Abstract
In the rodent whisker system, a key model for neural processing and behavioral choices during active sensing, whisker motion is increasingly recognized as only part of a broader motor repertoire employed by rodents during active touch. In particular, recent studies suggest whisker and head motions are tightly coordinated. However, conditions governing the selection and temporal organization of such coordinated sensing strategies remain poorly understood. We videographically reconstructed head and whisker motions of freely moving mice searching for a randomly located rewarded aperture, focusing on trials in which animals appeared to rapidly “correct” their trajectory under tactile guidance. Mice orienting after unilateral contact repositioned their whiskers similarly to previously reported head-turning asymmetry. However, whisker repositioning preceded head turn onsets and was not bilaterally symmetric. Moreover, mice selectively employed a strategy we term contact maintenance, with whisking modulated to counteract head motion and facilitate repeated contacts on subsequent whisks. Significantly, contact maintenance was not observed following initial contact with an aperture boundary, when the mouse needed to make a large corrective head motion to the front of the aperture, but only following contact by the same whisker field with the opposite aperture boundary, when the mouse needed to precisely align its head with the reward spout. Together these results suggest that mice can select from a diverse range of sensing strategies incorporating both knowledge of the task and whisk-by-whisk sensory information and, moreover, suggest the existence of high level control (not solely reflexive) of sensing motions coordinated between multiple body parts.
Keywords: vibrissa, somatosensory, decision, trigeminal, sensorimotor, vestibular
active motion is a prominent element of many sensory systems, often involving coordination of multiple body parts, for example, coordinated eye and head motions in vision or finger, hand, and arm motions during touch. The rodent whisker tactile system is one of the key models for active sensing (reviewed in Petersen 2007; Ahissar and Knutsen 2008; Diamond et al. 2008; Diamond 2010; Kleinfeld and Deschênes 2011; Feldmeyer et al. 2013) and has been especially prominent in studies of closed loops through sensory and motor areas back to peripheral sensors (Kleinfeld et al. 1999). However, research on sensing motions is often focused on whisker motion alone (Bermejo et al. 2005; Carvell and Simons 1990, 1995; Gao et al. 2003; Deutsch et al. 2012), while growing evidence suggests such motions are strongly coupled to head motions (Krupa et al. 2001; Sellien et al. 2005; Knutsen et al. 2006; Towal and Hartmann 2006; Godde et al. 2010; Grant and Prescott 2012; Arkley et al. 2014; Saraf-Sinik et al. 2015; Voigts et al. 2015). It remains unclear if whisker motion control is predominately reflexive, perhaps mediated by brainstem or other subcortical areas (Towal and Hartmann 2006; Mitchinson et al. 2007; Grant et al. 2009), or involves cortical areas also implicated in perceptual processing (Matyas et al. 2010; Smith and Alloway 2013). In particular, if the behavioral repertoire of rodent active touch involves tight coordination between whiskers, head, and body, then a focus on neural pathways constrained within whisker areas may be incomplete.
There have been many behavioral studies of whisker sensing, but most have been designed in ways that we believe suppress understanding of “naturalistic” full body sensing strategies. To investigate specifically the animal's selection of coordinated sensing motions, a behavioral paradigm should have the properties that the animal 1) is freely moving [e.g., as opposed to head fixed (Harvey et al. 2001; Bermejo et al. 2005; Crochet and Petersen 2006; Matyas et al. 2010; O'Connor et al. 2010a,b, 2013; Stüttgen and Schwarz 2010; Clack et al. 2012; Deutsch et al. 2012)]; 2) is not trained to exhibit prescribed head and whisker motions [e.g., as opposed to requiring placement of the nose at a particular location (Krupa et al. 2001; Shuler et al. 2002; Knutsen et al. 2006; Mehta et al. 2007; Wolfe et al. 2008; Venkatraman and Carmena 2011; Saraf-Sinik et al. 2015)] or requiring craning over a ledge (Towal and Hartmann 2006; von Heimendahl et al. 2007; Curtis and Kleinfeld 2009; Voigts et al. 2015); and 3) is goal oriented [e.g., as opposed to spontaneous exploration (Mitchinson et al. 2007; Grant et al. 2009; Prescott et al. 2011)]. Moreover, if contacted objects are not goal related but instead irrelevant or surprising obstacles (Sellien et al. 2005; Jenks et al. 2010; Arkley et al. 2014), it may be possible to determine important properties like reaction times but become difficult to interpret motions in terms of choice of sensing strategies. For example, animals may be ignoring whatever happens to their whiskers or even trying to avoid contacts. In addition to these task properties, high resolution quantitative analysis should be made not only of whiskers but also of head motions and object contacts [e.g., as opposed to contact-only tracking (Hutson and Masterton 1986; Brecht et al. 1997; von Heimendahl et al. 2007)]. The above studies each meet some of these criteria, but each (appropriately) limited some aspects of behavior to isolate a particular phenomena of interest. Our interest here is integrative, in the sense of asking what choices rodents make when all of the above criteria are met in a single task.
We quantitatively examined head-whisker coordination in freely moving mice searching for randomly located reward ports. We examine an apparent tension between “anticipatory” and “maximal contact” sensing strategies (Towal and Hartmann 2006; Mitchinson et al. 2007, 2011; Grant et al. 2009; Voigts et al. 2015) and find mice can switch between these strategies in a situation-dependent manner. Moreover, we find whisker repositioning can begin before corrective head turns during reward search. The observed timing and situation dependence suggest a volitional contribution to behavior not explained by simple reflexive models. In natural exploratory settings, motion for sensory acquisition may be accompanied by motions with overlapping use in navigation, threat assessment, or reward seeking. We suggest whisker repositioning may at times serve to counteract orienting head motions to maintain useful sensory input despite underlying large-scale motion.
MATERIALS AND METHODS
All procedures were approved by the Boston University Institutional Animal Care and Use Committee.
Task structure.
Six male water-restricted mice were trained to repeatedly traverse a polycarbonate track (31 cm by 9 cm) in alternating directions to receive a water reward (Fig. 1, A and B). Rewards were dispensed from two spouts located at the center of apertures with a 1.4-cm opening. The arena was enclosed by vertical walls (5 cm high), and a polycarbonate cover wrapped in black tape that prevented rearing and provided a dark background for videography. During initial training, apertures on each side of the track were fixed in a central location. The initial training consisted of two 20 min sessions per day for each mouse. The task was rapidly learned, and typically within 7–10 days the mice exceeded 80 trials per day, at which point one aperture became moveable.
Fig. 1.
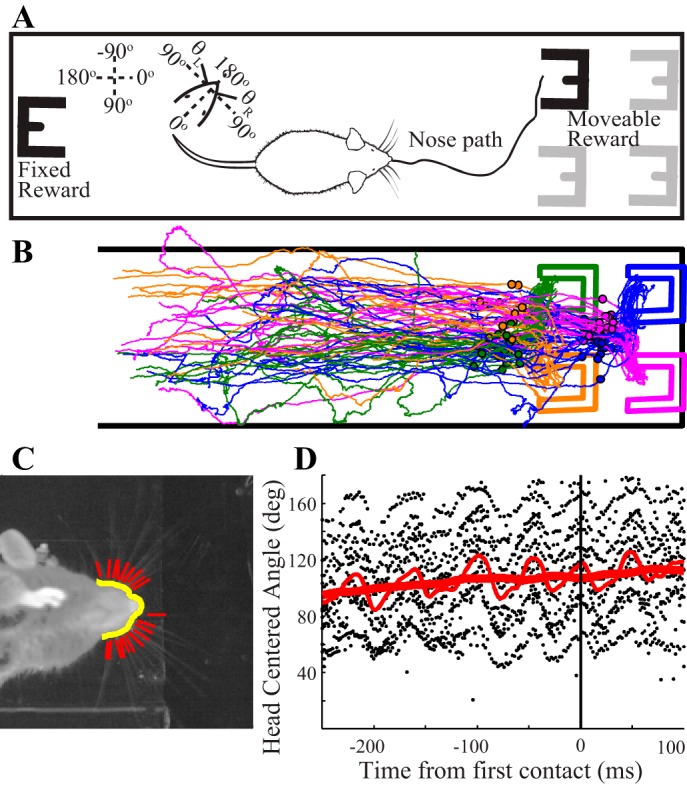
Freely exploring mice with intact whisker fields performing a tactile search task. A: mice were trained to repeatedly shuttle across a linear track under infrared illumination in search of a water reward dispensed within an aperture located in 1 of 4 possible locations. Insets: whisker position defined relative to the arena (counterclockwise), or relative to the head (180° corresponding to full protraction). Head angles were always arena centered, with 0° corresponding to rightward in the frame. B: thin lines show nose paths on a subset of trials (n = 85) for which the mouse made large head turns around the time of initial whisker contact with the aperture; paths are color coded to match the trial's aperture position (thick outlines). Dots show nose tip location in the frame identified as first aperture-whisker contact. C: sample high speed video frame showing the locations of automatically tracked snout and whiskers. D: sample whisker tracking for a single trial is shown aligned to first contact time. Black dots indicate the head-centered angle of all tracked whiskers in each frame, the thin solid line indicates the robust mean whisker angle, and the thick line indicates the set point (see materials and methods for definitions).
On each trial, the aperture was moved to one of four possible locations, determined from a random ordering of positions that ensured equal sampling of each reward location within each block of 16 trials. The aperture was positioned by means of a vertical pole suspended from two stepper motor driven translation stages (Newmark System), located 20 cm above the arena top (the pole entered through a small sliding cover). Aperture repositioning occurred when the mouse was moving in the opposite direction (towards the fixed reward end of the track) as determined by IR photodiodes placed along the track. Data collection began once mice progressed to the moveable aperture task. Mice regularly performed upwards of 100 trials per 30-min session, and the median trial duration was 1.1 s.
