Abstract
It is well established that permanent or transient reduction of somatosensory inputs, following hand deafferentation or anesthesia, induces plastic changes across the hand-face border, supposedly responsible for some altered perceptual phenomena such as tactile sensations being referred from the face to the phantom hand. It is also known that transient increase of hand somatosensory inputs, via repetitive somatosensory stimulation (RSS) at a fingertip, induces local somatosensory discriminative improvement accompanied by cortical representational changes in the primary somatosensory cortex (SI). We recently demonstrated that RSS at the tip of the right index finger induces similar training-independent perceptual learning across the hand-face border, improving somatosensory perception at the lips (Muret D, Dinse HR, Macchione S, Urquizar C, Farnè A, Reilly KT. Curr Biol 24: R736–R737, 2014). Whether neural plastic changes across the hand-face border accompany such remote and adaptive perceptual plasticity remains unknown. Here we used magnetoencephalography to investigate the electrophysiological correlates underlying RSS-induced behavioral changes across the hand-face border. The results highlight significant changes in dipole location after RSS both for the stimulated finger and for the lips. These findings reveal plastic changes that cross the hand-face border after an increase, instead of a decrease, in somatosensory inputs.
Keywords: somatosensory input, cortical plasticity, learning, spatial discrimination, dipole, MEG
most of our knowledge about large-scale plasticity in the primary somatosensory cortex (SI) comes from cases of permanent or transient reduction of inputs. In particular, massive cortical reorganization has been repeatedly observed across the border separating the hand and face representations (Dutta et al. 2013; Pons et al. 1991; Weiss et al. 2004). This input-deprivation model of cortical plasticity has been largely influential, with the representation of the face expanding and shifting toward that of the deprived hand having been thought of as being at the origin of phantom sensations and pain (Flor et al. 1995; Ramachandran et al. 1992). While more recent evidence calls for a reassessment of such a view in both the somatosensory (Makin et al. 2015) and motor (Gagné et al. 2011) domains, it is important to emphasize that input deprivation is not the only model for assessing somatosensory plasticity. In fact, input augmentation via active training (Braun et al. 2000a; Recanzone et al. 1992; Spengler et al. 1997) or even short passive exposure (Beste and Dinse 2013; Braun et al. 2000b; Jenkins et al. 1990) has been widely documented to improve tactile performance and induce cortical plasticity. Whether large-scale changes accompany input augmentation-based plasticity remains poorly understood, despite its potential impact in promoting adaptive plasticity remotely.
To first address this question at a behavioral level, we recently used a repetitive somatosensory stimulation (RSS) protocol known to induce both local plastic changes within SI and a local improvement in tactile acuity (for reviews see Beste and Dinse 2013; Parianen Lesemann et al. 2015). After RSS was applied to the right index fingertip we observed that spatial tactile acuity, rigorously measured by means of two-point discrimination threshold (2PDT), improved not only locally at the stimulated finger but also remotely at the face (Muret et al. 2014). While the RSS-induced improvement at the right but not the left index is compatible with local contralateral plastic changes, long-range cortico-cortical connections crossing the hand-face border (Fang et al. 2002; Florence et al. 1998; Manger et al. 1997), which can undergo Hebbian-based plastic changes (Marik and Hickmott 2009; Paullus and Hickmott 2011), might be involved in the transfer of improvement to the cortically adjacent face region, suggesting a novel form of adaptive somatosensory plasticity at distance. It is currently unknown, however, whether these cross-border perceptual changes are paralleled by cortical plastic changes.
To address this, we used magnetoencephalography (MEG) to extract the sources of the cortical activity evoked by mechanically touching the fingers and upper lips of healthy participants before and after RSS of the right index finger (right-D2). MEG provides a direct measure of neuronal activity with a sufficiently high spatial resolution to distinguish between lip and finger somatosensory sources (Nakamura et al. 1998). Given that the cortical plasticity induced locally in the representation of the stimulated finger within SI has been reported as a shift of its equivalent current dipole (ECD) toward that of the thumb (Godde et al. 2003; Pleger et al. 2001), we investigated whether RSS also alters the configuration of neuromagnetic dipole sources of the lips.
MATERIALS AND METHODS
Participants.
Twenty-five healthy volunteers [mean age = 22.24 ± 2.71 (SD) yr; 13 women, 12 men] were tested, and four participants were removed from analyses because of poor signal-to-noise ratio (see Magnetoencephalography data analysis for details). All participants were right-handed according to the Edinburgh Handedness Inventory (Oldfield 1971; mean score = 82.93% ± 18.37 SD), and they all gave written informed consent before participating. The protocol was approved by the ethics committee of Lyon and was performed in accordance with the Declaration of Helsinki.
Experimental time course.
The experiment took place over two consecutive days. On the first day, participants underwent a practice session to become familiar with the tactile discrimination task before undergoing the first assessment of their tactile spatial acuity as measured by 2PDT. The next day an additional measure of 2PDT was acquired before a first session of MEG recordings that was followed by 3 h of RSS of right-D2. Immediately after the RSS procedure, a second set of MEG recordings and of 2PDT assessments were performed, the order between these two last sessions being counterbalanced across participants. The structural MRI of each participant was acquired in the days after the experiment, usually the next day.
Repetitive somatosensory stimulation protocol.
A standard mechanical RSS procedure was used (Godde et al. 2000; Hodzic et al. 2004; Pleger et al. 2001, 2003). A small (8-mm diameter) solenoid controlled by an MP3 player was taped to the volar surface of the right-D2 fingertip. This solenoid delivered brief (10 ms) suprathreshold tactile stimuli for 3 h, with interstimulus intervals ranging from 100 to 3,000 ms and following a Poisson distribution (average stimulation frequency of 1 Hz). During the RSS procedure, conducted outside the MEG, participants were instructed to continue with their daily activities without paying attention to the device but to avoid intensive use of their fingers.
Tactile spatial acuity assessment.
2PDTs were measured with a two-alternative forced-choice task and force-controlled devices at both index fingertips (left-/right-D2), both little fingertips (left-/right-D5), and both sides of the upper lip region, three times before (Practice, S1, and S2) and once after (S3) 3 h of RSS applied to right-D2. Because of time constraints, and on the basis of previous studies reporting no threshold change at left-D2 following RSS on right-D2 (Godde et al. 2000, 2003; Pleger et al. 2001, 2003), 2PDTs at left-D2 and left-D5 were assessed in S2 and S3 only. To ensure that assessments were performed on a constant location throughout sessions, the skin regions of the 2PDT assessments [distance from fingertip: 5.17 ± 1.74 mm and distance from finger edge: 5.19 ± 1.13 mm (mean ± SD); distance from midlip: 14.88 ± 0.82 mm (mean ± SD), midway between the upper lip and the base of the nose] were marked on the first day with a 10-mm circular stamp soaked in an invisible ink visible with a UV lamp. These marks were also used to position the pneumatically driven stimulators used during the MEG mapping procedure.
2PDTs were assessed with the procedure described in Muret et al. (2014). In brief, eight probes were used, one with a single tip and seven with two tips separated by various distances. Because of differences in absolute sensitivity, different sets of distances were used for index fingertips (0.7, 1.0, 1.3, 1.6, 1.9, 2.2, 2.5 mm), little fingertips (1.0, 1.4, 1.8, 2.2, 2.6, 3.2, 4 mm), and lips (2, 3, 4, 5, 6, 7, 8 mm). After the participant was allowed to feel the extreme separation distances three times to make sure he/she clearly felt the difference between the two tips, the testing began. Each probe was tested 8 times in a pseudorandomized order, resulting in 64 trials per body site. Tips were always presented parallel to the longitudinal axis of the fingers and face. Two specially designed spring-mounted apparatuses were used to ensure almost constant application force across trials (see Muret et al. 2014 supplemental data for details).
