Abstract
Exposure to loud sounds damages the auditory periphery and induces maladaptive changes in central parts of the auditory system. Diminished peripheral afferentation and altered inhibition influence the processing of sounds in the auditory cortex. It is unclear, however, which types of inhibitory interneurons are affected by acoustic trauma. Here we used single-unit electrophysiological recording and two-photon calcium imaging in anesthetized mice to evaluate the effects of acute acoustic trauma (125 dB SPL, white noise, 5 min) on the response properties of neurons in the core auditory cortex. Electrophysiological measurements suggested the selective impact of acoustic trauma on inhibitory interneurons in the auditory cortex. To further investigate which interneuronal types were affected, we used two-photon calcium imaging to record the activity of neurons in cortical layers 2/3 and 4, specifically focusing on parvalbumin-positive (PV+) and somatostatin-positive (SST+) interneurons. Spontaneous and pure-tone-evoked firing rates of SST+ interneurons increased in layer 4 immediately after acoustic trauma and remained almost unchanged in layer 2/3. Furthermore, PV+ interneurons with high best frequencies increased their evoked-to-spontaneous firing rate ratios only in layer 2/3 and did not change in layer 4. Finally, acoustic trauma unmasked low-frequency excitatory inputs only in layer 2/3. Our results demonstrate layer-specific changes in the activity of auditory cortical inhibitory interneurons within minutes after acoustic trauma.
Keywords: calcium imaging, interneurons, inhibition, plasticity, broadband noise
acute acoustic trauma (AAT) results in complex alterations of central auditory processing that do not simply reflect the traumatically diminished peripheral input. Central alterations may contribute to the development of tinnitus (Roberts et al. 2010), a clinical condition with unclear pathogenesis and no available effective treatments.
In the primary auditory cortex (AC), exposure to loud sound is immediately followed by increased stimulus-driven activity, synchrony, and bursting, accompanied by complex changes of the receptive fields (RFs) usually associated with downward shifts of characteristic frequencies (Norena et al. 2003; Syka et al. 1994). RF modifications can be explained by unmasking latent inputs that are normally suppressed by feedforward lateral inhibition (Eggermont and Roberts 2004). Acoustic trauma can, however, increase and decrease inhibition in distinct parts of the RFs, which cannot be explained by unmasking alone (Scholl and Wehr 2008). Changes in inhibition also follow partial deafferentation caused by exposure to ototoxic drugs or by aging (Llano et al. 2012). Both of these conditions can lead to the onset of tinnitus, which suggests that maladaptive inhibitory plasticity might be a shared mechanism in the development of the condition (Wang et al. 2011). A homeostatic increase of gain compensating for the diminished input has been proposed as a common purpose of such plasticity (Norena 2011; Yang et al. 2011).
Inhibitory microcircuits in the auditory cortex are involved in shaping both temporal and spectral selectivity and intensity tuning (Zhang et al. 2011). Recent studies have shown specific computational functions and target specificity for two major inhibitory interneuron subclasses in the brain, parvalbumin (PV)- and somatostatin (SST)-positive neurons (Kepecs and Fishell 2014). PV cells participate in gain modulation across sensory systems (Atallah et al. 2012; Hamilton et al. 2013; Kato et al. 2013) and influence feature selectivity and perceptual discrimination in the visual cortex (Lee et al. 2012). SST+ cells suppress the activity of PV+ cells (Cottam et al. 2013), and their input synapses show unique short-term facilitation (Beierlein et al. 2003). In the auditory cortex, PV interneurons seem to be involved mainly in providing broadly tuned feedforward inhibition and SST neurons probably mediate more narrowly tuned feedback inhibition (Li et al. 2014). Because of these functional differences it can be expected that PV+ and SST+ neurons will be differently affected by the AAT. However, the extent to which the different classes of cortical interneurons are affected by acoustic trauma is unknown.
Various types of cortical neurons are organized into specific circuits positioned across the cortical layers. Cortical interneurons likely serve layer-dependent distinct functions (Li et al. 2014). Their input/output patterns often differ substantially (Atencio and Schreiner 2010; Liu et al. 2007) as well as the amount of direct thalamocortical afferentation they receive (Beierlein et al. 2003). PV+ neurons receive direct thalamic input (Moore and Wehr 2013); however, at least in the AC, there is currently no evidence for the direct thalamic input to SST+ neurons. Both classes of interneurons are innervated by axons of cortical pyramidal cells (Harris and Shepherd 2015). PV+ and SST+ neurons synapse mainly on excitatory cells, but with different subcellular localization, onto soma and onto dendrites, respectively. PV+ neurons also inhibit other PV+ neurons (Pfeffer et al. 2013), and SST+ neurons synapse on PV+ cells in layer (L)2/3 of the visual cortex (Cottam et al. 2013; Pfeffer et al. 2013). PV+ and SST+ neurons constitute a large portion of all GABAergic interneurons in the AC. In the rat AC PV+ and SST+ interneurons constitute 30 ± 4% and 25 ± 2%, respectively, of interneurons in L2/3 and 66 ± 5% and 27 ± 2% in L4 (Ouellet and de Villers-Sidani 2014). The functional architecture of the AC thus implies different patterns of noise-induced changes across cortical layers.
Here we evaluated the impact of AAT on the activity of different neuronal populations across the upper layers of the AC. Using single-unit recordings and calcium imaging in vivo we were able to determine the subtype- and layer-specific effects of AAT on the sensory processing of sounds in the AC.
MATERIALS AND METHODS
Experimental Animals
For electrophysiological experiments and measurements of auditory brain stem responses (ABRs) we used C57BL/6J mice (n = 25, Jackson Laboratory, stock no. 000664). To selectively label interneurons for imaging experiments in vivo we crossed tdTomato reporter mice (Jackson Laboratory, stock no. 007908) with mice expressing cre-recombinase under specific promoters characteristic for given classes of interneurons. PV-Cre/tdT mice (n = 25) with selectively labeled PV+ interneurons were generated by crossing Pvalb-IRES-Cre mice (Jackson Laboratory, stock no. 008069) with tdTomato mice. Analogously, SST-Cre/tdT mice (n = 30) with selectively labeled SST+ interneurons were generated by crossing Sst-IRES-Cre mice (Jackson Laboratory, stock no. 013044) with tdTomato mice. We used young adult (6–12 wk, 25–35 g) male and female mice: 25 PV-Cre/tdT mice and 30 SST-Cre/tdT mice in total.
Surgical Procedures
All experimental procedures were approved by the Ethics Committee of the Institute of Experimental Medicine, Academy of Sciences of the Czech Republic, and followed the guidelines of the Declaration of Helsinki.
Anesthesia was induced with 2% isoflurane with oxygen, and animals were then injected subcutaneously with a mixture of ketamine (35 mg/kg) and xylazine (6 mg/kg). The animal was placed onto a heated pad (38°C), and its eyes were covered with eye-protective ointment. To facilitate breathing and prevent endotracheal secretion in the animal, atropine (1 mg/kg) was injected subcutaneously. Throughout the entire experiment, pure oxygen was delivered via a custom-made gas mask (Smith and Bolon 2006) to maintain high oxygen saturation (>95%), which was continuously monitored with a pulse oximeter (mouseSTAT, Kent Scientific). To prevent brain swelling, dexamethasone (2.5 mg/kg) was injected subcutaneously.
Lidocaine (2%, 40 μl) was injected subcutaneously over the parietal area, skin was removed, and a custom-made head holder was attached to the skull with cyanoacrylate glue (UHU Super Glue). For electrophysiological experiments, the temporal muscle was removed and a craniotomy (∼4-mm diameter) and durotomy were performed over the right AC. For imaging experiments, after removal of the temporal muscle a plastic protective well was attached to the right temporal bone with cyanoacrylate glue and a small craniotomy (∼1.5- to 2-mm diameter) was performed over the right AC.
Auditory Brain Stem Response and Distortion Product Otoacoustic Emission Measurement
ABR and distortion product otoacoustic emission recordings.
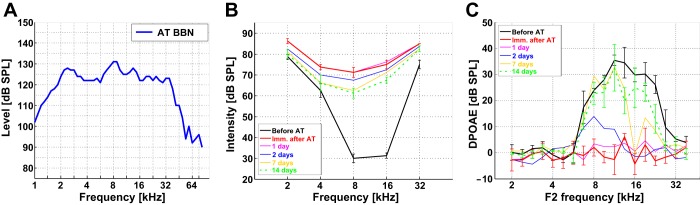
Sound-evoked ABRs were recorded before AAT, immediately after AAT, and 24 h, 2 days, 7 days, and 14 days after AAT in an anechoic chamber. The animal was placed onto a heated pad (38°C) in the middle of the chamber, and its head was fixed. Three stainless steel needlelike recording electrodes were placed subcutaneously. The positive recording electrode was placed over the interparietal and occipital bones (vertex), and the negative and grounding electrodes were placed at the neck muscles. A TDT (Tucker Davis Technologies) System III setup was used for acoustic stimulation and signal acquisition. The data were amplified and digitized with a Medusa RA16PA preamplifier and RA4LI headstage (A/D sample rate 25 kHz). The digitized signal was sent to a Pentusa RX5-2 base station via optic fibers and processed further in a computer. Free-field tone stimuli were generated in the TDT system, amplified with a custom-made amplifier, and presented from a two-way loudspeaker system (Jamo woofer and SEAS T25CF 002 tweeter with smooth responses; ±5 dB). Speakers were placed 70 cm in front of the animal's head. AAT was induced by loud noise generated by a custom-made broadband noise (BBN) source, amplified with a Transiwatt 140P amplifier, and presented with a piezoelectric speaker (Motorola KSN1025A, ±5 dB in the frequency range 2–33 kHz; Fig. 1A), which was positioned above the animal's head. The acoustic system was calibrated with a Brüel & Kjaer (B&K) 4939 microphone, a ZC0020 preamplifier, and a B&K 2231 Sound Level Meter.
Fig. 1.
Assessment of peripheral auditory changes induced by acute acoustic trauma (AAT). A: frequency spectrum of the traumatic broadband noise. B and C: auditory brain stem response (ABR) thresholds (B) and distortion product otoacoustic emission (DPOAE; C) measured before the AAT and immediately (0 h), 24 h, 2 days, 7 days, and 14 days after the AAT (n = 4 mice).
