Abstract
Changes in the environment require rapid modification or inhibition of ongoing behavior. We used the stop-signal paradigm and intracranial recordings to investigate response preparation, inhibition, and monitoring of task-relevant information. Electrocorticographic data were recorded in eight patients with electrodes covering frontal, temporal, and parietal cortex, and time-frequency analysis was used to examine power differences in the beta (13–30 Hz) and high-gamma bands (60–180 Hz). Over motor cortex, beta power decreased, and high-gamma power increased during motor preparation for both go trials (Go) and unsuccessful stops (US). For successful stops (SS), beta increased, and high-gamma was reduced, indexing the cancellation of the prepared response. In the middle frontal gyrus (MFG), stop signals elicited a transient high-gamma increase. The MFG response occurred before the estimated stop-signal reaction time but did not distinguish between SS and US trials, likely signaling attention to the salient stop stimulus. A postresponse high-gamma increase in MFG was stronger for US compared with SS and absent in Go, supporting a role in behavior monitoring. These results provide evidence for differential contributions of frontal subregions to response inhibition, including motor preparation and inhibitory control in motor cortex and cognitive control and action evaluation in lateral prefrontal cortex.
Keywords: beta oscillations, electrocorticography, high gamma, motor control, stop-signal task
response inhibition is an essential cognitive control function needed to withhold unwanted motor behavior. Deficits in response inhibition are observed in many neuropsychiatric disorders, including attention-deficit/hyperactivity disorder or obsessive-compulsive disorder, as well as in patients with damage to prefrontal cortex (PFC) (Aron et al. 2004; Chamberlain et al. 2006; Krämer et al. 2013; Lijffijt et al. 2005). Functional imaging, electrophysiological data, and lesion studies implicate a network of prefrontal, parietal, and subcortical regions in response inhibition (Aron 2011; Aron and Verbruggen 2008; Boucher et al. 2007). However, the precise role and temporal dynamics of activity in these different regions are uncertain (Munakata et al. 2011).
Attempts to elucidate the specific role of different brain regions can be guided by a separation of the agent, i.e., the source of inhibitory activity or where the inhibitory process is instigated, the site at which inhibitory activity is exerted, and the manifestation, i.e., where and how the result of the inhibitory activity can be measured (Band and van Boxtel 1999). Whereas the agent of inhibition is usually assumed to be within the PFC or basal ganglia, the site of inhibition might be within thalamus or the primary motor cortex (M1) and be manifested in a change of activity in inhibited trials compared with uninhibited trials (Band and van Boxtel 1999; Mattia et al. 2012). One influential model proposes a three-pronged network comprising right inferior frontal gyrus (rIFG), subthalamic nucleus (STN), and the presupplementary motor area (pre-SMA) as critical agents for stopping (Aron et al. 2007, 2014). Others have stressed the role of the pre-SMA and STN together with the striatum, rather than the rIFG in stopping (Munakata et al. 2011; Schmidt et al. 2013). The picture might be even more complex, as some recent studies have shown that both STNs need to be activated to stop an action (Mirabella et al. 2012; van den Wildenberg et al. 2006). Finally, transcranial magnetic stimulation studies have demonstrated inhibitory activity within M1, suggesting GABAB-mediated intracortical inhibition as a possible manifestation of stopping (Coxon et al. 2006; van den Wildenberg et al. 2010). This observation fits with event-related potential findings from electrocorticographic (ECoG) recordings in patients performing a stop-signal task, revealing stopping-specific event-related potential components in M1 and premotor cortex (Mattia et al. 2012). Neurophysiological recordings in monkeys during a manual go/no-go or stop-signal task have also reported stopping-related activity in several areas implicated in response selection during goal-directed action, including the pre-SMA [Matsuzaka and Tanji (1996); Shima et al. (1996); but see Scangos and Stuphorn (2010)], STN (Isoda and Hikosaka 2008), and dorsal premotor cortex (Kalaska and Crammond 1995; Mattia et al. 2013; Mirabella et al. 2011). This suggests that there is considerable overlap between the networks involved in action preparation and action inhibition (Mirabella 2014). However, comparisons between species must be made with caution, as connectivity and functional specialization of prefrontal areas might differ.
Here, we used the unique spatio-temporal resolution of intracranial electrophysiological (ECoG) recordings in humans to investigate the dynamics of cortical neural activity during inhibitory motor control. Recordings were obtained from patients with refractory epilepsy who had electrode grids or strips implanted for 4–10 days to localize seizure foci and perform cortical stimulation mapping. Electrodes were located over prefrontal, motor, temporal, or parietal areas of either the right or left hemisphere. Patients performed a visual stop-signal paradigm (SSP) (Logan et al. 1984), which is a well-established paradigm used to study response inhibition. In the SSP, participants perform a choice reaction-time (RT) task, in which a stop signal is infrequently presented after the go signal, indicating the need to stop the already-prepared response. The latency of the stopping process, referred to as the stop-signal RT (SSRT), is estimated, based on the RT distribution and the likelihood of inhibition (Logan et al. 1984).
Previous ECoG studies using the stop-signal task focused on the rIFG (Swann et al. 2009) and on activity and connectivity between pre-SMA and rIFG (Swann et al. 2012a). Swann et al. (2009) reported a beta-power increase at rIFG electrodes in inhibited stop trials relative to failed inhibitions. In a second ECoG study (Swann et al. 2012a), a beta-power increase in stop trials was observed over pre-SMA and rIFG electrodes. Together with EEG and behavioral data from Parkinson's disease patients on and off deep-brain stimulation STN stimulation (Mirabella et al. 2012; Ray et al. 2009; Swann et al. 2011; van den Wildenberg et al. 2006), these researchers hypothesized an inhibitory network involving rIFG, STN, and pre-SMA, which is indexed by beta oscillations. This hypothesis has been questioned by others (Schall and Godlove 2012), because the rIFG beta response seemed to vary among patients. Previous ECoG studies on a cued stop-signal task have also focused on power changes in high gamma, which included stop-related activations in pre-SMA (Swann et al. 2012a) and task set-related activations in ventrolateral PFC and dorsolateral PFC after both the cue and the go signal (Swann et al. 2012b).
In the present study, we present a more extensive dataset (8 patients) with coverage of both left and right PFC. We assessed both changes in activity in the beta band, as well as power changes in the high-gamma range (60–180 Hz). High-gamma power changes have been shown to mark local cortical activity in various sensory, motor, and cognitive tasks (Crone et al. 1998a; Edwards et al. 2005; Flinker et al. 2011) and to correlate with neuronal spiking activity (Cardin et al. 2009; Ray and Maunsell 2011). High-gamma activations in PFC have been reported before in a cued stop-signal task (Swann et al. 2012b) in relation to the cue and go stimuli. In the current paper, we analyzed prefrontal high-gamma activity to the stop stimulus to assess more specifically the role of PFC regions in reactive motor inhibition.
We hypothesized that brain regions involved in inhibitory motor control, i.e., agent and site of inhibition, should show differential high-gamma activity for successful and unsuccessful stop trials (SS and US, respectively) in the time range before the estimated SSRT (Schall and Godlove 2012). To address this, we first examined responses in the M1 as the putative cortical site of inhibition, where we hypothesized a high-gamma power increase (Crone et al. 1998a) and a beta decrease (Swann et al. 2009; Zhang et al. 2008) related to the motor response. For SS, we predicted a reduced response in the high-gamma band, as local cortical activity in M1 should be suppressed as a result of the stopping process. We then examined high-gamma activity over PFC to assess the temporal dynamics of middle frontal gyrus (MFG) and IFG activity related to motor preparation, inhibition, and monitoring. We also examined beta changes over prefrontal sites to test the hypothesis that a beta-dependent fronto-basal ganglia network is involved in stopping (Swann et al. 2009, 2012a).
