Abstract
Cues from social partners trigger the activation of socially responsive neuromodulatory systems, priming brain regions including sensory systems to process these cues appropriately. The fidelity with which neuromodulators reflect the qualities of ongoing social interactions in sensory regions is unclear. We addressed this issue by using voltammetry to monitor serotonergic fluctuations in an auditory midbrain nucleus, the inferior colliculus (IC), of male mice (Mus musculus) paired with females, and by concurrently measuring behaviors of both social partners. Serotonergic activity strongly increased in male mice as they courted females, relative to serotonergic activity in the same males during trials with no social partners. Across individual males, average changes in serotonergic activity were negatively correlated with behaviors exhibited by female partners, including broadband squeaks, which relate to rejection of males. In contrast, serotonergic activity did not correlate with male behaviors, including ultrasonic vocalizations. These findings suggest that during courtship, the level of serotonergic activity in the IC of males reflects the valence of the social interaction from the perspective of the male (i.e., whether the female rejects the male or not). As a result, our findings are consistent with the hypothesis that neuromodulatory effects on neural responses in the IC may reflect the reception, rather than the production, of vocal signals.
Keywords: auditory, serotonin, inferior colliculus, courtship, sender-receiver matching
the exchange of information between conspecific partners forms the basis for making social decisions in many animals (Clutton-Brock and McAuliffe 2009; van Staaden et al. 2011). During interactions like courtship, behavioral signals between participants can change as an encounter unfolds (Akre and Ryan 2011; Patricelli et al. 2004). Neural mechanisms that both respond to the social milieu and optimize central processing of communication signals on a relatively short time scale could help individuals cope with variation among social encounters. In this article, we present evidence that variation in the level of activation of the serotonergic system parallels variation in the valence (positive or negative quality; Goodson and Wang 2006; Russell 1935) of social encounters and makes this information available to a primary auditory region.
Serotonin is an important component of the central nervous system's response to social interaction across many animal taxa. In multiple brain areas, serotonergic fluctuations reflect shifts in social context (Hall et al. 2011; Mas et al. 1995; Nakazato 2013; Watt et al. 2007). Serotonin is also a strong modulator of sensory responses to incoming social stimuli (Deemyad et al. 2013; Hurley and Sullivan 2012). The social responsiveness of the serotonergic system thus has a potentially important role in sensory processing during conspecific interaction. However, there has been little investigation of serotonergic fluctuations in sensory regions during ongoing social encounters, and whether these fluctuations reflect variation within a context like opposite-sex interaction.
We explored this issue by measuring serotonergic activity in the inferior colliculus (IC), an auditory midbrain nucleus that is richly innervated by serotonergic fibers and contains an array of serotonin receptors (Hurley 2006; Hurley and Sullivan 2012; Hurley and Thompson 2001; Klepper and Herbert 1991). We monitored changes in serotonergic activity in awake and behaving male mice (Mus musculus) during opposite-sex interactions, in which vocal signals are a rich source of social information. During courtship, the emission of different calls relates to the behavioral motivation of male and female mice. Male mice emit copious ultrasonic vocalizations (USVs) in the presence of females, and an increase in the use of a particular structural category of USV, the “harmonic” USV, corresponds in time to bouts of mounting (Hanson and Hurley 2012). Audible squeaks, produced by both sexes during distress, are emitted by females during courtship and have a negative significance for sexually motivated males (Lahvis et al. 2011; McGill 1962; Sales 1972), in that squeaks emitted by females are coupled with kicking at males (Lahvis et al. 2011; Nyby 2001; Sugimoto et al. 2011; Wang HR et al. 2008). These vocalizations and other copulatory behaviors show a clear progression over the courtship interaction. For example, mounting and harmonic USVs occur more frequently later on in a courtship encounter (Hanson and Hurley 2014; Hull and Dominguez 2007; Mosig and Dewsbury 1976). Mouse vocalizations therefore contain potentially useful information for an opposite-sex social partner.
Using this opposite-sex interaction paradigm, we addressed several specific objectives. First, we determined whether changes in serotonergic activity in the male IC occur during interactions with females. We then assessed the sensitivity of serotonergic activity to the relative valence of individual interactions for males (whether the encounter had a positive or negative significance) as monitored through female rejection behaviors, including the production of audible squeaks. On the basis of a previous same-sex study, we predicted that increases in serotonergic activity would be triggered in males by social interaction with females and correlate in magnitude with measures of behavioral arousal of the male subjects, including the production of USVs.
MATERIALS AND METHODS
Animals.
All procedures were approved by the Bloomington Institutional Animal Care and Use Committee (Indiana University). Simultaneous measurements of serotonergic activity and behavior were made using 15 adult (7–21 wk old) male mice (M. musculus, CBA/J). Ten adult (8–22 wk old) female CBA/J mice were used as social partners, unfamiliar to the voltammetry male subjects. Mice were individually housed in standard laboratory cages on a 14:10-h light-dark cycle and fed standard mouse chow.
Before being used as social partners, female mice were given three 15-min encounters with sexually experienced males not used as voltammetry subjects. Only females who were mounted were used as social partners. The estrous states of females were assessed on basis of the cytology of Giemsa-stained epithelial cells collected by vaginal lavage (Goldman et al. 2007; Hanson and Hurley 2012). Male subjects in voltammetry experiments did not participate in any social encounters after arrival in our laboratory at 5 wk of age, except for one voltammetric recording session. This was to exclude social experience of the male subjects as a factor, because previous work in our laboratory has suggested that experience influences the serotonergic signal (Hall et al. 2011; Hanson and Hurley 2014).
Carbon fiber voltammetry.
Social stimuli likely trigger activity in a range of neurochemical systems innervating the IC (Klepper and Herbert 1991; Tong et al. 2005). Fast cyclic voltammetry, which relies on the shape of a voltammogram to distinguish neurochemicals, would therefore likely not allow us to focus on the serotonergic system during natural social behavior. Instead, we used “slow-scan” cyclic staircase voltammetry, which allows neurochemical classes to be distinguished by their oxidation potentials. With this approach, voltage of an electrode relative to a reference is changed in incremental steps and the resulting current is measured after each step (Crespi 2011; Marsden et al. 1988). The resulting plot of voltage vs. the first derivative of the current clearly separates neurochemical classes into different peaks, consisting of 1) ascorbate (AA), 2) catecholamines and their metabolites, and 3) serotonin along with its metabolite, 5-hydroxyindoleacetic acid (5-HIAA), and uric acid (Feng et al. 1987).
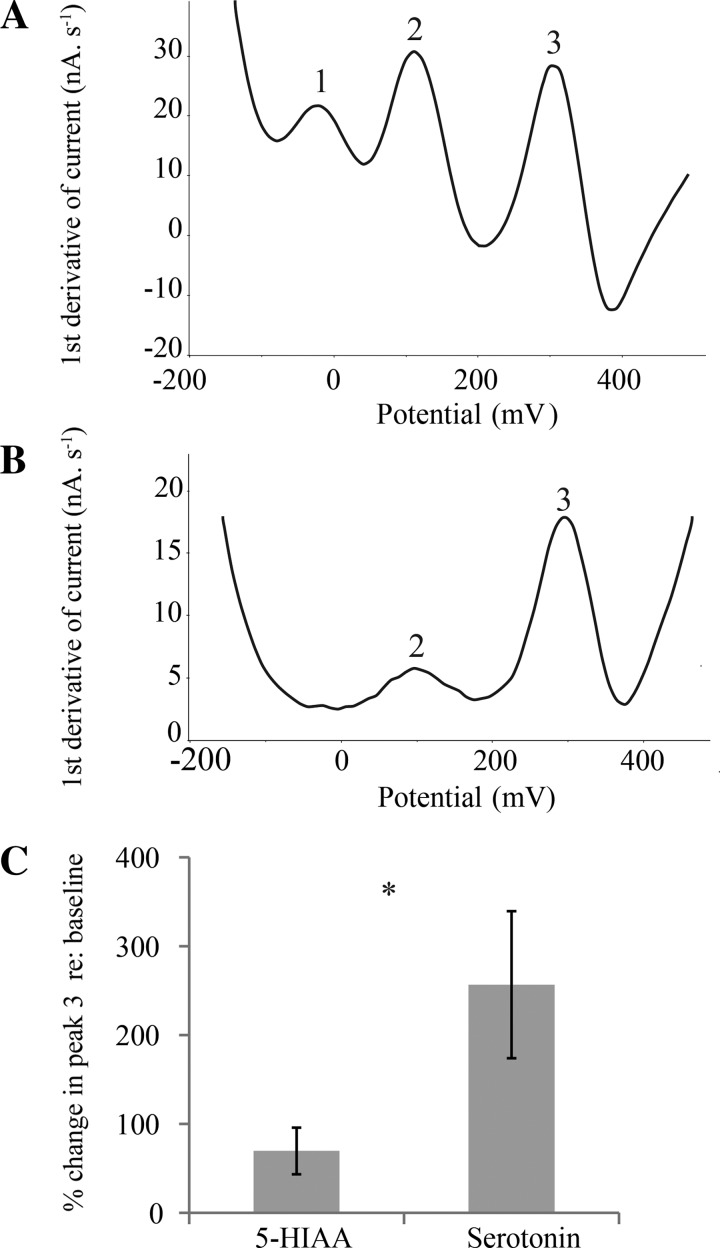
Carbon fiber electrodes (CFEs) were constructed as described in Hall et al. (2010). Tips of pulled capillary tubes (Flaming-Brown P-97; Sutter Instrument, Novato, CA) were broken to a 20- to 30-μm external diameter. An 11-μm-diameter carbon fiber (Thornell P25; Cytec Industries; West Paterson, NJ), protruding 120–130 μm from the glass tip, was secured with epoxy resin (Miller-Stephenson Chemical; Danbury, CT). An electrical pretreatment, consisting of the application of a 70-Hz, 0- to 3-V triangle waveform for 30 s, followed by a 1.5-V potential for 10 s, a −0.5-V potential for 5 s, and a 1.5-V potential for 8 s, was applied to all CFEs. This pretreatment separated the oxidation potentials of AA, catecholamines, and serotonin/5-HIAA as three peaks in the plot of voltage vs. the first derivative of current (Fig. 1A).
