Abstract
Saltatory conduction in mammalian myelinated axons was thought to be well understood before recent discoveries revealed unexpected subcellular distributions and molecular identities of the K+-conductance pathways that provide for rapid axonal repolarization. In this study, we visualize, identify, localize, quantify, and ultrastructurally characterize axonal KV1.1/KV1.2 channels in sciatic nerves of rodents. With the use of light microscopic immunocytochemistry and freeze-fracture replica immunogold labeling electron microscopy, KV1.1/KV1.2 channels are localized to three anatomically and compositionally distinct domains in the internodal axolemmas of large myelinated axons, where they form densely packed “rosettes” of 9-nm intramembrane particles. These axolemmal KV1.1/KV1.2 rosettes are precisely aligned with and ultrastructurally coupled to connexin29 (Cx29) channels, also in matching rosettes, in the surrounding juxtaparanodal myelin collars and along the inner mesaxon. As >98% of transmembrane proteins large enough to represent ion channels in these specialized domains, ∼500,000 KV1.1/KV1.2 channels define the paired juxtaparanodal regions as exclusive membrane domains for the voltage-gated K+ conductance that underlies rapid axonal repolarization in mammals. The 1:1 molecular linkage of KV1 channels to Cx29 channels in the apposed juxtaparanodal collars, plus their linkage to an additional 250,000–400,000 Cx29 channels along each inner mesaxon in every large-diameter myelinated axon examined, supports previously proposed K+ conductance directly from juxtaparanodal axoplasm into juxtaparanodal myeloplasm in mammalian axons. With neither Cx29 protein nor myelin rosettes detectable in frog myelinated axons, these data showing axon-to-myelin linkage by abundant KV1/Cx29 channels in rodent axons support renewed consideration of an electrically active role for myelin in increasing both saltatory conduction velocity and maximum propagation frequency in mammalian myelinated axons.
Keywords: Cx29 channels, inner mesaxon, juxtamesaxon, juxtaparanode, KV1.1/KV1.2 channels
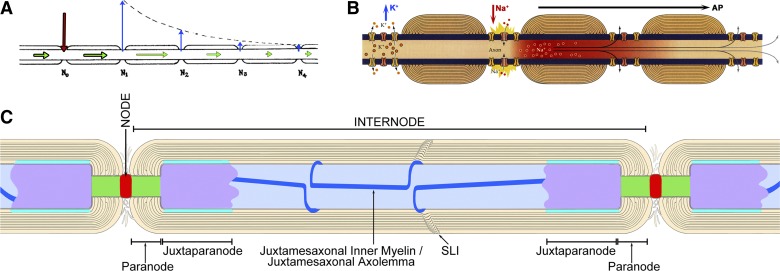
for almost a century, neuroscientists have sought to identify commonalities and differences that might be relevant to how large vertebrates and invertebrates separately increased axonal conduction velocity in their fastest conducting axons (Hodgkin and Huxley 1952). In their pioneering studies, Tasaki (1939) and Huxley and Stämpfli (1949) used dissected sciatic nerves of frogs to show that axonal action potentials in vertebrate myelinated axons result from inward electric current occurring solely at nodes of Ranvier (Fig. 1A; from Tasaki 1939). Parallel studies of unmyelinated squid giant axons (Hodgkin and Huxley 1952) and myelinated frog axons (Hodgkin 1951) revealed that depolarizing “action currents” occurred in both organisms via Na+-selective “carrier” molecules that were diffusely distributed along unmyelinated axons but localized solely to nodes of Ranvier in the myelinated axons of vertebrates (Hodgkin 1951; Huxley and Stämpfli 1949). Likewise, rapid axonal repolarization was shown to occur via separate carrier molecules for K+ that were diffusely distributed along squid giant axons but whose distribution in frog myelinated axons could not be determined by methods then available (Hodgkin 1951). Despite the caution of the original investigators, later investigators proposed that 1) voltage-gated K+ channels were colocalized with Na+ channels at nodes of Ranvier [Fig. 1B; from Purves (2012)]; 2) myelin passively increases conduction velocity by eliminating the internodal submyelinic extracellular space, thereby reducing axolemmal capacitance and, consequently, the amount of Na+ influx needed to overcome that capacitance during axonal depolarization [concepts summarized in Peles and Salzer (2000)]; and 3) the presumed absence of submyelinic extracellular space into which ions could flow was thought to preclude the occurrence of any ion channels in the internodal axolemma, thereby presumably minimizing internodal current loss and increasing the axonal “length constant” (Fig. 1, A and B, right).
Fig. 1.
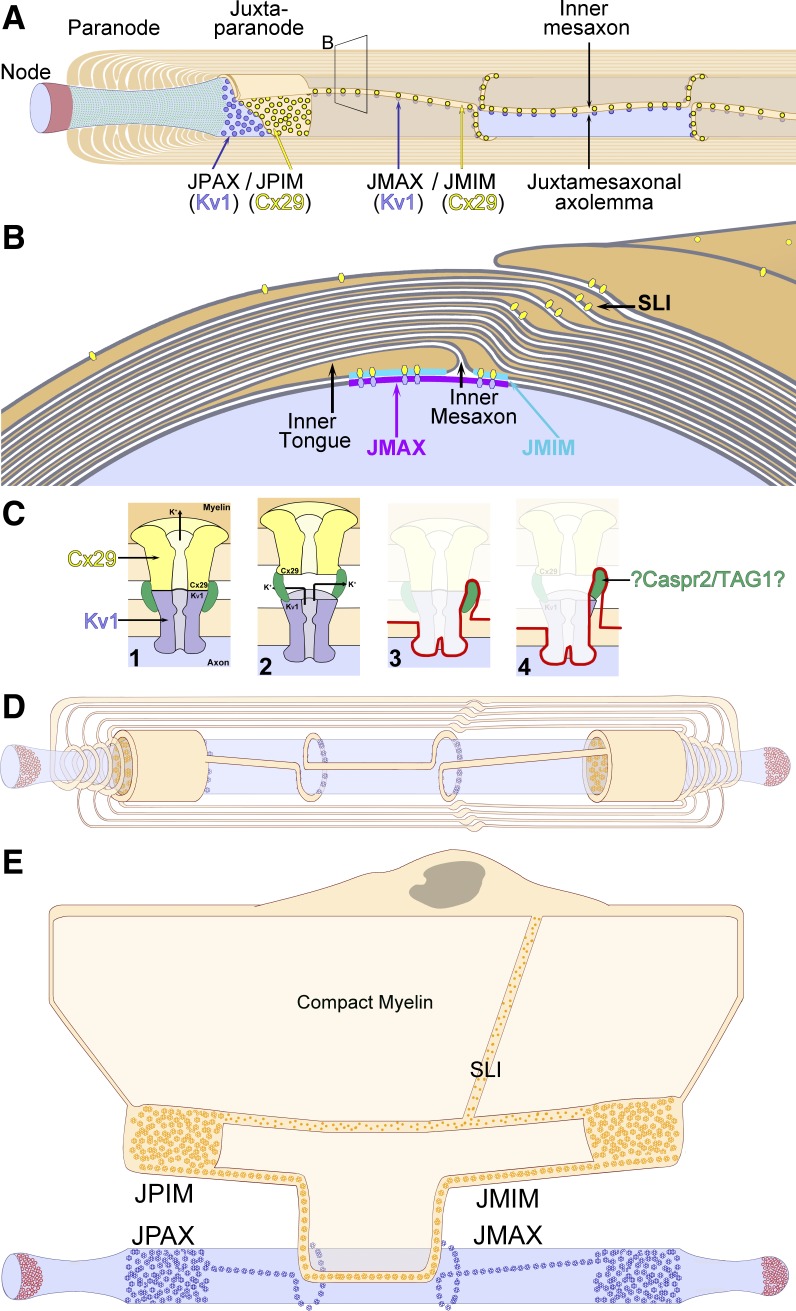
Early model of saltatory conduction in frogs (A), recent version attributed to frogs and mammals (B), and diagram of the major axonal regions described in this report (C). A: original model of saltatory conduction in frog sciatic nerve, illustrating inward current at nodes of Ranvier (red arrow), induced outward currents (blue arrows), also at nodes of Ranvier, and “nerve impulse” (green arrows) conducted in cytoplasm. [Modified from Tasaki (1939).] B: recent diagram showing colocalization of voltage-gated Na+ channels and voltage-gated K+ channels within nodes of Ranvier, with temporal but not spatial separation of Na+ and K+ currents. [Modified from Purves (2012)]. C: diagram delineating functional domains of mammalian myelinated axons described in this report. Node of Ranvier, red; paranode, green (para = both sides of); juxtaparanodal axolemma, purple (juxtaparanode = adjacent to both paranodes); internodal axolemma, light blue; inner mesaxon, dark blue line (mesaxon = mesentery-like support for axon). SLI, Schmidt-Lanterman incisure.
Three decades later, voltage-gated Na+ channels were localized solely to nodes of Ranvier in mammalian myelinated axons (Caldwell et al. 2000; Ritchie and Rogart 1977; Waxman and Ritchie 1985). However, neither voltage-gated K+ currents nor K+ channels could be detected at nodes of Ranvier in undamaged mammalian axons. Instead, voltage-gated K+ currents were demonstrated in rodent optic nerve prior to myelination but became undetectable immediately after the first myelin layers were laid down (Connors et al. 1982), suggesting that K+ efflux normally occurred within the internodes of adult mammals but was “masked” by even a few layers of myelin. In support, detachment of paranodal myelin loops by diverse mechanical and biochemical approaches (Bostock et al. 1981; Brismar 1979; Sherratt et al. 1980) allowed the first detection of internodal voltage-gated K+ currents (Chiu and Ritchie 1980). Moreover, upon acute demyelination, the previously-detected internodal K+ “leak” conductance (Chiu et al. 1979) appeared to be converted instantaneously into conventional voltage-gated K+ current (Chiu and Ritchie 1980), but no mechanism was proposed to account for that rapid conversion. Instead, submyelinic K+ currents were proposed to be masked by electrical barriers ascribed to the spiral septate junctions that link the paranodal loops of myelin to the axolemma (Livingston et al. 1973). However, that explanation was questioned because microperoxidase (1,900 mol wt) and colloidal lanthanum hydroxide applied outside intact mammalian axons rapidly diffused into and delineated the submyelinic extracellular space (Feder 1971; Hirano and Dembitzer 1969, 1982; MacKenzie et al. 1984), implying that smaller K+ ions should even more readily bypass those leaky barriers for easy detection.
To identify possible anatomical/molecular pathways for the newly discovered internodal K+ efflux (Chiu and Ritchie 1980), early freeze-fracture studies from rat sciatic nerve revealed distinctive hexagonal “rosettes” of 11-nm intramembrane particles (IMPs) in the internodal axolemma, restricted to and defining the “juxtaparanodal” region (Stolinski et al. 1981). Similar IMPs were also localized to two narrow bands that followed the inner mesaxon from node to node (Stolinski et al. 1981, 1985). (Anatomic regions of myelinated axons described in this report are illustrated in Fig. 1C.) Based on their structural similarity to known ion channels and their localization to the juxtaparanodal and inner mesaxonal axolemma, Stolinski et al. (1981) proposed that the axonal rosette particles corresponded to the newly detected internodal voltage-gated K+ channels (Chiu and Ritchie 1980). Remarkably, where the fracture plane stepped from axolemma to myelin within a rosette, the axolemmal rosette particles were directly apposed to and precisely aligned with matching 11-nm particles, also in rosettes, in innermost myelin, leading Stolinski et al. (1981) to propose direct ionic coupling of axoplasm with myeloplasm via these structurally coupled channels.
Subsequent immunofluorescence labeling revealed that KV1.1 and KV1.2, the primary voltage-gated K+ channels of fast-conducting axons in the mammalian central (CNS) and peripheral nervous systems (PNS), were not at nodes, as is still widely taught (Fig. 1C), but instead are restricted to the juxtaparanodal region and to a narrow ribbon that follows the inner mesaxon (Altevogt et al. 2002; Bhat et al. 2001; Chiu et al. 1999; Rasband and Shrager 2000; Rasband et al. 2001; Rios et al. 2003; Vabnick et al. 1999; Wang et al. 1993), precisely where Stolinski et al. (1981, 1985) had mapped the particle rosettes. Curiously, KV1.2 immunofluorescence was tightly colocalized with immunofluorescence for connexin29 (Cx29) (Altevogt et al. 2002), a 29-kDa connexin protein (equivalent to human GJC3) that is unique because it does not form gap junctions with itself or any other connexin (Ahn et al. 2008; Altevogt and Paul 2004) and thus has no known function. Independently, freeze-fracture replica immunogold labeling (FRIL) revealed that the rosettes in rodent myelin are composed of Cx29 (Li et al. 2002). However, the axolemmal rosette particles remained unidentified.
In the present study, we used immunofluorescence microscopy and FRIL to positively identify KV1.1 within the axonal rosette particles in rodent sciatic nerve and to demonstrate precise 1:1 molecular coalignment of KV1.1-containing channels with Cx29 channels in apposing myelin. Our data, in combination with published electrophysiological data, support the proposal of an electrically active role for myelin in axonal saltatory conduction in mammals and suggest that axo-glial KV1/Cx29 channels may underlie the faster axonal repolarizations and/or faster conduction velocity of mammalian myelinated axons.
METHODS
Animals.
Animals used in this study included a total of 12 adult Sprague-Dawley rats from which tissues were taken for analysis by immunofluorescence. Three male wild-type (WT) and three male Cx29 knockout (ko) C57BL/6 mice (Eiberger et al. 2006) were generously provided by Klaus Willecke (Berlin, Germany) for tests of specificity of Cx29 detection by anti-Cx29 antibodies. For FRIL, sciatic nerve from two WT C57BL/6 mice (1 male, 1 female) and from one male Cx29 ko mouse (courtesy of Dwight Bergles, Johns Hopkins University, Baltimore, MD) were fixed with 4% formaldehyde. In addition, segments of unfixed sciatic nerve were from a Cx32 ko C57BL/6 mouse from a colony established at the Colorado State University animal facility (2 breeding pairs provided by Carola Meier from animals provided by Klaus Willecke, then at the University of Bonn, Bonn, Germany) (Nelles et al. 1996), as reported previously (Meier et al. 2004). To assess possible species-specific or fixation-dependent differences in rosette morphology in FRIL samples in mice compared with the rosette morphology previously reported in glutaraldehyde-fixed sciatic nerve from rat (Stolinski et al. 1981), an additional WT mouse was fixed by perfusion with 2.5% glutaraldehyde for conventional freeze-fracture analysis. Animals were utilized according to protocols approved by the Central Animal Care Committee of the University of Manitoba and the Institutional Animal Care and Use Committees at Colorado State University and John Hopkins Medical Institutions, with minimization of pain and stress and minimization of the numbers of animals used.
Antibodies.
The antibodies used for immunofluorescence studies (with dilution or concentration used, catalog no., and source) include polyclonal anti-Cx29 (2 μg/ml; 48-7700; ThermoFisher, Grand Island, NY, formerly Invitrogen/Zymed Laboratories), monoclonal anti-KV1.2 and anti-KV1.1 (2 μg/ml; 75-008 and 75-105, respectively; Antibodies, Davis, CA), rabbit polyclonal pan anti-sodium channel antibody NaV (AB5210; Millipore, Temecula, CA), and monoclonal anti-caspr antibody, which was generously provided by Dr. E. Peles (Weizmann Institute of Science, Rehovot, Israel).
Fixation and sample preparation for light microscopic immunohistochemistry.
Rats were deeply anesthetized with Equithesin (3 ml/kg) and perfused transcardially with 40 ml of “prefixative” solution consisting of cold (4°C) 25 mM sodium phosphate buffer, pH 7.4, 0.9% NaCl (PBS), 0.1% sodium nitrite, and heparin (1 U/ml). This was followed by perfusion with 1 ml/g body wt of cold 0.16 M sodium phosphate buffer, pH 7.4, containing 4% formaldehyde, followed by perfusion with 40 ml of 25 mM phosphate buffer, pH 7.4, containing 10% sucrose. Sciatic nerves were removed and stored at 4°C for 48 h in cryoprotectant (10% sucrose in 25 mM phosphate buffer). Cryostat sections (10 μm thick) were collected on gelatinized glass slides and processed for immunofluorescence with primary and secondary antibodies diluted in 50 mM Tris·HCl, pH 7.4, containing 1.5% NaCl, 0.3% Triton X-100 (TBST), and 4% normal donkey serum.
Immunolabeling.
For single labeling, sections were incubated for 24 h at 4°C with primary antibody, washed for 1 h in TBST, and incubated for 1.5 h at room temperature with appropriate secondary antibody. For double immunofluorescence labeling, sections were incubated simultaneously with two different primary antibodies generated in different species, washed in TBST for 1 h at room temperature, and incubated simultaneously with appropriate secondary antibodies. Sections were washed for 20 min in TBST, followed by two 20-min washes in 50 mM Tris·HCl buffer, pH 7.4, and coverslipped with antifade medium Fluoromount-G (SouthernBiotech, Birmingham, AL). To establish the absence of inappropriate cross-reactions of primary with secondary antibodies, control procedures included omission of one of the primary antibodies with inclusion of each of the secondary antibodies. Secondary antibodies included Cy3-conjugated goat or donkey anti-mouse IgG (diluted 1:600; Jackson ImmunoResearch Laboratories, West Grove, PA), Alexa Flour 488-conjugated goat or donkey anti-rabbit and anti-mouse IgG (diluted 1:600; Molecular Probes, Eugene, OR), and Cy5-conjugated goat anti-mouse IgG (diluted 1:500).
Light microscopic analysis.
Immunofluorescence was examined on a Zeiss Axioskop2 fluorescence microscope, using Axiovision 3.0 software (Carl Zeiss Canada, Toronto, Canada) for capturing images, and on a Zeiss 710 laser scanning confocal microscope using ZEN 2010 image capture and analysis software. Data from wide-field and confocal microscopes were collected as either single-scan images or z-stack images, with multiple scans capturing a thickness of 2–6 μm of tissue at z scanning intervals of 0.4–0.6 μm. Final images were assembled according to appropriate size and adjusted for optimal signal-to-noise presentation using CorelDraw Graphics (Corel, Ottawa, Canada) and Adobe Photoshop CS software (Adobe Systems, San Jose, CA).
Freeze fracture and FRIL.