In distortion product otoacoustic emission (DPOAE) measurement, stimuli were presented to the ear canal with two custom-made piezoelectric stimulators connected to the probe with a low-noise microphone system (Etymotic probe ER-10B+, Etymotic Research). The signal from the microphone was analyzed with TDT System III (RP2 processor, sampling rate 100 kHz). DPOAEs were successively recorded in both ears of each animal.
Acoustic stimulation.
Baseline ABR measurement was followed by a 5-min period of intense BBN stimulation (125 dB SPL), followed by successive ABR measurements. During ABR measurements, the animals were stimulated with tone bursts (frequency 2–40 kHz, duration 5 ms with 2 ms rise-fall times) of decreasing intensity (10-dB decrements) from 100 dB SPL to 20 dB SPL. For each frequency-intensity combination, 300 tone stimuli were delivered at a 10-Hz repetition rate.
Cubic (2F1-F2) DPOAEs over the F2 frequency range from 4 to 38 kHz were recorded (ratio F2/F1 = 1.2, primary tone levels of 60 dB and 55 dB for F1 and F2, respectively).
Data analysis.
The signal was band-pass filtered (300-3,000 Hz) and averaged with TDT BioSig software. The hearing threshold at each frequency was determined as the lowest intensity at which the ABR signal was clearly distinguishable from noise. In some mice we did not observe brain stem responses after AAT at the highest frequency (40 kHz). Even at 100 dB SPL there was no signal that could be distinguished from noise. Therefore, we did not include the values for 40 kHz in further analysis.
Single-Unit Electrophysiology
Extracellular single-unit recordings.
Electrophysiological recordings were conducted in an anechoic chamber. Sound-evoked single-unit responses were recorded in the right AC of anesthetized mice. The location of the AC core was estimated from the position of the best frequency (BF) gradient (Stiebler et al. 1997); response latencies and responsiveness to tonal stimuli were obtained from coarse mapping performed at the beginning of a recording session. Neural activity was recorded from the middle layers (200–400 μm below pia) with 4 × 1 iridium tetrodes (150-μm intertetrode distances, 11-μm2 contact area, A4x1-tet-3mm-150-121, NeuroNexus Technologies).
The signal was amplified and digitized (sampling rate 25 kHz) by a Medusa RA16PA preamplifier and band-pass filtered (300-6,000 Hz) with Pentusa RX5-2 (TDT). Spikes from the raw recorded data traces were detected with thresholding (>4 SD of signal mean) and clustered with KlustaKwik (Harris et al. 2001) based on five spike waveform parameters (energy, peak, time, valley, and first principal component). Only those clusters that remained well separated throughout the entire experiment (BBN pulse stimulation, followed by acoustic trauma period and the second BBN stimulation) were included in the analysis. Single units were further checked manually with MClust (MClust-4.0, A. D. Redish; http://redishlab.neuroscience.umn.edu/MClust/MClust.html), and only clusters with an L ratio <0.5 and isolation distance >12 (Harris et al. 2001; Schmitzer-Torbert et al. 2005) were included in the analysis.
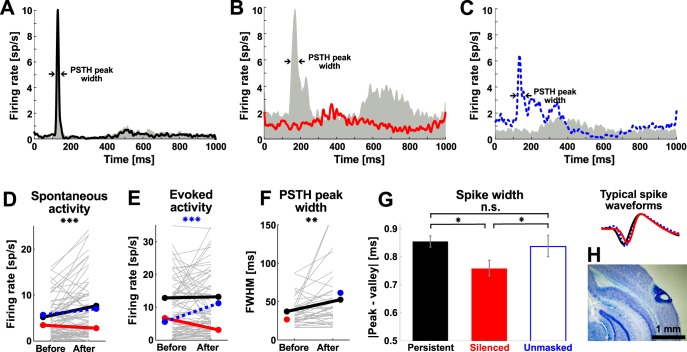
To confirm the recording depth estimates, we made small electrolytic lesions in a subset of mice (n = 4) with a custom-made device (current pulse 0.6 mA for 10 s). Mice were then transcardially perfused with 20 ml of saline followed by 40 ml of 4% paraformaldehyde, the brains were explanted, postfixed in 4% paraformaldehyde for 2–3 days, sliced with a cryostat into 40-μm thick sections, and stained with Nissl stain, and the recording depth was then evaluated by light microscopy (Fig. 2H).
Fig. 2.
Electrophysiological characterization of neuronal activity before and after the AAT. Example peristimulus time histograms (PSTHs) of neurons displaying persistent responsiveness (A, Persistent), cessation of significant responsiveness (B, Silenced), and emergence of significant responsiveness (C, Unmasked) before (gray areas) and after (colored lines) 5-min loud noise exposure. Arrows indicate where the full width at half-maximum (FWHM) was measured. D–F: changes in spontaneous activity (D), evoked activity (E), and response jitter, calculated as the FWHM (F), were evaluated for the 3 different groups of neurons. G: Silenced neurons had significantly narrower spike waveforms compared with other subgroups of neurons. Right: typical waveforms aligned according to their maxima. H: a coronal brain slice in Nissl staining (described in materials and methods) showing recording depths ∼200–400 μm below pia. *P < 0.05, **P < 0.01, ***P < 0.001. ns, Nonsignificant.
Acoustic stimulation and noise exposure.
The stimuli consisted of 100-ms BBN bursts (100 pulses, 65 dB SPL, 1,500-ms interstimulus interval). The AAT was induced with white noise (5-min duration, 125 dB SPL) delivered via a calibrated piezoelectric speaker (Motorola KSN1025A) positioned next to the animal's head. The acoustic system was calibrated with a B&K 4939 microphone, a ZC0020 preamplifier, and a B&K 2231 Sound Level Meter. Data recordings before and after AAT took ∼5 min. The after-AAT recordings started within 1 min after the AAT.
Data analysis.
Clustered data were analyzed with custom scripts written in MATLAB. Spontaneous activity was calculated as the mean firing rate [spikes/second (sp/s)] in a 100-ms window directly preceding stimulus presentation, and evoked activity was calculated as the mean firing rate in a 115-to 200-ms time window following stimulus presentation. The statistical significance of sound-evoked responses was evaluated with the Wilcoxon signed-rank test (P < 0.05). For each neuron we tested, on a trial-by-trial basis, whether the evoked activity increased significantly compared with the corresponding spontaneous activity. On the basis of significance of their responsiveness, neurons were divided into four groups, those that were significantly responsive both before and after the AAT (Persistent), those that were significantly responsive only before the AAT (Silenced), those that were significantly responsive only after the AAT (Unmasked), and neurons unresponsive under both conditions (unresponsive neurons were not included in further analysis). Response jitter was calculated as full width at half-maximum (FWHM) of a sound-evoked peristimulus time histogram (PSTH) computed in a 115- to 200-ms time window after stimulus onset. Spike width was defined as the time difference between the spike peak (maximum waveform deflection) and spike valley (minimum deflection following the peak) of the mean spike waveform for each neuron.
Two-Photon Calcium Imaging
Experimental layout.
Calcium imaging was performed with an Ultima IV two-photon laser scanning microscope (Prairie Technologies), controlled by PrairieView software, equipped with a Chameleon Ultra II laser (Coherent). Imaging experiments were performed with a ×20 objective (XLUMPLFLN 20XW, Olympus). The laser wavelength was set to 950 nm for all measurements, and the pulse precompensation was set accordingly. The laser pumping unit was positioned inside a separate custom-made soundproof box that minimized any ambient noise.
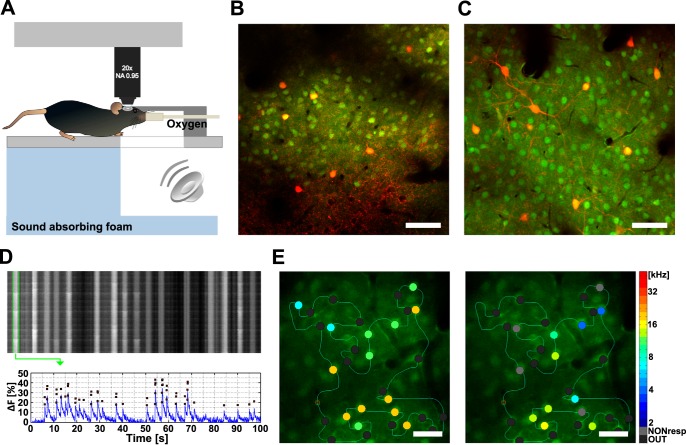
The mouse was placed onto a heated pad under the microscope, and its head was fixed to a holder. The calcium-sensitive fluorescent dye Oregon Green 488 BAPTA-1 (OGB-1 AM; Invitrogen; 50 μg) was dissolved in 4 μl of 20% Pluronic in DMSO (Invitrogen) and further diluted with artificial cerebrospinal fluid (in mM: 119 NaCl, 26.2 NaHCO3, 2.5 KCl, 1 NaH2PO4, 1.3 MgCl2, 10 glucose) containing 1 μM Alexa Fluor 594 (Invitrogen). The dye mixture was pressure-injected (Pneumatic PicoPump, WPI) into the cortex (MCBL method) to a depth of 300–500 μm with a pulled glass micropipette (diameter ∼5 μm). The craniotomy was then covered with 1.5% agarose (Sigma-Aldrich) and coverslipped. The first calcium imaging session began after 30 min. To find the core area of the AC, we quickly evaluated the responsiveness, BFs, shapes of RFs, and possible frequency gradient at several well-loaded locations (Issa et al. 2014). If the physiological parameters were consistent with the parameters of the AC core, we exposed the animal to acoustic trauma.
Calcium imaging data from L2/3 were obtained 130–220 μm below pia, and data from L4 were obtained 310–370 μm below pia. Neurons were scanned in line-scan mode (sampling frequency 70–115 Hz; mean 91 Hz); free-line closed-loop scan trajectory was determined by the experimenter to cover many neurons in the field of view (FOV; 300 × 300 μm, ∼0.29 μm/pixel). Dwell times ranged between 1.5 and 2.5 μm per pixel. The average laser power at the back aperture of the objective was maintained as low as possible (always <100 mW). Astrocytes were identified on the basis of their morphology and omitted from scanning. Before each measurement, we made a high-resolution dichromatic full-frame image to identify labeled interneurons.