Fig. 1.
A: in vitro voltammogram from a typical bare (i.e., without Nafion) electrically pretreated carbon fiber electrode (CFE), recorded in phosphate-citrate-buffered saline containing 50 μM ascorbic acid, 5 μM 3,4-dihydroxyphenylacetic acid (DOPAC), and 1 μM serotonin. This electrode demonstrates 3 oxidative peaks, corresponding to ascorbic acid (peak 1), DOPAC (peak 2), and serotonin (peak 3). B: in vitro pretesting of a Nafion-coated electrode in the same solution as electrode in A. This voltammogram represents a typical electrode with high selectivity for serotonin, as evidenced by an absence of peak 1, a small peak 2, and a large peak 3. C: in vitro comparison of responses of Nafion-coated CFEs to 5-hydroxyindoleacetic acid (5-HIAA) vs. serotonin. Nafion-coated CFEs (n = 10) demonstrate a greater percent change in peak 3 in response to serotonin compared with 5-HIAA (*P < 0.05, paired t-test).
To make the CFEs more selective for serotonin, they were coated with the ion-exchange resin Nafion to exclude anions such as 5-HIAA (Hashemi et al. 2009). Electrodes underwent a series of dips in a 5% solution of Nafion (Sigma-Aldrich) and were air-dried at least 24 h before use. Before its use during in vivo recording sessions, each CFE was pretested in phosphate-citrate-buffered saline containing 50 μM AA, 5 μM 3,4-dihydroxyphenylacetic acid (DOPAC), and 1 μM serotonin (Sigma-Aldrich). Nafion-coated CFEs were selected for use in voltammetric recording sessions on the basis of the sensitivity of the electrodes to serotonin relative to the anions AA and DOPAC, as assessed by the amplitude of the third oxidative peak compared with the first and second peaks (Fig. 1B). CFEs were used in vivo only if the third oxidative peak was larger than the first and second peaks, indicating reduced signals of anions (AA and DOPAC).
The resulting CFEs were more sensitive to serotonin than 5-HIAA (2 μM each; paired t-test, n = 10, P < 0.05; Fig. 1C). In previous in vivo validation experiments in our laboratory (Hall et al. 2010), intraperitoneal injection of the serotonergic precursor 5-hydroxytryptophan increased the size of the serotonergic peak, whereas injection of the monoamine oxidase A inhibitor clorgyline, which catalyzes the conversion of serotonin to its major metabolite, 5-HIAA, had little effect. These results are consistent with our electrodes exhibiting a relatively high selectivity for serotonin in vivo as well as in vitro. This previous study additionally demonstrated that coating our CFEs with Nafion is required to observe state-related changes in the serotonergic oxidative peak. Nafion-coated CFEs measured an expected large increase in serotonergic oxidation during recovery from anesthesia, but noncoated CFEs did not, potentially because any arousal-dependent signal was swamped out by high background levels of extracellular 5-HIAA.
For measurement of voltammetric current by CFEs against a Ag-AgCl reference electrode, a 1-min cyclic staircase waveform (−300 to +600 to −300 mV, 10-mV steps, 30 mV/s2) was applied every 5 min, yielding one measurement every 6 min. The resulting current was recorded using EChem software (eDAQ), which controlled the bipotentionstat (EI-400; Cypress Systems, Chelmsford, MA) and e-corder (eDAQ 821; eDAQ, Denistone East, NSW, Australia). The level of serotonergic oxidation was measured as the amplitude of the third oxidative peak (∼380 mV) on a plot of the first derivative of the current recorded after the voltage waveform was applied. Because of high variability among individual experiments, which could be due to variation in electrode sensitivity or intrinsic variation among male mice, measurements of oxidative current were normalized as percent change at each time point relative to the average of four baseline measurements made immediately before the onset of the experimental stimulus (female partner or control). We refer to these normalized measurements at distinct time points as “serotonergic activity” for several reasons. First, although voltammetric measurements reflect extracellular serotonin (e.g., Borland et al. 2005; Hall et al. 2010; Sanghera et al. 1990), serotonergic activity could be influenced by changes in reuptake mechanisms as well as in release. Additionally, although our electrodes were selective for serotonin, we cannot categorically state that the serotonergic metabolite 5-HIAA made no contribution to the signal. We therefore consider our measure of serotonergic activity to be an indication of functional serotonin availability, with a potential contribution of serotonin turnover.
Cannulation surgery.
Aseptic surgery was performed to fit custom-built Teflon hubs over both ICs to guide and secure electrodes during recording sessions. After the induction of anesthesia (ketamine, 100 mg/kg; xylazine, 5 mg/kg; acepromazine, 2 mg/kg; intraperitoneal), administration of analgesic (1 mg/kg; subcutaneous), and removal of hair from the surgical area, mice were positioned in a stereotaxic device. A sagittal incision followed by deflection of skin provided access to the skull. Two 1.1-mm-diameter holes were drilled in the skull and centered over each IC (1.1 mm posterior and 1.6 mm lateral from lambda). Surgical dental cement secured the hubs to two bone screws. The hubs were plugged with Teflon screws, and mice recovered for at least 5 days before recording sessions.
Voltammetric recording sessions.
On a recording session day, two Teflon microdrives, containing a pretested CFE and a reference electrode, were loaded into the hubs after the subject mouse was administered metacam (1 mg/kg) and ketamine/xylazine (60% surgical dose). After the mouse was returned to its home cage, voltammetric measurements were begun immediately to assess electrode performance, and the recording session started 2 h after recovery from anesthesia. During the recovery period, the serotonergic oxidative current was periodically monitored to ensure that the experiment began at a stable baseline. Four baseline measurements of serotonergic activity were recorded before introduction of the female mouse to the cage. The male subject and female partner interacted freely for 30 min, during which a measurement was made once every 6 min, yielding 5 measurements. After the fifth measurement, the female was removed and three additional measurements were made. Control sessions consisted of interactions with empty cages instead of females. These were performed in the IC contralateral to the experimental IC, with treatment order and laterality balanced across subjects and 5 days between treatments (experimental vs. control). Recordings were made in alternate ICs to minimize the possibility that electrode-induced damage would influence our measurements. Chronic recording through CFEs across multiple recording sessions would be difficult because of the fragility of the CFEs and because sensitivity is reduced by adhesion of organic material to the electrodes over time (Singh et al. 2011).
