Summary
Viral protein homeostasis depends entirely on the machinery of the infected cell. Accordingly, viruses can illuminate the interplay between cellular proteostasis components and their distinct substrates. Here we define how the Hsp70 chaperone network mediates the dengue virus life cycle. Cytosolic Hsp70 isoforms are required at distinct steps of the viral cycle, including entry, RNA replication and virion biogenesis. Hsp70 function at each step is specified by nine distinct DNAJ cofactors. Of these, DnaJB11 relocalizes to virus-induced replication complexes to promote RNA synthesis, while DnaJB6 associates with capsid protein and facilitates virion biogenesis. Importantly, an allosteric Hsp70 inhibitor, JG40, potently blocks infection of different dengue serotypes in human primary blood cells without eliciting viral resistance or exerting toxicity to the host cells. JG40 also blocks replication of other medically-important flaviviruses including yellow fever, West Nile and Japanese encephalitis viruses. Thus, targeting host Hsp70 subnetworks provides a path for broad-spectrum antivirals.
Introduction
A third of the world population is at risk of infection with the mosquito-borne dengue virus (DENV) (Bhatt et al., 2013; Shepard et al., 2014), with an estimated 390 million infections annually (Bhatt et al., 2013). Any of four serotypes causes a range of severe diseases; dengue fever (DF) is a debilitating acute flu-like illness, while dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), with about 500,000 cases annually, are life-threatening diseases typified by vascular leakage and circulatory shock (Halstead, 2007)(Bhatt et al., 2013). DENV's major in vivo targets in humans are myeloid cells, including dendritic cells (DCs) and macrophages (Schmid et al., 2014). The dysregulated overproduction of cytokines and chemokines during DENV infection is thought to contribute to the increased vascular permeability, disruption of the coagulation system and shock associated with DHF/DSS (Rothman, 2011). Despite its burden on global health, no specific antivirals or vaccines are licensed for human use (Lim et al., 2013).
DENV belongs to the genus Flavivirus of the family Flaviviridae, comprising other clinically relevant arthropod-borne viruses such as yellow fever virus (YFV), West Nile virus (WNV), Japanese encephalitis virus (JEV) and tick-borne encephalitis virus (TBEV) (Lindenbach BD, 2007). Flaviviruses have a capped positive-sense single-stranded RNA genome of ∼11kb that encodes a single polyprotein, which is co- and post-translationally cleaved by host and viral proteases into three structural (capsid, prM and E) and seven non-structural (NS1, NS2A/B, NS3, NS4A/B and NS5) proteins (Apte-Sengupta et al., 2014; Lindenbach, 2007). Capsid encapsidates the genomic RNA and is then enveloped by membranes containing prM and E to produce progeny virions (Perera and Kuhn, 2008). The non-structural proteins are involved in viral replication and modulate the host cell environment by, for example, remodeling cellular membranes or inhibiting immune responses (Rothman, 2011; Rodriguez-Madoz et al., 2010).
Like all RNA viruses, flaviviruses are dependent on the host cell machinery for replication., With only ten proteins. DENV completely remodels the cell and generates an endoplasmic reticulum (ER)-derived membranous web (Junjhon et al., 2014; Welsch et al., 2009), where viral replication complexes are assembled. DENV proteins also associate with lipid droplets (LD) (Fig. 1A) (Samsa et al., 2009). The ability of such a small genome to remodel the cell is linked to the structural and functional complexity of DENV proteins (Perera and Kuhn, 2008). This, in turn, presents a challenge to viral protein folding and assembly. Indeed, many viruses depend on host molecular chaperones for folding and proteostasis (Mayer, 2005; Nagy and Pogany, 2012). Chaperones are reported to facilitate flavivirus replication; ER chaperones associate with DENV E (Limjindaporn et al., 2009), and Hsp70 protects JEV NS3 and NS5 from degradation (Ye et al., 2013). However, the interplay between chaperone networks and the viral life cycle remains unclear. The structural complexity and accumulation to high levels of viral proteins may make viruses hyper-dependent on chaperones, and vulnerable to their inhibition (Neckers and Tatu, 2008; Geller et al., 2012). Pharmacological chaperone inhibitors are emerging as promising cancer therapies, particularly Hsp90 inhibitors (Neckers and Tatu, 2008; Trepel et al., 2010). A number of viruses are also sensitive to Hsp90 inhibitors (Geller et al., 2012), without evolving drug-resistant viral escape variants (Geller et al., 2013; Geller et al., 2007). Since drug resistance is a major stumbling block in antiviral treatment (De Clercq, 2007), the potential inability of viruses to become chaperone-independent makes chaperones attractive antiviral drug targets.
Figure 1. Cytosolic Hsp70 isoforms are required for DENV life cycle.
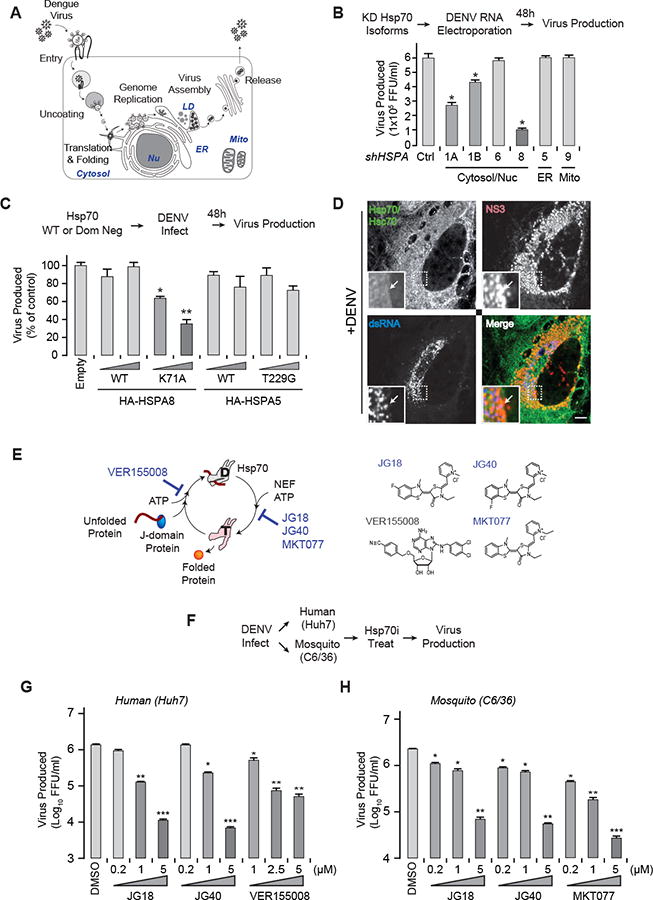
(A) DENV life cycle highlighting major steps and their subcellular localization. Nu, nucleus; LD, lipid droplet; Mito, mitochondria, ER; endoplasmic reticulum.