Acoustic stimulation and noise exposure.
Acoustic stimuli waveforms were created in MATLAB, generated by an E-MU 1212M sound card, and further amplified (Transiwatt 140P). Free-field stimuli were delivered from a TDT MF1 speaker positioned ∼15 cm from and pointing at the contralateral (left) ear and passed through a 7-cm-wide opening in the heated pad. The acoustic stimuli consisted of 100-ms BBN bursts (114 repetitions, 65 dB SPL, 5-ms linear ramps, 1,000-ms interstimulus interval) and pure tones (114 stimuli, 19 frequencies logarithmically spaced between 2 and 45 kHz presented at 6 intensity levels evenly spaced between 20 and 80 dB SPL, 5-ms linear ramps, 1,000-ms interstimulus interval) presented in a random order. The stimulation protocol consisted of, in order, five repetitions of the tonal set, one repetition of the BBN bursts, 5 min of loud noise stimulation (125 dB SPL), one repetition of the BBN bursts, and five repetitions of the tonal set. The system was calibrated (B&K 2231) throughout the entire range of frequencies, and the frequency spectrum was smoothed (±5 dB) with a digital correction. A calibrated piezoelectric speaker (Motorola KSN1025A) positioned near the animal's head was used to deliver the loud noise (125 dB SPL, ±5 dB across 2–33 kHz). Data recordings before and after the AAT took ∼15 min. The after-AAT recordings started immediately after the AAT. In three (of 20) mice we measured two FOVs before and after the AAT. In this case the imaging of the second FOV started ∼20 min after the AAT.
Data analysis.
Mice in which the loaded area of the cortex did not belong to the core AC and those with signs of cortical damage, such as unusually high (>3 sp/s) and markedly synchronized spontaneous activity of loaded neurons (usually accompanied by brain swelling) were excluded from further analysis. The selection of the animals included in the analysis was performed blindly with respect to the changes in neuronal response parameters that were evaluated in the study.
In PV-Cre/tdT mice calcium signals were analyzed from six L2/3 FOVs and six L4 FOVs (n = 11 mice). In SST-Cre/tdT mice calcium signals were analyzed from five L2/3 FOVs and six L4 FOVs (n = 9 mice). In one PV-Cre/tdT mouse and two SST-Cre/tdT mice we sufficiently stained both the granular and supragranular layers and were able to record data from both L2/3 and L4.
The raw imaging data were processed with a Two-Photon Processor (Tomek et al. 2013) and custom-written MATLAB routines.
To convert calcium traces to spike event data for further analysis, raw calcium traces were first band-pass filtered with a zero-phase digital FIR filter (filtfilt, MATLAB), using a Hamming window of length 11 and cutoff frequency set to 10% of sampling frequency. After the denoising filtration, the baseline was subtracted from the calcium trace and the calcium trace was transformed into a percentage representation. To extract spike-related calcium changes, we used the “peeling algorithm” except for the final onset fitting algorithm. High-threshold crossings determined the positions of action potential-related spikes. Using the terminology from Grewe et al. (2010), we used the following parameters: high threshold = 6%, low threshold = −1.5%, minimum event length = 70 ms, window before the current position = 3 s, integral ratio = 0.5, “back jump” = 100 ms, future fit range = 1.5. Amplitude and decay of the single action potential biexponential model were set to + 7.7%, 56 ms and 3.1%, 777 ms for the fast and slow components, respectively. The onset time constant was set to 8 ms.
A neuron was evaluated as responsive to a particular stimulation if there was a significant (Wilcoxon signed-rank test) increase of activity after the stimulus presentation (mean activity in 100-ms window preceding stimulus onset vs. mean activity during the stimulus 0–100 ms). Neurons were included in the analysis only if they were identified by the detection algorithm before and after the AAT in all cycles of stimulation, i.e., one presentation of the set of BBN stimuli and five presentations of the set of tonal stimuli. Neurons that were photobleached or drifted away from the scan line or from the focal plane were not included in the analysis.
The BF was defined as the frequency evoking the maximum mean spike count across all sound intensities. For each neuron, a raw frequency response area (FRA) was smoothed with a 2 × 3 Gaussian kernel (σ = 1). To compare changes of frequency tuning we summed the smoothed FRAs over intensity, normalized each pair of tuning curves to their corresponding pre-AAT peak, and aligned the normalized tuning curves of individual neurons according to their pre-AAT BFs. For each neuron we calculated several activity parameters: spontaneous firing rate (SFR) as the average firing rate in the second half (500-1,000 ms) of the interstimulus interval; noise-evoked firing rate (N-EFR) and pure-tone-evoked firing rate (PT-EFR), both as a mean response at the beginning (1–200 ms) of the interstimulus interval; noise-evoked to spontaneous ratio (N-ESR); and pure-tone-evoked to spontaneous ratio (PT-ESR). Evoked-to-spontaneous ratios were calculated as particular EFR divided by SFR.
Results of statistical tests were corrected for multiple comparisons with Bonferroni correction where necessary. Error bars in figures represent SE unless stated otherwise.
RESULTS
Distinct Effect of Acoustic Trauma on Peripheral Hearing Function
Acoustic trauma affects the entire auditory system. To estimate the extent to which trauma affected the afferentation into the central auditory system we first evaluated the effects of AAT (Fig. 1A) on the auditory periphery. We measured the thresholds of ABR and DPOAE before AAT—a 5-min exposure to 125 dB SPL BBN (Fig. 1, B and C)—and after 0 h, 24 h, 2 days, 7 days, and 14 days in four mice. Hearing thresholds were substantially elevated after AAT, especially at frequencies with the lowest before-AAT thresholds (Fig. 1B). DPOAE practically disappeared immediately after AAT (Fig. 1C). Over the following 14 days DPOAE almost returned to pre-AAT values, but ABR threshold improved only poorly (∼10 dB) and remained elevated (∼30 dB above pre-AAT values).
Despite the increase in thresholds, however, ABRs were still present, indicating a functional input into the central part of the auditory system.
Neurons Silenced After AAT Had Narrower Spikes
We then evaluated the effect of AAT on spiking activity of single neurons in the AC. We used 4 × 1 iridium tetrodes to record spiking activity of several neurons simultaneously. The intensity of the BBN pulses was set to 65 dB SPL. In contrast to the ABR thresholds, this intensity proved to be sufficient to evoke moderate responses in the AC (see discussion). Altogether we recorded spiking sound-evoked responses from 93 well-isolated and reliably reidentified neurons (from 20 mice, see materials and methods). In further analysis we included only neurons whose cluster quality fulfilled defined isolation criteria both before and after the AAT.
Individual neurons displayed different responses to BBN before and after the 5-min noise exposure. Almost half of the neurons (n = 42, 45%) responded significantly both before and after the AAT (Persistent neurons; Fig. 2A), a subset of neurons were silenced after the AAT, i.e., responded significantly only before the AAT (n = 13, 14%) (Silenced neurons; Fig. 2B), while another subset (n = 22, 24%) displayed response significance only after the AAT (Unmasked neurons; Fig. 2C). The rest of the neurons (n = 16, 17%) were not responsive to acoustic stimulation (unresponsive not shown).
Only neurons with persistent responsiveness displayed significantly increased spontaneous activity after the AAT (Fig. 2D; before: 5.14 ± 0.53 sp/s, after: 7.68 ± 0.93 sp/s; Wilcoxon signed-rank test, P < 0.01), while the spontaneous activity of Silenced and Unmasked neurons did not change significantly (Fig. 2D; before: 3.4 ± 1.1 sp/s and 5.7 ± 1.6 sp/s, after: 2.75 ± 0.80 sp/s and 6.97 ± 1.65 sp/s, respectively; Wilcoxon signed-rank test, P > 0.05). The evoked activity of Persistent neurons did not change (Wilcoxon signed-rank test, P > 0.05; Fig. 2E). The Silenced neurons decreased their evoked activity to the level of spontaneous activity, whereas Unmasked neurons displayed a significant increase in the evoked activity after AAT (Fig. 2E; before: 12.8 ± 1.3 sp/s, 6.7 ± 2.4 sp/s, and 5.5 ± 1.4 sp/s, after: 13.1 ± 1.5 sp/s, 3.07 ± 0.91 sp/s, and 11.2 ± 2.5 sp/s, respectively; Wilcoxon signed-rank test, P > 0.05, P > 0.05, and P < 0.001, respectively). The response jitter, estimated from the FWHM of sound-evoked PSTH, increased in neurons with persistent responsiveness (Fig. 2F; before: 37.2 ± 3.1 ms, after: 52.3 ± 6.2 ms; Wilcoxon signed-rank test, P < 0.01). Note that the assignment of neurons to different groups was based on their responsiveness before and after AAT (in terms of significant change of firing rate before and after the stimulus presentations), not on changes in evoked firing rates compared before and after AAT (see materials and methods). Overall changes in neuronal activity (Fig. 2, D–F) corresponded with the definition of each group of neurons.
Interestingly, Silenced neurons had significantly narrower spikes than other responsive neurons (Fig. 2G; for the Persistent, Silenced, and Unmasked groups: 0.855 ± 0.020 ms, 0.758 ± 0.030 ms, and 0.835 ± 0.038 ms, respectively; Wilcoxon rank sum test, Bonferroni correction, P < 0.013 for Persistent vs. Silenced, P < 0.013 for Silenced vs. Unmasked, P > 0.013 for Persistent vs. Unmasked), suggesting that neurons that stopped responding after the AAT were mostly fast-spiking interneurons (Beierlein et al. 2000; Moore and Wehr 2013). Electrophysiological identification of interneurons in vivo, however, can be rather ambiguous, and their precise identification requires a complementary method (Lima et al. 2009; Pinault 1996). Therefore, to detect the identity of cortical interneurons possibly affected by AAT, we then turned to calcium imaging in vivo.
