Abstract
We investigated the chronometry of neural processes in frontal eye fields of macaques performing double-step saccade visual search in which a conspicuous target changes location in the array on a random fraction of trials. Durations of computational processes producing a saccade to original and final target locations (GO1 and GO2, respectively) are derived from response times (RT) on different types of trials. In these data, GO2 tended to be faster than GO1, demonstrating that inhibition of the initial saccade did not delay production of the compensated saccade. Here, we measured the dynamics of visual, visuomovement, and movement neuron activity in relation to these processes by examining trials when neurons instantiated either process. First, we verified that saccades were initiated when the discharge rate of movement neurons reached a threshold that was invariant across RT and trial type. Second, the time when visual and visuomovement neurons selected the target and when movement neuron activity began to accumulate were not significantly different across trial type. Third, the interval from the beginning of accumulation to threshold of movement-related activity was significantly shorter when instantiating the GO2 relative to the GO1 process. Differences observed between monkeys are discussed. Fourth, random variation of RT was accounted for to some extent by random variation in both the onset and duration of selective activity of each neuron type but mostly by variation of movement neuron accumulation duration. These findings offer new insights into the sources of control of target selection and saccade production in dynamic environments.
Keywords: diffusion model, linking hypothesis, linking proposition, perceptual decision, decision making
when we are presented with a typical cluttered visual scene, the production of a gaze shift to the location of a target item entails neural and supervenient psychological processes. According to one theoretical framework, these processes are comprised of distinct computational stages accomplishing the dissociable subtasks needed to select the target and generate the gaze shift (e.g., Sato et al. 2001; Thompson et al. 1996; for review see Sternberg 2001). Here we investigated the neural basis of visual target selection and saccade preparation stages of processing through a new analysis of data collected in frontal eye fields (FEF) of monkeys performing a visual search double-step saccade task (Fig. 1). In this task, on a fraction of trials, the target for a saccade steps to a new location following a delay, and the subject is required to shift gaze to the final location of the target to earn a reward (Camalier et al. 2007; Murthy et al. 2001, 2007, 2009). The visual salience stage begins with the visual response to the search array and completes with the appearance of a pattern of activity representing the location of the target of a saccade in the FEF (Murthy et al. 2009; Thompson et al. 1996); this occurs as well in the superior colliculus (SC) (e.g., White and Munoz 2011), posterior parietal cortex (e.g., Katsuki and Constantinidis 2012; Shen and Paré 2014), and dorsolateral prefrontal cortex (Buschman and Miller 2007; Katsuki and Constantinidis 2012). The saccade preparation stage begins when movement-related neurons begin to accumulate activity and completes when it reaches a threshold that triggers a saccade (Woodman et al. 2008; see also Purcell et al. 2010, 2012).
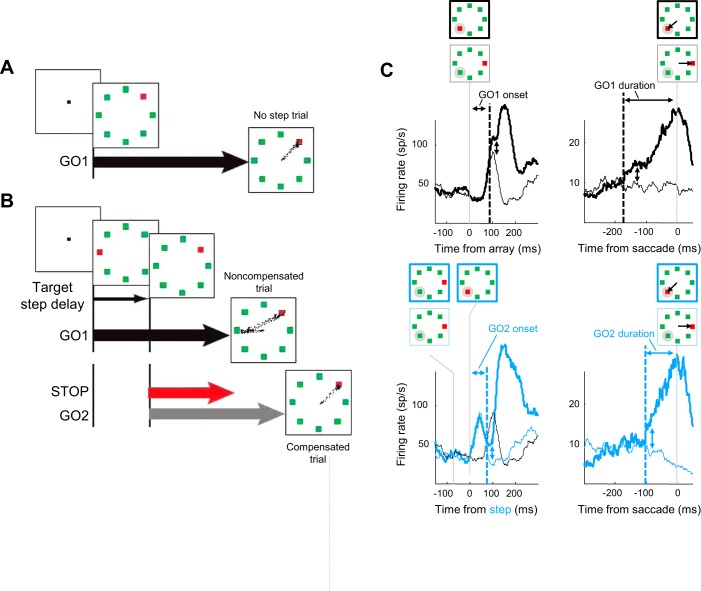
Fig. 1.
Search-step task with race model processes. A color singleton target appears with 7 distractors. A: on no-step trials reward is delivered for shifting gaze to the target. The response time on these trials is identified with the distribution of finish times of the GO1 process. B: on random trials the target steps to a different location after a variable target-step delay (TSD). Noncompensated gaze shifts to the original target location (top) were not rewarded even though participants commonly produce corrective saccades to the final target location. According to the race model, these were produced if the GO1 process finished early. Compensated gaze shifts to the final target location (bottom) were rewarded. According to the race model, these were produced if the GO1 process was interrupted by the STOP process, allowing the GO2 process to finish. C: schematic of the trial-type comparisons to measure the beginning and duration of the neural activity instantiating the GO1 and GO2 processes. Vertical arrows indicate the spike density functions being compared. Broken vertical lines mark the earliest difference. The beginning of the GO1 process (top left) was measured by comparing array-aligned activity in correct no-step trials when the target was in the response field (RF) (thick line) with array-aligned activity in correct no-step trials when the target was opposite the RF (thin line). The beginning of the GO2 process (bottom left) was measured by comparing activity in correctly compensated step trials when the target was initially opposite the RF and stepped into the RF (thick cyan line) with activity in correct no-step trials when a distractor remained in the RF (thin cyan line). This measurement must account for the fact that the activity of interest in compensated trials is elicited by the target step. Therefore, although the spike density from compensated trials was aligned on TSD, the spike density from the no-step trials was shifted backward in time by the mean TSD. For illustration, the spike density from no-step trials without this time adjustment is also plotted (black line). The duration of the GO1 process (top right) was measured by comparing activity in correct no-step trials when the target was in the RF (thick line) with activity in correct no-step trials when the target was opposite the RF (thin line), both aligned on saccade initiation; it is the interval from when spike density functions diverged until 10 ms before the saccade. Similarly, the duration of the GO2 process (bottom right) was measured by comparing activity in correctly compensated step trials when the target stepped into the movement field (thick cyan line) with activity in correct no-step trials when a distractor remained in the RF (thin cyan line), both aligned on saccade initiation. The data shown on the left and right are from the representative neurons illustrated in Figs. 4A and 3A, respectively.
These neural processes can be understood computationally as instantiating a stochastic race between three independent processes governing performance (Camalier et al. 2007). The GO1 process begins when the array is initially presented and concludes with a saccade to the initial target location. The GO2 process begins if and when the target steps to a new location and concludes with a saccade to the final target location. If the GO2 process finishes before GO1, then the trial ends with a successful compensated saccade. If the GO2 process finishes after GO1, then the trial ends with an errant noncompensated saccade. A STOP process is required to interrupt completion of the GO1 process without simultaneously initiating a compensated saccade to fit the patterns of response times (RTs) and proportions of noncompensated saccades toward the initial target on step trials (Camalier et al. 2007). These processes also can be modeled in a neural network instantiating interacting racing units (Ramakrishnan et al. 2012).
By analyzing selected trials according to this race model, neural activity can be identified with either the GO1 process (producing a saccade to the initial target location either correctly when no target is presented or erroneously if the target stepped to another location) or the GO2 process (producing a correct saccade to the final target location). The activity on the subset of trials when monkeys produce corrective saccades after errors has already been reported (Murthy et al. 2007). The present analysis is motivated by the finding that compensated saccade latencies relative to the target step were shorter than no-step saccade latencies relative to the array onset; in other words the duration of GO2 is shorter than that of GO1 (Camalier et al. 2007). This speeding of the compensated response is not observed for all participants or in all studies (e.g., Becker and Jürgens 1979), and the fact that compensated saccades were faster is not crucial for the logic of this analysis, only that they were measurably different from the saccade times on no-step trials.
With the behavior systematically varying between trial types in this manner, one can look to measures of neural activity that have been previously proposed to correspond to visual and movement processing stages to begin to answer the question of which stage differs between the two trial types and potentially gives rise to the differences in observed behavior. We approach this question in two specific ways. First, we perform a simple comparison across trial types to reveal which mean neural estimates of process timing differ the most in the direction consistent with the behavioral difference. Second, if these neural estimates do explain the systematic behavioral difference better than others, we would expect variations in them to explain variations in the behavioral estimates better than other neural estimates. We investigate this variance both across sessions and within sessions with the expectation that the same neural estimates that appear to underlie the systematic RT difference between GO1 and GO2 will also show the most covariance with random variations in RT.
