Abstract
We hypothesized that epilepsy affects the activity of the autonomic nervous system even in the absence of seizures, which should manifest as differences in heart rate variability (HRV) and cardiac cycle. To test this hypothesis, we investigated ECG traces of 91 children and adolescents with generalized epilepsy and 25 neurologically normal controls during 30 min of stage 2 sleep with interictal or normal EEG. Mean heart rate (HR) and high-frequency HRV corresponding to respiratory sinus arrhythmia (RSA) were quantified and compared. Blood pressure (BP) measurements from physical exams of all subjects were also collected and analyzed. RSA was on average significantly stronger in patients with epilepsy, whereas their mean HR was significantly lower after adjusting for age, body mass index, and sex, consistent with increased parasympathetic tone in these patients. In contrast, diastolic (and systolic) BP at rest was not significantly different, indicating that the sympathetic tone is similar. Remarkably, five additional subjects, initially diagnosed as neurologically normal but with enhanced RSA and lower HR, eventually developed epilepsy, suggesting that increased parasympathetic tone precedes the onset of epilepsy in children. ECG waveforms in epilepsy also displayed significantly longer TP intervals (ventricular diastole) relative to the RR interval. The relative TP interval correlated positively with RSA and negatively with HR, suggesting that these parameters are linked through a common mechanism, which we discuss. Altogether, our results provide evidence for imbalanced autonomic function in generalized epilepsy, which may be a key contributing factor to sudden unexpected death in epilepsy.
Keywords: SUDEP, heart rate variability, cardiac cycle, parasympathetic tone, children
epileptic seizures are known to have profound effects on autonomic function (Wyllie 2015). To quantify these effects, many studies have focused on heart rate (HR) (Behbahani et al. 2016; Jeppesen et al. 2015; Varon et al. 2015a, b; Zijlmans et al. 2002), cardiac abnormalities (Nei 2009; Smith and Delisle 2015; Varon et al. 2015a), respiration (Bulow and Ingvar 1963; Nashef et al. 1996), arterial blood pressure (BP) (Oztas and Turkel 2001), and oxygen saturation (Blum et al. 2000; Szurhaj et al. 2015) all during ictal periods. Whereas these and other numerous studies have helped us understand autonomic imbalance associated with seizures, less is understood about autonomic function during the considerably longer interictal periods, especially during sleep (Varon et al. 2015b). The closure of this critical gap in understanding has been suggested by researchers (Kothare and Singh 2014) as essential to obtaining a complete picture of autonomic regulation in epilepsy. This, in turn, would better inform us on the physiological mechanisms of sudden unexpected death in epilepsy (SUDEP) (Bozorgi and Lhatoo 2013; Lhatoo et al. 2015; Moghimi and Lhatoo 2013) and improve the efficacy of risk factors used to provide clinical prognoses for both epilepsy and SUDEP (Lhatoo et al. 2015; Varon et al. 2015b).
Furthermore, although clinical observations have shown that parasympathetic activity is often decreased during ictal periods in refractory or medication-resistant epilepsy (Kolsal et al. 2014), there is conflicting evidence regarding parasympathetic tone in interictal periods. For instance, studies on patients with Rolandic epilepsy have shown that vagal tone is enhanced compared with neurologically normal controls (Seri et al. 2012), whereas other studies have shown evidence of sympathetic overdrive and parasympathetic depression (Behbahani et al. 2016; Raju et al. 2012). In light of these opposing findings, it has been suggested that a more systematic investigation of sympathoparasympathetic balance in the absence of seizures is necessary to develop current approaches to treating epilepsy and preventing SUDEP (Sarkis et al. 2015).
A recent longitudinal study that followed children with epilepsy undergoing pharmacologic treatment over several years concluded that “SUDEP is not a rare event in children” (Terra et al. 2009). As 1.4% of the subjects succumbed to SUDEP during the study, the authors noted that premature mortality rates associated with SUDEP could diminish if more pediatric research were conducted. Researchers investigating both focal and generalized epilepsy have commented that data on children and young adults are scarce (El-Sayed et al. 2007; Seri et al. 2012), even though SUDEP makes up 14% of all reported cases of sudden unexpected death in children (Hesdorffer et al. 2015). Similarly, a Canadian pediatric surveillance study of children with epilepsy concluded that “risk factors for SUDEP in children are not well established” and citing the paucity of current research, called for more pediatric studies (Donner 2014).
According to Mostacci et al. (2015), “Most cases of SUDEP․ ․ ․occur while people are in bed, presumably sleeping.” In a clinical study, 58% of all reported SUDEP cases were found to have occurred during sleep; of these, 86% were unwitnessed but confirmed through autopsies (Lamberts et al. 2012). Systematic explorations of the linkages between sleep and SUDEP over the years have yielded interesting results: significant findings suggest that SUDEP most commonly occurs during the nonrapid eye movement (NREM) portion of sleep (Herman et al. 2001) and that epileptiform activity prone to triggering SUDEP is prevalent in shallower sleep stages (e.g., stage 2) or the transition to waking (Lamberts et al. 2012; Menezes Cordeiro et al. 2015). It has also been observed that when stage 2 sleep precedes REM sleep or wakefulness, it is accompanied by an activation of the adrenocorticotropic system that enhances sympathetic function; however, when stage 2 is followed by slow-wave sleep (stage 3), sympathetic tone decreases, in parallel with an activation of the renin-angiotensin system (Brandenberger et al. 2005). Because of this autonomic-endocrine duality, stage 2 sleep is of particular interest in the context of autonomic function in epilepsy, especially with regard to SUDEP. In addition, sleep is a consistent resting state and a good regime for study and comparison of autonomic function (Kanda et al. 2016).
Investigation of autonomic balance has often been done through the use of HR variability (HRV), via a set of well-validated, noninvasive measures of baroreflex sensitivity and vagal tone (Shaffer et al. 2014; Tobaldini et al. 2013). In practice, the low-frequency/high-frequency (LF/HF) spectral power ratio of HRV, a relative measure of the amplitude of LF (0.04–0.15 Hz) and HF (0.15–0.4 Hz) modulations of HR, is routinely used in clinical contexts to estimate sympathoparasympathetic balance. These LF and HF ranges have been used previously to examine HRV in children (Akinci et al. 1993; Blood et al. 2015). However, recent studies have cast doubt on the accuracy of the LF component of HRV in assessing sympathetic tone (Goldstein et al. 2011) and, consequently, the accuracy of the LF/HF ratio in measuring autonomic balance (Billman 2013). In this study, we will instead consider the non-normalized HF peak in the power spectral density (PSD) of HR, widely regarded as the vagal effect of respiratory sinus arrhythmia (RSA) (Shaffer et al. 2014; Yasuma and Hayano 2004). Use of the raw power of RSA affords interindividual comparisons of vagal activity in the natural, absolute units of power density (Indic et al. 2008).
Vagal tone has also been effectively characterized using time-domain measures of HRV, including mean HR (Meghana et al. 2015) and the SD of normal beat-to-beat, i.e., R-to-R (RR), intervals (Nayak et al. 2015). Decreased HR is often interpreted as sympathetic depression (Abukonna et al. 2013), but it may also be due to increased parasympathetic activity and is closely linked with arterial BP and baroreflex sensitivity (Taylor et al. 2015). Acute elevation of BP (unlike the chronic condition of hypertension) is considered to be primarily mediated by noradrenergic ganglionic pathways in the sympathetic nervous system (Guyenet 2006), and diastolic resting BP is a good measure of barosensitive sympathetic regulation (Joyner et al. 2010). In children, HRV-related parameters have been shown to correlate with age: specifically, systolic and diastolic BPs increase with age in young children (Riley and Bluhm 2012), whereas resting HR decreases quickly after infancy and continues to decline steadily through adolescence (Fleming et al. 2011). Pediatric analyses of autonomic function using these measures should account for age dependence and assess HR and BP contributions to sympathetic tone independently due to interdependent regulation involving the arterial baroreflex (Swenne 2013).
To address the mentioned gaps in the understanding of sympathoparasympathetic balance in children with epilepsy and at risk of SUDEP, we analyzed and compared HRV and ECG waveforms as noninvasive parameters of the autonomic tone from patients with generalized pediatric epilepsy and neurologically normal controls during interictal and normal EEG periods in stage 2 sleep.