All behavioral sessions were conducted in the dark in a sound and light attenuating cabinet. The arena was illuminated by custom infrared LED panels along both long sides of the track. To confound possible auditory cues of aperture location, the aperture moved through a central “home” position on its way to its new position for each trial, and stepper motors were activated for the same duration for each movement of the aperture. Trials where the mouse began an approach before the aperture reaching its new location were rejected. Water rewards were only dispensed once the snout triggered an IR-breambreak placed inside the aperture to prevent a possible olfactory cue if the reward were available early. All aspects of the behavioral session were fully automated to reduce the influence of a human operator on behavior.
High-speed videography tracking and analysis.
A subset of trials each day were recorded at 500 frames per second (HSV; pco.1200hs camera; Cooke) through a mirror underneath the transparent track. The field of view of the camera encompassed approximately three-fourths of the track length, focusing on the moveable aperture side of the track. Data storage was initiated by an infrared beam break located in the aperture, which triggered the download of the preceding frames from the camera's buffer using a custom Matlab acquisition GUI. The camera aquired the next trial after the previous clip had finished downloading, with no experimenter involvement, to minimize selection bias. We recorded ∼20–30 high-speed clips per session. The entire session was recorded using a standard camcorder (30 frames per second) to provide an overall record of behavior.
High speed videography was background normalized using the average of 50 frames before the mouse starting to traverse the track. Additionally, we identified the location of corner features on the track itself and used these locations for image registration. Preprocessed video was analyzed using the BIOTACT Whisker Tracking Tool (Perkon et al. 2011; http://bwtt.sourceforge.net), after importing video using a custom programmed loader for our filetypes. We first tracked snout position using the functions ppImageTransform, sdGeneric, and SStShapeSpaceKalman. After ensuring accurate snout tracking, and retracking any regions with errors, we tracked whisker positions using ppImageTransform and WdIgorMeanAngle. Parameters were selected for each clip iteratively with manual reviewing of tracking results and retracking as necessary to ensure visually accurate whisker identification. The algorithm identified each whisker in each frame but did not attempt to track an individual whisker from one frame to another. We used raw information on the angle of each tracked whisker relative to the snout midline for further analysis. We did not make use of the mean angle calculation included in the software but instead found it necessary to develop a robust estimate of the mean that was more reliable in our task condition.
Using the arena-centered angles of a large subset of whiskers (∼20 on each side of the face in each frame) found by automated videographic tracking (Fig. 1C) (Perkon et al. 2011), we combined the individual angles in each frame, separately on each side of the face, with iteratively reweighted least squares that reduced the impact of errors in tracking for the most posterior or most anterior whiskers, which functioned as outliers on the mean angles (Fig. 1D). Applying the least squares fit with a window of 15-ms temporally smoothed the two mean angles to produce “instantaneous” left and right pad angles (Fig. 1D, thin red). We defined the set point on each side as the angle after application of additional smoothing with a 100-ms window, which removed the contribution from individual whisks (Fig. 1D, thick red). Smoothing operations were computed using noncausal robust weighted liner least squares (Cleveland 1979).
We then defined the (acute angle) mean of arena-centered left and right set points as MSP, the “net direction” of bilateral whisker positioning. Note that this way of estimating net whisker angle is independent of head direction. We define the whisker positioning asymmetry, θASY, as the difference between MSP and the arena-centered head angle defined by the midline through the automated tracker's outline of the snout. This approach is robust to asynchronous whisking while still providing a measure of asymmetry on a frame by frame basis. Given that the anterior whiskers nearly always come into contact with the target first in our task, we also modified the analysis to provide estimates for anterior and posterior whiskers separately, by first sorting tracked angles in each frame into top and bottom quartiles, respectively, and then repeating the above analyses.
Identifying whisker contact and head turn onset frames.
We manually identified the frames of first whisker contact with the aperture (FC) and of first whisker contact with the opposite side of the aperture (OC) during orienting. To control for the inherent difficulties in identifying whisker tip locations and contacts, two observers independently assigned numerical scores (range: 1–3) for the likelihood of a contact in all potential first contact frames (possibly across multiple whisks) and separately chose which frame was most likely to be the first contact. The observers produced consistent results (median absolute difference 2 frames, or 4 ms, with interquartile range 16.25 frames). In addition, many of the subsequent analyses are robust to misidentification of first contact frames. For example, see that Fig. 2C does not depend on accurate knowledge of first contact time, and see that Fig. 5 depends only on identifying the correct contacting whisk, not the specific video frame where contact first occurs.
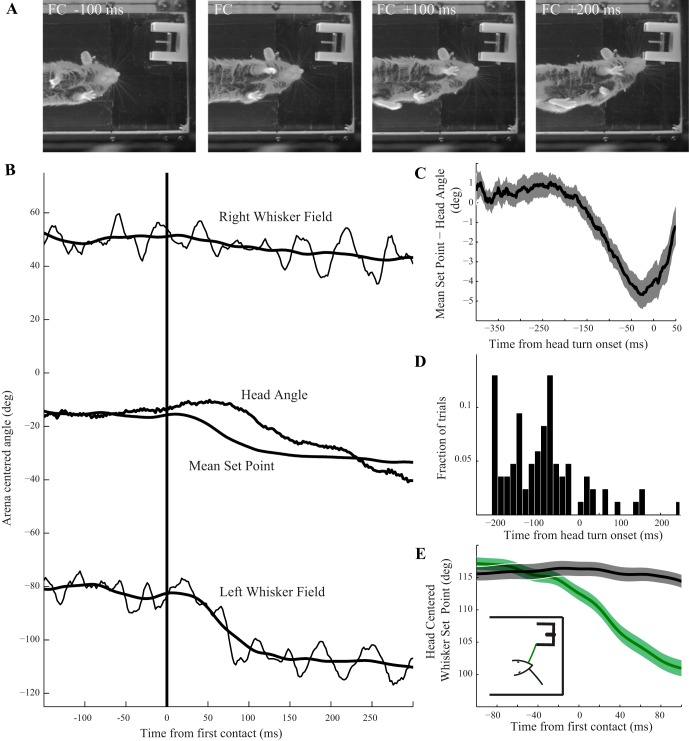
Fig. 2.
Whisker repositioning following first contact precedes head motion, is in the direction of the turn, and is mostly unilateral. A: high-speed video frames from an example trial illustrate large retraction of the left (contacting side) whisker field within 100 ms postcontact. B: arena-centered whisker and head angles are shown for the example trial in A, aligned to the time of the first whisker-aperture contact (unilaterally on the left side). The mean set point shows repositioning of whisker angle before the onset of a head turn, which begins ∼75 ms after first contact. Repositioning is dominated by retraction of the set point on the side the mouse is turning towards, with at most a small change of the contralateral set point. C: whisker asymmetry (difference between mean set point and head angle) develops ∼125 ms before the onset of a head turn, suggesting that repositioning cannot be fully explained by a reflex response to the turn itself (n = 85, mean ± SE). To combine data across trials, trials with rightward turns were reflected vertically so that all turns appear leftward. D: histogram of manually identified first contact times relative to turn onsets for the trials in C shows that the majority of turns follow contact, while in a small fraction of trials the mouse may initiate a turn spontaneously, possibly due to expected location of rewards (see results for further discussion). E: trial averaging of mean set points (n = 85, mean ± SE) shows that the asymmetry following first contact is dominated by set point retraction on the initially contacting side (mean change from 0 to 100 ms, 16.2°, green curve), with only small changes on the noncontacting side (mean change from 0 to 100 ms 1.1°, black curve). The mean differences between contacting and non-contacting side precontact (−100 to 0 ms) were not significant (P = 0.28), while the differences postcontact (0 to 100 ms) were (P = 2.0 × 10−13). Rightward turns were reflected as in C. Inset: schematic of mouse at first contact with whisker color convention.
Fig. 5.
Contact maintenance is an actively selected sensing strategy. A: dead-reckoning angles are defined by the line from the center of the head to a point of possible object contact, shown schematically by black and red dashed lines for the initial and opposite boundaries. The dead-reckoning angle is the arena-centered angle at which whiskers would point directly at the object, whether contacting or not, and accounts for contributions from both head translation and rotation. Note that since whisker angles are estimated near the whisker base, ignoring curvature (and in this figure are averaged across anterior whiskers), it is possible for whisker tips to make object contact at small deviations from the dead-reckoning angle. Inset: example frame of snout tracking with the calculated dead-reckoning line from the head to the initial contact point for that trial. B: an example trial shows that at first contact (FC; 0 ms; thin vertical line) the whisker angle (thin black curve) is close to the dead-reckoning angle to the initial boundary (thick red curve), but by the next whisk shifts closer to the opposite boundary (thick black curve). After contacting the opposite boundary (OC; ∼175 ms; thin vertical line, and thickened whisker angle curve), the protraction amplitude tracks the opposite boundary dead-reckoning angle. C: video frames corresponding to the example trial shown in B for times of FC, OC, and OC + 200 ms. Following opposite side contact, the snout trajectory passes very close to the near aperture boundary, resulting in the contralateral whiskers being mechanically forced backwards. D: trial averaged deviations from dead-reckoning are shown for peak protraction (green) and retraction (cyan) angles, in whisks around the first contact with the initial aperture boundary (n = 85, mean ± SE). Videos were vertically reflected as needed to make turns appear leftward. Contact maintenance with the first aperture boundary is not observed, as protraction angles move away from zero in the four whisks following contact (top). Contact maintenance is observed when the second aperture boundary is contacted (bottom; note alignment is still to first contact with the initial aperture boundary), as the green trace moves towards zero in the four whisks following contact. For comparison, a model whisker fixed relative to the head (brown) and a model whisker whose position is simulated to match published head-turning asymmetry (purple; see results) show that neither of these mechanisms is sufficient to account for the observed repositioning. E: head translation (top), arena-centered head rotation (middle), and whisker rotations (bottom; initially contacting whiskers in green, contralateral whiskers in black) are shown relative to time of first contact. Dead reckoning incorporates both head translation and rotation, to place the object in head-centered coordinates for direct comparison to whisker angle. Rightward turns were reflected as in D. F: summary of normalized deviations from dead reckoning for the whisk before and after initial boundary contact (the deviation during the contacting whisk is subtracted) show significant differences from dead reckoning on both the pre- and postcontact whisks and an absence of contact maintenance (n = 85, mean ± SE). G: same analysis as F for the whisks before and after contact with the opposite boundary shows small deviations postcontact and maintenance of contact angles (n = 85, mean ± SE).