Magnetoencephalography data acquisition.
Recordings were carried out with a 275-channel whole-head MEG system (CTF-275 by VSM Medtech) with a continuous sampling rate of 600 Hz, a 150-Hz low-pass filter, and third-order spatial gradient noise cancellation. During the two MEG sessions, participants lay supine in a magnetically shielded room with the head comfortably maintained by cushions to limit involuntary movements. Oblique electrooculograms (EOGs) were acquired with bipolar electrodes. The exact position of the head with respect to the sensors was determined with three indicator coils fixed to the nasion and the preauricular points (fiducial points). Their location was continuously recorded and checked at the beginning of each block to ensure that head movements did not exceed 5 mm. To maintain the exact same position across sessions, the coils and EOG electrodes remained on the participant's head between the two MEG sessions.
The stimulation system was pneumatically driven and electronically controlled from the command board outside of the shielded room. This system had eight individually controlled channels, each consisting of a pneumatic valve connected by a plastic tube to a membrane (8-mm diameter). A constant delay of 30 ms was found for the stimulation onset due to tube length and was taken into consideration for analyses. For each participant, a membrane was fixed at each of the tested body sites (D2, D5, and upper lip bilaterally) with double-adhesive tape combined with regular adhesive tape. At the beginning of each MEG session care was taken to position each stimulator exactly at the same location as 2PDT assessments thanks to the UV-visible marks made beforehand. This ensured a precise correspondence between the location of behavioral and MEG acquisitions throughout sessions. Tactile stimuli were delivered at constant suprathreshold intensity (0.8 bars) with 30-ms square-wave pulses interleaved by a delay ranging from 400 to 600 ms in 10-ms steps. Before each session participants were asked whether the tactile stimulation was clearly perceived at all body sites, with a comparable subjective intensity across the six body sites, and if necessary the fixation of the membranes was adjusted.
Each MEG session was subdivided into four blocks during which each body site was stimulated 125 times, resulting in 500 stimulations per area per session. The stimulated regions and intervals between stimulations were pseudorandomized. To mask the noise made by the stimulators, participants listened to 60-dB white noise presented binaurally through air-conducting tubes with foam ear tips. Participants were instructed to remain as still as possible, to fixate a cross drawn at the center of a board positioned 50 cm away from their eyes, and to avoid blinking during the stimulation period. Every 30 stimulations the white noise was interrupted for 3 s, indicating a rest period during which participants were instructed/allowed to blink. A few minutes of rest were also inserted between each block.
Behavioral data analysis.
For each participant and for each body site the mean of the verbal responses (“one” or “two”) was plotted as a function of distance between the probes and the psychometric function was fitted with a binary logistic regression. Threshold was determined from the fitted data and defined as the distance at which participants responded “two” 50% of the time. S1 and S2 thresholds were statistically analyzed for stability with four one-way repeated-measures ANOVAs (rmANOVAs) with factor Session (S1/S2). Two paired t-tests were performed to assess whether left-D2 and left-D5 thresholds (measured only at S2) were different from those of right-D2 and right-D5 at this same session. Pre (average of S1 and S2) and Post (S3) thresholds were analyzed with three two-way rmANOVAs with factors Side (left/right) and Session (Pre/Post). All statistical analyses were conducted with STATISTICA (v.10, StatSoft). Data were checked for normality with Kolmogorov-Smirnov tests. Post hoc analyses were performed with Bonferroni tests (referred to as PBonf). All group data are expressed as means ± SE.
Magnetoencephalography data analysis.
Neuromagnetic analyses were conducted with CTF software (VSM Medtech). After muscle artifact rejection, data sets were low-pass filtered to 100 Hz before being segmented into epochs of 350 ms including a prestimulus baseline of 100 ms. Trials coinciding with eye movements were automatically rejected on the basis of EOG recordings (threshold of 20 μV) as well as trials in which head motion exceeded the average head motion by 3 mm. After rejection of these trials the mean head position was recalculated separately for each condition and used to correct for head motion. These preprocessing steps resulted in an average of 404 (±15.72) artifact-free trials per area per session, which were averaged using the 100-ms prestimulus period for baseline correction. MEG data were coregistered to each participant's structural MRI using the position of the coils (3 fiducial points), which defined a head-based Cartesian coordinate system with the origin at the midpoint between the left and right preauricular points.
For source identification, ECDs were used to model local cerebral activity within a multispherical head model. For fingertip data a single ECD was fitted, as most of the activity was expected to be in the contralateral hemisphere. In contrast, two ECDs were fitted for the lips because of multiple MEG (Disbrow et al. 2003; Hoshiyama et al. 1996; Nevalainen et al. 2006) and fMRI (Eickhoff et al. 2008; Iannetti et al. 2003; Lin et al. 2010) reports showing a bilateral response following unilateral lip stimulation. Data from all MEG channels over a time interval of 7 ms around the peak of the first prominent component were used to solve the inverse problem. Given that lip sources were expected to be bilateral and symmetric, a minimal sphere was systematically used to exclude the center of the head model from the source space in order to minimize convergence of bilateral dipoles. The radius of this minimal sphere was set to 35% (i.e., 2.57 ± 0.02 cm) of each participant's head model radius. An extra dipole (excluded from further analyses) was added if the model did not explain >90% of the variance of the magnetic field (Wühle et al. 2010, 2011). Despite this additional dipole, three participants exhibited errors of fit >10% and one participant showed erroneous location of contralateral dipoles for at least one body site, in one session. They were thus excluded. These procedures resulted in ECDs explaining >95% of the variance (95.45 ± 0.47%) for the fingertips and >97% of the variance for the lips (97.25 ± 0.28%). Statistical analysis confirmed that the goodness of fit did not vary across sessions. Because of large volumes of confidence (209.20 ± 170.18 mm3), ipsilateral dipoles were excluded from further analyses. In contrast, contralateral dipoles exhibited reasonably sized volumes of confidence (i.e., 2.65 ± 0.96 mm3 for the lips and 2.60 ± 1.37 mm3 for the fingertips), with an error in dipole localization inferior to 1 mm in radius (0.78 ± 0.08 mm). Statistical analysis revealed that this uncertainty in localization did not vary across sessions. Dipole locations were analyzed on the basis of their Cartesian (x, y, z) and polar [the eccentricity or radius: r = , the theta angle: θ = cos−1(z/r), and the phi angle: φ = tan−1(y/x)] coordinates but also by computing the Euclidean distance (ED) between dipoles. Statistical analyses of peak latencies, dipole coordinates, and strengths were performed with rmANOVAs and post hoc Fisher's tests (referred to as PLSD).
RESULTS
Behavior.
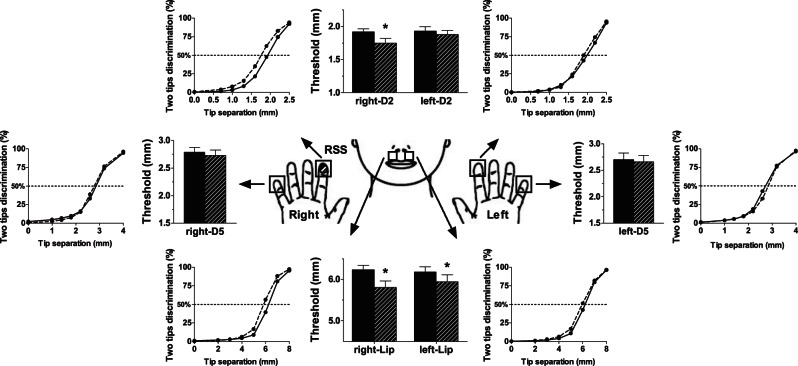
2PDTs obtained at baseline were stable (all P values > 0.12), and left-D2 and left-D5 thresholds at S2 were not statistically different from those obtained for their homologs at S2 (both P values > 0.7). Comparing thresholds obtained before and after RSS (Fig. 1), a significant interaction was found for the index fingertips [F(1.20) = 4.82, P = 0.040], with a significant decrease of right-D2 thresholds after RSS (PBonf = 0.002) while left-D2 thresholds remained stable (PBonf > 0.90). Similar threshold decreases were observed after RSS for both sides of the upper lip [F(1.20) = 11.47, P < 0.003]. In contrast, thresholds obtained at both little fingertips remained stable across sessions.