METHODS
Participants
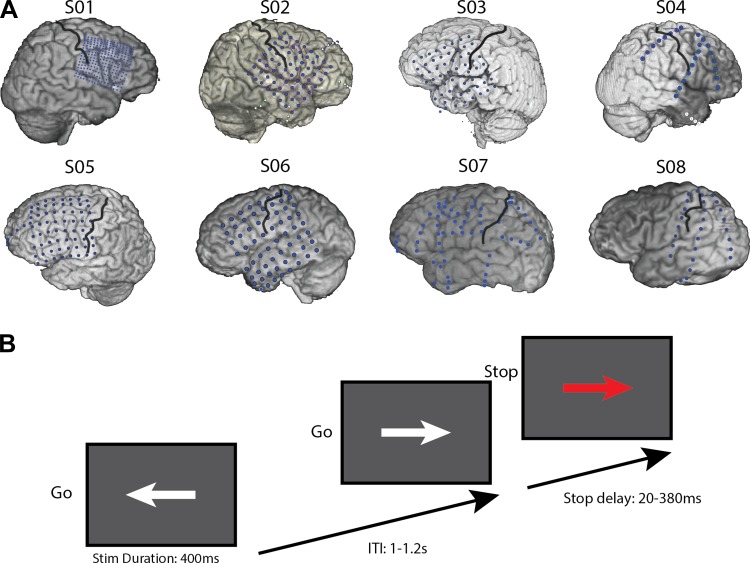
Eight patients (Fig. 1A and Table 1; 7 men; mean age, 27.5 yr), undergoing neurosurgical treatment for medically refractory epilepsy, participated in the study. Patients were implanted with one or more electrode arrays, with electrode placement and other medical decisions solely determined by the clinical needs of the patient. The patients were monitored for 4–7 days postimplantation. During lulls in clinical evaluation, the patients performed the SSP task while electrophysiological signals were recorded. Written and oral informed consent was obtained, according to the Declaration of Helsinki. Three subjects (S01–S03) were recruited from the University of California, San Francisco (UCSF), Hospital. Subjects had their epilepsy medications reduced or discontinued while being monitored. Four subjects (S04–S07) participated at Johns Hopkins Hospital, and one subject (S08) participated at Stanford Hospital. The study was approved by UCSF; Stanford; University of California, Berkeley; and Johns Hopkins Committees for Human Research. All subjects had normal or corrected-to-normal vision. All but one (S08) had some prefrontal coverage (four right, four left; Fig. 1A). Note that S04 had bilateral prefrontal coverage (left hemisphere not shown on figure). The location of the electrode grids and strips was reconstructed by realigning a preoperative anatomical MRI scan and a postimplant computed tomography (CT) scan using BioImage Suite software (Yale School of Medicine, New Haven, CT). Note that the precision of this method may vary by up to 1 cm due to small changes in the shape of the brain after grid electrode implantation.
Fig. 1.
A: CT and structural MRI reconstructions of individual subject electrode coverage. Black lines denote the central sulcus. Note that S04 had bilateral prefrontal strips (left side not shown). Note also that the grid for S03 has shifted ∼1 cm anterior after the CT was done, based on motor and auditory responses from a different task run in this subject (data not shown). The original CT–MR reconstruction shown here does not reflect this suspected shift. B: schematic of the stop-signal task used in the present study. Stim Duration, stimulus duration. ITI, intertrial interval.
Table 1.
Demographic and clinical data
| Subject | Age, yr | Sex | Handedness | Epileptic Focus |
|---|---|---|---|---|
| S01 | 23 | M | R | None found/unknown |
| S02 | 22 | M | R | Left parietal lesion, fronto-polar contusion |
| S03 | 42 | M | L | Left superior temporal cortex |
| S04 | 30 | M | R | Left orbitofrontal cortex |
| S05 | 14 | M | R | None found/unknown |
| S06 | 27 | F | R | Left anterior temporal cortex |
| S07 | 20 | M | L | None found/unknown |
| S08 | 42 | M | L | Posterior cingulate cortex |
M, male; R, right; L, left; F, female.
Paradigm and Experimental Procedures
The stop-signal task was a manual-choice RT task (Logan et al. 1984), wherein a visual stop signal is presented on 25% of the trials (Fig. 1B). The go signal was a white arrow pointing either to the right or to the left, requiring a corresponding mouse button press from the signaled hand. Patients performed the task with the hand contralateral to the electrode grid or strips (this was the dominant hand in 3 cases). In stop trials, the go signal was followed by a stop signal (arrow color changed to red) after a delay. Subjects were instructed to press the corresponding button when a go signal appeared and to inhibit this response when the stop signal was presented. They were instructed to react as fast and as accurately as possible. Stimulus duration was 400 ms and intertrial duration was jittered between 1.0 and 1.2 s. The stop-signal delay was initially set to 150 ms and adjusted online using a staircase-tracking algorithm to ensure a stopping probability of ∼50% (Aron et al. 2007; Levitt 1971). To achieve this, if the patient made an error, then the stop-signal delay increased by 16 ms, and it decreased by 16 ms after successful inhibitions. Stop-signal delays could range between 20 and 380 ms. The task was programmed in Presentation (Neurobehavioral Systems, Berkeley, CA) and was presented on a laptop. The task was practiced first to ensure that patients fully understood and followed the task instructions.
ECoG Recording
The data were recorded at three different sites using either a Tucker-Davis Technologies (Alachua, FL) recording system; patients recorded at Stanford and UCSF) or Stellate Harmony amplifier (Stellate Systems, Montreal, QC, Canada; Johns Hopkins patients). The electrophysiological channels were sampled at 3.051 kHz (Stanford, UCSF) or 1 kHz (Johns Hopkins). A subdural electrode was used as reference electrode, and a scalp electrode was used as ground during data acquisition. The electrode grids (platinum-iridium electrodes with 2 mm exposed diameter) had 1 cm interelectrode spacing, except the high-density grid of one patient (S01) with a 16 × 16 grid, with 4 mm spacing. The electrode grids were manufactured by Ad-Tech Medical Instrument (Racine, WI).
Data Analysis
Preprocessing.
For offline signal processing, we used functions from the EEGLAB toolbox (Delorme and Makeig 2004) and the Chronux toolbox (http://chronux.org) (Mitra and Bokil 2007), in combination with a custom-written Matlab (MathWorks, Natick, MA) code. The data were down sampled to 1 kHz after standard anti-alias filtering. Electrodes were excluded from the data if they showed 60 Hz line noise, epileptic activity, or other artifacts, such as excessive noise due to poor contact. Electrodes were also excluded if their variability exceeded the average variability across electrodes by >2 SD. All epochs with spread of epileptic activity from the primary epileptic site were also excluded from analysis. No corrections were made for eye movements or muscular artifacts, as no electrooculography or electromyography channels could be recorded. Although eye-movement artifacts may contaminate frontal ECoG electrodes, they mostly affect frequencies lower than the range on which we focused (13–200 Hz) (Ball et al. 2009). The data were subsequently de-trended with a high-pass filter of 0.5 Hz and segmented into three conditions: correct go trials (Go), SS, and US. Finally, the data were re-referenced using a common average reference method.
Time-frequency analysis.
Single-trial signal changes in the time-frequency domain were computed by estimating the analytic amplitude around a specific frequency through convolution with a complex Morlet wavelet with a spectral bandwidth of center frequency/2π, using 41 center frequencies logarithmically spaced between 2 and 200 Hz. We focused on the high-gamma band (60–180 Hz) and the beta band (13–30 Hz). For the spectrograms, the percent signal change in the beta or high-gamma range was computed relative to the prestimulus baseline (−200 to −50 ms). Differences between conditions were assessed after averaging the data in 10 ms time windows for a time window of 0–800 ms after the relevant stimulus and above-mentioned frequency bands. Statistical testing was done on raw (i.e., nonbaselined), single-trial power traces based on a within-subject design, and the number of trials was matched by randomly taking subset trials of the condition with the most trials. Note that the figures show the data in power percent change vs. baseline for visualization purposes only. Statistical significance was determined with a nonparametric statistical test (Wilcoxon signed-rank test) and corrected for multiple comparisons using a false discovery rate (FDR) of P < 0.05. All reported results were FDR corrected (Benjamini and Hochberg 1995) unless stated otherwise. As FDR correction is relatively conservative, we also reported uncorrected results, as to prevent false negatives. Uncorrected results were deemed significant and mentioned if three consecutive, 10 ms time bins had P < 0.01. Electrode selection was done by predefined anatomical regions, namely M1, IFG, and MFG. When multiple electrodes showed a similar effect, we chose to present the time courses of one representative electrode in the figures.