The noise in head angle as determined by the automated video tracker made it difficult to precisely determine head turn onsets. However, as the long axis of the arena over which animals travel is oriented horizontally in our images, the change in vertical position of the snout during turns provided another measure of onsets with smaller noise floor. We calculated the slope of a linear fit within a sliding 100-ms window (t:t + 100 ms) of the frame-by-frame absolute difference between nose and reward port vertical position. We chose the window size based on typical turn durations. We identified turns as the frame with minimum slope within ± 400 ms of identified first contacts (comprising most of the trial, but eliminating spurious detection of motions at either reward port). Identified turn onsets were visually consistent with video frames where observable head motion began.
RESULTS
Tactile search behaviors during the task.
We observed six mice performing a tactile search for a water reward located at the center of an aperture automatically positioned in one of four randomly selected locations before the start of each trial (Fig. 1A). Automated tracking of head and whisker positions in high-speed video was used to facilitate the analysis of coordinated sensing motions during this search task. We observed a variety of approach trajectories. In some cases, mice happen to approach the aperture straight on, while in others they tended to traverse the track along one wall, then pull away from the wall when nearing the reward area. We focused on a subset of trials where mice made large corrective head motions during the final stages of approach to the aperture (Fig. 1B; Supplemental Video S1; Supplemental Material for this article is available online at the Journal website). We selected trials where the distance (along the short axis of the arena) from the tip of the snout to the center of the aperture was greater than the width of the aperture opening at the time of first whisker contact. These trials each contain two situations of whisker contact: first contact with the near edge of the aperture, then, after repositioning, first contact by the same whisker field with the opposite aperture edge. Also, by construction, initial contact was made by whiskers on only one side of the face. This subset of trials exemplifies whisker-mediated sensory orienting and provides a rich environment to evaluate the selection of active sensing strategies that require coordinated head and whisker motions. We analyzed 85 such trials for a total of 105.3 s (52,664 frames) of tracked high-speed video data.
Asymmetric whisker positioning begins before head turns.
The first quantification of head-whisker coordination found that, during head turns in the absence of contact, rats can adjust the difference between left and right set points proportionally to head angular velocity (Towal and Hartmann 2006). For example, when turning to the left, the left setpoint retracts and the right setpoint protracts. A similar behavior was later reported for spontaneously exploring mice (Mitchinson et al. 2011). This head-turning asymmetry (HTA) serves to position the whiskers in the area towards which the head is moving, possibly as a reflexive response to head turns similar to the vestibulo-collic reflex (VCR), thus allowing the whiskers to “look ahead” in space (Towal and Hartmann 2006). HTA was defined in the above studies by measuring the correlation of instantaneous differences in whisker position (left minus right head-centered angles) with instantaneous head angular velocity relative to the arena. This definition has several limitations, notably the necessity of observing over large durations to obtain these distributions before HTA can be assessed. Additionally, the method encounters difficulties during periods of asynchronous whisking, which are especially prominent following contacts (Sachdev 2003; Towal and Hartmann 2006). This is an especially important concern in mice, which exhibit irregular whisking compared with the nearly periodic whisking of rats (Curtis and Kleinfeld 2009; Mitchinson et al. 2011; Schroeder and Ritt 2013). During asynchronous whisking, the instantaneous difference in whisker positions may not accurately reflect both fast whisking motions and slow set point adjustments. For these reasons, we developed a complementary analysis of HTA and similar relationships between head and whisker angles adapted for single trials and single whisks (see materials and methods).
A key question is whether the onset of whisker repositioning precedes head turning, suggesting a volitional or predictive sensing mechanism, in contrast to proposed analogy to vestibular reflexes (Towal and Hartmann 2006; Mitchinson et al. 2011). Figure 2, A and B, shows an example single trial in which the mean set point (MSP) begins to change within the first 20 ms following first contact, while the head angle does not begin to change until ∼75 ms following contact. In this example, the mouse is turning to the left and exhibits a retraction of the left whisker field, which drives MSP downward before the onset of head turning (note in Fig. 1 that arena-centered angles follow a clockwise convention to be consistent with the direction of head-centered angles on the animal's left). Visual inspection of the same trial highlights the whiskers adjustment before head motion (Fig. 2A).
We investigated the relative timing over many trials by assessing the timing of changes in asymmetry (θASY). Figure 2C shows summary data for 85 trials with θASY aligned to the time of head turn onset. To combine data over all trials, we vertically reflected videos with rightward turns so that all turns appear to be leftward. We find that asymmetric repositioning precedes turn onsets by ∼125 ms and that by the time an identifiable change in head angle occurs, the asymmetric repositioning of set points has already reached its maximum value. Whether the repositioning is a response to contact or an anticipatory preparation for a turn based on past experience, the timing argues against a reflex response to the turn itself.
The timing of initial contact is widely distributed with respect to turn onset but predominately occurs before the start of a turn (81%, 69/85 trials; Fig. 2D). In cases where head motion onset preceded whisker contact, 62.5% of trials (10 of 16) had the aperture located in one of the two rear positions, and for turns that preceded whisker contact by more than 50 ms, 90% of trials (9 of 10) had the aperture in a rear position. Thus mice may adjust their search trajectory once they know the aperture is not located in either of the two forward locations, which may account for the subset of trials in which head motions were observed before whisker contact. This possibility is further supported by the convergence of nose trajectories in rear aperture trials (Fig. 1B, blue and magenta traces) between the forward and rear aperture positions. It is also possible some first contact times were misidentified due to limitations in videography; however, we attempted to mitigate these issues though multiobserver identification of contact frames (see materials and methods).
Asymmetric repositioning is nearly unilateral.
In the simplest model of HTA, the whisker field on the turning side retracts and the field on the contralateral side protracts, inducing a bilateral shift towards the area the head is about to enter. Previous reports of HTA did not specifically evaluate the bilateralism of repositioning (Towal and Hartmann 2006; Mitchinson et al. 2011), although different adjustments of each whisker field, including pronounced contralateral side protractions, have been reported for rats responding to unilateral object contact (Mitchinson et al. 2007). In that study, the observed behavior appears consistent with the notion of positioning each whisker field to maximize the number of whiskers impinging lightly on the surface. Such a strategy may not be broadly applicable to all unilateral objects contacts, and so we asked whether repositioning in this task involved both whisker fields equally, and if not which side was responsible for the majority of the observed repositioning. Figure 2E shows the trial averaged whisker set points for the contacting (Fig. 2E, green) and noncontacting (Fig. 2E, black) sides of the face, aligned around first contact (n = 85 trials). To combine trials, we reflected trials with rightward turns, so that all turns appear to be leftward, before proceeding with analysis. Between the 100 ms before and 100 ms after contact, the whisker field on the side the mouse is turning towards adjusts by a mean 16.2°, compared with 1.1° on the contralateral side (note that since mice are likely to be protracting at first contact; t = 0), they are likely to have the whiskers relatively more retracted just before contact. Averaged set points then appear to “predict” contact (drop slightly before contact time) when this selection bias in precontact angle is smoothed with the decrease in set point after contact (contrast with the individual visible whisks; see Fig. 4A). The difference between the distributions on each side 100 ms following contact is significant (t-test, P = 2.0 × 10−13), while 100 ms before contact there is no significant difference between the two sides (P = 0.28). Additionally, the differences between the pre- and postcontact contralateral whisker angles were not significant (P = 0.45). This summary agrees with our informal observation in single trials that the majority of set point adjustment occurs on the turning side (as in the example in Fig. 2B). The precise nature of asymmetric repositioning following contact, and how much adjustment is provided by each whisker field, are likely to be situation dependent.
Fig. 4.