Fig. 1.
Mean psychometric curves and thresholds before and after the repetitive somatosensory stimulation (RSS) procedure at the 6 tested body sites. Psychometric curves correspond to the average over all individual regression curves, and bar plots represent the mean thresholds obtained respectively before (Pre, solid) and after (Post, hatched) RSS applied to the right-D2. *PBonf < 0.05.
Evoked responses.
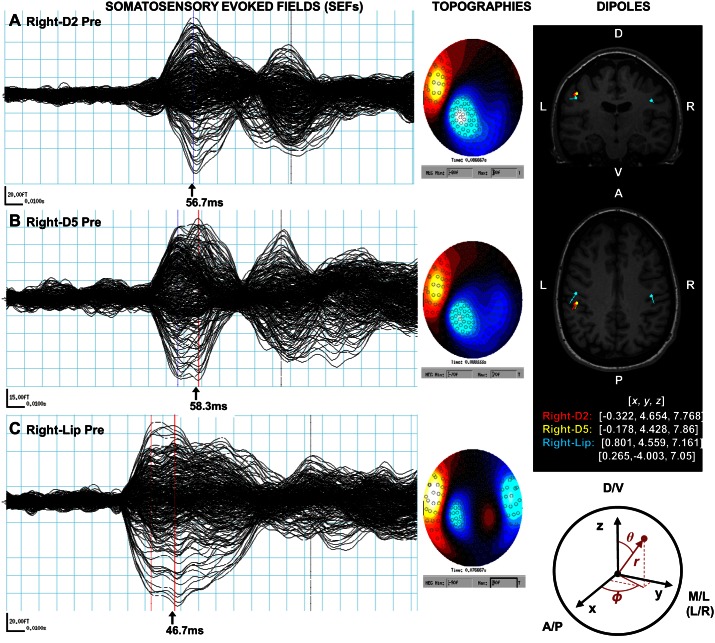
Somatosensory evoked fields (SEFs) were identified for all participants, with a first prominent peak observed on average at 60.10 (±1.86) ms after fingertip stimulation and at 47.38 (±1.51) ms after upper lip stimulation (see Table 1 for group data and Fig. 2 for an individual example).
Table 1.
Mean parameters of ECDs for each tested body site across sessions
| Peak Latency, ms |
Errors of Fit, % |
Dipole Coordinates, mm |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre |
Post |
|||||||||
| Body Site | Pre | Post | Pre | Post | x | y | z | x | y | z |
| Right-D2 | 63.7 ± 3.3 | 62.0 ± 2.9 | 4.2 ± 0.4 | 4.7 ± 0.5 | 0.1 ± 1.2 | 39.9 ± 1.3 | 26.9 ± 1.6 | 0.5 ± 1.4 | 39.8 ± 1.3 | 27.8 ± 1.5 |
| Left-D2 | 58.6 ± 0.8 | 58.1 ± 0.6 | 3.9 ± 0.5 | 4.3 ± 0.6 | 0.1 ± 1.1 | −43.6 ± 1.1 | 31.4 ± 1.0 | 0.6 ± 1.3 | −43.6 ± 1.2 | 31.3 ± 0.9 |
| Right-D5 | 61.7 ± 1.6 | 61.5 ± 1.7 | 5.1 ± 0.5 | 4.9 ± 0.3 | −1.5 ± 1.3 | 37.8 ± 1.3 | 35.1 ± 1.4 | −1.2 ± 1.3 | 36.8 ± 1.6 | 32.6 ± 1.5 |
| Left-D5 | 57.7 ± 0.8 | 57.4 ± 0.8 | 4.3 ± 0.5 | 5.0 ± 0.4 | −2.3 ± 1.3 | −42.6 ± 1.3 | 36.4 ± 1.2 | −2.0 ± 1.1 | −42.3 ± 1.1 | 35.7 ± 1.5 |
| Right-Lip | 47.5 ± 1.9 | 47.1 ± 1.8 | 2.8 ± 0.3 | 2.9 ± 0.3 | 2.7 ± 1.5 | 39.7 ± 1.0 | 17.0 ± 1.1 | 3.7 ± 1.6 | 41.1 ± 1.5 | 17.2 ± 1.1 |
| Left-Lip | 47.5 ± 1.0 | 47.5 ± 1.1 | 2.6 ± 0.3 | 2.7 ± 0.3 | 4.7 ± 1.2 | −43.8 ± 1.3 | 17.0 ± 1.1 | 4.1 ± 1.4 | −46.0 ± 1.3 | 17.2 ± 0.9 |
Values are mean ± SE peak latencies, errors of fit, and Cartesian coordinates of the equivalent current dipoles (ECDs) obtained for each body site across sessions.
Fig. 2.
Representative example of the data obtained after stimulation of right-D2 (A), right-D5 (B), and right-Lip (C) during the Pre session. Left: mean somatosensory evoked fields (SEFs), with the peak of the first prominent component and their respective latency. Center: topography of the magnetic fields observed at the peak and projected at the sensor level. Right: equivalent current dipoles (ECDs) modeling the 4 respective sources, overlaid on the structural MRI of the individual. Note the bilateral dipole obtained for the right-Lip and the somatotopic organization with D5-D2-Lip being sequentially more ventral and anterior. The different dipolar Cartesian (x, y, z) and polar (r, θ, φ) coordinates analyzed are represented on the schema below. A/P, antero-posterior; D/V, dorso-ventral; M/L, medio-lateral; L/R, left/right.
A three-way rmANOVA (Session × Area × Side) revealed significant differences between body sites [F(2,40) = 66.71, P < 10−6], with, as expected, shorter peak latencies for the lips than for the fingertips (both PLSD values < 10−6). It also revealed a very small (1 ms or less) significant decrease in peak latencies after RSS [F(1,20) = 6.14, P = 0.022], but because this difference is below the temporal resolution possible given our sampling rate it cannot be interpreted as a meaningful difference. The distribution of the magnetic fields observed at the sensor level for these components revealed a contralateral pattern for the fingertips (Fig. 2, A and B) and a bilateral pattern for the upper lips (Fig. 2C), suggesting bilateral sources. This confirmed the need for two ECDs to model lip activity.
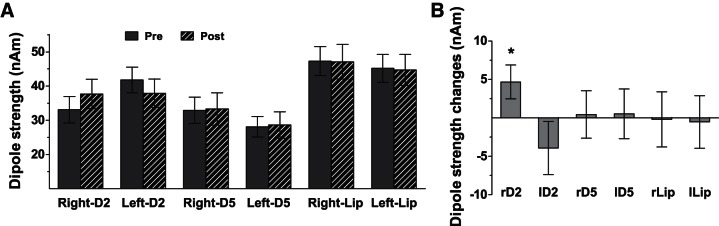
Strength of ECDs.