(B) Depletion of cytosolic Hsp70 isoforms reduces DENV replication. In vitro transcribed DENV RNA introduced by electroporation; 48 hours post infection (hpi), supernatants were harvested and extracellular virus titrated by focus forming assay (FFA). Ctrl: shRNA against luciferase as a control.
(C) Overexpression of dominant negative cytosolic Hsp70 but not ER Hsp70 BiP reduces DENV propagation. Wild type (WT) or dominant negatives (K71A or T229G) of cytosolic (HSPA8) or ER (BiP) Hsp70 were transduced into Huh7 cells. Transfected cells were infected with DENV2 at MOI 0.5 and extracellular virus quantified 36 hpi by FFA.
(D) Hsc70/Hsp70 colocalizes with DENV replication compartments. Huh7 cells infected with DENV2 were stained at 48 hpi with a pan cytosolic Hsc70/Hsp70 antibody (green), dsRNA (blue) and NS3 (red), marking viral replication sites. Scale bar, 10 μm
(E) Hsp70 chaperone and ATPase cycle is regulated by co-chaperone cofactors andtargeted by inhibitors. Hsp70 inhibitors used here: VER15508 blocks ATP binding toHsp70. JG18, JG40 and MKT077 block binding of Hsp70 to Nucleotide Exchange Factors (NEF).
(F-H) Hsp70 inhibitors suppress DENV propagation in human and mosquito cells in a dose dependent manner. (F) Experimental design: Huh7 (G) and C6/36 (H) cells infected with DENV2 at MOI 0.5 for 1 h, and inhibitors added for 36 h (Huh7 cells) or 48 h (C6/36 cells). Extracellular virus: determined by FFA. All data is expressed as means ± SD of three independent experiments each carried out in triplicate. * P < 0.01, ** P < 0.005.
We have a limited understanding of the chaperone network architecture, and its relationship to the organization of the cell (Hartl et al., 2011). One hallmark of the evolution of the chaperone machinery is the progressive diversification of chaperone isoforms and cofactors (Powers and Balch, 2013). In bacteria there is one Hsp70 chaperoneand one Hsp70 cofactor, DnaJ, while in humans there are 13 Hsp70s and over 45 DNAJ-like proteins (Kampinga and Craig, 2010; Powers and Balch, 2013). This diversification may allow more nuanced regulation of chaperone activity in an increasingly complex proteome. Understanding how chaperone systems facilitate a complex phenotype like viral infection could provide a window into the functional specialization of chaperone isoforms. Here we address the role of Hsp70 chaperones and their DnaJ cofactors in the DENV life cycle. We find that cytosolic Hsp70s, assisted by distinctly localized DnaJ's, are required at multiple steps of the viral life cycle. Importantly, Hsp70 provides a susceptible node for antiviral Hsp70 inhibitors, with negligible toxicity and no resistance. Hsp70 inhibitors block the replication of diverse DENV serotypes and other flaviviruses (WNV, YFV, TBEV). The multifaceted roles of Hsp70 subnetworks in flavivirus infection may provide the basis for broad-spectrum, resistance-free antivirals for a range of human diseases.
Results
Hsp70 is required for DENV infection of human and mosquito cells
To explore the involvement of Hsp70 in the DENV lifecycle (Fig. 1A), we established six Hsp70 isoform-specific shRNA knockdown (KD) cell lines (Fig. S1A), and confirmed reduction of mRNA (50-80%, Fig. S1A) and protein (50-95%, Fig. S1B). While reductions in some Hsp70 isoforms upregulated others, Hsp90 immunoblot confirmed that a general stress response was not induced (Fig. S1B). Depletion of cytosolic HSPA1A, 1B and 8, (also called Hsp70-1A, 1B and Hsc70 respectively) significantly reduced DENV infectious particle production (Fig. 1B). In contrast, depletion of ER-resident Hsp70 HSPA5 (BiP) or mitochondrial Hsp70 HSPA9 had no effect on DENV (Fig. 1B). KD of all cytosolic isoforms caused similar reductions in viral RNA (vRNA) levels (Fig. S1C) but HSPA8 had the most dramatic effect on virion production (Fig. 1B). Furthermore, overexpression of dominant-negative cytosolic Hsc70, mutated in the ATPase active site (HSPA8 K71A) (O'Brien et al., 1996), significantly and dose-dependently reduced vRNA replication and virion production (Fig. 1C, S1D). In contrast, dominant-negative BiP (HSPA5 T229G) had no effect. Immunofluorescence analysis showed that cytosolic Hsp70s, while ubiquitous, were concentrated in viral replication complexes containing double-stranded RNA (dsRNA) and NS3 (Fig. 1D; see S1E for uninfected control).
To overcome the confounding effects of functional redundancy between Hsp70 isoforms we employed a chemical biology approach. Hsp70 is a weak ATPase regulated by several sets of cellular cofactors (Fig. 1E) (Hartl et al., 2011). DnaJ-like proteins contain a J-domain that binds Hsp70 and promotes ATP hydrolysis, stabilizing the substrate-Hsp70 complex. Many DnaJ homologues also have domains that help deliver substrate proteins to Hsp70. Nucleotide exchange factors (NEFs) promote the release of Hsp70-bound substrates. Chemical modulators that interfere with these enzymatic activities and protein-protein interactions have been developed (Assimon et al., 2013). One class of molecules, exemplified by VER155008, compete with ATP for Hsp70 binding (Williamson et al., 2009), while MKT077 and its analogs target Hsp70-NEF interactions (Fig. 1E) (Assimon et al., 2013; Li et al., 2015; Li et al., 2013). We used both VER155008 and MKT077, and two improved MKT077 analogs, JG18 and JG40 (Fig. 1E). Of note, MKT077 analogs act similarly to the Hsp70 mutant K71A, since both stabilize Hsp70-substrate interactions by blocking NEF binding (Wang et al., 2013). The binding site of MKT077 analogs is known and conserved across eukaryotes and all Hsp70 isoforms (Assimon et al., 2013), thus the compounds function in both natural DENV hosts, humans and mosquitoes (Fig. 1F-H). Indeed, JG18, JG40 and VER155008, dose-dependently blocked DENV propagation in human (Huh7) cells (Fig. 1G), while MKT077, JG18 and JG40 suppressed DENV in mosquito (C6/36) cells (Fig. 1H). The fact that these chemically distinct compounds block DENV propagation is strong evidence for a role for Hsp70 in DENV replication. Furthermore, the structurally similar but inactive JG18 and JG40 derivatives JG19 and JG28 did not suppress DENV propagation (Fig. S1F). Thus, both genetic (Hsc70 K71A) and chemical (JG40, JG18) disruption of Hsp70-NEF function inhibited DENV replication, demonstrating the selectivity of the compounds and supporting a role for Hsp70 in DENV replication.