Because freeze-fracture and FRIL techniques have traditionally not been published in the Journal of Neurophysiology but are among the only current approaches that allow direct visualization, identification, quantification, ultrastructural characterization, and subcellular localization of membrane proteins such as those examined in the present study, we provide relatively more detailed information regarding pertinent aspects of replica formation, immunogold labeling, and image interpretation. In initial FRIL experiments, we used unfixed tissues remaining from previous FRIL studies of Cx32 and Cx29 in sciatic nerve of Cx32 ko mice (Li et al. 2002; Meier et al. 2004) wherein we made preliminary determinations of 1) the axonal types and ultrastructural locations of KV1.1 and Cx29 in myelinated axons, 2) whether KV1.1 forms either axolemmal P-face or E-face particles, or a mixture of particles on both E- and P-faces, 3) whether antibody labeling efficiency (LE) was significantly reduced by formaldehyde fixation compared with labeling in unfixed, rapidly frozen samples, and 4) whether formaldehyde fixation for FRIL accurately preserves the rosettes that previously had been demonstrated only in glutaraldehyde-fixed rat sciatic nerve (Stolinski et al. 1981, 1985). We then directly compared those unfixed Cx32 ko samples with samples from unfixed, formaldehyde-fixed, and glutaraldehyde-fixed WT mice vs. formaldehyde-fixed and glutaraldehyde-fixed Cx29 ko mice.
Perfusion fixation and cryoprotection.
Mice and rats were deeply anesthetized with ketamine and xylazine (80 and 8 mg/kg, respectively, supplemented to effect). To determine whether formaldehyde fixation reduces FRIL LE from that in unfixed samples, segments of either left or right sciatic nerve were removed before whole body perfusion with 1, 2, or 4% formaldehyde (Ted Pella, Redding, CA) in 0.15 M Sorensen's phosphate buffer (SPB), with the left and right sciatic nerves yielding both formaldehyde-fixed and unfixed samples from the same animal. Additional matched samples for high-resolution conventional freeze-fracture analysis were obtained by following the same procedures but using 2.5% glutaraldehyde instead of formaldehyde as the fixative. Because glutaraldehyde fixation results in formation of covalent bonds between cross-linked protein molecules (Johnson 1987), preventing most proteins from being washed from the replicas by SDS detergent, the resulting undigested tissue remnants completely block penetration of the electron beam that is used for image formation in transmission electron microscopy (Fujimoto 1995, 1997). Thus glutaraldehyde-fixed tissues cannot be used for FRIL. Instead, glutaraldehyde-fixed samples were used to determine whether the IMP rosette in axons or myelin were altered or disrupted by formaldehyde fixation or by the procedures used to cryoprotect and freeze unfixed tissue. In all cases, a 10-mm incision was made in the skin overlying the “sciatic notch” in the upper thigh, the sciatic nerve was exposed, and a 10-mm segment of nerve was dissected free and either infiltrated with 30% sucrose (unfixed samples) or placed into buffered formaldehyde or glutaraldehyde for 1 h at 4°C and then cryoprotected with 30% glycerol, as detailed below.
Immersion fixation of frog sciatic nerves.
Two adult leopard frogs (Rana pipiens; 3.5-in. body length) and three adult American green tree frogs (Hyla cinerea; 1.2–2.3 g) were anesthetized with MS222 (tricaine) in frog Ringer's solution. The sciatic nerves were exposed and immersed in 2.5% glutaraldehyde, 4% formaldehyde, or sucrose in Ringers' solution, dissected free, and prepared for freeze-fracture and FRIL by the same procedures used for mammalian tissues.
Determination of LE in formaldehyde-fixed vs. unfixed tissues.
To compare FRIL LE and labeling specificity for KV1 and Cx29 in formaldehyde-fixed vs. unfixed tissues, the sciatic nerves of one Cx32 ko mouse and one WT mouse (see below) were removed without fixation, immersed for 30 min in SPB containing 30% sucrose as a cryoprotectant, and slam-frozen. Other samples, including samples from both formaldehyde-fixed and unfixed WT mouse, were infiltrated at 4°C with increasing concentrations of sucrose or glycerol (to 30% in SPB) as a cryoprotectant and then rapidly frozen (Chandler and Heuser 1979; Heuser et al. 1981) by contact with a liquid nitrogen-cooled ultrapure copper block (Ultra-Freeze MF7000; RMC Products, Tucson, AZ). Cx32 ko samples had been stored for 10–15 years in liquid nitrogen.
High-resolution freeze-fracture replication to resolve molecular substructure.
Samples were freeze-fractured in a JEOL JFD-2 freeze-etch machine and replicated via unidirectional thermionic deposition of a 1- to 2-nm-thick “pre-carbon” coat, followed immediately by 1–1.5 nm of platinum-carbon, with both substances applied using separate electron beam guns. The pre-carbon coat slightly increases the diameter of IMPs and decreases the size of membrane pits. However, in addition to decreasing the granularity of the platinum replica film, thereby greatly improving imaging resolution (Kamasawa et al. 2006), it also has the advantage that it increases LE (Fujimoto 1995; Masugi-Tokita et al. 2007; Schlormann et al. 2007). After platinum shadowing, the replication process is completed by immediately coating the samples with ∼20 nm of “post-carbon” applied with constant tilting and rotation.
SDS washing and blocking of sites for nonspecific adsorption of antibodies.
Replicated samples were prepared for FRIL (name coined by Gruijters et al. 1987) according to our detailed procedures (Kamasawa et al. 2005, 2006; Rash and Yasumura 1999), as modified from Fujimoto (1995, 1997). After freeze-fracture replication, samples were digested in 1.7% SDS detergent in 30 mM sucrose and 15 mM Tris·HCl, pH 8.9 at 60°C for 12 h, rinsed briefly in distilled water, and enzymatically digested for 6–8 h in 4% collagenase D (Roche Applied Science, Indianapolis, IN) in 0.15 M SPB at 44°C, followed by washing for an additional 10–12 h in 3% SDS solution (no sucrose added), pH 8.9 at 80°C, with intermittent agitation. To reduce nonspecific adsorption of both primary and secondary antibody labels, washed replicas were rinsed for 3–12 h in “blocking buffer” (Dinchuk et al. 1987), consisting of 10% heat-inactivated goat serum plus 1.5% fish-gelatin digest (Sigma-Aldrich, St. Louis, MO) in 0.15 M SPB, and then immunogold labeled (Kamasawa et al. 2006; Rash and Yasumura 1999). [See Rash and Yasumura (1999) for methods to minimize background labeling “noise” and to discriminate “signal” from noise in FRIL.]
Analysis of primary antibodies directed against cytoplasmic vs. extracellular epitopes.
For FRIL, primary antibodies included polyclonal rabbit antibodies against cytoplasmic epitopes of Cx29 (48-7700; Invitrogen, Camarillo, CA) and monoclonal mouse anti-KV1.1 separately directed against extracellular epitopes (75-105) and cytoplasmic epitopes (75-007). Because KV1.1 and KV1.2 must be coexpressed for stability and functionality of channels (Al-Sabi et al. 2010; Dodson et al. 2002), because both KV1.1 and KV1.2 were confirmed by fluorescence microscopic immunocytochemistry to be equally colocalized at juxtaparanodes and along inner mesaxons (see Figs. 2–4), and because the antibodies currently available against KV1.2 proved inadequate for FRIL, we used FRIL to determine the ultrastructural distribution of KV1.1 as a proxy for both KV1.1 and KV1.2 in diverse axonal subdomains. In any case, ambiguities introduced by the “radius of uncertainty” of immunogold labeling (Kamasawa et al. 2006) do not yet permit determination of whether KV1.1 and KV1.2 are within the same intramembrane particle, or indeed within the same rosette. That determination is outside the scope of the current report.
Fig. 2.
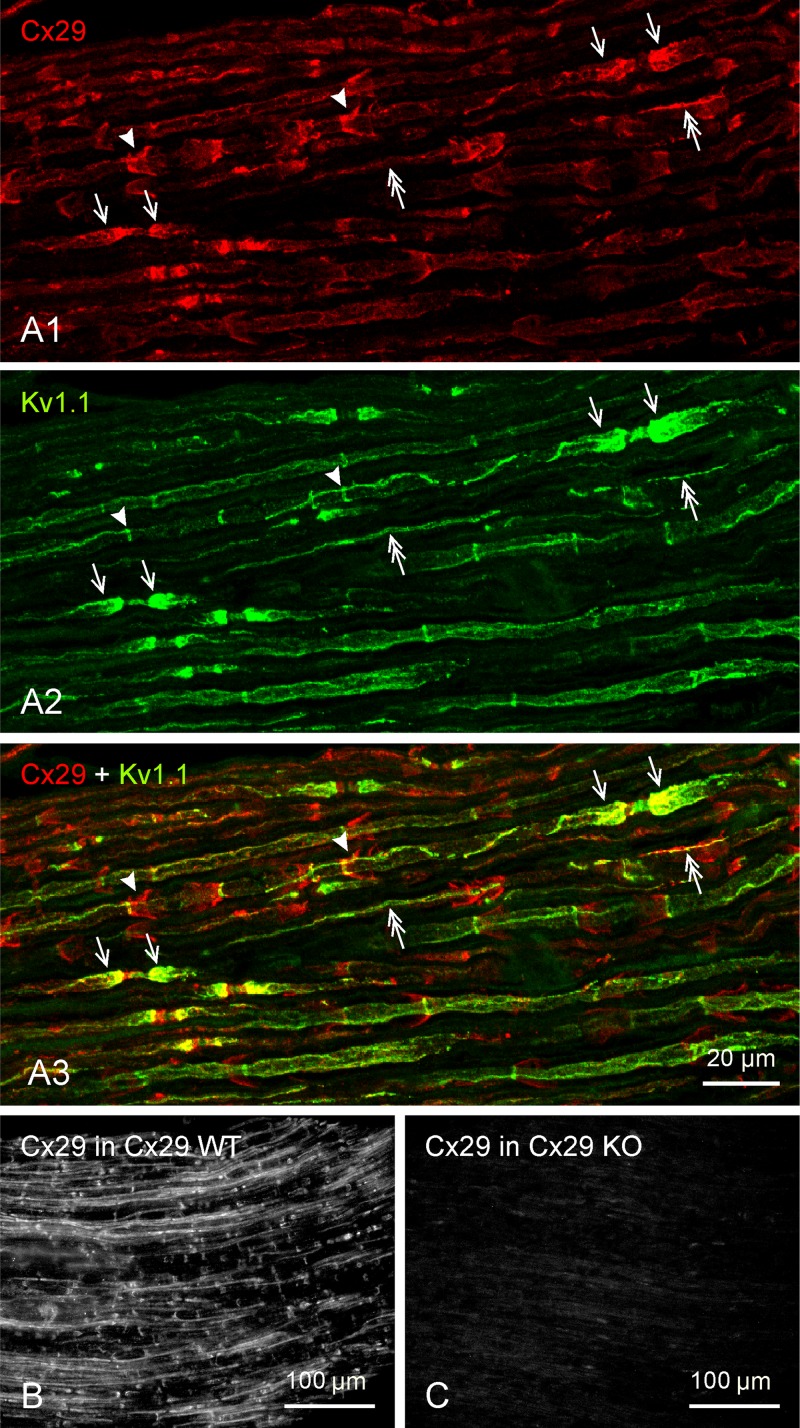
Low-magnification overview of immunofluorescence labeling of connexin29 (Cx29) and KV1.1 in adult rat sciatic nerve (A) and Cx29 channels in adult wild-type (WT) mouse and Cx29 knockout (ko) mouse (B and C). A: 3 images of the same field, showing similar patterns of labeling for Cx29 (A1) and KV1.1 (A2) at juxtaparanodal regions (arrows). Distinct patterns of labeling can be seen at SLIs (arrowheads), and overlapping patterns are visible along longitudinally oriented strands at internodal regions corresponding to locations of inner mesaxon (double arrows), as shown in the overlay (A3). B and C: immunofluorescence labeling of Cx29 shown in sciatic nerve of adult WT mouse (B) is absent in sciatic nerve of Cx29 ko mouse (C). Scale bars are as indicated on each lettered group of panels.
Fig. 4.
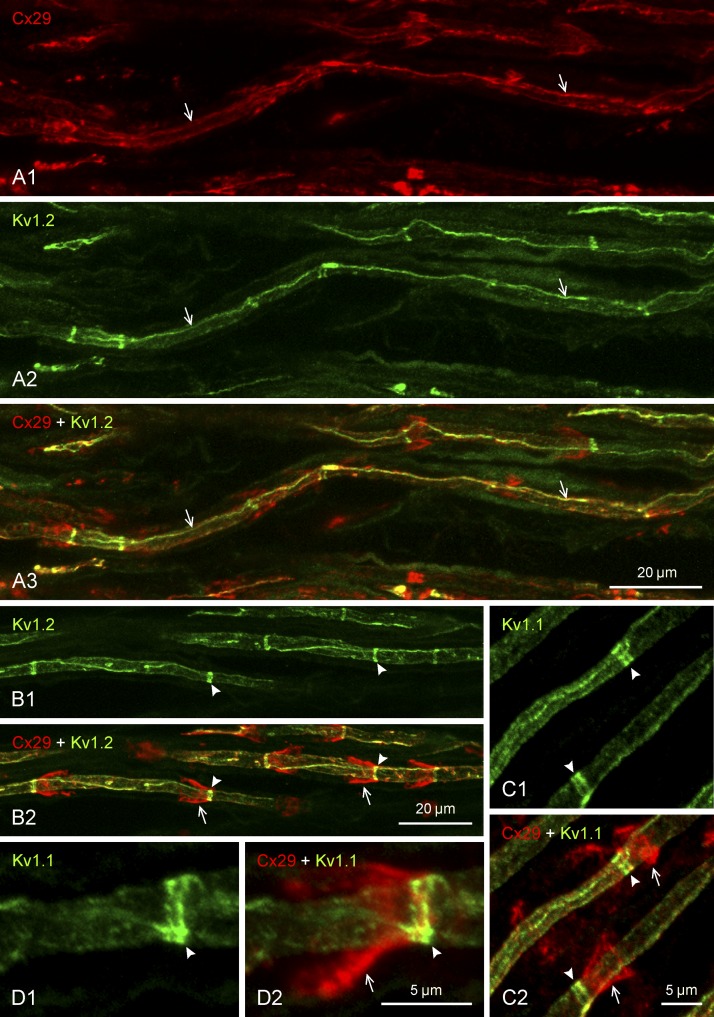
Immunofluorescence double labeling of Cx29 and KV channels along myelinated axons in sciatic nerve of adult rat. Proteins labeled and color code for labeling are indicated at top left of each panel. Images in panels with the same lettering show the same field. A: magnification of internodal regions, showing both Cx29 (A1) and KV1.2 (A2) localized at myelinated fibers as a single near-continuous linear strand of labeling (arrows) running along the internodal segment in an area overlying the axon, with Cx29/KV1.2 colocalization along these strands shown in overlay (A3, arrows). B: internodal regions of myelinated fibers, showing KV1.2 localized as intermittent narrow transverse bands along axons (B1, arrowheads) and Cx29 at funnel-shaped SLIs (B2, arrows), with the KV1.2 bands situated at the narrow ends of the Cx29-immunopositive incisures (B2, arrowheads). C and D: magnifications of SLIs, showing labeling for KV1.1 localized as transverse bands along axons (C1, arrowheads), with the bands consisting of a doublet of bars (arrowheads) overlapping with the narrow end of the incisures labeled for Cx29 (C2, arrows), as shown in overlay (arrowheads) and at higher magnification (D). Scale bars are as indicated on each group of panels.
Secondary antibodies and dual double labeling.
Secondary antibodies used in FRIL included goat anti-mouse IgG conjugated to 5- and 20-nm gold beads (dual labeling for a single protein) and goat anti-rabbit IgG conjugated to 10- and 30-nm gold beads (BBI Solutions, Madison, WI; dual labeling for a second protein), with the combination reflecting “dual double labeling.” The 20- and 30-nm gold beads in each labeling cocktail allowed larger labels to be detected at low magnification (×3,000–5,000), whereas the 5- and 10-nm gold beads, because of their much higher LE, were used to optimize LE and obtain high signal-to-noise ratios (Rash and Yasumura 1999). An additional advantage in using two sizes of gold beads for each labeled protein is that having two independent labels against each primary antibody provides for internal verification of labeling specificity of both primary and secondary antibodies, as well as allowing us to determine whether antibody clumping occurred for either the primary or secondary antibodies (Rash and Yasumura 1999).
Replicas were incubated simultaneously with both monoclonal (e.g., mouse anti-KV1) and polyclonal antibodies (e.g., rabbit anti-Cx29), washed in SPB for 1 h at room temperature, and incubated simultaneously with appropriate gold-conjugated species-specific secondary antibodies. After rinsing and air drying, samples were coated again with 20 nm of carbon on the labeled side to immobilize gold labels and to anneal thermal expansion cracks formed when the replicated samples were warmed from −170°C to +22°C for subsequent immunolabeling. This secondary carbon layer provides additional structural support for the replica during removal of the Lexan film by ∼10-h immersion of the grids in 60–80°C dichloroethane.
Determination of “sidedness” of labeling for KV1.1, KV1.2, and Cx29.
In SDS-washed FRIL replicas, antibodies cannot penetrate the atomic lattices of the thermionically deposited platinum-carbon replica and therefore cannot label the epitopes on the replicated side of exposed proteins, but instead are able to label only the unshadowed epitopes of those same proteins (Dinchuk et al. 1987; Fujimoto 1995, 1997; Rash and Yasumura 1999; Rash et al. 1989). Thus, to identify IMPs that might correspond to KV1.1-containing channels in sciatic nerve, it was first necessary to select appropriate antibodies for FRIL, which would depend on whether the corresponding IMPs remained adherent to the newly created extraplasmic (extracellular) membrane leaflet (or E-face), to the complementary “protoplasmic” membrane leaflet (P-face), or fractured randomly to both E- and P-faces. [Membrane P- and E-fracture faces are designated according to the internationally recognized terminology of Branton et al. (1975).] This topological feature provides a simple mnemonic: E-face particles label only with antibodies against extracellular epitopes, whereas P-face particles label only with antibodies against cytoplasmic epitopes.
“Complementarity” of membrane faces allows determination of total content of intramembrane proteins.
