Abstract
Voluntary limb modifications must be integrated with basic walking patterns during visually guided walking. In this study we tested whether voluntary gait modifications can become more automatic with practice. We challenged walking control by presenting visual stepping targets that instructed subjects to modify step length from one trial to the next. Our sequence learning paradigm is derived from the serial reaction-time (SRT) task that has been used in upper limb studies. Both random and ordered sequences of step lengths were used to measure sequence-specific and sequence-nonspecific learning during walking. In addition, we determined how age (i.e., healthy young adults vs. children) and biomechanical factors (i.e., walking speed) affected the rate and magnitude of locomotor sequence learning. The results showed that healthy young adults (age 24 ± 5 yr, n = 20) could learn a specific sequence of step lengths over 300 training steps. Younger children (age 6–10 yr, n = 8) had lower baseline performance, but their magnitude and rate of sequence learning were the same compared with those of older children (11–16 yr, n = 10) and healthy adults. In addition, learning capacity may be more limited at faster walking speeds. To our knowledge, this is the first study to demonstrate that spatial sequence learning can be integrated with a highly automatic task such as walking. These findings suggest that adults and children use implicit knowledge about the sequence to plan and execute leg movement during visually guided walking.
Keywords: human, learning, locomotion, vision, walking
the automatic control of walking is critical to our daily activities. We normally do not consciously think about walking unless we encounter novel situations where we have to make voluntary gait modifications (e.g., hiking across stepping stones). On the other hand, voluntary control of stepping patterns can become more automatic with practice (e.g., line dancing). Locomotor skills can be learned via visual cues from the environment or proprioceptive cues from the limbs during obstacle avoidance tasks (Erni and Dietz 2001; Lajoie et al. 2012). Moreover, there is some evidence that visual cue training could improve gait patterns that transfer to walking without cues in people with Parkinson's disease (Spaulding et al. 2013).
The classic serial reaction-time (SRT) task, first introduced by Nissen and Bullemer (1987), may be used to assess to what an extent a motor task has become automatic. The SRT task requires subjects to rapidly respond to different spatial cues by pressing corresponding buttons as fast and accurately as possible. With practice, subjects demonstrate learning by performing faster on the repeating sequence than on random sequences. This difference can only be accounted for by sequence-specific learning, which can occur with or without explicit knowledge (Cohen et al. 1990; Curran and Keele 1993; Nissen and Bullemer 1987).
The primary objective of this study was to test whether spatial sequence learning could be integrated with a highly automatic task such as walking. We challenged walking control by presenting stepping targets of varying spatial complexities (e.g., random vs. ordered). To our knowledge, this is the first study to investigate sequence learning in the lower limbs during walking. Our second objective was to determine how age (i.e., healthy young adults vs. children) and biomechanical factors (i.e., walking speed) affect the rate and magnitude of locomotor sequence learning. Cortical control of gait is not fully developed in children under the age of 10 yr (Petersen et al. 2010), so we therefore predicted that visuomotor control of walking might be more difficult in younger children compared with adults. Furthermore, biomechanical constraints may supersede the goal of precision, in which case we would see a drop in performance as we challenge subjects to walk at faster speeds.
METHODS
Subjects.
Twenty young adults (age 24 ± 5 yr) and 18 children (mean age 11 ± 3 yr, range 6–15 yr) with no known neurological disorders participated in this study. The study was approved by the local ethics committee (protocol #H-A-2008-029). All methods conformed to the Declaration of Helsinki. All subjects gave written informed consent prior to participation.
Motion capture.