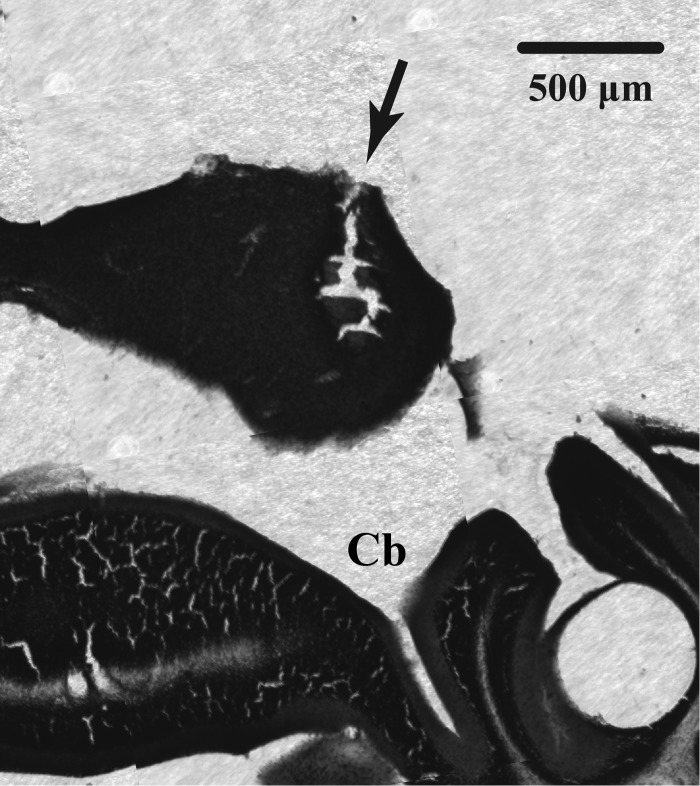
Immediately following an animal's second voltammetric recording session, a small current (0.3 mA) was passed through the carbon fiber to make an electrolytic lesion with a constant current lesion maker (Grass Instruments, Quincy, MA). Mice were neither restrained nor under anesthesia, but they did not show signs of responding to the small current. Mice were transcardially perfused 12–24 h later with 3.7% paraformaldehyde in phosphate-buffed saline, and Nissl-stained coronal sections of brain tissue were examined for lesions (Fig. 2). Electrolytic lesions or electrode tracks were typically centered on ∼250 μm below the dorsal extent of the IC. Because the active carbon fiber surfaces of the electrodes were 120–130 μm in length, the oxidation potentials represent integrated signals over a broad region of the IC.
Fig. 2.
Photomicrograph of a Nissl-stained coronal section of mouse brain, with inferior colliculus (IC) located dorsally. Arrow indicates the lesion left by a CFE used to measure serotonergic activity in the IC. Cb, cerebellum.
Behavioral analysis.
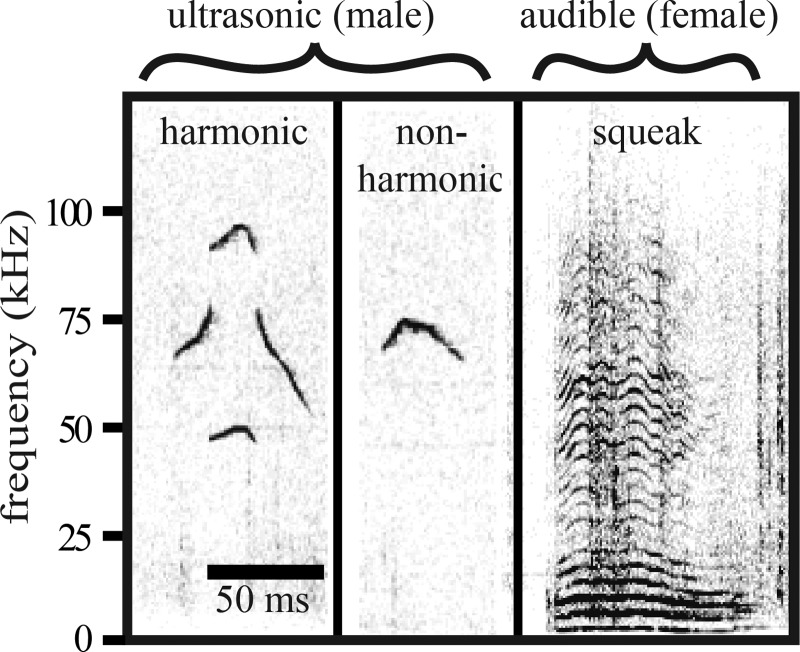
A charge-coupled device video camera (30 frames/s; Q-See 4-channel DVR PCI video capture card, SuperDVR software; Q-See, Digital Peripheral Solutions) and condenser microphone (CM16/CMPA; Avisoft Bioacoustics; 200-kHz maximum range) with a sound card (UltraSoundGate 116 Hb; Avisoft Bioacoustics; 250-kHz sample rate) were positioned above the cage to record social encounters. Avisoft SASlab Pro software (Avisoft Bioacoustics) generated spectrograms for vocalization analysis (FFT length of 512 and Hamming-style window with 50% overlap). Broadband (2–100 kHz) squeaks and narrowband, ultrasonic vocalizations (USVs) were counted (Fig. 3). USVs were categorized as “harmonic” if a call contained harmonic frequencies, a feature that is associated with mounting (Hanson and Hurley 2012). If USVs did not contain this harmonic element, they were categorized as “nonharmonic.”
Fig. 3.
Spectrograms of harmonic and nonharmonic ultrasonic vocalizations (USVs), primarily emitted by males, and a broadband squeak, emitted by females, during male-female courtship encounters.
Nonvocal social behaviors of both partners were measured using ODLog (Macropod Software, Eden Prairie, MN). Behaviors were defined as follows: “immobility” as the subject male performing no observable movement besides breathing; “anogenital investigation” as one mouse's nose contacting the posterior half of its partner; “nose-to-nose investigation” as either mouse's nose in contact with its partner's facial region; and “rejection” as females darting away from or kicking males. Rejections were used to assess inverse receptivity toward males, since female mice rarely display lordosis (Johansen et al. 2008). Although mounting did not occur, all males demonstrated behaviors indicative of interest in females.
Statistical analysis.
All statistical analyses were performed in SPSS version 20.0 (IBM). Linear mixed models, accounting for partial repeated measures due to electrode breakage or movement noise, were used with Bonferroni post hoc corrections (Wright 1992) to test effects of experimental treatment (whether females were present or not), session order, and female estrous state on serotonergic activity over time (at each of 8 measurements, minutes 6–48) relative to the control group. Recording sessions were included when at least two baseline measurements and at least two of five measurements after baseline were not obscured by movement-related noise.
Correlations between male serotonergic activity and behaviors, or the ages and masses of both social participants, were assessed with Spearman rank correlations using the Benjamini-Hochberg method (1995) for controlling false discovery rate. To obtain a single value per male for the change in oxidative current following female introduction, the mean of the four measurements (each a percent change from baseline) taken from 12 to 30 min after female presentation was calculated (the “serotonergic mean”). We used nonparametric Mann-Whitney U-tests to examine whether estrous state of the female partner had an effect on behaviors of the male or female.
To examine time courses of mouse vocal behaviors, we divided calls into 6-min time bins paralleling serotonergic measurements and used repeated-measures analyses of variance (ANOVAs). To ensure that males in our study were showing normal courtship behaviors, we used Welch's t-test to compare the total number of ultrasonic vocalizations emitted by males from this study with that of unmanipulated males from a previous study (Hanson and Hurley 2014). A two-way repeated-measures ANOVA was used to compare time courses in percent use of harmonic calls between this study and unmanipulated males from the same study.
We assessed whether patterns of increasing serotonergic activity and increasing proportional production of harmonic calls over time were associated with each other across males by quantifying the slopes of these measures between the final and initial values over the time of interaction with females (i.e., change in serotonergic activity per minute and change in percent harmonic calls per minute) for each male. For four males, because of missing time points from movement noise, serotonergic slopes were measured from time points immediately before or after the last measurement made during interaction with females, whichever was most consistent with the overall trend in serotonergic activity. We then conducted a Spearman's correlation on the slope of serotonergic progression vs. the slope of the percent usage of harmonic calls across males.
RESULTS
Serotonergic activity was elevated in the IC of males during encounters with females.
Fifteen male mice (7–21 wk old) and 10 female mice (7–22 wk old) were used to investigate serotonergic activity in the IC. Males prepared for voltammetric recording of serotonergic signals through CFEs were presented for a period of 30 min with unfamiliar female partners. In male subjects, voltammetric measurements of serotonergic oxidative currents were made every 6 min. Four baseline measurements were collected before the presentation of female partners, six measurements were collected while males interacted with females, and three additional measurements were collected after removal of females. To account for variability among CFEs or among males, the amplitude of oxidative current at each of these time points was normalized to the average of the four baseline measurements. This metric, serotonergic activity, represented the percent change in serotonin at a given time point relative to baseline. Male subjects also participated in parallel control experiments, in which CFEs were implanted into the contralateral IC and measurements were made in the same sequence, but no females were presented. The order of experimental treatment and the laterality of the experimental vs. control recordings were balanced across subjects.