We next focused on the second-generation, metabolically stable MKT077 analogs, JG18 and JG40 (Li et al., 2013). A concern with Hsp70 inhibitors is that they may block essential chaperone functions, leading to host cell toxicity. However, allosteric inhibitors of the Hsp70-NEF complex are surprisingly non-toxic in normal fibroblasts and animals (Assimon et al., 2013; Li et al., 2015; Li et al., 2013), likely because they leave other chaperone functions intact. Consistent with the idea that viral infection creates a hyper-dependence on distinct chaperone activities compared to host proteostasis, neither JG18 nor JG40 was toxic to host cells at concentrations that inhibit viral replication, as measured by the complementary MTT and LDH release assays (Fig. S1G).
Hsp70 acts at multiple steps in the DENV infectious cycle
The activity of Hsp70 inhibitors on DENV replication was comparable to a previously described NS5 polymerase inhibitor, 2′C-methyladenosine (2′CMA; Fig. S2A-E). Hsp70 inhibitors and 2′CMA suppressed vRNA synthesis (Fig. S2A, S2B) and viral protein expression (vProtein; Fig. S2C) in human and mosquito cells. To dissect which steps in the viral life cycle require Hsp70 in human cells, we performed an order-of-addition experiment using JG40, 2′CMA and the entry inhibitor heparin (HP). As expected, HP only blocked vRNA replication and viral production (Fig. 2A, series I-IV) when added prior to or concurrently with DENV infection (Fig. 2A, series V). In contrast, 2′CMA was effective only when added concurrently with or after infection (Fig. 2A, series III-V). JG40 inhibits DENV production and vRNA replication in any of these treatment regimes, indicating that Hsp70 is required both at entry and for post-entry steps (Fig. 2A, series I-V). To bypass viral entry, we directly electroporated in vitro transcribed genomic vRNA into Huh7 cells in the presence of these various compounds. As expected, HP no longer inhibited viral replication, but both 2′CMA and JG40 inhibited vRNA production to a similar extent (Fig. 2B). Thus, Hsp70 is required for both entry and post-entry steps of the viral life cycle.
Figure 2. Hsp70s facilitate multiple steps in the DENV viral life cycle.
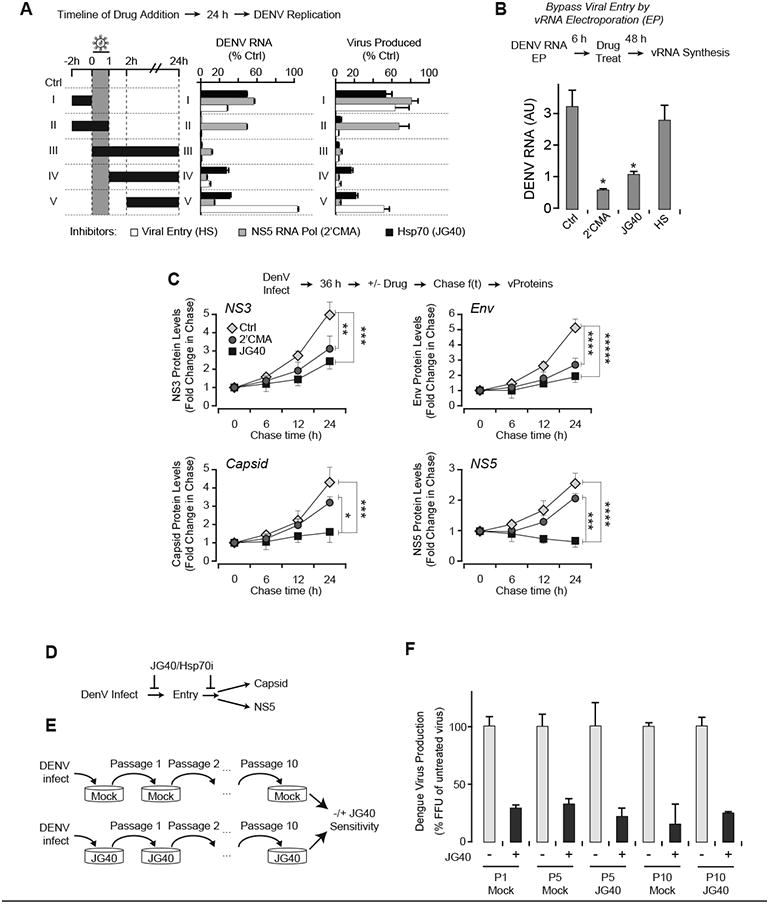
(A) Hsp70 inhibition blocks viral entry and post-entry steps. Time course of drug-addition experiment vis-a-vis DENV infection compared JG40 (Hsp70i); entry inhibitor heparin (HP) and NS5 polymerase inhibitor (2′CMA). Huh7 cells infected with DENV2 at MOI 0.5. Intracellular DENV RNA and extracellular viral production: measured by qRT-PCR and FFA respectively.
(B) JG40-treatment suppresses DENV replication post-entry. DENV RNA electroporated into Huh7 cells and inhibitors added 6 hpi. After 48 h, intracellular vRNA was quantified by qRT-PCR.
(C) Hsp70 inhibition decreases DENV capsid and NS5 protein levels. Huh7 cells infected with DENV2 at MOI 0.5 for 36 h. Inhibitors were then added and cells harvested as indicated. The levels of viral and host proteins during treatment were assessed by immunoblot using IR fluorescence on a Li-Cor Odyssey System (see Fig. S2F-S2G). (D) Hsp70 functions at different steps in the DENV lifecycle suggesting low chances of viral resistance to the drug.
(E) Serial passaging of DENV in JG40 to test development of drug resistance. At each passage, Huh7 cells were infected with DENV2 at an MOI of 0.5 in the presence or absence of JG40. Virus produced in each passage served as input in the next passage in the absence (Mock) or presence (JG40) of drug. (F) DENV remains JG40-sensitive following serial passage in the presence of JG40. JG40 sensitivity for the indicated virus passages obtained as above. Extracellular virus produced in the presence or absence of JG40 was quantified by FFU assay 36 hpi. All data is expressed as mean ± SD of three independent experiments.
* P < 0.05, ** P < 0.01, *** P < 0.005, **** P < 0.001, ***** P < 0.0005.
