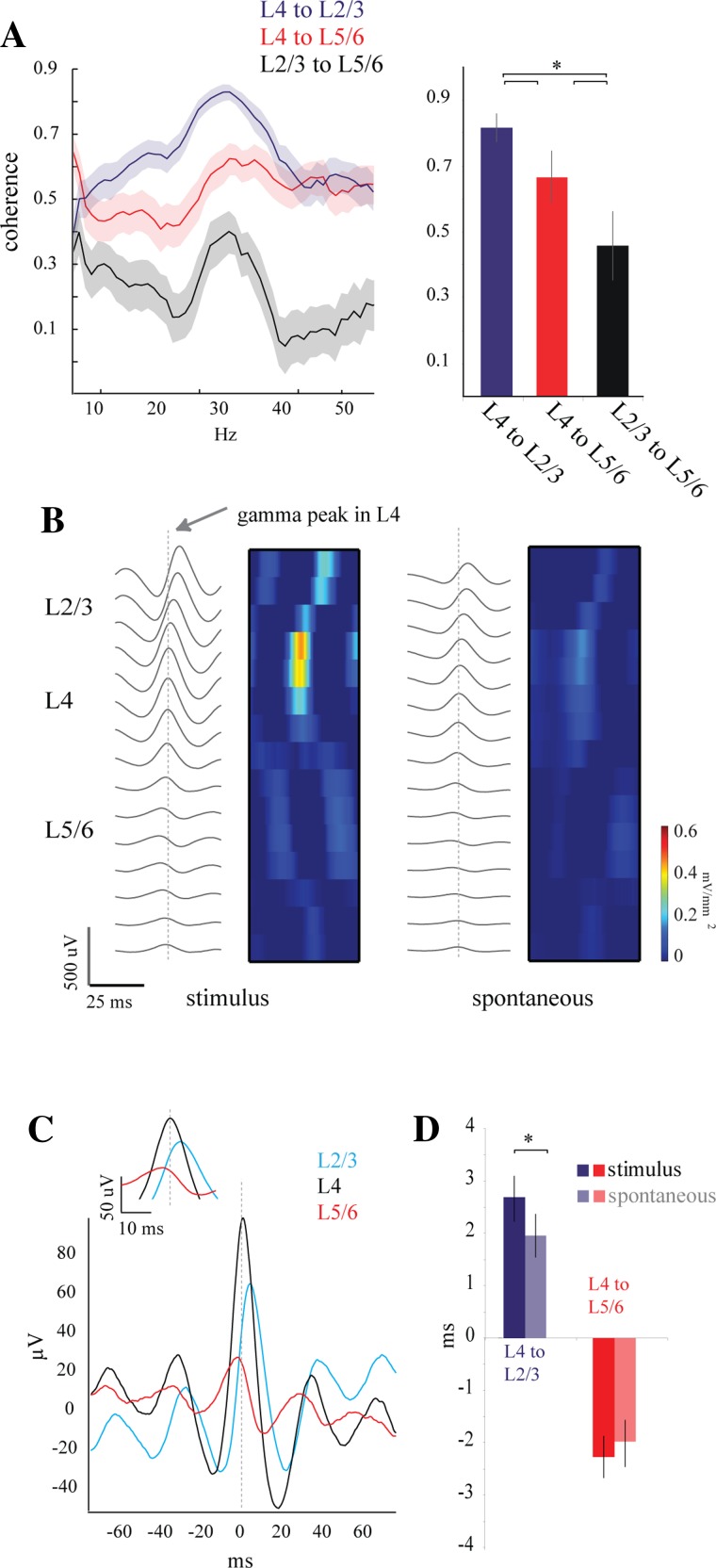
To further characterize the differences between spontaneous and visually driven gamma oscillations and their distribution in the cortical depth, we investigated the correlation of gamma phase and magnitude between the cortical layers. We performed coherence measurements (see materials and methods) between representative channels in L4, L2/3, and L5/6, in which the two nongranular channels were equidistant to L4. In the example of Fig. 3A, left, the coherence of the response to drifting gratings was strong between L4 and the other two layers along most of the frequency spectrum but was consistently larger between L4 and L2/3. The visually driven coherence between supra- and infragranular layers was considerably smaller throughout the spectrum. In all three cases, coherence increased in the gamma band (20–50 Hz) but was highest between L4 and L2/3 (Fig. 3A, left). We quantified coherence at the gamma frequency band for the population by averaging the coherence values for frequencies from 20 to 50 Hz across the population. The visually driven coherence between L4 and L2/3 (0.83 ± 0.1) was greater than the coherence between L4 and L5/6 (0.64 ± 0.2, P < 0.0001). Coherence between L2/3 and L5/6 (0.46 ± 0.2) was lower than that for either of the other pairs. The coherence analysis for the spontaneous gamma oscillations produced the same results as that for the stimulus-driven (L4 to L2/3 = 0.82 ± 0.1; L4 to L5/6 = 0.64 ± 0.2) oscillations. Thus our results show a large degree of broadband spectral coherence within the cortical column with a strong functional coupling between L4 and L2/3. More importantly, our results show that coherence is enhanced in the gamma frequency band for both spontaneous and visually driven gamma. The similar coherence values between spontaneous and visually driven oscillations suggest that, despite the differences in laminar distribution and frequency band, the phase relations across the cortical depth are equally stable over a wide spectral band.
Fig. 3.
Coherence and phase measurements between cortical laminae. A: coherence ± jackknife error measurements for 50 trials of drifting grating presentation. Coherence of L4 and L2/3 between 20 and 50 Hz (0.83 ± 0.1) was greater than the coherence of L4 and L5/6 (0.64 ± 0.17, *P < 0.0001); Coherence between L2/3 and L5/6 was lower than both of the other pairs (0.46 ± 0.23). These differences were significant over the population (right; n = 5 animals) and were similar between stimulus-driven and spontaneous gamma (not shown). B: wave-triggered averages (WTAs) using the peaks of gamma oscillations in L4 as time stamps show a distinct phase relationship through the cortical depth. The phase relationship is seen even more clearly in the CSD plots on right. Similar phase shifts are seen in both stimulus-driven (left) and spontaneous (right) gamma activity. C: detailed view of WTA averages seen in B for 1 channel from each lamina. L2/3 and L5/6 are equidistant to L4. D: quantification of the phase relationships shown in B and C for the population shows a larger phase precession through the layers during stimulus-driven gamma than spontaneous (n = 5 animals). The positive delay between L2/3 and L4 is larger for visually driven (2.7 ± 1.7 ms) than for spontaneous (1.9 ± 1.6 ms; *P = 0.029) gamma. The average phase between L4 and L5/6, in contrast, showed a negative delay (visually driven = −2.2 ± 1.5 ms; spontaneous = −1.95 ± 1.8 ms).