Layer- and Class-Dependent Differences in Sound-Evoked Responses After AAT
We used calcium imaging in transgenic mice to measure sound-evoked responses and resolve the identities of the two main types of interneurons positioned across the upper cortical layers of AC (Fig. 3). Neurons in the AC were bulk-loaded with OGB-1 for calcium imaging. To identify PV+ and SST+ cortical interneurons in vivo, we used transgenic PV-Cre/tdT and SST-Cre/tdT mice (see materials and methods), in which the respective subclasses of interneurons expressed tdTomato red fluorescent protein. Individual neurons loaded with OGB-1 were unambiguously reidentified after the AAT, and interneurons expressing the red fluorescent protein (PV+, SST+) were clearly distinguishable from the rest of the population (Fig. 3, B and C). Note that labeled interneurons were also loaded with OGB-1 and calcium activity was recorded in the green channel, whereas the red channel was used for interneuron identification only. In each field of view the green-only population of neurons (PV−, SST−) consisted mainly of pyramidal neurons. For example, any PV− population of cells in our FOVs—a complement population to the labeled PV+ cells—would contain ∼83% pyramidal neurons, ∼8% SST+ interneurons, and ∼9% other, less frequent types of interneurons (Pfeffer et al. 2013; Rudy et al. 2011).
Fig. 3.
Calcium imaging in the primary auditory cortex (AC) in vivo. A: experimental layout of free-field acoustic stimulation under 2-photon microscope. B: example field of view (FOV) of layer (L)2/3 in the right AC in vivo in a PV-Cre/tdT mouse. Parvalbumin-positive (PV+) cells are in red. C: similar FOV in a SST-Cre/tdT mouse with red somatostatin-positive (SST+) interneurons. Cells in B and C were loaded with Oregon Green 488 BAPTA-1 (OGB-1 AM, green); scale bars, 50 μm. D, top: example line scan data. Rows correspond to sequential scans through a custom-defined line scan trajectory. Vertical stripes correspond to individual neurons. Bottom: fluorescence trace of the neuron corresponding to the first vertical stripe was processed and plotted (blue) together with positions of putative action potentials (red squares) obtained by using a peeling algorithm. E: line-scanned neurons before and after the AAT. Identified neurons (circles) were color-coded according to their best frequency (BF). Gray circles represent neurons that did not respond significantly to sounds (NONresp); black circles represent out-of-plane neurons (OUT). Scale bars, 25 μm.
Tone-Responsive Neurons Shifted Their Best Frequencies After AAT
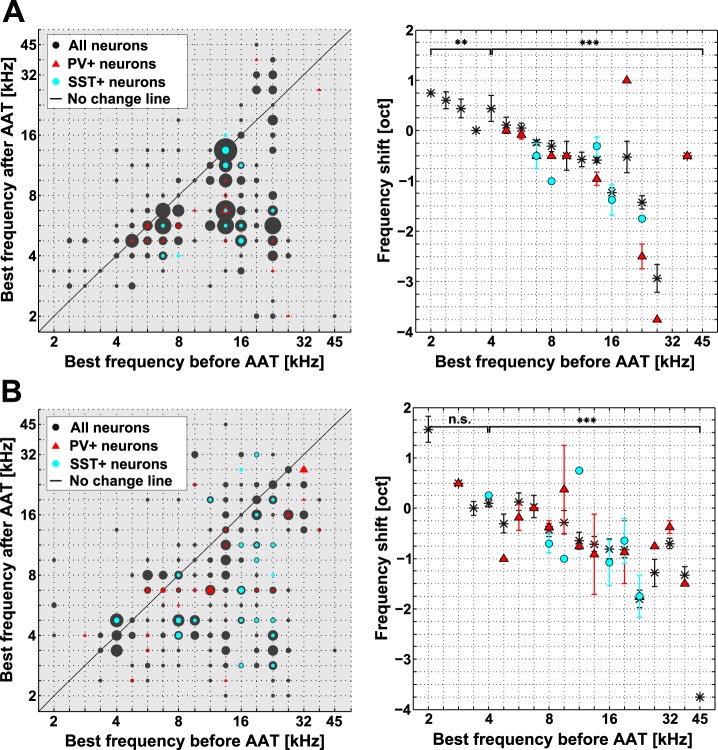
Neurons in the AC lowered their BFs after the AAT (Fig. 4). Across the whole population (n = 766 neurons), the mean BF shift was −0.65 oct (BF before 11.20 ± 0.13 kHz, BF after 8.6 ± 0.14 kHz; mean ± SE, P < 0.001, Wilcoxon signed-rank test). BF shifts induced by acoustic trauma were present in both L2/3 and L4. The mean BF shift in L2/3 (n = 406 neurons; Fig. 4A) was −0.66 oct (BF before 11.15 ± 0.16 kHz, BF after 8.52 ± 0.16 kHz; mean ± SE, P < 0.001, Wilcoxon signed-rank test). In L4, the mean BF shift across all neurons (n = 360; Fig. 4B) was −0.64 oct (BF before 11.25 ± 0.20 kHz, BF after 8.71 ± 0.22 kHz; mean ± SE, P < 0.001, Wilcoxon signed-rank test). The mean BF shifts of interneurons in both L2/3 and L4 (Fig. 4) differed neither from the mean BF shifts in respective layers nor from each other (P > 0.05).
Fig. 4.
Neuronal BFs were lower after AAT in L2/3 and L4. A: scatterplot of BFs in L2/3 before and after AAT (left) and dependence of the mean BF shift on the pre-AAT BF (right). Significance was evaluated with Wilcoxon signed-rank test, Bonferroni correction for multiple comparisons; n = 2. B: same format as in A, for L4. Values for all neurons (PV+, PV−, SST+, and SST−) are plotted as black dots or asterisks, PV+ neurons alone as red triangles, and SST+ neurons as blue dots. Circle sizes on left correspond to number of underlying data points.
Interestingly, neurons with the lowest BFs (BFs < 4 kHz) shifted their BFs toward higher frequencies in L2/3 (P < 0.01, Wilcoxon signed-rank test; Fig. 4A, right) but not in L4 (P > 0.05, Wilcoxon signed-rank test; Fig. 4B, right). BFs > 4 kHz shifted toward lower frequencies in both L2/3 and L4 (P < 0.001, Wilcoxon signed-rank test).
Acoustic Trauma Increased Tone-Evoked Responses Below BF
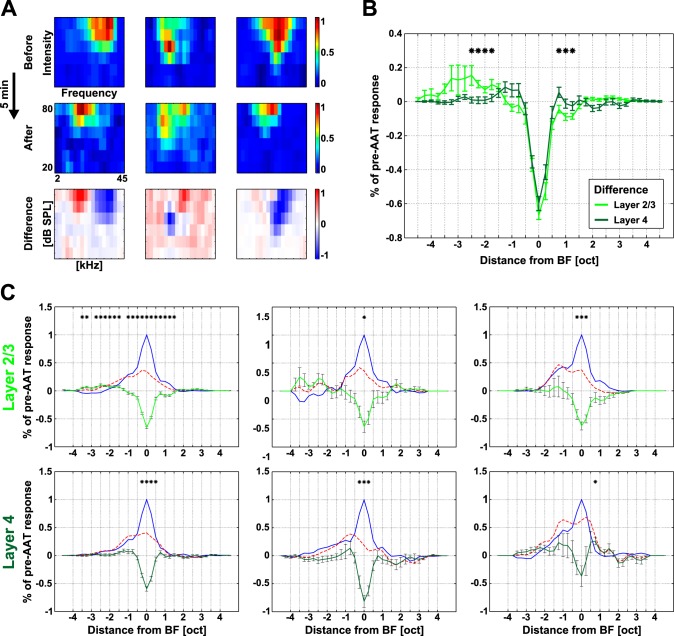
AAT also induced changes in the frequency tuning of individual neurons in the AC (Fig. 5). Individual neurons displayed pronounced disinhibition bands on the flanks of their V-shaped RFs after the AAT. Three example RFs are shown in Fig. 5A. Besides an increase in the response threshold around the BF, neurons in L2/3 typically increased their responses at the low-frequency side of their RF (Fig. 5A, left). In some L2/3 neurons responses increased at the high-frequency side of the RF (Fig. 5A, center). On the other hand, neurons in L4 mostly displayed smaller RFs after the AAT (Fig. 5A, right). These changes induced by the AAT were also apparent across neuronal populations in L2/3 and L4 (Fig. 5, B and C), mainly at and below the pre-AAT BF.
Fig. 5.
Frequency tuning changes in the AC after AAT. A: example receptive fields (RFs) before and after AAT: disinhibition at lower frequencies (left), disinhibition at higher frequencies (center), and no clear disinhibition (right). RFs became smaller in all 3 cases. B: mean difference in tuning curves before and after the AAT for L2/3 and L4 neurons. C: average pre-AAT tuning curves (blue), post-AAT tuning curves (dashed red), and differential curves (green) for both layers and 3 classes of neurons; Bonferroni correction for multiple comparisons. *P < 0.0014, Wilcoxon rank sum test (B) or Wilcoxon signed-rank test (C).
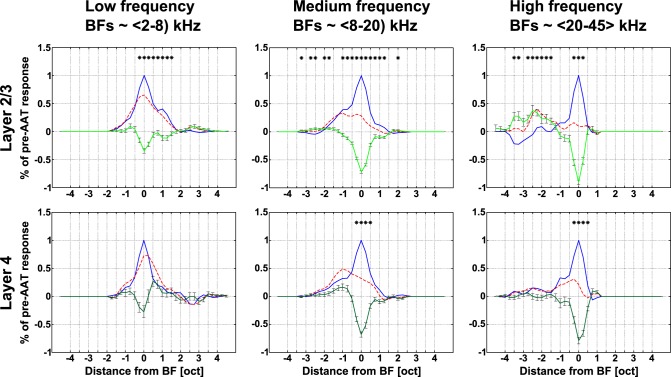
Neurons in L2/3 and L4 were affected differently by the AAT (Fig. 5B). The differential curve was narrower in L4; on the other hand, L2/3 neurons showed more pronounced disinhibition at lower frequencies. Consistent with the dominant BF shifts, responses to lower frequencies (∼2–2.5 oct below the BF) increased in L2/3 neurons (+10 ± 2%, averaged across intensities; Fig. 5C, left). All types of neurons decreased their responses around pre-AAT BF (Fig. 5C; Wilcoxon signed-rank test, P < 0.0014). Furthermore, we divided neurons within both layers into three groups according to their original BFs (Fig. 6). The AAT had the smallest impact on responses of low-frequency neurons in L2/3 and L4. Responses of medium- and high-frequency neurons, however, decreased overall after the AAT in both L2/3 and L4.
Fig. 6.