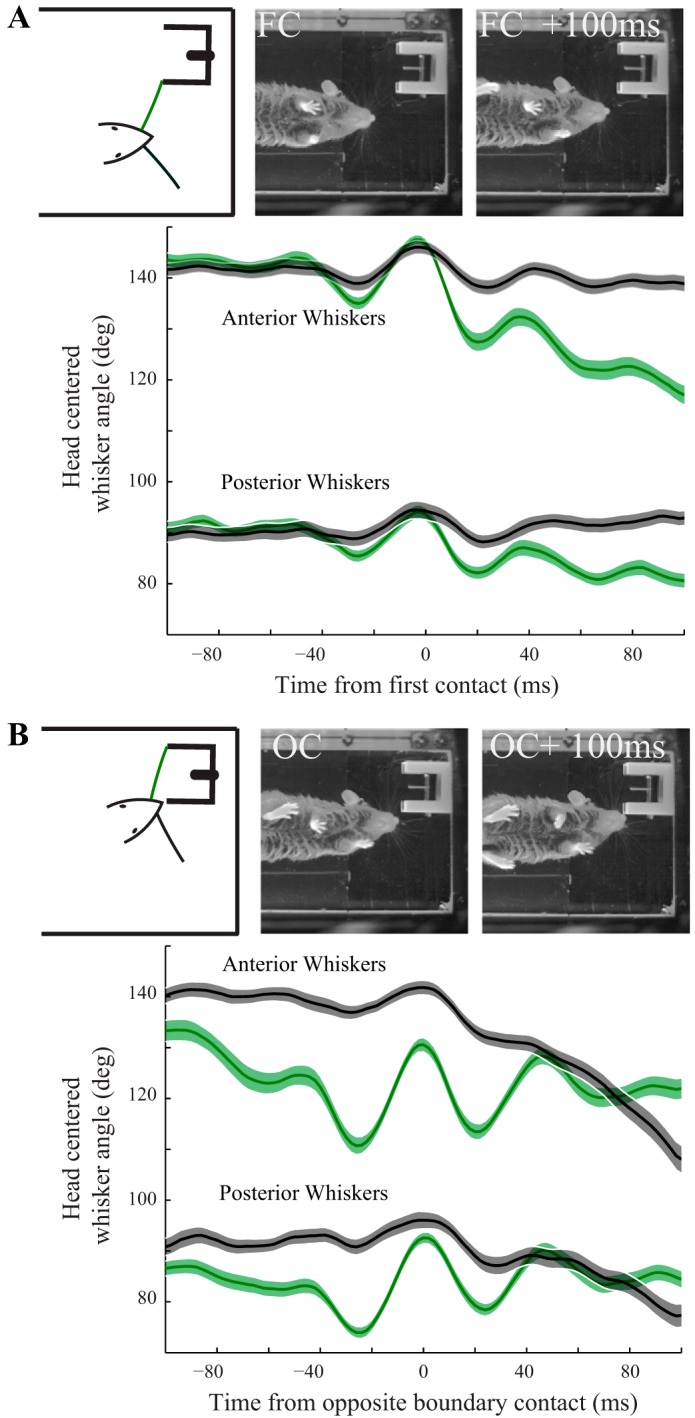
Asymmetric repositioning depends on the behavioral situation in which a whisker contact occurs. A: following initial, unilateral contact with the aperture (FC; whisker color convention in schematic), example video frames show pronounced contacting side retractions within 100 ms postcontact. Separate averages of anterior and posterior whisker angles (see materials and methods), aligned to first contact time, show rapid and pronounced contacting side retractions (green curves), with relatively minor contralateral adjustment (black curves) (n = 85 trials, mean ± SE). Anterior whiskers underwent greater adjustment than posterior whiskers. To combine data across trials, trials with rightward turns were vertically reflected so that all turns appear leftward. B: in contrast, when the same whisker field contacts the opposite aperture boundary (OC; whisker color convention in schematic), the contacting side whisker aligned average (green curves) reveals large amplitude whisking (temporally consistent across trials) around a nearly constant set point (n = 85 trials, mean ± SE). The angle of the contralateral whiskers (black curves) decreases as they are pushed against the outer edge of the aperture. Trials with rightward turns were reflected as in A.
Asymmetric repositioning allows whiskers to “look ahead.”
Asymmetric repositioning of whisker set points may serve to place the whisker field in a useful location for the imminent head motions (Towal and Hartmann 2006; Mitchinson et al. 2011). We estimated the amplitude by which MSP leads changes in the head angle by comparing linear regressions of the two quantities for 150 ms before and after initial whisker contact (Fig. 3). Both before and after contact, the regression slope is near one (0.86/0.90) with a strong correlation (r2 = 0.71/0.73), reflecting a strong relationship between head angle and (arena centered) whisker positioning. Before contact, the y-intercept is near zero (−0.03°), consistent with whisking at a fixed set point relative to the head (or a whisker held at a fixed angle relative to the head). Following contact, the vertical-intercept of the distribution shifts upwards to 6.3° ahead of the angle expected without repositioning, consistent with the idea that whisker repositioning serves to place the whiskers into the region that the head will soon enter. The observation of this shift only following contact, and not for changes in head angle before contact, suggests the animal is selecting an active sensing strategy for each situation and transitions from one state to another based on the information derived from aperture contact and knowledge of the arena. Note that our analysis compares whisker asymmetry against head position, in contrast to earlier analyses against head angular velocity (Towal and Hartmann 2006; Mitchinson et al. 2011).
Fig. 3.
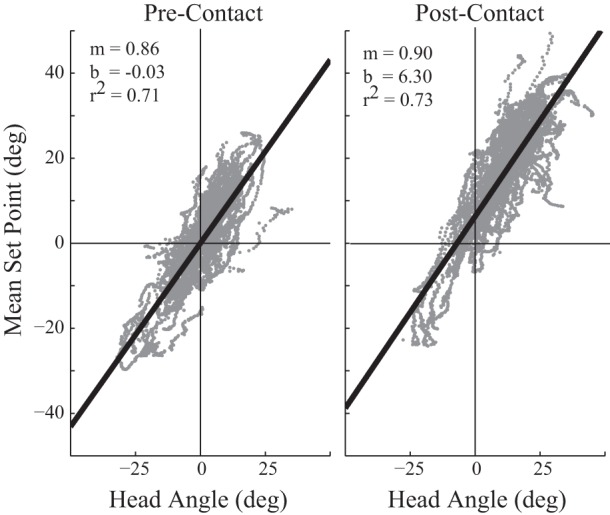
Active sensing strategy shifts following unilateral contact. The arena-centered mean set point on the contacting side is linearly correlated with head angle, shown by grey dots for each frame within 150 ms before (left) or after (right) first contact (n = 85 trials), and the best fit line (fit parameters in inset). To combine trials, videos were vertically reflected as needed to put all head turns in the same direction, as if to the animal's left (upward in the video frame). Following contact, the distribution shifts upward by ∼6°, indicating that the whiskers are repositioned in advance of head turns.
Responses to contact are situation dependent.
We next asked if there are differences in repositioning of the same whisker field in two different behavioral situations within a trial. The first situation occurs when the mouse makes initial unilateral whisker contact with one of the aperture boundaries (Fig. 4A, top left). The second occurs when, after changes in head position, that same whisker field makes contact with the far side of the aperture (Fig. 4B, top left). If responses to whisker contact are driven primarily by simple reflexes, we would expect to see similar whisker repositioning in each of these contact conditions and based on previous reports would predict contact induced retractions or suppression of protractions of the contacting side whisker field (Nguyen and Kleinfeld 2005; Mitchinson et al. 2007; Grant et al. 2009; Matyas et al. 2010). However, during the head turn the animal is likely trying to find the far side of the aperture and would instead need to counteract head rotation, a behavior we term contact maintenance.
Figure 4A shows the averaged head-centered field angles, similar to Fig. 2E for set points but here without additional smoothing and separated into anterior and posterior whiskers, demonstrating again a retraction of the contacting side whisker field (Fig. 4A, green), with a comparatively modest adjustment on the contralateral side (Fig. 4A, black). However, we did not observe a retraction of the contacting side whisker field later in the trial, when the same whiskers made contact with the far side of the aperture (Fig. 4B). Instead, these whiskers maintain a relatively consistent set point, and the appearance of periodic peaks in the contacting side aligned averages indicate that the mouse is whisking with a consistent temporal structure (across trials) around the time of initial contact with the opposite side. The different responses to putatively comparable whisker contacts suggest that repositioning could be sensitive to the specific situation of the contact within the larger task structure. The two different responses to contact are visually apparent in an example trial by comparing the retracted whisker position 100 ms after initial contact (Fig. 4A, top right) with the relatively protracted position 100 ms after contact with the opposite boundary (Fig. 4B, top right). While the entire whisker field is adjusted after contact, we observed a difference in magnitude across the pad, roughly similar to that reported by Grant et al. (2009), with larger adjustments of anterior than posterior whiskers (Fig. 4).
Contact maintenance as a parallel strategy to HTA.
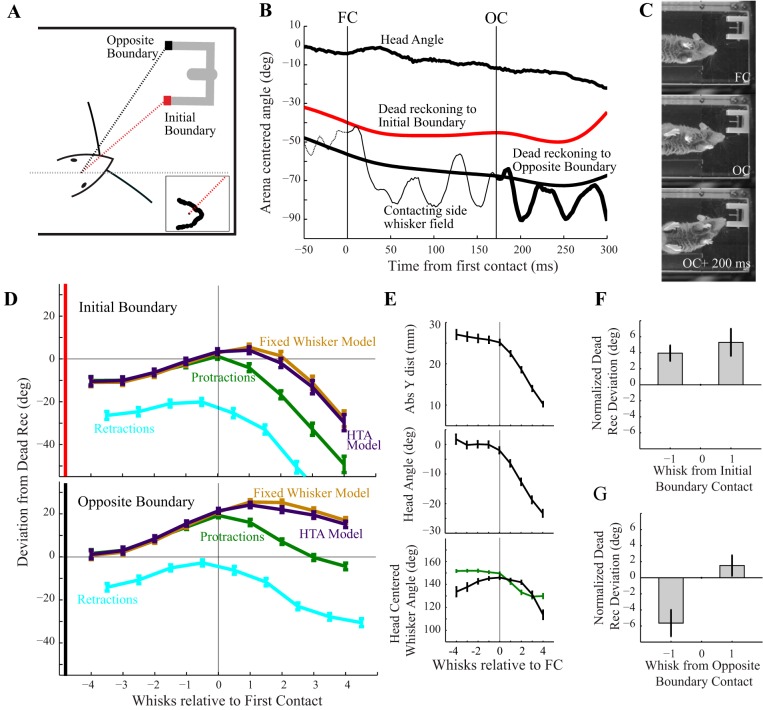
We defined contact maintenance as an adjustment of set point that counteracts head turning to sustain object contacts. In particular, during a turn towards an object of interest, contact maintenance would result in repositioning of whiskers that is in the opposite direction to the predictions based on HTA. To quantify the possible presence of this sensing strategy, we calculated the deviation between the arena-centered whisker angle and a “dead-reckoning angle.” The dead-reckoning angle is defined as the angle in arena-centered coordinates from the center of the head to the putative contact point on the aperture boundary. If the whisker angle matches the dead-reckoning angle (that is, the deviation is zero up to small whisker pad/base point displacements), the whisker field is pointing directly at the contact point, whether or not it is actually touching. The use of dead reckoning accounts for both translation and rotation of the head when comparing whisker positioning relative to the object. We evaluated the differences between the whisker angle and the dead-reckoning angle to the contact points on each side of the aperture (Fig. 5A).