A basic premise of freeze-fracture and FRIL, established by experiments spanning 30 years, is that all transmembrane proteins, including all ion channels, are cleaved intact, without breaking covalent bonds (Fisher and Yanagimoto 1986), leaving an IMP projecting from one fracture face and a corresponding pit in the fracture face that was cleaved away (Challcroft and Bullivant 1970; Li et al. 2008; Steere and Moseley 1969; Ting-Beall et al. 1986). [Energy released per unit area is insufficient to break covalent bonds: 50–250 vs. 0.1–1 kcal/mol to break Van der Waals and London dispersion forces during separation of transmembrane proteins from surrounding lipids and ice (Bailey et al. 1990; Lodish et al. 2000).] Moreover, by subtracting the platinum shadow thickness, the diameter of each IMP has been shown to be proportional to the square root of the number of transmembrane α-helices multiplied by 1.3–1.4 nm per α-helix (Eskandari et al. 1998; Rash et al. 2004a), ranging from very small IMPs [∼2–3 nm for replicated proteins having a single transmembrane α-helix (Dinchuk et al. 1987)] to 9- to 10-nm IMPs for 24- and 28-pass proteins, such as the hexameric arrangement of connexins within a connexon (Rash et al. 2004a), pentameric glutamate receptors (Rash et al. 2004b), and the tetrameric voltage-gated K+ channels in this study. In replicas made with 1.0–1.5 nm of platinum, examining both E- and P-fracture faces from the same subcellular location allows us to reliably discern, count, and measure ion channels as IMPs in one fracture face and to verify those counts by quantifying the corresponding pits in the complementary fracture face. Thus, based on identification of Cx29 as the primary or sole large-diameter protein in innermost myelin P-faces (Li et al. 2002), we were able for the first time to predict the morphology and then to find and quantify the complementary rosettes of pits in the E-face of innermost myelin. Equally important, based on the current identification of KV1.1/KV1.2 as the primary or sole large-diameter protein in axolemmal E-faces, we were able to identify the complementary P-face of the juxtaparanodal axolemma, and from those images, to visualize and quantify the unexpectedly complex molecular architecture of the juxtaparanodal axolemmal P-face and distinguish large-diameter ion channels from their small-diameter transmembrane, extracellularly projecting “tether” molecules.
Transmission electron microscopy and stereoscopic analysis.
Freeze-fracture and FRIL replicas were examined in JEOL JEM2000 EX-II and JEM1400 transmission electron microscopes, both operated at 100 kV. To establish sidedness of labels [as an aid to distinguishing signal from noise (Rash and Yasumura 1999)], analyze the three-dimensional topography, and optimize image contrast, replicas were tilted up to ±60° and photographed as stereoscopic pairs having an included angle of 8° (Steere and Rash 1979). Electron microscope negatives from the JEOL 2000 were digitized using an ArtixScan 2500f digital scanner (Microtek, Carson, CA), whereas digital images were obtained from the JEOL 1400 using an 11 MB Orius SC1000 camera (Gatan, Pleasanton, CA). All images were processed using Adobe Photoshop CS2 (Adobe Systems), with “levels” used for contrast expansion and “brightness/contrast” used to optimize image contrast. Extremes of photographic contrast of replica fragments and displaced sample debris (when present) were reduced using local area “dodging,” as indicated in appropriate images.
RESULTS
Light microscopic immunocytochemistry.
As shown in overview (Fig. 2), the general features of immunofluorescence labeling for Cx29, KV1.1, and KV1.2 in rat and mouse sciatic nerve were similar to previous descriptions (Altevogt et al. 2002; Bhat et al. 2001; Rios et al. 2003; Vabnick et al. 1999; Wang et al. 1993), but with several significant additional details revealed. Cx29 labeling was most concentrated at juxtaparanodal regions (Fig. 2, arrows), with moderate labeling at internodal funnel-shaped collars that extended from the surface of myelin at their expanded end to a convergence on the axon at their narrow end (Fig. 2A1, arrowheads), suggestive of labeling of Schmidt-Lanterman incisures, extending the findings of Altevogt et al. (2002). Additional internodal Cx29 was often localized to long filaments or strands of labeling associated with and running along the length of the axon (Fig. 2A1, double-headed arrows). Immunofluorescence labeling patterns of the two KV1 channels (KV1.1 and KV1.2) were indistinguishable one from the other. As shown for KV1.1 (Fig. 2A2), labeling was most dense at juxtaparanodes, followed by moderate labeling localized as narrow, intermittently occurring circumferential bands (“C-bands”) along internodal regions (arrowheads) and by labeling of variable intensity localized as thin strands running along internodal axons (double-headed arrows). Cx29 was colocalized with the KV channels at many but not all of these regions (Fig. 2A3) (details described below).
Labeling patterns for Cx29 and the KV1.1 and KV1.2 channels observed in sciatic nerve of rat were also evident in the sciatic nerve of mouse, and all labeling patterns of Cx29 observed in sciatic nerve of WT mice (Fig. 2B) were absent in Cx29 ko mice (Fig. 2C), indicating specificity of labeling with the anti-Cx29 antibodies used and complete absence of Cx29 protein in the ko mice.
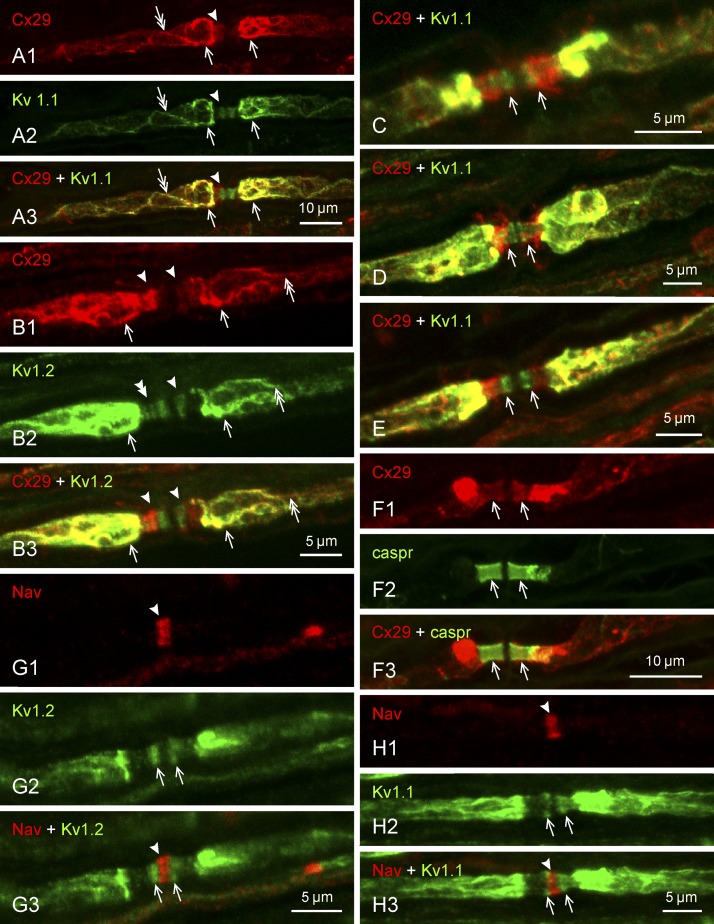
More detailed views of Cx29 and KV channel labeling at regions adjacent to nodes of Ranvier are shown in Fig. 3. In addition to the dense localization of Cx29 and KV1 channels at the juxtaparanodal region, labeling for these proteins along internodal strands corresponding to their localization to the inner mesaxon is seen extending into this region (Fig. 3A). Labeling for Cx29, together with faint labeling of both KV1.1 and KV1.2, is also seen in the paranodal region (Fig. 3B2, double arrowhead). Occasionally, KV1 labeling is resolved as one to three narrow bands straddling nodes of Ranvier (Fig. 3B2). The degree of Cx29 colocalization with the paranodal KV1 bands was variable, from nearly total colocalization (Fig. 3C), only partial colocalization (Fig. 3D), or apparently separate, with KV1 channels positioned closer to the node and Cx29 closer to juxtaparanode (Fig. 3E). Labeling of the transverse Cx29 bands was identified as intraparanodal based on its colocalization with the paranodal marker caspr (Gordon et al. 2014) (Fig. 3F). The paranodal KV1 labeling did not extend into the nodes of Ranvier, and no discernible labeling for either KV1.2 (Fig. 3G) or KV1.1 (Fig. 3H) could be detected at the nodal cleft, the exact location of which was identified by its dense labeling for NaV (Figs. 3G1 and 3H1). (Note: image halation at high fluorescence amplification can produce some minor overlap of fluorescence signals.)
Fig. 3.
Immunofluorescence labeling of Cx29 and KV1.1 at regions surrounding nodes of Ranvier in adult rat sciatic nerve. Proteins labeled and color code for labeling are indicated at top left of each panel. Images in panels with the same lettering show the same field. A: labeling for both Cx29 (A1) and KV1.1 (A2) is dense in the juxtaparanodal region (arrows), faint in the paranodal region (arrowheads), and can be seen extending up to and into the juxtaparanodal region along the inner mesaxon (double arrows), with overlap of labeling in most of these regions (A3). B: similar colocalized labeling of Cx29 (B1 and B3) in relation to KV1.2 (B2 and B3) is shown at juxtaparanodal (arrows), paranodal (arrowhead), and mesaxonal (double arrows) regions. At paranodes, labeling of KV1.2 is resolved as several transverse bands (B2, double arrowhead). C–E: overlays of labeling for Cx29 and KV1.1 at the paranodal/juxtaparanodal region, showing variable degrees of overlap between the transverse bands of KV1.1 labeling at paranodes and dense (C), moderate (D), or weak (E) labeling for Cx29 (arrows) extending into this region. F: labeling of Cx29 in the paranodal area (F1, arrows) overlaps with labeling for the paranodal marker caspr (F2, arrows), as shown in overlay (F3, arrows). G and H: labeling for KV1.2 (G1) and KV1.1 (H1) at paranodes (arrows) does not extend into nodes of Ranvier, identified by their labeling of sodium channels (NaV) (G2 and H2, arrowheads) sandwiched between the paranodal transverse KV bands (G3 and H3). Scale bars are as indicated on each lettered group of panels.
Localization of Cx29 and the KV1 channels along internodal regions is shown in greater detail in Fig. 4. Tortuous, often spiral strands of continuous labeling for these proteins, colocalized as thin filaments or ribbons at the inner mesaxon (Altevogt et al. 2002), could sometimes be followed for relatively long distances, including regions where the filaments seemed to maintain their continuity upon crossing the C-bands at the adaxonal extent of Schmidt-Lanterman incisures (Fig. 4, A and B2, right arrowhead). The funnel-shaped incisures, delineated by their labeling for Cx29, were oriented with adjacent incisures having their narrow ends either pointing away from each other or pointing in the same direction (both configurations illustrated in Fig. 4B). Internodal labeling of KV1 channels, localized as narrow C-bands encircling the axons (Fig. 4B1), were situated at the extreme narrow ends of the Cx29-positive Schmidt-Lanterman incisures (Fig. 4B2, arrows and arrowheads). The bands of KV1 channels at these locations actually consisted of a doublet of bands in the case of both KV1.1 (Fig. 4C1) and KV1.2 (not shown), and it appeared that only one of the pair of bands displayed overlap with labeling of Cx29 at the narrow end of incisures that converged on it (Fig. 4, C2 and D).
FRIL analysis of KV1.1 and Cx29.
In initial FRIL experiments, we first analyzed freeze-fractured samples of sciatic nerve that remained from an earlier study (Li et al. 2002; Meier et al. 2004) involving FRIL analysis of Cx32 and Cx29 in samples from sciatic nerve of unfixed Cx32 ko mice (Figs. 5B and 6, A and B) and directly compared those images from sciatic nerve of formaldehyde-fixed WT mice (Figs. 6C and 7, A and B) with images from unfixed sciatic nerve (not shown). All data presented are from large- and medium-diameter myelinated axons (primarily α-, β-, and γ-motor axons and large primary 1A afferent sensory fibers), all of which were previously established to express both KV1.1 and KV1.2, but not KV1.4, within their heterotetrameric channels, whereas the small-diameter A-δ myelinated “fast pain” fibers and unmyelinated C fibers express KV1.4 as their primary K+ channels, usually coexpressed with KV1.1 (Rasband et al. 2001).
Fig. 5.
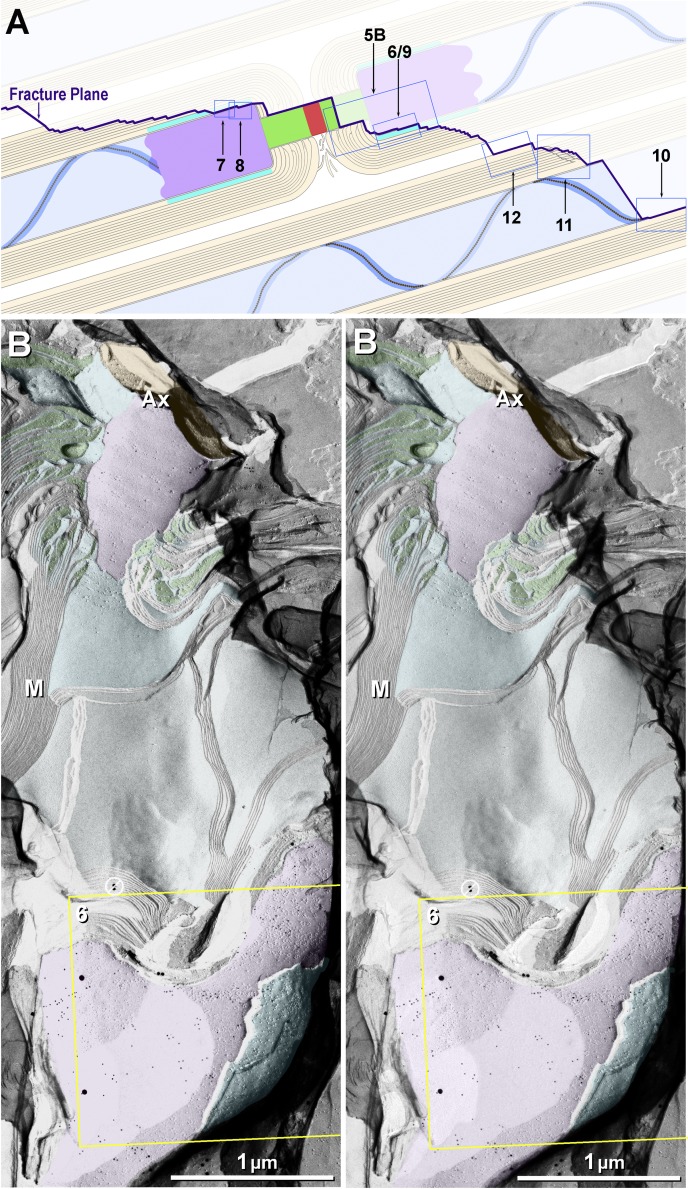
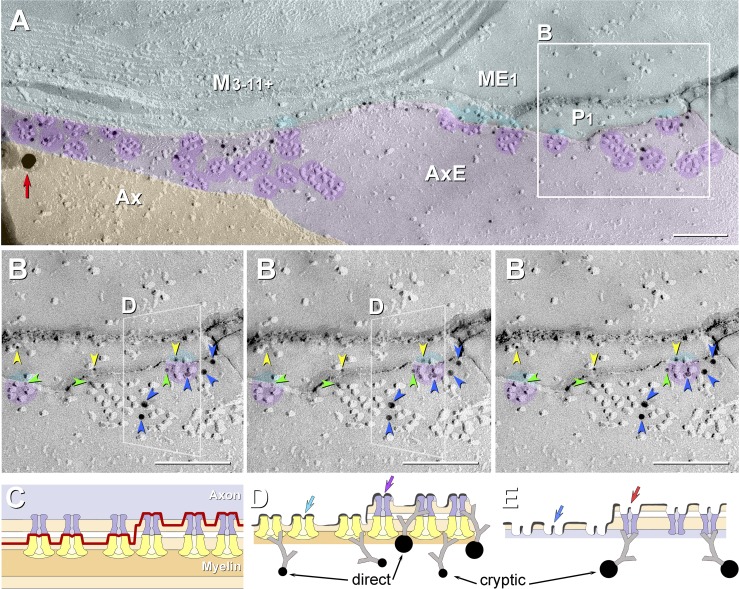
Eight of the 10 principal fracture planes examined in this study (A) and stereoscopic low-magnification image from dual-double-labeled freeze-fracture replica immunogold labeling (FRIL) replica from mouse sciatic nerve revealing the paranodal and juxtaparanodal membranes (B). A: 2 freeze-fractured myelinated axons (blue axoplasm), with the fracture plane (purple line) exposing a node of Ranvier, E-face of the paranodal axolemma, E-face of the juxtaparanodal axolemma (JPAX), and P-face of juxtaparanodal innermost myelin (JPIM; box 6/9), pitted E-face of juxtaparanodal myelin (box 7), P-face of the JPAX (box 8), E- and P-faces of inner mesaxonal myelin (IMAX; blue overlay and box 10), Schmidt-Lanterman incisures, and P-face of outer surface of myelin. Boxes correspond to numbered figures. For simplicity, only 10 of the usual 25–50 layers of myelin are indicated. Dark aqua and box 8, KV1.1-enriched JPAX; blue ribbon and box 10, IMAX; light blue, axon; beige, myelin; red, node; green, paranodes; purple, juxtaparanodes. B: low-magnification overview of the paranodal and juxtaparanodal membranes in a single axon; boxed area is shown at higher magnification in succeeding figures. At this magnification, 3 of the 4 sizes of gold beads used for dual double labeling are discernible (10- and 30-nm gold for KV1.1 and 20-nm gold for Cx29) inside the yellow box. Abundant 5-nm gold beads (also for Cx29) cannot be discerned but are detectable when box 6 is further enlarged as Fig. 6. A pair of gold beads (barred circles at top edge of yellow box) on top of the replica are positively identified as “noise.” Purple overlay at top is axolemmal E-face, with impressions of paranodal loops of myelin; purple overlay at bottom is E-face of the JPAX region within the same complexly fractured myelin sheath (M). Aqua overlays (middle and bottom) are portions of the underlying P-face of innermost myelin, including tips of paranodal loops. Green overlays are cytoplasm of paranodal loops. Local area “dodging” was used to reduce the photographic intensity of superimposed replica fragments and areas of folded replica. Ax, axoplasm. Scale bar, 1 μm.
Fig. 6.