Dipole strength was significantly higher for the lips than for the fingertips [F(2,40) = 9.36, P < 5 × 10−4, both PBonf values < 0.023; see Fig. 3A] and for right than left body sites but only after RSS [significant interaction between sessions and sides: F(1,20) = 4.50, P = 0.047, PLSD = 0.027]. However, a close look at the values observed for the various body parts (Fig. 3A) suggests that this interaction likely arises from large differences between sides in the Pre session for D2 and D5, which were maintained after RSS for D5 but decreased for D2, because of opposite changes for the two index fingers. Interestingly, these dipole strength changes at the index fingers were significantly different from zero only for the RSS-stimulated finger, namely, right-D2 [t(20) = 2.12, P = 0.046; Fig. 3B].
Fig. 3.
Mean dipole strength obtained for each of the tested body sites Pre and Post RSS (A) and their changes across sessions (Post − Pre) (B). r/l, right/left. Significant RSS-induced change: *P < 0.05.
Somatotopy of ECDs.
A somatotopic organization of the ECDs was observable at the individual level (Fig. 2, right) and also at the level of the group, with D5, D2, and Lip ECDs positioned sequentially more ventral [F(2,40) = 137.88, P < 10−6, 3 PLSD values < 10−5] and anterior [F(2,40) = 20.61, P < 10−6, 3 PLSD values < 0.022; see Table 1 for mean dipole coordinates]. Lip ECDs were also significantly more lateral than D5 ECDs [F(2,40) = 4.51, P = 0.017, PLSD = 0.005]. These results are consistent with the somatotopic organization described in the literature (Nakamura et al. 1998; Penfield and Boldrey 1937). In addition, ECDs were significantly more medial for right than left body sites [F(1,40) = 17.23, P < 5.10−4], and an interaction [F(2,40) = 3.69, P = 0.034] revealed that D2 (PLSD < 5.10−4) and D5 (PLSD = 0.037) ECDs were significantly more ventral in the left hemisphere than the right, while the location of lip dipoles along the dorsoventral axis did not differ between hemispheres (PLSD = 0.996). This pattern suggests a smaller distance between lip and finger ECDs in the hemisphere contralateral to the dominant hand. This was confirmed by a significant difference between sides for the Lip-D2 Δθ angle [F(1,20) = 4.91, P = 0.038], which was significantly smaller for right than left body sites. Interestingly, while a similar somatotopy was found when analyzing EDs [F(2,40) = 46.76, P < 10−6] with D2–D5 ED being smaller than Lip-D2 ED, itself smaller than Lip-D5 ED (all PLSD < 5 × 10−5), an interaction between body site pairs and side was also observed [F(2,40) = 6.65, P = 0.003]. The latter revealed a larger ED between D2 and D5 ECDs in the left hemisphere (PLSD = 0.018), while the other two body site pairs (Lip-D2 and Lip-D5) had similar EDs in both hemispheres (both PLSD values > 0.05), suggesting a larger representation of the dominant hand in its contralateral hemisphere.
RSS-induced changes in ECDs.
When considering Pre/Post changes, ECDs for all six body sites shifted significantly after RSS, by 6.32 (±0.82) mm on average [6 t-tests, all t(20) values > 6.80, all P values < 10−5 << PBonf = 0.008]. To investigate the direction of these shifts we analyzed RSS-induced changes in the EDs and polar coordinates between body site pairs using separate two-way rmANOVAs (Session × Side). These analyses revealed a significant increase in the ED [F(1,20) = 8.27, P = 0.009, Fig. 4A] and in the Δθ angle [F(1,20) = 6.40, P = 0.020, Fig. 4B] between the Lip and D2 dipoles after RSS for both sides of the body. Note, however, that ED changes were significantly different from zero only for right Lip-D2 [mean change right Lip-D2: 2.06 ± 0.75 mm, t(20) = 2.75, P = 0.012; mean change left Lip-D2: 1.09 ± 0.76 mm, t(20) = 1.44, P = 0.165]. In contrast to Lip-D2 ECDs, no changes were observed for D2–D5 and Lip-D5 EDs or Δθ angles.
Fig. 4.
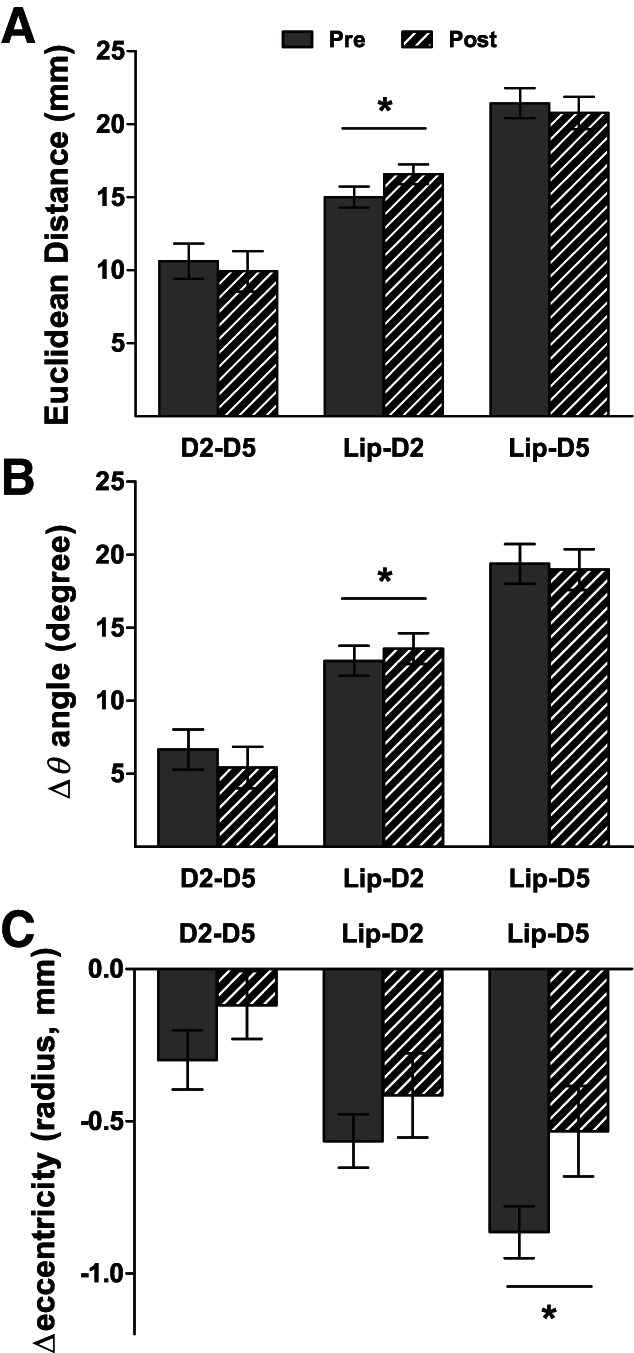
Mean relative distance or angle between ECDs of the tested body sites Pre and Post RSS. A: mean Euclidean distance. B: mean Δθ angle. C: mean Δeccentricity between D2 and D5 (D2–D5), Lip and D2 (Lip-D2), and Lip and D5 (Lip-D5). Significant RSS-induced changes: *P < 0.05.
A significant decrease in the relative eccentricity between the Lip and D5 dipoles was also observed after RSS [F(1,20) = 5.56, P = 0.029; Fig. 4C], but here again, eccentricity changes were significantly different from zero only for right Lip-D5 [mean change: 0.39 ± 0.15 mm, t(20) = 2.59, P = 0.017]. No significant changes were observed for Δφ angles, and similar analyses performed on Cartesian coordinates did not reveal any further effects. Finally, no significant linear correlation was found between changes in dipole location (ED, Δeccentricity, or Δθ angles) and 2PDT changes (all P values > 0.05).