A drug-chase experiment in infected cells next examined whether Hsp70 inhibitors specifically affect individual DENV proteins. Huh7 cells were infected with DENV for 36 hours; then either 2′CMA, JG40 or vehicle were added and the level of viral RNA and proteins examined during a 24 h time-course. Both 2′CMA and JG40 blocked vRNA production to a similar degree and no new vRNA was synthesized during the chase (Fig. S2A, 4F). Both drugs also decreased the expression of cytosolic and ER viral proteins (Fig. 2C, S2F-G; see Experimental Procedures). All DENV proteins are translated in an equimolar ratio as a single polyprotein, but their steady state levels are also determined by their half-life. For a given protein, the ratio between 2′CMA and JG40 treatment should reveal which proteins are further destabilized by Hsp70 inhibition, a hallmark of an Hsp70 substrate. The levels of cytosolic NS3 and membrane-bound E, prM, NS2B and NS4B all decreased to a similar extent upon 2′CMA or JG40 treatment (Fig. 2C, S2G). In contrast, JG40 disproportionately reduced the levels of NS5 and capsid (Fig. 2C, S2G). This is not due to positional effects on translation, since the capsid is the most N-terminal protein in the polyprotein and NS5 the most C-terminal. Thus, Hsp70 is required for folding and/or stabilization of capsid and NS5, suggesting these proteins are Hsp70 substrates (Fig. 2D).
Figure 4. Hsp70 interacts with capsid and participates in virion production.
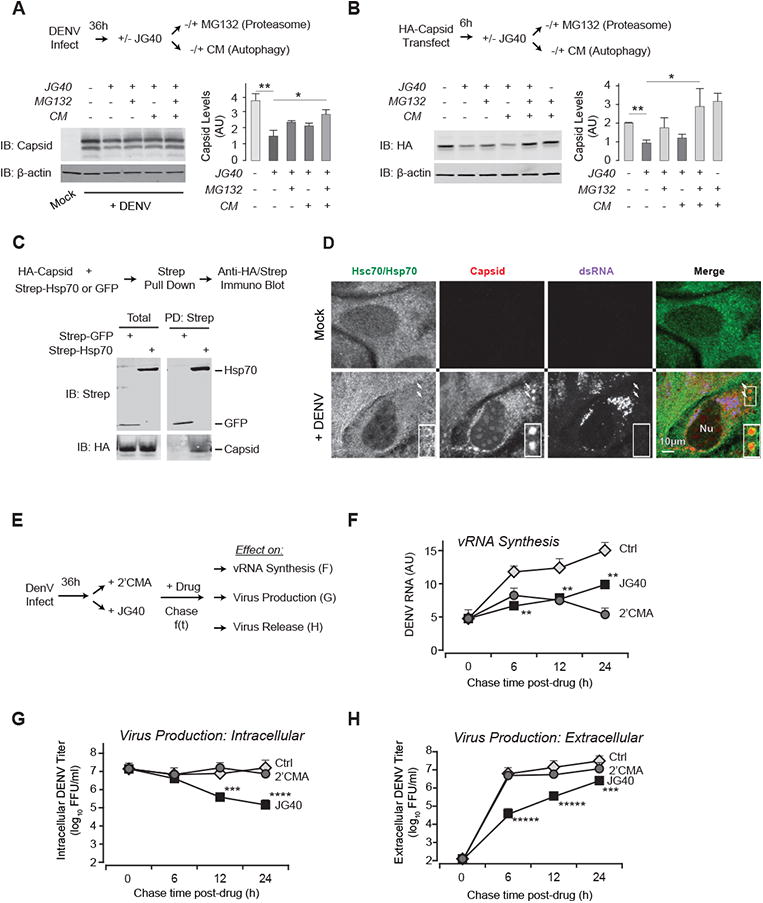
(A-B) JG40 treatment leads to capsid degradation through proteasome- and autophagy-dependent pathways. (A) Huh7 cells were infected with DENV2 at MOI 0.5. At 36 hpi, JG40 was added with or without proteasome inhibitor MG132 and/or lysosomal inhibitor CM for an additional 12 h. Immunoblot analysis (left panel) and quantification (right panel) show that the JG40-induced decrease in NS5 is rescued by MG132.
(B) 293T cells expressing HA-capsid were treated with JG40 with or without MG132 and/or CM for 36 h and analyzed as in (A).
(C) Hsp70 physically associates with capsid. Strep-tagged Hsp70 or a GFP control were expressed together with HA-tagged capsid in 293T cells, and their association assessed by coimmunoprecipitation and immunoblot.
(D) Hsp70 colocalizes with capsid in infected cells. Huh7 cells infected with DENV2 were immunostained for Hsc70/Hsp70 (green), dsRNA (blue) and capsid (red) 48 hpi. Insets: ring-shaped structures formed by capsid due to its association with LD and vesicles. Scale bar, 10 μm
(E-H) Hsp70 is required for infectious viral particle production. (E) Scheme of drug-chase experiment comparing the effect of the NS5 inhibitor (2′CMA) and the Hsp70i JG40 on vRNA production and virion production. Huh7 cells infected with DENV2 at MOI 0.5 were treated at 36 hpi with 3 μM 2′CMA or 3 μM JG40 and levels of intracellular DENV RNA was measured by qRT-PCR (F); intra- and extracellular virion levels (G and H) were measured by FFA. Data are expressed as mean ± SD of three independent experiments.
* P < 0.05, ** P < 0.01, *** P < 0.005, **** P < 0.001, ***** P < 0.0005.
Dengue virus cannot develop resistance to small molecule inhibitors of Hsp70
The mutational plasticity of viruses allows them to escape from most antiviral drugs targeting viral and even host factors (Dowd et al., 2014; Lauring et al., 2013). The spread of resistant viruses then renders drugs completely or partially ineffective. To determine if DENV can escape treatment with Hsp70 inhibitors, we serially passaged DENV in the presence of 2′CMA (Fig. S2D) or 3 μM JG40 (Fig. 1G). At each passage, the drug-sensitivity of the untreated and compound-passaged virus was tested in the presence of JG40 or 2′CMA (Fig. 2E, S2H). Even after ten passages in the presence of JG40, the virus remained as sensitive to JG40 as untreated or parental virus (Fig. 2F) while virus passaged in 2′CMA developed significant resistance against 2′CMA by passage 10 (Fig. S2H). Similar results were obtained in at least three independent biological replicates (not shown). These results, together with the low toxicity of JG40 in human cells, suggest Hsp70 inhibitors could provide an antiviral treatment against DENV replication that exhibit reduced emergence of drug resistant variants.