Stereoscopic images of particle rosettes in JPAX E-face and JPIM P-face from Cx32 ko mouse (A and B) and from WT mouse (C). A and B: E-face rosettes (purple overlays) in the axolemma are labeled for KV1.1, and P-face rosettes (aqua overlays) in innermost myelin are labeled for Cx29. Four sizes of gold beads are used: 2 for KV1.1 (10- and 30-nm gold) and 2 for Cx29 (5- and 20-nm gold). Double-ended red arrow compares 30- vs. 20-nm gold beads; double-ended yellow arrow compares 10- vs. 5-nm gold beads. Green overlays are tongues of myeloplasm at inner mesaxon. Apposing strips of axolemmal E-face (AxE; orange overlay) and myelin P-face (MyP; yellow overlay) are deficient in rosettes, whereas rosettes are abundant on either side of this band. B: High-magnification stereoscopic image of AxE (enlarged from yellow box in A). Most axonal rosettes are labeled by one to four 10-nm gold beads (blue arrowheads) for KV1.1. In bottom right quadrant, the fracture plane dropped from the AxE to the MyP, where the rosettes and clusters of 9-nm particles are labeled for Cx29 by 5-nm gold beads (yellow arrowheads). Purple overlays are rosettes of axolemmal 9-nm E-face particles. Rosettes of P-face particles (aqua overlays) in innermost myelin are labeled for Cx29. C: fracture from AxE to MyP in a WT sample that was dual-double-labeled for Cx29 (∼18 10-nm and six 30-nm gold beads) and for cytoplasmic epitopes of KV1.1 (5- and 20-nm gold; none present). As shown here, cytoplasmic epitopes of KV1.1-containing particle rosettes were never labeled. Purple overlays are unlabeled axonal rosettes. Aqua overlays are clusters and rosettes of 9-nm intramembrane particles (IMPs) labeled for Cx29. Left inset, myelin P-face particles labeled for Cx29 (from top yellow box), presented with black shadows to reveal the central “dimples” (yellow arrowhead). Right inset, axolemmal E-face rosette (from bottom yellow box), also presented with black shadows to reveal the central dimple in each KV1.1-containing IMP (yellow arrowhead). Scale bar, 0.1 μm (10 nm in insets).
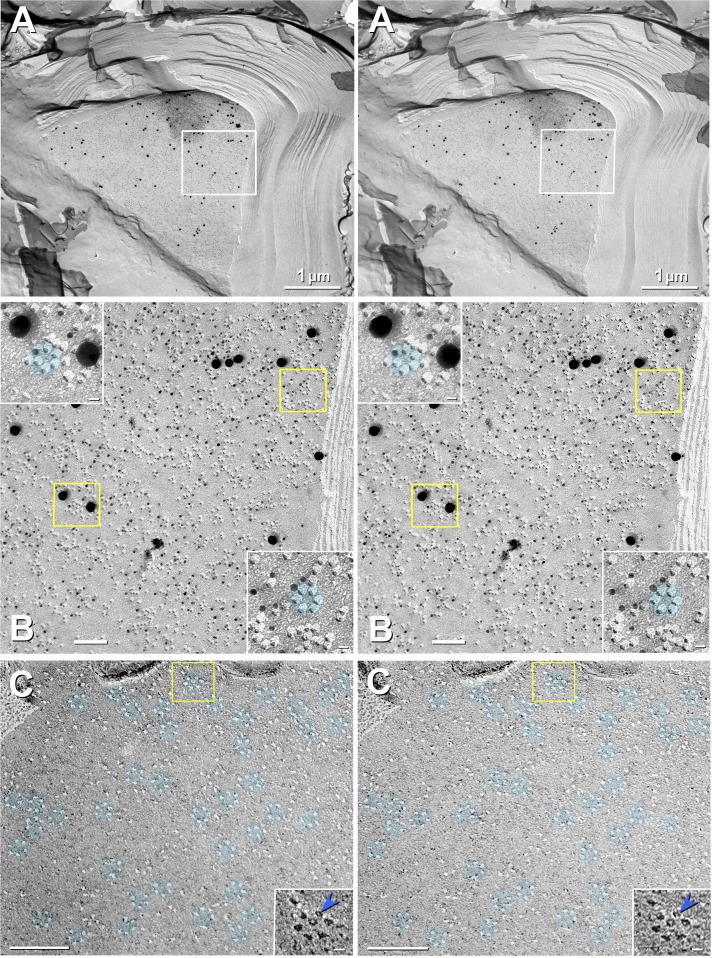
Fig. 7.
Stereoscopic images of labeled JPIM P-face (A and B) and unlabeled JPIM E-face (C). A: high labeling specificity, high labeling efficiency (LE; >1:3), and high signal-to-noise ratio for Cx29 in innermost adaxonal myelemma of a large-diameter axon in mouse sciatic nerve. Box in A is further magnified as B. Slight local area dodging was used to reduce the photographic intensity of the clump of antibodies outside the top left region of the box. B: at higher magnification, the excessively high LE is shown to result in potential obscuring of some particles. Yellow boxes are enlarged (insets) to reveal the greater LE for small gold beads. C: E-face imprints of densely packed Cx29 rosettes (aqua overlays) in JPIM of glutaraldehyde-fixed (i.e., not labeled) sciatic nerve from WT mouse. Most rosettes are complete rings, but a few incomplete rings contain only 4 or 5 pits. Most pits contain a central peg (blue arrowhead in inset; shown with black shadows). Pegs represent the frozen water-filled matrix extracted from the ion channel during cleaving (Hirokawa and Heuser 1982; Rash et al. 2004a), implying that most Cx29 channels are open when fixed. Paucity of E-face IMPs indicates the virtual absence of all other types of ion channels in E-faces. Likewise, in the complementary P-faces (B), there are few, if any, particles other than Cx29. Scale bars, 1 μm (A), 0.1 μm (B and C), and 10 nm (B and C, insets).
There are 50–100 potential fracture planes within each large-diameter myelinated axon, but there is only one potential fracture plane, along the lower surface of each axon (Fig. 5A), that exposes the E-face of the juxtaparanodal axolemma (JPAX), revealing its distinctive rosettes of E-face particles (Figs. 6 and 8A), and there is only one fracture plane, along the upper axonal membrane, that reveals the previously unrecognized JPAX P-face, with its unexpected ultrastructural complexity (detailed below). Likewise, there is only one fracture plane that exposes the P-face of the adjacent juxtaparanodal innermost myelin (JPIM), revealing its distinctive rosettes of P-face particles (Figs. 6A and 7B), and only one fracture plane that exposes the complementary JPIM E-face, with its distinctive rosettes of pits (Fig. 7C).
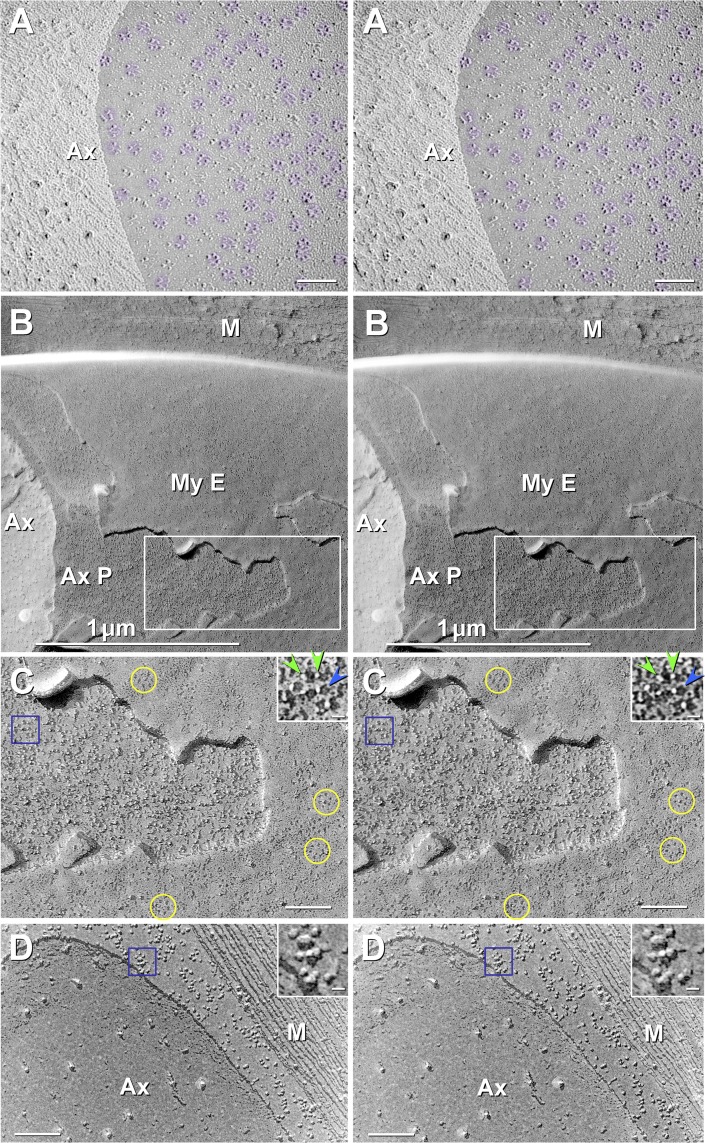
Fig. 8.
Stereoscopic images comparing JPAX E-face rosettes of particles (A), JPAX P-face rosettes of pits (B and C), and internodal axolemmal P-face particles (D). A: at high magnification, the JPAX E-face from formaldehyde-fixed WT mouse sciatic nerve contains densely packed rosettes of KV1.1/KV1.2 particles (purple overlays), with almost no additional free particles that are larger than 6 nm in diameter. WT sciatic nerve axon dual-double-labeled with antibodies against cytoplasmic epitopes of KV1.1 and KV1.2 (10- and 30-nm gold beads; none present) and Cx29 (not present here). Axonal E-face rosettes are densely packed in juxtaparanodal regions, typically ranging from 200 to 270 rosettes per μm2 (see also Stolinski et al. 1981, 1985). In this ∼0.4-μm2 area, there are 270 rosettes per μm2. B: low-magnification stereoscopic view of axon and surrounding myelin. Boxed area is shown at higher magnification in C. Ax, axoplasm; AxP, axolemmal P-face; MyE, innermost myelin E-face; M, cross-fracture myelin. C: JPIM E-face collar is recognized by its characteristic high density of E-face rosettes of pits (a few delineated by yellow circles). The underlying JPAX P-face is not smooth, as we originally expected based on the densely packed rosettes of IMPs in the JPAX E-face (A), but instead has a fine “stubble” of 2- to 3-nm-diameter stringlike IMPs that are interspersed with, and therefore partially disguise, the rosettes of pits (blue box; enlarged as inset). Stringlike IMPs (inset, green arrowheads) jut from the left side (leeward cleaving side) of each 7- to 9-nm pit (blue arrowhead). The vertically oriented stringlike IMPs are delineated by the extremely electron-opaque platinum coat as viewed along its vertical axis, shown as intense white dots in the black shadow image. Each pit has a faint central peg (blue arrowhead). (See Fig. 12C for diagram of “tether” proteins.) D: small clusters of large IMPs characterize the internodal axolemma, which has no rosettes of P-face pits, except along the juxtamesaxon (see Fig. 10A). Blue box (enlarged as inset) shows clustered medium- to large-diameter IMPs. Scale bars, 1 μm (B), 0.1 μm (A, C, and D), and 10 nm (insets).
From the few areas containing internodal axonal E-face and/or adaxonal myelin P-face that were encountered in each replica, only 1–2% of those were fractured within the 10- to 15-μm-long juxtaparanodal region, with the other 98% fracturing within the roughly 800-μm-long segment of internodal myelin or within deeper layers of compact myelin. Based on the relatively rare fractures within juxtaparanodal membranes, several anatomically instructive images containing almost all regions relevant to this study are from a fortuitous single small area from an unfixed sample from a Cx32 ko mouse sciatic nerve (Figs. 5B and 6, A and B). We utilize those images primarily for anatomical reference purposes, because as documented below, the axolemmal regions and molecules identified in this Cx32 ko mouse were indistinguishable from those from similar areas in WT mice (Figs. 6C and 7). Moreover, this replica from Cx32 ko mouse had additional advantages, including a large area of juxtaparanodal apposition containing contiguous areas of both axolemmal E-face and innermost myelin P-face (Figs. 5B and 6, A and B); an optimum shadow thickness, resulting in both high-resolution replication of IMPs and pits and high visibility of the smallest gold beads (Fig. 6B, yellow arrowheads); and optimum LE for both KV1.1 and Cx29 (i.e., ∼1:6, or ∼1 gold bead per rosette; Fig. 6, A and B). Additional replicas from WT mice were prepared so as to have higher LE for quantitative analysis (Fig. 7, A and B). Although high numbers of gold beads potentially could obscure important replica details, those replicas provided positive demonstration that virtually all 9-nm IMPs in JPIM P-faces were immunogold labeled (Fig. 7B), as detailed below.
Nodes of Ranvier, identified by imprints of paranodal loops that mark the proximal and distal boundaries of the nodal plasma membrane, were devoid of gold beads labeling for either extracellular or cytoplasmic epitopes of KV1.1 on either P- or E-faces, thereby documenting complete absence of KV1.1 from rodent nodes of Ranvier, further contradicting proposals for voltage-gated K+ conductance within nodes (Fig. 1B). In contrast, juxtaparanodal regions of the axolemma were labeled at high density for KV1.1 (detailed next), whereas the paranodes had only a few axolemmal rosettes between imprints of paranodal loops, many of which were labeled for KV1.1 (not shown), consistent with faint immunofluorescence labeling for this protein within some paranodes (Fig. 3B2).
A low-magnification stereoscopic overview image from the juxtaparanodal region (Fig. 5B) is enlarged to show a portion of the axon E-face (Fig. 6A) and a relatively large portion of the innermost myelin P-face (Fig. 6A), which is shown at still higher magnification in Fig. 6B. The JPAX domain has distinctive, densely-packed hexagonal “rosettes” and irregular clusters of 9-nm E-face particles, many of which were dual labeled by immunogold antibodies against extracellular epitopes of KV1.1 (10- and 30-nm gold beads; ∼1 gold bead per 12 IMPs). In this dual double-labeled sample, myelin P-faces exhibited densely-packed IMP rosettes that were dual labeled by antibodies against cytoplasmic epitopes of Cx29 (5- and 20-nm gold beads), consistent with our previous identification of the myelinic P-face rosette particles as Cx29 (Li et al. 2002). The red two-ended arrow in Fig. 6A distinguishes 20-nm from 30-nm gold beads, and the yellow two-ended arrow in Fig. 6, A and B, distinguishes 5-nm gold from 10-nm gold beads. The larger gold beads in each labeling combination acted as visual “flags” to facilitate finding labeled areas at ×3,000–5,000 “survey” magnifications, whereas the smaller gold beads, with their 10-fold higher LE but low detectability below ×30,000, were used for quantitative analysis. (See methods for additional rationale for dual double labeling.)
In the apposed JPIM P-face, densely packed rosettes and irregular clusters of 9-nm P-face particles are visible (Fig. 6, A–C, aqua overlays), many of which were labeled for Cx29 (mostly 5-nm and a few 20-nm gold beads). In the JPIM collar of a heavily labeled replica from a WT mouse (Fig. 7, A and B), the LE for Cx29 was ∼1:3 (i.e., ∼2 gold beads per rosette). Approximately the same high LE also occurred beneath both dispersed 9-nm IMPs and those still clustered in rosettes and strings, demonstrating that during formaldehyde fixation, the almost pure population of Cx29 rosettes seen in glutaraldehyde-fixed samples (Fig. 7C) is variably dispersed, often into strings, clusters and individual 9-nm IMPs, but invariably containing at least a few recognizable rosettes (Figs. 6, B and C, and 7, A and B).
KV1.1 rosettes label only with antibodies against extracellular epitopes.
In multiple replicas from WT and Cx32 ko mice, axonal E-face particle rosettes were abundantly labeled by antibodies against extracellular epitopes of KV1.1 (Figs. 5B and 6, A and B). To test whether antibodies directed against cytoplasmic epitopes of KV1.1 also labeled the juxtaparanodal axolemmal rosettes or labeled any other IMPs in nodes of Ranvier or in any other regions of the axolemma, we dual double-labeled other samples from WT mice using antibodies against cytoplasmic epitopes of KV1.1, in combination with our standard antibodies against cytoplasmic epitopes of Cx29. In the JPAX E-face, KV1.1-containing rosettes were abundant (Figs. 6C and 8A), but none were labeled by antibodies against their cytoplasmic epitopes, even though nearby myelin P-face rosettes were strongly labeled with antibodies against cytoplasmic epitopes of Cx29 (Fig. 6C). The latter area also served as an internal “control” to reveal that potentially excessive SDS washing had not removed other nearby proteins, which then remained for FRIL labeling. Also noteworthy, antibodies against cytoplasmic epitopes of KV1.1 did not label axonal P-face IMPs at nodes, paranodes, inner mesaxon, or internodal axolemma. Thus, combined with strong labeling of axolemmal E-face rosettes (Figs. 6 and 9, A and B), the absence of P-face labeling confirms that most, if not all, freeze-fractured KV1.1 channels remained as E-face particles within the internodal axolemmas in both the JPAX and juxtamesaxonal axolemma (JMAX), as further detailed below. Thus these immunogold-labeling data reveal that the “particle partitioning coefficient” (Satir and Satir 1979) for KV1.1-containing channels is 100% to the axolemmal E-face particles and 0% to any of the few particles found on the axolemmal P-faces. (JPAX P-face pits are described below). Because KV1.1 and KV1.2 must be coexpressed for stability and functionality of K+ channels (Al-Sabi et al. 2010; Dodson et al. 2002), and because both KV1.1 and KV1.2 were confirmed by immunocytochemistry to be colocalized at juxtaparanodes and inner mesaxons (Figs. 2–4), we used FRIL of KV1.1 as a proxy for both proteins.
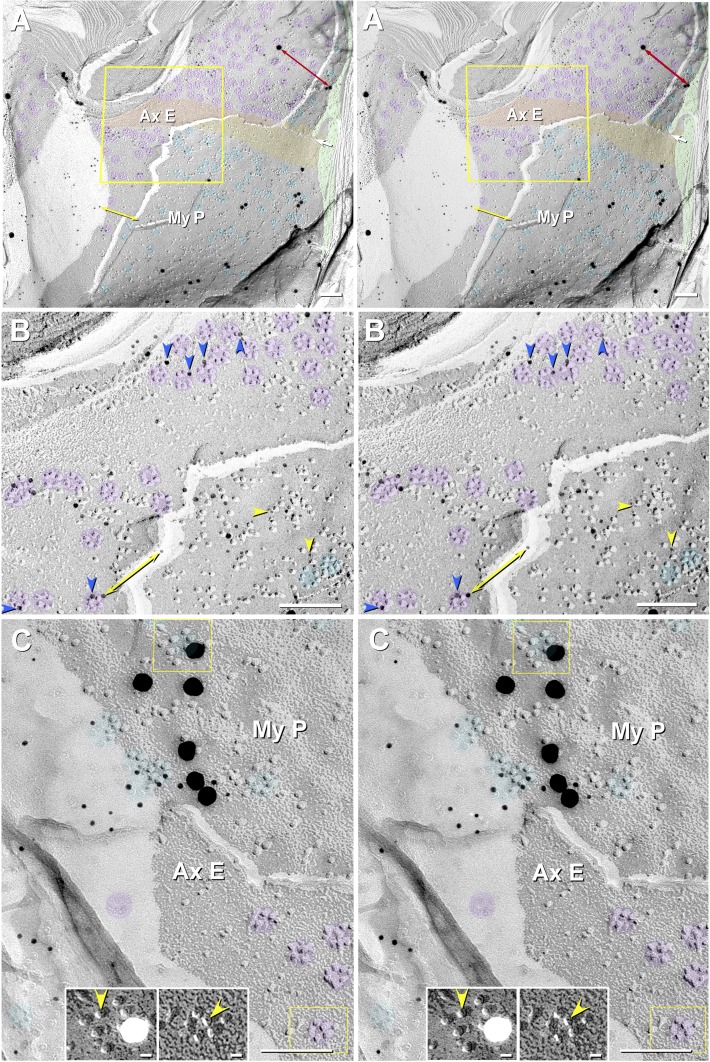
Fig. 9.