DISCUSSION
The purpose of this study was to assess the neurophysiological substrates underlying the RSS-induced perceptual changes recently observed across the hand-face border (Muret et al. 2014). In agreement with our previous report, we found that 3 h of RSS at right-D2 improved tactile spatial acuity at this finger and at both sides of the upper lip, without affecting left-D2. Here we additionally show that tactile perception improvement does not occur on either the right or left little finger. These findings suggest that RSS-induced perceptual changes within the hands are present at the stimulated finger but do not spread to distant fingers within the same hand (for similar results for the D3 adjacent finger see Godde et al. 2000) or the other hand, while they do spread to both sides of the upper lip. The absence of within-hand spread, here replicated and extended to a nonadjacent finger (D5), may possibly be explained by the existence of intracortical inhibitory connections underlying lateral inhibition (Négyessy et al. 2013; Simões et al. 2001), which might prevent plastic changes from transferring locally.
Before considering the RSS-induced changes, several differences between body parts should be emphasized. First, the overall topography of body sites that we assessed in this study (right/left upper lips and D2/D5 fingertips) was consistent with the classical somatotopic organization of SI (Nakamura et al. 1998; Penfield and Rasmussen 1950). Indeed, analysis of the D5, D2, and Lip ECDs revealed a clear somatotopic organization in both hemispheres, with D5, D2, and Lip represented sequentially more ventral and anterior. The largest differences between body sites were observed along the dorso-ventral direction (i.e., y-axis and θ angle). The stable presence of this global organization across sessions, together with the high goodness of fit and small uncertainty in dipole localization, attests to the reliability of our dipoles across body sites and sessions, thus ensuring that any change in our electrophysiological variables could be reliably ascribed to the RSS procedure. Also consistent with previous reports (Imai et al. 2003), we found that the ED between D2 and D5 dipoles was significantly larger for the right than for the left side of the body, suggesting a comparatively larger somatosensory representation of the dominant hand. Supporting this view, it has been reported that the rostral area of the postcentral gyrus is more extended in the left than in the right hemisphere (Jung et al. 2003). An asymmetry in dipole location was also found between Lip and D2, as Δθ measures revealed a smaller angle between their dipoles for the right body sites. Together with the larger D2–D5 distance mentioned above and the absence of Lip-D5 asymmetry, this novel result suggests a closer proximity between the Lip and D2 representations in the left hemisphere, which could come from a smaller D1 representation between them, a smaller Lip representation, or, more likely, from more overlapping representations than in the right hemisphere. However, this remains to be investigated. Finally, still regarding differences between body parts, dipole strength was higher for the lips than for the fingertips. Given that the current dipole strength is usually considered as an indicator of the net strength of cortical polarization, which reflects the total number of synchronously firing neurons contributing to the stimulus-driven cortical response (Williamson and Kaufman 1990), these differences may reflect a larger neuronal population (i.e., representation) for the lip than for each fingertip. Such differences in cortical magnification are thought to be related to the differential sensitivity/acuity across body sites, arising from the differential densities of receptors embedded in the skin.
Considering the RSS-induced changes, the analysis of dipole model sources revealed several shifts of body site ECDs after RSS. Before discussing these, it is worth noting that these changes in dipole location are unlikely to arise from methodological issues such as altered repositioning of the participant's head inside the MEG helmet, coregistration errors, or systematic head motion, since for a given participant body sites stimulated within the same block were not all similarly affected. In addition, a systematic shift would have left the relative distances between ECDs unaltered, which is not the case. One other possibility is that the stimulators might have been attached slightly differently across body sites and sessions, resulting in slightly different stimulation intensities, but stimulation intensity changes typically alter dipole strength and latency (the higher the intensity, the higher the dipole strength and the lower the latency) rather than localization (see, e.g., Hoshiyama and Kakigi 2001; Otsuru et al. 2011). Thus our results can be interpreted as evidence that RSS induced reliable, but multidirectional, shifts at the tested body sites. These shifts resulted in an increased distance between Lip and D2 dipoles (ED and Δθ angle) as well as a decrease in the relative eccentricity between the Lip and D5 dipoles, which were both more pronounced on the right side of the body. In addition to these shifts, we also observed side-specific differences after RSS, with higher dipole strength for the right compared with the left body sites, this effect being mainly driven by opposite changes at both index fingers.
Among these results, the increased distance between the Lip and D2 dipoles seems the most relevant with respect to the RSS-induced acuity improvement observed at right-D2 and both upper lips (here and in Muret et al. 2014). Given the previously reported enlargement of the cortical representation of right-D2 within SI (Hodzic et al. 2004; Pleger et al. 2003), the increased distance between the Lip and D2 dipoles could arise from either 1) a symmetric expansion of right-D2, which could have passively displaced the Lip representation away from right-D2, or 2) an asymmetric expansion of right-D2, resulting in a shift of its representation away from the Lip representation. The latter option seems more likely since D2 expansion has been associated with a shift in the fMRI activation peak (Pleger et al. 2003), and since with EEG or MEG this plasticity has been expressed in terms of a shift of the RSS-stimulated right-D2 ECD (Dinse et al. 2003; Godde et al. 2003; Pleger et al. 2001), with an increase in its dipole strength (Dinse et al. 2003; Pleger et al. 2001). In the present study, the higher dipole strength reported after RSS for right than for left body sites is consistent with this literature, especially given the fact that the change in dipole strength was significantly different from zero only for right-D2. Together with the enlargement of right-D2's representation previously reported by fMRI (Hodzic et al. 2004; Pleger et al. 2003), this increased dipole strength is compatible with an increase in the amount of cortical resources contributing to the stimulus-driven response (Williamson and Kaufman 1990), which could in turn explain the improved discrimination. This interpretation should be taken with caution, however, as dipole strength can also be altered by changes in dipole orientation or eccentricity. Altogether, these different pieces of evidence point toward an asymmetric enlargement of right-D2's representation.
It is important to note that while this plasticity could explain the dipole shifts observed within the hemisphere contralateral to the RSS-stimulated body site, this may not be the only mechanism involved, as it cannot account for the bilateral increase in Lip-D2 distance. Rather, it would appear that plastic changes also affected the Lip ECDs. As a passive displacement of the Lip ECDs due to right-D2 expansion is unlikely, Lip ECDs may have shifted actively or as a result of an expansion of lip representations. While these two options cannot be disentangled in the present study, the lack of dipole strength changes for the lips tends to support a shift rather than an expansion. In addition, this hypothesis is consistent with plastic changes reported in monkeys that received an extensive stimulation of the fingertips with a rotating grooved disk (Jenkins et al. 1990). Using microelectrode mapping techniques, Jenkins and colleagues revealed an expansion of the cortical representations of the stimulated fingertips, similar to that reported in humans for the RSS-stimulated right-D2 (Hodzic et al. 2004; Pleger et al. 2003). In addition, the borders between the representations of individual digits and digit segments shifted in parallel. This resembles the unspecific and multidirectional shifts of dipoles observed in the present study. Interestingly, Jenkins and colleagues also reported a significant lateral translocation of the borders between the representations of the hand and the face, consistent with the increased Lip-D2 distance. Finally, it is worth noting that the possible shift of lip representations, combined with an asymmetric expansion of right-D2 representation, could explain the larger effect found for the right side of the body (as supported by the t-tests against zero). While the plastic changes observed within the right hemisphere could at first appear surprising, they are consistent with our behavioral data showing improved perception at the left upper lip. In addition to the increase in Lip-D2 distance, an increased eccentricity was found between the Lip and D5 ECDs. This effect might come from the shift of Lip dipoles or, alternatively, from a rotation of Lip and D5 dipoles around that of D2. It should be noted, however, that mapping an evolution of source strength and/or source eccentricity to some quantitative and mechanistic plastic phenomenon is nontrivial; therefore such changes should be interpreted with caution.