METHODS
Data were collected from five adult monkeys (4 Macaca mulatta and 1 M. radiata). All animal care procedures were in compliance with the Guide for the Care and Use of Laboratory Animals as stated in a protocol approved by the Vanderbilt Animal Care and Use Committee.
We used neural and behavioral data collected from three monkeys that have been the basis for previous publications (Camalier et al. 2007; Murthy et al. 2001, 2007, 2009), as well as behavioral data from one monkey that have appeared in a previous publication (Nelson et al. 2010) plus behavioral data from another monkey that has not yet appeared in any publications. Because all methods have been described in the previous publications, we will only introduce necessary concepts and focus on analyses unique for this study.
Behavioral task.
In the search-step task, no-step and target-step trials were randomly interleaved (Fig. 1). On no-step trials, monkeys were required to make a saccade to an oddball color singleton, either a red target among green distractors or a green target among red distractors. The color of the singleton typically switched between consecutive sessions (i.e., days in which the monkey performed the task). On target-step trials, the target stepped to a different location in the array following a variable delay after its appearance in its initial location. We refer to this variable delay as the target-step delay (TSD). On these trials subjects were supposed to compensate for the target step and make a saccade to the final target location. We refer to these trials as compensated trials. Monkeys were rewarded following both compensated target-step trials and correct no-signal trials. Because the occurrence, timing, and location of the steps were unpredictable, on some trials subjects could not compensate for the step and made a saccade toward the initial target location. We refer to these error trials as noncompensated trials. Monkeys were not rewarded following these trials.
The probability of executing a compensated or noncompensated saccade varied with TSD, which was titrated to ensure an approximately equal number of compensated (correct) and noncompensated (error) responses. Given idiosyncrasies across monkeys and demand differences across tasks, TSDs typically varied between 50 and 300 ms to achieve 50% correct performance on target-step trials. The target step consisted of a target and a distractor swapping locations through isoluminant color changes at the initial and final locations of the target.
The target appeared with equal probability at each of the possible array positions. During neurophysiological data collection, however, the initial and final location on step trials was restricted to maximize the number of trials with the target stepping into or out of the neuron's response/receptive field (RF). Typically two or three target locations were considered to be in the RF of the neuron being monitored during the recordings. On step trials, the target stepped to and from the three target positions centered on the neuron's RF and the three target positions directly opposite of the neuron's RF. The target never stepped within or beside the RF. This amounts to 18 target-step combinations that were used (6 possible initial target locations × 3 possible final target locations for each initial target location). Target-step combinations were randomized and interleaved with no-step trials. The behavioral data indicated that the monkeys could not predict the location of the target or the occurrence of target-step trials.
Neural recordings.
FEF units were recorded from the rostral bank of the arcuate sulcus, which was determined by sulcal landmarks during craniotomies for monkeys C and F, confirmed with structural magnetic resonance imaging of monkey L, histology of monkeys C and F, and microstimulation in all monkeys. In all, 76 neurons exhibiting task-related modulation were recorded from five hemispheres while the three monkeys performed the search-step task. Neurons were classified into three types using an offline assessment of patterns of modulation before visually guided saccades and the conventional memory-guided saccade task (Bruce and Goldberg 1985b; Hikosaka and Wurtz 1983), which separates the visual and movement components of visually guided saccadic activity. Visual neurons displayed a brisk response following the presentation of a flashed visual stimulus, but no response to movements. Movement neurons displayed a progressively increasing response preceding saccade initiation with no response to the flashed stimulus. Visuomovement neurons displayed both components of activity. Of the neurons we analyzed, 24 were classified as visual neurons, 38 were classified as visuomovement neurons, and 14 were classified as movement neurons. Further details about the methods used for classification of the neurons we present here have been described previously (Murthy et al. 2007, 2009).
Analysis of neural activity.
Measurements of neural discharge were accomplished by converting discrete spike times to continuous functions by convolving spike trains with a combination of growth and decay exponential functions that resembled a postsynaptic potential given by R(t) = [1 − exp(−t/τg)] × [exp(−t/τd)] where rate as a function of time [R(t)] varies according to the time constant for the growth phase (τg) and the time constant for the decay phase (τd). Physiological data from excitatory synapses indicate that τg = 1 ms and τd = 20 ms (Kim and Connors 1993; Sayer et al. 1990). The rationale for this approach was to derive physiologically plausible spike density functions as described elsewhere (Hanes et al. 1998; Thompson et al. 1996). The result of this convolution was then averaged across trials to yield the spike density function, which we use for further analysis.
We defined the onset time of significant differential activity between two conditions, a “target” condition and a “reference” condition, as the instant when the difference between the two spike density functions first exceeded 2 standard deviations (SDs) of the differential activity measured in the baseline interval of the 200 ms immediately preceding array presentation, provided that the difference ultimately reached 6 SDs and was maintained above 2 SDs for at least 50 ms. The SD is measured across time bins sampled every 1 ms over the length of the baseline interval on the average spike density function differences between the target and reference condition. By these definitions, one movement neuron, one visual neuron, and one visuomovement neuron were not selective for compensated trials and were necessarily excluded from analyses that required this selectivity.
The definition of RF corresponded to that used previously (Murthy et al. 2001, 2007, 2009). Typically two or three of the eight stimulus locations were defined as in the target's RF based on the neuron's responsiveness, whereas typically four locations were outside the RF. Throughout this article, when we refer to “in the RF” or “out of the RF,” we refer to these particular stimulus locations for each neuron. Locations yielding intermediate responses (typically 1 or 2 locations/neuron) were excluded from the analysis. The mean (± SD) RF widths did not differ between the neuron types; in units of polar angle, they were for visual neurons: 118.1°(± 41.6°), for visuomovement neurons: 110.1°(± 45.2°), and for movement neurons: 110.6°( ± 44.0°).
We determined when neural activity distinguished whether a target or distractor was in the RF with trials aligned on stimulus presentation time (Fig. 1). Activity in the 200-ms interval preceding initial array presentation was taken as the baseline for comparison. The beginning of the GO1 process was derived from comparing activity in the correct no-step trials with the target in the RF with that in the correct no-step trials with a distractor in the neuron's RF. The beginning of the GO2 process was derived from comparing activity in the compensated trials when the target steps into the RF with that from correct no-step trials with a distractor in the neuron's RF aligned on the target step rather than initial array presentation, that is, the GO2 process is isolated by contrasting activity when a distractor in the RF becomes the target compared with remaining a distractor. By shifting the no-step trials back in time by the mean TSD occurring in the compensated trials, we equate the time course of responses to the initial search array. Note that other compensated trials could not have been used as the reference trial type because in our experimental design the target always stepped either from out of the RF into the RF, or vice versa. The plots in Fig. 1C show that this shift in time results in the nonselective visual response of the reference no-step trials aligning with the initial response observed in the target compensated trials, both resulting from the initial presence of the distractor in the RF. Thus the data show that subtracting the mean TSD was sufficient to align the neural activity in the comparisons appropriately.
The duration of movement activity was the interval between the onset of movement neuron activity calculated when aligned to the saccade initiation (Fig. 1C, right) and 15 ms before saccade initiation because the threshold firing rate preceding the saccade is measured in the interval from 10 to 20 ms preceding the initiation of a saccade into the neuron's RF on correct no-step trials (Hanes and Schall 1996). By these definitions, one movement neuron, four visual neurons, and six visuomovement neurons were not selective for no-step and/or compensated trials and were necessarily excluded from analyses that required this selectivity.
Trial group analyses.
Trials were sorted into 10 RT deciles. Because of the low number of trials within each decile for some cells, trial groups were occasionally unselective for a given measurement based on the criteria described above. Such null values were not included in the regression of the given measure against RT. Any neuron with fewer than five trial groups providing a particular timing measurement was excluded from the regression for that measurement. Rare instances of the peak of activity for a neuron occurring before its identified onset for a given trial group were treated as that trial group being nonselective for the given measurement, and the trial group was excluded for that measurement.