Coherence analysis quantifies the stability of the phase relationships between waveforms but does not provide a measure of the phase value. To measure the phase delay (in ms) of gamma oscillations between layers, we averaged the gamma oscillations in all channels around the threshold-detected positive gamma cycles from one chosen electrode in L4. As illustrated by the example of Fig. 3B, these WTAs centered on L4 showed a positive phase shift in supragranular layers progressively increasing toward the pia and a negative phase shift that progressively decreased toward the white matter. These peaks can be seen clearly in the corresponding CSD (Fig. 3B, pseudocolor plots). To obtain a population measure of phase shift we measured the delay of the central peak with respect to L4 from the same two representative channels (L2/3 and L5/6) as for the coherence analysis (Fig. 3C). We measured the phase shift for both stimulus-driven and spontaneous gamma. The example in Fig. 3C of stimulus-driven gamma showed a L4-L5/6 negative delay of −2.6 ms and a L4-L2/3 positive delay of 2.8 ms. Population averages revealed a positive delay in L2/3 during visually driven gamma (2.7 ± 1.7 ms) that was larger than the positive delay during spontaneous gamma (1.9 ± 1.6 ms; P = 0.029), as shown in Fig. 3D. The average phase between L4 and L5/6, in contrast, showed a negative delay (visually driven = −2.2 ± 1.5 ms, spontaneous = −1.9 ± 1.8 ms). In addition, the WTA analysis showed a much larger central peak for L2/3 than L5/6, emphasizing the functional coupling of the superficial layers with L4. Thus the large coherence values and the small phase shifts of the gamma positive peaks between layers ensure effective interactions in the gamma band along the vertical axis of the cortical column, with particular interaction between granular and supragranular layers. In addition, sensory-driven gamma oscillations have a better-defined spatial and temporal structure than spontaneous oscillations.
Single units entrain to gamma differently through cortical layers.
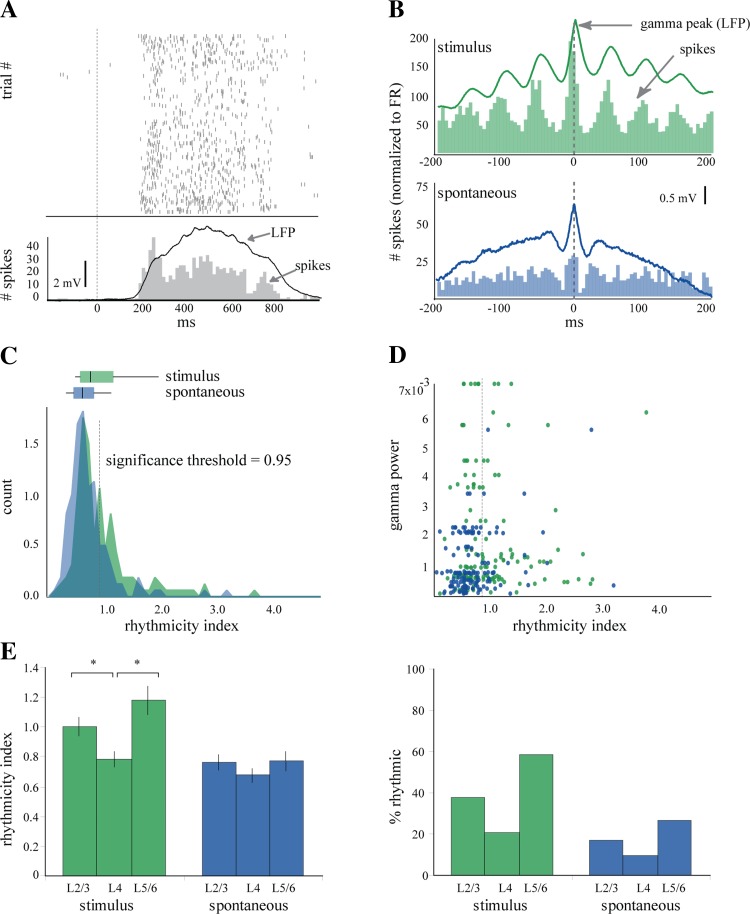
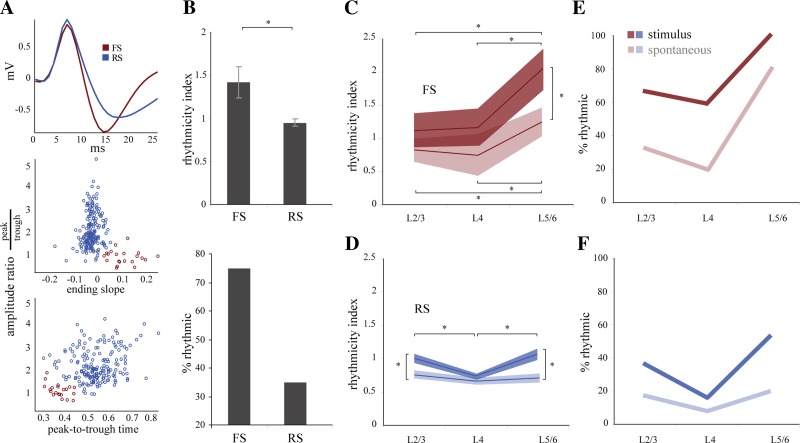
To determine whether the laminar structure of the LFP oscillations was reflected in the spike output of V1, we recorded single units and LFPs throughout V1 with independently movable tetrodes (n = 215 units). Single units in the mouse V1 responded robustly to full-screen drifting gratings (mean firing rate to visual stimulation = 2.2 ± 3.2 Hz; baseline firing rate = 0.5 ± 1.1; n = 176 cells). However, since response firing rates rarely reached firing rates in the gamma range, responsive cells typically fired only once every several gamma cycles. An example of a cell responding to various orientations of a full-screen drifting grating is illustrated by the rastergram in Fig. 4A. The spike response of this example cell was robust as shown by the peristimulus histogram (PSTH) in Fig. 4A, bottom, and it faithfully followed the evoked potential recorded in the LFP from the same electrode (superimposed trace, polarity inverted for clarity). However, despite the strong responses and the presence of gamma oscillations in the LFP no gamma frequency activity was visible in the PSTH. Therefore, to quantify the rhythmicity of cells given their low firing rates, we generated PEHs, which are spike time histograms around the threshold-detected positive gamma peaks in the LFP (Fig. 4B). From the PEHs we calculated a rhythmicity index (RI; see materials and methods) as the average distance between trough and peak of the center three peaks of the PEH (Popescu et al. 2009). We set a criterion for significance at 95% over the level of the shuffled PEH (Fig. 4C, significance threshold) and found that 38% of single units showed a significant entrainment to stimulus-driven gamma-frequency fluctuations in the LFP (mean RI 0.99 ± 0.6) but only 15% to the spontaneous gamma oscillations (mean RI 0.74 ± 0.4) (Fig. 4C). For example, the PEH of the cell in Fig. 4B (Fig. 4B, green histogram, RI = 2.59) showed a strong entrainment to the visually driven gamma oscillations, which were very robust as shown by its WTA (green line; vertical dashed line indicates the zero time of the central gamma peak). The same cell showed a much smaller entrainment to the spontaneous gamma oscillations (Fig. 4B, blue histogram, RI = 1.03), which in this example also produced a less robust WTA (blue line). Variations in the degree of entrainment of the spikes to the LFP could be a trivial result of variations in the magnitude of the gamma oscillations. However, the entrainment of a given cell could not be predicted by the overall magnitude of gamma activity in the LFP rhythm (Fig. 4D) or the RI of other cells in very close spatial proximity (data not shown). In contrast, cell rhythmicity was dependent on the laminar position of the cell during visually driven gamma (Fig. 4E). The mean RIs were highest in L2/3 (1 ± 0.6, P = 0.025) and L5/6 (1.18 ± 0.6, P = 0.0039) compared with L4 (0.78 ± 0.3). During spontaneous gamma, there were no significant differences in RI (L2/3 = 0.76 ± 0.5, L4 = 0.67 ± 0.3, L5/6 = 0.77 ± 0.4) throughout the cortical depth (Fig. 4E, left). Furthermore, compared with spontaneous gamma, visual stimulation only triggered significant increases in gamma RI in L2/3 and L5/6 and not in L4 (evoked vs. spontaneous for L2/3, P = 0.003872; L4, P = 0.12634; L5/6, P = 0.000413).