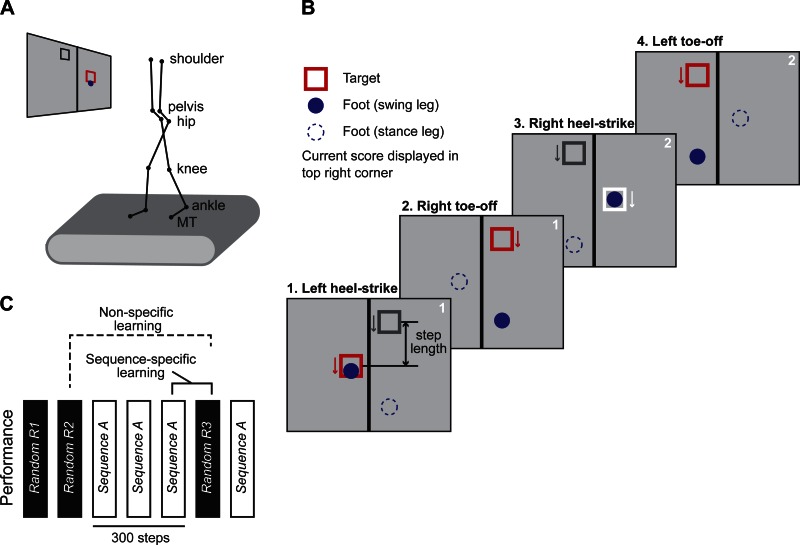
Reflective markers were placed bilaterally on the toe (5th metatarsal head, 5MT), ankle (lateral malleolus), knee (lateral joint space), hip (greater trochanter), pelvis (iliac crest), and shoulder (acromion process) to capture walking kinematics (Fig. 1A). A six-camera ProReflex motion capture system (Qualisys, Gothenburg, Sweden) was used to collect three-dimensional (3-D) marker data at 100 Hz. Identification of markers was performed using the AIM module in the Qualisys Track Manager software.
Fig. 1.
A: visual feedback was projected on a screen in front of the treadmill. A 12-marker setup was used for motion capture. MT, metatarsal. B: target (open square) and foot position of the swing leg (filled circle) were displayed during the walking task. The targets moved down the screen at a speed corresponding to the treadmill speed (arrows). Stance leg foot position (open circle) was not visible on the display. The vertical distance between the current target (red square) and the next target (gray square) indicated the desired step length (1). The position of the swing leg appeared after ipsilateral toe off (2). The current target turned from red to white color on a successful hit (3). Scores were displayed on the upper right corner of the screen. The current target and foot position disappeared after ipsilateral heel strike (4). C: the order of random (R1–R3) and sequence blocks (S1–S4) in the sequence learning paradigm. Each block consisted of 100 steps. Sequence-specific learning was calculated as the difference in performance between S3 and R3 (solid line); nonspecific learning was calculated as the difference between R2 and R3 (dotted line).
Experimental setup and visual feedback.
We created a visuomotor walking task where subjects must change their step length to hit visual targets while walking on a treadmill (Fig. 1A). Stepping targets and foot position were projected on a screen in front of the treadmill. The projector (Toshiba TDP-T 355) was mounted on the ceiling. The projection distance was 148 cm away from the screen, which resulted in a screen size of 125 cm high by 167 cm wide. This setup allowed us to manipulate the sequence of target locations step by step and to provide real-time visual feedback to the subject during the experiment. A custom-made computer program controlled the position of the targets while the QTM real-time server provided the position of the foot (i.e., 5MT marker) during the walking task.
Targets were represented by 16 × 16-cm open squares on the visual display (Fig. 1B). The swing leg's foot position was displayed as an 8-cm-diameter circle. Stepping targets for the left foot and right foot appeared on the left and right of midline, respectively. The targets first appeared near the top of the screen and moved down at a speed corresponding to the treadmill speed (arrow). The subject saw both the current target (red square) and the next target (gray square). The vertical distance between the two targets indicated the required step length (Fig. 1B, 1). The position of the swing leg appears at ipsilateral toe off (Fig. 1B, 2). Subjects were instructed to step on the targets as accurately as possible. A successful hit was one where the center of the foot (circle) lay within 8 cm of the center of the target (square) after heel strike (Fig. 1B, 3). The current target turned white on each successful hit. The score was updated and displayed on the top right corner of the screen. The current target and foot position disappeared after ipsilateral heel strike (Fig. 1B, 4). The next target turned red and the foot position of the contralateral leg appeared. See Supplementary Video S1 for a replay of the visual display during the visuomotor task. (Supplemental material for this article is available online at the Journal of Neurophysiology website.)