High-magnification FRIL images (A and B) and correlative diagrams (C–E) showing molecular coalignment of immunogold-labeled KV1 and Cx29 channels along the juxtamesaxonal axolemma/juxtamesaxonal innermost myelin (JMAX/JMIM). A: rosettes of axonal E-face particles (purple overlays) are labeled by ∼15 10-nm gold beads and by one 30-nm gold bead (red arrow at left edge). At the step from axolemmal E-face to myelin P-face, both proteins remain adsorbed following SDS washing, as inferred from presence of labels for both KV1 and Cx29. Ax, axoplasm (orange overlay); AxE, axolemmal E-face (light purple overlay); P1, innermost myelin P-face; ME1 and M3-11+, succeeding layers of myelin (light aqua overlay). B: stereoscopic (left pair of images) and reverse stereoscopic images (right pair of images) from boxed area in A, revealing “direct” labeling of Cx29 (yellow arrowheads) and KV1.1 IMPs (blue arrowheads) vs. “cryptic” labeling for Cx29 (green arrowheads) beneath KV1.1 rosettes. In reverse stereo, gold beads appear to be on top of the replica, making them easier to discern. Where the fracture plane stepped from axonal E-face to myelin P-face within a rosette (left and right sides of B), axonal E-face particles form one side of the rosette (purple portions of circular overlays), and underlying myelin P-face particles complete the rosette pattern (aqua portions of same circular overlays). The fracture step in box D is diagrammed as Fig. 10D. C–E: diagrams showing complementarity of composite KV1.1/Cx29 particles and their respective pits, with the icy pegs (E; arrows) corresponding to the central dimple (mouth of ion channel) in each IMP (D; arrows). C: diagram of fracture plane (red line) in B. D and E: complementary fracture faces. Unreplicated proteins remain attached to the replicated IMPs, allowing cryptic labeling (Fujimoto 1995, 1997; Rash and Yasumura 1999). Axonal E-face particles (blue channels) directly appose myelin P-face particles (yellow channels). Direct labeling occurs where the target protein is visualized by replication with platinum-carbon, whereas cryptic labeling occurs where the target protein is not visualized by replication but, nonetheless, is inferred on the basis of immunogold labeling of its strongly bound and platinum-replicated coupling partner. Thus some axonal E-face particles appear to be labeled for both KV1 and Cx29. Y-shaped gray linkers are primary and secondary antibodies. Scale bars, 0.1 μm.
Complementarity of membrane faces allows quantification of all ion channels in JPAX and JPIM.
To investigate the basis for the inability of previous investigators to find the complementary pit images of the JPAX and JPIM rosettes (Miller and Pinto da Silva 1977; Stolinski et al. 1981, 1985), and moreover, to determine if any other large transmembrane proteins were present in those previously unrecognized membrane faces, we examined conventional freeze-fracture replicas of glutaraldehyde-fixed (i.e., not labeled) sciatic nerve from WT mice. In stereoscopic images of the JPIM E-faces, we discovered densely packed rosettes of myelin E-face pits (Fig. 7C), for the first time revealing the complementary impressions of the Cx29 P-face rosettes. These new images were extremely informative, revealing molecular details that could otherwise not be determined. Especially noteworthy was that very few IMPs of any size class were intermixed with the E-face rosettes of pits in the juxtaparanodal collars (Fig. 7C). Overall, there were 0–30 unidentified 6- to 10-nm-diameter IMPs per square micrometer in innermost JPIM collar E-faces compared with 3,000 Cx29 particles per square micrometer in the complementary P-face. This means that the JPIM is a molecularly homogeneous membrane domain, with Cx29 representing >98% of all particles large enough to be ion channels in the JPIM collars.
Based on these newly recognized myelin E-face views, we surmise that replicas of JPIM E-face pits were not recognized in 1980-era replicas because the rosettes of membrane pits were partially filled in, either by a thin layer of water vapor contamination (Rash et al. 1979) or by the thicker platinum coats commonly used at that time. Either of those shadowing defects would have resulted in smooth, essentially particle-free myelin E-faces that would have resembled (and likely been mistaken for) one of the many deeper layers of particle-free compact myelin.
Although the JPAX E-face particle rosettes were abundant and easily recognized (Figs. 6 and 8A), JPAX P-faces were especially difficult to recognize because we initially searched for membrane faces that were anticipated to be as smooth and as replete with rosettes of pits as were the JPIM E-faces (Fig. 7C). However, JPAX P-faces (Fig. 8, B and C) had almost no large-diameter IMPs, but instead exhibited a fine “stubble” of 3-nm-diameter × 10- to 15-nm-long stringlike IMPs, interspersed with densely packed rosettes of pits (Fig. 8C, inset, blue arrowheads). Thus the JPAX P-face pits were difficult to recognize, even in stereoscopic images, because the pits were almost always camouflaged by an abundance of these 3-nm-diameter, stringlike particles (Fig. 8C, inset, green arrowheads) that projected upward from the edge of almost all of the hexagonally arranged 9-nm membrane pits. Based on their 3-nm diameter (presumably slightly enlarged by the 1- to 1.5-nm platinum coat), each stringlike molecule is likely formed by a single transmembrane α-helix that extends from the axoplasm, through the protoplasmic leaflet, around each axolemmal KV1.1 particle, and across the extracellular space, apparently to link to Cx29 IMPs in apposed myelin (detailed below). Moreover, at the step from myelin E-face to JPAX P-face, the stringlike IMPs often spanned the extracellular space, suggesting that these slender IMPs act as extracellular tether molecules that link and align the Cx29- and KV1.1-containing channels. Thus we presume, but have not yet confirmed by FRIL, that these stringlike IMPs correspond to the Caspr2/Tag-1 complex that is thought to localize KV1.1/KV1.2 subunits to the JPAX membrane (Poliak et al. 2003; Rasband 2004). Also noteworthy, the absence of any other large-diameter IMPs in either JPAX P- or E-faces means that there can be no significant numbers of ion channels other than KV1.1/KV1.2 in the JPAX domain. In contrast, small clusters of large-diameter IMPs characterize the internodal axolemma (Fig. 8D).
Molecular coalignment of KV1.1 and Cx29 channels.
As originally shown by Stolinski et al. (1981), the fracture plane occasionally steps from axolemmal E-face to myelin P-face within a rosette (Fig. 9, A and B, composite purple-aqua overlays). There, myelin P-face particles are located precisely (±2 nm) where an axonal E-face rosette particle would have been predicted to overlie the visualized myelin P-face particle had the axonal E-face particle not been fractured away (Fig. 9B, yellow arrowhead in obliquely transecting box). This intercellular alignment of rosette IMPs, seen in multiple examples, means either 1) that the apposed molecules are structurally linked via short intercellular molecular tethers that maintain molecular alignment (Fig. 8C, inset, green arrowheads) or 2) that the mouths of the two types of apposed channels are in direct molecular contact across the slightly narrowed extracellular space, as also occurs when connexons align across a narrowed extracellular space during formation of gap junctions [see Fig. 3C, red and blue arrowheads, and Fig. 4A in Johnson et al. (2012)].
Cryptic labeling.
In multiple areas where the fracture plane stepped from axolemmal E-face to myelin P-face within individual rosettes (Fig. 9B, purple overlays), the rosettes often were simultaneously double-labeled for KV1.1 (10-nm gold beads; blue arrowheads) and Cx29 (5-nm gold beads; yellow arrowheads). Of the 19 gold labels shown, three 5-nm gold beads for Cx29 (green arrowheads) beneath KV1.1/KV1.2 channels apparently represent “cryptic” labeling of the unvisualized Cx29 channels that remained adsorbed beneath the overlying and replicated KV1.1 channels (interpretive drawing, Fig. 9, C and D). [Note: cryptic labeling of unvisualized connexin proteins beneath gap junction E-face pits is well documented (Fujimoto 1995, 1997), as confirmed in matched double replicas (Li et al. 2008).]
If each paired rosette particle forms a composite channel, as proposed by Stolinski et al. (1981), these asymmetric KV1/Cx29 channels would constitute an intercellular ion conductance pathway that would likely have unique gating, ion selectivity, and ion conductance properties. Moreover, the proposed conductance properties of KV1/Cx29 channels in mammals would likely be asymmetrically regulated by the cell expressing each protein: KV1.1/KV1.2 by the axon and Cx29 by myelin. Although one might envision difficulties to be created by coupling a four-subunit channel (Wang et al. 1999) to a six-subunit connexon channel (Cx29; Unwin and Zampighi 1980), it should be noted that the four KV1 channel monomers are “six-pass proteins,” whereas connexons are composed of hexamers of “four-pass proteins,” making both KV1.1 and Cx29 equally large “24-pass channels.” With both of these 24-pass channels having 12 extracellular loops, this also creates the potential for unique intercellular molecular coupling, potentially mediated by one or more of the cell-adhesion molecules localized to the JPAX/JPIM and JMAX/JMIM (juxtamesaxonal innermost myelin) in mammalian myelinated axons (Ogawa et al. 2008; Peles and Salzer 2000; Poliak et al. 2003; Rasband 2004). In any case, the intercellular junctions formed by KV1/Cx29 channels are “xenotypic” (i.e., unrelated proteins linking to form intercellular junctions); however, demonstration of electrical coupling via KV1/Cx29 channels and elucidation of their biophysical properties must await analysis of apposing cells separately expressing these proteins.
KV1.1 and Cx29 resolved as tubular IMPs; their complementary pits have central pegs.
Based on improvements in affordable high-vacuum and high-resolution shadowing technology (Rash and Yasumura 1992, 1999), two- to fourfold finer details are resolved in these replicas, approaching 0.7-nm resolution (Rash et al. 1997, 2004a). In the absence of a detectable layer of water vapor contamination, which otherwise obscures membrane pits and enlarges IMPs (Rash et al. 1979), these thinner and finer grained platinum shadows revealed new details of molecular morphology: 1) the 11- to 12-nm rosette particles previously described (Miller and Pinto da Silva 1977; Stolinski et al. 1981, 1985) are now resolved as 9-nm IMPs; 2) the rosettes of myelin E-face pits (Figs. 7C and 8C), which previously had never been observed, are now revealed to be complementary to the rosettes of Cx29-containing P-face particles (Figs. 6, 7B, and 9, A and B); and 3) these higher resolution complementary views reveal both the ice “peg” (Fig. 7C, insets, blue arrowheads; and explanatory drawing, Fig. 9, C–E) extracted from within the pore of each aqueous ion channel, as well as the complementary “dimple” in the P-face IMP that represents the mouth of the pore of each ion channel (Fig. 6C, insets, yellow arrowheads). [For details regarding visualization of open vs. closed connexons in deep-etched samples and of the “etchability” of the water-filled central pegs in the complementary E-face pits of both gap junctions and aquaporin 4 (AQP4) arrays, see Hirokawa and Heuser (1982) and Rash et al. (2004a), respectively.] The insets are presented with both black shadows (Figs. 6C, 7C, and 8, C and D) and “white shadows” (Fig. 7B), because at high magnification, “life-like” black shadows are more easily interpreted than images with unnatural white shadows (Steere et al. 1980).
KV1.1 and Cx29 form rosettes along the inner mesaxon.
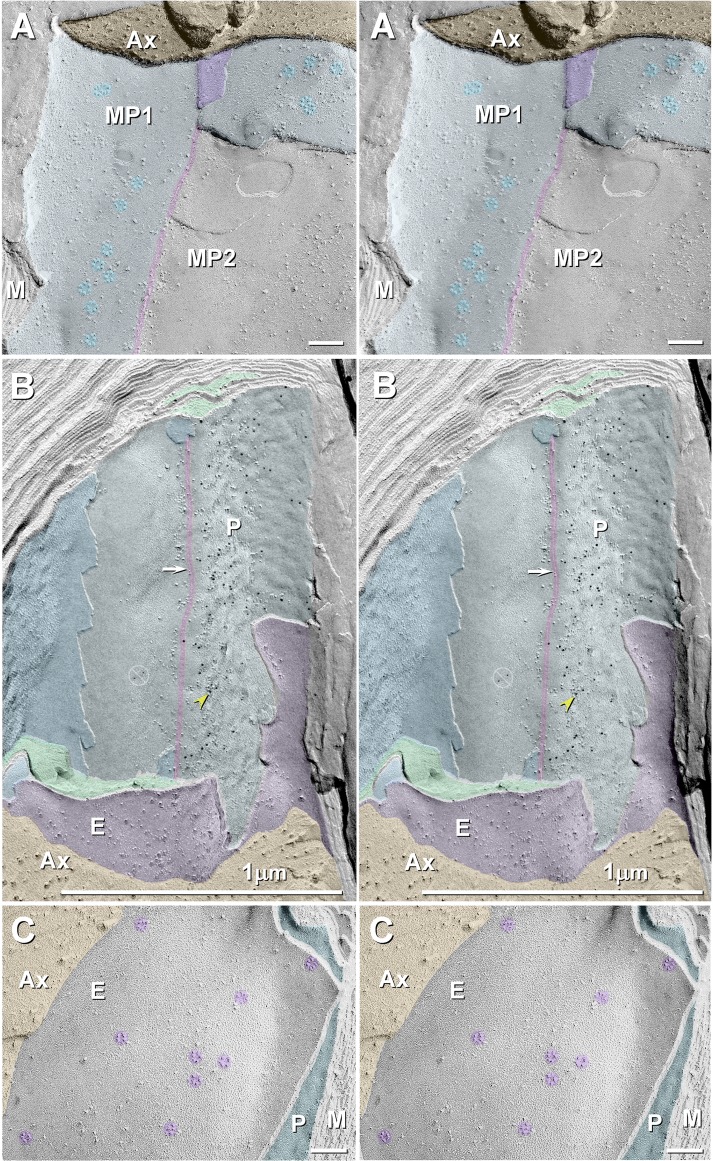
Along the entire internodal region of myelinated axons, the inner mesaxon represents the anatomic location where the membrane of the innermost cytoplasmic tongue of myelin abuts the membrane of the cytoplasmic shoulder formed within the first complete layer of myelin (Fig. 10, A and B). At this apposition, myelin is not compact, but instead forms two small, variable-bore cytoplasmic conduits, the juxtamesaxonal innermost myelin (JMIM) canals, which link the proximal JPIM collar to the distal JPIM collar (Mugnaini et al. 1977; Schnapp and Mugnaini 1978; Stolinski et al. 1985). [The physiological basis for the often noted variation in volume of the JMIM inner tongue, which on occasion can extend around the entire circumference of the axon and become continuous with and terminate at the cytoplasmic shoulder of the first complete wrapping (see Figs. 6.4 and 6.5 in Peters et al. 1991) is not yet established.] In FRIL images, the JMIM conduit is recognized by the presence of one to three tight junction strands that link the inner tongue of myelin to the next overlying continuous layer of myelin (Fig. 10, A and B), as described by others (Livingston et al. 1973; Mugnaini et al. 1977; Schnapp and Mugnaini 1978; Stolinski et al. 1985). In glutaraldehyde-fixed sciatic nerve axons, almost all of the 9-nm IMPs were present in rosettes in both inner mesaxonal axolemmal and inner mesaxonal myelin (Fig. 10A), confirming the original descriptions of Stolinski et al. (1985). In contrast, formaldehyde fixation resulted in fewer rosettes, most of which were replaced by irregular clusters of 9-nm IMPs that, nevertheless, were immunogold labeled (Fig. 10B), thereby documenting that the rosettes disassemble into clustered 9-nm particles during fixation with formaldehyde. Except for localization of Cx29 at Schmidt-Lanterman incisures (see below), Cx29 was not detected elsewhere within compact myelin.
Fig. 10.
Stereoscopic images of juxtamesaxonal membranes in glutaraldehyde-fixed (A) and formaldehyde-fixed WT mouse sciatic nerve (B) and in glutaraldehyde-fixed Cx29 ko mouse (C). A: rosettes of myelin P-face particles in glutaraldehyde-fixed JMIM (MP1; light aqua overlay) and absence of rosettes where the second wrapping of myelin is exposed (MP2). Orange overlay, axoplasm; pink overlay, tight junction; purple overlay, axon E-face; M, compact myelin. B: abundant 9-nm IMPs along the JMIM expansion (to right side of the tight junction, arrow and pink overlay) but almost no 9-nm IMPs on the continuation of the same membrane, which becomes the second wrapping of myelin to the left of the tight junction. Cx29 is labeled with 10-nm gold beads (yellow arrowhead). Ax, axoplasm (orange overlay); E, E-face of the JMAX (purple overlay); P, myelin P-face; dark aqua, myelin E-face; bottom green overlay, cytoplasm of myelin inner tongue; top green overlay, juxtamesaxonal myeloplasm. C: axolemma of myelinated axon from formaldehyde-fixed Cx29 ko mouse. Rosettes of 9-nm KV1 E-face particles (purple overlays) are present, even though their normal coupling partners (Cx29 rosettes) are absent from the apposed myelin P-face (aqua overlay) (none present here or elsewhere). Also noteworthy, there are essentially no other large P-face particles (<50 per μm2) in the innermost myelin juxtaparanodal membrane, consistent with the proposal that, normally, there are virtually no channels other than Cx29 in the JPIM collars. Scale bars, 0.1 and 1 μm.