Fig. 4.
Single units are modulated by gamma oscillations differently through the cortex. A: raster and peristimulus histogram (PSTH) from a regular-spiking (RS) cell in L2/3 during presentation of a drifting grating. The evoked response in the LFP (black trace, inverted polarity) follows a time course similar to the PSTH (gray) of the unit. B: perievent histograms for stimulus-driven (green) and spontaneous (blue) spiking events, calculated with respect to the positive peaks of gamma oscillations that crossed the significance threshold (2.5 SD above mean). C: distribution of rhythmicity indexes (RIs) for 176 cells during stimulus-driven (green) and spontaneous (blue) gamma. RIs for 1/3 (38%) of the neurons were greater than the significance threshold during stimulus-driven gamma (mean RI 0.99 ± 0.6), and RI for 15% of the neurons crossed threshold during spontaneous gamma (mean RI 0.742 ± 0.4). Box and whisker plots demonstrate the median and interquartile values for each condition (stimulus median = 0.79, lower quartile = 0.62, upper quartile = 1.16; spontaneous median = 0.66, lower quartile = 0.51, upper quartile = 0.84). D: RI did not correlate with the magnitude of gamma oscillations in the LFP from the same tetrode for either spontaneous or stimulus-driven gamma. E: distinct laminar differences in rhythmicity were observed during stimulus-driven but not spontaneous gamma. Neurons in both L2/3 (1 ± 0.6, *P = 0.025) and L5/6 (1.18 ± 0.6, *P = 0.0039) had significantly higher mean RI than L4 neurons (0.78 ± 0.3) (left). During spontaneous gamma there were no differences between the mean RIs for each layer (L2/3 = 0.76 ± 0.45; L4 = 0.67 ± 0.33; L5/6 = 0.77 ± 0.4). Similarly, a greater percentage of neurons in L2/3(38%) and L5/6 (58.5%) were rhythmic compared with L4 (20.9%) during stimulus-driven gamma (right). There was a similar trend during spontaneous gamma, but the differences between layers were smaller than during stimulus-driven gamma (L2/3 = 17.1%, L4 = 9.3%, L5/6 = 26.8%).
Similar results were obtained when examining the percentage of rhythmic cells by layer. During stimulus-driven gamma L2/3 and L5/6 had a greater percentage of rhythmic cells (38% and 58.5%, respectively) than L4 (20.9%). During spontaneous gamma, the differences were much smaller, although L4 still had the smallest percentage of rhythmic cells (9.3% compared with 17.1% in L2/3 and 26.8% in L5/6; Fig. 4E, right). To provide additional insight, we performed an alternative quantification to measure the correlation between spikes and the local field signal, spike field coherence. The spike field coherence for L2/3 (0.28 ± 0.1) was significantly greater than that of L4 (0.18 ± 0.06, P = 0.0001), while the spike field coherence for L5/6 (0.22 ± 0.1) trended toward an increase over L4 but did not reach significance (P = 0.07). In sum, cells in L2/3 and L5/6 showed higher entrainment to visually driven gamma oscillations than those in L4 and compared with spontaneous oscillations.
RS and FS cells entrain differently during spontaneous and stimulus-driven gamma.
In cortex, gamma oscillations rely strongly on excitatory and inhibitory network interactions. To determine the potential influence of excitatory and inhibitory cells on rhythmogenesis throughout the cortex, we classified all 215 units as putative excitatory (regular spiking, RS) or putative inhibitory (fast spiking, FS) cells on the basis of the shape of their waveform (Fig. 5A) (n = 195 RS, 20 FS) (Niell and Stryker 2008). Unlike what has been observed in other species (Bruno and Simons 2002), FS and RS cells both showed high variability in firing rates and very similar mean rates during visual stimulation (FS = 2.65 ± 2.8 Hz, RS = 2.13 ± 3.3 Hz). However, the average rhythmicity of FS cells was much more pronounced than that of RS cells (RI: FS = 1.42 ± 0.7, RS = 0.95 ± 0.5; P = 0.002; Fig. 5B, top), and a higher percentage of FS cells were rhythmic compared with RS cells (FS = 75%, RS = 35%; Fig. 5B, bottom). Similarly, spike field coherence was greater for FS cells (0.32 ± 0.1 vs. RS 0.22 ± 0.1; P = 0.009). Both FS and RS cells showed laminar structure in their rhythmicity during stimulus-driven gamma oscillations. FS cells showed no differences in average rhythmicity between L2/3 (1.12 ± 0.6) and L4 (1.16 ± 0.6), but rhythmicity in L5/6 was significantly greater than in either of the superficial layers (2.03 ± 0.67, P = 0.02, P = 0.022; Fig. 5C). Similarly, the percentage of rhythmic FS cells in L5/6 (100%) was higher than in L2/3 (66.7%) or L4 (60%; Fig. 5E). The laminar profile of the rhythmicity of RS cells during stimulus-driven gamma was distinct from that of FS cells (Fig. 5D). RS cells had significantly higher rhythmicity in L2/3 (1 ± 0.6, P = 0.006) and L5/6 (1.06 ± 0.46, P = 0.0006) than in L4. (0.73 ± 0.25) and a similar pattern in the percentage of rhythmic cells across layers (L2/3 = 36%, L5/6 = 52.8%, L4 = 15.8%; Fig. 5F).
Fig. 5.
Fast-spiking (FS) and RS cell classification and modulation through the cortex. A: FS and RS cells were well separated on the basis of their peak-to-trough amplitude ratio, peak-to-trough time, and ending slope of the trough. B: FS cells showed significantly greater rhythmicity than RS cells in terms of both mean RI (FS 1.42 ± 0.7; RS 0.95 ± 0.5, *P = 0.002), and % of rhythmic cells (75% FS, 35% RS). C: FS cells displayed a laminar structure to their rhythmicity during both stimulus-driven and spontaneous gamma. During stimulus-driven gamma (dark lines) FS cells in L5/6 were more rhythmic (2.03 ± 0.7) than those in L4 (1.16 ± 0.6, *P = 0.022) or L2/3 (1.12 ± 0.6; *P = 0.02). FS cells had a similar laminar profile during spontaneous gamma (light lines) (L5/6 1.24 ± 0.7; L4 0.75 ± 0.7, *P = 0.038; L2/3 0.82 ± .042, *P = 0.033). The rhythmicity in L5/6 was greater during stimulus-driven than spontaneous gamma activity (*P = 0.0217). D: RS cells in L2/3 (0.998 ± 0.6) and L5/6 (1.06 ± 0.46) showed greater rhythmicity than cells in L4 (0.734 ± 0.25, *P < 0.0001). During spontaneous gamma, RS cells showed no distinct changes in rhythmicity through the depth of the cortex (L2/3 0.76 ± 0.5, L4 0.66 ± 0.27, L5/6 0.71 ± 0.34). RS cell rhythmicity in both L2/3 and L5/6 was greater during stimulus-driven than spontaneous gamma activity (*P < 0.0001). E: similar to mean RI shown in C, more FS cells were rhythmic in L5/6 (100%) than in L2/3 (66.7%) or L4 (60%) during stimulus-driven gamma. This same relationship held true for spontaneous gamma (L5/6 = 80%; L2/3 = 33.3%; L4 = 20%). F: like the data presented in D, more RS cells were rhythmic in L2/3 (36%) and L5/6 (52.8%) than in L4 (15.8%) during stimulus-driven gamma. During spontaneous gamma there was a trend in the same direction, but the difference between layers was much smaller (L2/3 = 17%, L4 = 7.9%, L5/6 = 19.4%).