METHODS
Subject cohort.
Data collection from the EEG/ECG database of the Pediatric Epilepsy Unit in the Pediatric Neurology Department at University Hospitals in Cleveland, Ohio, was approved and conducted in accordance with the ethical standard guidelines and regulations set by the Institutional Review Board of University Hospitals and Case Western Reserve University. Subjects were selected based on availability of sleep ECG and EEG data as well as diagnosis of epilepsy (either generalized or no epilepsy diagnosis) from all subjects who had an overnight EEG conducted between January 1, 2008, and November 1, 2014, at the unit. Records were reviewed retrospectively. A total of 151 subjects was initially selected, 106 with generalized epilepsy and 45 neurologically normal (control group), with ages ranging from neonates to 22 yr. Monitoring reports from those subjects included medical history, demographic information, and results from routine clinical tests performed on children in the Epilepsy Unit. Sex, height, weight, date of birth, date of physical exam, EEG/ECG evaluation, as well as resting systolic and diastolic BPs were obtained from these reports. The age of each subject was determined as the difference between the date of data acquisition and the date of birth.
Epochs of ECG and EEG data from overnight EEG sleep studies at University Hospitals were obtained for each subject. These clips contained 30 uninterrupted min of stage 2 sleep, as determined by a hospital technician, who also verified, along with a pediatric neurologist, that the EEG activity recorded was normal (or interictal in epilepsy). All subjects with epilepsy who were selected had generalized epilepsy, with the majority of seizures generalized as tonic-clonic seizures. Other types of seizures included absence seizures, myoclonic seizures, and spasms. Subjects were grouped according to final diagnosis at the time of discharge.
Subjects from the control group were children who had visited the Epilepsy Unit due to sleeping problems, staring spells, or recent head trauma and/or who had family members (particularly siblings or parents) with epilepsy, which indicated a potential inherited risk for developing epilepsy. It is common practice at the Epilepsy Unit that children coming in for a sleep evaluation also receive a routine EEG to confirm healthy brain activity. Subjects in the control group were advised to be monitored for a sleep study to rule out epilepsy as a diagnosis and were diagnosed by a pediatric neurologist specialized in epilepsy not to have epilepsy, according to their overnight EEG examination.
All patients already diagnosed with epilepsy before the EEG/ECG evaluation were on a version of the ketogenic diet and had been taking anti-epileptic medications. For the overnight EEG/ECG evaluation itself, patients were asked not to take their medications during this period to minimize their effect on the recorded signals. Commonly taken anti-epileptic medications included enhancers of GABAergic signaling (clonazepam, tiagabine), modulators of sodium and calcium channels (carbamazepine, lamotrigine, zonisamide), and antagonists of glutamate receptors (clobazam, phenobarbital) and medications that may decrease blood pH (carbonic anhydrase inhibitors, such as topiramate); more frequently, patients took combinations of these medications. Specific details regarding dosages (amount, frequency, timing, etc.) were not available to the data collection.
Exclusion criteria and group characteristics.
Subjects with unusable ECG data due to movement artifacts, interruptions, or technical issues were obviously excluded from the analyses. Subjects with monitoring reports but without corresponding EEG or ECG recordings on or immediately after the date of admission into the Pediatric Epileptic Unit were also excluded from the study. Subjects with multiple dates of admission and multiple sleep evaluations were identified, and only the most recently collected 30-min clip from each of these patients was selected for analysis. Neither the nature of anti-epileptic medications nor their possible combination or dose was considered as an exclusion criterion.
While finding subjects based on our selection criteria, we discovered five subjects who were initially diagnosed as neurologically normal but developed epilepsy after the first sleep study. Of these five subjects, two had not been taking any medications regularly by the time of the overnight sleep study, and the other three were taking a sleep aid (melatonin), medication for attention deficit disorder (amphetamine), asthma medications (albuterol, montekulast, budesonide), and/or anxiety medications (risperidone, sertraline) but no anti-epileptic medications. None of these five subjects had reported experiencing any kind of seizure before the time of data acquisition nor had they been diagnosed previously with epilepsy; additionally, the EEG data collected during the sleep study were deemed to be normal by a pediatric neurologist. However, hospital records more recent than the time frame of this retrospective study confirm that these subjects later developed and were diagnosed with epilepsy. We also observed that seven neurologically normal subjects, according to the EEG evaluation, were taking anti-epileptic medication at the time of data acquisition as a preventive measure to treat psychogenic, nonepileptic seizures.
Both the group of five subjects who later developed epilepsy and the group of seven nonepileptic patients taking anti-epileptic medication were excluded from the main cohort but were analyzed separately to determine the potential of RSA and HR as prognostic biomarkers for epilepsy (see results). The demographic composition of these two groups did not appear to be essentially different from the rest of the cohort.
After application of all of the exclusionary criteria, 25 control subjects and 91 patients with generalized epilepsy remained in the main cohort for analysis. Subjects in the control group had a mean age of 7.5 yr, with an SD of 6.4 yr, whereas patients with epilepsy had a mean age of 10.5 yr, with an SD of 5.0 yr. Of the control subjects, 5 (20%) were male, and 20 (80%) were female, whereas 64 patients (70%) were male, and 27 (30%) were female in the epilepsy group. To minimize the effect of these asymmetric proportions on our results, we adjusted all measured parameters of interest for age, body mass index (BMI), and sex (see below).
Analysis of HRV.
ECG data were imported into MATLAB (version 2015a; MathWorks, Natick, MA) for analysis with custom-written software (developed by S. S. Sivakumar and R. F. Galán). ECG spike detection (R wave) was performed on ECG traces (with sampling frequency 200 Hz) and their time derivatives with a double-threshold set at the 98th percentile of the mean-subtracted rectified ECG and 98th percentile of the rectified time derivative of the ECG, over a sliding time window of 10 s. Each signal fluctuation exceeding both thresholds within a margin of 0.2 s was registered as a single beat, marked at the maximum point of the ECG trace within that margin. Histograms of the interbeat interval distribution were manually inspected for high variability and outliers due to missed or incorrectly detected beats. Errors in the automatic beat detection algorithm (<5%) were either due to sharp P or T waves that were erroneously identified as beats or to blunt R waves that were not detected. These errors were manually corrected and blinded to the subject's identity and diagnosis.
HR series were calculated as the reciprocal of each interbeat interval and plotted against the time point of the initial beat in each interval. Cubic-spline interpolation was then used to generate the instantaneous HR at regular time intervals with a sampling rate of 200 Hz over the duration of the 30-min recording. For each subject, the mean HR was calculated as the time average of the instantaneous HR.
A zero-phase, digital high-pass filter with a cutoff frequency of 0.1 Hz was applied to the instantaneous HR to eliminate LF variability uniformly across subjects. MATLAB’s implementation of the multitaper PSD estimate (Thomson 1982) was then used to compute the PSD of the HR for each subject. The height of the peak in the PSD and its location were recorded for each subject, which correspond to the power density and frequency of the RSA.
The power density unit of a signal measured in units of U is U2/Hz; thus the instantaneous HR, measured in hertz, has power density measured in units of Hz2/Hz = Hz, and therefore, both power density and frequency of RSA were measured in units of hertz. When assessing correlations between power density of RSA and other parameters, power density was instead expressed in units of decibels via the formula RSAdB = 10·log10 (RSAHz/1 Hz).
ECG waveform analysis.
A cycle-triggered average ECG was computed for each subject as follows: the mean-subtracted ECG waveform during each RR interval was mapped onto a fixed interval of 360 linearly spaced samples, such that one sample corresponds to 1° in the cardiac cycle. We then averaged these cycles degree by degree to obtain the average ECG waveform over the RR interval.
Adjustment of measured parameters for age, BMI, and sex.
When comparing parameters of HRV and ECG waveforms between groups, we investigated the covariation of these parameters with age, BMI, and sex, with the intent to adjust for these correlations if present. BMI was computed using the formula mass/height2, with mass measured in units of kilograms and height measured in units of meters; height (in centimeters) and weight (in kilograms) measurements were taken during the clinical check-up at the time of ECG data acquisition. Sex was coded as a binary variable, with zero representing male and one representing female.