In formaldehyde-fixed WT mice viewed toward the JMIM P-face (Fig. 10B), abundant 5-nm gold beads for Cx29 are present, paralleling the inner mesaxon (Fig. 10B, yellow arrowhead), reflecting the FRIL correlate of the immunofluorescence ribbons along the inner mesaxon (Figs. 2 and 3). Notably, immunogold labeling is absent where the second layer of myelin is overlapped and covered by the inner tongue of myelin (Fig. 10B, to the left of the tight junctions). This confirms that Cx29 rosettes and Cx29 labeling occurs only in the areas of direct apposition of the axolemma with the adaxonal layer of myelin on either side of the inner mesaxon, where direct structural coupling of axonal KV1 to myelin Cx29 can occur. Overall, we estimate that there were 30–50 rosettes per linear micrometer of inner mesaxon or 240,000–400,000 KV1/Cx29 channels per 800-μm-long JMIM, which in addition to the JPIM provides a second, stereotypically distributed configuration of axo-glial junctions in mammals.
Cx29 ko mice: KV1 rosettes occur without apposed Cx29 rosettes.
To begin our investigations of factors regulating structural assembly of axolemmal and myelin rosettes, we examined FRIL replicas of sciatic nerve from Cx29 ko mice. Notably, internodal axolemmas of myelinated axons of Cx29 ko mice exhibited E-face KV1 rosettes (Fig. 10C) that had particle sizes and particle spacings identical to those in WT mice (Figs. 6C, 8A, and 10A). However, apposed JMIM P-faces of Cx29-deficient mice were devoid of the normally abundant rosettes of 9-nm IMPs (Fig. 10C), and there appeared to be no compensatory migration into or insertion of other classes of ion channels into the JPIM collar of Cx29 ko mice, which remained remarkably particle free. Thus the assembly of KV1.1-containing rosettes in Cx29 ko mice reveals that the formation of axolemmal rosettes is an inherent property of KV1.1/KV1.2 channels and does not depend on linkage with Cx29.
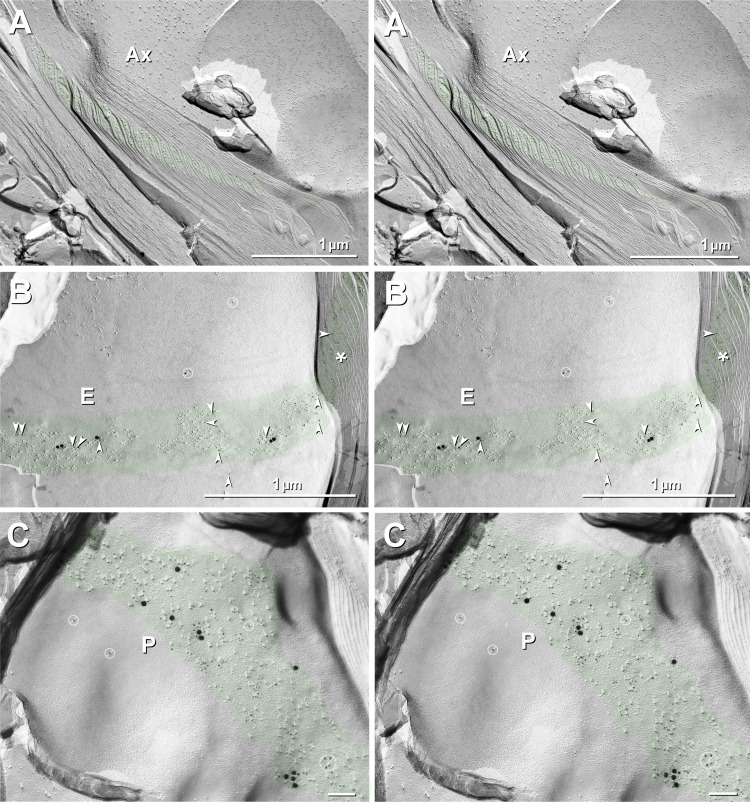
Cx29 but not KV1.1 in Schmidt-Lanterman incisures.
Cross fractures of myelin frequently exposed the stair-stepped Schmidt-Lanterman incisures. In en face views of myelin that included one or more steps within a Schmidt-Lanterman incisure (Fig. 11), labeling for Cx29 (5- and 20-nm gold beads) was robust on P-faces (Fig. 11C), which were enriched in 9-nm IMPs, and moderate on E-faces (Fig. 11B), where 9-nm IMPs were at low density. In contrast, labeling for KV1.1 (10- and 30-nm gold beads) was entirely absent on both E- and P-faces of the Schmidt-Lanterman incisure, accounting for the funnel-like distribution of Cx29 and the absence of immunofluorescence labeling for KV1 in immunofluorescence images of Schmidt-Lanterman incisures (Figs. 2 and 4). Although Cx29 immunogold-labeled 9-nm IMPs are abundant in Schmidt-Lanterman incisures, these particles are not arranged in hexagonal rosettes, even in glutaraldehyde-fixed tissues, as noted by Stolinski et al. (1985), further supporting the conjecture that Cx29 does not form rosettes without apposed KV1.1/KV1.2 coupling partners. Notably, however, clusters of hexagonally arranged 9-nm P-face IMPs and corresponding E-face pits, both labeled for Cx32 and therefore positively identified as “reflexive” gap junctions (Larsen 1983), were occasionally encountered in Schmidt-Lanterman incisures in both formaldehyde-fixed and unfixed sciatic nerve (Meier et al. 2004). These regular hexagonal arrays of Cx32-containing gap junction particles in both glutaraldehyde-fixed and formaldehyde-fixed sciatic nerve are thus easily distinguished from the irregularly distributed Cx29 particles described above.
Fig. 11.
Cross fracture (A) and en face fractures (B and C) through SLIs (green overlays) from dual-double-labeled sciatic nerve of Cx32 ko mouse. A: cross-fractured SLIs are only weakly immunogold labeled for Cx29. B and C: fractures that include en face views of one of the steps in the SLI E-face (B) and P-face (C) are strongly labeled for Cx29 [5-nm (arrowheads) and 20-nm gold beads], but they are not at all labeled for KV1 (10- and 30-nm gold beads; none present in either E- or P-face views). (See Figs. 2 and 4 for immunofluorescence imaging correlates.) E-face labeling represents “cryptic” labeling of Cx29 in the subjacent membrane of another step in the SLIs. No rosettes are present in E- or P-faces of SLIs in either formaldehyde-fixed (present study) or glutaraldehyde-fixed tissue (see also Stolinski et al. 1985), suggesting that Cx29 is present as singlet IMPs that do not form rosettes without KV1.1-containing coupling partners. Gold beads in barred circles in B and C are identified as “noise” because they are on top of the replica, where no labeling is possible. Green overlays (and asterisk in B) mark cytoplasmic expansions that characterize the SLIs. Scale bar, 0.1 μm (C).
Cx29 but not KV1.1 in the outer surface of myelin.
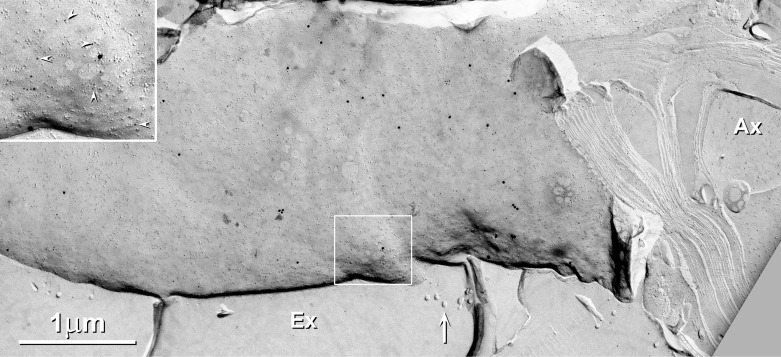
In WT and Cx32 ko mice, P-faces of outermost myelin of large-diameter axons were immunogold labeled at moderate density for Cx29 (Fig. 12). Overall, gold beads for Cx29 were at a density of ∼10–50/μm2 of Schwann cell outer P-face in large-diameter myelinated fibers, as compared with ∼0.1/μm2 as background noise in other areas, such as nodes of Ranvier, E- and P-faces of fibroblasts, and on replicated extracellular ice. Thus the signal-to-noise ratio for Cx29 in outer plasma membrane P-faces of large-diameter myelinating Schwann cell was 100:1 to 1,000:1 (compared with >5,000:1 in the JPIM and JMIM). Thus diffuse immunofluorescence labeling for Cx29 along the surface of fibers was considered to be authentic based on the low level of labeling for Cx29 in WT mice vs. a large depletion of labeling in Cx29 ko mice (Fig. 2, B vs. C). In contrast, there was no detectable FRIL labeling for KV1.1 on either P- or E-faces of the outermost layer of myelin. In support, the area of overlap of the outermost tongue of myelin, adjacent to the tight junctions of the outer mesaxon (Meier et al. 2004), was not labeled for Cx29 (not shown), with the abrupt termination of labeling at this normal biological landmark further supporting specificity of labeling of Cx29 in the outer surface of myelin. However, none of the 9-nm IMPs in the outer myelin P-face were arranged in rosettes in either glutaraldehyde-fixed or formaldehyde-fixed samples. Thus the absence of rosettes in outermost myelin is further consistent with the proposed inability of Cx29 to form rosettes in the absence of apposed KV1 coupling partners.
Fig. 12.
Outer surface of myelin, showing moderate labeling for Cx29 (5-and 20-nm gold beads) but absence of immunogold labeling for KV1.1 (10-and 30-nm gold beads; none present). Box enlarged as inset shows relative abundance of 5-nm (arrowheads) vs. 20-nm gold beads. Note the absence of gold beads on extracellular ice (Ex) and on cross-fractured myelin and cross-fractured axoplasm (Ax). Arrows indicate collagen fibers. Scale bars, 1 μm.
The overall density of labeling for Cx29 was moderately high over the entire length of outermost myelin (∼10/μm2 × 800 μm long × 10 μm in diameter × π = 240,000 labels per internode outer surface). Thus we estimate that the number of immunogold labels on the outer surface of myelin is ∼35% of the total number of immunogold labels in innermost myelin. This moderately high density of Cx29 channels along myelinated fibers could imply that in PNS myelin, a portion of the ionic and osmotic load arising from high-frequency axonal activity may be dispersed temporally and spatially into the surrounding endoneurial space by Cx29 hemichannels in the Schwann cell outer plasma membrane. [This potential for local release of K+ in the PNS is to be contrasted with proposed long-distance K+ siphoning via the panglial syncytium in the CNS (Rash 2010).]
Axolemmal E-face rosettes are abundant in lower vertebrates, but P-face rosettes are not detected in their myelin.
Teleost myelinated axons [from Electrophorus electricus, see Fig. 4A in Rash (2010); and from Salmo truta (Shinowara N, personal communication)] have abundant axolemmal KV1-like rosettes in their juxtaparanodes and along their internodal axolemmas. However, they apparently lack myelin Cx29 rosettes, either at JPIM or along the JMIM. Likewise, the antibodies against Cx29 used for both immunocytochemistry and FRIL do not detect Cx29 in frog myelinated axons, due to either 1) the absence of the Cx29 gene in frogs (see discussion) or 2) to complete lack of cross-reactivity of the antibodies used with the frog ortholog of mammalian Cx29. If the former is true, Cx29 would represent a late evolutionary addition to myelinated axons, where its direct structural coupling to KV1.1 channels may contribute to the faster axonal conduction velocity and/or faster repolarization/higher frequency of axonal action potential generation that characterizes the myelinated axons of mammals (see discussion). With frog myelinated axons apparently lacking Cx29 yet propagating action potentials at 13–20 m/s (Raymond 1979) [compared with up to 120 m/s in mammals (Buchthal and Rosenfalck 1966; Waxman 1980)], it is not surprising that deletion of Cx29 in mice (Eiberger et al. 2006) apparently had no detectable effect on myelin formation and no statistically significant effect on the few electrophysiological properties of axons that were monitored. Rather, measurements pertinent to K+ conductance into myelin, as proposed by Stolinski et al. (1981), were not monitored in that study. Based on additional data provided above, we suggest that future studies of Cx29 ko mice include 1) analysis of the maximum sustainable rate of impulse conduction (related to altered rate of axonal repolarization by interrupting the flow of K+ into myelin) and 2) resistance to axonal fatigue during repetitive stimulation (as related to proposed rapid sequestration of K+ in myelin).
DISCUSSION
Anatomical and molecular underpinnings of faster saltatory conduction velocity in mammals.
Voltage-gated Na+ and K+ channels, Cx29 hemichannels, and their anchoring, scaffolding, and intercellular adhesion molecules (Komada and Soriano 2002; Kordeli et al. 1995; Ogawa et al. 2008; Peles and Salzer 2000; Poliak et al. 2003; Rasband 2004; Srinivasan et al. 1988) are localized to and define 11 distinct subcellular domains of myelinated axons (Fig. 13, A and B): voltage-gated Na+ channels are localized solely to nodes of Ranvier (1) (Caldwell et al. 2000; Ritchie and Rogart 1977; Rosenbluth 1976); KV1.1/KV1.2 voltage-gated K+ channels, plus cell adhesion molecules such as Caspr2 and Tag-1, are localized to the proximal and distal JPAX (2 and 3) and to two thin bands (4 and 5) along the JMAX (Gordon et al. 2014) and to the internodal C-bands (Poliak et al. 2003), whereas Cx29 channels are localized to the proximal and distal JPIM collars (6 and 7) and to the twin-bore JMIM conduits (8 and 9) formed by the inner tongue of myelin and by the cytoplasmic shoulder of myelin to which the inner tongue is attached. Finally, immunofluorescence labeling for Caspr, contactin, and NF155 [reviewed in Peles and Salzer (2000)] are used to define the paranodal axolemma and paranodal loops of myelin (10 and 11).
Fig. 13.
Diagrams showing relationship of JPAX to JPIM and JMAX to JMIM (A), comparative sizes of JMIM conduits (B), proposed coupling of KV1 to Cx29 rosettes (C), proposed intramyelinic pathways for K+ along the JMIM conduits that link proximal and distal JPIM collars (D), and myelin unwrapped from its axon (E). A: distribution of NaV channels (red) at nodes of Ranvier, intercellular contactin/Caspr proteins (green) that link paranodal loops (beige) to the axolemma (blue), and axonal KV1 channels (dark blue circles) concentrated in the JPAX region and in 2 thin bands along the JMAX. Cx29 channels (yellow rosettes) are restricted to the JPIM and JMIM. The cross section in the blue box in A is diagrammed in B. B: cytoplasmic expansions of myelin on both sides of the mesaxon (i.e., the paired JMIM) are continuous with the stair-stepped cytoplasmic expansions of the SLIs. (See Figs. 2–4 for immunofluorescence images.) The JMAX (purple arrow to purple segment of axolemma), opposite the inner mesaxon, is defined by its content of KV1 rosettes (blue ovals), whereas the JMIM (aqua arrow to aqua line segments) is defined by its high density of Cx29 rosettes (yellow ovals) and by tight junctions linking the inner tongue with the second layer of myelin (Fig. 10). C: models for tight vs. loose coupling of Cx29 to KV1 in mammals (C1 vs. C2) and their corresponding freeze-fracture images (C3 vs. C4). Cx29 channel (yellow) aligns precisely with KV1.1-containing channel (blue). It is not yet determined whether the channels are in molecular contact (C1) or whether there are tether proteins loosely linking the 2 channels (C2), where they may contribute to a molecular seal. D and E: wrapped (D) and unwrapped myelin (E) showing the cytoplasmic expansions (beige cylinders) surrounding the axolemma (blue), with its densely packed NaV channels (red) at nodes of Ranvier. Axolemmal KV1 channels (dark blue rosettes) are concentrated in the JPAX and in 2 thin bands within the JMAX. Cx29 channels (yellow rosettes) are concentrated in the JPIM and JMIM. SLIs are stair-stepped expansions of myeloplasm that usually extend from the juxtamesaxonal expansions to outermost cytoplasmic myelin.
Although axolemmal E-face rosettes similar to those identified in mammals as KV1 are abundant at juxtaparanodes in fish axons [see Fig. 4A in Rash (2010)], similar rosettes have not been detected along fish JMAX (Shinowara N, personal communication), nor have we detected them along the JMAX of frog axons. Likewise, immunofluorescence labeling for KV1.2 was robust at frog juxtaparanodes (Rasband 2004) but was not visible along the JMAX of those same axons. Also noteworthy, we detected neither Cx29 immunolabeling nor Cx29 rosettes along the JMIM in frog myelin. With no direct genomic evidence so far for the presence of a Cx29 gene ortholog in fish, amphibia, birds, or reptiles, with synteny analyses suggesting an absence of the Cx29 ortholog in fish [Braasch I, personal communication], and with Cx29 protein not reported in the axons of those inframammalian species, mammals appear to have evolved an additional axo-glial coupling mechanism that is not present in more basal vertebrates. If so, the molecular mechanisms underlying saltatory conduction in mammals appear to be modified from those in frogs, potentially accounting for the faster conduction velocity, higher sustainable rates of firing, and/or greater metabolic efficiency of saltatory conduction in mammalian myelinated axons.
The immunocytochemical and ultrastructural data provided in this and recent reports, combined with electrophysiological observations made over the past two decades proposing electrical coupling of axoplasm to myeloplasm in mammalian myelinated axons (summarized below), require revision of the traditional “nodal conduction/passive myelin” model that had been derived from studies of frog axons, and reconsideration of an alternative “dual-circuit/active myelin” model that incorporates a direct axo-glial pathway for K+ ions (Stolinski et al. 1981, 1985). Based on the current demonstration of the abundance and highly reproducible distribution of molecularly linked KV1/Cx29 channels in the internodal axonal and glial membranes of mammalian myelinated axons, we propose the rudiments of a modified model for saltatory conduction in mammals in which KV1/Cx29 axo-glial junctions contribute to one or more of the following: 1) increased speed of axonal repolarization/shorter absolute refractory period, 2) local (intrasegmental) recycling of K+, and 3) precocious activation of multiple successive nodes of Ranvier via K+ flux from distal JPIM into distal JPAX/nodal axoplasm, resulting in increased conduction velocity.
Juxtaparanodal domains further defined.