Spike field coherence measurements revealed similar trends, although differences between infragranular and granular layers failed to reach significance. For FS cells, there is a trend toward a larger coherence in L5/6 (0.37 ± 0.1) compared with L4 (0.29 ± 0.1) and L2/3 (0.31 ± 0.2). RS cells were more coherent in L2/3 (0.27 ± 0.1) than in L4 (0.17 ± 0.04, P = 0.00002) and showed a greater coherence in L5/6 cells [0.2 ± 0.1, not significant (n.s.)] compared with L4.
During spontaneous gamma, the mean RI decreased in both cell classes and across all layers. However, the RI of FS cells maintained its laminar structure during spontaneous gamma, with L5/6 cells showing significantly greater rhythmicity than cells in more superficial layers (L5/6 = 1.24 ± 0.47; L4 = 0.75 ± 0.7, P = 0.038; L2/3 = 0.82 ± .042, P = 0.033) (Fig. 5C). In contrast, despite the significant entrainment of cells throughout the cortex, RS cells did not show laminar specificity during spontaneous gamma (L2/3 = 0.76 ± 0.5, L4 = 0.66 ± 0.27, L5/6 = 0.71 ± 0.34; Fig. 5D). The percentage of rhythmic cells followed the same trends as the mean RI for both FS and RS cells during spontaneous gamma (Fig. 5, E and F). Taken together, these results show that the RS cells in L2/3 and L5/6 are specifically engaged during stimulus-driven gamma activity and that both types of gamma activity more powerfully engage FS cells in the deep layers.
Strong entrainment of deep-layer cells is in apparent contradiction to the relatively lower amplitude of gamma oscillation in deep layers compared with superficial layers (see Fig. 2E and Fig. 5, C–F). To ensure that this contradiction was not due to contamination of the LFP by spike waveforms, we removed spike waveforms of rhythmic cells from the LFP and recalculated gamma spectral measurements (see materials and methods). The gamma power ratio measurements changed <1.5% from those reported in Fig. 2E. As an additional confirmation, we calculated the correlation between the LFP gamma power ratio and the number of cells entrained to gamma and found no relationship, allowing us to conclude that the spike waveforms are not contributing significantly to the measured gamma power of the LFP.
Stimulus-driven, but not spontaneous, gamma frequency activity depends on GABAergic transmission between RS cells.
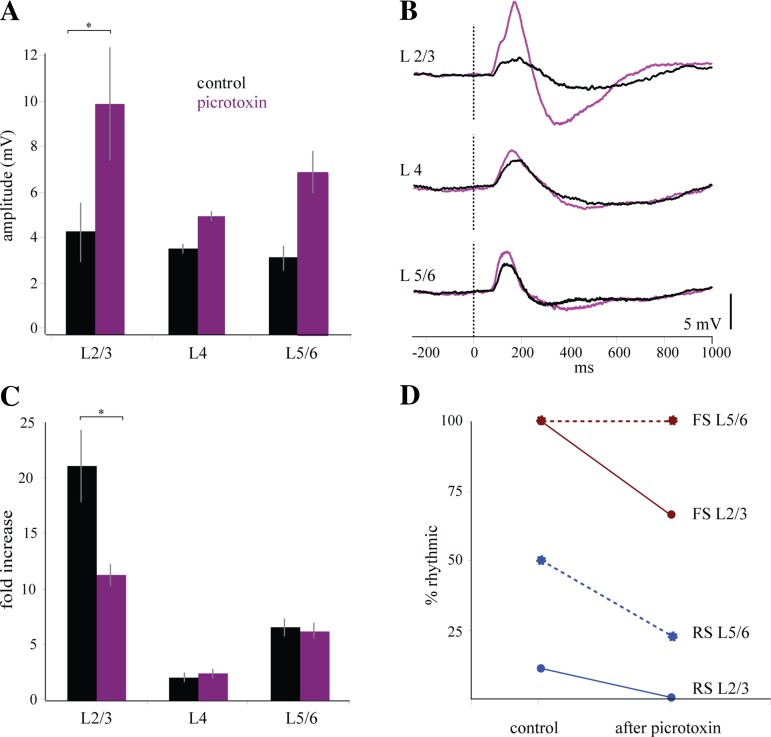
Anatomical and electrophysiological studies of cortical circuits have found a strong efferent drive from L2/3 to L5/6 (Binzegger et al. 2009; Thomson et al. 2002). We hypothesized that the increase in gamma frequency activity in the LFP and single units of L2/3 during stimulus-associated gamma might be driving the increased rhythmicity seen in FS and RS cells in L5/6. To test this hypothesis, we specifically reduced gamma-frequency activity in the superficial layers through topical application of a weak concentration of picrotoxin (0.75 μM, 75 μl) to the surface of the cortex and presented drifting gratings over the course of 20–30 min. Within 5–10 min, the picrotoxin had noticeably increased the size of the evoked response in the LFP throughout all cortical layers (L2/3 increased from 0.004 mV to 0.01 mV, P = 0.02; L4 increased from 0.004 mV to 0.005 mV, n.s.; L5/6 increased from 0.003 mV to 0.007 mV, n.s.) (Fig. 6A; for example voltage traces, see Fig. 6B). Correspondingly, the magnitude of stimulus-driven gamma activity in L2/3 was selectively reduced (L2/3 gamma ratio value dropped from 21.1 ± 7.9 to 11.4 ± 2.4, P = 0.02; L4 rose from 2.1 to 2.5; L5/6 dropped from 6.5 ± 2.2 to 6.3 ± 2, n.s.) (Fig. 6C). The reduction of the GABAA-dependent transmission also reduced the percentage of rhythmic cells in L2/3 (50% reduction) and L5/6 (15%) (Fig. 6D). FS and RS cells were identified on the basis of their waveform shape, as described above (n = 30 RS, 7 FS). In L2/3, the number of rhythmic cells was reduced for both FS cells (33% reduction, n = 3) and RS cells (100% reduction, n = 9). In L5/6 the percentage of RS cells with significant rhythmicity was reduced (88% reduction, n = 18), but the number of rhythmic FS cells in L5/6 was unchanged (n = 3).
Fig. 6.