A linear model of the form y ∼ β0 + β1x1 + β2x2 + β3x3 was then computed for each parameter of interest, e.g., HR (y) as a function of age (x1), BMI (x2), and sex (x3), using the MATLAB “fitlm” function. The validity of the model was assessed with three sequential tests, whose outputs are reported (see Tables 1–4). 1) An F-test is used for the null hypothesis that the regression coefficients β1, β2, β3 are all equal to zero or, equivalently, that the model is constant; i.e., y ∼ β0. We report (see Tables 1–4) the F-statistic, its critical value for significance (F#), and its P value. If F < F#, then parameter y does not significantly covary with age, BMI, or sex and does not require further adjustment. 2) If F ≥ F#, then a t-test for each individual regression coefficient determines if the covariation of its associated variable with the parameter of interest (y) is significant. 3) The residuals of the model are then computed as the differences y − (β0 + β1x1 + β2x2 + β3x3) across subjects and represent the adjustment of parameter y for age, BMI, and sex. If the adjustment is meaningful, then the residuals must be distributed normally; otherwise, the model does not capture relevant covariations in the dataset. We tested the null hypothesis of normality of the residuals with the Lilliefors test (Lilliefors 1967). We provide the D-statistic of this test, its threshold value to reject the null hypothesis (D#), and its P value (see Tables 1–4). The null hypothesis was not rejected for any of the parameters that required adjustment.
Table 1.
Regression analyses for power of RSA
|
F-test for nonconstant model | |||
|---|---|---|---|
| F-test | F = 1.09 | F# = 3.077 | P = 0.355 |
| Estimated coefficients of linear regression model |
||||
|---|---|---|---|---|
| Estimate | SE | t-Statistic | P | |
| Intercept | 0.0697 | 0.0430 | 1.621 | 0.108 |
| Age | −0.0021 | 0.0023 | −0.934 | 0.353 |
| BMI | 0.0022 | 0.0024 | 0.900 | 0.370 |
| Sex | −0.0342 | 0.0215 | −1.590 | 0.115 |
| Lilliefors test for non-normality of residuals | |||
|---|---|---|---|
| Lilliefors test | D = 0.2510 | D# = 0.0819 | P = 0** |
Respiratory sinus arrhythmia (RSA) power is independent of age, body mass index (BMI), and sex. F#, critical value for significance of F-statistic; D#, threshold value to reject the null hypothesis of D-statistic of Lilliefors test.
P < 0.01 (highly significant).
Table 4.
Regression analyses for TP/RR
|
F-test for nonconstant model | |||
|---|---|---|---|
| F-test | F = 11.4 | F# = 3.077 | P = 1.44e-6** |
| Estimated coefficients of linear regression model |
||||
|---|---|---|---|---|
| Estimate | SE | t-Statistic | P | |
| Intercept | 47.296 | 2.5909 | 18.254 | 2.37e-35** |
| Age | 0.5381 | 0.1352 | 3.979 | 1.23e-4** |
| BMI | −0.0940 | 0.1460 | −0.644 | 0.521 |
| Sex | −4.7402 | 1.2949 | −3.661 | 3.85e-4** |
| Lilliefors test for non-normality of residuals | |||
|---|---|---|---|
| Lilliefors test | D = 0.0581 | D# = 0.0827 | P = 0.436 |
The relative TP interval significantly covaries with age and sex but not BMI.
P < 0.01 (highly significant).
Statistical tests for group comparisons and correlation coefficients.
Statistical analyses for HRV and waveform measures between groups were conducted using MATLAB's implementation of Wilcoxon's rank sum test, a nonparametric test that compares the medians of two one-dimensional distributions through a ranking process (Wilcoxon 1950). Correlation coefficients (r) were computed, according to Pearson's formula (Press et al. 1992). The value of r was then transformed as t = r and compared with Student's t-distribution with N − 2 degrees of freedom, where N is the number of subjects, to determine the P value (Press et al. 1992).
In the figures and tables, lack of statistical significance is indicated as not significant (n.s.), whereas statistical significance is indicated with a single asterisk for P values between 0.05 and 0.01 (significant) and with two asterisks for P values below 0.01 (highly significant).
Sensitivity, specificity, accuracy, and receiver-operating characteristic.
Sensitivity (SE) and specificity (SP) measure the performance of a binary classification test (i.e., whether or not the subject has epilepsy). SE is defined as the proportion of positive cases that are correctly classified as such (i.e., fraction of subjects with epilepsy that are classified as epileptic). Similarly, SP is defined as the proportion of negative cases that are correctly classified as such (i.e., fraction of subjects who are not epileptic and are classified as controls). Accuracy (AC) is a “compromise” between SE and SP, defined as the ratio of correctly labeled cases out of the total number of cases. The receiver-operating characteristic (ROC) was obtained by plotting the SE and SP at increasing values of the parameter for binary classification (Florkowski 2008). The optimal discriminating threshold was determined as the value of the parameter that maximized the sum of SE and SP.
RESULTS
HRV in epilepsy.
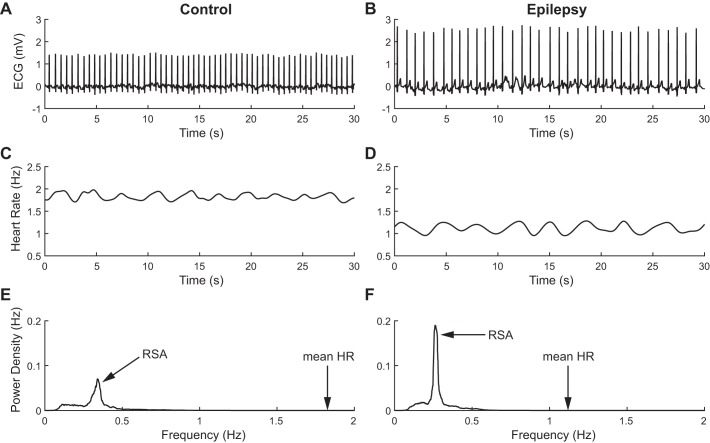
Clear differences in both mean HR and HRV are readily observed in patients with epilepsy compared with control subjects. Figure 1, A and B, shows two representative ECG traces during sleep in a control subject and a patient with epilepsy, respectively. It is apparent that the mean HR is lower in the patient with epilepsy, as evidenced by the increased RR intervals. In addition, the instantaneous HR displays a stronger oscillation, corresponding to RSA over the short 30-s window depicted (Fig. 1, C and D). The amplitude of the oscillation is quantified by the HF peak in the power spectrum of instantaneous HR computed over the 30-min epoch; the amplitude is much higher in the subject with epilepsy (Fig. 1, E and F). The combination of lower HR and stronger RSA points to an enhancement of parasympathetic activity in epilepsy during interictal periods in stage 2 sleep.
Fig. 1.
Heart rate variability (HRV) in representative control (left) and epilepsy (right) subjects. A and B: raw ECG traces over periods of 30 s; decreased HR is readily apparent in the subject with epilepsy. C and D: instantaneous HR over the same 30-s periods as in A and B; lower baseline and increased variability are visible in the patient with epilepsy. E and F: power spectral densities of HR over a 30-min period, filtered above 0.1 Hz; high-frequency modulation of HR, corresponding to respiratory sinus arrhythmia (RSA), is enhanced in epilepsy.