Learning paradigm.
Each sequence was composed of three different step lengths (e.g., short, medium, long). The step lengths were adjusted proportionally to each subject's leg length. The medium step length was defined as two-thirds of leg length. The short step length was set at 80% of medium step length, and the long step length at 120% of medium step length. The average leg length was 0.85 ± 0.06 m in adults and 0.77 ± 0.12 m in children who participated in this study.
Two speed conditions were tested in the adult subjects. Ten adult subjects walked at slower treadmill speeds (2.3 ± 0.16 km/h), and another 10 adult subjects walked at faster treadmill speeds (3.2 ± 0.16 km/h). All children were tested at slower treadmill speeds (1.8 ± 0.32 km/h). The treadmill speeds for individuals were set to match cadence between adults and children.
Subjects performed a total of 7 blocks that each consisted of 100 steps (Fig. 1C). In random blocks, the targets appeared randomly at locations that required different step lengths (i.e., short, medium, long). In sequence blocks, subjects were presented with a repeating sequence of step lengths (i.e., short-long-medium-long-short-medium). The first random block (R1) was used to familiarize the subject to the task. The second random block (R2) provided a measure of final baseline performance. In subsequent training blocks (S1–S3), subjects were presented with the repeating sequence. Sequence-specific learning was calculated as the difference in performance between the last training block (S3) and the last random block (R3); nonspecific learning was calculated as the difference between blocks R2 and R3. The final block (S4) tested whether subjects could immediately recall the learned sequence after reexposure to the random sequence (R3). At the end, subjects were asked whether they “noticed any patterns or repeating sequence?”
Sequence-by-sequence success rate was calculated as the fraction of hits within each six-step sequence. The score (number of successful hits) and mean error were calculated to measure performance within each block. Error was defined as the absolute difference between the foot position and target position (i.e., | center of the circle − center of the square |) and was normalized to each subject's leg length. Error in the anterior-posterior directions was analyzed. To evaluate sequence-specific learning, we subtracted the mean score in R3 from the mean score in the S3 block (Perez et al. 2007; Willingham et al. 2000). Nonspecific learning was measured by subtracting the mean score in R3 from the mean score in R2 (Perez et al. 2007).
Statistical analysis.
Between-groups repeated-measures ANOVA was used to compare performance changes over the experimental blocks between groups (e.g., 7 blocks × 2 age groups) and speed conditions (e.g., 7 blocks × 2 speeds). When ANOVAs showed significant effects, the Tukey's honest significant difference test was used for post hoc pairwise analysis (i.e., R3 and S3 for sequence-specific learning, R2 and R3 for nonspecific learning).
RESULTS
Locomotor sequence learning.
Subjects improved their score over the seven blocks of testing, performing better in sequence blocks compared with random blocks. A typical subject hit about 50% of the targets in the first random block, R1, and improved his score on the second random block, R2, when he became more familiar with the task (Fig. 2A). Subjects had more difficulty shortening than lengthening step length. The number of hits on short steps was significantly lower compared with long steps (P < 0.001). Learning continued over the subsequent sequence blocks, S1–S3, as the score improved further. To determine whether the learning was sequence specific, we exposed the subject to another random block, R3. A drop in score in R3 compared with S3 indicates that the learning was sequence specific. That is, the subject did not simply learn to react to the visual stimuli. Moreover, the subject was able to recall the learned sequence on reexposure in S4. We also quantified sequence-by-sequence performance as the rate of success within each six-step sequence. Sequence-by-sequence performance is plotted on top of the score from the same subject to show variability in performance within a block (Fig. 2A).
Fig. 2.