Gamma activity is reduced after the application of picrotoxin. A: GABAA receptor antagonist picrotoxin increases the amplitude of the evoked response in the LFP during presentation of the drifting grating through all layers of cortex, with the largest increase in L2/3 (n = 3 animals, *P = 0.0215). B: single traces of evoked responses per layer showing that the effect of picrotoxin is to increase the amplitude of the visually evoked response only in supragranular layers. C: picrotoxin reduces the gamma power (20–50 Hz) during the presentation of the visual stimulus in L2/3 (*P = 0.0313). D: % of rhythmic RS cells (n = 30) in both L2/3 and L5/6 decreases after the application of picrotoxin (100% and 88% decrease); % of rhythmic FS cells (n = 7) falls in L2/3 (33% decrease) but not in L5/6 (0% decrease).
These results suggest that networks of FS and RS cells in L2/3 generated gamma oscillations driven by sensory stimulation through a mechanism that was dependent on GABAergic transmission. This network activity underlying gamma generation in L2/3 during visual stimulation contributed to the rhythmicity of RS but not FS cells in L5/6. This result suggests a mechanism for L5/6 RS cell entrainment based either on the location of their apical dendrites in L2/3 or on excitatory vertical connectivity and suggests that networks of FS cells in L5/6 are capable of generating gamma oscillations independently of their RS neighbors and of afferent drive from L2/3. Furthermore, the magnitude of stimulus-driven gamma in L5/6 was unchanged after the application of picrotoxin (Fig. 6C), suggesting that L5/6 stimulus-driven gamma at a population level does not depend on activity in L2/3 or RS cell entrainment in L5/6. This suggestion is consistent with the hypothesis that FS cells are the primary drivers of gamma-frequency activity in L5/6.
DISCUSSION
Our goal was to compare the laminar distribution of gamma-frequency activity driven by visual stimuli with that occurring spontaneously in the V1 of the mouse. We use the term “spontaneous” simply to indicate oscillations that occur in the absence of a visual stimulus. We do not imply that gamma-band activity that is not directly triggered by a sensory stimulus is devoid of function or information, as abundant evidence indicates that ongoing gamma oscillations may underlie a multiplicity of information processing operations (Llinas et al. 1998, 1999, 2007, 2015; Llinas and Ribary 1998; Llinas and Steriade 2006; Roy and Llinas 2008; Sekar et al. 2013). Our results show that visual stimuli drive a 4- to 20-fold increase of a narrow spectral band of gamma oscillations (peak = 29 Hz, width = 13 Hz) in the LFP of granular and supragranular layers, which is 1.5–2 times larger than the fold increase in gamma in infragranular layers. In contrast, spontaneous gamma is spectrally wider (23 Hz), albeit with the same peak frequency (29 Hz), with a nonsignificant tendency for an increase in gamma power from deep to superficial layers.
Surprisingly, the study of single units revealed discrepancies between the distribution of LFP gamma and rhythmic units. These differences were much more pronounced for evoked than spontaneous gamma. During spontaneous gamma, the percentage of rhythmic units was highest in infragranular layers (27%), followed by supragranular (17%) and granular (9%), while RI was not different among layers, consistent with the evenly distributed LFP gamma power. Visual stimulation drove the highest percentage of rhythmic cells and mean RI in infragranular (59%, 1.2) layers, followed by supragranular (38%, 1.0) and granular (20%, 0.8) layers, which is exactly the inverse of the arrangement of LFP gamma power (granular > supragranular ≫ infragranular). Thus, compared with spontaneous gamma, visual stimuli drove many more units into gamma oscillations and tightened their phase relations with the local field (i.e., increased their RI). However, both types of gamma led to an uneven distribution of cellular rhythmicity, with higher values in infragranular and supragranular compared with granular layers.
Breaking the number of rhythmic cells into RS (putative excitatory) and FS (putative inhibitory) cells showed a higher percentage and a larger RI of FS cells in infragranular layers compared with the other layers for both spontaneous and evoked gamma. In contrast, rhythmic RS cells were equally distributed across depth during spontaneous gamma but were more rhythmic in supra- and infragranular than granular layers during visual stimulation. Thus overall a higher percentage of cells demonstrating higher RI values were present in infragranular and supragranular layers than in granular layer.
This apparent discrepancy between units and fields may originate from their different levels of spatial resolution. Single-unit recordings report, by definition, from a single cell, but LFPs represent the average synaptic activity from a surrounding volume of tissue much larger than a single cell. Even when recorded by the same electrode as a single unit, the LFP represents an unknown number of activity sources that introduce noise and blur the correlation between the cell and the LFP activity. As the LFP captures a more diverse set of voltage fluctuations, synchrony between these sources is likely to decrease, resulting in increased “spectral noise,” which represents an increase in the SD of the power spectrum. Increased spectral noise appears to have an inverse relationship with cellular rhythmicity. For example, in Fig. 2B L4 has the largest spectral noise and shows proportionately the least neuronal rhythmicity, despite having the largest fold increase in gamma power. Infragranular layers, in contrast, have the least amount of noise and the largest cellular rhythmicity, despite demonstrating the smallest fold increase in gamma. Supragranular layers have intermediate levels of noise and neuronal rhythmicity.
Thus the discrepancy between laminar distribution of rhythmic cells and LFP gamma power may result from the existence of overlapping ensembles of oscillating cells in granular and supragranular layers with different and shifting phase relations with the average gamma oscillations recorded in the LFP. By representing the average of synaptic potentials in the local vicinity, the LFP may mask the fine-grain synaptic structure underlying the entrainment of specific population of cells into synchronized ensembles. Moreover, individual neurons may rapidly switch between ensembles and participate in synchronized gamma networks having different phase relations to our “average” gamma LFP, thus leading to lower rhythmicity. In contrast, in infragranular layers, the almost absent spectral noise suggests quieter networks in response to visual stimulation and, consequently, a cleaner LFP average. Less noisy networks would lead to an average LFP that is more representative of the cell recorded by the same electrode, thus leading to higher RI and larger percentage of rhythmic cells. This interpretation implies that gamma activity would be tighter, or less flexible, in terms of participating neurons in infragranular compared with granular and supragranular layers. If our interpretation is correct, the signal-to-noise ratio is a critical factor in understanding and interpreting measurement of gamma power and neuronal rhythmicity.
The different population dynamics between supra- and infragranular layers is compatible with their diverse roles in cortical function, which in turn is in agreement with the evolutionary view of the thalamus (Llinas et al. 1998, 1999, 2007, 2015; Llinas and Ribary 1998; Llinas and Steriade 2006; Roy and Llinas 2008; Sekar et al. 2013). Supragranular layers integrate cortico-cortical information with rapidly changing thalamocortical input, while infragranular layers are responsible for generating subcortical outputs.
This work demonstrates that the spatiotemporal characteristics of gamma oscillations may undergo state-specific modulation and suggests that aspects of gamma oscillation laminar geometry are a component of the sensory response in primary cortical areas.
Gamma oscillations are ubiquitous during sensory processing and spontaneous background activity.