Changes in frequency tuning of AC neurons after the AAT with respect to their BFs. Average pre-AAT tuning curves (blue), post-AAT tuning curves (dashed red), and differential curves (green) across all neurons are shown for L2/3 and L4; Bonferroni correction for multiple comparisons. *P < 0.0014, Wilcoxon signed-rank test.
Only medium- and high-frequency neurons in L2/3 consistently displayed increased tone-evoked responses below BF. The greatest overall increase was present in L2/3 high-frequency neurons (+37 ± 9%, averaged across the used intensities).
Distinct Groups of AC Inhibitory Interneurons Were Differentially Affected by AAT
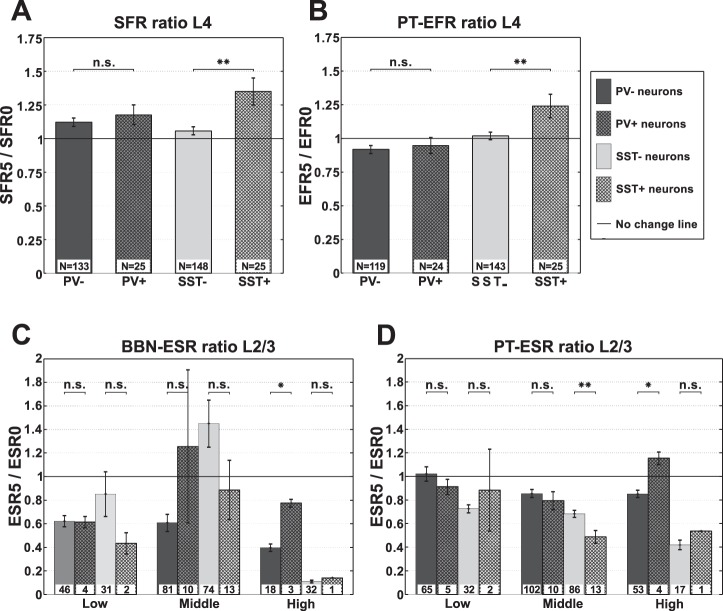
Finally, we evaluated changes in five different parameters of neuronal activity after the AAT (see materials and methods): SFR, PT-EFR, BBN-EFR, PT-ESR, and BBN-ESR.
To compare changes in the activity of PV+ and SST+ neurons among different mice we used PV− and SST− (tdTomato negative) neuronal populations as an internal control in each mouse. We divided our data according to layer and BF. The main significant results are shown in Fig. 7 and further in the tables.
Fig. 7.
Changes in activity parameters of AC neurons after AAT. A: changes in spontaneous firing rate (SFR) of PV−, PV+, SST−, and SST+ neurons in L4. B: changes in pure-tone-evoked firing rate (PT-EFR) of PV−, PV+, SST−, and SST+ neurons in L4. C: changes in broadband noise evoked-to-spontaneous firing rate (BBN-ESR) of PV−, PV+, SST−, and SST+ neurons in L2/3. D: changes in pure-tone evoked-to-spontaneous ratio (PT-ESR) of PV−, PV+, SST−, and SST+ neurons in L2/3. Two-tailed two-sample permutation test, Bonferroni correction for multiple comparisons; n = 2 for A and B and n = 6 for C and D. *P < 0.05, **P < 0.01.
SFRs increased significantly in SST+ neurons (P < 0.05; Table 1). Across all frequencies SFRs increased significantly only in L4 (P < 0.01) and but not in L2/3 (Fig. 7A, Table 1). With respect to the three frequency bands, the increase in SFR was significant only for SST+ neurons with low- and mid-frequency BFs (Table 2). SFRs of PV+ neurons did not change significantly after the AAT.
Table 1.
AAT-induced changes in SFR, PT-EFR, BBN-EFR, PT-ESR, and BBN-ESR of PV+ and SST+ neurons compared with PV− and SST− neurons
| SFR |
PT-EFR |
BBN-EFR |
PT-ESR |
BBN-ESR |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFR5/SFR0 |
SFR5/SFR0 |
EFR5/EFR0 |
EFR5/EFR0 |
EFR5/EFR0 |
EFR5/EFR0 |
ESR5/ESR0 |
ESR5/ESR0 |
ESR5/ESR0 |
ESR5/ESR0 |
|||||||||||
| PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | |
| L2/3 | 1.12 | 1.15ns | 1.44 | 2.13ns | 0.95 | 0.91ns | 0.78 | 0.99ns | 0.64 | 0.80ns | 0.89 | 0.78ns | 0.90 | 0.90ns | 0.66 | 0.53ns | 0.55 | 1.02* | 1.13 | 0.79ns |
| L4 | 1.12 | 1.17ns | 1.06 | 1.35*** | 0.92 | 0.94ns | 1.02 | 1.24** | 1.03 | 1.02ns | 1.13 | 1.13ns | 0.84 | 0.82ns | 0.98 | 0.95ns | 0.84 | 0.81ns | 1.45 | 1.66ns |
| L2/3/4 | 1.12 | 1.17ns | 1.23 | 1.67* | 0.94 | 0.93ns | 0.90 | 1.15** | 0.78 | 0.90ns | 1.00 | 0.96ns | 0.88 | 0.85ns | 0.82 | 0.78ns | 0.65 | 0.90ns | 1.28 | 1.26ns |
Values are acute acoustic trauma (AAT)-induced changes in spontaneous firing rate (SFR), pure-tone-evoked firing rate (PT-EFR), broadband-noise-evoked firing rate (BBN-EFR), pure-tone evoked-to-spontaneous firing rate (PT-ESR), and broadband-noise evoked-to-spontaneous firing rate (BBN-ESR) of parvalbumin (PV+)- and somatostatin (SST+)-positive neurons compared with parvalbumin (PV−)- and somatostatin (SST−)-negative neurons, respectively. Significant differences:
P < 0.05,
P < 0.01. ns, Nonsignificant.
Table 2.
AAT-induced changes in SFR of PV+ and SST+ neurons compared with PV− and SST− neurons
| Low Frequencies |
Middle Frequencies |
High Frequencies |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFR5/SFR0 Count |
SFR5/SFR0 Count |
SFR5/SFR0 Count |
SFR5/SFR0 Count |
SFR5/SFR0 Count |
SFR5/SFR0 Count |
|||||||
| PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | |
| L2/3 | 1.16 | 1.00ns | 0.96 | 1.13ns | 0.96 | 1.29ns | 1.16 | 2.33* | 1.32 | 1.01ns | 3.82 | 1.23ns |
| n | 67 | 5 | 32 | 2 | 105 | 10 | 87 | 15 | 66 | 4 | 17 | 1 |
| L4 | 1.41 | 1.39ns | 0.94 | 1.87** | 1.03 | 1.20ns | 1.08 | 1.43** | 0.96 | 0.87ns | 1.26 | 0.95ns |
| n | 28 | 5 | 33 | 2 | 67 | 12 | 84 | 17 | 38 | 8 | 31 | 6 |
| L2/3/4 | 1.26 | 1.26ns | 0.97 | 1.50** | 0.99 | 1.24ns | 1.11 | 1.84*** | 1.19 | 0.92ns | 2.02 | 0.99ns |
| n | 95 | 10 | 65 | 4 | 172 | 22 | 171 | 32 | 104 | 12 | 48 | 7 |
Values are AAT-induced changes in SFR of PV+ and SST+ neurons compared with PV− and SST− neurons, respectively. Neurons were classified according to their best frequencies. Significant differences:
P < 0.05,
P < 0.01,
P < 0.001. ns, Nonsignificant.
Along with SFRs SST+ neurons increased their PT-EFRs in L4 (P < 0.01; Fig. 7B, Table 3). Interestingly, BBN-EFR of SST+ neurons did not change (Table 4). Evoked firing rates of PV+ neurons remained unchanged (Fig. 7B, Table 3). The only changes in PV+ neurons were observed in PT-ESR and BBN-ESR of high-frequency neurons (P < 0.05; Fig. 7, C and D, Tables 5 and 6).
Table 3.
AAT-induced changes in PT-EFR of PV+ and SST+ neurons compared with PV− and SST− neurons
| Low Frequencies |
Middle Frequencies |
High Frequencies |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EFR5/EFR0 Count |
EFR5/EFR0 Count |
EFR5/EFR0 Count |
EFR5/EFR0 Count |
EFR5/EFR0 Count |
EFR5/EFR0 Count |
|||||||
| PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | |
| L2/3 | 1.15 | 0.94ns | 0.66 | 0.89* | 0.75 | 0.79ns | 0.71 | 1.04ns | 1.08 | 1.18ns | 1.39 | 0.66ns |
| n | 65 | 5 | 32 | 2 | 102 | 10 | 86 | 13 | 53 | 4 | 17 | 1 |
| L4 | 1.17 | 1.19ns | 1.14 | 1.73** | 0.78 | 0.85ns | 1.01 | 1.31** | 0.85 | 0.82ns | 0.90 | 0.86ns |
| n | 27 | 4 | 32 | 2 | 57 | 12 | 83 | 17 | 35 | 8 | 28 | 6 |
| L2/3/4 | 1.15 | 1.10ns | 0.90 | 1.31* | 0.76 | 0.82ns | 0.86 | 1.20*** | 0.99 | 0.94ns | 1.09 | 0.83ns |
| n | 92 | 9 | 64 | 4 | 159 | 22 | 169 | 30 | 88 | 12 | 45 | 7 |
Values are AAT-induced changes in PT-EFR of PV+ and SST+ neurons compared with PV− and SST− neurons, respectively. Neurons were classified according to their best frequencies. Significant differences:
P < 0.05,
P < 0.01,
P < 0.001. ns, Nonsignificant.
Table 4.