For these analyses of durations of processing for visual, visuomovement, and movement neuron types, we measured the duration from the onset of an activity increase to the time of the peak activity occurring before saccade initiation, which we refer to as the postselection duration. Trials were aligned to the initiation of the saccade for both measures. The activity peak was chosen for these comparisons because the concept of a threshold firing rate for visual and visuomovement neurons is not interpretable. The applicability of this duration definition to discriminate between the contributions of visually dominated and movement-dominated neurons is apparent from viewing the histograms of saccade-aligned visual and movement neurons (Fig. 6). For movement neurons, this peak essentially always coincided with times near the initiation of the saccade, although this usually occurred at other times for visual and visuomovement neurons. The same comparisons of compensated- and no-step trials described above were performed to determine the onset-to-peak durations for the GO1 and GO2 processes separately.
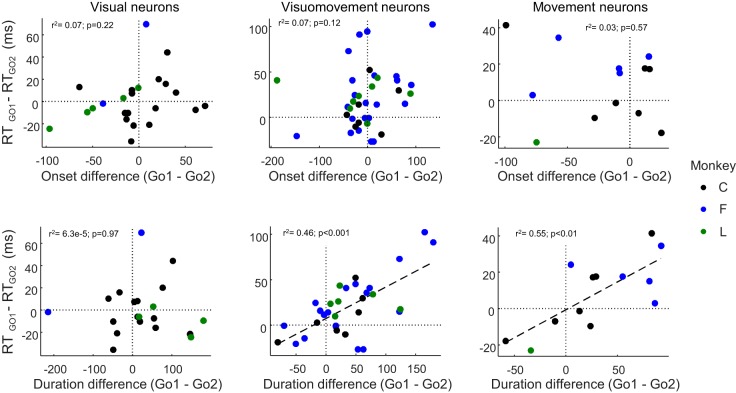
Fig. 6.
Random timing variation across sessions. Row on top shows the difference between RTGO1 and RTGO2 plotted against the difference in target selection time in no-step trials (GO1 process) and compensated-step trials (GO2 process) for visual (left), visuomovement (middle), and movement (right) cells. Row on bottom shows in the same format the difference between RTGO1 and RTGO2 plotted against the difference in the duration of accumulation of activity for the same neurons.
Visual-movement index.
We calculated a visual-movement index (VMI) for each neuron, using activity during a separate memory-guided saccade task as described previously (Murthy et al. 2009). This task separates the visual and movement components of visually guided saccades and thus serves as an ideal task to separate their respective contributions to a neuron's activity. Visual activity (VA) was defined as the mean firing rate 0–200 ms after stimulus onset minus the spontaneous activity firing rate. Movement activity (MA) was defined as the mean firing rate 50-0 ms before saccade initiation minus the same spontaneous activity firing rate. The spontaneous activity firing rate was measured as the mean firing rate from 800 to 400 ms before the stimulus and affected the vales of both MA and VA. VMI was the contrast ratio (MA − VA)/(MA + VA). Neurons with comparatively higher VA yield negative VMIs, whereas neurons with higher movement activity yield positive VMIs.
RESULTS
Note that we define the duration of the GO1 process as the time of the response relative to the time of initial array presentation on correct no-step trials and the duration of the GO2 process as the time of the response relative to the target step on compensated-step trials.
Response times.
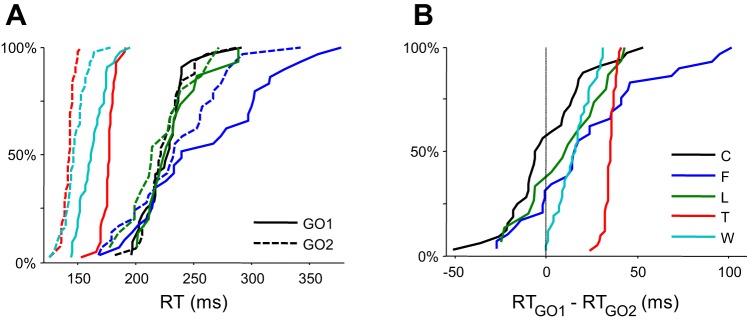
RTs were the duration between the presentation of the search array and the initiation of a saccade. According to the race model formulation (Camalier et al. 2007), the RT of saccades to the initial target location relative to the presentation of the initial search array measures the duration of the GO1 process. The RT of compensated saccades to the final target location relative to the target step measures the duration of the GO2 process. As reported previously, RTs for compensated saccades (RTGO2) were generally faster than RTs for saccades on no-step trials (RTGO1) (Fig. 2 and Table 1). Despite individual differences, across monkeys RTGO2 was significantly less than RTGO1 [mean(median) difference: 17.3(14.9) ms, paired t-test, t(4) = 2.96, P < 0.05].
Fig. 2.
Distributions of response times (RT) for G01 (RTGO1) and G02 (RTGO2) (A) and their pairwise differences (B) across sessions for each monkey. Note the consistently shorter durations of RTGO2 relative to RTGO1 plotted as positive values in B.
Table 1.
For each monkey the mean (median) values of the GO1 and GO2 finish times, as well as their differences, are shown along with the results of a paired t-test of these mean values across sessions
| Monkey | GO1 RT | GO2 RT | GO1 RT − GO2 RT | No. of Sessions | t | P Value |
|---|---|---|---|---|---|---|
| C | 226.0 (228.0) | 225.5 (222.0) | 0.4 (−5.0) | 32 | 0.10 | 0.92 |
| F | 260.0 (240.0) | 235.9 (233.0) | 24.2 (16.0) | 29 | 3.66 | <0.01 |
| L | 233.1 (226.0) | 221.7 (214.0) | 11.5 (12.1) | 15 | 2.00 | 0.065 |
| T | 176.5 (177.0) | 141.6 (142.5) | 35.0 (35.0) | 42 | 67.54 | <0.001 |
| W | 162.5 (161.5) | 147.1 (146.0) | 15.5 (16.4) | 42 | 10.39 | <0.001 |
GO1, original target location; GO2, final target location; RT, response time.
Movement neuron threshold invariance.
As described previously in a similar task (Hanes and Schall 1996), we determined whether the activity of FEF movement neurons at the time of saccade initiation was invariant across RT and also trial types. Figure 3 illustrates results from a representative movement neuron and from the entire sample. The average activation level in the 10- to 20-ms interval before saccade initiation was invariant across RT deciles for both no-step and compensated saccades [no step: b = 0.003, F(1,8) = 0.004, P = 0.95; compensated: b = −0.008, F(1,8) = 0.013, P = 0.91]. Thus, threshold activation was invariant across RT when it was instantiating both the GO1 and the GO2 processes.
Fig. 3.
Saccade initiation threshold. A: average discharge rate of representative frontal eye field (FEF) movement neuron on no-step (black lines) and compensated-step (cyan lines) trials (combined across TSD) when the target was (thick lines) or was not (thin lines) in the RF aligned on array presentation for no-step trials and on target step for compensated trials (left) and saccade initiation (right). The beginning of accumulation of activity is shown for no-step trials (broken black vertical) and for compensated-step trials (broken cyan vertical lines). Average RTGO1 (thin black vertical lines) and RTGO2 (thin cyan vertical lines) are also indicated. On the right, the onsets of accumulation are marked for no-step (broken black vertical lines) and compensated (broken cyan vertical lines) trials. B: trials were sorted using RTGO1 and RTGO2 into 10 deciles of RT. The average activation level during the interval −20 to −10 ms before saccade initiation is plotted against the average RT for each decile for GO1 (black circles) and GO2 (cyan circles) processes. C and D: analysis averaged across neuron sample. The terminal discharge rate at saccade initiation was invariant across RT when the neurons instantiated GO1 and GO2.
Visual and movement stage timing.
Reduced RTGO2 could result from either less time to accomplish target selection, less time to complete response preparation and initiate the saccade, or some combination of the two. By analyzing the activity of FEF visual and movement neurons during execution of the task, we measured the respective contributions of these processing stages to the difference between RTGO1 and RTGO2.