Over the last several decades, many studies have produced evidence that gamma oscillations in neocortex are relevant to information representation. Initial work demonstrated increased synchrony in gamma-frequency activity in single units in V1 (Gray et al. 1989; Gray and Singer 1989). Later studies revealed that the gamma component of LFPs in V1 is directly modulated by stimulus characteristics (Frien et al. 2000; Henrie and Shapley 2005). Work in humans showed that gamma oscillations are modulated by perceptual processes, such as the interpretation of gestalt stimuli (Keil et al. 1999; Tallon-Baudry and Bertrand 1999) and are structured differently in patients with psychiatric disorders such as schizophrenia (Kwon et al. 1999; Spencer et al. 2004). Furthermore, results from magnetoencephalography (MEG) have shown that gamma oscillations display a specific spatiotemporal organization over the entire human cortex that is reset by sensory stimuli (Ribary et al. 1999).
However, gamma-frequency activity is not only present during information processing-related activity but also occurs spontaneously as part of the background activity of the brain. Spontaneous gamma activity is found during the depolarization phases of anesthesia and slow-wave oscillations, REM sleep, and brain activation by stimulation of neuromodulatory systems of the brain stem and in the waking state not in association with motor or sensory activity (Steriade et al. 1996b; reviewed in Steriade 2006). The ubiquity of gamma oscillations during a wide variety of brain states brings into question whether gamma-frequency activity is stereotypic or state specific, regarding both its spatiotemporal properties and the cellular networks it entrains.
Spatiotemporal structure of gamma oscillations during background activity and sensory processing.
While there are few studies that directly compare the structure of gamma oscillations in different functional or behavioral contexts, a number of studies have described the structure of spontaneous gamma activity. It was shown that spontaneous gamma oscillations have limited spatial synchrony in the cortex of awake cats (Steriade et al. 1996a) and rats (Sirota et al. 2008) and there are multiple distributed current sinks and sources through the depth of the cortex (Steriade and Amzica 1996). The study by Steriade and Amzica (1996) reported for the first time that gamma oscillations occur in tight phase relations across the depth of the cortex and that single-cell firing was tightly locked to the oscillations, indicative of local generation rather than volume conduction. That LFPs are indeed local in nature has been quantified in cat V1 (Katzner et al. 2009; Xing et al. 2009). We have found a similar tight relation between cell firing and LFPs as quantified by PEHs centered on the peaks of gamma oscillations in the LFP. Furthermore, in our study we quantified the phase shift of visually driven gamma across layers and found a systematic positive shift from L4 to L2/3 of approximately 3 ms and a systematic negative phase shift from L4 to L5/6 of approximately −2 ms. Thus gamma oscillations occur with very small (<6 ms) shifts of their positive peaks across the cortical depth, which may allow effective communication between layers of gamma frequency spikes.
Gamma oscillations produced by stimulation of brain stem cholinergic systems have a homogeneous amplitude distribution through the layers of the cortex (Steriade and Amzica 1996), similar to our results for spontaneous gamma activity. Brain stem cholinergic systems depolarize thalamic nonspecific nuclei, which in turn globally activate the neocortex through glutamatergic transmission (Steriade 2006). A study comparing spontaneous gamma oscillations with those evoked by stimulation of an intralaminar, nonspecific thalamic nucleus showed a similar and homogeneous distribution through the depth of the cortex (Sukov and Barth 1998). The similarity between gamma oscillations due to activation of diffusely projecting thalamic nuclei and those occurring spontaneously is consistent with the hypothesis that nonspecific thalamic inputs may be an important component of the background gamma activity that characterizes functional brain states (Jones 2002; Llinás and Pare 1997; Steriade 2000) and which is altered in several pathological conditions (Llinás et al. 1999). Furthermore, it clearly distinguishes spontaneous gamma oscillations with a nonspecific thalamic contribution from those generated by specific sensory inputs as reported in this study and suggests that the origin and structure of excitatory inputs to cortex may produce the state-specific modification of gamma oscillations. Finally, despite the differences in structure, our study showed that spontaneous and sensory-driven gamma share a common mean frequency and spectral amplitude that are in agreement with a similar comparison made in the primary auditory cortex of the rat (Lakatos et al. 2005).
Our results show an increase in gamma oscillation magnitude in the superficial layers that occurs only during stimulus-driven gamma. Similar findings of enhanced gamma activity in superficial layers during visual stimulation have been reported previously in visual cortex of awake macaque (van Kerkoerle et al. 2014; Xing et al. 2012). Specific activation of L4 and L2/3 gamma oscillations with high interlayer coherence during the processing of sensory stimuli is consistent with the known functional architecture of the cortex. Pyramidal cells in L2/3 have widespread horizontal connections that are known to be critical for cortico-cortical information processing (Barbas et al. 2005; Binzegger et al. 2009; Felleman and Van Essen 1991), and strong excitatory projections from thalamorecipient L4 neurons drive excitatory activity in L2/3 during the representation of sensory stimuli. Furthermore, the coherence at gamma frequency along the vertical axis of the cortex is highly increased during visual responses but mostly between L4 and L2/3.
In apparent contrast to our findings, Maier et al. (2011) report higher gamma oscillation magnitude in superficial than deep cortical layers during spontaneous activity in awake monkeys. However, their study did not compare the magnitude of stimulus-driven and spontaneously driven gamma oscillations, making these results difficult to compare directly to ours. In addition, this work may be consistent with the slight trend toward larger gamma oscillation magnitude in superficial layers during spontaneous activity (see Fig. 2E). Finally, a portion of this difference in our findings and those of Maier et al. may be attributable to the reduction in top-down activity in anesthetized animals, which may decrease excitatory input to the superficial layers of cortex (Barbas et al. 2005).
It has been suggested that gamma oscillations may “bind” together assemblies of neurons that are representing a single percept, but recent studies have shown that gamma activity depends instead on stimulus intensity, not perceptual grouping (Chalk et al. 2010; Henrie and Shapley 2005; Ray and Maunsell 2011). We find that gamma oscillations that are produced during sensory representation in the primary sensory areas are distinct from those occurring in the absence of sensory stimulation. Our work does not reflect a role for “binding” of a percept but does suggest that gamma activity is a distinct component of the sensory response in V1.
In vitro cortical slice studies have suggested that there may be two independent gamma-generating circuits in the cortex, one in superficial layers and one in deep layers, because a cut in L4 does not reduce the power of pharmacologically induced gamma in either L2/3 or L5/6 (van Aerde et al. 2009; Roopun et al. 2006). Similarly, in vivo experiments demonstrate coherence in the gamma band either among superficial-layer LFPs or among deep-layer LFPs, with a distinct transition between the two zones in the thalamorecipient layers (Maier et al. 2010). Supra- and infragranular-layer gamma oscillations were differentially modulated by ongoing alpha oscillations in the awake, behaving macaque, providing evidence for laminar specificity in gamma activity (Spaak et al. 2012). Additionally, in both in vitro studies, superficial-layer gamma was dependent on chloride GABAergic transmission. The laminar functional architecture of sensory-driven gamma reported here depends on GABAergic transmission as well, since superficial application of picrotoxin abolished gamma activity in L2/3. Furthermore, superficial reduction of gamma oscillation amplitude by picrotoxin did not reduce gamma activity in the LFP of deep layers but did reduce the RI of RS cells, suggesting that in vivo as well deep and superficial layers of cortex may have distinct, but related, networks for generating oscillations.