RESULTS
Behavior
RTs in Go ranged from 430 to 660 ms (526 ± 34 ms, mean and SE). The RT for US (445 ± 25 ms; t6 = 5.26, P < 0.01, paired t-test) was shorter compared with Go trials for each subject, which is to be expected, since it is more likely to make an error when responding faster. The staircase algorithm adapted the stop-signal delay to, on average, 185 ± 26 ms for SS and 204 ± 25 ms for US. The average probability of failure was 49.4% (41–53%). The latency of stopping (or SSRT) was 302 ± 31 ms, which is still within the range observed in the stop-signal task in previous ECoG studies (Swann et al. 2009, 2012a). We also calculated RT changes after stop compared with Go trials as the difference in response time after Go or stop trials relative to the preceding trial (Krämer et al. 2011). All subjects showed post-error slowing, i.e., how much the subject slows down in Go trials after compared with before stop trials (average difference in RT is 59 ms ± 10 SE; t6 = 5.8, P < 0.01, paired t-test), and were faster after SS trials (average difference in RT is −64 ms ± 21 SE; t6 = −3.1, P < 0.05, paired t-test). Note that faster RTs after successful inhibitions are in line with previous findings from our lab (Krämer et al. 2011) but are not consistently found. In fact, whether a postinhibition slowing or speeding is found seems to depend on the way the post-stop slowing is calculated, i.e., as difference relative to average Go trials or difference relative to stop preceding Go trials [see Krämer et al. (2011) for further discussion]. Individual behavioral results are reported in Table 2.
Table 2.
Behavioral data
| Subject | Go-RT | US-RT | SSD-SS | SSD-US | SSRT | Probability of Failure, % | PostUS-RT change | PostSS-RT change |
|---|---|---|---|---|---|---|---|---|
| S01 | 635 | 495 | 351 | 355 | 219 | 41 | 66 | −133 |
| S02 | 517 | 454 | 183 | 202 | 316 | 49 | 49 | −36 |
| S03 | 502 | 427 | 212 | 235 | 256 | 52 | 97 | −109 |
| S04 | 501 | 374 | 192 | 213 | 268 | 53 | 90 | −94 |
| S05 | 140 | 160 | 49 | |||||
| S06 | 430 | 406 | 108 | 128 | 294 | 50 | 51 | 24 |
| S07 | 443 | 392 | 141 | 166 | 271 | 49 | 21 | −21 |
| S08 | 661 | 560 | 155 | 170 | 474 | 52 | 41 | −76 |
| Mean ± SE | 526 ± 34 | 445 ± 25 | 185 ± 26 | 204 ± 25 | 302 ± 31 | 49.4 ± 1.3 | 59 ± 10 | −64 ± 21 |
Go reaction time (Go-RT), stop-error reaction time in unsuccessful (US-RT), stop-signal delay in unsuccessful (SSD-US), successful stop trials (SSD-SS), stop-signal reaction time (SSRT), and the difference in go-response time after compared with before an unsuccessful stop trial (PostUS-RT change; slowing) or a successfully inhibited stop trial (PostSS-RT change; slowing) in milliseconds. Due to technical issues, some of the behavioral data for S05 was lost.
Motor-Related Changes in the beta and High-gamma Bands in M1
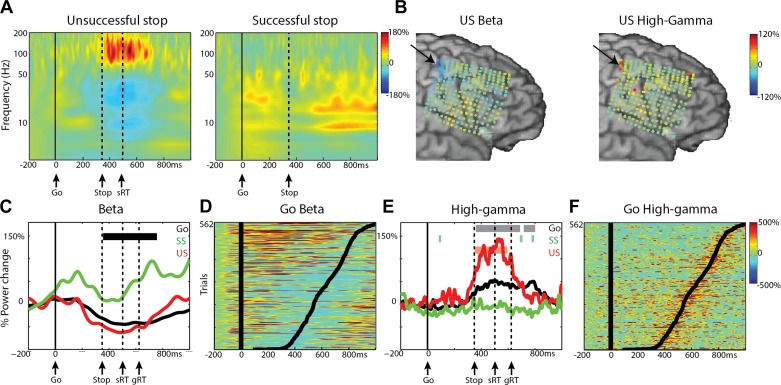
We first investigated motor-related beta and high-gamma changes in the M1. Four patients had coverage of the M1 hand area (1 over right hemisphere), and the results for an M1 electrode and the topography of power changes across all electrodes from a patient with a high-density grid (4 mm spacing) are shown in Fig. 2. Data for the other subjects with 1 cm electrode separation are shown in Fig. 3. In trials with a motor response (Go and US), we observed a high-gamma (60–180 Hz) power increase and a beta (13–30 Hz) desynchronization in the time window of 200 ms before the motor response until 200 ms after the motor response (relative to a 200-ms baseline before the go stimulus). As can be observed in Fig. 2B, the beta decrease was more widespread across the sensorimotor cortex, whereas the high-gamma increase was focused at only very few electrodes. We first discuss effects for both frequency bands relative to prestimulus baseline (−200 to −50 ms before the go or stop stimulus) and then present the results of condition differences. The reported effects were significant after FDR correction at P < 0.05, unless stated otherwise (see also methods). Note that the topographic maps in Fig. 2B (and the following figures) show power changes for all electrodes independently of statistical significance. For Go trials, effects were significant for all four subjects, and for three of four subjects, they were significant in US events (P < 0.01, uncorrected, for US high gamma in S06). For SS trials, the high-gamma response was reduced or absent. Beta power increased in SS trials relative to baseline (S01, P < 0.01, uncorrected; S08, P < 0.05, FDR corrected) did not change from baseline (S06), or a decrease relative to baseline was observed instead (S03, P < 0.05, FDR corrected; Fig. 3).
Fig. 2.
High-gamma and beta effects in M1 for patient S01. A: time-frequency average for the 3 conditions, time locked to the go signal. Dashed, vertical lines show the mean reaction time for Go (gRT) or US (sRT) trials and the mean stop-signal delay (Stop). B: beta (left) and high-gamma (right) power changes for US shown for all electrodes. Data shown are time locked to the response and averaged over −50 to 50 ms relative to the response time. Arrows indicate the location of electrodes shown in other panels in this figure. C: time course for mean beta power in the 3 conditions: Go (black), successful stop (SS; green), and unsuccessful stop (US; red). The horizontal black bar indicates the time window with a significant difference between US and SS (P < 0.05, FDR corrected). D: beta power for single trials for the Go condition, sorted by reaction time (black lines). E: time-course plot for high-gamma power in the 3 conditions. Colored bars indicate the time windows with significant differences compared with baseline (P < 0.05, FDR corrected). F: high-gamma power for single trials for the Go condition, sorted by reaction time (black lines).
Fig. 3.
Beta and high-gamma time courses for M1 for subjects S03, S06, and S08. A: time-course plots for beta power in the 3 conditions: Go (black), successful stop (SS; green), and unsuccessful stop (US; red). Horizontal black bar signifies the time window with a significant difference between US and SS (P < 0.05, FDR corrected). B: time-course plots for high-gamma power in the 3 conditions. Dashed, vertical lines indicate the average time point of the stop signal, the reaction time in unsuccessful stop trials (sRT), and reaction time for Go trials (gRT). Colored bars signify the time window with significant differences compared with baseline (P < 0.05, FDR corrected). C: beta (left) and high-gamma (right) power changes for US shown for all electrodes. Data shown are time locked to the response and averaged over −50 to 50 ms relative to the response time. The arrows indicate the location of electrodes shown in other panels in this figure.