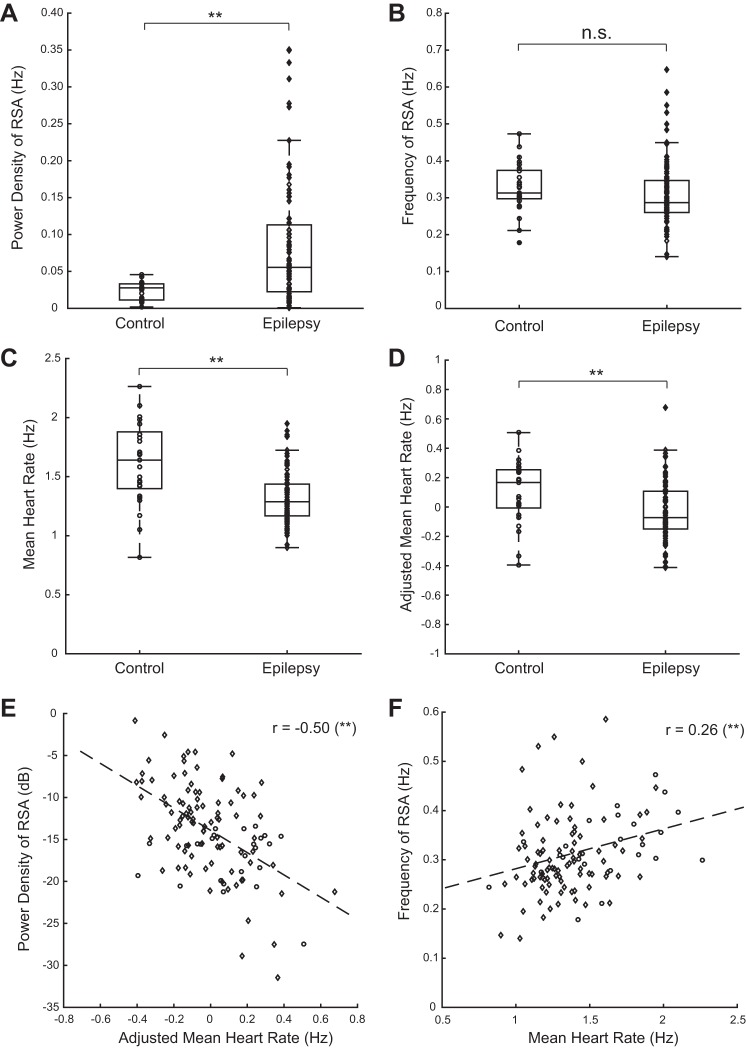
These observations are consistent across subjects. Figure 2A demonstrates that the median power of RSA is increased by 97% in epilepsy (medians: 0.028 Hz for control, 0.055 Hz for epilepsy; P = 3e-4, Wilcoxon's rank sum test), whereas the frequency of RSA, a measure of the respiratory frequency, is not significantly different between groups (medians: 0.31 Hz for control, 0.87 Hz for epilepsy; P = 0.06, Wilcoxon's rank sum test; Fig. 2B). In contrast, Fig. 2C shows that the HR is reduced by 21% (medians: 1.64 Hz for control, 1.29 Hz for epilepsy; P = 4e-5, Wilcoxon's rank sum test) during interictal periods in stage 2 sleep.
Fig. 2.
Analysis of HRV across control (circles) and epilepsy (diamonds) subjects. A: RSA tends to be increased in patients with epilepsy. B: frequency of RSA (a measure of the respiratory frequency) is not different in epilepsy compared with the control group. C: mean HR tends to be lower in epilepsy. D: after adjusting for age, body mass index (BMI), and sex, HR is still significantly lower in epilepsy. E: RSA and HR are negatively correlated, consistent with their being mediated by parasympathetic activity. F: frequency of RSA and HR are positively correlated, as expected. r, correlation coefficient. **P < 0.01 (highly significant); n.s., not significant.
These three HRV parameters (power of RSA, frequency of RSA, and HR) were regressed on age, BMI, and sex to correct for possible effects of these variables, as described in methods. Table 1 displays the F-test for the linear model, the regression parameters, their significance based on t-tests, and Lilliefors test values for the regression model for RSA (see methods). The regression models for power of RSA (F = 1.09, P = 0.355, F-test for nonconstant model) and frequency of RSA (F = 1.87, P = 0.139, F-test for nonconstant model) were both not significant. However, the model outlined in Table 2 reveals that HR significantly covaries with age and sex (F = 28.8, P = 7e-14, F-test for nonconstant model).
Table 2.
Regression analyses for HR
|
F-test for nonconstant model | |||
|---|---|---|---|
| F-test | F = 28.8 | F# = 3.077 | P = 7.02e-14** |
| Estimated coefficients of linear regression model |
||||
|---|---|---|---|---|
| Estimate | SE | t-Statistic | P | |
| Intercept | 1.6095 | 0.0813 | 19.797 | 2.25e-38** |
| Age | −0.0341 | 0.0042 | −8.028 | 1.08e-12** |
| BMI | 0.0038 | 0.0046 | 0.839 | 0.406 |
| Sex | 0.0930 | 0.0406 | 2.288 | 0.024* |
| Lilliefors test for non-normality of residuals | |||
|---|---|---|---|
| Lilliefors test | D = 0.0724 | D# = 0.0828 | P = 0.141 |
The heart rate (HR) significantly covaries with age and sex but not BMI.
P < 0.01 (highly significant); 0.05 >
P > 0.01 (significant).
Importantly, the effect of decreased HR in epilepsy is still highly significant after adjusting for age, BMI, and sex (P = 2e-3, Wilcoxon's rank sum test; Fig. 2D). Since these covariates do not affect the significance of the difference in HR between groups, we used the raw HR for most of the analyses. In addition, we show that RSA power and adjusted mean HR are negatively correlated (r = −0.50, P = 1e-8, Pearson's correlation; Fig. 2E), consistent with their being simultaneously modulated by parasympathetic activity. This correlation is mostly accounted for by the subjects with epilepsy, as it loses significance when considering the controls only (r = −0.11, P = 0.62, Pearson's correlation). Finally, frequency of RSA and HR share a significant positive correlation (r = 0.26, P = 4e-3, Pearson's correlation; Fig. 2F), a well-documented trend (Fleming et al. 2011; Pitzalis et al. 1998; Song and Lehrer 2003).
HRV as biomarker for epilepsy.
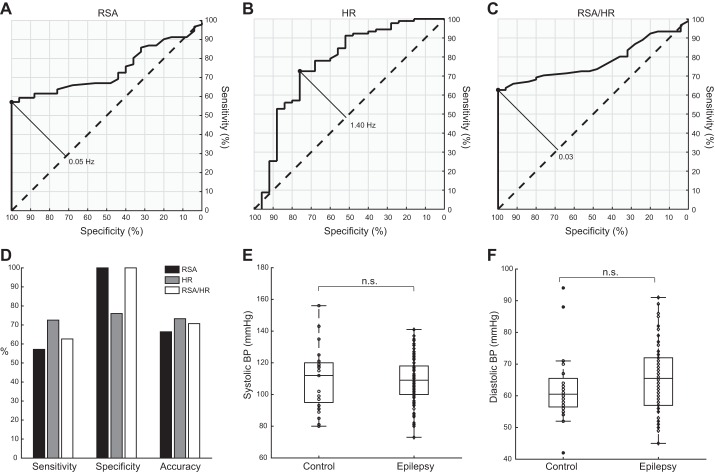
Can RSA power and mean HR during interictal sleep serve as sensitive clinical metrics? To test this possibility, we computed the SE, SP, and AC of these measures (see methods) to identify individuals at risk for developing epilepsy and potentially, experiencing SUDEP. To this end, we plotted the ROC curves for each parameter (Fig. 3, A and B) and determined their optimal discriminating thresholds (see methods). We note that the optimal threshold for RSA power (0.05 Hz) labels more than one-half of the subjects with epilepsy in our cohort as epileptic (SE = 57%) but correctly labels all of the neurologically normal controls (SP = 100%). The optimal threshold for mean HR (1.40 Hz) is more sensitive (SE = 73%) but less specific (SP = 76%). Both measures have a moderately high AC (AC = 66% for power of RSA; AC = 73% for mean HR). A combination of both RSA power and mean HR appears even more promising in the context of classification. In particular, we found the ratio of power of RSA/mean HR (conveniently unitless) also to be significantly different between groups (P = 2e-5, Wilcoxon's rank sum test), with ROC curve as shown in Fig. 3C. Its optimal discriminating threshold (0.03) was 63% sensitive, 100% specific, and 71% accurate. Figure 3D displays for comparison the SE, SP, and AC for RSA, HR, and RSA/HR.
Fig. 3.
Sensitivity (SE) and specificity (SP) of HRV parameters and comparison of blood pressures (BPs). A–C: receiver-operating characteristic curves for power of RSA, mean HR, and the RSA/HR ratio, respectively. Solid diagonal lines mark the optimal thresholds for classification, and their numerical values are shown. D: comparison of SE, SP, and accuracy for RSA, HR, and the RSA/HR ratio. E and F: systolic and diastolic BPs at rest are similar in both control (circles) and epilepsy (diamonds) groups. n.s., not significant.