AAT-induced changes in BBN-EFR of PV+ and SST+ neurons compared with PV− and SST− neurons
| Low Frequencies |
Middle Frequencies |
High Frequencies |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EFR5/EFR0 Count |
EFR5/EFR0 Count |
EFR5/EFR0 Count |
EFR5/EFR0 Count |
EFR5/EFR0 Count |
EFR5/EFR0 Count |
|||||||
| PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | |
| L2/3 | 0.69 | 0.85ns | 0.60 | 0.57ns | 0.56 | 0.67ns | 1.03 | 0.85ns | 0.86 | 1.17ns | 0.82 | 0.31ns |
| n | 46 | 4 | 32 | 2 | 81 | 10 | 74 | 13 | 18 | 3 | 17 | 1 |
| L4 | 1.44 | 0.96ns | 1.47 | 1.68ns | 0.83 | 1.01ns | 1.07 | 1.09ns | 1.00 | 1.09ns | 0.81 | 0.98ns |
| n | 21 | 4 | 31 | 2 | 40 | 7 | 56 | 10 | 18 | 4 | 21 | 5 |
| L2/3/4 | 0.93 | 0.90ns | 1.02 | 1.12ns | 0.65 | 0.81ns | 1.05 | 0.96ns | 0.93 | 1.13ns | 0.82 | 0.87ns |
| n | 67 | 8 | 63 | 4 | 121 | 17 | 13 | 23 | 36 | 7 | 38 | 6 |
Values are AAT-induced changes in BBN-EFR of PV+ and SST+ neurons compared with PV− and SST− neurons, respectively. Neurons were classified according to their best frequencies. ns, Nonsignificant.
Table 5.
AAT-induced changes in PT-ESR of PV+ and SST+ neurons compared with PV− and SST− neurons
| Low Frequencies |
Middle Frequencies |
High Frequencies |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESR5/ESR0 |
ESR5/ESR0 |
ESR5/ESR0 |
ESR5/ESR0 |
ESR5/ESR0 |
ESR5/ESR0 |
|||||||
| PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | |
| L2/3 | 1.02 | 0.91ns | 0.72 | 0.88ns | 0.85 | 0.79ns | 0.68 | 0.49** | 0.85 | 1.15* | 0.42 | 0.53ns |
| L4 | 0.86 | 0.83ns | 1.19 | 0.94ns | 0.78 | 0.73ns | 0.94 | 0.97ns | 0.92 | 0.94ns | 0.85 | 0.88ns |
| L2/3/4 | 0.96 | 0.86ns | 0.96 | 0.91ns | 0.82 | 0.76ns | 0.81 | 0.75ns | 0.88 | 1.01* | 0.70 | 0.83ns |
Values are AAT-induced changes in PT-ESR of PV+ and SST+ neurons compared with PV− and SST− neurons, respectively. Neurons were classified according to their best frequencies. Significant differences:
P < 0.05,
P < 0.01. ns, Nonsignificant.
Table 6.
AAT-induced changes in BBN-ESR of PV+ and SST+ neurons compared with PV− and SST− neurons
| Low Frequencies |
Middle Frequencies |
High Frequencies |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESR5/ESR0 |
ESR5/ESR0 |
ESR5/ESR0 |
ESR5/ESR0 |
ESR5/ESR0 |
ESR5/ESR0 |
|||||||
| PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | PV− | PV+ | SST− | SST+ | |
| L2/3 | 0.62 | 0.61ns | 0.85 | 0.43ns | 0.61 | 1.26ns | 1.45 | 0.89ns | 0.40 | 0.77* | 0.11 | 0.14ns |
| L4 | 1.06 | 0.75ns | 1.74 | 1.46ns | 0.76 | 0.67ns | 1.57 | 1.96ns | 0.83 | 1.21* | 0.76 | 0.99ns |
| L2/3/4 | 0.74 | 0.69ns | 1.28 | 0.95ns | 0.67 | 0.94ns | 1.50 | 1.40ns | 0.54 | 1.05** | 0.49 | 0.85ns |
Values are AAT-induced changes in BBN-ESR of PV+ and SST+ neurons compared with PV− and SST− neurons, respectively. Neurons were classified according to their best frequencies. Significant differences:
P < 0.05,
P < 0.01. ns, Nonsignificant.
DISCUSSION
Results Overview
We studied the effect of acute AAT on the activity of defined neuronal populations in the mouse AC. Using single-unit electrophysiological recordings we demonstrated that cortical neurons that were silenced after the AAT had significantly narrower spikes than other sound-responsive neurons. Tone-responsive neurons in the AC lowered their BFs after the AAT, which was caused both by partial peripheral deafferentation in the vicinity of the pre-AAT BF and disinhibition at lower frequencies. Our main results include the observed rise in SFR and EFR of SST+ interneurons in L4 at lower and middle frequencies and a rise in ESR of PV+ interneurons in L2/3 on higher frequencies. Our results suggest a selective layer-dependent impact of the AAT on the activity of cortical interneurons.
Calcium Imaging In Vivo
In vivo two-photon calcium imaging is a widely used technique for simultaneously recording the activity of many neurons (Denk et al. 1990; Huber et al. 2012; Issa et al. 2014; Svoboda and Yasuda 2006). Here we used OGB-1, a calcium indicator characterized by relatively bright basal fluorescence and high signal-to-noise ratio. Thanks to its low Ca2+ dissociation constant, Kd ∼ 170–180 nM (Gersbach et al. 2009), OGB-1 reliably detects single action potentials (Bandyopadhyay et al. 2010). We used a Two-Photon Processor (Tomek et al. 2013), a freely available software package, to process data from two-photon calcium imaging and estimate positions and numbers of action potentials. The software uses the peeling algorithm (Grewe et al. 2010) to detect action potential-related events. Using the optimal set of internal parameters of the used algorithm, we reduced the risk of data contamination with subthreshold-event-related calcium fluxes (Bandyopadhyay et al. 2010). To minimize any possible contamination of experimental data by signal from neuropil (Lutcke and Helmchen 2011) we used a high-numerical aperture objective (NA = 0.95) whose back aperture was overfilled with the laser beam. Therefore, the contamination from neuropil should be generally low, <10% in neurons sectioned in the middle (Gobel and Helmchen 2007).
Impact of Acoustic Trauma on Auditory Processing
Most previous studies used loud pure tones as a traumatic stimulus (Kimura and Eggermont 1999; Norena et al. 2003; Norena and Eggermont 2003). Loud pure-tone stimulation damages regions of the cochlea that respond to frequencies equal or higher than that of the traumatic frequency (Ruettiger et al. 2013). We chose 125-dB BBN as it corresponds better to blunt real-world traumatic acoustic exposures than a pure tone-induced trauma. BBN was used only in a few studies, and in none of them was the activity in the AC recorded with a single-cell resolution (Groeschel et al. 2011; Kim et al. 2007; Syka et al. 1994; Syka and Rybalko 2000).
We performed our study in mice under ketamine-xylazine anesthesia. The anesthesia could influence excitability and the presumed central effects of the AAT, such as overstimulation and possible accompanying plasticity. Ketamine itself has a rather complex influence on the spontaneous and evoked activity in the auditory thalamus and cortex (Zurita et al. 1994); however, administration of a mixture of ketamine and xylazine decreases both spontaneous and evoked activity (Kisley and Gerstein 1999; Syka et al. 2005). Therefore, anesthesia during the AAT should have a rather protective influence on neuronal activity. We kept the level of anesthesia constant during the experiment, and thus the effects of the AAT described in our study were recorded under comparable anesthetic conditions.
We observed broad ABR threshold shifts, mostly manifested at higher frequencies (Fig. 1B). The magnitude of the ABR threshold shift with a maximum of ∼40 dB at 8–16 kHz was comparable to previously published results (Komiya and Eggermont 2000; Norena et al. 2003). DPOAE practically disappeared immediately after the AAT. During the following 14 days DPOAE almost returned to pre-AAT values, but the ABR threshold improved only slightly (∼10 dB) and remained elevated (∼30 dB above the pre-AAT values). Although the noise intensity we used for AAT induction was similar to intensities used in other studies, we used a much shorter traumatic duration. Most other studies used traumatic exposure lasting 1 h or more (Norena and Eggermont 2003; Sun et al. 2008; Tan et al. 2007). All components of the auditory periphery could be affected by the AAT during the short period in which we recorded data from the AC. The peripheral deterioration is most likely a mix of the temporary and persistent effects of acoustic trauma. The observed persistence of ABR threshold elevation and the recovery of DPOAE suggest that our acoustic trauma protocol also caused some primary degeneration with spared outer hair cells (Kujawa and Liberman 2009).
Interestingly, the elevated peripheral threshold exceeds the thresholds of neurons in the AC in our data. This is probably caused by the fact that ABR measurement generally shows lower sensitivity compared with, e.g., behavioral estimation of the thresholds. ABR measurements tend to overestimate the hearing threshold by up to 20 dB SPL, especially at lower frequencies (Stapells and Oates 1997; Werner et al. 1993).
Heterogeneous reactions of cortical neurons after acoustic trauma have been consistently reported (Norena et al. 2010). Together with altered thalamocortical afferentation such effect of AAT on cortical neurons is probably also a consequence of altered interactions between different inhibitory interneuron subtypes and excitatory neurons as proposed previously (Norena and Eggermont 2003), which is further supported by our data showing layer dependency of the AAT in the AC. Increased spontaneous and evoked activities in the Persistent group of our electrophysiological data (Fig. 2) may be caused by diminished tonic and phasic perisomatic inhibition, respectively, provided by PV+ cells (Hu et al. 2014). In vivo cortical whole cell recordings revealed no change in SFR up to 1 h after the AAT (Scholl and Wehr 2008). The discrepancy may be caused by the fact that the authors recorded neuronal activity across most cortical layers (135–770 μm deep). To the best of our knowledge, changes in spike-timing precision after AAT have not been addressed previously. The decreased spike-timing precision and prolonged firing may reflect altered PV neuron responses (Hu et al. 2014) or may be a manifestation of changes in subcortical processing (Eggermont and Roberts 2004). Spike widths for the Silenced neuronal subgroup (putative fast-spiking neurons) are similar to widths recorded from optogenetically identified PV+ cells (Moore and Wehr 2013).
Downward shifts of preferred frequencies were repeatedly reported previously (Kimura and Eggermont 1999; Komiya and Eggermont 2000; Norena et al. 2003, 2010). At first glance, this might seem to be a sole consequence of the peripheral threshold shift at higher frequencies. However, several studies provided evidence that a decrease of inhibition at lower frequencies is also involved (Norena and Eggermont 2003; Scholl and Wehr 2008). Our data are consistent with these results and extend them further. Using calcium imaging we observed mostly downward shifts of BFs accompanied by disinhibition at a lower-frequency part of the RF (Figs. 4–6). The shifted RFs thus occupied parts of the stimulus space that had not been included in the initial RFs, suggesting that the post-AAT BF shifts are not a simple result of peripheral threshold shifts. All neurons underwent similar BF shifts; the BF shifts of PV+ and SST+ neurons were the same as in the rest of the neuronal population. We observed marked disinhibition in L2/3, especially in high-frequency neurons, but we did not observe any disinhibition in L4. These layer-dependent differences had not been described previously. The observed effect might be related to opposite AAT impact on lower frequencies (Norena et al. 2010) and more prominent intralaminar connections between regular-spiking cells in L2/3 (Douglas and Martin 2007; Watkins et al. 2014). This model was previously proposed in Eggermont and Roberts (2004).