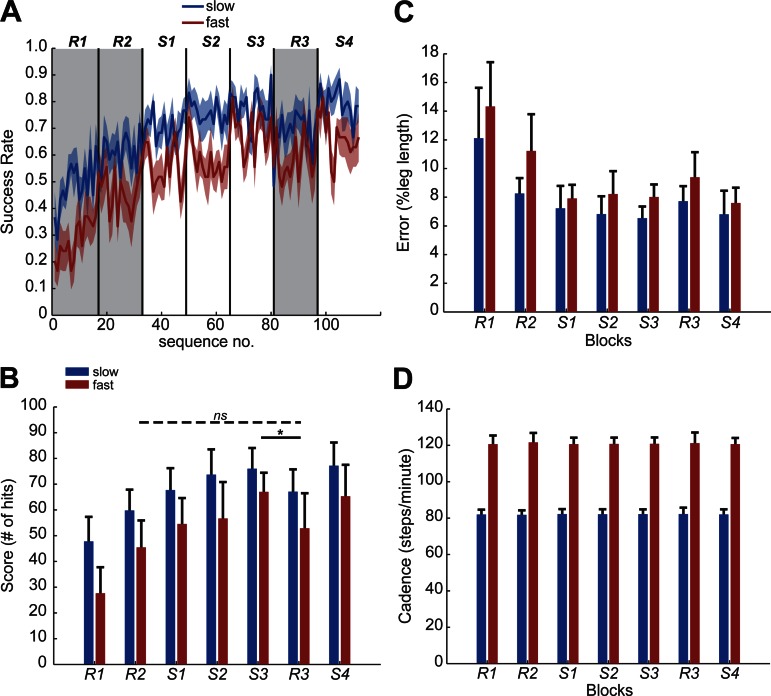
Locomotor sequence learning. Each sequence consisted of 6 steps, and each block consisted of 16 complete sequences. A: performance in a typical subject over 7 blocks. Score (left y-axis, maximum score = 100) is the total number of hits in each block (top x-axis). Success rate (right y-axis) is the fraction of hits within each 6-step sequence (bottom x-axis). Score (bar graph) and success rate (line graph) for the same subject are overlaid on the plot to show variability within each block. B: mean success rate for each sequence averaged across subjects (n = 10). Shaded area of the curve represents standard error (SE). C: mean score (number of hits) over 7 blocks of testing averaged across subjects (n = 10). D: nonspecific learning (R3 − R2) and sequence-specific learning (S3 − R3) for individual subjects. E: group average mean error (n = 10) in the anterior-posterior (AP) direction, calculated as the foot position (5th MT) relative to the target position. Error was normalized to each subject's leg length. F: group average cadence (n = 10). *P < 0.05; ns, not significant.
Learning was evident in both the average sequence-by-sequence success rate (Fig. 2B) and the average change in score across subjects (Fig. 2C). Statistical analysis was performed on the change in score across blocks. Significant performance changes were observed over the seven blocks of testing [repeated-measures ANOVA: effect of block, F(6,54) = 28.7, P < 0.001]. Importantly, the score decreased from S3 to R3, indicating that the learning was sequence specific (P = 0.03). Moreover, the amount of nonspecific learning (i.e., R3 − R2) was not significant (P = 0.1), suggesting that improved performance (after becoming accustomed to the walking task in R1) was mostly accounted for by sequence-specific learning. The amounts of nonspecific and sequence-specific learning for individual subjects are illustrated in Fig. 2D. At the end, subjects were asked whether they “noticed any patterns or repeating sequence?” None of the subjects gave perfect descriptions of the six-step repeating sequence. Thus the step length sequence appears to be learned without conscious awareness.
The group average (n = 10) mean error is plotted in Fig. 2E. The magnitude of errors decreased as the score improved [repeated-measures ANOVA: F(6,54) = 16.8, P < 0.001; Fig. 2E]. Post hoc analysis showed a significant decrease in error from R1 to R2 (P < 0.001). The same cadence was maintained across all blocks [repeated-measures ANOVA: F(6,54) = 0.2, P = 0.9; Fig. 2F].
Younger children performed worse in baseline but showed the same amount of sequence learning compared with older children.
To compare the learning rates from early to late childhood, children were divided into two age groups: 6–10 and 11–16 yr old (Fig. 3, A and B). The groups reflect the development in cortical control of gait, which is not fully mature in children under the age of 10 yr (Petersen et al. 2010). Figure 3A shows the average sequence-by-sequence success rate across the two age groups. Younger children had lower success rate in baseline (R1, R2), but they were able to improve performance over each block of training. Children in both age groups showed similar success rates by the third sequence block, S3.