To test the classification performance of RSA, HR, and their ratio as prognostic biomarkers, we used the optimal thresholds for the three parameters to attempt classification of two groups of subjects previously excluded from analysis (see Exclusion criteria and group characteristics in methods): 1) five subjects that developed epilepsy at a later point and 2) seven subjects that were taking anti-epileptic medication at the time of data acquisition to prevent nonepileptic, psychogenic seizures, along with other abnormalities, including tics, spells, and fainting. We determined that the optimal thresholds for both power of RSA and RSA/HR independently identified 10 out of 12 (83%) of these subjects as those with epilepsy, whereas the optimal threshold for mean HR classified 7 out of 12 (58%) of these subjects as those with epilepsy.
Normal BP and sympathetic tone at rest.
Up to this point, our results are consistent with an enhancement of parasympathetic tone in generalized epilepsy, but the decrease in HR is also consistent with withdrawal of sympathetic tone. To test this, we analyzed the resting diastolic BP and for the sake of completeness, the systolic BP. These measurements were taken at rest before sleep trials but serve as a control for comparing sympathetic tone in a nonsleep resting state, as is routinely done in clinical settings (Joyner et al. 2010). We found that neither systolic BP (medians: 112 mmHg for control, 109 mmHg for epilepsy; P = 0.97, Wilcoxon's rank sum test) nor diastolic BP (medians: 60.5 mmHg for control, 65.5 mmHg for epilepsy; P = 0.14, Wilcoxon's rank sum test) was significantly different between groups (Fig. 3, E and F). In the process of adjusting for natural covariates, both BP measures were found to be positively correlated with age; systolic BP was additionally correlated with BMI (F = 19.8, P = 2e-10, F-test for nonconstant model), and diastolic BP was additionally correlated with sex (F = 11.0, P = 2e-6, F-test for nonconstant model). Neither adjusted systolic BP (P = 0.15, Wilcoxon's rank sum test) nor adjusted diastolic BP (P = 0.75, Wilcoxon's rank sum test) was found to be significantly different between groups. These results suggest that sympathetic tone in pediatric epilepsy during interictal periods, at least during wakefulness at rest, is comparable with normal sympathetic tone.
Lengthened ventricular diastole relative to cardiac cycle.
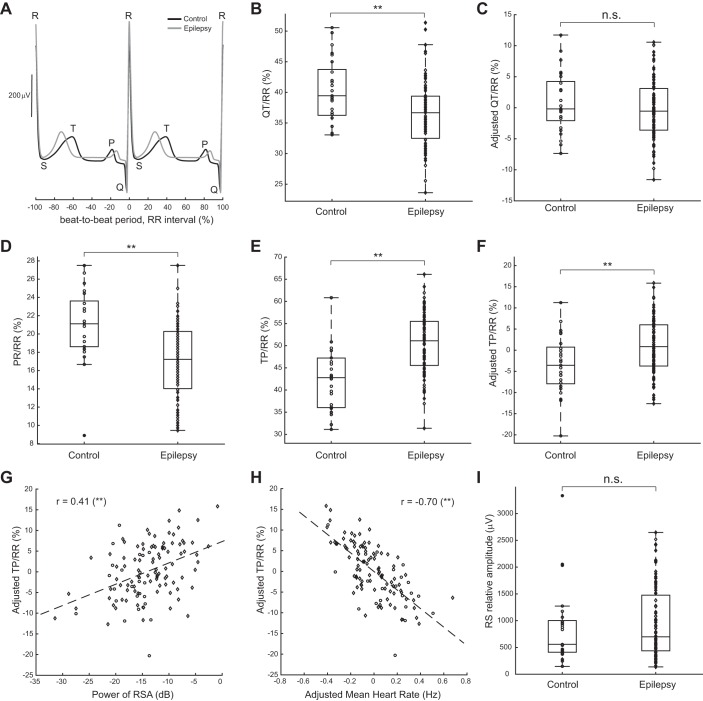
All significant effects reported thus far are chronotropic; i.e., they refer to the cardiac rhythm. We then wanted to investigate whether inotropic effects (related to cardiac contraction) and dromotropic effects (related to conduction of electrical activity in the heart) may be affected by epilepsy. To this end, we analyzed canonical features of the ECG waveforms. Two representative cycle-triggered ECG traces (Fig. 4A) show that the relevant waves are similar in amplitude in epilepsy and control but that cardiac intervals of interest seem to be different. We thus analyzed these amplitudes and durations across subjects.
Fig. 4.
ECG waveform analyses. A: comparison of mean-subtracted, cycle-triggered, average ECG traces centered at R wave and plotted over 2 beat-to-beat periods (RR interval). B and C: the QT interval relative to the RR interval appears to be shortened in epilepsy (diamonds) compared with controls (circles); however, after adjusting for age, BMI, and sex, there is no significant difference in QT interval between groups. D: in contrast, the relative PR interval (atrial systole) is significantly shorter and does not depend on age, BMI, or sex. E and F: this relative shortening occurs at the expense of a longer relative ventricular diastole (TP/RR). G and H: the adjusted relative ventricular diastole correlates positively with the RSA power and negatively with the adjusted HR. I: the RS relative amplitude is not significantly different between groups. **P < 0.01 (highly significant); n.s., not significant.
We focused first on the QT interval, a measure of the duration of ventricular systole, using standard methodology to identify its start and end (van Noord et al. 2010) and normalized it relative to the RR interval (see methods). When comparing between groups, the percentage of the RR interval that is QT appeared to be significantly shorter in epilepsy (medians: 39.4% for control, 36.7% for epilepsy; P = 3e-3, Wilcoxon's rank sum test; Fig. 4B). However, regression of the QT interval on age, BMI, and sex revealed a negative correlation with age and a strong correlation with sex (Table 3), both of which have been reported previously in the literature (Rautaharju et al. 1992). After adjusting the QT/RR parameter (see methods), we found that this shortening was no longer significant between groups (P = 0.36, Wilcoxon's rank sum test; Fig. 4C).
Table 3.
Regression analyses for QT/RR
|
F-test for nonconstant model | |||
|---|---|---|---|
| F-test | F = 11.7 | F# = 3.077 | P = 1.01e-6** |
| Estimated coefficients of linear regression model |
||||
|---|---|---|---|---|
| Estimate | SE | t-Statistic | P | |
| Intercept | 40.13 | 1.8352 | 21.866 | 3.19e-42** |
| Age | −0.3247 | 0.0958 | −3.390 | 9.65e-4** |
| BMI | −0.0582 | 0.1034 | −0.563 | 0.575 |
| Sex | 3.5831 | 0.9172 | 3.907 | 1.61e-4** |
| Lilliefors test for non-normality of residuals | |||
| Lilliefors test | D = 0.0798 | D# = 0.0826 | P = 0.068 |
The relative QT interval significantly covaries with age and sex but not BMI.
P < 0.01 (highly significant).
We also analyzed the percentage of the RR interval that is PR or the duration of atrial systole. This interval was significantly shorter in epilepsy (medians: 21% for control, 17% for epilepsy; P = 6e-5, Wilcoxon's rank sum test; Fig. 4D) and did not produce a significant regression with age, BMI, or sex (F = 2.4, P = 0.07, F-test for nonconstant model) and consequently, does not appear to be due to covariations with those three variables. This result indicates that in addition to chronotropic affects, generalized epilepsy is associated with dromotropic effects, at least during stage 2 sleep.
A shortening in atrial (PR) but not ventricular (QT) systole relative to the whole cardiac cycle must occur at the expense of lengthening the diastole. We thus investigated the ventricular diastole via the relative duration of the TP interval, and indeed, the TP/RR parameter was found to be significantly longer in epilepsy (medians: 43% for control, 51% for epilepsy; P = 4e-6, Wilcoxon's rank sum test; Fig. 4E). After adjustment for significant correlations with age and sex (Table 4), the relative TP interval was still significantly longer in epilepsy (P = 5e-3, Wilcoxon's rank sum test; Fig. 4F). Importantly, the lengthening of the relative TP interval was positively correlated with RSA power (r = 0.41, P = 4e-4, Pearson's correlation; Fig. 4G) and negatively correlated with adjusted HR (r = −0.70, P = 1e-18, Pearson's correlation; Fig. 4H), suggesting that these three parameters are mechanistically linked.