Cytosolic Hsp70 is required for NS5 stability and its polymerase function
To examine whether the observed decrease in NS5 upon Hsp70 inhibition is due to proteasomal degradation, cells infected with DENV for 36 h were treated with JG40 for an additional 12 h with or without the proteasome inhibitor MG132 (Fig. 3A). Indeed, the JG40-induced reduction in NS5 was abrogated by proteasome inhibition (Fig. 3A). Furthermore, Hsp70 appears to act directly on NS5 in the absence of other viral proteins, as transfected HA-NS5 was also degraded upon addition of JG40 in a proteasome-dependent manner (Fig. 3B). NS5 also co-immunoprecipitated with Hsp70 (Fig. 3C). Even though MG132 abrogated the JG40-induced NS5 reduction (Fig. 3A, B), restoring NS5 levels did not rescue vRNA synthesis or virus production (Fig. 3D). Since MG132 does not block DENV replication under the conditions of the experiment, it appears that Hsp70 is not only required for NS5 stability, but also for the acquisition of its folded, functional state. To test this, we isolated active crude replication complexes (RCs) from DENV-infected cells. These RCs produce vRNA in an NS5-dependent manner (Fig. 3E), allowing us to examine the effect of JG40 on the activity of NS5 that has already folded and assembled into the large multi-component replication complex. α-amanitin was added to inhibit host RNA polymerase, and DENV vRNA synthesis monitored by incorporation of radioactive 32P-nucleotides into dsRNA duplexes (replicative form; RF) and replication intermediates (RI) (Fig. 3F). No radioactive species were detected using uninfected cells (no DENV, Fig. 3F ii), confirming their dependence on DENV RCs. As expected, the NS5 inhibitor 2′CMA blocked production of vRNA species (Fig. 3F i). Of note, JG40 also caused a reduction in vRNA synthesis (Fig. 3F i), and incubating the RCs with anti-Hsp70 antibodies blocked vRNA production in a concentration-dependent manner (Fig. 3F ii). We conclude that the large 100 kDa protein NS5 is a direct substrate of Hsp70, which is required for both stabilizing NS5 and for continued NS5 activity after replication complexes assemble.
Figure 3. Hsp70 required for polymerase NS5 biogenesis and function.
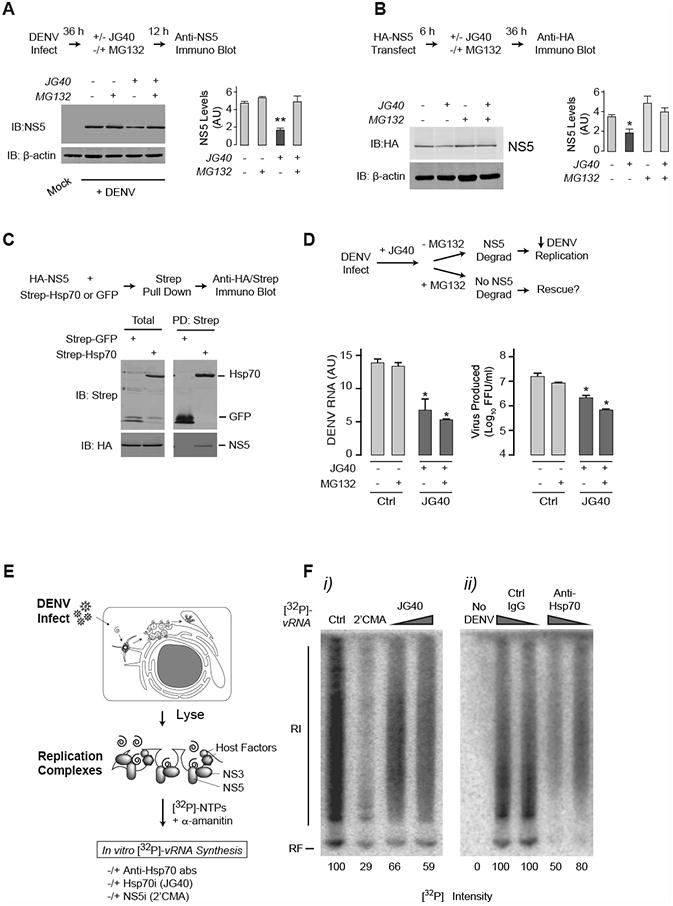
(A) Hsp70 inhibition leads to proteasomal degradation of NS5. Huh7 cells were infected with DENV2 at MOI 0.5. At 36 hpi, JG40 was added with or without proteasome inhibitor MG132 for an additional 12 h. Immunoblot analysis (left panel) and quantification (right panel) show that JG40-induced NS5 decrease is rescued by MG132.
(B) Hsp70 inhibition directly leads to proteasomal degradation of NS5. 293T cells transfected with HA-NS5 were treated with JG40 with or without proteasome inhibitor MG132 for 36 h and analyzed as in (A).
(C) Hsp70 physically associates with NS5. Strep-tagged Hsp70 or a GFP control were expressed together with HA-tagged NS5 in 293T cells, and their association assessed by coimmunoprecipitation and immunoblot. Total: represents 20% of the IP input.
(D) Rescue of NS5 levels by MG132 is not enough to restore DENV RNA replication and viral progeny production. NS5 degradation was blocked as in Fig. 3A, and levels of intracellular viral RNA and extracellular virus production measured by qRT-PCR (left panel) and FFA (right panel) respectively.
(A, B and C) Data is expressed as mean ± SD of three independent experiments. * P < 0.05. ** P < 0.01
(E) In vitro RNA replication assay to test the effect of Hsp70 inhibition on activity of preassembled, folded DENV replication complexes. Crude replication complexes were harvested from infected cells 48 hpi, and processed as described.
(F) Hsp70 is required for NS5 polymerase activity. [32P]-vRNA synthesized in vitro in isolated replication complexes in the presence of NS5 or Hsp70 inhibitors (i) or following addition of control or anti-Hsp70 antibody (ii). No vRNA is synthesized in extracts from uninfected cells. The data shown are representative of three independent experiments.
Hsp70 associates with capsid protein and promotes virus production
A parallel analysis of the role of Hsp70 in capsid stability showed MG132 did not fully restore the drop in capsid levels observed with JG40 (Fig. 4A). Since capsid associates with LDs (Samsa et al., 2009) and vesicles, we considered lysosomal degradation as an alternative degradation route. Despite the confounding fact that the lysosome inhibitor concanamycin A (CM) reduces overall DENV replication (not shown), co-incubation of JG40 with CM did partially restore capsid levels. We also observed an additive effect of blocking both proteasomal and lysosomal degradation (Fig. 4A). JG40 also reduced levels of transfected HA-capsid, circumventing the effect of these inhibitors on DENV replication (Fig. 4B). This reduction was partially abrogated by independent treatment with MG132 and CM, and additively blocked when both pathways were inhibited (Fig. 4B). Thus, JG40-induces capsid degradation via proteasomal and lysosomal routes.
Hsp70 also associated with capsid in co-immunoprecipitation experiments (Fig. 4C). Immunofluorescence analysis demonstrated that, as reported (Balinsky et al., 2013; Samsa et al., 2009), some of the capsid pool is directed to nucleoli, and in the cytoplasm capsid associates with LDs and nearby vesicles, forming puncta and ring-like structures distinct from sites containing viral dsRNA (Fig. 4D, S3). We therefore questioned whether Hsp70 is required for the assembly of viral particles. 2′CMA or JG40 were used to inhibit vRNA production 36 hpi to compare the effect of the drugs on encapsidation of preexisting vRNA (Fig. 4E). Both inhibitors rapidly blocked vRNA synthesis to a similar extent (Fig. 4F) but had very different effects on the accumulation of intracellular (Fig. 4G) and extracellular infectious virus (Fig. 4H). Cells treated with 2′CMA continue to produce viral particles and secrete them into the medium for at least 24 h post-treatment. This suggests that by 36 hpi, DENV-infected cells contain sufficient vRNA and proteins to support the assembly of new virus particles. In contrast, JG40 treatment did reduce intracellular and extracellular virus accumulation, suggesting Hsp70 plays an additional role in virion biogenesis.