Figure 5B shows an example trial. First contact occurs at t = 0 ms, at which point the actual whisker field angle (Fig. 5B, thin black trace) is very close to the dead-reckoning angle to the right side (Fig. 5B, red trace). By the next whisk after initial contact, however, the left whisker angle deviates from the dead-reckoning angle to the initial aperture boundary. The left whisker field contacts the opposite aperture boundary at around t = 175 ms (Fig. 5B, vertical black indicator). In the whisks following contact with the opposite boundary, whisking parameters are adjusted to compensate for head motions such that the protractions on subsequent whisks track changes in the dead-reckoning angle to the opposite aperture boundary (Fig. 5B, thick black); note head angle continues to change. In other words, the compensatory changes in whisker motion that define contact maintenance are observed only in response to contact with the opposite boundary, when the mouse is attempting to align its snout with the aperture interior to reach the reward spout. Figure 5C shows representative video frames corresponding to the example trial (Fig. 5B) for first contact (FC; Fig. 5B, top), opposite contact (OC; Fig. 5B, middle), and 200 ms after opposite contact (OC + 200 ms; Fig. 5B, bottom).
To examine this effect across all trials, we identified individual whisks between angular local maxima and minima and evaluated the angles at protraction and retraction for the four whisks before and after initial contact. This whisk-by-whisk analysis facilitates comparisons across a range of trial durations, where simple averaging of continuous time series is ill defined. We examined four whisks preceding and following contact because the mice typically make two to three contacts with the far aperture boundary before the snout enters the aperture. Past this point the whiskers tend to be mechanically forced backwards. We evaluated the difference between the anterior field angle and the dead-reckoning angle at both peak protractions (Fig. 5D, green) and retractions (Fig. 5D, blue) for the four whisks before and after the initially contacting whisk (Fig. 5D). With the use of this metric, contact maintenance would be evident if the difference from dead reckoning at protractions (Fig. 5D, green) moves closer to zero following contact. Initial contact does not lead to contact maintenance; by the next whisk after first contact, anterior whiskers pull away from the dead-reckoning angle (Fig. 5D, top, green curve). Within the next four whisks, however, whisker repositioning reduces the difference between the anterior angle and the dead-reckoning angle to the opposite aperture boundary (Fig. 5D, bottom, green curve).
If it were the case that the mouse ceased whisking and the changing field angle was due to passive mechanics of the whiskers held against the object as the head moved, the difference from dead-reckoning angles at observed protraction and retraction would be the same. Instead, we see the difference between protractions and retractions increase following contact, indicating that the mouse is on average increasing the amplitude of whisking on the ipsilateral side. Rather than protractions being cut off by contact with the aperture and being unable to advance further, the set point is being adjusted such that large amplitude whisking continues but with the protraction amplitude modified based on object location. This analysis highlights that the mouse is not simply leaving the whiskers pressed against the contact point once locating the aperture but continuing to whisk throughout the approach.
To further explore the question of active repositioning, we simulated a fixed whisker model (Fig. 5D, brown curve) where the angle is fixed relative to the head (at the average angle within the 500 ms preceding contact). Angle changes in the fixed whisker model are wholly due to head motions and are insufficient to account for the observed repositioning with either the first or the second aperture boundary, as seen by the differences between the green and brown curves in Fig. 5D. Furthermore, we evaluated whether a model of HTA was sufficient to explain the repositioning (Fig. 5D, purple curve), without requiring any further retraction. We found the average whisker angle during the precontact period by subtracting the anticipated contribution from HTA in each precontact frame (head angular velocity × HTA coefficient) from the mean precontact whisker angle. We then estimated an expected amount of asymmetry for each frame following contact by multiplying the instantaneous head angular velocity by the largest HTA coefficient (115 ms) reported in the literature (Towal and Hartmann 2006). We then adjusted the whisker angles on both sides from their precontact means by the equal amounts with opposite signs to achieve the predicted value of asymmetry for each frame. We found that at the head angular velocities experienced in this task, the degree of repositioning far exceeds what would be expected from reports of HTA.
The comparison between arena-centered whisker angles and the dead-reckoning angle incorporates changes introduced by head translation, head rotation, and whisker rotations. Figure 5E shows that head translation (measured as the absolute distance in the Y-direction from the snout to the aperture), head angle, and contacting side whisker angle are all modulated in the whisks immediately following first contact. In contrast, the contralateral whiskers (Fig. 5E, bottom, black trace) do not show adjustment until approximately the third whisk postcontact. The mice generally adopt a strategy where the snout remains close to the near side aperture boundary during repositioning, as opposed to pulling the entire head backwards and approaching again. As a result, once the animal begins entering the reward aperture, the contralateral side whiskers are mechanically forced backwards by the near side aperture boundary, rather than engaging in an active repositioning strategy such as contact maintenance (example shown in Fig. 5C, bottom).
We further quantified dead-reckoning deviations for the whisks immediately preceding and following initial contact (Fig. 5F) and preceding and following contact with the opposite aperture boundary (Fig. 5G). The deviation on the frame of contact is subtracted (hence the deviation at whisk “0” is 0°) from the deviations before and after; this normalization accounts for small errors between the true contact location in each trial and the point selected to compute dead reckoning. We find that around the initial contact, both the preceding and following whisks showed large differences from dead reckoning (3.9 ± 0.11 and 5.3 ± 0.18°, respectively), both significantly different from zero (t-test, P = 7.7 × 10−5 and P = 0.002, respectively). Around contact with the opposite side, however, a large deviation on the preceding whisk (−5.6 ± 0.18°, significantly different from zero, t-test, P = 7.4 × 10−4) is followed by a smaller deviation after opposite boundary contact (1.5 ± 0.14°, not significantly different than zero, t-test, P = 0.23). This reduction in deviation only to the opposite boundary contact point supports the hypothesis that contact maintenance is employed selectively and is not a fixed motor response to contact.
We also asked if the magnitude of initial angular adjustment following first contact appeared directed towards the opposite boundary. If mice are able to estimate the distance to the aperture (for example, by sensing radial distance of whisker contact; Pammer et al. 2013), they could use that information along with knowledge of the arena to predict the opposite boundary location. To address this question, we compared the magnitude of the retraction following first contact to the size of retraction that would have been needed to match the whisker angle to the opposite boundary dead-reckoning angle (Fig. 6A). Values less than zero show retractions that exceeded the size needed to match the dead-reckoning angle. We observed mice typically exhibited larger retractions (−9.1 ± 1.9°) than would have been expected if they were modulating the retraction towards an expected, learned position. To test whether mice adjusted their retraction amplitudes by their location relative to the aperture, we regressed the size of the retraction after first contact vs. the (vertical) distance from the snout to the near aperture boundary (Fig. 6B, left). We observed a wide range of retraction amplitudes (typically 15–35°) but found no relationship between the distance at first contact and the size of retraction. For comparison, we examined what this relationship would look like for a simulated retraction made equal to the size needed to match dead reckoning (Fig. 6B, right) and found a correlation between distance and retraction size (r2 = 0.39). These results together suggest that the mouse is not using contact distance to adjust the size of retraction immediately towards the opposite boundary. Instead, the large postcontact retractions appear similar to HTA during the head turn towards the aperture, followed by upregulation of whisking amplitude until opposite contact occurs.
Fig. 6.
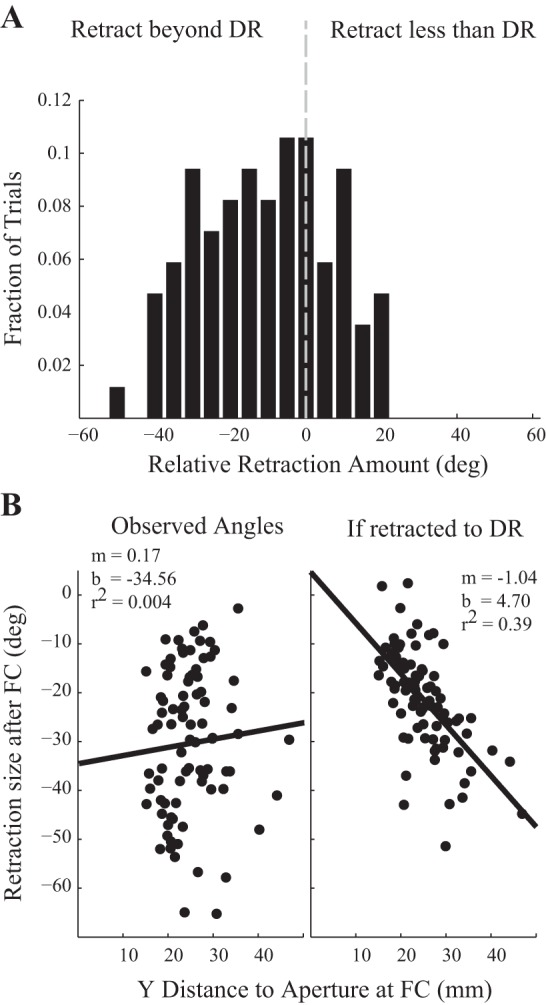
Retraction following first contact (FC) is not directed towards the opposite boundary. A: histogram showing the magnitude of post-first contact retraction, relative to the size of retraction that would have placed the whisker field on the dead-reckoning line to the opposite boundary (n = 85 trials). Values less than zero indicate retraction magnitudes larger than necessary to match dead reckoning. B: scatterplots of the size of post-first contact retraction against the distance (along the short axis of the arena) from the snout to the aperture at first contact time (n = 85 trials). Real data (left) shows no correlation. There would be a negative correlation if the retraction magnitude equaled the dead-reckoning angle (simulated at right).