RSS is hypothesized to rely on Hebbian-like mechanisms (Godde et al. 1996; see Beste and Dinse 2013; Parianen Lesemann et al. 2015 for recent reviews on RSS), the repetitive stimulation of several cutaneous receptive fields (RFs) leading to long-term potentiation (LTP)-like plasticity. This hypothesis is supported by several lines of evidence showing 1) the pharmacological sensitivity of RSS effects (Bliem et al. 2008; Dinse et al. 2003), 2) the requirement of a Hebbian-like “costimulation” of several cutaneous RFs (Pleger et al. 2003; Ragert et al. 2008), 3) an inverse modulation of RSS behavioral effects depending on the stimulation frequency (Ragert et al. 2008), and 4) the short timescale over which RSS cortical and behavioral effects occur (3 h) and last (up to 8 h; Godde et al. 2000), the final point being consistent with transient changes in synaptic efficiency. This RSS-induced plasticity is thus thought to trigger cortical plastic changes that may take place at, but are possibly not limited to, the level of the efficiency of the synaptic transmission, which could underlie the enlarged representation of the stimulated finger within SI (Hodzic et al. 2004; Pleger et al. 2003). In turn, this enlarged representation would provide more resources available to process tactile information arising from the finger, which could explain the improvement in tactile discrimination.
Regarding the remote changes observed at both the perceptual and cortical levels after RSS of right-D2, while the present study cannot disentangle whether RSS-induced plastic changes affected the cortical representation of other fingers, an improved tactile discrimination was found at the lips but not at D5 fingertips. Together with the increased distance between Lip and D2 ECDs, this pattern of transfer suggests that RSS effects spread more easily to the face than to other fingers. As previously hypothesized by us (Muret et al. 2014), this preferential transfer to the face could arise from the presence of long-range cortico-cortical connections across the hand and face representations in SI (Fang et al. 2002; Florence et al. 1998; Manger et al. 1997; Marik and Hickmott 2009; Paullus and Hickmott 2011), which may have intrinsic properties different from those connecting the finger representations (Jones and Powell 1970), which are thought to underlie lateral inhibition (Négyessy et al. 2013; Simões et al. 2001) through GABAergic activity (Li et al. 2002). RSS may also modulate this lateral inhibition since it has already been shown to affect intracortical inhibition in a visuo-motor task (Wilimzig et al. 2012) and paired-pulse inhibition (Höffken et al. 2007). Finally, any contribution from subcortical and/or subthalamic plastic changes affecting these two body parts (see Kambi et al. 2014), then magnified at the cortical level, cannot be ruled out and needs further investigation. Therefore, depending on the origin of RSS-induced plastic changes, the transfer to the ipsilateral hemisphere (i.e., left lip representation) can be accounted for either by bilateral thalamo-cortical projections or by transcallosal connections, as the lips have been reported to be bilaterally represented within SI (Blatow et al. 2007; Disbrow et al. 2003; Nagamatsu et al. 2000; Nash et al. 2010; Nevalainen et al. 2006). Clearly, further investigations are needed to unravel the processes at stake in the cortical reorganization reported here.
From a more functional perspective, one may wonder why such perceptual improvement would transfer (apparently more easily) to the lip (than to the other fingers). While this question requires further investigation, one may speculate that improving perception at both the hand and the face might be beneficial for primitive behaviors such as eating (i.e., bringing food to the mouth). From this perspective, “transferring” an improved perception from the finger to the face may help haptic perception (already reported at the hand level; see Kalisch et al. 2008, 2010; Kowalewski et al. 2012), and eventually precision grip involving hand and mouth coordination.
Finally, the present results may appear contradictory with respect to some well-known clinical cases, such as hand-arm vibration syndrome (HAVS; also known as vibration-induced neuropathy or white finger). Indeed, while this syndrome is known to occur after extensive use of vibrating tools (thus inducing an extensive vibratory stimulation that may appear similar to RSS), unlike RSS HAVS is associated with negative neurological symptoms such as tingling, paresthesia, sensory loss, and decreased dexterity (see Heaver et al. 2011 for a review). But despite some similarities, the tactile stimulation produced by RSS and vibrating tools differs drastically not only in terms of nature, frequency, and pattern of stimulation but also in terms of duration. Indeed, RSS consists of a more “on/off” repeated tapping stimulation than a vibration and its average frequency is 1 Hz, whereas the dominant frequencies for HAVS to occur are between 25 and 320 Hz (Heaver et al. 2011). Furthermore, RSS stimulation is jittered to avoid adaptation processes, while HAVS vibratory stimulation is continuous. Finally, most of the RSS effects have been studied after short stimulation durations (i.e., 3 h), whereas HAVS is usually triggered after decades of protracted daily stimulation. In addition, while a cortical reorganization similar to that induced by RSS (i.e., enlarged representations in SI) has been reported in HAVS patients (Björkman et al. 2010), the pattern of perceptual changes associated with this plasticity is different from that observed after RSS. Indeed, HAVS is associated with increased tactile detection thresholds but preserved two-point discrimination and vibration perception (Björkman et al. 2010), while RSS is associated with unaltered tactile detection thresholds (Kalisch et al. 2007; Ladda et al. 2014) but improved two-point discrimination (for reviews see Beste and Dinse 2013; Parianen Lesemann et al. 2015) and impaired frequency discrimination (Hodzic et al. 2004). These discrepancies are likely to arise from differences in the underlying mechanisms triggering cortical plastic changes, since HAVS has also been associated with sensory nerve damage (Goldsmith et al. 1994; Hirata and Sakakibara 2007) and various vascular and musculoskeletal disorders (see Bovenzi 1998; Bovenzi et al. 2000; Gemne 1997; Schweigert 2002), making this pathology largely multifactorial. Altogether, while these different features suggest that HAVS and RSS may rely on different mechanisms, since the present study provides evidence of remote adaptive plastic changes, it would be interesting to investigate whether RSS could be used to improve somatosensory deficits of either peripheral (e.g., HAVS patients) or central (e.g., stroke patients) origin.
Last but not least, the discrepancy between RSS and HAVS in terms of perceptual changes also highlights the complex relationship between cortical reorganization and behavior, similar cortical changes being associated with opposite perceptual changes. This raises the point of technical and analytical limitations, with the possibility that MEG does not capture all the signal of interest and that imaging analyses always restrict the data to a few features in order to take into account a number of variables as minimal as possible. Regarding the technical limitation, the use of other imaging techniques such as EEG and fMRI could provide complementary information. For instance, using both fMRI and EEG to investigate the spatiotemporal characteristics of visual cortex activity, Whittingstall and colleagues (2008) found that the location of dipole sources and positive BOLD responses were similarly affected by changes in stimulus location, whereas this was not the case for negative BOLD responses. This suggests that negative BOLD (measured with fMRI) might provide complementary information (see also Amedi et al. 2005; Zeharia et al. 2012) not captured by dipole source analysis.
Taken together, these results show that the pattern of changes induced by RSS of the right index finger in SI is much more complex than previously thought. Indeed, in line with our behavioral results showing improved tactile acuity at the right-D2 and both upper lips, we observed changes in the representation of these areas, suggesting the existence of hand-face cross-border changes following RSS at a fingertip. Interestingly, changes were also observed in the hemisphere ipsilateral to the stimulated finger, suggesting that RSS induces widespread changes also reaching the other hemisphere.