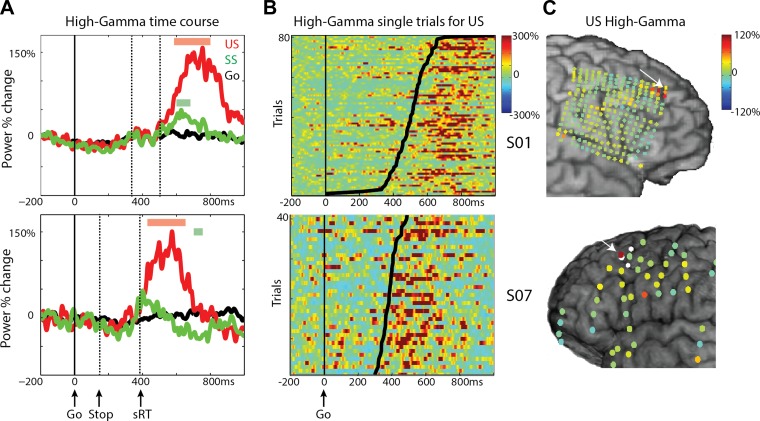
Next, we looked at significant differences among conditions. For S01 and S08, we observed an early increase of beta power ∼100 ms and a beta rebound ∼700 ms after the go signal; the latter was significantly different between SS and US (Figs. 2C and 3A), as well as between SS and Go trials. Notably, the power in the alpha band (8–13 Hz) (Pfurtscheller and Neuper 1994) shows a similar time course as the beta band (Fig. 2A), as well as a similar topography as the beta band (beta topography shown in Fig. 8). High-gamma increases in Go and US were significant compared with SS in all subjects (P < 0.01, uncorrected for S03 and S06). Single-trial power traces for Go trials sorted by RT (Fig. 2, D and F) show that the effects in both frequency bands are consistent over trials and centered around the RT. The high-gamma response was transient and started 125 ± 83 ms (latency of significant high-gamma power vs. baseline, n = 4) before the response. Note that these motor-preparation and execution high-gamma effects are not necessarily limited to one electrode, especially as seen in the high-density grid (Fig. 2B). The effects reported here confirm previous findings of beta desynchronization and high-gamma power increases in M1 during motor preparation and execution (Babiloni et al. 2016; Crone et al. 1998a, b; Pfurtscheller and Lopes da Silva 1999; Swann et al. 2009). Notably, the effects were quite consistent for high gamma, whereas the beta effects were more heterogeneous, which has been reported previously (Crone et al. 1998a, b).
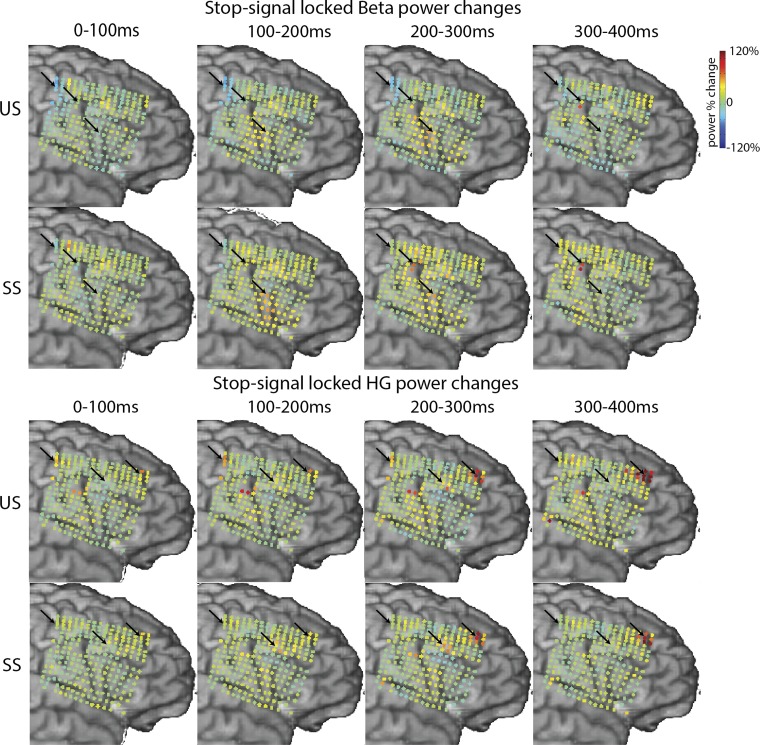
Fig. 8.
Beta and high-gamma (HG) power changes overview for subject S01. The data are time locked to the stop signal (0 ms) and averaged >100 ms for 4 consecutive time windows. The marked electrodes correspond to the electrodes presented in Figs. 2 and 7 for the beta panels and Figs. 2, 4, 5, and 6 for HG panels. The effects described in the main text are highlighted with arrows.
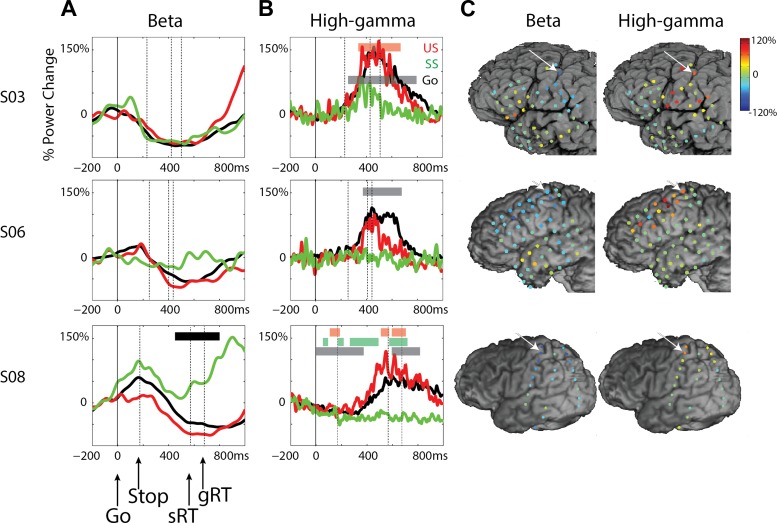
Early Stop-Related High-gamma Responses in MFG
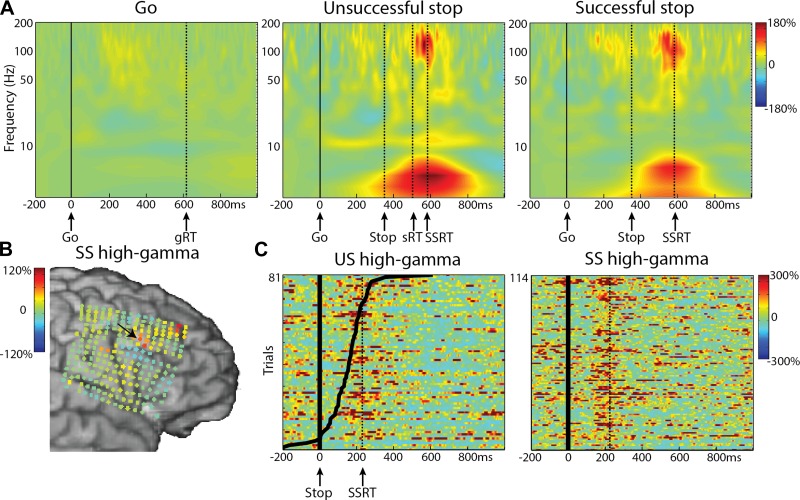
In stop trials, we observed a high-gamma increase over both right and left MFG that was significant vs. baseline. This effect was found in all five subjects with substantial frontal coverage (P < 0.01 uncorrected for SS in S03). As an example, time-frequency plots in Fig. 4A show this transient high-gamma increase for both SS and US conditions for the subject with the high-density, 4-mm grid (S01). This was consistent over trials and occurred after the stop stimulus, as can be assessed from the stop-signal locked data (Fig. 4C). Note that the high-gamma response was accompanied by an increase in the theta band. Figure 4B shows the high-gamma power change in the time window of −50 to 50 ms around the estimated SSRT in stop-signal locked data for SS across all electrodes. The high-gamma increase is localized to a few electrodes close to the inferior frontal sulcus. Note also the high-gamma increase in the anterior-superior corner of the grid, which is described further later in this paper (Fig. 6).