Although whisker contacts between the two sides of the aperture are unlikely to be mechanically identical, they are similar in the respect that they involve contact with an identically shaped and textured boundary by the same whisker field, and the two different situations of unilateral whisker contact are available for comparison within a given trial. The proposed selection between alternative sensing strategies in response to similar unilateral whisker contacts is summarized in Fig. 7. The strategy of contact maintenance requires set point adjustments that oppose those expected by HTA, and we observe this strategy in some behavioral situations, where such contact maintenance may be most useful for the fine positioning needed to reach reward.
Fig. 7.
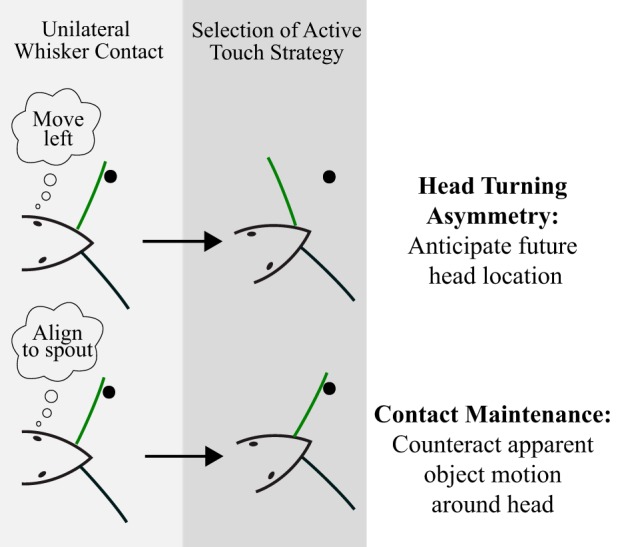
Conceptual summary of active sensing strategy selection. Mice may exhibit a range of sensing behaviors following similar unilateral whisker contact, depending on the behavioral situation. When moving the head into a new area (e.g., reorienting after contact with the initial boundary), we observe contacting side set point retractions expected from previous reports of head-turning asymmetry (Towal and Hartmann 2006; Mitchinson et al. 2011). In contrast, when keeping whiskers on a surface is useful for task completion (e.g., contacting the far side aperture boundary), mice might employ a strategy of contact maintenance, where set point adjustments counteract head motion to ensure repeated contact on subsequent protractions. The latter is consistent with a maximal contact strategy (Mitchinson et al. 2007), now conditionally situated within a goal-oriented task, and not necessarily dependent on force feedback from contacts. The ability of mice to select from diverse sensing strategies based on the behavioral context of unilateral whisker contact suggests possible higher level whisk-by-whisk control, beyond contact induced or vestibular reflexes.
DISCUSSION
The information available in neural pathways during active touch will depend on behavioral choices involving multiple body locations (e.g., feet, head, and whiskers), so that neural function occurs within the context of a “global” sensing strategy. We investigated how multiple degree of freedom coordination is organized in mouse whisker touch, specifically head-whisker coupling.
Previous reports showed mean whisker angles to be negatively correlated with head angular velocity (Towal and Hartmann 2006; Mitchinson et al. 2011), without determining the individual contributions of each whisker field or the timing of whisker repositioning relative to turn onset. We estimated the turn onset time in our task by a change in head position, which provided a more precise and conservative (early) measure than head angle change. The timing could in principle by improved by electromyography (e.g., in neck muscles), however, with additional challenges related to muscle response times and interpretation of noisy EMG data. We observed mice asymmetrically reposition their whiskers ∼100 ms before the onset of head turning, suggesting head-whisker coupling would not be due solely to a vestibular reflex and may be under more sophisticated control. This would be true whether the turns were in response to unilateral whisker contact, the expectation of contact, or a central efferent signal.
HTA was reported for whisking in air (Towal and Hartmann 2006) and spontaneous head turns during exploration of a largely featureless arena (Mitchinson et al. 2011). While persistent floor contacts occurred in the second study, no obvious benefit would accrue from orienting based on those contacts. Unsurprisingly, rats trained to make large sweeping head turns while craning from a platform and whisking into empty space (Towal and Hartmann 2006) exhibited a larger degree of HTA than rats making spontaneous turns while exploring an enclosed arena containing no objects and minimal features but including floor and wall contacts (Mitchinson et al. 2011; Grant and Prescott 2012). These differences in effect size, along with our finding of different repositioning in different circumstances (Figs. 4 and 5), suggest that rodents make positioning decisions influenced by informational factors. The conclusion that simple, feed-forward motor programs or reflex responses are insufficient to explain the observed repositioning seen here, combined with previously described efferent signals in S1 (Curtis and Kleinfeld 2009; Hill et al. 2011; Kleinfeld and Deschênes 2011), the presence of sensory information in M1 to S1 projections (Petreanu et al. 2012; Chen et al. 2013), the reported use of contact phase as an error signal to update estimates of distance (Voigts et al. 2015), and short latency changes in whisking following S1 stimulation (Matyas et al. 2010; Schroeder et al. 2013), all support a whisk-by-whisk role for S1 during selection of sensing motions.
A widely considered hypothesis is that bilateral control places each whisker field where it will acquire the most useful information, for example, HTA repositioning whiskers to “look ahead” into the region the head is moving (Towal and Hartmann 2006) or mice-modulating protraction amplitudes based on expected object locations (Voigts et al. 2015). We found both whisker fields need not contribute equally to repositioning, finding adjustment primarily of the turning and contacting whisker field. A mouse searching for a single object in a known arena, such as in our experiment, may have no reason for contralateral repositioning following unilateral contact. In a prior study where rats showed contralateral protractions when orienting towards a unilateral contact (Mitchinson et al. 2007), the absence of repositioning in trials without orienting was attributed to attentional choice and is consistent with our finding that repositioning responses are situation dependent. Natural sensing strategies are complex and likely include reflexive contributions as well as central control. We have identified several aspects of sensing motions that do not appear to be explained solely by low-level feedback loops.
Experiments that independently manipulate the number and relevance (i.e., target/distractor) of anticipated object contacts would help clarify the mechanisms underlying detailed differences in whisker sensing strategies. It is worth contrasting search tasks with sensory discrimination tasks typically designed to study limits on sensory capabilities (Ritt et al. 2008; Wolfe et al. 2008; von Heimendahl et al. 2007; Hutson and Masterton 1986; Guić-Robles et al. 1989; Carvell and Simons 1990). While the sensing strategy may influence the quality of acquired information, and hence task success (Carvell and Simons 1995; Knutsen et al. 2006), such studies likely underplay the closed loop iteration that drives subsequent sensing motions. In contrast, here each contact is expected to be highly salient, and only minimal demands are placed on discrimination capabilities. Since the choice of active sensing behavior is of primary interest, the emphasis is placed on the sequence of acquired pieces of information and the associated iterative decision process. We suggest such designs should become more common for whisker based tasks, as a potentially powerful complement to studies of sensory fidelity, overt and covert attention, and decision making investigated extensively in visual and other systems (Somers et al. 1999; Celikel and Sakmann 2007; Diamond et al. 2008; Carrasco 2011; Shadlen and Kiani 2013; Somers and Sheremata 2013). Comparison of humans and rats in a simple localization task demonstrated that both used an iterative, convergent process to identify object location (Horev et al. 2011), and extensions of this paradigm could benefit from explicitly considering integration of other inputs (i.e., head or body motions) into the establishment of object perception. A distinct literature with overlapping conceptual content is sensor planning in robotics, which has also been informative to and informed by whisker touch paradigms (Mihaylova et al. 2002; Seth et al. 2004; Fend 2005; Solomon and Hartmann 2010; Fox et al. 2011; Schroeder and Hartmann 2012).
We found that a similar contact by the same whisker field on the same trial can produce different repositioning in different situations (first contact with object, vs. first contact on the opposite side of the object). Mice positioned their whiskers to maintain contact on subsequent whisks, but only when doing so was likely to be useful for task completion (Fig. 7). Significantly, we saw adjustments on the next whisk following contact (Grant et al. 2009; Deutsch et al. 2012), in contrast to reports of rats taking three to six contacts to localize an object (Knutsen et al. 2006; Celikel and Sakmann 2007). These differences may depend to some degree on task difficultly. A recent study of head-fixed mice (Deutsch et al. 2012) showed larger amplitude motor changes after initial compared with subsequent contacts with the same object (a pole). The differences we found between contacts with the near and far aperture boundaries reveal coupling to head motion that is not accessible for study in a head fixed experiment. An additional confound in the head-fixed study was that the animal often responded to acoustic noise as the pole had to be moved into the whisker field and not contact alone (Deutsch et al. 2012). Another recent study (Saraf-Sinik et al. 2015) showed that rats are able to modulate sensing motions to maintain stable perception in the face of perturbations (i.e., artificial wind); however, the animals needed only to determine relative pole locations while walking towards reward ports. Since maintaining contact with a located pole would not provide additional information, the strategy they observed of short initial contacts followed by a change in head trajectory toward an appropriate port is consistent with our view that animals select from available active sensing strategies in part based on behavioral goal. We find whisker motions are actively modulated throughout the approach, in a task that relies on tighter head-whisker coupling and in which the contacted object is the goal. The divergence from fixed whisker or simple HTA models reinforces that active repositioning is dependent on whisker contacts. Mice engage in different repositioning behaviors given the situation in which a particular whisker contact occurs, from which we infer that repositioning incorporates contact information with past experience. Future interventional studies that manipulate activity in S1 through stimulation timed to whisker motions (Schroeder et al. 2013) and measure resulting shifts in whisker and head motion could help clarify this relationship.