GRANTS
This work was supported by the Labex/Idex ANR-11-LABX-0042, ANR-DFG (ANR-14-CE35-0014-01) to K. T. Reilly and H. R. Dinse, by grants from Fondation pour la Recherche Médicale (FRM), and by a James S. McDonnell Foundation Scholar Award to A. Farnè. D. Muret was supported by the Rhône-Alpes region (ExploraDoc 2013) and the Progamme Avenir Lyon Saint-Etienne (Idex).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.M., C.D., J.M., K.T.R., and A.F. conception and design of research; D.M. and S.D. performed experiments; D.M. and S.D. analyzed data; D.M., K.T.R., and A.F. interpreted results of experiments; D.M. prepared figures; D.M. drafted manuscript; D.M., S.D., H.R.D., C.D., J.M., K.T.R., and A.F. edited and revised manuscript; D.M., S.D., H.R.D., C.D., J.M., K.T.R., and A.F. approved final version of manuscript.
REFERENCES
- Amedi A, Malach R, Pascual-Leone A. Negative BOLD differentiates visual imagery and perception. Neuron 48: 859–872, 2005. [DOI] [PubMed] [Google Scholar]
- Beste C, Dinse HR. Learning without training. Curr Biol 23: R489–R499, 2013. [DOI] [PubMed] [Google Scholar]
- Björkman A, Weibull A, Svensson J, Balogh I, Rosén B. Cortical changes in dental technicians exposed to vibrating tools. Neuroreport 21: 722–726, 2010. [DOI] [PubMed] [Google Scholar]
- Blatow M, Nennig E, Durst A, Sartor K, Stippich C. fMRI reflects functional connectivity of human somatosensory cortex. Neuroimage 37: 927–936, 2007. [DOI] [PubMed] [Google Scholar]
- Bliem B, Tegenthoff M, Dinse HR. Cholinergic gating of improvement of tactile acuity induced by peripheral tactile stimulation. Neurosci Lett 434: 129–132, 2008. [DOI] [PubMed] [Google Scholar]
- Bovenzi M. Exposure-response relationship in the hand-arm vibration syndrome: an overview of current epidemiology research. Int Arch Occup Environ Health 71: 509–519, 1998. [DOI] [PubMed] [Google Scholar]
- Bovenzi M, Giannini F, Rossi S. Vibration-induced multifocal neuropathy in forestry workers: electrophysiological findings in relation to vibration exposure and finger circulation. Int Arch Occup Environ Health 73: 519–527, 2000. [DOI] [PubMed] [Google Scholar]
- Braun C, Schweizer R, Elbert T, Birbaumer N, Taub E. Differential activation in somatosensory cortex for different discrimination tasks. J Neurosci 20: 446–450, 2000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C, Wilms A, Schweizer R, Godde B, Preissl H, Birbaumer N. Activity patterns of human somatosensory cortex adapt dynamically to stimulus properties. Neuroreport 11: 2977–2980, 2000b. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science 301: 91–94, 2003. [DOI] [PubMed] [Google Scholar]
- Disbrow EA, Hinkley LB, Roberts TP. Ipsilateral representation of oral structures in human anterior parietal somatosensory cortex and integration of inputs across the midline. J Comp Neurol 467: 487–495, 2003. [DOI] [PubMed] [Google Scholar]
- Dutta A, Kambi N, Raghunathan P, Khushu S, Jain N. Large-scale reorganization of the somatosensory cortex of adult macaque monkeys revealed by fMRI. Brain Struct Funct 219: 1305–1320, 2013. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Fink GR, Zilles K. Functional lateralization of face, hand, and trunk representation in anatomically defined human somatosensory areas. Cereb Cortex 18: 2820–2830, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Jain N, Kaas JH. Few intrinsic connections cross the hand-face border of area 3b of New World monkeys. J Comp Neurol 454: 310–319, 2002. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375: 482–484, 1995. [DOI] [PubMed] [Google Scholar]
- Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science 282: 1117–1121, 1998. [DOI] [PubMed] [Google Scholar]
- Gagné M, Hétu S, Reilly KT, Mercier C. The map is not the territory: motor system reorganization in upper limb amputees. Hum Brain Mapp 32: 509–519, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemne G. Diagnostics of hand-arm system disorders in workers who use vibrating tools. Occup Environ Med 54: 90–95, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde B, Ehrhardt J, Braun C. Behavioral significance of input-dependent plasticity of human somatosensory cortex. Neuroreport 14: 543–546, 2003. [DOI] [PubMed] [Google Scholar]
- Godde B, Spengler F, Dinse HR. Associative pairing of tactile stimulation induces somatosensory cortical reorganization in rats and humans. Neuroreport 8: 281–285, 1996. [DOI] [PubMed] [Google Scholar]
- Godde B, Stauffenberg B, Spengler F, Dinse HR. Tactile coactivation-induced changes in spatial discrimination performance. J Neurosci 20: 1597–1604, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith PC, Molina FA, Bunker CB, Terenghi G, Leslie TA, Fowler CJ, Polak JM, Dowd PM. Cutaneous nerve fibre depletion in vibration white finger. J R Soc Med 87: 377–381, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaver C, Goonetilleke KS, Ferguson H, Shiralkar S. Hand-arm vibration syndrome: a common occupational hazard in industrialized countries. J Hand Surg Eur Vol 36: 354–363, 2011. [DOI] [PubMed] [Google Scholar]
- Hirata M, Sakakibara H. Sensory nerve conduction velocities of median, ulnar and radial nerves in patients with vibration syndrome. Int Arch Occup Environ Health 80: 273–280, 2007. [DOI] [PubMed] [Google Scholar]
- Hodzic A, Veit R, Karim AA, Erb M, Godde B. Improvement and decline in tactile discrimination behavior after cortical plasticity induced by passive tactile coactivation. J Neurosci 24: 442–446, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffken O, Veit M, Knossalla F, Lissek S, Bliem B, Ragert P, Dinse HR, Tegenthoff M, Oliver H, Veit M, Knossalla F, Lissek S, Bliem B, Ragert P, Dinse HR, Tegenthoff M. Sustained increase of somatosensory cortex excitability by tactile coactivation studied by paired median nerve stimulation in humans correlates with perceptual gain. J Physiol 584: 463–471, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R. Two evoked responses with different recovery functions in the primary somatosensory cortex in humans. Clin Neurophysiol 112: 1334–1342, 2001. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R, Koyama S, Kitamura Y, Shimojo M, Watanabe S, Ryusuke K, Koyama S, Kitamura Y, Shimojo M, Watanabe S, Kakigi R. Somatosensory evoked magnetic fields following stimulation of the lip in humans. Electroencephalogr Clin Neurophysiol 100: 96–104, 1996. [DOI] [PubMed] [Google Scholar]
- Iannetti G, Porro C, Pantano P, Romanelli P, Galeotti F, Cruccu G. Representation of different trigeminal divisions within the primary and secondary human somatosensory cortex. Neuroimage 19: 906–912, 2003. [DOI] [PubMed] [Google Scholar]
- Imai T, Kamping S, Breitenstein C, Pantev C, Lütkenhöner B, Knecht S. Learning of tactile frequency discrimination in humans. Hum Brain Mapp 18: 260–271, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guíc-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol 63: 82–104, 1990. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP. Connexions of the somatic sensory cortex of the rhesus monkey. 3. Thalamic connexions. Brain 93: 37–56, 1970. [DOI] [PubMed] [Google Scholar]
- Jung P, Baumgärtner U, Bauermann T, Magerl W, Gawehn J, Stoeter P, Treede R. Asymmetry in the human primary somatosensory cortex and handedness. Neuroimage 19: 913–923, 2003. [DOI] [PubMed] [Google Scholar]