Fig. 4.
Stop-related high-gamma effects in the middle frontal gyrus (MFG) for S01. A: time-frequency plots for the 3 conditions, time locked to the go signal. Dashed, vertical lines reflect the mean reaction time for Go (gRT) or US (sRT), mean stop-signal delay (Stop), and the stop-signal reaction time (SSRT). B: high-gamma power changes for SS shown for all electrodes. Data shown are time locked to the stop signal and averaged over −50 to 50 ms relative to the SSRT. Arrow indicates the location of electrode shown in other panels in this figure. C: high-gamma power for single trials for both stop conditions, time locked to the stop signal, sorted by reaction time (black lines). Dotted, vertical lines are the SSRT.
Fig. 6.
Late high-gamma response in MFG for S01 and S07. A: time courses for high-gamma power aligned to the go signal for 3 conditions: Go (black), successful stops (SS; green), and unsuccessful stops (US; red). Dashed, vertical lines reflect the mean stop-signal delay (Stop) and the reaction time for US (sRT). Colored bars indicate significant high-gamma increases compared with baseline for US (red) and SS (green; P < 0.05, FDR corrected). B: high-gamma power for single trials for US, aligned to the go signal, sorted by reaction time (black lines). C: high-gamma power changes for US shown for all electrodes. Data shown are time locked to the response time and averaged over 0–200 ms relative to the motor response. Arrows indicate the location of electrodes shown in other panels in this figure.
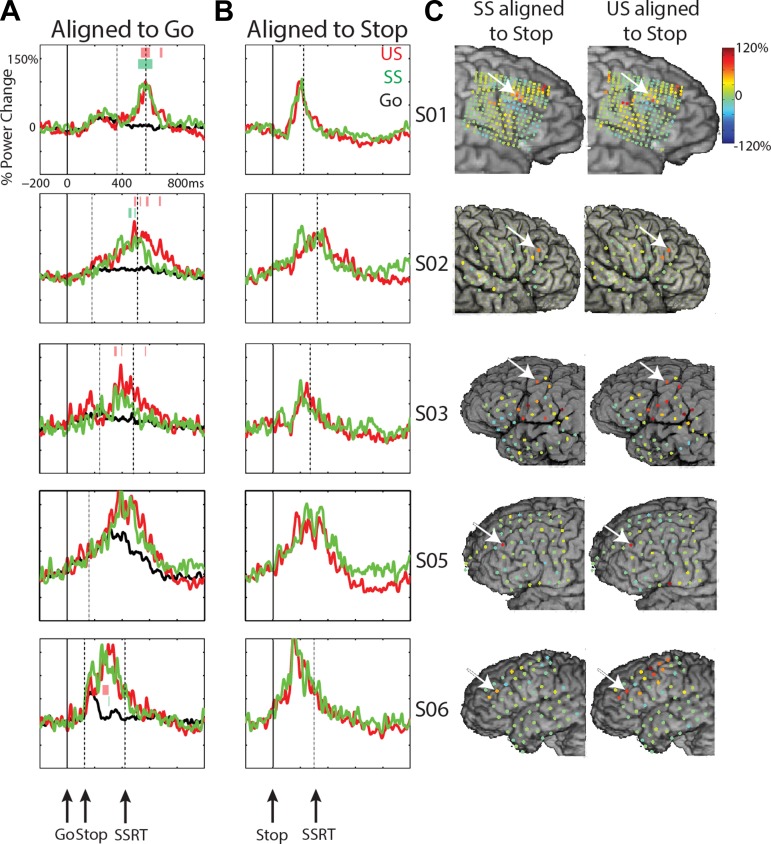
In Fig. 5, the mean high-gamma time course is shown for all five subjects. The stop-related high-gamma response was observed in electrodes located in the MFG, varying in exact location across subjects (Fig. 5C). The high-gamma response was absent in Go trials (S01, S02, S03) or diminished (S05, S06). The high-gamma response for stop trials was larger compared with Go trials for all subjects (Fig. 5A; P < 0.05, FDR corrected; this was only significant at an uncorrected level of P < 0.01 in S03 SS), but high-gamma amplitude did not differ between SS and US trials. Note also that the stop-signal-related high-gamma power increase started ∼120 ms before the end of the estimated SSRT (high gamma was significant vs. baseline, P < 0.05, FDR corrected, at 111 ± 58 ms before SSRT for US; 136 ± 62 ms for SS; t3 = 3.9, P = 0.03) and was maximal before the end of the SSRT (Fig. 5, A and B). For one patient (S03), this effect was observed in a more posterior site compared with other patients. We may be missing an activation site that occurs more anterior, where we do not have coverage. For S03, a high-gamma increase was also observed at more inferior frontal and parietal sites (Fig. 5C), but this was not seen in the other two patients with coverage of these areas (S05, S06).
Fig. 5.
Stop-related high-gamma effects in the MFG for S01, S02, S03, S05, and S06. A: time courses for high-gamma power aligned to the go signal for the 3 conditions: Go (black), successful stops (SS; green), and unsuccessful stops (US; red). Dashed, vertical lines reflect the mean stop-signal delay (Stop) and the stop-signal reaction time (SSRT). Colored bars signify time windows for differences between SS and Go (green) and US and Go (red; P < 0.05, FDR corrected). B: time courses for high-gamma power aligned to the stop signal for SS and US. C: high-gamma power changes for SS and US shown for all electrodes. Data shown are time locked to the stop signal and averaged over −50 to 50 ms relative to the SSRT. For S05, the data were taken from 150 to 250 ms after the stop signal for visualization purposes only, as the actual SSRT is not known. Arrows indicate the location of electrodes shown in other panels in this figure.
Stop signals in the SSP are less frequent than go signals, and neural responses to the stop signal might be modulated by this difference in stimulus probability. To investigate whether the early MFG response reflected the tracking and updating of local stimulus probabilities, we performed a post hoc analysis of the relationship between the high-gamma amplitude in these electrodes and the number of Go trials preceding the stop trial. This analysis revealed no significant correlations (t499 = 0.28, P = 0.63, linear mixed-effects model, with high-gamma amplitude as the dependent variable, number of Go trials preceding the stop trial as the independent variable, and subject as random effect).
Postresponse, Stop-Related High gamma in MFG
A longer latency high-gamma increase for stop trials was recorded in the MFG in two subjects (S01, S07). Figure 6A shows that the high-gamma increase was absent in Go trials but increased vs. baseline for both stop conditions for S01 and for US in S07. The high-gamma signal was also increased for US vs. SS and US vs. Go (P < 0.01, uncorrected for S07). This high-gamma response occurred after the motor response in US trials (Fig. 6B). The electrode showing the effect in S01 was located more anterior in the MFG (and close to the superior frontal sulcus) compared with the electrode site for S07. Figure 6C shows the topography of high-gamma power changes in the time window of 0–200 ms after the response, demonstrating the very focal high-gamma response at few electrodes in S01 and one electrode in S07. This late MFG response was strongest after failed inhibitions, suggesting a behavioral monitoring effect. Such monitoring of performance in the MFG in stop trials might be related to behavioral adaptation after stop trials, particularly after unsuccessful inhibitions. We examined a possible relation between the MFG high-gamma response and the amount of post-error slowing across trials. This post hoc analysis showed no significant differences [t56 = −0.21, P = 0.83, linear mixed-effects model, with high-gamma amplitude as the dependent variable, the amount of post-error slowing as the independent variable, and subject as a random effect (2 subjects)]. Note also that the high-gamma response was almost absent in SS (Fig. 6). In fact, in S07, only a late high-gamma decrease relative to baseline yielded significance. Interestingly, patients showed a decrease of RTs after SS in contrast to an RT increase after stop errors (see Table 2).