Figure 4 shows the activity of a representative visual neuron and the average from the sample of such neurons in no-step and compensated-step trials. No significant difference was measured in the times of target selection between no-step and compensated trials in which the GO1 and GO2 processes were instantiated across the population of visual neurons [no-step onset time − compensated onset time mean(median) difference: −4.4(−7.0) ms, paired t-test, t(22) = −0.53, P = 0.60]. Likewise, target selection times in no-step and compensated trials were not significantly different for visuomovement neurons [no-step onset time − compensated onset time mean(median) difference is −2.08(−3.00) ms, paired t-test, t(36) = −0.22; P = 0.83]. Thus, we cannot conclude that a systematic difference in visual target selection time accounted for the different durations of the GO1 and GO2 processes.
Fig. 4.
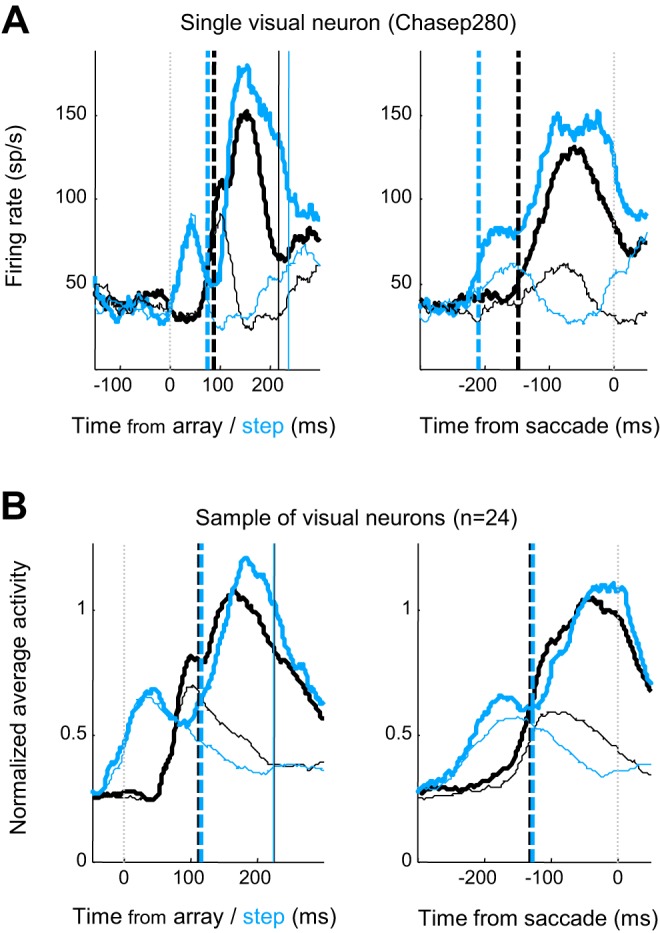
Visual selection stage. Average discharge rate of a representative FEF visual neuron (A) and the sample of 24 neurons (B). Conventions as in Fig. 3 except the broken vertical lines on the left indicate target selection time. Duration of target selection stage did not vary across GO1 and GO2 processes.
We measured the contribution of movement neuron timing to the different durations of the GO1 and GO2 processes. Figure 3 also shows the beginning of accumulation of activity of a representative neuron and the average of the sample of movement neurons in no-step and compensated-step trials. The onset of movement activity accumulation was not significantly different between trial types [no-step − compensated onset time mean (median)difference: −22.3(−9.0) ms, paired t-test, t(12) = −1.94, P = 0.08]. Thus, we cannot conclude that the onset time of movement preparation activity differed between the GO1 and GO2 processes, and the delay to begin movement preparation appears not to contribute considerably to the faster RTGO2. This corroborates the null result from the analysis of visual neurons and further indicates that the target selection stage did not contribute to the observed RT difference.
An analysis comparing the times when selective activity began across the three neuron types found no significant variation between them [mean(median) average of GO1 and GO2 onset times: visual neuron = 110.5(112.0) ms, visuomovement neuron = 116.7(116.5) ms, movement neuron = 114.9(124.0) ms; between-factors ANOVA F(2,73) = 0.30, P = 0.74], suggesting that the onset of selective activity in all three neuron types may result from a response to the same neural event. The recently formulated gated accumulator model explains how this can be so (Purcell et al. 2010, 2012).
We measured the duration of the movement processing stage instantiating both the GO1 and GO2 processes. This was defined as the interval between the beginning of accumulation until 15 ms before the saccade, corresponding to the moment when movement neurons reach threshold and brain stem circuits initiate the saccade. Figure 5 plots the distributions of these durations and the pairwise differences for all movement neurons sampled. This duration was significantly shorter during compensated- relative to no-step trials [median(mean) difference: 30.2(26.0) ms, paired t-test, t(12) = 2.27, P < 0.05]. The same results were observed when comparing the movement neurons' duration to reach their peak firing rate as well [mean(median) GO1 − GO2 difference is 28.4(23.0), paired t-test, t(12) = 2.26; P < 0.05].
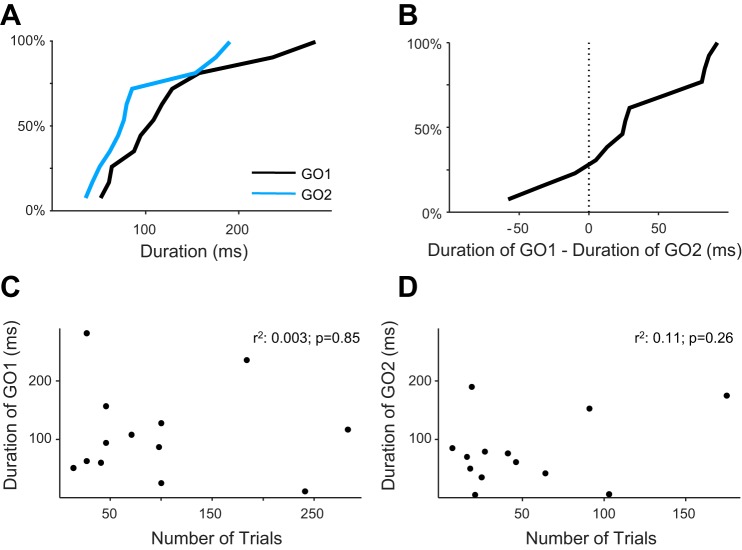
Fig. 5.
Duration of movement neuron activity across the population. A: cumulative distribution over the population of movement neurons of the duration from neural activity onset to saccade initiation threshold for no-step trials (when the neuron instantiated GO1, shown in black) and compensated-step trials (when the neuron instantiated GO2, shown in blue). B: cumulative distribution of the neuron-by-neuron difference of the durations in A. Values to the left (right) of zero indicate durations that were longer (shorter) during the GO2 than the GO1 process. C and D: scatterplots of the durations in A as a function of the number of target trials for each neuron for no-step and compensated-step trials, respectively.
We investigated this finding further to ascertain that it did not result from the difference in the number of no-step and compensated trials sampling the GO1 and GO2 processes, respectively. Figure 5, C and D, shows the duration of movement neuron activity accumulation corresponding to the GO1 and GO2 processes, respectively, plotted against the number of trials sampled with a saccade in the neuron's movement field. We find no correlation between the number of trials and process duration [GO1: Spearman correlation, ρ(11) = 0.03, P = 0.929; GO2: Spearman correlation, ρ(11) = −5.4−3, P = 0.993; pooled GO1 and GO2: Spearman correlation, ρ(24) = 0.16, P = 0.435, GO1 − GO2/cell as a function of the difference in the numbers of trials: Spearman correlation, ρ(11) = −0.31, P = 0.297]. To further ensure that the number of trials did not affect this conclusion, we applied a repeated-measures general linear model to the data using the number of no-step (GO1) and compensated (GO2) trials as between-session factors and GO1 vs. GO2 as a within-session factor. The within-session factor of GO1 vs. GO2 was significant [adjusted mean(median) difference: 29.3(25.1) ms, F(1,10) = 5.47, P < 0.05]. Similar results were obtained using the average number of trials between the GO1 and GO2 as a single between-session covariate. We further found no correlation between the number of trials and measures of onset duration [GO1: Spearman correlation, ρ(12) = −0.27, P = 0.36; GO2: Spearman correlation, ρ(11) = −0.34, P = 0.26; pooled GO1 and GO2: Spearman correlation, ρ(25) = −0.30, P = 0.13; GO1 − GO2/cell vs. the difference in the numbers of trials: Spearman correlation, ρ(11) = 0.44, P = 0.14]. These analyses show that the number of sampled trials did not contribute to the observed mean duration difference between the GO1 and GO2 processes.