Fig. 3.
Locomotor sequence learning in healthy adults vs. children. A: mean success rate for each sequence averaged across children in two age groups: 6–10 yr (n = 8) and 11–16 yr (n = 10). Shaded area of the curve represents SE. B–D: group average mean score (B), normalized AP error (C), and cadence (D). Bars represent average data across 7 blocks of testing within each age group: 6–10 yr (n = 8), 11–16 yr (n = 10), and adults (n = 10). E–G: individual data for each child plotted against age. Pearson's correlation (r) is shown for baseline score (E), nonspecific learning (F) and sequence-specific learning (G). *P < 0.05.
Statistical analysis was performed on the group average change in score (Fig. 3B). The results showed a significant difference between age groups [repeated-measures ANOVA: F(2,15) = 6.01, P = 0.02; block × age, F(12,150) = 0.9, P = 0.5]. Post hoc analysis showed a significant difference between younger and older children (P < 0.001) and between younger children and adults (P < 0.001). All age groups (6–10 yr, 11–16 yr, adults) showed sequence-specific learning (P < 0.001), as indicated by a decrease in score from S3 to R3. There was no increase in score from R3 to R2 (P ≥ 0.5), which indicates little nonspecific learning beyond R2.
The magnitude of error was significantly reduced over the seven blocks of training [repeated-measures ANOVA: effect of blocks, F(6,144) = 32.5, P < 0.001]. Error was normalized to each subject's leg length; the average leg length is 0.6 ± 0.05 m in younger children, 0.79 ± 0.08 m in older children, and 0.89 ± 0.05 m in adults. The normalized errors were largest in younger children [repeated-measures ANOVA: effect of age, F(2,24) = 21.0, P <0.001; blocks × age, F(12,144) = 2.2, P = 0.01; Fig. 3C]. Post hoc analysis showed a significant difference in normalized error between younger and older children (P < 0.001) and between younger children and adults (P < 0.001).
Subjects maintained the same cadence across all blocks [repeated-measures ANOVA: effect of blocks, F(6,150) = 1.0, P = 0.4; Fig. 3D]. Note that cadence was matched across age groups [repeated-measures ANOVA: effect of age, F(2,25) = 0.07, P = 0.8].
Regression analysis of individual data was performed to confirm the results from group average data. The baseline (R2) score for each child is plotted against age (Fig. 3E). There was a significant correlation between age and baseline score (r = 0.71, P = 0.001); performance improved from early to late childhood. There was no significant correlation between age and nonspecific learning (Fig. 3F; r = 0.22, P = 0.37) or sequence-specific learning (Fig. 3G; r = 0.05, P = 0.85). Thus younger children were able to learn and improve performance, despite starting with lower baseline score compared with older children.
Learning capacity is dependent on walking speed.
Locomotor patterns are characterized by distinct regularity in spatial and temporal parameters; the percentage of stride time spent in stance phase decreases as walking speed increases (Dietz et al. 1994). At faster walking speeds, subjects have less time to increase (or decrease) push-off forces to shorten (or lengthen) a step. Therefore, we predicted that subjects would perform worse during fast walking compared with slow walking.
Figure 4, A and B, shows the performance of adults walking at two different speeds. The score was lower at the fast walking speed [repeated-measures ANOVA: effect of speed, F(1,18) = 19.1, P < 0.001]. The score improved over seven blocks of testing at both walking speeds [repeated-measures ANOVA: effect of block, F(6,108) = 46.7, P < 0.001; block × speed, F(6,108) = 1.2, P = 0.3]. Post hoc analysis showed a significant amount of sequence-specific learning (P < 0.01) at both speeds. There was no significant difference between blocks R3 and R2 (P = 0.1), indicating little nonspecific learning beyond the first random block R2. Subjects reached a lower final score at the fast speed than at the slow speed, which suggests that learning capacity may be more limited when the task becomes more challenging.