Selected J-domain proteins are required for DENV replication
How and why chaperones select their substrates in vivo is an important and poorly understood question. Substrate selection and stable binding to Hsp70 is thought to rely on DNAJ proteins (Fig. 5A), which in humans constitute the largest and most diverse sub-group of chaperones. All DNAJs contain a J-domain and additional domains confer distinct subcellular localization and recruitment to specific complexes. Depending on their additional domains DNAJs are classified into three types: Type I, also called DNAJA; Type II, or DNAJB; and Type III, or DNAJC (Kampinga and Craig, 2010). The diversification of J-domain proteins likely underlies the multifaceted regulation of Hsp70, however, few examples exist of the division of labor among different DnaJs.
Figure 5. A subset of human DNAJ cochaperones is required for the DENV life cycle.
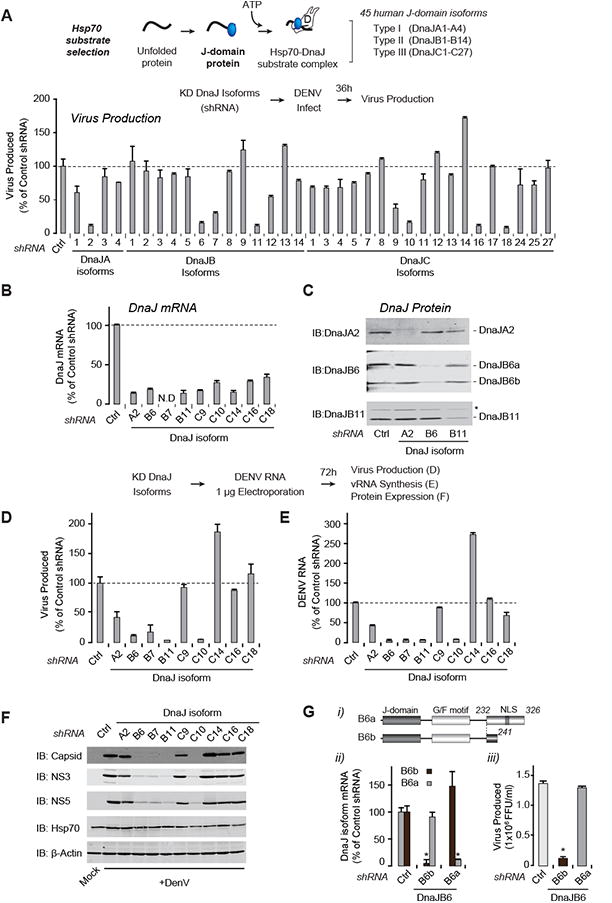
(A) Screen for human DNAJ involvement in DENV replication. Forty-five human DNAJs were tested for effect on DENV by KD using three different shRNAs. Indicated DNAJ-KD cells were established as for Hsp70s in Fig. 1, and infected with DENV2 for 36 h. Extracellular virus production was measured by FFA. Ctrl: shRNA against luciferase.
(B-C) Validation of KD of different DNAJ isoforms by assessing mRNA levels (B) and DNAJ protein (C).
(D-F) Identification of DNAJs involved in post-entry stages of the DENV life cycle. DENV vRNA was electroporated into DNAJ-KD cells. Extracellular viral infectivity (D); intracellular viral RNA (E) and viral proteins (F) were measured after 72 h, by FFA, qRT-PCR and immunoblotting respectively.
(G) Isoform DnaJB6b is required for DENV propagation. (i) Splicing creates DnaJB6 variants. KD cells were established for each variant, and infected with DENV2 for 36 h. The levels of each variant transcript (ii) and extracellular viral production (iii) were measured by qRT-PCR with variant-specific primers and FFA respectively. Data are representative of three independent experiments and expressed as mean ± SD of triplicates. * P < 0.01.
To test if the distinct function of Hsp70 in the DENV life cycle is driven by different DnaJ proteins, we conducted a comprehensive screen using two or three shRNAs per J-domain protein in human cells. Thirty-five of the KD cells were viable (Fig. 5A, S4A, S4B), and KD of DNAJs affecting DENV replication was confirmed by qRT-PCR and immunoblot (Fig. 5B, 5C). DENV infection was significantly reduced upon depletion of eight DNAJ proteins (Fig. 5A, S4A), and enhanced upon depletion of the known DENV restriction factor DnaJC14 (Yi et al., 2011). Following electroporation of vRNA, four of the DNAJ proteins no longer inhibited infectious virion production, suggesting they participate in viral entry (Fig. 5D-F). In contrast, five DNAJs still inhibited DENV particle production (Fig. 5D) and vRNA synthesis (Fig. 5E), which we independently confirmed using enzymatically-produced esiRNAs (Fig. S4C). The distinction between DNAJs required in entry versus post-entry steps was also confirmed by immunoblot for viral proteins following vRNA electroporation (Fig. 5F). The DNAJ proteins required for DENV replication exhibit distinct domain structures and subcellular localizations (Fig. S4D) (Kampinga and Craig, 2010). Those involved in entry include DnaJC9, reportedly nuclear-localized; as well as the membrane-anchored DnaJC16 and DnaJC18, which expose the J-domain into the cytosol. Post-entry steps require DnaJA2, DnaJB6, DnaJB7, DnaJB11, and DnaJC10. The cytosolic DnaJA2 significantly inhibited virus production and vRNA synthesis, with only a minor impact on viral proteins. The closely related DnaJA1 and DnaJA3 had little or no effect on DENV replication, indicating that related isoforms can have different substrate specificities. DnaJC10, a lumenal ER chaperone (Kampinga and Craig, 2010), may participate in folding ER-bound DENV proteins, or alternatively, host proteins required for DENV replication. Membrane-anchored DnaJB11 is also ER-localized (Kampinga and Craig, 2010). DnaJB6 exists as two alternatively spliced isoforms: DnaJ6Ba is predominantly nuclear and DnaJB6b is nuclear/cytoplasmic (Fig. 5G i). We used shRNAs to selectively KD these splice-variants (Fig. 5G ii, S4E). DENV RNA replication, protein expression and virion production were all significantly reduced in DnaJB6b KD cells but unaffected by DnaJB6a KD (Fig 5G iii, S4E-F). Thus, closely related DnaJs, and even splice-variants, have divergent specificities and functions. We conclude that the distributed action of DNAJ cofactors localized in distinct cellular compartments orchestrates the many processes leading to productive DENV infection.