Thus, whereas the duration of the visual target selection stage was invariant across trial types, the duration of the saccade preparation stage as estimated by the duration of accumulation of movement activity was significantly shorter when instantiating the GO2 vs. the GO1 process.
Correlations of neural and behavioral differences across sessions.
The preceding analyses potentially imply that movement neuron accumulation duration underlies the difference in RTs between no-step and compensated trials, with limitations stemming from the sample size of movement neurons available. This link could be more firmly established if we found that fluctuations of the neural effect covaried with fluctuations of the behavioral effect. We first investigated this across sessions. Figure 6 plots the differences in onset time and in processing duration between no-step trials (when instantiating the GO1 process) and compensated trials (when instantiating the GO2 process) for the different types of cells as a function of the difference in the GO1 and GO2 RTs for each session. Variation in the duration of the visual processing stage was not correlated with the difference between RTGO1 and RTGO2 for the session in which each neuron was recorded [Spearman correlation, ρ(21) = 0.34, P = 0.11]. Similarly, variation in the beginning of saccade preparation as measured by the onset of accumulation of visuomovement, and movement activity was not correlated with the difference between RTGO1 and RTGO2 [visuomovement: Spearman correlation, ρ(35) = 0.26, P = 0.12; movement: Spearman correlation, ρ(11) = −0.12, P = 0.71]. However, variation in the duration of saccade preparation measured as the interval between the onset of movement neuron accumulation and trigger threshold was significantly correlated with the difference between RTGO1 and RTGO2 [Spearman correlation, ρ(11) = 0.69, P < 0.05]. Variation in the duration of visuomovement cell processing also correlated with the difference between RTGO1 and RTGO2 [Spearman correlation, ρ(30) = 0.56, P < 0.001] but not visual cell processing [Spearman correlation, ρ(18) = −0.07, P = 0.78]. In a linear regression analysis, variation in the duration of movement and visuomovement durations accounted for 55 and 46%, respectively, of the variation between RTGO1 and RTGO2 across sessions, whereas variation in target selection time in visual and visuomotor cells and the beginning of saccade preparation in movement cells accounted for only 7, 7, and 3%, respectively, of the variation in the difference between RTGO1 and RTGO2.
We note, however, that the slopes for both visuomovement and movement neurons as shown in Fig. 6 are less than one (slope ± 95% confidence interval: visuomovement neurons, 0.35 ± 0.14; movement neurons, 0.31 ± 0.18). For both, the neural event timing difference was larger than the behavioral difference. Thus, we show that there is a positive relation between these processes as we are estimating them here and the RT difference between no-step and compensated trials, although these processes may not directly underlie the behavioral difference in a serial-order fashion.
Correlations of timing of target selection and response preparation with RT within sessions.
Next we investigated covariation of these neural estimates and RT within a session. Here we cannot measure both GO processes within the same trials as we did for each session in the above analysis, so instead we measured the neural-behavioral covariance across trials for each GO process individually. We divided no-step and compensated trials into quantiles based on RTGO1 and RTGO2, respectively. For each quantile, we measured both the stimulus-aligned onset time of target-selective activity, and the duration between the onset, and the peak in the saccade-aligned activity. We then performed a linear regression across trial quantiles of both the onset times and the onset-to-peak durations relative to the quantile RT for no-step and compensated trials individually. Neural events more closely tied to and potentially driving the RT should yield a higher slope, closer to one.
Note that in this case it is not the differences between the GO1 and GO2 processes that motivate the analyses, but largely the similar properties between the two. What we expect to find given the preceding results is that movement neuron duration will tend to show larger slopes for both no-step and compensated trials and hence closer relations to both RTGO1 and RTGO2. When plotting the RT and neural timing estimates for each quantile against each other (see Fig. 7), for movement neuron durations in particular, we expect that no-step and compensated trials will lie along the same line, with the mean difference between them manifested by a translation along that common line. For completeness, we also analyze and interpret potential differences in slopes between no-step and compensated trials, which, if observed, could reflect differences between the processes occurring between no-step and compensated trials (i.e., the presence or absence of the STOP process, the different overall duration before making a saccade, etc.).
Fig. 7.
Analysis of onset and duration from onset to peak firing rate for an example visual (A) and visuomovement (B) neuron plus 3 example movement neurons (C). For each neuron, the plots on top show the spike density functions on no-step trials with the target in its RF (thick lines) or out of its RF (thin line) aligned on array (left) and saccade (right) from trials in the 2nd (earlier, light gray), 4th (middle gray), and 8th (later, black) quantiles of the RT distribution. Panels on the bottom show the onset time (left) and the duration from onset to peak of activity (right) as a function of mean RT for each RT quantile for the GO1 (black circles) and GO2 (cyan circles) processes. Regression lines are shown for individually significant regressions.
Figure 7 shows results for representative visual, visuomovement, and movement neurons. The visual neuron exhibits little variation of target selection time and postselection duration with the variation of RT in either no-step or compensated trials. The visuomovement neuron exhibits little variation of target selection time as well for either trial type, but the postselection duration increases with RT. The movement neurons' firing rates rise at slower rates across trial quantiles as RT increases, arriving at the same threshold firing rate for each neuron as the saccade is initiated. The result of this is that the processing durations increase as RT increases. The selection time aligned on array presentation for only one of the three example movement neurons also showed a significant increase with RT, with a comparatively smaller slope than the slope for processing duration. In all cases, slopes are similar between no-step trials (instantiating GO1) and compensated trials (instantiating GO2).
Figure 8 shows the histograms of slopes and slope differences across the population, with the results numerically summarized in Table 2. For onset times, the slopes vs. RT significantly exceeded zero across the population for no-step and compensated trials for all neuron types, with the exception of visual neurons in no-step trials. For postselection durations, the slopes vs. RT showed positive slopes across the population for no-step trials across all cell types, with movement cells showing the largest slopes overall. For compensated trials, only movement cells showed significantly positive slopes across the population. Overall, the neural event that accounted for the most variability in RT was the postselection duration of movement neuron activity, with a mean slope of 0.66 averaged across the no-step and compensated trial values, accounting for on average 44% of the variance of the mean RTs across deciles. Moreover, only movement neurons showed significantly higher slopes of duration vs. RT than onset vs. RT when averaged across the no-step and compensated trial values, although this difference was also significant in visual cells for no-step trials only (Table 2).
Fig. 8.
Variation of measures with RT. A: distributions of rate of change of onset of activity in no step (top), compensated step (middle) with RT, and their difference (bottom). Filled values signify neurons with statistically significant differences between the GO1 and GO2 process measures. *Distributions with mean value significantly different from 0 (1-sample t-test). B: relation of duration from onset to peak activity to RT.
Table 2.