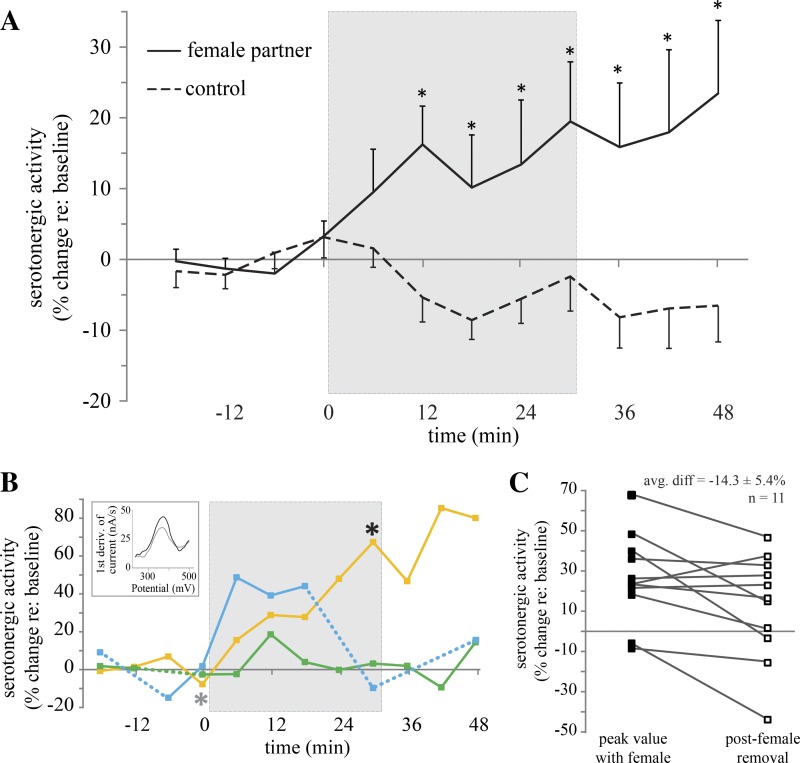
During these staged encounters with females, serotonergic activity in the IC of male mice increased, compared with that during encounters using the same males in which no social partners were present (female partner, n = 11; control, n = 11; mixed-model F = 4.421, P = 0.037; Fig. 4A). After 12 min of social interaction, serotonergic activity was significantly increased compared with that during control trials (female partner, n = 10; control, n = 9; P = 0.003). Serotonergic activity remained elevated throughout the social encounter at 18 min (female partner, n = 9; control, n = 10; P = 0.009), 24 min (female partner, n = 9; control, n = 10; P = 0.006), 30 min (female partner, n = 8; control, n = 10; P = 0.003), and for the 18 min after female removal (female partner, n = 10; control; n = 10; P < 0.001). There was substantial variability among males in this average pattern, however. Some males showed large and consistent increases in serotonergic activity throughout the encounter with a female, but some showed no increases, or even peaks followed by decreases while females were still present (Fig. 4B). There was similar variability in serotonergic activity following female removal. Although some males showed continued increases in serotonergic activity in the time following female removal, others did not. A comparison of the maximum serotonergic activity while females were present and the first measurement made after female removal showed a significant decline of 14.3 ± 5.4% (Fig. 4C; 2-tailed t-test, P < 0.05).
Fig. 4.
Time courses of serotonergic activity in the IC of male mice when paired with a female partner. A: mean serotonergic activity significantly increased in male mice during an encounter with a female partner (n = 11; solid line) compared with no-partner control trials (n = 11; dashed line). Gray box indicates presentation of the female partner. Values are means ± SE. *P < 0.05, significant difference between female interaction and control treatments. B: time courses of serotonergic activity in 3 different male mice during encounters with females, demonstrating the diversity of serotonergic activity during courtship. Dashed portions of the line indicate interpolation of missing time points due to movement noise; each marker represents measured time points. Inset depicts 2 overlying voltammograms, recorded from the male subject represented by the yellow line at time 0 (gray line, indicated by gray asterisk on time course) and at time 30 (black line, indicated by black asterisk on time course). C: peak values of serotonergic activity in males during encounters with females were significantly greater than the first measure of serotonergic activity after the female was removed (P < 0.05). 1st deriv., first derivative; avg. diff, average difference.
Whether males first underwent a social interaction with females or a control trial had no effect on serotonergic activity during either trials with females (first week, n = 5; second week, n = 6; mixed-model F = 0.622, P = 0.451) or control trials (first week, n = 4; second week, n = 7; mixed-model F = 0.524; P = 0.483).
Serotonergic means of males correlated with female partner's behavior.
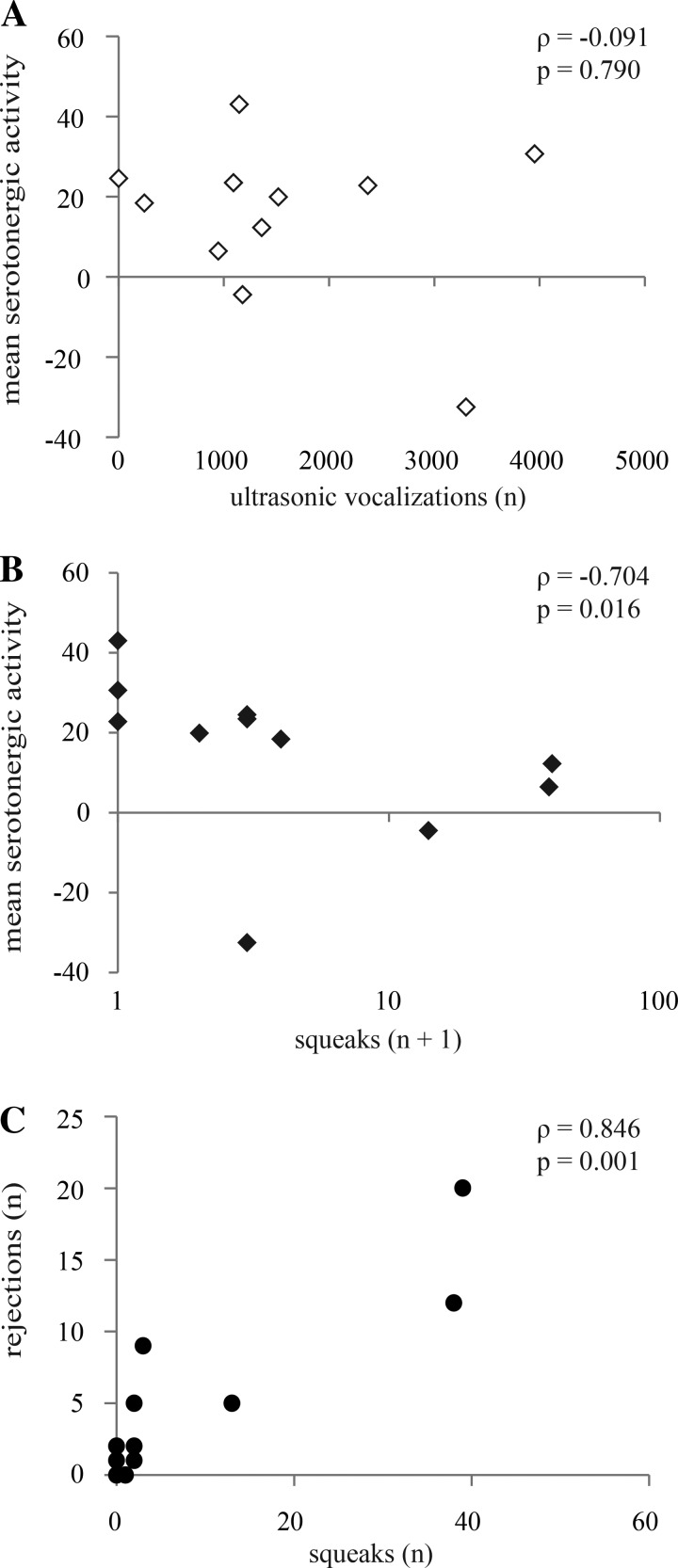
We tested the prediction that serotonergic activity would correspond to the behavior of the male subjects. To facilitate comparison among males, we created a single value for each individual male by averaging the last four measurements of serotonergic activity (the serotonergic mean), since these time points had values for serotonergic activity that were significantly different from those of controls, on average. The serotonergic mean therefore represented the integrated serotonergic response to presentation of a female relative to baseline. Males showed great interest in females, physically investigating females and producing USVs at an even higher rate (324 ± 68 USVs in the first 5 min) than previously reported for unmanipulated males (161 ± 96 USVs in 5 min; t15 = 2.16, P = 0.047; Hanson and Hurley 2012). However, the serotonergic mean was not correlated with anogenital investigation performed by the males (Spearman ρ = −0.418, P = 0.201) or to the total number of all types of USVs produced by males across an entire interaction (Spearman's ρ = −0.091, P = 0.790; Fig. 5A). The percentage of USVs that were harmonic in each interaction also was not correlated with the serotonergic mean (Spearman's ρ = −0.291, P = 0.385). We found in past work that serotonergic measurements correspond to overall behavioral activity levels for both male and female subjects in social interactions, but the serotonergic mean did not correlate with immobility in the males in the current study (Spearman's ρ = 0.245, P = 0.467). Males sometimes exhibited prolonged calling and movement around their cages after female removal, but the serotonergic mean after the female was removed was not related to male immobility after female removal (Spearman's ρ = 0.327, P = 0.326) or to USVs emitted after female removal (Spearman's ρ = 0.127, P = 0.726). Finally, the serotonergic mean was not related to the nonbehavioral qualities we measured for either partner: female age (Spearman's ρ = 0.638, P = 0.14) or weight (Spearman's ρ = 0.718, P = 0.051), and male age (Spearman's ρ = 0.082, P = 0.81) or weight (Spearman's ρ = 0.518, P = 0.41).