Similarly, head fixed experiments necessarily remove the contributions of head motion, which is likely important to navigation and sensory orienting, as suggested by evidence from the rodent head direction system (Taube et al. 1996; Taube 1998; Sharp et al. 2001). The ability of rats to perform a bilateral discrimination task that requires intact whiskers, but in which they did not whisk (Krupa et al. 2001), further highlights the importance of considering head and body positioning in addition to whisker motions. A highly interesting emerging technique simulates body motion in head fixed mice using a virtual reality track ball system (Sofroniew et al. 2014). However, mice still are unable to significantly move their head relative to their body and likely adopt other compensatory behaviors. While head fixed and virtual reality experiments have strong utility for intracellular recordings and in vivo imaging (Harvey et al. 2009; Dombeck et al. 2010; Leinweber et al. 2014), we believe such experiments complement but cannot replace studies of unrestrained animals. As mice orient to the reward in this task, it is clear that both head and whisker motions are critical to their strategy.
We chose to train mice with the full whisker field intact rather than trimmed animals. The mice are freely exploring and repositioning based on contacts that are relevant to a behavioral goal. While trimming to a single row of whiskers may aid videographic tracking, we believe that trimming is also likely to induce nonnatural sensing strategies as the mouse seeks to cope with newly limited sensors. Additionally, the extensive plasticity induced by modifying the whisker field (Diamond et al. 1993; Maier et al. 2003; Polley et al. 2004; Margolis et al. 2012; Kätzel and Miesenböck 2014) may also alter whisking strategies.
We note that evaluating contact times from videography can be challenging. We mitigated the difficulty in visualizing whisker tips by using multiple observers and analyses robust to small errors in contact frame identification (e.g., Figs. 2C and 5). Importantly, untracked microvibrissae were often in contact with the aperture during orienting. Microvibrissae likely enhance object recognition (Brecht et al. 1997) and rats may alter head position to bring them into contact with a surface (Hartmann 2001). Future work should clarify what role is played by the microvibrissae.
An important question addressed by the concept of contact maintenance is that previously described repositioning behaviors sometimes predict whisker motions in opposite directions. One key issue is whether the whiskers “anticipate” head placement, as in HTA, or “counteract” head motion, as in the minimal impingement/maximal contact strategy. There are really two hypothesized mechanisms at issue. First, the animal selects the mode of head-whisker coupling (to anticipate or counteract). Second, even in the latter case, contact maintenance/minimal impingement could be employed without direct coupling to head motion; for example, the Prescott group (2011) initially proposed negative feedback from contact force to whisking amplitude as a basis for the behavior. With the term contact maintenance, we are seeking to describe a broader concept of combined head and whisker behavior that volitionally brings the whiskers into repeated contact with objects of interest, possibly driven by a combination of the animal's expectations with actual object contact. For example, the anticipatory aspect of HTA could apply also to alignment of the whiskers with an object of interest, as we have addressed through our dead-reckoning analysis. Prescott has recently proposed a more general framework of viewing whisker motions as a form of covert attention, using behavioral observations as motivation, but implemented in simulation and robotics (Mitchinson and Prescott 2013). We have presented data consistent with this broader view of whisker motions, with a particular emphasis on head whisker coupling and how its changes over time align with task demands. We stress again that both HTA and minimal impingement/maximal contact were revealed and described in contexts without goal-relevant contact, as if they are autonomous behaviors. We suggest that analyzing these behaviors in a true localization task, postcontact, is more informative of the underlying active sensing strategies of the animal and advances our understanding of the nature and extent of sensing choices during active whisker touch.
Our results show that mice can select from a diverse range of sensing behaviors, that the selection is driven at least in part by the behavioral situation, and that the timing of changes is incompatible with a purely reflexive mechanism. Significantly, the responses we observed in different situations often require the animal to act counter to the predictions of a previously observed sensing strategy (HTA or minimal impingement/maximal contact), while in another situation they may obey those predictions, suggesting a level of volitional control over the selection of sensing strategy. Considering both sensor (e.g., whisker) and more general body motions, within a goal-defined context, is likely to be critical to understanding the organization of neural activity underlying perceptual events during active sensing.
GRANTS
J. B. Schroeder was supported by a Quantitative Biology and Physiology Training Program (National Institute of General Medical Sciences Grant 5-T32-GM-8764-10). J. T. Ritt holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.B.S. and J.T.R. conception and design of research; J.B.S. performed experiments; J.B.S. and J.T.R. analyzed data; J.B.S. and J.T.R. interpreted results of experiments; J.B.S. and J.T.R. prepared figures; J.B.S. and J.T.R. drafted manuscript; J.B.S. and J.T.R. edited and revised manuscript; J.B.S. and J.T.R. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gregory Telian, David Freedman, and Benjamin Perrone for assistance with the design and implementation of the behavioral arena and Gregory Telian, Vincent Mariano, Allicia Imada, Michael Palmiere, and Paul Zhang for assistance with training animals.
REFERENCES
- Ahissar E, Knutsen PM. Object localization with whiskers. Biol Cybern 98: 449–458, 2008. [DOI] [PubMed] [Google Scholar]
- Arkley K, Grant RA, Mitchinson B, Prescott TJ. Strategy change in vibrissal active sensing during rat locomotion. Curr Biol 24: 1507–1512, 2014. [DOI] [PubMed] [Google Scholar]
- Bermejo R, Friedman W, Zeigler HP. Topography of whisking II: interaction of whisker and pad. Somatosens Mot Res 22: 213–220, 2005. [DOI] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res 84: 81–97, 1997. [DOI] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: the past 25 years. Vision Res 51: 1484–1525, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Task- and subject-related differences in sensorimotor behavior during active touch. Somatosens Mot Res 12: 1–9, 1995. [DOI] [PubMed] [Google Scholar]
- Carvell G, Simons D. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci 10: 2638–2648, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celikel T, Sakmann B. Sensory integration across space and in time for decision making in the somatosensory system of rodents. Proc Natl Acad Sci USA 104: 1395–1400, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Carta S, Soldado-Magraner J, Schneider BL, Helmchen F. Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex. Nature 499: 336–340, 2013. [DOI] [PubMed] [Google Scholar]
- Clack NG, O'Connor DH, Huber D, Petreanu L, Hires A, Peron S, Svoboda K, Myers EW. Automated tracking of whiskers in videos of head fixed rodents. PLoS Comput Biol 8: e1002591, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 74: 829–836, 1979. [Google Scholar]
- Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci 9: 608–610, 2006. [DOI] [PubMed] [Google Scholar]
- Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat Neurosci 12: 492–501, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D, Pietr M, Knutsen PM, Ahissar E, Schneidman E. Fast feedback in active sensing: touch-induced changes to whisker-object interaction. PLoS One 7: e44272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME. Texture sensation through the fingertips and the whiskers. Curr Opin Neurobiol 20: 319–327, 2010. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci USA 90: 2082–2086, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. “Where” and “what” in the whisker sensorimotor system. Nat Rev Neurosci 9: 601–612, 2008. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci 13: 1433–1440, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Brecht M, Helmchen F, Petersen CC, Poulet JF, Staiger JF, Luhmann HJ, Schwarz C. Barrel cortex function. Prog Neurobiol 103: 3–27, 2013. [DOI] [PubMed] [Google Scholar]
- Fend M. Whisker-based texture discrimination on a mobile robot. In: Advances in Artificial Life, edited by Capcarrère MS, Freitas AA, Bentley PJ, Johnson CG, Timmis J. Berlin, Germany: Springer, Lecture Notes in Computer Science, 2005, p. 302–311. [Google Scholar]
- Fox CW, Evans MH, Lepora NF, Pearson M, Ham A, Prescott TJ. CrunchBot: a mobile whiskered robot platform. In: Towards Autonomous Robotic Systems, edited by Groß R, Alboul L, Melhuish C, Witkowski M, Prescott TJ, Penders J. Berlin, Germany: Springer, Lecture Notes in Computer Science, 2011, p. 102–113. [Google Scholar]
- Gao P, Hattox AM, Jones LM, Keller A, Zeigler HP. Whisker motor cortex ablation and whisker movement patterns. Somatosens Mot Res 20: 191–198, 2003. [DOI] [PubMed] [Google Scholar]
- Godde B, Diamond ME, Braun C. Feeling for space or for time: task-dependent modulation of the cortical representation of identical vibrotactile stimuli. Neurosci Lett 480: 143–147, 2010. [DOI] [PubMed] [Google Scholar]
- Grant RA, Mitchinson B, Fox CW, Prescott TJ. Active touch sensing in the rat: anticipatory and regulatory control of whisker movements during surface exploration. J Neurophysiol 101: 862–874, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RA, Prescott TJ. The role of orienting in vibrissal touch sensing. Front Behav Neurosci 6: 39, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guić-Robles E, Valdivieso C, Guajardo G. Rats can learn a roughness discrimination using only their vibrissal system. Behav Brain Res 31: 285–289, 1989. [DOI] [PubMed] [Google Scholar]