Results from population analyses of the regression slopes shown in Fig. 8
| Visual Neurons |
Visuomovement Neurons |
Movement Neurons |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slopes | P value | r2 | No. of cells | Slopes | P value | r2 | No. of cells | Slopes | P value | r2 | No. of cells | ||
| Onset | GO1 | 0.14 (0.15) | 0.08 | 0.24 | 15 | 0.34 (0.28) | 9.6−7 | 0.35 | 33 | 0.24 (0.20) | 0.006 | 0.29 | 13 |
| GO2 | 0.28 (0.10) | 0.03 | 0.32 | 19 | 0.29 (0.17) | 2.9−4 | 0.31 | 34 | 0.40 (0.37) | 0.012 | 0.40 | 12 | |
| GO1GO2 Avg | 0.19 (0.13) | 0.017 | 0.29 | 15 | 0.33 (0.31) | 7.2−7 | 0.33 | 33 | 0.32 (0.31) | 0.003 | 0.36 | 12 | |
| GO1GO2 Diff | −0.11 (−0.08) | 0.17 | 15 | 0.03 (0.05) | 0.86 | 33 | −0.14 (−0.06) | 0.26 | 12 | ||||
| Duration | GO1 | 0.30 (0.31) | 0.001 | 0.27 | 22 | 0.41 (0.42) | 3.1−6 | 0.38 | 36 | 0.53 (0.59) | 0.0003 | 0.48 | 12 |
| GO2 | 0.06 (0.09) | 0.28 | 0.19 | 21 | 0.14 (0.10) | 0.18 | 0.21 | 34 | 0.61 (0.66) | 0.010 | 0.30 | 10 | |
| GO1GO2 Avg | 0.21 (0.31) | 0.008 | 0.25 | 20 | 0.28 (0.24) | 1.4−4 | 0.30 | 34 | 0.66 (0.59) | 5.4−5 | 0.44 | 9 | |
| GO1GO2 Diff | 0.28 (0.16) | 0.31 | 20 | 0.28 (0.27) | 0.01 | 34 | −0.05 (−0.19) | 0.93 | 9 | ||||
| Onset-Duration | GO1 | −0.29 (−0.43) | 0.03 | 15 | −0.09 (−0.30) | 0.37 | 33 | −0.32 (−0.27) | 0.056 | 12 | |||
| GO2 | 0.06 (0.09) | 0.22 | 19 | 0.15 (0.18) | 0.29 | 34 | −0.23 (−0.49) | 0.35 | 10 | ||||
| GO1GO2 Avg | −0.20 (−0.33) | 0.19 | 15 | 0.04 (−0.03) | 0.75 | 33 | −0.46 (−0.44) | 7.6−4 | 9 | ||||
| GO1GO2 Diff | −0.18 (−0.35) | 0.39 | 15 | −0.25 (−0.34) | 0.17 | 33 | −0.05 (−0.02) | 0.81 | 9 | ||||
Results from population analyses of the regression slopes shown in Fig. 8. Mean(median) values of the slopes across neurons for each cell-type sample are shown for the GO1 and GO2 processes, as well as their averages (Avg) and differences (Diff) within each neuron in the corresponding columns. The resulting P values from a 1-sample t-test across neurons are shown. These tests were performed across the z-scores of the slopes, except for the onset-duration comparison, which was performed on the raw slopes for each cell. The mean r2 values over the cells satisfying the criteria for inclusion for each category are also shown. Note that the no. of cells for the average and difference correspond to cells with slopes that met criteria for inclusion for both the GO1 and GO2 processes; cells contributing only to the individual GO1 and GO2 process results were not included. Thus, the GO1GO2 average values need not correspond to the midpoint between the GO1 and GO2 values.
A significant difference between no-step and compensated trials (instantiating the GO1 and GO2 processes, respectively) did occur for the postselection duration slopes of visuomovement neurons, which were significantly steeper for the no-step than for the compensated trials [mean(median) difference: 0.28(0.27), paired t-test, t(33) = 2.64, P < 0.05]. Visual neurons showed a similar difference where slopes were significantly positive for no-step but not compensated trials, although the mean difference between them was not significant. The median difference did approach significance though [signed-rank test, W(19) = 156; P = 0.058]. This suggests that the postselection duration activity in visual and visuomovement neurons contributes less to compensated saccade RT than to no-step RT, whereas the movement neuron duration contributed as much to compensated saccade RT as to no-step RT. This is consistent with the finding that the mean duration of postselection movement neuron activity accumulation for the GO2 process is primarily responsible for the shorter RTGO2.
In comparisons across neuron types for both onset and duration slopes, the duration vs. RT slopes for movement neurons were higher than those for visual neurons [mean(median) difference: −0.45(−0.28), Mann-Whitney U = 251, n = 9, visual neurons = 20, P < 0.05] and for visuomovement neurons [mean(median) difference: −0.37(−0.35), Mann-Whitney U = 670, n movement neurons = 9, n visuomovement neurons = 34, P < 0.05]. No other comparison showed a significant difference.
Results for individual monkeys are shown in Fig. 9. While some differences were observed, for each monkey the slopes for movement cell duration tend to be the same as or greater than the slopes for movement cell onset. Also, for each monkey, duration slopes tend to increase from visual to visuomovement to movement cells.
Fig. 9.
Relationships of Fig. 8 separated by monkey.
We investigated the effects of the number of trials on the slope estimates in Fig. 8. We first performed a linear regression of the regression slopes in each subpanel of Fig. 8 against the number of trials giving rise to each estimate. There was no significant linear regression of the slopes against the number of trials for any measurement, although there were some nonsignificant tendencies of more trials leading to lower-onset slopes but higher-duration slopes.
In addition to the previous analyses sorting neurons into the three types, we also compared across the visual-movement spectrum continuously using the VMI. The average VMI ± SE for visual, visuomovement, and movement neurons in our sample was −0.17 ± 0.07, −0.09 ± 0.06, and 0.25 ± 0.09, respectively. We performed a multiple-regression analysis using VMI as an experimental factor and the number of trials recorded with the target in each cell's RF as a control factor. Figure 10 shows that there was a significant effect of VMI on postselection duration slopes for no-step trials, when neurons instantiate the GO1 process [t(67) = 2.93, P < 0.01; Fig. 10, bottom left]. Thus, the more movement dominated a neuron's activity is as assessed in an independent memory-guided saccade task, the more the neuron contributes to the variation of RT, although the effect was not significant for the noisier GO2 process. For the onset times, there was no significant effect of the number of trials or the VMI for either no-step or compensated trials, but there was a significant negative effect of the VMI on the differences of the slopes between no-step and compensated trials [t(57) = −2.23, P < 0.05; Fig. 10, top right]. This means that the correlation between onset time and RT tends to show stronger GO1 vs. GO2 values for visually dominated neurons than for movement-dominated neurons.
Fig. 10.
Variation of timing relationships with visual-movement index (VMI). Panels on top show scatterplots of rate of change of onset of activity in no step (left), compensated step (middle) with RT, and their difference (right) as a function of VMI. Panels on bottom show scatterplots of rate of change of duration from onset to peak in no step (left), compensated step (middle) with RT, and their difference (right) as a function of VMI. Different markers distinguish data from each monkey as indicated. Regression lines in plots with significant effects of VMI when controlling for the number of trials.
DISCUSSION
We investigated the contribution of neural processes instantiating different stages of processing to the systematic and random variation in saccade latency observed in a double-step saccade visual search task. We found that the duration of FEF movement neuron accumulation but not of target selection time or the onset of movement accumulation potentially accounted for systematically shorter latencies of saccades compensating for infrequent changes of target location, with a few caveats discussed below that prevent stronger conclusions from being reached. We showed that the movement and visuomovement neuron accumulation duration difference covaried with the behavioral effect difference across sessions. We also demonstrated that the movement neuron discharge rate at saccade initiation, equated to a trigger threshold, was invariant across RT in both no-step and step trials. Looking across RT-sorted trial groups within no-step and compensated trials individually, we found that random variation in movement neuron accumulation duration contributed more to RT variation than variation in target selection duration or the onset of movement neuron accumulation, although both of the latter did contribute to a significant portion of the RT variation as well. This relation between movement neuron accumulation and RT was evident in trials when the neuron instantiated the GO1 process and in other trials when the neuron instantiated the GO2 process.
Possible limitations of analysis.
Here we consider several limitations of the data that limit strong conclusions.
First, we had available a smaller collection of data than would be preferred, but unfortunately further data collection was not possible by these authors.
Second, individual variability of performance and of neural sampling across monkeys produced inconsistencies. Two of the three monkeys that contributed neural data to these analyses did not show the RT difference, although for one of these (monkey L) the behavioral effect approached significance. Notably, monkey C contributed many of the movement neurons of the sample yet showed no overall behavioral effect. Still, this monkey showed a significant correlation between the neural and behavioral effect across sessions, and the large range of RTGO1 − RTGO2 differences for this monkey strengthen this important observation. Visuomovement neurons for both monkey C and F appear to contribute to the overall result, although the movement neurons from monkey F alone do not appear to concur with the resulting behavior-neural correlation. Finally, the onset times for visual neurons of monkey L appear to show a strong linear relationship with RTGO1 − RTGO2 across sessions. However, data from other monkeys and the onset times of all other neurons do not show this, even though the overall onset times for all three neurons were similar, indicating this uniform onset may comprise a global event across the three neuron types in FEF. This effect that seems strong in monkey L is further mitigated by the lack of within-session regression effects for the visual cells of this monkey while the timing of visuomovement neuron activity in the same monkey does show within-session relationships to RT. This caveat is further mitigated by Fig. 11 showing a similar (if not the same) relationship between GO2 RT and TSD in all three monkeys. Thus there is mixed evidence of potential behavioral differences between the monkeys that some readers may interpret as preventing strong conclusions about the accumulation duration in movement neurons driving the systematic RT differences between the GO1 and GO2 process.