Fig. 4.
Locomotor sequence learning at different walking speeds. A: mean success rate for each sequence averaged across subjects for two walking speeds: slow (2.3 ± 0.16 km/h) and fast (3.2 ± 0.16 km/h). B–D: group average mean score (B), normalized AP error (C), and cadence (D). Bars represent average data across 7 blocks of testing at 2 different walking speeds: slow (n = 10) and fast (n = 10). *P < 0.05.
Subjects made larger errors at the fast walking speed [repeated-measures ANOVA: effect of speed, F(1,18) = 539.6, P <0.001; Fig. 4C]. The magnitude of the error decreased over seven blocks of training [repeated-measures ANOVA: effect of block, F(6,108) = 25.8, P < 0.001; block × speed, F(6,108) = 3.4, P = 0.004]. Cadence was higher at the fast walking speed [repeated-measures ANOVA: effect of speed, F(1,18) = 18,285.6, P < 0.001; Fig. 4D]. This is expected, because the same step length was imposed at the slow and fast walking speeds. Subjects used the same cadence across the seven blocks of testing [repeated-measures ANOVA: effect of block, F(6,108) = 0.2, P = 0.9].
DISCUSSION
We used a novel paradigm to study spatial sequence learning during walking. The results showed that visually guided step length modification was improved through training. After training, subjects performed better on the repeating sequence compared with random sequences, suggesting that subjects used knowledge about the sequence to plan and control foot placement (rather than simply reacting to the visual stimuli). Moreover, none of the subjects were able to describe the repeating sequence correctly, suggesting that the step length sequence could be learned implicitly without conscious awareness.
Visuomotor coordination in locomotion.
Vision provides information about the position of objects within the environment and our own movement within the environment. The nervous system makes anticipatory adjustments to the control of walking in response to visual cues (Matthis and Fajen 2014; Warren et al. 1986). Patla et al. (1989) have reported the effects of the timing of visual cues to regulate step length during overground walking. Subjects were able to shorten step length when the visual cue was given before the contralateral heel contact, but not when the visual cue was given after the contralateral heel contact (Patla et al. 1989). When the visual cue was given at or before the contralateral heel contact, subjects had enough time to regulate step length by changing the contralateral push-off force. Subjects reduced push-off force to shorten step length and increased push-off force to increase step length (Patla et al. 1989). Consistent with previous reports on step length modification (Patla et al. 1989), subjects had more difficulty shortening step length during our visuomotor walking paradigm. Moreover, we found that subjects were less accurate at the fast walking speeds because there is less time to change push-off forces to shorten (or lengthen) a step.
Locomotor sequence learning.
We applied principles of sensorimotor learning to establish a new paradigm for studying visually guided sequence learning during walking. Learning can be defined as the storage of new motor patterns through repeated practice. Once a movement is learned, it could be immediately used in the appropriate context. Motor sequence learning involves integrating a series of movements that are executed in a specific order. The SRT task from the original study by Nissen and Bullemer (1987) has been used to study age-related changes in this type of procedural learning (Janacsek et al. 2012; King et al. 2013; Thomas et al. 2004). However, there are no studies in the literature that have explored sequence learning in lower limb movements during walking. We found that healthy adults and children improved their performance over 7 blocks of testing and learned a specific walking sequence over 300 training steps. In this case, improved performance and accuracy were achieved without changing movement timing (e.g., cadence). This demonstrates that sequence learning can be integrated with human locomotor pattern generation.
Neural control of gait modifications.
Visually guided limb modifications must be integrated into the locomotor pattern during ongoing movements (Georgopoulos and Grillner 1989; Rossignol 1996). Cortical mechanisms are involved in the planning and execution of movements during visually guided walking (Drew et al. 2008; Drew and Marigold 2015). The motor cortex of cats is not strongly modulated during unobstructed level walking but becomes more active when precise adjustments in paw placement or end-point control is required (Beloozerova and Sirota 1993; Drew 1993). Lesions of the pyramidal tract or the corticospinal tract can cause marked deficits in voluntary modifications to the walking pattern, such as in obstacle avoidance (Drew et al. 2002).