Fig. 5.
Serotonin measured in males correlated with behaviors of the female partner. A: mean serotonergic activity was not correlated with USVs. B: mean serotonergic activity measured in males was negatively correlated with the number of broadband squeaks emitted by the female partner. C: female squeaks were significantly related to female rejection (darting away and kicking the male).
In contrast to the lack of expected correlation with male behaviors, the serotonergic mean correlated with mutual social behavior and with behaviors performed by the female partner. The serotonergic mean was negatively correlated with both the total number of nose-to-nose investigations (Spearman ρ = −0.736, P = 0.010) and female rejections, defined as females either darting away from or kicking the male (Spearman ρ = −0.679, P = 0.022). Thus males in interactions with females who performed low levels of both nose-to-nose investigation and rejection behaviors showed the largest serotonergic responses. Because we measured serotonergic activity in an auditory region, we were particularly interested in how the serotonergic mean related to potential auditory cues. As expected from the relationship between the serotonergic mean and nonvocal female rejection behaviors, the serotonergic mean of males was negatively correlated with the number of squeaks emitted by the female partner so that males in interactions with females who produced high numbers of squeaks showed the smallest serotonergic responses (Spearman's ρ = −0.704, P = 0.016; Fig. 5B). Confirming previous reports that squeaks relate to the acute rejection of males, female squeaks and rejection behavior were positively correlated with each other (Spearman's ρ = 0.846, P = 0.001; Fig. 5C; Nyby 2001; Sales 1972; Sugimoto et al. 2011; Wang HR et al. 2008).
Although female behavior correlated with the serotonergic mean of males, female estrous state had no effect on the serotonergic mean (proestrus/estrus, n = 7; diestrus, n = 4; mixed-model F = 0.094, P = 0.911). There was also no effect of female estrous state (proestrus/estrus = 7; diestrus = 4) on either female behaviors (kicking: Mann-Whitney U = 22.5, P = 0.851; squeaking: Mann-Whitney U = 29.0, P = 0.3951; female anogenital investigation: Mann-Whitney U = 20.0, P = 0.508) or male behaviors (total USVs: Mann-Whitney U = 18.0, P = 0.2986; %harmonic USVs: Mann-Whitney U = 21.0, P = 0.637; male anogenital investigation: Mann-Whitney U = 29.0, P = 0.395; immobility: Mann-Whitney U = 23.0, P = 0.925).
Matching time courses of serotonergic activity and vocalizations.
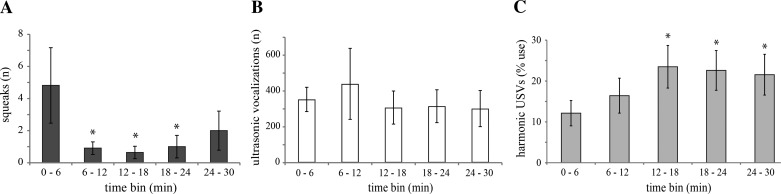
Behaviors in mouse opposite-sex interactions, including vocalization behaviors, change over time as an interaction progresses (Hanson and Hurley 2012, 2014; Hull and Dominguez 2007; McGill 1962; Mosig and Dewsbury 1976), so we assessed the progression of vocal behavior while males and females were paired. Female squeaks were produced throughout the encounters, but most squeaks occurred in the first 6 min, compared with second, third, and fourth time bins (F4,27 = 1.911, P = 0.123 overall; P < 0.05 for pairwise comparisons between first time bin and the last 3 time bins; Fig. 6A). The total number of USVs did not vary over the course of 30-min interactions (F4,65 = 0.46, P = 0.86; Fig. 6B). However, proportional use of harmonic USVs rose in the last three time bins, compared with the first time bin (F4,36 = 2.358, P = 0.07 overall; P < 0.05 for last 3 time bins; Fig. 6C). This pattern was quantitatively indistinguishable from that exhibited by nonvoltammetry subjects in an earlier study (F1,72 = 0.031, P = 0.861; Hanson and Hurley 2014).
Fig. 6.
Time courses of vocal behavior exhibited during 30-min encounters between male and female mice. A: emission of broadband squeaks by females varied over time; the majority of squeaks occurred within the first 6 min (n = no. of squeaks). B: total emission of USVs by males did not vary over time (n = no. of vocalizations). C: significantly more harmonic USVs were emitted later on in encounters. For A–C, values are means ± SE. *P < 0.05, significant difference compared with first time bin.
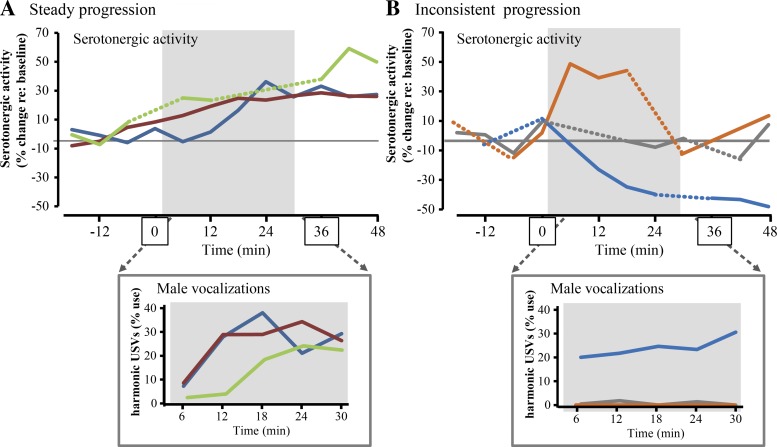
Increases in the proportion of harmonic calling by males occurred over roughly the same time course as increases in serotonergic activity. As for serotonergic activity, there was also substantial variability among males in the increase in the percent usage of harmonic USVs over time. We therefore assessed whether the increasing serotonergic activity and increasing proportional production of harmonic USVs were associated with each other across males. Figure 7A highlights examples of three males in which serotonergic activity steadily increased during, and even after, interaction with females, and in which the percentage of USVs that were harmonic increased from an initial low level to a higher level (“steady progression”). Figure 7B highlights examples of three males in which serotonergic activity never increased, increased but subsequently decreased in the continued presence of females, or decreased during the entire time of female interaction (“inconsistent progression”). For these males, harmonic calling either never emerged, or began and remained at a high level.
Fig. 7.
Comparison of the time course in serotonergic activity and harmonic vocalization over time. A: example traces of serotonergic activity (top plot) in 3 sample males exhibiting a steady progression over time. Bottom plot shows progression of harmonic calling during female presence in the same males as the percentage of harmonic USVs out of all USVs. The same colors represent the same males in each plot. Gray boxes represent presence of a female. B: plots of the progression of serotonergic activity and harmonic calling in 3 males showing an inconsistent progression in serotonergic activity, as defined in the text. As in A, colors represent male identity and gray boxes represent female presence.
We quantitatively assessed the relationship between the progressions in serotonergic activity and harmonic calling over time by correlating the slopes of changes in serotonergic activity and percent harmonic calling from the start to the end of male-female interaction (Fig. 8). Although some males showed high levels of progression in both of these values (top right quadrant), the overall correlation between harmonic and serotonergic progression was not significant (Spearman's ρ = 0.273, P = 0.417). Furthermore, there was no close correspondence between the progression of harmonic calling and female estrous state. Some males with strong progression in serotonergic activity interacted with females in estrus or proestrus (asterisks in Fig. 8), but males with low or negative values for serotonergic or call progression also interacted with estrous females.