Fig. 11.

Histograms of regression slopes of GO2 RT vs. TSD. A significant negative correlation was found in the sessions for monkeys F and L and in the sessions pooled across monkeys.
Third, fewer trials contribute to the measurement of the duration of the GO2 process than that of the GO1 process. This will introduce more noise but should not create a systematic bias in measurements.
Fourth, the temporal shift of the activity in no-step trials to account for the TSD entails some care in interpretation. As a result of the shift, saccades out of the movement field in the reference trials will happen sooner than saccades into the movement field of the “target trials.” If movement neurons had pronounced nonselective activity for saccades away from the movement field, then this comparison would have been compromised. However, an inspection of the example neuron and population histograms reveals an absence of such confounding activity.
Processing stages vs. continuous processing frameworks.
We note that these analyses assume serial stages of processing, but we do not wish to imply that alternative processing architectures are excluded by the results. While the serial processing framework usefully describes many observed phenomena, alternative frameworks (e.g., continuous processing) may provide useful perspective on these data as well.
Origin of RT variability.
Because the RT in this task is primarily the sum of visual target selection and movement preparation stages (Sato et al. 2001; Thompson et al. 1996), either or both stages could contribute to the reduced RT in compensated-step trials. We found that the duration of visual target selection did not differ between no-step and compensated-step trials. Furthermore, the time when movement activity began to accumulate did not differ between the GO1 and GO2 processes, indicating that the STOP process interruption of the GO1 process did not delay the accumulation of movement activity instantiating the GO2 process. Taken together, the shorter GO2 process must be explained by a shorter duration of the movement preparation stage, as we found. In other words, the rate of accumulation to the trigger threshold was on average higher when instantiating GO2 than when instantiating GO1.
The finding that the onset of accumulation of movement activity was the same for compensated and no-step saccades has two interesting implications. First, this suggests that the STOP process mechanism may be selective and not global (but see Wessel et al. 2013). This suggestion is supported by the model fits applied to microstimulation data in Ramakrishnan et al. (2012), as well as by a countermanding saccade experiment where monkeys exhibited different stop-signal reaction times to different target locations when the fraction of stop-signal trials varied between the locations (Poitou and Pouget 2012), raising the possibility of the existence of multiple spatially specific STOP processes. It may also be possible that some aspects of the STOP processes, like whether a successful STOP acts globally or with specificity, can differ between countermanding and double-step tasks. Corticospinal excitability measurements like those from Wessel et al. (2013) made on subjects performing the search-step task should reveal if this is the case.
Second, regardless of the potential directional specificity, this finding provides important support for preferring the parallel GO-GO + STOP model over the sequential GO-STOP-GO model presented in Camalier et al. (2007). Camalier et al. (2007) showed mimicry of these two architectures in accounting for task performance. The present finding identifying the GO2 process with the accumulation of activity in movement neurons resolves this mimicry in favor of the parallel GO-GO + STOP model. Camalier et al. (2007) also showed that the parallel architecture could account for the incidence and latency of unrewarded rapid corrective saccades produced on noncompensated error trials, and we showed previously how FEF movement neurons contribute to the corrective saccades (Murthy et al. 2007).
The lack of variation of target selection time was not unexpected because target-distractor similarity was the same in no-step and step trials. If discrimination is more difficult, target selection time is elevated (Cohen et al. 2007; Sato et al. 2001) with a concomitant increase in the time needed to react to the target step (Camalier et al. 2007).
This analysis has emphasized the sequence of visual selection followed by saccade preparation, which can explain performance of visual search in terms of the successive neural operations (Purcell et al. 2010, 2012). However, it is well known that in other conditions responses can be produced without or in spite of evidence. For example, in the compelled saccade task, saccade preparation precedes the resolution of visual target information (Stanford et al. 2010; Costello et al. 2013). Similarly, the noncompensated error trials observed in the double-step search task arise because saccade production ignores the accurate representation of target location accomplished by FEF visual neurons (Murthy et al. 2009). Finally, our earlier analysis of corrective saccades also showed that the FEF movement neurons can become active before reafferent visual processing can provide any information about actual target location (Murthy et al. 2007). In general, the distinction of computational stages need not be a commitment to the order and relation of their operations. Importantly, this distinction does not affect our hypotheses or results quantifying the contributions of target selection, movement activity onset, and movement activity accumulation to the RT within and between the two GO processes.
Possible causes of shorter GO2 process.
Classic studies of double-step saccade performance reported longer RTGO2 (e.g., Becker and Jürgens 1979). This is found for some participants in the search-step task (Camalier et al. 2007), but for many monkeys and some humans we found RTGO2 consistently faster than RTGO1. This finding is inconsistent with that of a recent study investigating how countermanding saccades influences cortical excitability associated with limb movements (Wessel et al. 2013). These authors concluded that stopping a saccade entails a global inhibition of all action. If that were always the case, then we could not find RTGO2 faster than RTGO1.
One possible mechanism is a general facilitation of all saccade preparation when the system is preparing the first saccade that happened to be inhibited after the target step occurred. Perhaps this is mediated by a reduction of activity of gaze-holding fixation neurons in FEF and SC that occurs during saccade countermanding (Hanes et al. 1998; Paré and Hanes 2003). Another possible mechanism is increased attention or executive control following successful correction of a potential error (e.g., Ray et al. 2004).
A third possible contribution is strategic slowing of the GO1 process given the likelihood of the target stepping. Earlier research with the closely related countermanding task showed that average RTGO1 is progressively longer with increasing fractions of stop trials (Bissett and Logan 2011; Emeric et al. 2007) and is increased after a step trial in a double-step task (Farooqui et al. 2011). In other words, RTGO2 may not be particularly fast, but RTGO1 was unusually slow.
Whatever the underlying mechanism(s) of the shorter GO2 process, our results indicate that it operates through movement-related activity more than through the visual target selection process.
Linking proposition implications.
Empirical and modeling studies over the last 20 years have described the activity of neurons in structures like FEF, SC, and posterior parietal cortex in terms of a stochastic accumulator process defined by mathematical models of decision making and response control (e.g., Bollimunta et al. 2012; Boucher et al. 2007; Ding and Gold 2010; Hanes and Schall 1996; Lo et al. 2009; Purcell et al. 2010, 2012; Ratcliff et al. 2007; Roitman et al. 2002; Salinas and Stanford 2013). While broadly accepted, the validity of such linking propositions cannot be taken for granted. Recent findings seem to contradict the identity mapping of the most basic stochastic decision process onto neural activity (Heitz and Schall 2012, 2013; Pouget et al. 2011). Nevertheless, computational models articulate how movement neurons in FEF and SC can instantiate the GO processes of race models (Boucher et al. 2007; Logan et al. 2015). This linking proposition is supported by anatomical connectivity to the oculomotor regions of the brain stem (Raybourn and Keller 1977; Segraves 1992) and a pattern of accumulating activity to a threshold discharge rate at the instant that brain stem circuitry initiates saccades (Hanes and Schall 1996). The differences in duration of the GO1 and GO2 processes provide a further independent test of this linking proposition. The shorter duration of accumulating movement neuron activity on compensated-step trials is consistent with the linking proposition that these neurons instantiate the GO2 process characterized by the race model. We expect that such a link will be established for movement neurons in the thalamus (Sommer 2003; Tanaka 2007) and the basal ganglia (Hikosaka et al. 2000; Schmidt et al. 2013). However, the activity of visual and visuomovement neurons disqualifies them from inclusion in the bridge locus for the GO1 or GO2 processes (see also Costello et al. 2013; Ding and Gold 2010; Meister et al. 2013; Ray et al. 2009). Other computational models articulate how the activity of visual and visuomovement neurons can represent the evidence that is accumulated by the movement neurons (Purcell et al. 2010, 2012). We do not yet know if such multistage models can account for performance of this double-step search task.