Finally, we analyzed the relative amplitude between R and S waves (the largest voltage difference between the two peaks) and found that this quantity is not significantly different between groups (medians: 558 μV for control, 699 μV for epilepsy; P = 0.49, Wilcoxon's rank sum test; Fig. 4I). Other relative amplitudes displayed similar nonsignificance and are omitted for simplicity. These results were maintained after adjusting for age, BMI, and sex, suggesting that inotropic effects are negligible in generalized epilepsy.
DISCUSSION
Summary.
In light of the relatively sparse literature on interictal autonomic control in epilepsy (Sarkis et al. 2015) and inconsistent results regarding epileptic parasympathetic activity during these periods (Behbahani et al. 2016; Raju et al. 2012; Seri et al. 2012), we analyzed HRV of children and adolescents during sleep in the absence of seizures and found two key indicators of increased vagal tone: enhanced RSA and decreased HR. These results are consistent with previous findings (Seri et al. 2012) and extend them to pediatric patients with generalized forms of epilepsy other than focal benign Rolandic epilepsy. We also investigated parameters of the ECG waveforms and found that the ventricular diastole normalized by the RR interval was significantly longer in generalized epilepsy at the expense of shorter atrial systole, demonstrating abnormal conduction of electrical activity in the heart. Other physiological parameters, including frequency of RSA (corresponding to respiratory frequency), systolic and diastolic BP, and the amplitudes of the P, Q, R, S, and T waveforms of the ECG, were similar between controls and patients with generalized epilepsy.
Possible physiological mechanisms.
The three main findings of this paper—enhanced RSA, decreased HR, and lengthened ventricular diastole—can be readily explained by increased parasympathetic tone, leading to enhanced cholinergic neuromodulation of the cardiac cycle. The question of why the parasympathetic tone is enhanced demands further and deeper investigation. It may be due to a homeostatic mechanism, secondary to the primary cause of epilepsy, or perhaps more intriguingly, increased parasympathetic tone may be a leading factor in the etiology and development of the disease. The fact that enhanced RSA and lower HR preceded epilepsy in five subjects in our cohort who were originally diagnosed as neurologically normal lends support to the latter interpretation.
As for the homeostatic interpretation, we note that the increased parasympathetic tone during sleep in the absence of epileptiform activity contrasts with the sympathetic overexpression frequently observed during and after ictal events (Behbahani et al. 2016; Kolsal et al. 2014; Nagai 2015; Poh et al. 2012). These discrepant observations suggest that the function of the autonomic nervous system is fundamentally different during ictal and interictal periods in children with generalized epilepsy and that assessment of the risk of multifactorial conditions, such as SUDEP, requires a more nuanced understanding of the mechanisms of autonomic activity both during and in the absence of seizures.
Is abnormal HRV in epilepsy iatrogenic?
An alternative mechanistic interpretation of our results is that abnormal HRV in generalized epilepsy is caused by the anti-epileptic medications themselves. We believe that this possibility is unlikely, because patients with higher values of RSA power (>0.1 Hz, i.e., greater than twice the optimal discriminating threshold) took anti-epileptic medications that were also taken by patients with RSA values comparable with the controls. In fact, there were only three epileptic patients with high values of RSA power who took medications (cefdinir, azithromycin, and midazolam) that no other patient took (each patient took only one of these medications, so there was no overlap); the first two are nonpenicillin antibiotics, and the third is a sleep aid of the benzodiazepine class, which is not used primarily to treat epilepsy.
Perhaps the strongest argument against the interpretation that our findings are iatrogenic is that the five subjects who were neurologically normal at the time of the ECG recordings and who did not take any anti-epileptic medications at that time, already presented with abnormally higher RSA and lower HR before developing epilepsy at a later point. Two of them did not take any medications at all, and the other three took medications for attention deficits (e.g., amphetamine), asthma (e.g., albuterol), and anxiety (e.g., sertraline).
Cautionary remarks on vagal nerve stimulation.
A more detailed understanding of autonomic function in epilepsy is crucial to administering therapies effectively for epilepsy, such as vagal nerve stimulation (VNS), which has more recently become an established method of treating drug-resistant epilepsy (Connor et al. 2012). It is well known that VNS affects chronotropic and inotropic regulation of cardiac activity and that controlling specific parameters of vagal stimulation (such as current and frequency) can elicit different changes to cardiorespiratory function (Rousselet et al. 2014). However, numerous authors have questioned whether VNS in its current form is an appropriate treatment for epilepsy, as its mechanism of action is still unclear, and it does not appear to lower the mortality rate due to complications, including SUDEP (Granbichler et al. 2015). In fact, VNS may even increase the risk of SUDEP (Annegers et al. 1998). VNS has been shown to regulate sympathetic activation of the sinoatrial node (Zhou et al. 2016) and reduce resting HR (Mulders et al. 2015) and is believed to enhance parasympathetic activity (Kampusch et al. 2015). Additionally, VNS is hypothesized to worsen sleep-breathing disorders via altered laryngeal motility (Zambrelli et al. 2016) and has been shown to trigger obstructive sleep apnea, a condition often coupled with an overexpression of parasympathetic activity (Ebben et al. 2008; Parhizgar et al. 2011; Vollono et al. 2015).
In the context of our findings, VNS may further increase the abnormally enhanced vagal tone that we observe in patients with epilepsy, thereby enhancing the respiratory modulation of HR and incidence of apnea during sleep when VNS is often activated (Ebben et al. 2008) and decreasing the HR, which potentially increases the risk of SUDEP. Our results suggest that a more thorough investigation into the autonomic effects of vagal neurostimulation during sleep is needed, especially in pediatric epilepsy, where consequences of autonomic perturbation may be more severe and perhaps fatal (Annegers et al. 1998).
Alternative therapeutic targets for epilepsy.
With the consideration of alternative treatments for epilepsy, the use of parasympathetic modulators appears to have been overlooked as well. Common anti-epileptic medications in use are known to impact sympathetic regulation, but the short- and long-term effects of these medications on parasympathetic function are poorly understood. For instance, benzodiazepines are an often-prescribed family of drugs that allosterically enhance GABAergic function to enhance inhibition and hence, manage epileptic conditions (Riss et al. 2008). They ostensibly inhibit sympathetic neuronal activity, but a mechanistic understanding remains elusive (Zahner et al. 2007), and the discussion regarding the impact of benzodiazepines on parasympathetic function is meager. Further research into parasympathetic routes to control seizures and prevent SUDEP may be fruitful, provided the following: 1) that the mechanisms of action of drugs that affect autonomic function are better understood and 2) that care is taken to regulate the parasympathetic tone appropriately in epilepsy during interictal periods.
HRV as a prognostic biomarker.
The consideration of RSA and HR as potential biomarkers for the development of epilepsy is a natural ramification of the significant alterations to autonomic balance observed in our study. We find that the use of power of RSA and mean HR (either before or after, correcting for age effects) as predictors for epilepsy is moderately accurate, and the ratio of power of RSA/mean HR is highly specific and as accurate as either parameter independently. Most remarkably, all three measures classified more than one-half of subjects not diagnosed with epilepsy but with a history of seizures as having epilepsy, whereas power of RSA and the RSA/HR ratio both correctly identified five subjects as having epilepsy before clinical diagnosis. This demonstrates the potential of HRV as a noninvasive, prognostic biomarker for epilepsy, which has been overlooked previously.
Limitations.
We analyzed data retrospectively from a single pediatric unit. As is the case for any study with a geographically/temporally constrained dataset, it will be important for future studies to expand the analyses to an even more diverse set of subjects, such as those with focal epilepsy. Our study is also constrained to stage 2 sleep (the only one systematically available for subjects in the database) as the regime for comparison. Whereas studies have shown that stage 2 sleep and its transition between slow-wave sleep and REM or wakefulness are of high interest in the context of autonomic function in SUDEP (Kanda et al. 2016; Menezes Cordeiro et al. 2015), we did not have control either over the selected portion of stage 2 sleep available for each subject or over which epoch of stage 2 sleep was selected over the course of the overnight observation period at the Pediatric Epilepsy Unit. Consequently, we could not determine whether stage 2 sleep EEG and ECG data were taken immediately following or preceding stage 1 sleep, stage 3 sleep, REM sleep, or awakening. This information would be useful in light of a previous study demonstrating autonomic-endocrine profiles in stage 2, depending on its occurrence relative to contiguous stages of sleep (Brandenberger et al. 2005). Further studies may address all of these concerns by collecting and maintaining ECG and EEG data throughout the sleep period and analyzing each epoch of interest with respect to the total elapsed time of sleep.