- Hartmann MJ. Active sensing capabilities of the rat whisker system. Auton Robots 11: 249–254, 2001. [Google Scholar]
- Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461: 941–946, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Bermejo R, Zeigler HP. Discriminative whisking in the head-fixed rat: optoelectronic monitoring during tactile detection and discrimination tasks. Somatosens Mot Res 18: 211–222, 2001. [DOI] [PubMed] [Google Scholar]
- Hill DN, Curtis JC, Moore JD, Kleinfeld D. Primary motor cortex reports efferent control of vibrissa motion on multiple timescales. Neuron 72: 344–356, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horev G, Saig A, Knutsen PM, Pietr M, Yu C, Ahissar E. Motor-sensory convergence in object localization: a comparative study in rats and humans. Philos Trans R Soc B Biol Sci 366: 3070–3076, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson KA, Masterton RB. The sensory contribution of a single vibrissa's cortical barrel. J Neurophysiol 56: 1196–1223, 1986. [DOI] [PubMed] [Google Scholar]
- Jenks RA, Vaziri A, Boloori AR, Stanley GB. Self-motion and the shaping of sensory signals. J Neurophysiol 103: 2195–2207, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kätzel D, Miesenböck G. Experience-dependent rewiring of specific inhibitory connections in adult neocortex. PLoS Biol 12: e1001798, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Berg RW, O'Connor SM. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens Mot Res 16: 69–88, 1999. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Deschênes M. Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron 72: 455–468, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen PM, Pietr M, Ahissar E. Haptic object localization in the vibrissal system: behavior and performance. J Neurosci 26: 8451–8464, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Matell MS, Brisben AJ, Oliveira LM, Nicolelis MA. Behavioral properties of the trigeminal somatosensory system in rats performing whisker-dependent tactile discriminations. J Neurosci 21: 5752–5763, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinweber M, Zmarz P, Buchmann P, Argast P, Hübener M, Bonhoeffer T, Keller GB. Two-photon calcium imaging in mice navigating a virtual reality environment. J Vis Exp: e50885, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier DL, Grieb GM, Stelzner DJ, McCasland JS. Large-scale plasticity in barrel cortex following repeated whisker trimming in young adult hamsters. Exp Neurol 184: 737–745, 2003. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Lütcke H, Schulz K, Haiss F, Weber B, Kügler S, Hasan MT, Helmchen F. Reorganization of cortical population activity imaged throughout long-term sensory deprivation. Nat Neurosci 15: 1539–1546, 2012. [DOI] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CC. Motor control by sensory cortex. Science 330: 1240–1243, 2010. [DOI] [PubMed] [Google Scholar]
- Mehta SB, Whitmer D, Figueroa R, Williams BA, Kleinfeld D. Active spatial perception in the vibrissa scanning sensorimotor system. PLoS Biol 5: e15, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova L, Lefebvre T, Bruyninckx H, Gadeyne K, Schutter JD. Active sensing for robotics–a survey. In: Proceedings 5th of International Conference On Numerical Methods and Applications. Berlin, Germany: Springer, Lecture Notes in Computer Science, 2002, p. 316–324. [Google Scholar]
- Mitchinson B, Grant RA, Arkley K, Rankov V, Perkon I, Prescott TJ. Active vibrissal sensing in rodents and marsupials. Philos Trans R Soc B Biol Sci 366: 3037–3048, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchinson B, Martin CJ, Grant RA, Prescott TJ. Feedback control in active sensing: rat exploratory whisking is modulated by environmental contact. Proc R Soc B Biol Sci 274: 1035–1041, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchinson B, Prescott TJ. Whisker movements reveal spatial attention: a unified computational model of active sensing control in the rat. PLoS Comput Biol 9: e1003236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QT, Kleinfeld D. Positive feedback in a brainstem tactile sensorimotor loop. Neuron 45: 447–457, 2005. [DOI] [PubMed] [Google Scholar]
- O'Connor DH, Clack NG, Huber D, Komiyama T, Myers EW, Svoboda K. Vibrissa-based object localization in head-fixed mice. J Neurosci 30: 1947–1967, 2010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Hires SA, Guo ZV, Li N, Yu J, Sun QQ, Huber D, Svoboda K. Neural coding during active somatosensation revealed using illusory touch. Nat Neurosci 16: 958–965, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 67: 1048–1061, 2010b. [DOI] [PubMed] [Google Scholar]
- Pammer L, O'Connor DH, Hires SA, Clack NG, Huber D, Myers EW, Svoboda K. The mechanical variables underlying object localization along the axis of the whisker. J Neurosci 33: 6726–6741, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkon I, Košir A, Itskov PM, Tasič J, Diamond ME. Unsupervised quantification of whisking and head movement in freely moving rodents. J Neurophysiol 105: 1950–1962, 2011. [DOI] [PubMed] [Google Scholar]
- Petersen CC. The Functional organization of the barrel cortex. Neuron 56: 339–355, 2007. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Gutnisky DA, Huber D, Xu N, O'Connor DH, Tian L, Looger L, Svoboda K. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature 489: 299–303, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Kvasnák E, Frostig RD. Naturalistic experience transforms sensory maps in the adult cortex of caged animals. Nature 429: 67–71, 2004. [DOI] [PubMed] [Google Scholar]
- Prescott TJ, Diamond ME, Wing AM. Active touch sensing. Philos Trans R Soc B Biol Sci 366: 2989–2995, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritt JT, Andermann ML, Moore CI. Embodied information processing: vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron 57: 599–613, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev RN. Unilateral vibrissa contact: changes in amplitude but not timing of rhythmic whisking. Somatosens Mot Res 20: 163, 2003. [DOI] [PubMed] [Google Scholar]
- Saraf-Sinik I, Assa E, Ahissar E. Motion makes sense: an adaptive motor-sensory strategy underlies the perception of object location in rats. J Neurosci 35: 8777–8789, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CL, Hartmann MJ. Sensory prediction on a whiskered robot: a tactile analogy to “optical flow”. Front Neurorobotics 6: 9, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JB, Mariano VJ, Telian GI, Ritt JT. Stimulation of somatosensory cortex locked to whisker motions in a mouse model of active sensing. In: 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER). San Diego, CA: IEEE/EMBS, 2013, p. 637–640. [Google Scholar]
- Schroeder JB, Ritt JT. Extraction of intended palpation times from facial EMGs in a mouse model of active sensing. In: 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). Osaka, Japan: IEEE, 2013, p. 2016–2019. [DOI] [PubMed] [Google Scholar]
- Sellien H, Eshenroder DS, Ebner FF. Comparison of bilateral whisker movement in freely exploring and head-fixed adult rats. Somatosens Mot Res 22: 97–114, 2005. [DOI] [PubMed] [Google Scholar]
- Seth AK, McKinstry JL, Edelman GM, Krichmar JL. Texture discrimination by an autonomous mobile brain-based device with whiskers. In: 2004 IEEE International Conference on Robotics and Automation Proceedings. Barcelona, Spain: IEEE, 2005, vol. 5, p. 4925–4930. [Google Scholar]
- Shadlen MN, Kiani R. Decision making as a window on cognition. Neuron 80: 791–806, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PE, Blair HT, Cho J. The anatomical and computational basis of the rat head-direction cell signal. Trends Neurosci 24: 289–294, 2001. [DOI] [PubMed] [Google Scholar]
- Shuler MG, Krupa DJ, Nicolelis MA. Integration of bilateral whisker stimuli in rats: role of the whisker barrel cortices. Cereb Cortex 12: 86–97, 2002. [DOI] [PubMed] [Google Scholar]
- Smith JB, Alloway KD. Rat whisker motor cortex is subdivided into sensory-input and motor-output areas. Front Neural Circuits 7: 4, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew NJ, Cohen JD, Lee AK, Svoboda K. Natural whisker-guided behavior by head-fixed mice in tactile virtual reality. J Neurosci 34: 9537–9550, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JH, Hartmann MJ. Extracting object contours with the sweep of a robotic whisker using torque information. Int J Rob Res 29: 1233–1245, 2010. [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA 96: 1663–1668, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DC, Sheremata SL. Attention maps in the brain. Wiley Interdiscip Rev Cogn Sci 4: 327–340, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüttgen MC, Schwarz C. Integration of vibrotactile signals for whisker-related perception in rats is governed by short time constants: comparison of neurometric and psychometric detection performance. J Neurosci 30: 2060–2069, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol 55: 225–256, 1998. [DOI] [PubMed] [Google Scholar]
- Taube JS, Goodridge JP, Golob EJ, Dudchenko PA, Stackman RW. Processing the head direction cell signal: a review and commentary. Brain Res Bull 40: 477–484; discussion 484–486, 1996. [DOI] [PubMed] [Google Scholar]
- Towal RB, Hartmann MJ. Right-left asymmetries in the whisking behavior of rats anticipate head movements. J Neurosci 26: 8838–8846, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman S, Carmena JM. Active sensing of target location encoded by cortical microstimulation. IEEE Trans Neural Syst Rehabil Eng 19: 317–324, 2011. [DOI] [PubMed] [Google Scholar]
- Voigts J, Herman DH, Celikel T. Tactile object localization by anticipatory whisker motion. J Neurophysiol 113: 620–632, 2015. [DOI] [PubMed] [Google Scholar]
- von Heimendahl M, Itskov PM, Arabzadeh E, Diamond ME. Neuronal activity in rat barrel cortex underlying texture discrimination. PLoS Biol 5: e305, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J, Hill DN, Pahlavan S, Drew PJ, Kleinfeld D, Feldman DE. Texture coding in the rat whisker system: slip-stick versus differential resonance. PLoS Biol 6: e215, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.