GRANTS
This work was supported by National Eye Institute Grants R01-EY-8890 and P30-EY-08126 and Robin and Richard Patton through the E. Bronson Ingram Chair of Neuroscience.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.N. and A.M. analyzed data; M.J.N., A.M., and J.D.S. interpreted results of experiments; M.J.N., A.M., and J.D.S. prepared figures; M.J.N. and A.M. drafted manuscript; M.J.N., A.M., and J.D.S. edited and revised manuscript; M.J.N., A.M., and J.D.S. approved final version of manuscript; A.M. and J.D.S. conception and design of research; A.M. performed experiments.
ACKNOWLEDGMENTS
We thank Kirk Thompson for sharing data, Supriya Ray for helpful discussions, and the reviewers for insightful suggestions.
Present address for M. J. Nelson: Neurospin (Inserm U992), CEA, Gif-sur-Yvette, France.
REFERENCES
- Becker W, Jürgens R. An analysis of the saccadic system by means of double step stimuli. Vis Res 19: 976–983, 1979. [DOI] [PubMed] [Google Scholar]
- Bissett PG, Logan GD. Balancing cognitive demands: control adjustments in the stop-signal paradigm. J Exp Psychol Learn Mem Cogn 37: 392–404, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Totten D, Ditterich J. Neural dynamics of choice: single-trial analysis of decision-related activity in parietal cortex. J Neurosci 32: 12684–12701, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol Rev 114: 376–397, 2007. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol 53: 603–635, 1985. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315: 1860–1862, 2007. [DOI] [PubMed] [Google Scholar]
- Camalier CR, Gotler A, Murthy A, Thompson KG, Logan GD, Palmeri TJ, Schall JD. Dynamics of saccade target selection: Race model analysis of double step and search step saccade production in human and macaque. Vision Res 47: 2187–2211, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Pouget P, Woodman GF, Subraveti CR, Schall JD, Rossi AF. Difficulty of visual search modulates neuronal interactions and response variability in the frontal eye field. J Neurophysiol 98: 2580–2587, 2007. [DOI] [PubMed] [Google Scholar]
- Costello MG, Zhu D, Salinas E, Stanford TR. Perceptual modulation of motor–but not visual–responses in the frontal eye field during an urgent-decision task. J Neurosci 33: 16394–16408, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Gold JI. Caudate encodes multiple computations for perceptual decisions. J Neurosci 30: 15747–15759, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Boucher L, Carpenter RH, Hanes DP, Harris R, Logan GD, Mashru RN, Paré M, Pouget P, Stuphorn V, Taylor TL, Schall JD. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vis Res 47: 35–49, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui A, Bhutani N, Kulashekhar S, Behari M, Goel V, Murthy A. Impaired conflict monitoring in Parkinson's disease patients during an oculomotor redirect task. Exp Brain Res 208: 1–10, 2011. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol 79: 817–834, 1998. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996. [DOI] [PubMed] [Google Scholar]
- Heitz R, Schall JD. Neural chronometry and coherency across speed-accuracy demands reveal lack of homomorphism between computational and neural mechanisms of evidence accumulation. Phil Trans R Soc B 368: 20130071, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz R, Schall JD. Neural mechanisms of speed-accuracy tradeoff. Neuron 76: 616–628, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol 49: 1268–1284, 1983. [DOI] [PubMed] [Google Scholar]
- Katsuki F, Constantinidis C. Early involvement of prefrontal cortex in visual bottom-up attention. Nat Neurosci 15: 1160–1166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Connors BW. Apical dendrites of the neocortex: correlation between sodium-and calcium-dependent spiking and pyramidal cell morphology. J Neurosci 13: 5301–5311, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CC, Boucher L, Paré M, Schall JD, Wang XJ. Proactive inhibitory control and attractor dynamics in countermanding action: a spiking neural circuit model. J Neurosci 29: 9059–9071, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister ML, Hennig JA, Huk AC. Signal multiplexing and single-neuron computations in lateral intraparietal area during decision-making. J Neurosci 33: 2254–2267, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A, Ray S, Shorter SM, Priddy EG, Schall JD, Thompson KG. Frontal eye field contributions to rapid corrective saccades. J Neurophysiol 97: 1457–1469, 2007. [DOI] [PubMed] [Google Scholar]
- Murthy A, Ray S, Shorter SM, Schall JD, Thompson KG. Neural control of visual search by frontal eye field: Effects of unexpected target displacement on visual selection and saccade preparation. J Neurophysiol 101: 2485–2506, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A, Thompson KG, Schall JD. Dynamic dissociation of visual selection from saccade programming in frontal eye field. J Neurophysiol 86: 2634–2637, 2001. [DOI] [PubMed] [Google Scholar]
- Nelson MJ, Boucher L, Logan GD, Palmeri TJ, Schall JD. Nonindependent and nonstationary response times in stopping and stepping saccade tasks. Atten Percept Psychophys 72: 1913–1929, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci 23: 6480–6489, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitou T, Pouget P. Saccadometry and movement inhibition. Biocybern Biomed Eng 32: 33–47, 2012. [Google Scholar]
- Pouget P, Logan GD, Palmeri TJ, Boucher L, Paré M, Schall JD. Neural basis of adaptive response time adjustment during saccade countermanding. J Neurosci 31: 12604–12612, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell BA, Heitz RP, Cohen JY, Schall JD, Logan GD, Palmeri TJ. Neurally constrained modeling of perceptual decision making. Psychol Rev 117: 1113–1143, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell BA, Schall JD, Logan GD, Palmeri TJ. From salience to saccades: multiple-alternative gated stochastic accumulator model of visual search. J Neurosci 32: 3433–3446, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan A, Sureshbabu R, Murthy A. Understanding how the brain changes its mind: Microstimulation in the macaque frontal eye field reveals how saccade plans are changed. J Neurosci 32: 4457–4472, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Hasegawa YT, Hasegawa RP, Smith PL, Segraves MA. Dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. J Neurophysiol 97: 1756–1774, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Pouget P, Schall JD. Functional distinction between visuomovement and movement neurons in macaque frontal eye field during saccade countermanding. J Neurophysiol 102: 3091–3100, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Schall JD, Murthy A. Programming of double-step saccade sequences: modulation by cognitive control. Vis Res 44: 2707–2718, 2004. [DOI] [PubMed] [Google Scholar]
- Raybourn MS, Keller EL. Colliculoreticular organization in primate oculomotor system. J Neurophysiol 40: 861–878, 1977. [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22: 9475–9489, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Stanford TR. The countermanding task revisited: fast stimulus detection is a key determinant of psychophysical performance. J Neurosci 33: 5668–5685, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Murthy A, Thompson KG, Schall JD. Effect of search efficiency but not response interference on visual selection in frontal eye field. Neuron 30: 583–591, 2001. [DOI] [PubMed] [Google Scholar]
- Sayer RJ, Friedlander MJ, Redman SJ. The time course and amplitude of EPSPs evoked at synapses between pairs of CA3:CA1 neurons in the hippocampal slice. J Neurosci 10: 826–836, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci 16: 1118–1124, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves MA. Activity of monkey frontal eye field neurons projecting to oculomotor regions of the pons. J Neurophysiol 68: 1967–1985, 1992. [DOI] [PubMed] [Google Scholar]
- Shen K, Paré M. Predictive saccade target selection in superior colliculus during visual search. J Neurosci 34: 5640–5648, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA. The role of the thalamus in motor control. Curr Opin Neurobiol 13: 663–670, 2003. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Shankar S, Massoglia DP, Costello MG, Salinas E. Perceptual decision making in less than 30 milliseconds. Nat Neurosci 13: 379–385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. Separate modifiability, mental modules, and the use of pure and composite measures to reveal them. Acta Psychol (Amst) 106: 147–246, 2001. [DOI] [PubMed] [Google Scholar]
- Tanaka M. Cognitive signals in the primate motor thalamus predict saccade timing. J Neurosci 27: 12109–12118, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol 76: 4040–4055, 1996. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Reynoso HS, Aron AR. Saccade suppression exerts global effects on the motor system. J Neurophysiol 110: 883–890, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BJ, Munoz DP. Separate visual signals for saccade initiation during target selection in the primate superior colliculus. J Neurosci 31: 1570–1578, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Kang MS, Thompson K, Schall JD. The effect of visual search efficiency on response preparation: neurophysiological evidence for discrete flow. Psychol Sci 19: 128–136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]