Finally, we investigated changes in response parameters of different classes of neurons after the AAT. The most prominent changes were observed in low- and middle-frequency SST+ neurons in L4 and in high-frequency PV+ neurons in L2/3. The difference in the observed impact of the AAT on these two distinct classes of interneurons might be related to previously published frequency-dependent increase/decrease of inhibition after AAT (Scholl and Wehr 2008).
Possible Mechanisms Underlying Changes in Neuronal Activity After AAT
AAT generally impairs all levels of the auditory system (Eggermont and Roberts 2004), and its impact should always be considered throughout the whole pathway. Although the auditory periphery is somewhat protected against excessive auditory stimulation (Peterson and Liden 1972; Wen et al. 2009; Zheng et al. 1997, 1999), a traumatic stimulus, such as 125-dB BBN, probably still evokes excessive firing rates across auditory nerve fibers and the activity spreads further within the ascending auditory pathway. However, only firing characteristics of spiral ganglion neurons during the traumatic stimulus were described (Cody and Johnstone 1980). The maximum discharge rate of an auditory nerve fiber does not depend on its characteristic frequency (Taberner and Liberman 2005). Within the subcortical part of the central auditory pathway, however, sound stimuli are processed extensively and the RFs of individual neurons are shaped by local inhibitory circuits (Backoff et al. 1999; Cotillon-Williams et al. 2008; Egorova et al. 2001; Grecova et al. 2009; Ingham et al. 2006; LeBeau et al. 2001; Oertel et al. 2011; Peruzzi et al. 1997). Given the complexity of the subcortical part of the auditory pathway, which is also affected by AAT (Bures et al. 2010; Dong et al. 2010; Grecova et al. 2009; Kaltenbach et al. 1998; Popelar and Syka 1982), it is challenging to determine the specific impact of AAT on thalamocortical inputs. Nonetheless, many classic publications describe the effect of AAT on response characteristics of neurons in the AC (Norena et al. 2003, 2010; Norena and Eggermont 2003; Tomita et al. 2004; for review see Eggermont 2015). These studies have demonstrated that changes in responsiveness of the AC neurons after AAT are particularly attributable to alterations in both peripheral afferentation and local cortical inhibition (Norena et al. 2003; Norena and Eggermont 2003; Scholl and Wehr 2008). We have previously described a difference between the central and the peripheral effect of AAT in guinea pigs and rats (Popelar et al. 1987; Syka and Rybalko 2000), and we have also shown that there is a time and dosage dependency influencing the central effect (Popelar et al. 2008; Syka et al. 1994). Interestingly, the alteration in inhibition seems to be more complex, as inhibition can be both decreased and increased with respect to stimulus frequency (Norena et al. 2003; Scholl and Wehr 2008).
Here we focused on the role of PV+ and SST+ cells in changes in cortical activity induced by AAT. Although recent evidence suggests that at least some patterns of canonical microcircuits are shared between different sensory cortices (Kepecs and Fishell 2014), some current data on the role of PV+ and SST+ neurons are available only from the somatosensory or visual cortex and might not be easily extrapolated to the auditory cortex. The two types of interneurons have distinct profiles of postsynaptic partners. SST+ interneurons nonreciprocally inhibit PV+ interneurons, as has been shown in the somatosensory (Xu et al. 2013) and visual (Pfeffer et al. 2013) cortices. During high cortical activity SST+ interneurons inhibit regular spiking neurons as well (Beierlein et al. 2003; Kapfer et al. 2007). PV+ fast-spiking basket cells are involved in feedforward inhibition, notably in regulation of thalamocortical transmission onto L4 regular-spiking cells (Cruikshank et al. 2007; Isaacson and Scanziani 2011). Other substantial differences between SST+ and PV+ interneurons are in short-term plasticity on both input and output synapses. While most cortical synapses desensitize, excitatory synapses onto SST+ neurons strongly facilitate (Beierlein et al. 2003; Reyes et al. 1998). This facilitation is persistent even for longer periods, >10 min (Chen et al. 2009; Lu et al. 2007). Paradoxically, synapses of thalamocortical axons targeting L4 PV+ interneurons, the cells regulating the timing and amount of thalamocortical input (Cruikshank et al. 2007), desensitize most strongly (Beierlein et al. 2003). SST+ neurons probably do not receive any direct thalamic afferentation (Beierlein et al. 2003; Reyes 2011).
Here we hypothesize that SST+ cells could serve as a key component to counteract excessive cortical activity because of their unique features: the supralinearity of their recruitment and facilitation of input synapses from regular-spiking neurons. During the traumatic acoustic stimulus SST+ neurons and their synapses might be affected. Dendrite-targeting inhibitory cells, for example, deteriorate in experimental epilepsy models (Binaschi et al. 2003; Cossart et al. 2001; Silberberg and Markram 2007). We observed higher reactivity (higher SFR and PT-EFR) in low- and middle-frequency SST+ interneurons in L4 after AAT, compared with complementary neurons. These cells could suppress the activity of PV+ cells in similar frequency bands in L4 and might disinhibit the low-frequency part of RF. However, we did not observe any significant change in activity of PV+ neurons in L4, although the absolute evoked PV+ activity in L2/3 was presumably lower at specific frequencies after the AAT (Tables 2–6). On the higher frequencies, the effect was the opposite.
Acoustic trauma is tightly associated with the onset of tinnitus, probably via altered inhibition (Roberts et al. 2010). Our results show that distinct types of inhibitory interneurons are differentially affected by intense noise exposure. Whether the changes in inhibitory subsystems are adaptive and compensatory or caused by detrimental overactivation of the AC during intense noise exposure is unclear. We observed neither immediate cell death nor morphological changes of the investigated interneurons. Our data point at a possible effect of maladaptive plasticity in the following circuit: thalamocortical relay cell → regular-spiking cell in L4 → SST+ cell in L4 → PV+ cell in L2/3 → pyramidal cell in L2/3. Addressing the precise nature of circuit changes after or even during acoustic trauma is, however, beyond the scope of this study. Whether the traumatic changes in cortical processing are transient or persistent also remains an open question that can be answered with the use of the chronic cranial window technique, which enables the study of properties of individual neurons over weeks or months with a cellular or even dendritic resolution (Holtmaat et al. 2009).
GRANTS
This work was supported by the Grant Agency of the Czech Republic (P303/12/1347 and P304/12/G069).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: O.N. and O.Z. performed experiments; O.N., O.Z., and T.H. analyzed data; O.N., O.Z., and J.S. interpreted results of experiments; O.N. and T.H. prepared figures; O.N. and O.Z. drafted manuscript; O.N., T.H., and J.S. edited and revised manuscript; O.N., O.Z., T.H., and J.S. approved final version of manuscript; J.S. conception and design of research.