Stop-Related Changes in the beta Band
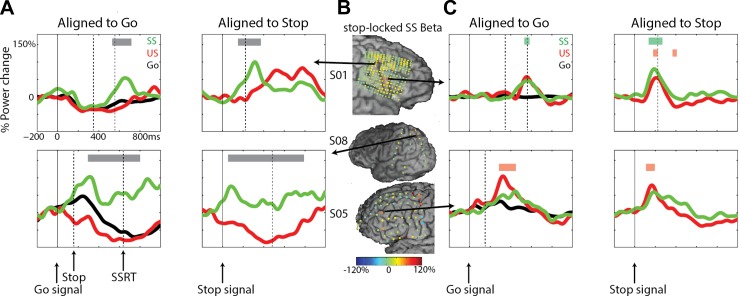
In addition to the beta changes in M1, we observed beta-band effects for three subjects in sites around the central and precentral sulci (S01) and parietal cortex (S08; Fig. 7A) and in the IFG/MFG in both left and right hemispheres (S01, S05; Fig. 7B). Generally, stopping-related beta effects in the PFC were more heterogeneous across subjects than the high-gamma response. In the central sulcus/parietal sites, beta increased during SS. For S01, beta power increased after the SSRT, whereas in S08, beta power was increased throughout the trial. In both subjects, this increase was stronger in SS compared with US and Go conditions. Note that the electrode in S01 is several electrode rows anterior and inferior to the M1 site reported in this paper.
Fig. 7.
Changes in beta power for subjects S01, S05, and S08. A: time courses for beta power aligned to go and stop signals for peri-central sulcus sites for 3 conditions: Go (black), successful stops (SS; green), and unsuccessful stops (US; red). Dashed, vertical lines reflect the stop signal and stop-signal reaction time (SSRT). Gray bars signify time windows for differences between SS and US (P < 0.05, FDR corrected). B: beta power changes for SS shown for all electrodes. Data shown are time locked to the stop signal and averaged over −50 to 50 ms relative to the SSRT. Arrows indicate the location of electrodes shown in other panels in this figure. C: time course for beta power aligned to go and stop signals for IFG/MFG sites for 3 conditions. Colored bars indicate time windows for differences between SS (green) and US (red) vs. baseline (P < 0.05, FDR corrected).
The beta increase over IFG/MFG sites was apparent in both stop conditions, and no significant differences between SS and US were found. Figure 7B shows the beta power changes for all electrodes in S01, S05, and S08. The beta effects for both subjects were significant vs. baseline for SS (P < 0.01, uncorrected for S05) and for US (P < 0.05, FDR corrected for S01, stop locked only; S05, both go and stop locked). A third subject with extensive rIFG coverage (S02) showed no significant beta changes in any of the IFG electrodes (data not shown). Note in Fig. 7C that the beta response for subject S05 was found in the same electrode as the stop-related high-gamma response described earlier (Fig. 5). Finally, to provide an overview of the complete stop-related time course of beta and high-gamma changes, Fig. 8 shows the averaged relative power changes for patient S01 separately for SS and US.
DISCUSSION
We examined oscillatory dynamics over lateral frontal cortex related to inhibitory motor control in a stop-signal task in patients with subdural grid electrodes. We observed two distinct effects in lateral PFC during stop trials. A transient high-gamma increase, maximal before the SSRT, was found in MFG. This response did not differ between SS and US and may reflect an attention process triggered by the salient, behaviorally relevant stop signals. A later high-gamma increase in more rostral MFG was evident after motor responses and was larger for US compared with SS, suggestive of action monitoring of incorrect behavior. We confirmed previous findings in the M1, showing a beta decrease and high-gamma increase related to motor preparation and execution and a beta rebound and absence of high-gamma changes for successfully inhibited responses. In contrast to previous findings, analyses of stop-related beta changes in IFG or MFG could not reveal significant differences between SS and US (Swann et al. 2009).
Motor Preparation Activity and Inhibitory Effects in M1
Motor preparation in M1 sites was associated with a beta-band decrease and a high-gamma increase (Figs. 2 and 3) relative to a prestimulus baseline and maximal around the time of movement execution. This is in line with findings from noninvasive studies [as reviewed by Pfurtscheller and Lopes da Silva (1999)], as well as ECoG studies (Crone et al. 1998a, b; Miller et al. 2007). For SS, our data confirmed a beta rebound, i.e., a power increase after an initial decrease, similar to that shown before in human ECoG (Swann et al. 2009) and animal studies (Zhang et al. 2008). With an expansion on this, we observed a smaller or absent high-gamma response in SS. Note that the beta rebound in SS was found in two patients only and was not observed for patient S03, who also showed a small, yet statistically not significant increase in high gamma in this condition. This patient may not have fully inhibited the motor response but inhibited it sufficiently enough to prevent a button press. However, since we did not record electromyography, this remains speculative.
The observed differences between SS and US indicate that M1 is either downstream of the site of inhibition, which may be located in subcortical sites (Mirabella et al. 2012; van den Wildenberg et al. 2006; Zandbelt and Vink 2010) or premotor cortex (Mattia et al. 2012, 2013; Mirabella et al. 2011), or is the site of inhibition itself (Band and van Boxtel 1999). This would be in line with recent empirical and theoretical work, attributing the causal role in motor inhibition to the (pre)motor cortex and suggesting that response preparation and inhibition are subserved by specific interactions in strongly overlapping networks (Mattia et al. 2013; Mirabella 2014; Mirabella et al. 2011).
Attentional Control and Monitoring of Behavior in the MFG
The first prefrontal effect that we observed was a transient, stop-related high-gamma power increase (Figs. 4 and 5) in both the right and left MFG. This increase was seen for stop trials compared with Go trials in all subjects with extensive coverage of the PFC (n = 5). In two patients, a weaker high-gamma response was also observed during Go trials. The high-gamma effect occurred before the estimated SSRT but did not differ between SS and US conditions. Brain regions critical for stopping should show differential activity between SS and US in a time range before the SSRT. Our results are more in line with an attention signal, which is reduced or absent in the more frequent, less attention-demanding go signals (Chikazoe 2010; Erika-Florence et al. 2014; Swick et al. 2011). The absence of stopping-specific effects in PFC electrodes is consistent with data from PFC lesion patients who had comparable SSRTs with healthy controls (Krämer et al. 2013). One suggestion is that the MFG may facilitate the stopping process by signaling task-relevant stimulus probabilities. In patient S01, an additional go/no-go task was run with blocks of either 50% or 20% no-go signal probability. In that task, a similar high-gamma increase was observed for No-go trials in the same MFG electrode, which was significantly higher for the 20% vs. the 50% conditions (E. Tzvi, Y. M. Fonken, N. E. Crone, E. F. Chang, J. Parvizi, R. T. Knight, U. M. Krämer, unpublished observations). In the present study, a post hoc analysis, investigating the correlation between the high-gamma amplitude and the number of Go trials preceding it, showed no relationship. Perhaps this MFG site is not involved with directly tracking stimulus probabilities, and the differential stimulus probability effects are due to task-set changes influenced by the stimulus probability.