Conclusions.
Our results provide novel evidence that parasympathetic tone is enhanced during interictal periods in stage 2 NREM sleep in children with generalized epilepsy. We show that certain measures of HRV during interictal activity in sleep, including RSA and mean HR, may be overlooked risk factors for epilepsy and that the RSA/HR ratio may be a potential prognostic biomarker for epilepsy itself. These results set the stage for developing a more detailed understanding of autonomic dysfunction in patients with epilepsy by highlighting the dissimilarity between sympathovagal imbalance during ictal and interictal periods. Our findings also shed light onto a possible autonomic mechanism for SUDEP and promote investigation into parasympathetic modulators as potential treatments for epilepsy, particularly in children and adolescents.
GRANTS
Support for this work has been provided by a Biomedical Researcher Award of The Hartwell Foundation (to R. F. Galán).
DISCLOSURES
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Author contributions: R.F.G. conception and design of research; S.S.S., A.G.N., and R.F.G. analyzed data; S.S.S., A.G.N., I.E.T., S.J.L., and R.F.G. interpreted results of experiments; S.S.S. and R.F.G. prepared figures; S.S.S. and R.F.G. drafted manuscript; S.S.S., A.G.N., I.E.T., S.J.L., and R.F.G. edited and revised manuscript; S.S.S., A.G.N., I.E.T., S.J.L., and R.F.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Ahmad Zrik for his assistance with clinical EEG reading and interpretation. The authors also thank Prof. Hillel J. Chiel for helpful suggestions on the manuscript.
REFERENCES
- Abukonna A, Yu X, Zhang C, Zhang J. Volitional control of the heart rate. Int J Psychophysiol 90: 143–148, 2013. [DOI] [PubMed] [Google Scholar]
- Akinci A, Celiker A, Baykal E, Tezic T. Heart rate variability in diabetic children: sensitivity of the time- and frequency-domain methods. Pediatr Cardiol 14: 140–146, 1993. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Coan SP, Hauser WA, Leestma J, Duffell W, Tarver B. Epilepsy, vagal nerve stimulation by the NCP system, mortality, and sudden, unexpected, unexplained death. Epilepsia 39: 206–212, 1998. [DOI] [PubMed] [Google Scholar]
- Behbahani S, Dabanloo NJ, Nasrabadi AM, Dourado A. Classification of ictal and seizure-free HRV signals with focus on lateralization of epilepsy. Technol Health Care 24: 43–56, 2016. [DOI] [PubMed] [Google Scholar]
- Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 4: 26, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood JD, Wu J, Chaplin TM, Hommer R, Vazquez L, Rutherford HJ, Mayes LC, Crowley MJ. The variable heart: high frequency and very low frequency correlates of depressive symptoms in children and adolescents. J Affect Disord 186: 119–126, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum AS, Ives JR, Goldberger AL, Al-Aweel IC, Krishnamurthy KB, Drislane FW, Schomer DL. Oxygen desaturations triggered by partial seizures: implications for cardiopulmonary instability in epilepsy. Epilepsia 41: 536–541, 2000. [DOI] [PubMed] [Google Scholar]
- Bozorgi A, Lhatoo SD. Seizures, cerebral shutdown, and SUDEP. Epilepsy Curr 13: 236–240, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger G, Ehrhart J, Buchheit M. Sleep stage 2: an electroencephalographic, autonomic, and hormonal duality. Sleep 28: 1535–1540, 2005. [DOI] [PubMed] [Google Scholar]
- Bulow K, Ingvar DH. Respiration in petit mal epilepsy. Neurology 13: 201–206, 1963. [DOI] [PubMed] [Google Scholar]
- Connor DE Jr, Nixon M, Nanda A, Guthikonda B. Vagal nerve stimulation for the treatment of medically refractory epilepsy: a review of the current literature. Neurosurg Focus 32: E12, 2012. [DOI] [PubMed] [Google Scholar]
- Donner EJ. Sudden unexpected death in epilepsy: who are the children at risk? Paediatr Child Health 19: 389, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebben MR, Sethi NK, Conte M, Pollak CP, Labar D. Vagus nerve stimulation, sleep apnea, and CPAP titration. J Clin Sleep Med 4: 471–473, 2008. [PMC free article] [PubMed] [Google Scholar]
- El-Sayed HL, Kotby AA, Tomoum HY, El-Hadidi ES, El Behery SE, El-Ganzory AM. Non-invasive assessment of cardioregulatory autonomic functions in children with epilepsy. Acta Neurol Scand 115: 377–384, 2007. [DOI] [PubMed] [Google Scholar]
- Fleming S, Thompson M, Stevens R, Heneghan C, Pluddemann A, Maconochie I, Tarassenko L, Mant D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 377: 1011–1018, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkowski CM. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: communicating the performance of diagnostic tests. Clin Biochem Rev 29, Suppl 1: S83–S87, 2008. [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol 96: 1255–1261, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granbichler CA, Nashef L, Selway R, Polkey CE. Mortality and SUDEP in epilepsy patients treated with vagus nerve stimulation. Epilepsia 56: 291–296, 2015. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. [DOI] [PubMed] [Google Scholar]
- Herman ST, Walczak TS, Bazil CW. Distribution of partial seizures during the sleep–wake cycle: differences by seizure onset site. Neurology 56: 1453–1459, 2001. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Crandall LA, Friedman D, Devinsky O. Sudden unexplained death in childhood: a comparison of cases with and without a febrile seizure history. Epilepsia 56: 1294–1300, 2015. [DOI] [PubMed] [Google Scholar]
- Indic P, Salisbury E, Paydarfar D, Brown E, Barbieri R. Interaction between heart rate variability and respiration in preterm infants. Comput Cardiol 35: 57–60, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen J, Beniczky S, Johansen P, Sidenius P, Fuglsang-Frederiksen A. Detection of epileptic seizures with a modified heart rate variability algorithm based on Lorenz plot. Seizure 24: 1–7, 2015. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension 56: 10–16, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampusch S, Kaniusas E, Szeles JC. Modulation of muscle tone and sympathovagal balance in cervical dystonia using percutaneous stimulation of the auricular vagus nerve. Artif Organs 39: E202–E212, 2015. [DOI] [PubMed] [Google Scholar]
- Kanda T, Tsujino N, Kuramoto E, Koyama Y, Susaki EA, Chikahisa S, Funato H. Sleep as a biological problem: an overview of frontiers in sleep research. J Physiol Sci 66: 1–13, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsal E, Serdaroglu A, Cilsal E, Kula S, Soysal AS, Kurt AN, Arhan E. Can heart rate variability in children with epilepsy be used to predict seizures? Seizure 23: 357–362, 2014. [DOI] [PubMed] [Google Scholar]
- Kothare SV, Singh K. Cardiorespiratory abnormalities during epileptic seizures. Sleep Med 15: 1433–1439, 2014. [DOI] [PubMed] [Google Scholar]
- Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia 53: 253–257, 2012. [DOI] [PubMed] [Google Scholar]
- Lhatoo S, Noebels J, Whittemore V, NINDS Center for SUDEP Research . Sudden unexpected death in epilepsy: identifying risk and preventing mortality. Epilepsia 56: 1700–1706, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilliefors H. On the Kolmogorov-Smirnov test for normality with mean and variance unknown. J Amer Statist Assoc 62: 399–402, 1967. [Google Scholar]
- Meghana A, Sriranjini SJ, Sathyaprabha T, Sanjib S, Prathyusha V, Satishchandra P. Autonomic function in reflex and non-reflex epilepsy—an exploratory study. Acta Neurol Scand. First published September 15, 2015; doi: 10.1111/ane.12486. [DOI] [PubMed] [Google Scholar]