- Kalisch T, Tegenthoff M, Dinse HR. Differential effects of synchronous and asynchronous multifinger coactivation on human tactile performance. BMC Neurosci 8: 58, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch T, Tegenthoff M, Dinse HR. Improvement of sensorimotor functions in old age by passive sensory stimulation. Clin Interv Aging 3: 673–690, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch T, Tegenthoff M, Dinse HR. Repetitive electric stimulation elicits enduring improvement of sensorimotor performance in seniors. Neural Plast 2010: 690531, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambi N, Halder P, Rajan R, Arora V, Chand P, Arora M, Jain N. Large-scale reorganization of the somatosensory cortex following spinal cord injuries is due to brainstem plasticity. Nat Commun 5: 3602, 2014. [DOI] [PubMed] [Google Scholar]
- Kowalewski R, Kattenstroth J, Kalisch T, Dinse HR. Improved acuity and dexterity but unchanged touch and pain thresholds following repetitive sensory stimulation of the fingers. Neural Plast 2012: 974504, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladda AM, Pfannmoeller JP, Kalisch T, Roschka S, Platz T, Dinse HR, Lotze M. Effects of combining 2 weeks of passive sensory stimulation with active hand motor training in healthy adults. PLoS One 9: e84402, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Callaway JC, Waters RS. Removal of GABAergic inhibition alters subthreshold input in neurons in forepaw barrel subfield (FBS) in rat first somatosensory cortex (SI) after digit stimulation. Exp Brain Res 145: 411–428, 2002. [DOI] [PubMed] [Google Scholar]
- Lin CC, Sun Y, Huang CI, Yu CY, Ju MS. Cortical activation by tactile stimulation to face and anterior neck areas: an fMRI study with three analytic methods. Hum Brain Mapp 31: 1876–1885, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin TR, Scholz J, Henderson Slater D, Johansen-Berg H, Tracey I. Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain 138: 2140–2146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger PR, Woods TM, Muñoz A, Jones EG. Hand/face border as a limiting boundary in the body representation in monkey somatosensory cortex. J Neurosci 17: 6338–6351, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik SA, Hickmott PW. Plasticity of horizontal connections at a functional border in adult rat somatosensory cortex. Neural Plast 2009: 294192, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muret D, Dinse HR, Macchione S, Urquizar C, Farnè A, Reilly KT. Touch improvement at the hand transfers to the face. Curr Biol 24: R736–R737, 2014. [DOI] [PubMed] [Google Scholar]
- Nagamatsu K, Nakasato N, Hatanaka K, Kanno A, Iwasaki M, Yoshimoto T. Neuromagnetic localization of N15, the initial cortical response to lip stimulus. Neuroreport 12: 1–5, 2000. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Yamada T, Goto A, Kato T, Ito K, Abe Y, Kachi T, Kakigi R. Somatosensory homunculus as drawn by MEG. Neuroimage 7: 377–386, 1998. [DOI] [PubMed] [Google Scholar]
- Nash PG, MacEfield VG, Klineberg IJ, Gustin SM, Murray GM, Henderson LA. Bilateral activation of the trigeminothalamic tract by acute orofacial cutaneous and muscle pain in humans. Pain 151: 384–393, 2010. [DOI] [PubMed] [Google Scholar]
- Négyessy L, Pálfi E, Ashaber M, Palmer C, Jákli B, Friedman RM, Chen LM, Roe AW. Intrinsic horizontal connections process global tactile features in the primary somatosensory cortex: neuroanatomical evidence. J Comp Neurol 521: 2798–2817, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen P, Ramstad R, Isotalo E, Haapanen ML, Lauronen L. Trigeminal somatosensory evoked magnetic fields to tactile stimulation. Clin Neurophysiol 117: 2007–2015, 2006. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Otsuru N, Inui K, Yamashiro K, Urakawa T, Keceli S, Kakigi R. Effects of prior sustained tactile stimulation on the somatosensory response to the sudden change of intensity in humans: an magnetoencephalography study. Neuroscience 182: 115–124, 2011. [DOI] [PubMed] [Google Scholar]
- Parianen Lesemann FH, Reuter EM, Godde B. Tactile stimulation interventions: influence of stimulation parameters on sensorimotor behavior and neurophysiological correlates in healthy and clinical samples. Neurosci Biobehav Rev 51: 126–137, 2015. [DOI] [PubMed] [Google Scholar]
- Paullus JR, Hickmott PW. Diverse excitatory and inhibitory synaptic plasticity outcomes in complex horizontal circuits near a functional border of adult neocortex. Brain Res 1416: 10–25, 2011. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443, 1937. [Google Scholar]
- Penfield W, Rasmussen T. The Cerebral Cortex of Man: a Clinical Study of Localization of Function. New York: Macmillan, 1950. [Google Scholar]
- Pleger B, Dinse HR, Ragert P, Schwenkreis P, Malin JP, Tegenthoff M. Shifts in cortical representations predict human discrimination improvement. Proc Natl Acad Sci USA 98: 12255–12260, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Foerster AF, Ragert P, Dinse HR, Schwenkreis P, Malin JP, Nicolas V, Tegenthoff M. Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron 40: 643–653, 2003. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science 252: 1857–1860, 1991. [DOI] [PubMed] [Google Scholar]
- Ragert P, Kalisch T, Bliem B, Franzkowiak S, Dinse HR. Differential effects of tactile high- and low-frequency stimulation on tactile discrimination in human subjects. BMC Neurosci 23: 9, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science 258: 1159–1160, 1992. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol 67: 1031–1056, 1992. [DOI] [PubMed] [Google Scholar]
- Schweigert M. The relationship between hand-arm vibration and lower extremity clinical manifestations: a review of the literature. Int Arch Occup Environ Health 75: 179–185, 2002. [DOI] [PubMed] [Google Scholar]
- Simões C, Mertens M, Forss N, Jousmäki V, Lange J, Lütkenhöner B, Hari R. Functional overlap of finger representations in human SI and SII cortices. J Neurophysiol 86: 1661–1665, 2001. [DOI] [PubMed] [Google Scholar]
- Spengler F, Roberts TP, Poeppel D, Byl N, Wang X, Rowley HA, Merzenich MM. Learning transfer and neuronal plasticity in humans trained in tactile discrimination. Neurosci Lett 232: 151–154, 1997. [DOI] [PubMed] [Google Scholar]
- Weiss T, Miltner WH, Liepert J, Meissner W, Taub E. Rapid functional plasticity in the primary somatomotor cortex and perceptual changes after nerve block. Eur J Neurosci 20: 3413–3423, 2004. [DOI] [PubMed] [Google Scholar]
- Whittingstall K, Wilson D, Schmidt M, Stroink G. Correspondence of visual evoked potentials with FMRI signals in human visual cortex. Brain Topogr 21: 86–92, 2008. [DOI] [PubMed] [Google Scholar]
- Wilimzig C, Ragert P, Dinse HR. Cortical topography of intracortical inhibition influences the speed of decision making. Proc Natl Acad Sci USA 109: 3107–3112, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson SJ, Kaufman L. Evolution of neuromagnetic topographic mapping. Brain Topogr 3: 113–127, 1990. [DOI] [PubMed] [Google Scholar]
- Wühle A, Mertiens L, Rüter J, Ostwald D, Braun C. Cortical processing of near-threshold tactile stimuli: an MEG study. Psychophysiology 47: 523–534, 2010. [DOI] [PubMed] [Google Scholar]
- Wühle A, Preissl H, Braun C. Cortical processing of near-threshold tactile stimuli in a paired-stimulus paradigm—an MEG study. Eur J Neurosci 34: 641–651, 2011. [DOI] [PubMed] [Google Scholar]
- Zeharia N, Hertz U, Flash T, Amedi A. Negative blood oxygenation level dependent homunculus and somatotopic information in primary motor cortex and supplementary motor area. Proc Natl Acad Sci USA 109: 18565–18570, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]