Juxtaparanodal domains were first defined in freeze-fracture images showing abundant rosettes of IMPs linking the JPAX with the surrounding JPIM collar (Stolinski et al. 1981, 1985). Subsequently, immunocytochemistry revealed the localized distribution of KV1.1 and KV1.2 to the juxtaparanodal domains in mammals (Altevogt et al. 2002; Bhat et al. 2001; Chiu et al. 1999; Rasband 2004; Rios et al. 2003; Vabnick et al. 1999; Wang et al. 1993). However, it remained unknown whether significant numbers of any other ion channels might also be present in those specialized domains. In the present study, we have shown that in adult mice, as in rats (Stolinski et al. 1981, 1985), KV1.1 channels that define the JPAX, and Cx29 channels that define the JPIM collars, are densely packed to the virtual exclusion of all other classes of transmembrane proteins large enough to be ion channels. Based on the presence of almost a million KV1/Cx29 channels per internode and their highly reproducible localization to the JPAX/JPIM and JMAX/JMIM in mammalian myelinated axons, we suggest that these axo-glial junctions are sufficiently abundant and appropriately localized to provide for the more rapid axonal depolarizations and repolarizations of mammals. By way of comparison to mammalian neuronal gap junctions, whose high-speed electrical communications are critically important for a host of neuronal functions in a wide variety of brain regions, neuronal gap junctions contain 10-4,000 connexons (Nagy et al. 2004; Rash et al. 2005, 2007), whereas there are 750,000–900,000 KV1/Cx29 channels per internode, or >100 to 10,000 times more KV1/Cx29 channels than connexin channels in a typical neuronal gap junction, suggesting the importance of the function(s) subserved.
Electrophysiological evidence for K+ conductance from axoplasm to myeloplasm.
David et al. (1993) used their newly developed “ultrasharp” electrodes to discover that precisely coincident with axonal action potentials, strong action potential-like depolarizations occurred within the deepest layers of myelin, decreasing in amplitude in successively external myelin layers. Those “prolonged negative potentials” (PNPs) in inner myelin persisted for hundreds of milliseconds after each action potential, resulting in virtually continuous myelin depolarization during repetitive axonal firing. Importantly, the myelin PNPs were blocked by tetraethylammonium (TEA), a specific blocker of voltage-gated K+ channels, thereby demonstrating that 1) PNPs resulted from axon-to-myelin K+ flux, 2) through pharmacologically identified voltage-gated K+ channels, and 3) that these K+ channels remained conductive for hundreds of milliseconds after the axonal resting potential was restored, effectively voltage-clamping axoplasm to an undefined compartment within deepest myelin. Based on the present results, we reinterpret those findings to suggest that these continuously open, TEA/4-aminopyridine (4-AP)-sensitive K+ channels correspond to KV1/Cx29 channels within the internode. Moreover, the prolonged “leak” conductance, occurring even at resting potential (also Chiu et al. 1979), implies that these K+ channels are not “voltage gated” in the classical sense and that most remain conductive at rest. This apparent lack of voltage-dependent gating of KV1 channels may have profound implications for axonal physiology in mammals.
Evidence for bidirectional conductance between axoplasm and myeloplasm.
The TEA-sensitive K+ channels of mammalian myelinated axons were shown to be bidirectionally conductive for multiple cations, including K+, Rb+, and Na+ (David et al. 1993), and apparently even Ca2+ (Zhang et al. 2006). In mouse optic nerve axons [which, although CNS, have similar juxtaparanodal and internodal distributions of KV1.1, KV1.2, and Cx29 (Altevogt et al. 2002; Bhat et al. 2001; Rios et al. 2003)], Zhang et al. (2006) noted that “during each action potential, ‘retrograde’ Ca2+ conductance occurs from myeloplasm into axoplasm.” Moreover, this intercellular Ca2+ conductance was not blocked by ryanodine or nifedipine, which are specific blockers of Ca2+-induced Ca2+-release channels and L-type voltage-gated Ca2+ channels, respectively. Instead, myelin-to-axon Ca2+ conductance was blocked by TEA and 4-AP, both of which are blockers of voltage-gated K+ channels. Thus, bidirectional conductance of Ca2+ appears to occur via the classical K+-conductance pathway. Current FRIL data show that KV1.1/Cx29 channels are distributed in the JPAX/JPIM domains and in the JMAX/JMIM along the entire internode, where internodal Ca2+ influx was reported. Moreover, based on the general cation permeability of other connexons (Bennett and Goodenough 1978; Kanaporis et al. 2010; Palacios-Prado and Bukauskas 2009), we propose broad ion permissiveness of the KV1.1/Cx29 channels, as determined in part by the conductance and gating properties of the connexon-side of these ultrastructurally linked xenotypic channels.
Rudiments of a modified model for electrically active myelin in mammalian saltatory conduction.
Based on the explicit presumption that tight structural coupling of KV1.1 channels with Cx29 channels connotes functional coupling, we support the proposal (Stolinski et al. 1981, 1985) that during an axonal action potential, KV1.1/Cx29 rosettes provide pathways for conventional outward K+ currents, with K+ efflux occurring from juxtaparanodal axoplasm directly into the JPIM collar (Fig. 13D). Moreover, we propose that during depolarization toward threshold, the rapidly increasing voltage difference between JPAX and JPIM augments K+ efflux from the depolarizing axoplasm into the less depolarized proximal JPIM collar. The proposed capacitance-augmented K+ efflux would likely reduce the time between successive depolarizations (i.e., reduce the absolute refractory period) and correspondingly increase the maximum attainable frequency of action potential generation.
Does increased conduction velocity in mammalian myelinated axons arise in part from axo-glial coupling?
The speed of saltatory conduction in frogs was originally proposed to be limited primarily by distance between nodes [2.3 mm in frogs (Tasaki et al. 1943; see also Raymond 1979)] and the time needed to reach threshold at each succeeding node [100 μs (Marks and Loeb 1976; Tasaki and Frank 1955)], with that simple numerical ratio proposed to account for the observed conduction velocity of 23 m/s in frogs (i.e., 2.3 mm/node ÷ 0.1 ms/node = 23 m/s). However, that numerical relationship likely was a mere coincidence, because in humans, nodal separations average ∼0.8 mm (Friede et al. 1981; Hiscoe 1947), and the time to reach threshold is 80 μs (Marks and Loeb 1976; Paintal 1966). Thus the same simple numerical ratio would predict a maximum conduction velocity of only 10 m/s in humans (i.e., 0.8 mm/node ÷ 0.08 ms/node = 10 m/s). Yet conduction velocity of large myelinated fibers in human PNS is an order of magnitude faster than predicted by that numerical ratio [i.e., ∼120 vs. 10 m/s (Buchthal and Rosenfalck 1966; Waxman 1980)]. Also relevant, this 120 m/s conduction velocity corresponds to an average delay of 8 μs between successive nodes, which would seem to require that 10 nodes [to as many as 30 nodes (Debanne et al. 2011)] be activated simultaneously under a nominal 80-μs nodal depolarization to threshold. Such microsecond events are too fast to be measured by current electrophysiological methods using either sharp electrodes or patch-clamp electrodes (Sakmann and Neher 2009; Sontheimer and Ransom 2002). Thus, to analyze microsecond changes in voltage within innermost myeloplasm during saltatory conduction, we envision using high-speed optical detection methods (>100,000 frames/s or >1,000,000 line scans/s) to measure changes in fluorescence intensity of voltage indicator dyes injected into or genetically expressed in the innermost cytoplasm of oligodendrocytes and Schwann cells.
Evidence that K+ efflux in mammals does not occur into the submyelinic extracellular space.
Early suggestions that K+ efflux in mammals occurs into the submyelinic extracellular space (Chiu et al. 1979) were contradicted by experiments investigating the effects of repetitive stimulation and of K+ channel activation on saltatory conduction, neither of which produced the predicted swelling of the submyelinic extracellular space, but instead resulted in swelling of innermost and outermost myelin cytoplasm (see below) or in swelling of the nodal axoplasm (Love and Cruz-Höfling 1986). As predicted by the “electrically active myelin” model, when K+ transport pathways within the “panglial syncytium” (Rash 2010) were disrupted by mutations or genetic knockouts of glial connexins (Cx32, Cx43, Cx26), glial fibrillary acidic protein (GFAP), or KIR4.1, swelling of innermost myelin cytoplasm was substantial, often resulting in myelin sclerosis (Lennon et al. 2004; Lutz et al. 2009; Menichella et al. 2003, 2006; Odermatt et al. 2003; Senderek et al. 1999). Those observations are consistent with the proposal (Rash 2010) that K+ and obligatorily associated osmotic water normally utilize KV1.1/Cx29 channels to enter innermost myeloplasm, with disruption of K+ exit pathways resulting in the observed myelin swelling of CNS myelin.
Proposed local recycling of K+.
In early models of saltatory conduction, K+ efflux was presumed to occur into the perinodal extracellular space, where K+ was thought to diffuse away freely, thereby requiring axoplasmic replacement, presumably by the action of Na+-K+ATPase (Ariyasu et al. 1985; Mata et al. 1991). As another alternative, K+ efflux into the submyelinic extracellular space (Chiu and Ritchie 1980; David et al. 1993), with either diffusion across the paranodal septate junctions or direct return to the axoplasm via axolemmal Na+-K+ATPase, would require equally substantial expenditure of energy. In mammals, however, linked KV1/Cx29 channels provide a plausible axo-glial coupling pathway for efficient intrasegmental recycling of K+, from axoplasm to myeloplasm to axoplasm, which would greatly reduce this energy expenditure.
Alternative explanation for the detection of voltage-gated K+ conductance after acute demyelination.
Structural breakage of linked KV1.1/Cx29 by diverse demyelinating procedures (Bostock et al. 1981; Brismar 1979; Sherratt et al. 1980) would result in K+ efflux through the newly separated KV1 channels, directly into the peri-internodal extracellular space and its immediate detectability by K+-sensitive electrodes. The proposed separation of KV1 channels from Cx29 channels, with resultant removal of allosteric influence by Cx29, would account for the reported instantaneous conversion of submyelinic K+ leak current into voltage-gated K+ current (Chiu and Ritchie 1980).
Potential function(s) for two JPAX/JPIM domains in each internode.
In PNS axons, which normally conduct only unidirectionally, it is relevant to consider the invariant presence of two ionically active juxtaparanodal domains, one on each side of each node of Ranvier. One explanation could be that this arrangement would provide twice as many KV1/Cx29 channels in close proximity to each node for more rapid repolarization. However, that would not explain why in mammals, but not in frogs, the axoplasm is linked to the internodal myeloplasm along the entire internode via the KV1/Cx29 channels of the JPAX/JMIM (Figs. 10, A and B, and 13, A, B, and E; also see Figs. 2A3 and 4A3). As one potential additional explanation, we propose that at the distal JPIM, return K+ flux occurs directly into the distal juxtaparanodal axoplasm (Fig. 13, D and E, right). In turn, this would provide for precocious, capacitance-driven initiation of depolarization (“K+ kindling”) of the next node, even before the preceding node had reached threshold. In the resulting wave of depolarization, occurring almost simultaneously across a dozen or more nodes, this proposed long-distance K+ kindling would be expected to increase conduction velocity severalfold over that permitted by separate, temporally delayed saltatory activations of successive individual nodes, as previously envisioned.
An active role proposed for myelin in increasing velocity of saltatory conduction.
We propose that in each internode, myelin is segmented into two biological capacitors (the proximal and distal JPIM collars), linked in series by the twin-bore JMIM conduits. These cylindrical capacitors are envisioned to store and release potassium ions, which flow into and between at least three distinct cytoplasmic myelin compartments (Fig. 13, B, D, and E): 1) During initial depolarization toward threshold, capacitance-augmented K+ efflux is proposed to occur from proximal JPAX into the proximal JPIM via the estimated 250,000 KV1/Cx29 channels within each JPIM collar. In contrast to electronic circuits, where capacitance discharge via electrons is virtually instantaneous, limited only by the resistance and capacitance of the discharging circuit and the rate of flux of electrons in copper conductors, biological systems utilize Na+ and K+ ions as charge carriers and cytoplasm as the conductive medium. As a result, ionic capacitance discharge of the proximal JPIM through abundant but tiny conductors having finite resistance (i.e., KV1/Cx29 channels) may be slightly slower than in conventional electronic circuits, perhaps requiring several microseconds to sequentially depolarize and repolarize each successive JPIM compartment. Likewise, even though they are electrically connected by the narrow twin-bore inner mesaxonal cytoplasms, the relatively long distances between proximal and distal JPIM collars (nominally 800 μm) likely means that these two partially isolated compartments will be depolarized and repolarized rapidly, but not at precisely the same instant. This relatively brief delay arising from proposed capacitance-augmented repolarization in mammalian axons may have profound implications for both velocity and maximum frequency of saltatory conduction. As envisioned, the depolarization of the proximal JPIM collar during an action potential is proposed to lag the depolarization of the proximal JPAX by a few microseconds. Likewise, the rapidly rising depolarization of the distal JPIM collar is envisioned to slightly lead (by a few microseconds) the depolarization of the distal JPAX. Also according to this model, capacitance-augmented discharge of the distal JPIM collar would occur faster than the action potential depolarization could be conducted through the internodal axoplasm.
Proposed additional role for the distal JPIM collars: K+ kindling.
We envision that an approaching action potential (still many nodes upstream) would first be manifest as increased perinodal axoplasmic K+ influx, from the upstream distal JPIM collar via continuously open KV1/Cx29 channels, creating a small inward “K+ kindling current” sufficient to begin depolarizing the upstream node by a few millivolts, even before arrival of the axoplasmically conducted action potential. On reaching threshold (approximately −40 mV; requiring ∼80 ms) and before formation of a full-amplitude action potential at the upstream node, axonal K+ efflux into the upstream proximal JPIM collar would be strongly augmented by its nominal −70-mV resting potential. Nearly simultaneously at the downstream distal juxtaparanode, as electrically linked by the JMIM cytoplasm (Fig. 13, D and E), we envision that capacitance-augmented K+ efflux occurs from the now rapidly depolarizing distal JPIM collar into the still polarized downstream distal JPAX axoplasm, thereby precociously initiating depolarization (K+ kindling) of the next node. With each successive distal JPAX axoplasm proposed to be capacitance coupled via abundant KV1/Cx29 channels to its surrounding JPIM collar, rapid K+ kindling would also be initiated in a graded fashion at many successive downstream nodes, thereby almost simultaneously kindling multiple additional nodes in advance of the axonal action potential and increasing conduction velocity of large-diameter myelinated axons in mammals.
Summary.
In mammals, KV1.1/KV1.2 channels are confined to the internodal axolemmal and not to nodes of Ranvier, as previously proposed. Moreover, K+ conductance apparently does not occur into the submyelinic extracellular space, as previously envisioned, but instead is proposed to occur into innermost myeloplasm, which is strongly electrically isolated from the nodal extracellular space by the myelin plasma membrane. Using FRIL, we have shown that the KV1.1-containing channels are tightly structurally coupled and therefore are likely functionally coupled to Cx29 channels in innermost myelin, creating xenotypic intercellular junctions between the JPAX/JPIM and JMAX/JMIM domains. These xenotypic channels would provide direct, possibly bidirectional, intercellular molecular pathways for K+, from axoplasm into myeloplasm and return. KV1/Cx29 channels, pulled tightly together and potentially sealed by surrounding Caspr2/Tag-1 cell adhesion molecules, would account for failure to detect K+ efflux at intact nodes but its ready detection following acute detachment of myelin and consequent physical separation of KV1 channels from Cx29 channels, which would result in pathological release of K+ into the submyelinic extracellular space. In addition, we propose that KV1/Cx29 channels provide for downstream, capacitance-augmented K+ kindling that precociously and almost simultaneously activates depolarizations at multiple successive nodes, thereby increasing both velocity and maximum frequency of saltatory conduction in mammalian myelinated axons. Finally, return flux of K+ from myeloplasm to axoplasm would provide for efficient local recycling of all or a portion of the K+ used for axonal repolarization.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS31027, NS44010, and NS44295 (to J. E. Rash) and Canadian Institutes of Health Research Grant MOP 106598 (to J. I. Nagy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.E.R., K.G.V., and J.I.N. conception and design of research; J.E.R., K.G.V., T.Y., J.H., J.T.B., and J.I.N. performed experiments; J.E.R., K.G.V., and J.I.N. analyzed data; J.E.R., K.G.V., and J.I.N. interpreted results of experiments; J.E.R., K.G.V., and J.I.N. prepared figures; J.E.R. drafted manuscript; J.E.R., K.G.V., T.Y., and J.I.N. edited and revised manuscript; J.E.R., K.G.V., T.Y., J.H., J.T.B., and J.I.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brett McLean for excellent technical assistance, Klaus Willecke (Berlin, Germany) for providing Cx29 knockout mice and their wild-type counterparts, Carola Meier (Saarland University, Hamburg, Germany) and Klaus Willecke for providing Cx32 knockout mice, Dwight Bergles and Valerie Larson (Johns Hopkins Medical Institutions, Baltimore, MD) for providing formaldehyde-fixed sciatic nerve from Cx29 knockout mice and glutaraldehyde-fixed sciatic nerve from wild-type mice, and Elior Peles (Weizmann Institute of Science, Rehovot, Israel) for providing monoclonal anti-caspr antibody. We also thank Michael Gutnick (Tel Aviv, Israel) and Ilya Fleidervish (Rehovot) for critiquing early versions of this manuscript.