- Menezes Cordeiro I, von Ellenrieder N, Zazubovits N, Dubeau F, Gotman J, Frauscher B. Sleep influences the intracerebral EEG pattern of focal cortical dysplasia. Epilepsy Res 113: 132–139, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi N, Lhatoo SD. Sudden unexpected death in epilepsy or voodoo heart: analysis of heart/brain connections. Curr Cardiol Rep 15: 424, 2013. [DOI] [PubMed] [Google Scholar]
- Mostacci B, Bisulli F, Vignatelli L, Licchetta L, Di Vito L, Rinaldi C, Trippi I, Ferri L, Plazzi G, Provini F, Tinuper P. Incidence of sudden unexpected death in nocturnal frontal lobe epilepsy: a cohort study. Sleep Med 16: 232–236, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders DM, de Vos CC, Vosman I, van Putten MJ. The effect of vagus nerve stimulation on cardiorespiratory parameters during rest and exercise. Seizure 33: 24–28, 2015. [DOI] [PubMed] [Google Scholar]
- Nagai Y. Modulation of autonomic activity in neurological conditions: epilepsy and tourette syndrome. Front Neurosci 9: 278, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, Fish DR. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry 60: 297–300, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak C, Sinha S, Nagappa M, Thennarasu K, Taly AB. Lack of heart rate variability during apnea in patients with juvenile myoclonic epilepsy (JME). Sleep Breath 19: 1175–1183, 2015. [DOI] [PubMed] [Google Scholar]
- Nei M. Cardiac effects of seizures. Epilepsy Curr 9: 91–95, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztas B, Turkel N. Influence of an abrupt increase in blood pressure on the blood-brain barrier permeability during acute hypertension and epileptic seizures. Pharmacol Res 44: 209–212, 2001. [DOI] [PubMed] [Google Scholar]
- Parhizgar F, Nugent K, Raj R. Obstructive sleep apnea and respiratory complications associated with vagus nerve stimulators. J Clin Sleep Med 7: 401–407, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis MV, Mastropasqua F, Massari F, Passantino A, Colombo R, Mannarini A, Forleo C, Rizzon P. Effect of respiratory rate on the relationships between RR interval and systolic blood pressure fluctuations: a frequency-dependent phenomenon. Cardiovasc Res 38: 332–339, 1998. [DOI] [PubMed] [Google Scholar]
- Poh MZ, Loddenkemper T, Reinsberger C, Swenson NC, Goyal S, Madsen JR, Picard RW. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology 78: 1868–1876, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes in C: the Art of Scientific Computing. Cambridge, UK: Cambridge University Press, 1992. [Google Scholar]
- Raju KN, Choudhary N, Gulati S, Kabra M, Jaryal AK, Deepak KK, Pandey RM. Comparison of heart rate variability among children with well controlled versus refractory epilepsy: a cross-sectional study. Epilepsy Res 101: 88–91, 2012. [DOI] [PubMed] [Google Scholar]
- Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol 8: 690–695, 1992. [PubMed] [Google Scholar]
- Riley M, Bluhm B. High blood pressure in children and adolescents. Am Fam Physician 85: 693–700, 2012. [PubMed] [Google Scholar]
- Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand 118: 69–86, 2008. [DOI] [PubMed] [Google Scholar]
- Rousselet L, Le Rolle V, Ojeda D, Guiraud D, Hagege A, Bel A, Bonnet JL, Mabo P, Carrault G, Hernandez AI. Influence of vagus nerve stimulation parameters on chronotropism and inotropism in heart failure. Conf Proc IEEE Eng Med Biol Soc 2014: 526–529, 2014. [DOI] [PubMed] [Google Scholar]
- Sarkis RA, Thome-Souza S, Poh MZ, Llewellyn N, Klehm J, Madsen JR, Picard R, Pennell PB, Dworetzky BA, Loddenkemper T, Reinsberger C. Autonomic changes following generalized tonic clonic seizures: an analysis of adult and pediatric patients with epilepsy. Epilepsy Res 115: 113–118, 2015. [DOI] [PubMed] [Google Scholar]
- Seri S, Di Lorenzo G, Pisano T, Pinci M, Brazzo D, Betteridge H, Cerquiglini A. Interictal autonomic abnormalities in idiopathic Rolandic epilepsy. Epilepsy Behav 24: 241–245, 2012. [DOI] [PubMed] [Google Scholar]
- Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol 5: 1040, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BN, Delisle BP. The long and the short of it: seizures induce cardiac remodeling and arrhythmia. Epilepsy Curr 15: 90–91, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HS, Lehrer PM. The effects of specific respiratory rates on heart rate and heart rate variability. Appl Psychophysiol Biofeedback 28: 13–23, 2003. [DOI] [PubMed] [Google Scholar]
- Swenne CA. Baroreflex sensitivity: mechanisms and measurement. Neth Heart J 21: 58–60, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurhaj W, Troussiere AC, Logier R, Derambure P, Tyvaert L, Semah F, Ryvlin P, De Jonckheere J. Ictal changes in parasympathetic tone: prediction of postictal oxygen desaturation. Neurology 85: 1233–1239, 2015. [DOI] [PubMed] [Google Scholar]
- Taylor CE, Witter T, El Sayed K, Hissen SL, Johnson AW, Macefield VG. Relationship between spontaneous sympathetic baroreflex sensitivity and cardiac baroreflex sensitivity in healthy young individuals. Physiol Rep 3: pii: e12536, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terra VC, Scorza FA, Sakamoto AC, Pinto KG, Fernandes RM, Arida RM, Cavalheiro EA, Machado HR. Does sudden unexpected death in children with epilepsy occur more frequently in those with high seizure frequency? Arq Neuropsiquiatr 67: 1001–1002, 2009. [DOI] [PubMed] [Google Scholar]
- Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE 70: 1055–1096, 1982. [Google Scholar]
- Tobaldini E, Nobili L, Strada S, Casali KR, Braghiroli A, Montano N. Heart rate variability in normal and pathological sleep. Front Physiol 4: 294, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noord C, Eijgelsheim M, Stricker BH. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol 70: 16–23, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon C, Jansen K, Lagae L, Van Huffel S. Can ECG monitoring identify seizures? J Electrocardiol 48: 1069–1074, 2015a. [DOI] [PubMed] [Google Scholar]
- Varon C, Montalto A, Jansen K, Lagae L, Marinazzo D, Faes L, Van Huffel S. Interictal cardiorespiratory variability in temporal lobe and absence epilepsy in childhood. Physiol Meas 36: 845–856, 2015b. [DOI] [PubMed] [Google Scholar]
- Vollono C, Fuggetta F, Cioni B, Della Marca G. Teaching NeuroImages: obstructive sleep apnea triggered by vagus nerve stimulation. Neurology 85: e140, 2015. [DOI] [PubMed] [Google Scholar]
- Wilcoxon F. Some rapid approximate statistical procedures. Ann N Y Acad Sci 52: 808–814, 1950. [Google Scholar]
- Wyllie E. Wyllie's Treatment of Epilepsy: Principles and Practice. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2015, chapt. 13. [Google Scholar]
- Yasuma F, Hayano J. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest 125: 683–690, 2004. [DOI] [PubMed] [Google Scholar]
- Zahner MR, Li DP, Pan HL. Benzodiazepine inhibits hypothalamic presympathetic neurons by potentiation of GABAergic synaptic input. Neuropharmacology 52: 467–475, 2007. [DOI] [PubMed] [Google Scholar]
- Zambrelli E, Saibene AM, Furia F, Chiesa V, Vignoli A, Pipolo C, Felisati G, Canevini MP. Laryngeal motility alteration: a missing link between sleep apnea and vagus nerve stimulation for epilepsy. Epilepsia 57: e24–e27, 2016. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhou L, Wang S, Yu L, Wang Z, Huang B, Chen M, Wan J, Jiang H. The use of noninvasive vagal nerve stimulation to inhibit sympathetically induced sinus node acceleration: a potential therapeutic approach for inappropriate sinus tachycardia. J Cardiovasc Electrophysiol 27: 217–223, 2016. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Flanagan D, Gotman J. Heart rate changes and ECG abnormalities during epileptic seizures: prevalence and definition of an objective clinical sign. Epilepsia 43: 847–854, 2002. [DOI] [PubMed] [Google Scholar]