REFERENCES
- Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73: 159–170, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atencio CA, Schreiner CE. Columnar connectivity and laminar processing in cat primary auditory cortex. Plos One 5: e9521, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backoff PM, Palombi PS, Caspary DM. Gamma-aminobutyric acidergic and glycinergic inputs shape coding of amplitude modulation in the chinchilla cochlear nucleus. Hear Res 134: 77–88, 1999. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Shamma SA, Kanold PO. Dichotomy of functional organization in the mouse auditory cortex. Nat Neurosci 13: 361–368, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci 3: 904–910, 2000. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol 90: 2987–3000, 2003. [DOI] [PubMed] [Google Scholar]
- Binaschi A, Bregola G, Simonato M. On the role of somatostatin in seizure control: clues from the hippocampus. Rev Neurosci 14: 285–301, 2003. [DOI] [PubMed] [Google Scholar]
- Bures Z, Grecova J, Popelar J, Syka J. Noise exposure during early development impairs the processing of sound intensity in adult rats. Eur J Neurosci 32: 155–164, 2010. [DOI] [PubMed] [Google Scholar]
- Chen HX, Jiang M, Akakin D, Roper SN. Long-term potentiation of excitatory synapses on neocortical somatostatin-expressing interneurons. J Neurophysiol 102: 3251–3259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody AR, Johnstone BM. Single auditory neuron response during acute acoustic trauma. Hear Res 3: 3–16, 1980. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci 4: 52–62, 2001. [DOI] [PubMed] [Google Scholar]
- Cotillon-Williams N, Huetz C, Hennevin E, Edeline JM. Tonotopic control of auditory thalamus frequency tuning by reticular thalamic neurons. J Neurophysiol 99: 1137–1151, 2008. [DOI] [PubMed] [Google Scholar]
- Cottam JC, Smith SL, Haeusser M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J Neurosci 33: 19567–19578, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10: 462–468, 2007. [DOI] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science 248: 73–76, 1990. [DOI] [PubMed] [Google Scholar]
- Dong SY, Mulders W, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J Neurosci 31: 1616–1628, 2010. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Recurrent neuronal circuits in the neocortex. Curr Biol 17: R496–R500, 2007. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. The auditory cortex and tinnitus—a review of animal and human studies. Eur J Neurosci 41: 665–676, 2015. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci 27: 676–682, 2004. [DOI] [PubMed] [Google Scholar]
- Egorova M, Ehret G, Vartanian I, Esser KH. Frequency response areas of neurons in the mouse inferior colliculus. I. Threshold and tuning characteristics. Exp Brain Res 140: 145–161, 2001. [DOI] [PubMed] [Google Scholar]
- Gersbach M, Boiko DL, Niclass C, Petersen CC, Charbon E. Fast-fluorescence dynamics in nonratiometric calcium indicators. Opt Lett 34: 362–364, 2009. [DOI] [PubMed] [Google Scholar]
- Gobel W, Helmchen F. In vivo calcium imaging of neural network function. Physiology 22: 358–365, 2007. [DOI] [PubMed] [Google Scholar]
- Grecova J, Bures Z, Popelar J, Suta D, Syka J. Brief exposure of juvenile rats to noise impairs the development of the response properties of inferior colliculus neurons. Eur J Neurosci 29: 1921–1930, 2009. [DOI] [PubMed] [Google Scholar]
- Grewe BF, Langer D, Kasper H, Kampa BM, Helmchen F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nat Methods 7: 399–405, 2010. [DOI] [PubMed] [Google Scholar]
- Groeschel M, Mueller S, Goetze R, Ernst A, Basta D. The possible impact of noise-induced Ca2+-dependent activity in the central auditory pathway: a manganese-enhanced MRI study. Neuroimage 57: 190–197, 2011. [DOI] [PubMed] [Google Scholar]
- Hamilton LS, Sohl-Dickstein J, Huth AG, Carels VM, Deisseroth K, Bao S. Optogenetic activation of an inhibitory network enhances feedforward functional connectivity in auditory cortex. Neuron 80: 1066–1076, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Hirase H, Leinekugel X, Henze DA, Buzsaki G. Temporal interaction between single spikes and complex spike bursts in hippocampal pyramidal cells. Neuron 32: 141–149, 2001. [DOI] [PubMed] [Google Scholar]
- Harris KD, Shepherd GM. The neocortical circuit: themes and variations. Nat Neurosci 18: 170–181, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hubener M, Keck T, Knott G, Lee WC, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc 4: 1128–1144, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345: 1255263, 2014. [DOI] [PubMed] [Google Scholar]
- Huber D, Gutnisky DA, Peron S, O'Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature 484: 473–478, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham NJ, Bleeck S, Winter IM. Contralateral inhibitory and excitatory frequency response maps in the mammalian cochlear nucleus. Eur J Neurosci 24: 2515–2529, 2006. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron 72: 231–243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa JB, Haeffele BD, Agarwal A, Bergles DE, Young ED, Yue DT. Multiscale optical Ca2+ imaging of tonal organization in mouse auditory cortex. Neuron 83: 944–959, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang JS. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res 124: 78–84, 1998. [DOI] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci 10: 743–753, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Gillet SN, Peters AJ, Isaacson JS, Komiyama T. Parvalbumin-expressing interneurons linearly control olfactory bulb output. Neuron 80: 1218–1231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature 505: 318–326, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kang HH, Ahn JH, Chung JW. Hypoxic changes in the central nervous system of noise-exposed mice. Acta Otolaryngol Suppl 127: 73–77, 2007. [DOI] [PubMed] [Google Scholar]
- Kimura M, Eggermont JJ. Effects of acute pure tone induced hearing loss on response properties in three auditory cortical fields in cat. Hear Res 135: 146–162, 1999. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Gerstein GL. Trial-to-trial variability and state-dependent modulation of auditory-evoked responses in cortex. J Neurosci 19: 10451–10460, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya H, Eggermont JJ. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol 120: 750–756, 2000. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29: 14077–14085, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau FE, Malmierca MS, Rees A. Iontophoresis in vivo demonstrates a key role for GABAA and glycinergic inhibition in shaping frequency response areas in the inferior colliculus of guinea pig. J Neurosci 21: 7303–7312, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, Deisseroth K, Dan Y. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488: 379–383, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LY, Ji XY, Liang FX, Li YT, Xiao ZJ, Tao HZ, Zhang LI. A feedforward inhibitory circuit mediates lateral refinement of sensory representation in upper layer 2/3 of mouse primary auditory cortex. J Neurosci 34: 13670–13683, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PloS One 4: e6099, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BH, Wu GK, Arbuckle R, Tao HW, Zhang LI. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat Neurosci 10: 1594–1600, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Turner J, Caspary DM. Diminished cortical inhibition in an aging mouse model of chronic tinnitus. J Neurosci 32: 16141–16148, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JT, Li CY, Zhao JP, Poo MM, Zhang XH. Spike-timing-dependent plasticity of neocortical excitatory synapses on inhibitory interneurons depends on target cell type. J Neurosci 27: 9711–9720, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutcke H, Helmchen F. Two-photon imaging and analysis of neural network dynamics. Rep Prog Phys 74: 086602, 2011. [Google Scholar]
- Moore AK, Wehr M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J Neurosci 33: 13713–13723, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev 35: 1089–1109, 2011. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res 183: 137–153, 2003. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Moffat G, Blanc JL, Pezard L, Cazals Y. Neural changes in the auditory cortex of awake guinea pigs after two tinnitus inducers: salicylate and acoustic trauma. Neuroscience 166: 1194–1209, 2010. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol 90: 2387–2401, 2003. [DOI] [PubMed] [Google Scholar]
- Oertel D, Wright S, Cao XJ, Ferragamo M, Bal R. The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus. Hear Res 276: 61–69, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet L, de Villers-Sidani E. Trajectory of the main GABAergic interneuron populations from early development to old age in the rat primary auditory cortex. Front Neuroanat 8: 40, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzi D, Bartlett E, Smith PH, Oliver DL. A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. J Neurosci 17: 3766–3777, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JL, Liden G. Some static characteristics of stapedial muscle reflex. Audiology 11: 97–114, 1972. [DOI] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 16: 1068–1076, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods 65: 113–136, 1996. [DOI] [PubMed] [Google Scholar]
- Popelar J, Grecova J, Rybalko N, Syka J. Comparison of noise-induced changes of auditory brainstem and middle latency response amplitudes in rats. Hear Res 245: 82–91, 2008. [DOI] [PubMed] [Google Scholar]
- Popelar J, Syka J. Noise impairment in the guinea pig. II. Changes of single unit responses in the inferior colliculus. Hear Res 8: 273–283, 1982. [DOI] [PubMed] [Google Scholar]
- Popelar J, Syka J, Berndt H. Effect of noise on auditory evoked responses in awake guinea pigs. Hear Res 26: 239–247, 1987. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci 1: 279–285, 1998. [DOI] [PubMed] [Google Scholar]
- Reyes AD. Synaptic short-term plasticity in auditory cortical circuits. Hear Res 279: 60–66, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci 30: 14972–14979, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71: 45–61, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruettiger L, Singer W, Panford-Walsh R, Matsumoto M, Lee SC, Zuccotti A, Zimmermann U, Jaumann M, Rohbock K, Xiong H, Knipper M. The reduced cochlear output and the failure to adapt the central auditory response causes tinnitus in noise exposed rats. PloS One 8: e57247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131: 1–11, 2005. [DOI] [PubMed] [Google Scholar]
- Scholl B, Wehr M. Disruption of balanced cortical excitation and inhibition by acoustic trauma. J Neurophysiol 100: 646–656, 2008. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53: 735–746, 2007. [DOI] [PubMed] [Google Scholar]
- Smith JC, Bolon B. Isoflurane leakage from non-rebreathing rodent anaesthesia circuits: comparison of emissions from conventional and modified ports. Lab Anim 40: 200–209, 2006. [DOI] [PubMed] [Google Scholar]
- Stapells DR, Oates P. Estimation of the pure-tone audiogram by the auditory brainstem response: a review. Audiol Neurootol 2: 257–280, 1997. [DOI] [PubMed] [Google Scholar]
- Stiebler I, Neulist R, Fichtel I, Ehret G. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. J Comp Physiol A 181: 559–571, 1997. [DOI] [PubMed] [Google Scholar]
- Sun W, Zhang L, Lu J, Yang G, Laundrie E, Salvi R. Noise exposure-induced enhancement of auditory cortex response and changes in gene expression. Neuroscience 156: 374–380, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron 50: 823–839, 2006. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N. Threshold shifts and enhancement of cortical evoked responses after noise exposure in rats. Hear Res 139: 59–68, 2000. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Popelar J. Enhancement of the auditory-cortex evoked responses in awake guinea pigs after noise exposure. Hear Res 78: 158–168, 1994. [DOI] [PubMed] [Google Scholar]
- Syka J, Suta D, Popelar J. Responses to species-specific vocalizations in the auditory cortex of awake and anesthetized guinea pigs. Hear Res 206: 177–184, 2005. [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol 93: 557–569, 2005. [DOI] [PubMed] [Google Scholar]
- Tan J, Ruettiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Koepschall I, Rohbock K, Knipper M. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience 145: 715–726, 2007. [DOI] [PubMed] [Google Scholar]
- Tomek J, Novak O, Syka J. Two-Photon Processor and SeNeCA: a freely available software package to process data from two-photon calcium imaging at speeds down to several milliseconds per frame. J Neurophysiol 110: 243–256, 2013. [DOI] [PubMed] [Google Scholar]
- Tomita M, Norena AJ, Eggermont JJ. Effects of an acute acoustic trauma on the representation of a voice onset time continuum in cat primary auditory cortex. Hear Res 193: 39–50, 2004. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Caspary DM. Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity. Hear Res 279: 111–117, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PV, Kao JP, Kanold PO. Spatial pattern of intra-laminar connectivity in supragranular mouse auditory cortex. Front Neural Circuits 8: 15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen B, Wang GI, Dean I, Delgutte B. Dynamic range adaptation to sound level statistics in the auditory nerve. J Neurosci 29: 13797–13808, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner LA, Folsom RC, Mancl LR. The relationship between auditory brain-stem response and behavioral thresholds in normal-hearing infants and adults. Hear Res 68: 131–141, 1993. [DOI] [PubMed] [Google Scholar]
- Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron 77: 155–167, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci USA 108: 14974–14979, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Zhou Y, Tao HW. Inhibitory synaptic mechanisms underlying functional diversity in auditory cortex. J Gen Physiol 138: 311–320, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XY, Henderson D, McFadden SL, Ding DL, Salvi RJ. Auditory nerve fiber responses following chronic cochlear de-efferentation. J Comp Neurol 406: 72–86, 1999. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Henderson D, McFadden SL, Hu BH. The role of the cochlear efferent system in acquired resistance to noise-induced hearing loss. Hear Res 104: 191–203, 1997. [DOI] [PubMed] [Google Scholar]
- Zurita P, Villa AE, Deribaupierre Y, Deribaupierre F, Rouiller EM. Changes of single-unit activity in the cat's auditory thalamus and cortex associated to different anesthetic conditions. Neurosci Res 19: 303–316, 1994. [DOI] [PubMed] [Google Scholar]