Fig. 8.
Scatterplot quantifying the progression of serotonergic activity relative to baseline and percent harmonic calling as the slopes of these values during female presence [(final − initial values)/time]. Asterisks represent males interacting with females in estrus or proestrus.
DISCUSSION
During social interactions, neurochemical systems that respond to social stimuli can serve a potent adaptive function by matching sensory physiology to the signals of a social partner (e.g., Castelino and Schmidt 2010; Remage-Healey et al. 2013). The current study investigated sensory physiology in male mice paired with female social partners, comparing the amount of serotonergic activity in an auditory midbrain nucleus, the IC, with vocal and nonvocal social behaviors. These measurements confirmed an initial prediction that serotonergic activity would increase in the IC of male mice interacting with females. We also found that male serotonergic physiology closely corresponded to female behavior such that mean serotonergic activity was inversely correlated with several measures of female behavior that were indicative of negative valence for males, including nonvocal rejections and broadband squeaks. These findings suggest that the serotonergic system is one mechanism for matching the neurochemical environment of the auditory system to the social milieu. Below, we describe the relationships between serotonergic activity and male vs. female behaviors, assess how these relationships align with a multiphase model of mouse courtship, and outline a model of how courtship-related neuromodulation in primary sensory regions such as the IC could interact with parallel events in social behavioral centers.
Serotonergic fluctuations in social contexts.
Two past studies of serotonergic activity in the IC have focused on different types of social contexts, making measurements of serotonergic activity in male or female mice placed with conspecific male social partners (Hall et al. 2011; Hanson and Hurley 2014). In both of these studies, serotonergic activity increased following the presentation of male partners. Combined with the current findings, these studies support the hypothesis that serotonergic activity in the IC signals multiple types of social contexts. An important difference in our current results from these previous studies is in the correlation of serotonergic activity with behavior. In the past studies, the levels of serotonergic signaling positively correlated with levels of overall behavioral activity in both the male and female subjects placed with male intruders. In males presented with male social partners, serotonergic activity further positively correlated with a measure of social investigation, the anogenital inspection of intruders by the male subjects, although not with anogenital inspection of the male subjects by the intruders (Hall et al. 2011). In contrast with these findings, the level of serotonergic activity in the current study was directly correlated not with the behaviors of male subjects, but with the behaviors of their female partners.
Because this finding was incongruent with previous findings, we assessed multiple aspects of the behavior of male subjects. However, neither male vocalization, social investigation, overall activity, nor male or female age or weight were significantly correlated with the serotonergic mean. Our inability to measure a direct correlation is certainly not conclusive evidence of a lack of correlation; nuances of male behaviors that we did not measure could be correlated with measurements of serotonin on finer spatial or temporal scales.
It is further possible that male responsiveness to females has a relationship to serotonergic activity that is not best described by an analysis of correlation. For example, male arousal could have a permissive influence allowing serotonin levels to rise, depending on the specific social signals in a given interaction. Since all of our males showed signs of sexual arousal, we were not able to assess this specific possibility. Overall, the conclusion that best fits our experimental paradigm is that the level of serotonergic activity in the male IC is correlated with female rejection behavior, but not with our measures of male behavior.
A correlation between male physiology and female behavior could be useful from the perspective of a sexually motivated male, because audible squeaks and associated behaviors produced by females are signals with potentially high relevance for males in courtship interactions. Increases in serotonergic activity were related to the presence of female partners, in that they did not occur in the absence of females, and all of the males in our study showed sexual motivation through production of USVs and investigation of females. As previously reported, squeaks produced by females in the current study were tightly coupled in time and number to females kicking at or abruptly darting away from males (Lupanova and Egorova 2015; Nyby 2001; Sugimoto et al. 2011). This supports the hypothesis that female squeaks have an unambiguous meaning for sexually motivated males, signaling acute rejection by females. The fact that the serotonergic mean correlated with female signaling suggests that serotonin may serve as an indication of the valence of an interaction for the courting males. In this view, a positive sexual valence is signaled by fewer female squeaks and other rejection behaviors, and is accompanied by higher levels of serotonergic activity in the IC.
Female estrous state has an important influence on the reproductive behaviors of male mice, notably via olfactory cues (e.g., Dixon and Mackintosh 1975; Haga-Yamanaka et al. 2014; Ingersoll and Weinhold 1987; Jakupovic et al. 2008). In terms of vocal behavior, we have found that male mice show subtle shifts in the spectrotemporal structure of their USVs across the estrous phases of their female partners (Hanson and Hurley 2012). In the current study, however, neither male nor female behavior corresponded to the estrous states of females. Therefore, we found no evidence for a deterministic effect of female estrous state on male behavior or serotonergic activity in our specific behavioral paradigm.
Relevance to mouse courtship.
A prominent feature of the rise in male serotonergic activity was its gradual time course, paralleling changes in male and female vocal behavior. This finding complements previous descriptions of mouse courtship as a multiphased progression. It has long been noted that male mice begin to produce USVs rapidly after encountering females, or even after being presented with a limited range of female cues, such as female urine (Holy and Guo 2005; Nyby et al. 1977; Sipos et al. 1992). However, males usually engage in mounting attempts after an initial period of calling and investigative behavior, and perform multiple intromissions with long ejaculation latencies (38.9–120.8 min; Hull and Dominguez 2007; Mosig and Dewsbury 1976). As more recently reported, and as replicated in the current study, the composition of male vocalizations also changes to include increased proportions of harmonic calls (also called “40-kHz” calls: White et al. 1998) later in interactions, corresponding in time with male mounting attempts (Hanson and Hurley 2012). Our findings suggest that physiological changes in the male IC match the time course of these reproductively crucial events, with serotonergic activity increasing on the same general timescale as increased mounting and changing vocal repertoire of males.
The relative timing of female rejection behavior compared with the timing of both male serotonergic activity and harmonic USVs is also suggestive of an important role for female signaling in opposite-sex interactions of mice. Most female calls and other rejection behaviors in the current study occurred early in the time period of interaction, whereas male elevations in serotonergic activity and proportional harmonic calling, if they occurred, happened later in interactions. Female vocal and nonvocal rejection behavior was therefore in a position to act as a signal to males, influencing later male behavior and physiology. A hypothesis that results from this observation is that early signals of female rejection could suppress later serotonergic activity in the male IC, but an important caveat is that the design of our study does not allow us to definitively conclude that female behaviors have a causal relationship to male serotonergic activity.
Consequences of changing serotonergic activity.
In the IC, the outcome of increasing serotonergic activity during social encounters for the perception of vocalizations is unknown. However, the serotonergic elevations measured in this study are likely to modulate the responses to acoustic stimuli by IC neurons, which express a range of different serotonin receptor types, including receptors in the metabotropic 5-HT1 and 5-HT2 families as well as ionotropic 5-HT3 receptors (Hurley and Sullivan 2012). Activation of different types of serotonin receptors may decrease or increase excitability in the IC by presynaptic or postsynaptic mechanisms (Hurley 2007; Hurley et al. 2008; Wang HT et al. 2008). One receptor type that is prominently expressed in the IC is the 5-HT1A receptor (Peruzzi and Dut 2004, Smith et al. 2014; Thompson et al. 1994). Since this receptor type decreases auditory and spontaneous responsiveness and narrows frequency tuning in the IC (Hurley 2006, 2007), it may in part account for a prominent effect of exogenously applied serotonin, which is to increase the selectivity of IC neurons for both simple acoustic stimuli and conspecific calls (Hurley and Pollak 2001, 2005). Given the inverse correlation between female rejection behaviors and male serotonergic activity, less rejection from females should be associated with enhanced selectivity for vocalizations. Although females emitted fewer calls during later stages of encounters, processing of the intermittent calls may be particularly important for males, since female vocalizations are frequently concomitant with male mounting attempts (Wang HR et al. 2008). Modulation of auditory processing also may be important for other courtship-relevant sounds, including self-produced male USVs, which are abundant throughout the interaction.
The IC is an auditory region of great significance for the processing of vocalizations. Neural responses for vocalizations are much more selective in the IC than in an immediately upstream nucleus, the dorsal nucleus of the lateral lemniscus, likely because of strong inhibitory inputs received by IC neurons (Bauer et al. 2002; Klug et al. 2002). Neural activity in the IC, or in its homologs in nonmammalian species of vertebrates, is also dependent on many factors important in establishing behavioral context, such as the salience of acoustic signals and an animal's reproductive state (Hoke et al. 2004; Lynch and Wilczynski 2008; Maney et al. 2006). However, the auditory midbrain reflects some features of social behavior less accurately than later sensory or sensorimotor regions (Hoke et al. 2007). This illustrates the important point that activity in the IC is a snapshot of events within the ascending auditory system, with downstream regions further refining the representation of vocal communication signals (Suta et al. 2008). Auditory regions both upstream and downstream of the IC are also innervated by serotonergic projections (DeFelipe et al. 1991; Klepper and Herbert 1991), so the ultimate effect of serotonin on the representation of vocalizations is likely to reflect integration of the effects of serotonin in all of these auditory areas.