Distinct roles of DnaJB11 and DnaJB6b in DENV replication
We next focused on DnaJB11 and DnaJB6b, since their KD resulted in strong post-entry effects. To distinguish between a role in vRNA synthesis and viral particle assembly we electroporated an excess of vRNA into cells depleted of DnaJB6b or DnaJB11. This incoming vRNA should provide enough template for viral protein synthesis and biogenesis, reducing the requirement for vRNA synthesis (Fig. 6A). Depletion of DnaJB6b and DnaJB11 resulted in comparable reductions in vProtein (Fig. 6A i) and vRNA (Fig. 6A ii). However, viral particle production was virtually unaffected in DnaJB11-depleted cells, suggesting that significant reductions in vRNA and vProtein levels can have only modest effects on the output of viral particles. In contrast, DnaJB6b depletion significantly reduced infectious particle release (Fig. 6A iii). Therefore, DnaJB6b participates in viral particle biogenesis, while DnaJB11 is not required if sufficient vRNA and vProteins are present.
Figure 6. Spatial and functional diversity of DNAJs in DENV propagation(A).
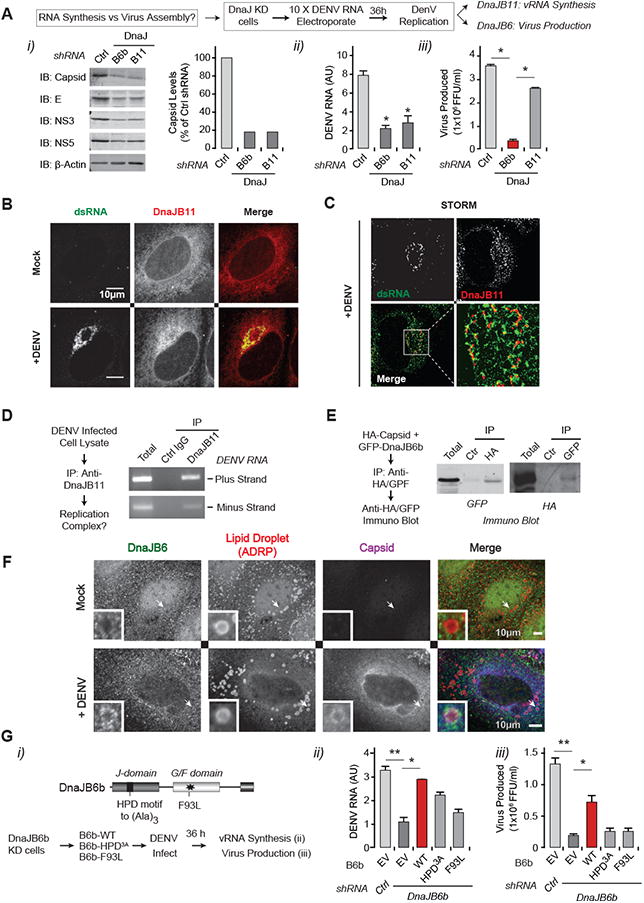
Introduction of excess DENV RNA by electroporation rescues DENV production in DnaJB11-KD, but not in DnaJB6-KD cells. Intracellular proteins (i), viral RNA (ii), and extracellular viral infectivity (iii) were measured by immunoblot, qRT-PCR and FFA respectively at 36 hpi. * P < 0.05
(B-C) DnaJB11 relocalizes to DENV replication sites. Uninfected Huh7 cells or cells infected with DENV2 for 48 h were immunostained for DnaJB11 (red) and dsRNA (green) and imaged using confocal microscopy (B) and super-resolution microscopy by STORM (C). Scale bar, 10 μm
(D) DnaJB11 physically associates with DENV vRNA. DENV-infected cell lysates were subjected to immunoprecipitation with DnaJB11 antibodies. vRNA was extracted and positive-strand and negative strand detected by RT-PCR.
(E) DnaJB6b associates with capsid in the absence of infection. GFP-tagged DnaJB6b or GFP were overexpressed together with HA-tagged capsid in 293T cells and association measured by immunoprecipitation and immunoblot.
(F) DnaJB6 colocalizes with capsid in infected cells. Uninfected Huh7 cells or cells infected with DENV2 for 48 h were immunostained for DnaJB6 (green), ADRP (a marker of lipid droplets; red) and capsid (blue). Inset: magnification of capsid containing cellular structure. Scale bar, 10 μm
(G) Wild Type DnaJB6b, but not its mutants can rescue the reduced DENV propagation in DnaJB6b KD cells. (i) DnaJB6b domain structure and position of HDP-AAA mutation, disrupting Hsp70 binding, and disease associated F39L mutation. DnaJB6b or its mutants were transfected into KD cells 48 h prior to infection with DENV2 at MOI 0.5. At 36 hpi, intracellular viral RNA (ii) and extracellular virus production (iii) were measured as described. Data are representative of three independent experiments and expressed as mean ± SD of triplicate. * P < 0.05, ** P < 0.01.
The observed reduction in viral infectivity upon DnaJB6 KD could be due to reduced particle production or impaired particle maturation. These possibilities make distinct predictions as to how DnaJB6 KD affects the ratio of infectious versus total virions (Fig. S5A). To address this, we quantified vRNA genomes from equivalent amounts of focus forming units (FFUs) from the supernatants of infected DnaJB6 KD or control cells. A reduction in infectivity would predict that more viral particles, i.e. more RNA genomes, will be required for the same number of FFU in DnaJB6 KD cells. This was not the case (Fig. S5A), confirming that DnaJB6 is required for viral particle production itself.
Immunofluorescence analysis using confocal microscopy and super-resolution STochastic Optical Reconstruction Microscopy (STORM) (Fig. 6B, 6C, S5B) indicated that DnaJB11 is distributed throughout the cell, colocalizing with the ER, in uninfected cells. Upon infection, DnaJB11 was additionally enriched in dsRNA-containing DENV replication complexes (Fig. 6B, S5B, Fig. 6C). Immunoprecipitation of DnaJB11 followed by RT-PCR analysis (Fig. 6D) confirmed that both positive and negative vRNA strands associate with DnaJB11, probably in DENV replication complexes.