The notion of inhibition-specific modules in PFC has been questioned recently (Erika-Florence et al. 2014; Mirabella 2014). Based on functional MRI data of different variants of the stop-signal task, the authors argued that PFC regions associated with inhibitory functions are part of spatially distributed networks activated when processing infrequent stimuli or learning new tasks (Erika-Florence et al. 2014). Our results support the results in Erika-Florence et al. (2014), as the MFG response to the stop signal could reflect attention toward task-relevant sensory input. The effect might be similar to the dorsolateral PFC high-gamma activity observed in Swann et al. (2012b), which was interpreted as related to the task representation. The activity in their study was observed in response to the task cue but also to the go cue in a stop-signal task, with a “maybe stop-go” condition (Swann et al. 2012b). However, the observed neural response in this study was more generalized compared with ours, since we only observed the MFG activation following the stop signal. The results described here, as well as in the study by Swann et al. (2012b), are consistent with the MFG high-gamma activity reflecting attention toward behaviorally relevant signals or retrieval of task goals, which could generalize to other tasks unrelated to response inhibition (Erika-Florence et al. 2014).
Since this stop-related high-gamma effect in MFG did not distinguish between SS and US, signals related to the decision process may be found in basal ganglia (Schmidt et al. 2013) or possibly motor cortex (van den Wildenberg et al. 2010). Our observations of clear stop-related effects in M1 indicate that the decision process possibly occurs in this brain region. Since we did not measure striatal activity, we cannot determine if this indeed is the decision process itself or whether it is a downstream effect from processes in basal ganglia. Regarding the framework proposed by Band and van Boxtel (1999), our results confirmed that M1 is either the site of inhibition or where the inhibitory process manifests itself [see also Mirabella (2014)]. The activations that we observed in MFG do not support a role as the inhibition agent. Rather, this signal may be evidence of attentional control, which may potentially be modulating activity of an inhibitory agent in the basal ganglia.
We also observed a later high-gamma increase over more anterior and dorsal PFC sites during stop trials, which occurred after the motor response for US trials. This effect was observed in two out of eight subjects who had electrodes in more frontal and superior sites. For patient S01, this effect was more anterior to the early MFG response, and it was also observed over the left MFG at a slightly more posterior site (patient S07). The late timing indicates that this effect cannot be involved in the stopping process itself. This increase in high-gamma power was enhanced for errors and may reflect behavioral monitoring. Activity in dorsolateral PFC has been consistently linked to top-down control and behavioral adaptation after action errors or response conflicts (Botvinick et al. 2001; Kerns et al. 2004; Marco-Pallares et al. 2008). Notably, the late MFG high-gamma response was considerably weaker (in S01) or reversed (in S07) in SS. At the same time, patients even showed faster response times after SS trials relative to before. Whereas post-error slowing in the stop-signal task is a robust finding, behavioral after-effects of SS trials are more variable, with many studies reporting no RT change (Beyer et al. 2012; Verbruggen et al. 2008), some reporting an RT increase (Boehler et al. 2011), and some observing an RT decrease (Krämer et al. 2011). A postinhibition RT decrease with a reduced or absent MFG high-gamma effect supports an interpretation in terms of behavioral adaptation. A post hoc correlation analysis between the amount of post-error slowing and the high-gamma amplitude did not confirm a relationship between the MFG response and behavioral adaptation. However, RT changes after SS or US might reflect not only adaptation and enhanced cognitive control but also simple repetition priming effects (Beyer et al. 2012; Verbruggen et al. 2008). The post-error slowing might thus be a mixture of priming and adaptation effects and not related to the high-gamma MFG response.
Dynamics in the beta Band over PFC and Sensorimotor Cortex
Engel and Fries (2010) suggested that beta frequencies might signal the maintenance of the sensorimotor and cognitive set and are involved in the suppression of novel or unexpected external events. The beta rhythm has also been referred to as an idling rhythm (Engel and Fries 2010; Miller et al. 2012), potentially fulfilling a role in suppressing local cortical activity, whereas beta desynchronization might enable the transition into an active processing state (Miller et al. 2012).
We observed two different beta-power effects. In peri-central sulcus sites, we observed a beta increase for SS, which sometimes coincided with a beta decrease for motor response trials (Go and US; see Fig. 7A). This is in line with a motor maintenance study in macaques that showed that activity in peri-central sulcus and parietal cortex was organized in a large-scale network characterized by beta-band coherence (Brovelli et al. 2004). In the present study, we found changes in beta power in similar peri-central sulcus and superior parietal sites, possibly reflecting different nodes of the sensorimotor network.
We also analyzed changes in beta power specific to stop trials to test the hypothesis that rIFG beta activity mediates inhibition in a fronto-basal ganglia network involved in stopping (Swann et al. 2009, 2012a). We found beta effects in both left and right IFG or MFG, which resembled results reported by Swann et al. (2009, 2012b) in rIFG. However, we did not find a difference between SS and US conditions, confirming observations that these beta changes in IFG may be variable across patients (Schall and Godlove 2012). An explanation for this could be that the stop-signal tasks used previously were more complex (Swann et al. 2009, 2012a), possibly causing an increased involvement of the PFC compared with the simpler stop-signal task used in the present study. Another explanation might be that the intertrial interval in the study by Swann et al. (2009) was not jittered, whereas it was in the present study. A recent magnetoencephalography study in humans, using a cued anti-saccade task, observed increased beta power over prefrontal sites when preparing for an anti-saccade compared with a pro-saccade (Hwang et al. 2014). Perhaps the increased predictability of when to be ready for response inhibition is reflected by increased beta-band synchronization.
We also found stop-related beta effects in the left PFC, which is not in accord with a strict lateralization of response inhibition to the right PFC (Aron et al. 2014; Swann et al. 2009, 2012a). In agreement with our ECoG findings, a recent study with PFC lesion patients (Krämer et al. 2013) showed that patients with lesions in either left or right PFC had a similar speed of stopping compared with age-matched controls but increased commission error rate in No-go trials. This result can be explained by an attentional control function of the lateral PFC rather than by inhibition implementation (Erika-Florence et al. 2014; Swick et al. 2011).
Conclusions
Our findings argue for a role of monitoring for task-relevant sensory signals and of task performance in subregions of the lateral PFC during motor inhibition. The early stop-related activity over right and left MFG likely reflects attention-related activity to the behaviorally relevant stop signals, whereas the late stop related in rostral PFC activity tracks behavioral performance. Inhibition-related modulation of beta oscillatory activity was found over sensorimotor areas, including M1, supporting the notion of a beta-mediated network of motor control.
GRANTS
Support for this work was provided by the Deutsche Forschungsgemeinschaft (KR3691/1-1 to U. M. Krämer); National Institute of Neurological Disorders and Stroke (2R37RO1NS21135 to R. T. Knight, 1R01NS078396 to J. Parvizi, and 1R01NS40596 to N. E. Crone); Fulbright Fellowship to Y. M. Fonken; Howard Hughes Medical Institute fellowship to Y. M. Fonken; Nielsen Corporation to R. T. Knight; Defense Advanced Research Projects Agency; National Institute on Deafness and Other Communication Disorders (R01-DC012379); National Institute of Neurological Disorders and Stroke (R00-NS065120); U.S. National Institutes of Health Director's New Innovator Award Program grant (DP2-OD00862); McKnight Foundation; and Ester A. and Joseph Klingenstein Foundation to E. Chang.
DISCLOSURES
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.T.K. and U.M.K. conception and design of research; Y.M.F., N.E.C., E.C., J.P., and U.M.K. performed experiments; Y.M.F., J.W.R., and E.T. analyzed data; Y.M.F., R.T.K, and U.M.K interpreted results of experiments; Y.M.F. prepared figures; Y.M.F. drafted manuscript; R.T.K. and U.M.K. edited and revised manuscript; Y.M.F., J.W.R., E.T., N.E.C., E.C., J.P., R.T.K., and U.M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Nicki Swann and the members of the Knight lab for their feedback and helpful discussions. In addition, the authors thank the following people for their help in data acquisition: Brian Pasley, Sara Szcepanski, Avgusta Shestyuk, Aurelie Bidet-Caulet, Leon Deouell, Adeen Flinker, Matar Haller, and Maya Cano, as well as the technical support staff at the recording sites.