In humans, the corticospinal tract directly modulates leg muscle activation during steady-state walking (Petersen et al. 2001, 2010). Increase in muscular coherence in developing children reflects the maturation level of the corticospinal drive (Petersen et al. 2010). In addition, corticospinal input facilitates ankle muscle activation during foot placements in visual walking tasks (Schubert et al. 1999). Thus the human corticospinal tract likely contributes to the precision of foot placement control during human walking.
Locomotor patterns in young children (age < 4 yr) develop rapidly, resulting in greater specificity in muscle activation patterns on foot contact (Dominici et al. 2011). More gradual development in walking occurs until 8–10 yr of age (Lacquaniti et al. 2012). In general, the walking pattern of children shows greater variability compared with that of adults (Dominici et al. 2010; Petersen et al. 2010). The corticospinal drive is not fully mature in children under the age of 10 yr (Petersen et al. 2010). We therefore predicted that visuomotor control of walking might be more difficult in younger children compared with adults.
Our results are consistent with the hypothesis that the development of the corticospinal tract contributes to the control of foot placement. Children up to 10 yr of age performed worse at the task compared with older children. Interesting, we found that the ability to learn in healthy children aged 6–15 yr is comparable to that of healthy adults during visually guided step length modifications. There was no correlation in the amount of sequence-specific learning and nonspecific learning with age. We speculate that this may be related to participation in sports and other activities, which often involve changes in step length (e.g., soccer, hopscotch). The result is interesting because it suggests that mechanisms that underlie the planning and execution of voluntary gait modifications are intact before the locomotor system is fully mature. The ability to make gait modifications may facilitate the learning of new walking patterns. In fact, our results showed that the magnitude and rate of locomotor sequence learning are similar between children and adults.
Conclusions.
In summary, we have developed and used a novel paradigm to study sequence learning during visually guided walking in healthy subjects. Our results demonstrate that implicit sequence learning can be integrated with a highly automatic task such as walking. We conclude that visuomotor coordination in walking is dependent on the development of the corticospinal tract in children.
GRANTS
This work was supported by the Danish Medical Research Council (11–107721/FSS) and the Whitaker International Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.T.C. and J.B.N. conception and design of research; J.T.C. and P.J. performed experiments; J.T.C. and P.J. analyzed data; J.T.C., P.J., and J.B.N. interpreted results of experiments; J.T.C. prepared figures; J.T.C. drafted manuscript; J.T.C., P.J., and J.B.N. edited and revised manuscript; J.T.C., P.J., and J.B.N. approved final version of manuscript.
Supplementary Material
REFERENCES
- Andujar JE, Lajoie K, Drew T. A contribution of area 5 of the posterior parietal cortex to the planning of visually guided locomotion: limb-specific and limb-independent effects. J Neurophysiol 103: 986–1006, 2010. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG. The role of the motor cortex in the control of vigour of locomotor movements in the cat. J Physiol 461: 27–46, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Ivry RI, Keele SW. Attention and structure in sequence learning. J Exp Psychol Learn Mem Cogn 16: 17–30, 1990. [Google Scholar]
- Curran T, Keele SW. Attentional and nonattentional forms of sequence learning. J Exp Psychol Learn Mem Cogn 19: 189–202, 1993. [Google Scholar]
- Dietz V, Zijlstra W, Duysens J. Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 101: 513–520, 1994. [DOI] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, d'Avella A, Mondi V, Cicchese M, Fabiano A, Silei T, Di Paolo A, Giannini C, Poppele RE, Lacquaniti F. Locomotor primitives in newborn babies and their development. Science 334: 997–999, 2011. [DOI] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, Zampagni ML, Lacquaniti F. Kinematic strategies in newly walking toddlers stepping over different support surfaces. J Neurophysiol 103: 1673–1684, 2010. [DOI] [PubMed] [Google Scholar]