On a broader scale, these auditory events occur against the backdrop of widespread changes in neural activity triggered by social interaction, which coordinate signal production and reception between senders and receivers. Multiple classes of chemical signals regulate socially triggered neural activity on different temporal scales. On the timescale of reproductive cycles, gonadal hormones both increase the production of context-appropriate social signals and alter receiver sensory systems either peripherally or centrally to match these signals (Ball and Balthazart 2010; Chakraborty and Burmeister 2015; Miranda and Wilczynski 2009; Sisneros et al. 2004). On the timescale of individual social interactions, neuromodulatory systems producing neuroestrogens or catecholamines (as well as serotonin) respond to social cues and may reflect the performance of social behaviors (Bharati and Goodson 2006; Gale and Perkel 2010; Goodson et al. 2009; Matragrano et al. 2012; Remage-Healey et al. 2008). These modulatory systems then alter neural responses to, and behavioral preferences for, particular types of social signals (e.g., Appeltants et al. 2002; Deemyad et al. 2013; Lynch and Ball 2008; Pawlisch et al. 2011; Remage-Healey et al. 2010; Velho et al. 2012).
An important target for these regulatory mechanisms is an interacting set of brain regions orchestrating a range of social behaviors across vertebrates, the social behavior network, which includes regions such as the medial amygdala and hypothalamic nuclei (SBN; Newman 1999). Activity in the SBN has some interesting parallels to the serotonergic activity we measured in the IC. One such similarity is in sensitivity to interaction valence. For example, a population of peptidergic neurons in the bed nucleus of the stria terminalis, closely associated with the medial amygdala, shows activity that is related to the valence of a social situation for subject animals (Goodson and Wang 2006). More specifically, socially induced elevations in serotonergic activity in at least one node of the SBN important in regulating male sexual behavior, the medial preoptic area (Rubio-Casillas et al. 2015), resemble those in the IC. In the medial preoptic area of male rats, serotonergic activity increases in males placed with females, shows a delayed time course similar to what we have observed in the IC, and likewise corresponds to female sexual availability (Fumero et al. 1994; Mas et al. 1995). Serotonin in this region is thought to signal the consummatory phase of a sexual interaction and to promote either some types of consummatory sexual behaviors or sexual inhibition/satiety, depending on the types of serotonin receptors that are activated (Rubio-Casillas et al. 2015).
These similarities suggest several possibilities for how social events coordinate auditory processing in the IC with activity in other neural centers important to social behavior. One possibility is that activity in primary sensory regions such as the IC and in social behavior nodes such as the medial preoptic area are coregulated by the serotonergic system, along with other neuromodulatory systems, during specific behavioral contexts. A second possibility is that ultimate sources of information to the IC on the valence of social interactions could be higher order social processing centers such as the SBN. These possibilities are consistent with a view of the serotonergic activity in the IC representing feedback from higher order social centers to early-stage sensory regions, which would have the effect of filtering the sensory representation of social signals. These sensory representations would then serve as context-biased sources of information on the social milieu to these social centers. The context-dependent coordination of neural activity at all of these levels by diffuse regulatory systems could therefore be important in ensuring appropriate social behavior, given the dynamics of a particular social interaction.
GRANTS
This work was funded by the Center for the Integrative Study of Animal Behavior at Indiana University and National Institute of Deafness and Other Communications Disorders Grant DC008963.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.K. and L.M.H. conception and design of research; S.M.K. performed experiments; S.M.K. and L.M.H. analyzed data; S.M.K. and L.M.H. interpreted results of experiments; S.M.K. and L.M.H. prepared figures; S.M.K. and L.M.H. drafted manuscript; S.M.K. and L.M.H. edited and revised manuscript; S.M.K. and L.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank K. Bunner, J. Hanson, S. Huguenard, B. Wise, and C. Woods for technical assistance.
REFERENCES
- Akre KL, Ryan MJ. Female túngara frogs elicit more complex mating signals from males. Behav Ecol 22: 846–853, 2011. [Google Scholar]
- Appeltants D, Del Negro C, Balthazart J. Noradrenergic control of auditory information processing in female canaries. Behav Brain Res 133: 221–235, 2002. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Japanese quail as a model system for the study of the neuroendocrine control of reproductive and social behaviors. ILAR J 51: 310–325, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EE, Klug A, Pollak GD. Spectral determination of responses to species-specific calls in the dorsal nucleus of the lateral lemniscus. J Neurophysiol 88: 1955–1967, 2002. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300, 1995. [Google Scholar]
- Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience 143: 661–670, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland LM, Shi G, Yang H, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Methods 146: 149–158, 2005. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Schmidt MF. What birdsong can teach us about the central noradrenergic system. J Chem Neuroanat 39: 96–111, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M, Burmeister SS. Effects of estradiol on neural responses to social signals in female túngara frogs. J Exp Biol 218: 3671–3677, 2015. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T, McAuliffe K. Female mate choice in mammals. Q Rev Biol 84: 3–27, 2009. [DOI] [PubMed] [Google Scholar]
- Crespi F. Influence of Neuropeptide Y and antidepressants upon cerebral monoamines involved in depression: an in vivo electrochemical study. Brain Res 1407: 27–37, 2011. [DOI] [PubMed] [Google Scholar]
- Deemyad T, Metzen MG, Pan YZ, Chacron MJ. Serotonin selectively enhances perception and sensory neural responses to stimuli generated by same-sex conspecifics. Proc Natl Acad Sci USA 110: 19609–19614, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Hashikawa T, Jones EG. Synapatic relationships of serotonin-immunoreactive terminal baskets on GABA neurons in the cat auditory cortex. Cereb Cortex 1: 117–133, 1991. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Mackintosh JH. The relationship between the physiological condition of female mice and the effects of their urine on the social behaviour of adult males. Anim Behav 23: 513–520, 1975. [DOI] [PubMed] [Google Scholar]
- Feng JX, Brazell M, Renner K, Kasser R, Adams RN. Electrical pretreatment of carbon-fibers for in vivo electrochemistry–effects on sensitivity and response-time. Anal Chem 59: 1863–1867, 1987. [DOI] [PubMed] [Google Scholar]
- Fumero B, Fernandez-Vera JR, Gonzalez-Mora JL, Mas M. Changes in monoamine turnover in forebrain areas associated with masculine sexual behavior: a microdialysis study. Brain Res 662: 233–239, 1994. [DOI] [PubMed] [Google Scholar]
- Gale SD, Perkel DJ. A basal ganglia pathway drives selective auditory responses in songbird dopaminergic neurons via disinhibition. J Neurosci 30: 1027–1037, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80: 84–97, 2007. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci USA 106: 8737–8742, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci USA 103: 17013–17017, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga-Yamanaka S, Ma L, He J, Qiu Q, Lavis LD, Looger LL, Yu CR. Integrated action of pheromone signals in promoting courtship behavior in male mice. Elife 3: e03025, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IC, Rebec GV, Hurley LM. Serotonin in the inferior colliculus fluctuates with behavioral state and environmental stimuli. J Exp Biol 213: 1009–1017, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IC, Sell GL, Hurley LM. Social regulation of serotonin in the auditory midbrain. Behav Neurosci 125: 501–511, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hurley LM. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS One 7: e40782, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hurley LM. Context-dependent fluctuation of serotonin in the auditory midbrain: the influence of sex, reproductive state and experience. J Exp Biol 217: 526–535, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi P, Dankoski EC, Petrovic J, Keithley RB, Wightman RM. Voltammetric detection of 5-hydroxytryptamine release in the rat brain. Anal Chem 81: 9462–9471, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Burmeister SS, Fernald RD, Rand AS, Ryan MJ, Wilczynski W. Functional mapping of the auditory midbrain during mate call reception. J Neurosci 24: 11264–11272, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Integration of sensory and motor processing underlying social behavior in túngara frogs. Proc Biol Sci 274: 641–649, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol 3: e386, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav 52: 45–55, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. Different serotonin receptor agonists have distinct effects on sound-evoked responses in inferior colliculus. J Neurophysiol 96: 2177–2188, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Res 1181: 21–29, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Bohorquez A, Tracy J. The serotonin 1B receptor modulates frequency response curves and spectral integration in the inferior colliculus by reducing GABAergic inhibition. J Neurophysiol 100: 1656–1667, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin effects on frequency tuning of inferior colliculus neurons. J Neurophysiol 85: 828–842, 2001. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin modulates responses to species-specific vocalizations in the inferior colliculus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 535–546, 2005. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Sullivan MR. From behavioral context to receptors: serotonergic modulatory pathways in the IC. Front Neural Circuits 6: 58, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM. Serotonergic innervation of the auditory brainstem of the Mexican free-tailed bat, Tadarida brasiliensis. J Comp Neurol 435: 78–88, 2001. [DOI] [PubMed] [Google Scholar]
- Ingersoll DW, Weinhold LL. Modulation of male mouse sniff, attack, and mount behaviors by estrous cycle-dependent urinary cues. Behav Neural Biol 48: 24–42, 1987. [DOI] [PubMed] [Google Scholar]
- Jakupovic J, Kang N, Baum MJ. Effect of bilateral accessory olfactory bulb lesions on volatile urinary odor discrimination and investigation as well as mating behavior in male mice. Physiol Behav 93: 467–773, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JA, Clemens LG, Nunez AA. Characterization of copulatory behavior in female mice: evidence for paced mating. Physiol Behav 95: 425–429, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res 557: 190–201, 1991. [DOI] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Hanson JT, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. J Neurophysiol 88: 1941–1954, 2002. [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Alleva E, Scattoni ML. Translating mouse vocalizations: prosody and frequency modulation. Genes Brain Behav 10: 4–16, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupanova AS, Egorova MA. Vocalization of sex partners in the house mouse (Mus musculus). J Evol Biochem Phys 51: 324–333, 2015. [PubMed] [Google Scholar]
- Lynch KS, Ball GF. Noradrenergic deficits alter processing of communication signals in female songbirds. Brain Behav Evol 72: 207–214, 2008. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilcynski W. Reproductive hormones modify reception of species-typical communication signals in a female anuran. Brain Behav Evol 71: 143–150, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci 23: 1523–1529, 2006. [DOI] [PubMed] [Google Scholar]
- Marsden CA, Joseph MH, Kruk ZL, Maidment NT, O'Neill RD, Schenk JO, Stamford JA. In vivo voltammetry–present electrodes and methods. Neuroscience 25: 389–400, 1988. [DOI] [PubMed] [Google Scholar]
- Mas M, Fumero B, González-Mora JL. Voltammetric and microdialysis monitoring of brain monoamine neurotransmitter release during sociosexual interactions. Behav Brain Res 71: 69–79, 1995. [DOI] [PubMed] [Google Scholar]
- Matragrano LL, Beaulieu M, Phillip JO, Rae AI, Sanford SE, Sockman KW, Maney DL. Rapid effects of hearing song on catecholaminergic activity in the songbird auditory pathway. PLoS One 7: e39388, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TE. Sexual behavior in three inbred strains of mice. Behaviour 19: 341–350, 1962. [Google Scholar]
- Miranda JA, Wilcynski W. Female reproductive state influences the auditory midbrain response. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195: 341–349, 2009. [DOI] [PubMed] [Google Scholar]
- Mosig DW, Dewsbury DA. Studies of the copulatory behavior of house mice (Mus musculus). Behav Biol 16: 463–473, 1976. [DOI] [PubMed] [Google Scholar]
- Nakazato T. Dual modes of extracellular serotonin changes in the rat ventral striatum modulate adaptation to a social stress environment, studied with wireless voltammetry. Exp Brain Res 230: 583–596, 2013. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Ann NY Acad Sci 877: 242–257, 1999. [DOI] [PubMed] [Google Scholar]
- Nyby J. Auditory communication among adults. In: Handbook of Mouse Auditory Research: From Behavior to Molecular Biology, edited by Willott JF. Boca Raton, FL, CRC, 2001, p. 3–18. [Google Scholar]
- Nyby J, Wysocki CJ, Whitney G, Dizinno G. Pheromonal regulation of male mouse ultrasonic courtship (Mus musculus). Anim Behav 25: 333–341, 1977. [DOI] [PubMed] [Google Scholar]
- Patricelli GL, Uy JA, Borgia G. Female signals enhance the efficiency of mate assessment in satin bowerbirds (Ptilonorhynchus violaceus). Behav Ecol 15: 297–304, 2004. [Google Scholar]
- Pawlisch BA, Stevenson SA, Riters LV. α1-Noradrenergic receptor antagonism disrupts female songbird responses to male song. Neurosci Lett 496: 20–24, 2011. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Dut A. GABA, serotonin and serotonin receptors in the rat inferior colliculus. Brain Res 998: 247–250, 2004. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci 11: 1327–1334, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman ME, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci USA 107: 3852–3857, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Jeon SD, Joshi NR. Recent evidence for rapid synthesis and action of estrogens during auditory processing in a songbird. J Neuroendocrinol 25: 1024–1031, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Casillas A, Rodríguez-Quintero CM, Rodríguez-Manzo G, Fernández-Guasti A. Unraveling the modulatory actions of serotonin on male rat sexual responses. Neurosci Biobehav Rev 55: 234–246, 2015. [DOI] [PubMed] [Google Scholar]
- Russell ES. Valence and attention in animal behavior. Acta Biotheor 1: 91–99, 1935. [Google Scholar]
- Sales GD. Ultrasound and mating behavior in rodents with some observations on other behavioural situations. J Zool Lond 168: 149–164, 1972. [Google Scholar]
- Sanghera MK, Crespi F, Martin KF, Heal DJ, Buckett WR, Marsden CA. Biochemical and in vivo voltammetric evidence for differences in striatal dopamine levels in inbred strains of mice. Neuroscience 29: 649–656, 1990. [DOI] [PubMed] [Google Scholar]
- Singh YS, Sawarynski LE, Dabiri PD, Choi WR, Andrews AM. Head-to-head comparisons of carbon fiber microelectrode coatings for sensitive and selective neurotransmitter detection by voltammetry. Anal Chem 83: 6658–6666, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos ML, Kerchner M, Nyby JG. An ephemeral sex pheromone in the urine of female house mice (Mus domesticus). Behav Neural Biol 58: 138–143, 1992. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen Comp Endocrinol 136: 101–116, 2004. [DOI] [PubMed] [Google Scholar]
- Smith AR, Kwon JH, Navarro M, Hurley LM. Acoustic trauma triggers upregulation of serotonin receptor genes. Hear Res 315: 40–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Okabe S, Kato M, Koshida N, Shiroishi T, Mogi K, Kikusui T, Koide T. A role for strain differences in waveforms of ultrasonic vocalizations during male-female interaction. PLoS One 6: e22093, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suta D, Popelár J, Syka J. Coding of communication calls in the subcortical and cortical structures of the auditory system. Physiol Res 57: S149–S159, 2008. [DOI] [PubMed] [Google Scholar]
- Thompson GC, Thompson AM, Garrett KM, Britton BH. Serotonin and serotonin receptors in the central auditory system. Otolaryngol Head Neck Surg 110: 93–102, 1994. [DOI] [PubMed] [Google Scholar]
- Tong L, Altschuler RA, Holt AG. Tyrosine hydroxylase in rat auditory midbrain: distribution and changes following deafness. Hear Res 206: 28–41, 2005. [DOI] [PubMed] [Google Scholar]
- van Staaden MJ, Searcy WA, Hanlon RT. Signaling aggression. In: Aggression, edited by Huber R, Bannasch DL, Brennan P, and Frishman K. San Diego, CA: Elsevier Academic, 2011, p. 23–49. [DOI] [PubMed] [Google Scholar]
- Velho TA, Lu K, Ribeiro S, Pinaud R, Vicario D, Mello CV. Noradrenergic control of gene expression and long-term neuronal adaptation evoked by learned vocalizations in songbirds. PLoS One 7: e36276, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HR, Liang SY, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One 3: e1893, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HT, Luo B, Huang YN, Zhou KQ, Chen L. Sodium salicylate suppresses serotonin induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear Res 236: 42–51, 2008. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Forster GL, Korzan WJ, Renner KJ, Summers CH. Rapid neuroendocrine responses evoked at the onset of social challenge. Physiol Behav 90: 567–575, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NR, Prasad M, Barfield RJ, Nyby JG. 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiol Behav 63: 467–473, 1998. [DOI] [PubMed] [Google Scholar]
- Wright SP. Adjusted p-values for simultaneous inference. Biometrics 48: 1005–1013, 1992. [Google Scholar]