REFERENCES
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry 69: e55–e68, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27: 3743–3752, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177, 2004. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18: 177–185, 2014. [DOI] [PubMed] [Google Scholar]
- Aron AR, Verbruggen F. Stop the presses; dissociating a selective from a global mechanism for stopping. Psychol Sci 19: 1146–1153, 2008. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Del Percio C, Vecchio F, Sebastiano F, Di Gennaro G, Quarato PP, Morace R, Pavone L, Soricelli A, Noce G, Esposito V, Rossini PM, Gallese V, Mirabella G. Alpha, beta and gamma electrocorticographic rhythms in somatosensory, motor, premotor and prefrontal cortical areas differ in movement execution and observation in humans. Clin Neurophysiol 127: 641–654, 2016. [DOI] [PubMed] [Google Scholar]
- Ball T, Kern M, Mutschler I, Aertsen A, Schulze-Bonhage A. Signal quality of simultaneously recorded invasive and non-invasive EEG. Neuroimage 46: 708–716, 2009. [DOI] [PubMed] [Google Scholar]
- Band GP, van Boxtel GJ. Inhibitory motor control in stop paradigms: review and reinterpretation of neural mechanisms. Acta Psychol (Amst) 101: 179–211, 1999. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300, 1995. [Google Scholar]
- Beyer F, Münte TF, Fischer J, Krämer UM. Neural aftereffects of errors in a stop-signal task. Neuropsychologia 50: 3304–3312, 2012. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Bunzeck N, Krebs RM, Noesselt T, Schoenfeld MA, Heinze HJ, Münte TF, Woldorff MG, Hopf JM. Substantia nigra activity level predicts trial-to-trial adjustments in cognitive control. J Cogn Neurosci 23: 362–373, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev 108: 624–652, 2001. [DOI] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol Rev 114: 376–397, 2007. [DOI] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci USA 101: 9849–9854, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry 163: 1282–1284, 2006. [DOI] [PubMed] [Google Scholar]
- Chikazoe J. Localizing performance of go/no-go tasks to prefrontal cortical subregions. Curr Opin Psychiatry 23: 267–272, 2010. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 95: 3371–3383, 2006. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain 121: 2301–2315, 1998a. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain 121: 2271–2299, 1998b. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol 94: 4269–4280, 2005. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol 20: 156–165, 2010. [DOI] [PubMed] [Google Scholar]
- Erika-Florence M, Leech R, Hampshire A. A functional network perspective on response inhibition and attentional control. Nat Commun 5: 4073, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinker A, Chang EF, Barbaro NM, Berger MS, Knight RT. Sub-centimeter language organization in the human temporal lobe. Brain Lang 117: 103–109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Ghuman AS, Manoach DS, Jones SR, Luna B. Cortical neurodynamics of inhibitory control. J Neurosci 34: 9551–9561, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci 28: 7209–7218, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Deciding not to GO: neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cereb Cortex 5: 410–428, 1995. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026, 2004. [DOI] [PubMed] [Google Scholar]
- Krämer UM, Knight RT, Münte TF. Electrophysiological evidence for different inhibitory mechanisms when stopping or changing a planned response. J Cogn Neurosci 23: 2481–2493, 2011. [DOI] [PubMed] [Google Scholar]
- Krämer UM, Solbakk AK, Funderud I, Løvstad M, Endestad T, Knight RT. The role of the lateral prefrontal cortex in inhibitory motor control. Cortex 49: 837–849, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am 49: 467–477, 1971. [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol 114: 216–222, 2005. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform 10: 276–291, 1984. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J, Camara E, Mu TF, Rodrı A. Neural mechanisms underlying adaptive actions after slips. J Cogn Neurosci 20: 1595–1610, 2008. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Tanji J. Changing directions of forthcoming arm movements: neuronal activity in the presupplementary and supplementary motor area of monkey cerebral cortex. J Neurophysiol 76: 2327–2342, 1996. [DOI] [PubMed] [Google Scholar]
- Mattia M, Pani P, Mirabella G, Costa S, Del Giudice P, Ferraina S. Heterogeneous attractor cell assemblies for motor planning in premotor cortex. J Neurosci 33: 11155–11168, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia M, Spadacenta S, Pavone L, Quarato P, Esposito V, Sparano A, Sebastiano F, Di Gennaro G, Morace R, Cantore G, Mirabella G. Stop-event-related potentials from intracranial electrodes reveal a key role of premotor and motor cortices in stopping ongoing movements. Front Neuroeng 5: 1–13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Hermes D, Honey CJ, Hebb AO, Ramsey NF, Knight RT, Ojemann JG, Fetz EE. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput Biol 8: e1002655, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG. Spectral changes in cortical surface potentials during motor movement. J Neurosci 27: 2424–2432, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G. Should I stay or should I go? Conceptual underpinnings of goal-directed actions. Front Syst Neurosci 8: 1–21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella G, Iaconelli S, Romanelli P, Modugno N, Lena F, Manfredi M, Cantore G. Deep brain stimulation of subthalamic nuclei affects arm response inhibition in Parkinson's patients. Cereb Cortex 22: 1124–1132, 2012. [DOI] [PubMed] [Google Scholar]
- Mirabella G, Pani P, Ferraina S. Neural correlates of cognitive control of reaching movements in the dorsal premotor cortex of rhesus monkeys. J Neurophysiol 106: 1454–1466, 2011. [DOI] [PubMed] [Google Scholar]
- Mitra PP, Bokil H. Observed Brain Dynamics. Oxford, UK: Oxford University Press, 2007. [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O'Reilly RC. A unified framework for inhibitory control. Trends Cogn Sci 15: 453–459, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857, 1999. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Event-related synchronization of mu rhythm in the EEG over the cortical hand area in man. Neurosci Lett 174: 93–96, 1994. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Brittain J, Holland P, Joint C, Nandi D, Bain PG, Yousif N, Green A, Stein JS, Aziz TZ. The role of the subthalamic nucleus in response inhibition: evidence from deep brain stimulation for Parkinson's disease. Neuropsychologia 47: 2828–2834, 2009. [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol 9: e1000610, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos KW, Stuphorn V. Medial frontal cortex motivates but does not control movement initiation in the countermanding task. J Neurosci 30: 1968–1982, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Godlove DC. Current advances and pressing problems in studies of stopping. Curr Opin Neurobiol 22: 1012–1021, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci 16: 1118–1124, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Mushiake H, Saito N, Tanji J. Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci USA 93: 8694–8698, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Poizner H, Houser M, Gould S, Greenhouse I, Cai W, Strunk J, George J, Aron AR. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson's disease. J Neurosci 31: 5721–5729, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci 29: 12675–12685, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, Aron AR, Tandon N. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. Neuroimage 59: 2860–2870, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Tandon N, Pieters TA, Aron AR. Intracranial electroencephalography reveals different temporal profiles for dorsal- and ventro-lateral prefrontal cortex in preparing to stop action. Cereb Cortex 23: 2479–2488, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 56: 1655–1665, 2011. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, Burle B, Vidal F, van der Molen MW, Ridderinkhof KR, Hasbroucq T. Mechanisms and dynamics of cortical motor inhibition in the stop-signal paradigm: a TMS study. J Cogn Neurosci 22: 225–239, 2010. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson's disease. J Cogn Neurosci 18: 626–636, 2006. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD, Liefooghe B, Vandierendonck A. Short-term aftereffects of response inhibition: repetition priming or between-trial control adjustments? J Exp Psychol Hum Percept Perform 34: 413–426, 2008. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M. On the role of the striatum in response inhibition. PLoS One 5, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Bressler SL, Ding M. Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm. Neuroscience 156: 238–246, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]