REFERENCES
- Ahn M, Lee J, Gustafsson A, Enriquez A, Lancaster E, Sul JY, Haydon PG, Paul DL, Huang Y, Abrams CK, Scherer S. Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct. J Neurosci Res 86: 992–1006, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sabi A, Shamotienko O, Dhochartaigh SN, Muniyappa N, Le Berre M, Shaban H, Wang J, Sack JT, Dolly JO. Arrangement of KV1 α subunits dictates sensitivity to tetraethylammonium. J Gen Physiol 136: 273–282, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altevogt BM, Kleopas KA, Postma FR, Scherer SS, Paul D. Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J Neurosci 22: 6458–6470, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altevogt BM, Paul DL. Four classes of intercellular channels between glial cells in the CNS. J Neurosci 24: 4313–4323, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyasu RG, Nichol JA, Ellisman MH. Localization of sodium/potassium adenosine triphosphatase in multiple cell types of the murine nervous system with antibodies raised against the enzyme from kidney. J Neurosci 5: 2581–2596, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SM, Chiruvolu S, Israelachvili JN, Zasadzinski JA. Measurements of forces involved in vesicle adhesion using freeze-fracture electron microscopy. Langmuir 6: 1326–1329, 1990. [Google Scholar]
- Bennett MV, Goodenough DA. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Program Bull 16: 1–486, 1978. [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, Martin MS, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/caspr/paranodin. Neuron 30: 369–383, 2001. [DOI] [PubMed] [Google Scholar]
- Bostock H, Sears TA, Sherratt RM. The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J Physiol 313: 301–315, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D, Bullivant S, Gilula NB, Karnovsky MJ, Moor H, Northcote DH, Packer L, Satir B, Satir P, Speth V, Staehelin LA, Steere RL, Weinstein RS. Freeze-etching nomenclature. Science 190: 54–56, 1975. [DOI] [PubMed] [Google Scholar]
- Brismar T. Potential clamp experiments on myelinated nerve fibres from alloxan diabetic rats. Acta Physiol Scand 105: 384–386, 1979. [DOI] [PubMed] [Google Scholar]
- Buchthal F, Rosenfalck A. Evoked action potentials and conduction velocity in human sensory nerves. Brain Res 3: v-122, 1966. [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Nav1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc Natl Acad Sci USA 97: 5616–5620, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challcroft JP, Bullivant S. An interpretation of liver cell membrane and junction structure based on observation of freeze-fracture replicas of both sides of the fracture. J Cell Biol 47: 49–60, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DE, Heuser J. Membrane fusion during secretion: cortical granule exocytosis in sea urchin eggs as studied by quick-freezing and freeze-fracture. J Cell Biol 83: 91–108, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SY, Ritchie JM. Potassium channels in nodal and internodal axonal membrane of mammalian myelinated fibres. Nature 284: 170–171, 1980. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Ritchie JM, Rogart RB, Stagg D. A quantitative description of membrane currents in rabbit myelinated nerve. J Physiol 292: 149–166, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SY, Zhou L, Zhang CL, Messing A. Analysis of potassium channel functions in mammalian axons by gene knockouts. J Neurocytol 28: 349–364, 1999. [DOI] [PubMed] [Google Scholar]
- Connors BW, Ransom BR, Kunis DM, Gutnick MJ. Activity-dependent K+ accumulation in the developing rat optic nerve. Science 216: 1341–1343, 1982. [DOI] [PubMed] [Google Scholar]
- David G, Barrett JN, Barrett EF. Activation of internodal potassium conductance in rat myelinated axons. J Physiol 472: 177–202, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. Axon physiology. Physiol Rev 91: 555–602, 2011. [DOI] [PubMed] [Google Scholar]
- Dinchuk JE, Johnson TJA, Rash JE. Postreplication labeling of E-leaflet molecules: membrane immunoglobulins localized in sectioned labeled replicas examined by TEM and HVEM. J Electron Microsc Tech 7: 1–16, 1987. [DOI] [PubMed] [Google Scholar]
- Dodson PD, Barker MC, Forsythe ID. Two heteromeric KV1 potassium channels differentially regulate action potential firing. J Neurosci 22: 6953–6961, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiberger J, Kibschull M, Strenzke N, Schober A, Büssow H, Wessig C, Djahed S, Reucher H, Koch DA, Lautermann J, Moser T, Winterhager E, Willecke K. Expression pattern and functional characterization of connexin29 in transgenic mice. Glia 53: 601–611, 2006. [DOI] [PubMed] [Google Scholar]
- Eskandari S, Wright EM, Kreman M, Sterace DM, Zampighi GA. Structural analysis of cloned plasma membrane proteins by freeze-fracture electron microscopy. Proc Natl Acad Sci USA 95: 11235–11240, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder N. Microperoxidase an ultrastructural tracer of low molecular weight. J Cell Biol 51: 339–343, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KA, Yanagimoto KC. Effect of membrane splitting on transmembrane polypeptides. J Cell Biol 102: 551–559, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede RL, Meier T, Diem M. How is the exact length of an internode determined? J Neurol Sci 50: 217–228, 1981. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci 108: 3443–3449, 1995. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. SDS-digested freeze-fracture replica labeling electron microscopy to study the two-dimensional distribution of integral membrane proteins and phospholipids in biomembranes: practical procedure, interpretation and application. Histochem Cell Biol 107: 87–96, 1997. [DOI] [PubMed] [Google Scholar]
- Gordon A, Adamsky K, Vainshtein A, Frechter S, Dupree JL, Rosenbluth J, Peles E. Caspr and Caspr2 are required for both radial and longitudinal organization of myelinated axons. J Neurosci 34: 14820–14826, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruijters WT, Kistler J, Bullivant S, Goodenough DA. Immunolocalization of MP70 in lens fiber 16–17-nm intercellular junctions. J Cell Biol 104: 565–572, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol 275: 275–300, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A, Dembitzer HM. The transverse bands as a means of access to the periaxonal space of the central myelinated nerve fiber. J Ultrastruct Res 28: 141–149, 1969. [DOI] [PubMed] [Google Scholar]
- Hirano A, Dembitzer HM. Further studies on the transverse bands. J Neurocytol 11: 861–866, 1982. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Heuser J. The inside and outside of gap-junction membranes visualized by deep etching. Cell 30: 395–406, 1982. [DOI] [PubMed] [Google Scholar]
- Hiscoe HB. Distribution of nodes and incisures in normal and regenerated nerve fibers. Anat Rec 99: 447–475, 1947. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL. The ionic basis of electrical activity in nerve and muscle. Biol Rev 26: 339–409, 1951. [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117: 500–544, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF, Stämpfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J Physiol 108: 315–339, 1949. [PubMed] [Google Scholar]
- Johnson RG, Reynhout J, TenBroek E, Quade BJ, Yasumura T, Davidson KGV, Sheridan JD, Rash JE. Gap junction assembly: roles for the formation plaque and regulation by the C-terminus of connexin43. Mol Biol Cell 23: 71–86, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TJ. Glutaraldehyde fixation chemistry: oxygen-consuming reactions. Eur J Cell Biol 45: 160–169, 1987. [PubMed] [Google Scholar]
- Kamasawa N, Furman CS, Davidson KG, Sampson JA, Magnie AR, Gebhardt B, Kamasawa M, Morita M, Yasumura T, Pieper M, Zumbrunnen JR, Pickard GE, Nagy JI, Rash JE. Abundance and ultrastructural diversity of neuronal gap junctions in the OFF and ON sublaminae of the inner plexiform layer of rat and mouse retina. Neuroscience 142: 1093–1117, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamasawa N, Sik A, Morita M, Yasumura T, Davidson KG, Nagy JI, Rash JE. Connexin47 and connexin32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops, and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience 136: 65–86, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaporis G, Brink PR, Valiunas V. Gap junction permeability: selectivity for anionic and cationic probes. Am J Physiol Cell Physiol 300: C600–C609, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, Soriano P. bIV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol 156: 337–348, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett Ankyrin V. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem 270: 2352–2359, 1995. [DOI] [PubMed] [Google Scholar]
- Larsen WJ. Biological implications of gap junction structure, distribution and composition: a review. Tissue Cell 15: 645–671, 1983. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364: 2106–2112, 2004. [DOI] [PubMed] [Google Scholar]
- Li X, Kamasawa N, Ciolofan C, Olson CO, Lu S, Davidson KG, Yasumura T, Shigemoto R, Rash JE, Nagy JI. Connexin45-containing neuronal gap junctions in rodent retina also contain connexin36 in both apposing hemiplaques, forming bi-homotypic gap junctions, with scaffolding contributed by zonula occludens-1. J Neurosci 28: 9769–9789, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lynn BD, Olson C, Meier C, Davidson KGV, Yasumura T, Rash JE, Nagy JI. Connexin29 expression, immunocytochemistry and freeze-fracture replica immunogold labeling (FRIL) in sciatic nerve. Eur J Neurosci 16: 795–806, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston RB, Pfenninger K, Moor H, Akert K. Specialized paranodal and interparanodal glial-axonal junctions in the peripheral and central nervous system: a freeze-etching study. Brain Res 58: 1–24, 1973. [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Molecular Cell Biology (4th ed). New York: WH Freeman, 2000. [Google Scholar]
- Love S, Cruz-Höfling MA. Acute swelling of nodes of Ranvier caused by venoms which slow inactivation of sodium channels. Acta Neuropathol 70: 1–9, 1986. [DOI] [PubMed] [Google Scholar]
- Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J Neurosci 29: 7743–7752, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie ML, Ghabriel MN, Allt G. Nodes of Ranvier and Schmidt-Lanterman incisures: an in vivo lanthanum tracer study. J Neurocytol 13: 1043–1055, 1984. [DOI] [PubMed] [Google Scholar]
- Marks WB, Loeb GE. Action currents, internodal potentials, and extracellular records of myelinated mammalian nerve fibers derived from node potentials. Biophys J 16: 655–668, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masugi-Tokita M, Tarusawa E, Watanabe M, Molnar E, Fujimoto K, Shigemoto R. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J Neurosci 27: 2135–2144, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata M, Fink DJ, Ernst SA, Siegal GJ. Immunocytochemical demonstration of Na+,K+-ATPase in internodal axolemma of myelinated fibers of rat sciatic and optic nerves. J Neurochem 57: 184–192, 1991. [DOI] [PubMed] [Google Scholar]
- Meier C, Dermietzel R, Davidson KGV, Yasumura T, Rash JE. Connexin32-containing gap junctions in Schwann cells at the internodal zone of partial myelin compaction and in Schmidt-Lanterman incisures. J Neurosci 24: 3186–3198, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J Neurosci 23: 5963–5973, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Majdan M, Awatramani R, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J Neurosci 26: 10984–10991, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RG, Pinto da Silva P. particle rosettes in the periaxonal Schwann cell membrane and particle clusters in the axolemma of rat sciatic nerve. Brain Res 130: 135–141, 1977. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Olsen KK, Schnapp B, Friedrich VL Jr. Distribution of Schwann cell cytoplasm and plasmalemmal vesicles (caveolae) in peripheral myelin sheaths. An electron microscopic study with thin sections and freeze-fracturing. J Neurocytol 6: 647–668, 1977. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian central nervous system. Brain Res Brain Res Rev 47: 191–215, 2004. [DOI] [PubMed] [Google Scholar]
- Nelles E, Butzler C, Jung D, Temme A, Gabriel HD, Dahl U, Traub O, Stumpel F, Jungermann K, Zielasek J, Toyka KV, Dermietzel R, Willecke K. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc Natl Acad Sci USA 93: 9565–9570, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci 23: 4549–4559, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Horresh I, Trimmer JS, Bredt DS, Peles E, Rasband MN. Postsynaptic density-93 clusters KV1 channels at axon initial segments independently of Caspr2. J Neurosci 28: 5731–5739, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal AS. The influence of diameter of medullated nerve fibres of cats on the rising and falling phases of the spike and its recovery. J Physiol 184: 791–811, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N, Bukauskas FF. Heterotypic gap junction channels as voltage-sensitive valves for intercellular signaling. Proc Natl Acad Sci USA 106: 14855–14860, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Salzer JL. Molecular domains of myelinated axons. Curr Opin Neurobiol 10: 558–565, 2000. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System. Neurons and Their Supporting Cells. New York: Oxford University Press, 1991. [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol 162: 1149–1160, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Hall WC, Mantia AS, White LE. Neuroscience. Sunderland, MA: Sinauer, 2012. [Google Scholar]
- Rasband MN. It's “juxta” potassium channel! J Neurosci Res 76: 749–757, 2004. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci USA 98: 13373–13378, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Shrager P. Ion channel sequestration in central nervous system axons. J Physiol 525: 63–73, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE. Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system. Neuroscience 168: 982–1008, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Davidson KG, Kamasawa N, Yasumura T, Kamasawa M, Zhang C, Michaels R, Restrepo D, Ottersen OP, Olson C, Nagy JI. Ultrastructural localization of connexins (Cx36, Cx43, Cx45), glutamate receptors and aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J Neurocytol 34: 307–341, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Davidson KG, Yasumura T, Furman CS. Freeze-fracture and immunogold analysis of aquaporin-4 (AQP4) square arrays, with models of AQP4 lattice assembly. Neuroscience 129: 915–934, 2004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Duffy HS, Dudek FE, Bilhartz BL, Whalen LR, Yasumura T. Grid-mapped freeze-fracture analysis of gap junctions in gray and white matter of adult rat central nervous system, with evidence for a “panglial syncytium” that is not coupled to neurons. J Comp Neurol 388: 265–292, 1997. [DOI] [PubMed] [Google Scholar]
- Rash JE, Graham WF, Hudson CS. Sources and rates of contamination in a conventional Balzers freeze-etch device. In: Freeze Fracture: Methods, Artifacts, and Interpretations, edited by Rash JE, Hudson CS. New York: Raven, 1979, p. 111–122. [Google Scholar]
- Rash JE, Johnson TJ, Dinchuk JE, Duch DS, Levinson SR. Labeling intramembrane particles in freeze-fracture replicas. In: Freeze -Fracture Studies of Membranes, edited by Hui SW. Boca Raton, FL: CRC, 1989, p. 41–59. [Google Scholar]
- Rash JE, Olson CO, Pouliot WA, Davidson KG, Yasumura T, Furman CS, Royer S, Kamasawa N, Nagy JI, Dudek FE. Connexin36, miniature neuronal gap junctions, and limited electrotonic coupling in rodent suprachiasmatic nucleus. Neuroscience 149: 350–371, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Pereda A, Kamasawa N, Furman CS, Yasumura T, Davidson KG, Dudek FE, Olson C, Nagy JI. High-resolution proteomic mapping in the vertebrate central nervous system: close proximity of connexin35 to NMDA glutamate receptor clusters and co-localization of connexin36 with immunoreactivity for zonula occludens protein-1 (ZO-1). J Neurocytol 33: 131–152, 2004b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T. Improved structural detail in freeze-fracture replicas: high-angle shadowing of gap junctions cooled below −170°C and protected by liquid nitrogen-cooled shrouds. Microsc Res Tech 20: 187–204, 1992. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T. Direct immunogold labeling of connexins and aquaporin4 in freeze-fracture replicas of liver, brain and spinal cord: factors limiting quantitative analysis. Cell Tissue Res 296: 307–321, 1999. [DOI] [PubMed] [Google Scholar]
- Raymond SA. Effects of nerve impulses on threshold of frog sciatic nerve fibres. J Physiol 290: 273–303, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios JC, Rubin M, Martin M, Downey RT, Einheber S, Rosenbluth J, Levinson SR, Bhat M, Salzer JL. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J Neurosci 23: 7001–7011, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM, Rogart RB. Density of sodium channels in mammalian myelinated nerve fibers and nature of the axonal membrane under the myelin sheath. Proc Natl Acad Sci USA 74: 211–215, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain. J Neurocytol 5: 731–745, 1976. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Neher E, editors. Single-Channel Recording. New York: Springer Science & Business Media, 2009. [Google Scholar]
- Satir BH, Satir P. Partitioning of intramembrane particles during the freeze-fracture procedure. In: Freeze Fracture: Methods, Artifacts, and Interpretations, edited by Rash JE, Hudson CS. New York: Raven, 1979, p. 43–49. [Google Scholar]
- Schlormann W, John M, Steiniger F, Westermann M, Richter W. Improved antigen retrieval in freeze-fracture cytochemistry by evaporation of carbon as first replication layer. Histochem Cell Biol 127: 633–639, 2007. [DOI] [PubMed] [Google Scholar]
- Schnapp B, Mugnaini E. Membrane architecture of myelinated fibers as seen by freeze fracture. In: Physiology and Pathobiology of Axons, edited by Waxman S. New York: Raven, 1978, p. 83–123. [Google Scholar]
- Senderek J, Hermanns B, Bergmann C, Boroojerdi B, Bajbouj M, Hungs M, Ramaekers VT, Quasthoff S, Karch D, Schroder JM. X-linked dominant Charcot-Marie-Tooth neuropathy: clinical, electrophysiological, and morphological phenotype in four families with different connexin32 mutations. J Neurol Sci 167: 90–101, 1999. [DOI] [PubMed] [Google Scholar]
- Sherratt RM, Bostock H, Sears TA. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature 283: 570–572, 1980. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Ransom BR. Whole-cell patch-clamp recordings. In: Patch-Clamp Analysis: Advanced Techniques, edited by Walz W, Boulton AA, and Baker GB. New York: Springer Science & Business Media, 2002, p. 35–67. [Google Scholar]
- Srinivasan Y, Elmer L, Davis J, Bennett V, Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature 333: 177–180, 1988. [DOI] [PubMed] [Google Scholar]
- Steere RL, Erbe EF, Moseley JM. Prefracture and cold-fracture images of yeast plasma membranes. J Cell Biol 86: 113–122, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere RL, Moseley M. New dimensions in freeze-etching. In: Proceedings of the 27th Meeting of the Electron Microscopy Society of America. Baton Rouge, LA: Claitor's, 1969, p. 202–203. [Google Scholar]
- Steere RL, Rash JE. Use of double-tilt device (goniometer) to obtain optimum contrast in freeze-fracture replicas. In: Freeze Fracture: Methods, Artifacts, and Interpretations, edited by Rash JE, Hudson CS. New York: Raven Press, 1979, p. 161–167. [Google Scholar]
- Stolinski C, Breathnach AS, Martin B, Thomas PK, King RH, Gabriel G. Associated particle aggregates in juxtaparanodal axolemma and adaxonal Schwann cell membrane of rat peripheral nerve. J Neurocytol 10: 679–691, 1981. [DOI] [PubMed] [Google Scholar]
- Stolinski C, Breathnach AS, Thomas PK, Gabriel G, King RH. Distribution of particle aggregates in the internodal axolemma and adaxonal schwann cell membrane of rodent peripheral nerve. J Neurol Sci 67: 213–222, 1985. [DOI] [PubMed] [Google Scholar]
- Tasaki I. The electro-saltatory transmission of the nerve impulse and the effect of narcosis upon the nerve fiber. Am J Physiol 127: 211–227, 1939. [Google Scholar]
- Tasaki I, Frank K. Measurement of the action potential of myelinated nerve fiber. Am J Physiol 182: 572–578, 1955. [DOI] [PubMed] [Google Scholar]
- Tasaki I, Ishii K, Ito H. On the relation between the conduction-rate, the fiber-diameter and the internodal distance of the medullated nerve fiber. Jpn J Med Sci Biophys 9: 189–199, 1943. [Google Scholar]
- Ting-Beall HP, Burgess F, Robertson JD. Particles and pits matched in native membranes. J Microsc 142: 311–316, 1986. [DOI] [PubMed] [Google Scholar]
- Unwin PNT, Zampighi G. Structure of the junction between communicating cells. Nature 283: 545–549, 1980. [DOI] [PubMed] [Google Scholar]
- Vabnick I, Trimmer JS, Schwarz TL, Levinson SR, Risal D, Shrager P. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J Neurosci 19: 747–758, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FC, Parcej DN, Dolly JO. α Subunit compositions of KV1.1-containing K+ channel subtypes fractionated from rat brain using dendrotoxins. Eur J Biochem 263: 230–237, 1999. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature 365: 75–79, 1993. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve 3: 141–150, 1980. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Ritchie JM. Organization of ion channels in the myelinated nerve fiber. Science 228: 1502–1507, 1985. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Wilson JA, Williams J, Chiu SY. Action potentials induce uniform calcium influx in mammalian myelinated optic nerves. J Neurophysiol 96: 695–709, 2006. [DOI] [PubMed] [Google Scholar]