Rhythmicity in supragranular layers may be related to infragranular layers either by direct projections to L5 or by contacting L5 apical dendrites in L2/3; this second possibility is supported by the much stronger LFP gamma in supragranular layers. In addition, picrotoxin in superficial layers reduced the RI of deep RS cells (which presumably would have apical dendrites in superficial layers), further supporting the notion that such cells receive entraining input in their apical dendrites. From the preceding arguments it follows that not all L5 cells would be entrained by supragranular input but only those with apical dendrites in L2/3.
Excitatory and inhibitory network contribution to gamma oscillations in cortex.
A strong case has been made for the importance of the FS cell network, mediated by gap junctions, in the generation of cortical gamma oscillations. Inhibitory transmission is thought to carry a large portion of the gamma rhythm (Hasenstaub et al. 2005), and rhythmic depolarization of FS cells produces gamma activity in the LFP and increases spike precision (Cardin et al. 2005). Inhibitory interneurons are necessary for the robust generation of gamma oscillations in cortex (Cardin et al. 2009; Sohal et al. 2009; Takada et al. 2014). In addition, in vitro studies have shown the generation of cortical gamma to be abolished by the blockade of GABAA transmission (Roopun et al. 2006; Traub et al. 2005). However, excitatory neurons do contribute to the generation of gamma oscillations through increased excitatory drive, as demonstrated by the depolarization of superficial RS cells that produced gamma activity in the LFP and drove the firing of RS cells in L5/6 (Adesnik and Scanziani 2010), consistent with the known excitatory drive from L2/3 to L5/6 in sensory cortex (Binzegger et al. 2009; Thomson et al. 2002).
Our work highlights the involvement of both putative excitatory and inhibitory networks in gamma rhythmogenesis but suggests that the entrainment of excitatory and inhibitory cells varies with the context in which gamma oscillations are generated. RS cells in L2/3 and L5/6 show increased rhythmicity during stimulus-driven activity compared with ongoing gamma oscillations. Furthermore, disruption of rhythmic activity in the superficial layers by the GABAA receptor antagonist picrotoxin produces a corresponding decrease in the entrainment of all superficial neurons, along with a specific decrease in the entrainment of only RS cells in the deep layers. This is consistent with the hypothesis put forward by Adesnik and Scanziani, that gamma activity in L2/3 contributes to the entrainment of RS cells in L5/6. Thus the excitatory cell network seems to be specifically engaged by stimulus-driven gamma oscillations in superficial and deep cortex.
In contrast, putative inhibitory cells are more rhythmic than putative excitatory cells throughout the cortex but only display context-specific modulation of rhythmicity in the deep layers. Superficial FS cells display similar rhythmicity regardless of context and always are less rhythmic than their deep-layer FS counterparts. In contrast, FS cells in deep layers show increased overall rhythmicity during stimulus-driven gamma but also maintain high rhythmicity during spontaneous activity. The specific reduction of superficial gamma oscillations with picrotoxin does not produce a corresponding reduction in deep-layer gamma oscillation amplitude or FS cell entrainment to gamma activity, even as the rhythmicity of deep-layer RS neurons declines. The relative independence of deep-layer FS neurons from superficial excitatory drive is consistent with in vitro studies showing that FS-RS connected pairs of neurons in L5/6 are less likely to receive strong excitatory input than non-FS-RS connected pairs (Otsuka and Kawaguchi 2009). These results suggest an independent gamma generation mechanism in deep layers that may depend primarily on FS interneuron contribution.
Taken together, these findings show that the dynamic geometry of gamma oscillations through the cortical laminae is influenced by the state in which they occur. The spatiotemporal distribution observed may reflect the divergent sources of excitatory drive to the cortex inherent to different brain states. In addition, excitatory and inhibitory cell networks contribute differently depending on context of the oscillations, with putative excitatory neurons generally showing greater context specificity than putative inhibitory neurons. Our results also are consistent with other reports suggesting that superficial and deep layers of cortex have related but independent networks for generating gamma oscillations. In addition, they suggest that the GABAergic-dependent mechanism for the generation of gamma oscillations in the superficial cortex is a component of the representation of sensory information in the cortex.
GRANTS
This work was supported by National Institutes of Health Grant R01 EY-020765, Conte Center P50 MH-064045, and Vision Training Grant T32 EY-00735.
DISCLAIMERS
The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.G.W. and D.C. conception and design of research; C.G.W. performed experiments; C.G.W. analyzed data; C.G.W. and D.C. interpreted results of experiments; C.G.W. prepared figures; C.G.W. drafted manuscript; C.G.W. and D.C. edited and revised manuscript; C.G.W. and D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Daniel Denman for his help with writing code and for providing some experimental data. We thank Noah Roy and Ashlan Reid for providing analysis code and Larry Palmer, Jason Wester, Leif Vigeland, Jessica Cardin, and other members of the Contreras lab for their helpful discussion of this work. We also thank Eugene Civillico and Victor Krauthamer for their critical review of the manuscript.
Present address of C. G. Welle: Depts. of Neurosurgery and Bioengineering, Univ. of Colorado School of Medicine, Aurora, CO.