- Drew T. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. J Neurophysiol 70: 179–199, 1993. [DOI] [PubMed] [Google Scholar]
- Drew T, Andujar JE, Lajoie K, Yakovenko S. Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res Rev 57: 199–211, 2008. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res Rev 40: 178–191, 2002. [DOI] [PubMed] [Google Scholar]
- Drew T, Marigold DS. Taking the next step: cortical contributions to the control of locomotion. Curr Opin Neurobiol 33C: 25–33, 2015. [DOI] [PubMed] [Google Scholar]
- Erni T, Dietz V. Obstacle avoidance during human walking: learning rate and cross-modal transfer. J Physiol 534: 303–312, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Grillner S. Visuomotor coordination in reaching and locomotion. Science 245: 1209–1210, 1989. [DOI] [PubMed] [Google Scholar]
- Janacsek K, Fiser J, Nemeth D. The best time to acquire new skills: age-related differences in implicit sequence learning across the human lifespan. Dev Sci 15: 496–505, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, Fogel SM, Albouy G, Doyon J. Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front Hum Neurosci 7: 142, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacquaniti F, Ivanenko YP, Zago M. Development of human locomotion. Curr Opin Neurobiol 22: 822–828, 2012. [DOI] [PubMed] [Google Scholar]
- Lajoie K, Bloomfield LW, Nelson FJ, Suh JJ, Marigold DS. The contribution of vision, proprioception, and efference copy in storing a neural representation for guiding trail leg trajectory over an obstacle. J Neurophysiol 107: 2283–2293, 2012. [DOI] [PubMed] [Google Scholar]
- Lajoie K, Drew T. Lesions of area 5 of the posterior parietal cortex in the cat produce errors in the accuracy of paw placement during visually guided locomotion. J Neurophysiol 97: 2339–2354, 2007. [DOI] [PubMed] [Google Scholar]
- Matthis JS, Fajen BR. Visual control of foot placement when walking over complex terrain. J Exp Psychol Hum Percept Perform 40: 106–115, 2014. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cogn Psychol 19: 1–32, 1987. [Google Scholar]
- Patla AE, Robinson C, Samways M, Armstrong CJ. Visual control of step length during overground locomotion–task-specific modulation of the locomotor synergy. J Exp Psychol Hum Percept Perform 15: 603–617, 1989. [Google Scholar]
- Perez MA, Wise SP, Willingham DT, Cohen LG. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci 27: 1045–1053, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Butler JE, Marchand-Pauvert V, Fisher R, Ledebt A, Pyndt HS, Hansen NL, Nielsen JB. Suppression of EMG activity by transcranial magnetic stimulation in human subjects during walking. J Physiol 537: 651–656, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TH, Kliim-Due M, Farmer SF, Nielsen JB. Childhood development of common drive to a human leg muscle during ankle dorsiflexion and gait. J Physiol 588: 4387–4400, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S. Visuomotor regulation of locomotion. Can J Physiol Pharmacol 74: 418–425, 1996. [PubMed] [Google Scholar]
- Schubert M, Curt A, Colombo G, Berger W, Dietz V. Voluntary control of human gait: conditioning of magnetically evoked motor responses in a precision stepping task. Exp Brain Res 126: 583–588, 1999. [DOI] [PubMed] [Google Scholar]
- Spaulding SJ, Barber B, Colby M, Cormack B, Mick T, Jenkins ME. Cueing and gait improvement among people with Parkinson's disease: a meta-analysis. Arch Phys Med Rehabil 94: 562–570, 2013. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Hunt RH, Vizueta N, Sommer T, Durston S, Yang YH, Worden MS. Evidence of developmental differences in implicit sequence learning: an fMRI study of children and adults. J Cogn Neurosci 16: 1339–1351, 2004. [DOI] [PubMed] [Google Scholar]
- Warren WH Jr, Young DS, Lee DN. Visual control of step length during running over irregular terrain. J Exp Psychol Hum Percept Perform 12: 259–266, 1986. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Wells LA, Farrell JM, Stemwedel ME. Implicit motor sequence learning is represented in response locations. Mem Cognit 28: 366–375, 2000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.