REFERENCES
- Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature 464: 1155–1160, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Aerde KI, Mann EO, Canto CB, Heistek TS, Linkenkaer-Hansen K, Mulder AB, van der Roest M, Paulsen O, Brussaard AB, Mansvelder HD. Flexible spike timing of layer 5 neurons during dynamic beta oscillation shifts in rat prefrontal cortex. J Physiol 587: 5177–5196, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273: 1868–1871, 1996. [DOI] [PubMed] [Google Scholar]
- Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex 15: 1356–1370, 2005. [DOI] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ, Martin KA. Topology and dynamics of the canonical circuit of cat V1. Neural Netw 22: 1071–1078, 2009. [DOI] [PubMed] [Google Scholar]
- Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci 22: 10966–10975, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Palmer LA, Contreras D. Stimulus-dependent gamma (30–50 Hz) oscillations in simple and complex fast rhythmic bursting cells in primary visual cortex. J Neurosci 25: 5339–5350, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalk M, Herrero JL, Gieselmann MA, Delicato LS, Gotthardt S, Thiele A. Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1. Neuron 66: 114–125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Timofeev I, Steriade M. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol 494: 251–264, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK. Top-down attentional processing enhances auditory evoked gamma band activity. Neuroreport 14: 683–686, 2003. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci 19: 4595–4608, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager UC. Receptive fields of single cells and topography in mouse visual cortex. J Comp Neurol 160: 269–290, 1975. [DOI] [PubMed] [Google Scholar]
- Eckhorn R, Frien A, Bauer R, Woelbern T, Kehr H. High frequency (60–90 Hz) oscillations in primary visual cortex of awake monkey. Neuroreport 4: 243–246, 1993. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1–47, 1991. [DOI] [PubMed] [Google Scholar]
- Freeman JA, Nicholson C. Experimental optimization of current source-density technique for anuran cerebellum. J Neurophysiol 38: 369–382, 1975. [DOI] [PubMed] [Google Scholar]
- Frien A, Eckhorn R, Bauer R, Woelbern T, Gabriel A. Fast oscillations display sharper orientation tuning than slower components of the same recordings in striate cortex of the awake monkey. Eur J Neurosci 12: 1453–1465, 2000. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291: 1560–1563, 2001. [DOI] [PubMed] [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338: 334–337, 1989. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA 86: 1698–1702, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber T, Tsivilis D, Montaldi D, Müller MM. Induced gamma band responses: an early marker of memory encoding and retrieval. Neuroreport 15: 1837–1841, 2004. [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsáki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84: 401–414, 2000. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron 47: 423–435, 2005. [DOI] [PubMed] [Google Scholar]
- Henrie JA, Shapley R. LFP power spectra in V1 cortex: the graded effect of stimulus contrast. J Neurophysiol 94: 479–490, 2005. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci 8: 347–355, 2004. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Bear MF. Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo. J Neurosci 21: 9801–9813, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Thalamic circuitry and thalamocortical synchrony. Philos Trans R Soc Lond B Biol Sci 357: 1659–1673, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron 61: 35–41, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Müller MM, Ray WJ, Gruber T, Elbert T. Human gamma band activity and perception of a gestalt. J Neurosci 19: 7152–7161, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature 425: 954–956, 2003. [DOI] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, van der Togt C, Roelfsema PR. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci USA 111: 14332–14341, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry 56: 1001–1005, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol 94: 1904–1911, 2005. [DOI] [PubMed] [Google Scholar]
- Laurent G. Dynamical representation of odors by oscillating and evolving neural assemblies. Trends Neurosci 19: 489–496, 1996. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Choi S, Urbano FJ, Shin HS. Gamma-band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Natl Acad Sci USA 104: 17819–17824, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Pare D. Coherent Oscillations in Specific and Non-Specific Thalamocortical Networks and Their Role in Cognition. Amsterdam: Elsevier, 1997. [Google Scholar]
- Llinas RR, Ribary U. Temporal conjunction in thalamocortical transactions. Adv Neurol 77: 95–102, 1998. [PubMed] [Google Scholar]
- Llinas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1841–1849, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA 96: 15222–15227, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol 95: 3297–3308, 2006. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Ustinin MN, Rykunov SD, Boyko AI, Sychev VV, Walton KD, Rabello GM, Garcia J. Reconstruction of human brain spontaneous activity based on frequency-pattern analysis of magnetoencephalography data. Front Neurosci 9: 373, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A, Barthó P, Harris KD. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron 62: 413–425, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A, MacLean JN. Default activity patterns at the neocortical microcircuit level. Front Integr Neurosci 6: 30, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KD, Brett B, Barth DS. Inter- and intra-hemispheric spatiotemporal organization of spontaneous electrocortical oscillations. J Neurophysiol 76: 423–437, 1996. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron 48: 811–823, 2005. [DOI] [PubMed] [Google Scholar]
- Maier A, Adams GK, Aura C, Leopold DA. Distinct superficial and deep laminar domains of activity in the visual cortex during rest and stimulation. Front Syst Neurosci 4: 31, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Aura CJ, Leopold DA. Infragranular sources of sustained local field potential responses in macaque primary visual cortex. J Neurosci 31: 1971–1980, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed Brain Dynamics. New York: Oxford Univ. Press, 2007. [Google Scholar]
- Nase G, Singer W, Monyer H, Engel AK. Features of neuronal synchrony in mouse visual cortex. J Neurophysiol 90: 1115–1123, 2003. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci 28: 7520–7536, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons. J Neurosci 29: 10533–10540, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu AT, Popa D, Paré D. Coherent gamma oscillations couple the amygdala and striatum during learning. Nat Neurosci 12: 801–807, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol 9: e1000610, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribary U. Dynamics of thalamo-cortical network oscillations and human perception. Prog Brain Res 150: 127–142, 2005. [DOI] [PubMed] [Google Scholar]
- Ribary U, Cappell J, Mogilner A, Hund-Georgiadis M, Kronberg E, Llinás R. Functional imaging of plastic changes in the human brain. Adv Neurol 81: 49–56, 1999. [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, Mogilner A, Llinás R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci USA 88: 11037–11041, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun AK, Middleton SJ, Cunningham MO, LeBeau FE, Bibbig A, Whittington MA, Traub RD. A beta2-frequency (20–30 Hz) oscillation in nonsynaptic networks of somatosensory cortex. Proc Natl Acad Sci USA 103: 15646–15650, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Llinas R. Dynamic geometry, brain function modeling, and consciousness. Prog Brain Res 168: 133–144, 2008. [DOI] [PubMed] [Google Scholar]
- Sakata S, Harris KD. Laminar-dependent effects of cortical state on auditory cortical spontaneous activity. Front Neural Circuits 6: 109, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci USA 90: 4470–4474, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131: 1–11, 2005. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Poort J, Oostenveld R, Fries P. Selective movement preparation is subserved by selective increases in corticomuscular gamma-band coherence. J Neurosci 31: 6750–6758, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci 23: 10809–10814, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar K, Findley WM, Poeppel D, Llinas RR. Cortical response tracking the conscious experience of threshold duration visual stimuli indicates visual perception is all or none. Proc Natl Acad Sci USA 110: 5642–5647, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60: 683–697, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaak E, Bonnefond M, Maier A, Leopold DA, Jensen O. Layer-specific entrainment of γ-band neural activity by the α rhythm in monkey visual cortex. Curr Biol 22: 2313–2318, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA 101: 17288–17293, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience 101: 243–276, 2000. [DOI] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience 137: 1087–1106, 2006. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Intracortical and corticothalamic coherency of fast spontaneous oscillations. Proc Natl Acad Sci USA 93: 2533–2538, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci 16: 392–417, 1996a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Amzica F, Timofeev I. Synchronization of fast (30–40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J Neurosci 16: 2788–2808, 1996b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukov W, Barth DS. Three-dimensional analysis of spontaneous and thalamically evoked gamma oscillations in auditory cortex. J Neurophysiol 79: 2875–2884, 1998. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG, Bezdudnaya T. Activation of a cortical column by a thalamocortical impulse. J Neurosci 22: 7766–7773, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski FD, Garcia-Lazaro JA, Schnupp JW. Current source density profiles of stimulus-specific adaptation in rat auditory cortex. J Neurophysiol 102: 1483–1490, 2009. [DOI] [PubMed] [Google Scholar]
- Takada N, Pi HJ, Sousa VH, Waters J, Fishell G, Kepecs A, Osten P. A developmental cell-type switch in cortical interneurons leads to a selective defect in cortical oscillations. Nat Commun 5: 5333, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3: 151–162, 1999. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2–5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex 12: 936–953, 2002. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, LeBeau FE, Cunningham MO, Whittington MA. Persistent gamma oscillations in superficial layers of rat auditory neocortex: experiment and model. J Physiol 562: 3–8, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 286: 1943–1946, 1999. [DOI] [PubMed] [Google Scholar]
- Xing D, Yeh CI, Burns S, Shapley RM. Laminar analysis of visually evoked activity in the primary visual cortex. Proc Natl Acad Sci USA 109: 13871–13876, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Yeh CI, Shapley RM. Spatial spread of the local field potential and its laminar variation in visual cortex. J Neurosci 29: 11540–11549, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]