Since DnaJB6b is cytosolic and facilitates viral particle biogenesis, we tested its association with capsid, the cytosolic component of virions. DnaJB6b co-immunoprecipitated with transfected HA-capsid (Fig. 6E). In DENV-infected cells, capsid was distributed throughout the cell and displayed a previously-described association with LDs (white arrow, shown in inset) and other vesicular structures (Fig. 6F) (Samsa et al., 2009). While DnaJB6 localized to the cytosol and nucleus in uninfected cells (Fig. 6F, S5C), DnaJB6 colocalized with capsid on vesicular structures and on the surface of LDs after infection, and the proportion of nuclear DnaJB6 was slightly reduced. These results suggest that DnaJB6 associates with capsid to facilitate viral particle biogenesis. To test whether DnaJB6b acts only through Hsp70, we employed two mutations known to disrupt different aspects of DnaJB6b activity (Fig. 6G i). The Hsp70 recruitment ability of DNAJB6 was disrupted by replacing its J-domain HPD motif with an AAA sequence. DnaJB6 is mutated in inherited myofibrillar myopathy and limb-girdle muscular dystrophy (Stein et al., 2014), and we also tested the effect of a disease-linked DnaJB6b mutation on DENV replication (Fig. 6G, S5D, F93L). In DnaJ-depleted cells, only wild-type (WT) DnaJB6b fully restored viral production (Fig. 6G i-iii, S5D). However, the HPD-AAA mutation, but not the F93L mutation, had a modest effect in restoring vRNA synthesis (Fig. 6G ii). Thus, the interaction of DnaJB6b with Hsp70 is important to promote viral particle biogenesis, though DnaJB6b may also possess intrinsic chaperone activity.
JG40 inhibits the replication of divergent flaviviruses in human dendritic cells
Primary human monocyte-derived dendritic cells (MDDCs) are an established ex vivo model system for DENV (Fig. 7A) (Aguirre et al., 2012; Rodriguez-Madoz et al., 2010), allowing us to examine the therapeutic potential of Hsp70 inhibitors (Fig. 7, S6). JG40 dose-dependently inhibited DENV2 replication, to undetectable levels at 5 μM (Fig. 7C), but did not reduce MDDC viability at these concentrations (Fig. 7B). JG40 also reduced vRNA accumulation (Fig. S6A). JG40's higher potency in primary MDDCs compared to Huh7 cells is interesting, since transformed cells have disregulated chaperone activity, making primary cells more responsive to chaperone inhibition (Trepel et al., 2010). The therapeutic window for antiviral use of chaperone modulators is also greater in primary cells (Geller et al., 2013; Geller et al., 2007). JG40 also inhibited DENV4, the most divergent of the four DENV serotypes (Fig. 7C, S6A). We next examined the antiviral effects of JG40 on the flaviviruses WNV (strain Kunjin; KUNV), YFV, and the TBEV model Langat virus (LGTV). All three viruses were dose-dependently inhibited by JG40 (Fig. 7D, S6A, S6B), and in all cases infectious virus was undetectable with 5 μM JG40 (Fig. 7D). JG40 is therefore a broad spectrum antiviral active against distantly related flaviviruses, many of which have limited treatment options.
Figure 7. JG40 inhibits the replication of DENV and other flaviviruses in primary human MDDCs and modulates cytokine induction during DENV infection.
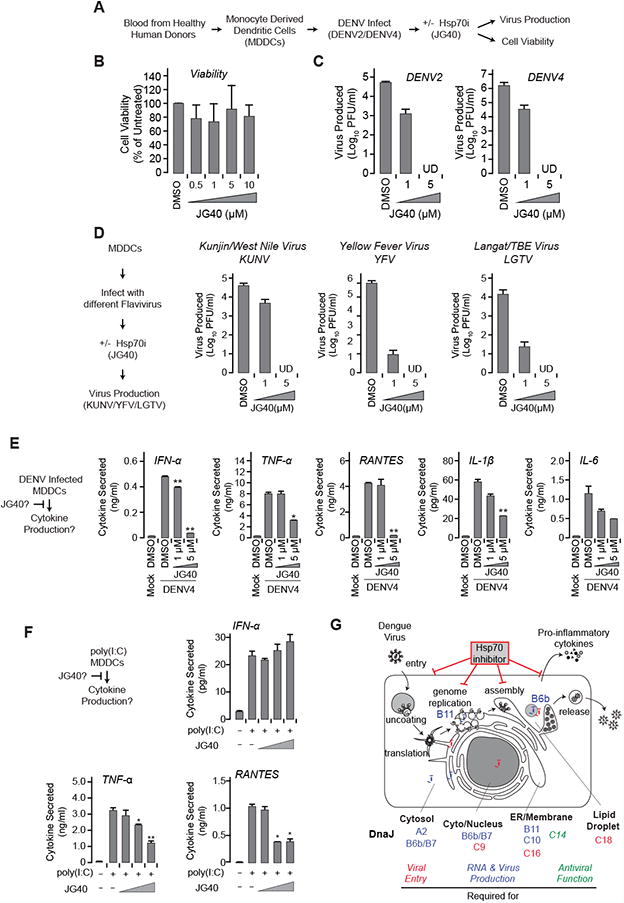
(A-C) JG40 potently inhibits different DENV serotypes at concentrations without toxicity to the host cell. (A) Effects of JG40 in primary human MDDCs at 36 h post-treatment. (B) Host toxicity of JG40 was determined by LDH release into the culture supernatant. Differences are not statistically significant. (C) MDDCs infected with DENV2 or DENV4 at MOI 0.5 and subsequently treated with JG40 for 36 h. The level of extracellular viral infectivity was measured by plaque assay. UD, undetectable. Data are the mean of three independent donors, expressed as mean ± SD.
(D) Broad Spectrum action of JG40 inhibiting the replication of diverse flaviviruses. MDDCs were infected with KUNV, YFV or LGTV at MOI 0.5 and subsequently treated with JG40 for 36 h. The level of extracellular viral infectivity was measured by plaque assay. UD, undetectable. Data are the mean of three independent donors, expressed as mean ± SD.
(E) JG40 can suppress cytokine induction during DENV infection. Human MDDCs were infected with DENV4 (MOI 0.5) and subsequently treated with JG40 for 36 h. Cytokine levels in the culture supernatant were measured by ELISA. * P < 0.05, ** P < 0.01. One representative donor of three shown.
(F) JG40 directly affects the production or secretion of a subset of cytokines. Human MDDCs were treated with increasing amounts (0, 1, 5 or 10 μM) of JG40, and subsequently transfected with 5 μg/ml poly(I:C). Cytokine induction was measured by ELISA. * P < 0.05, ** P < 0.01 (one-tailed Student's t-test with Bonferroni correction). One representative donor of three shown.
(G) Role of the Hsp70/DNAJ network at various steps in the DENV life cycle, highlighting the therapeutic potential of Hsp70 inhibitors (see text for details).
Effects of JG40 on cytokine and chemokine induction in DENV-infected MDDCs
An ideal dengue therapy should attenuate the overproduction of cytokines and chemokines associated with DHF/DSS as well as viral replication. We therefore measured the effect of JG40 on relevant cytokines and chemokines produced during DENV2 or DENV4 infection in MDDCs (Fig. 7E, S6C). The pro-inflammatory cytokines TNF-α, IL-6 and IL-1β induce fever and may cause vascular permeability during DENV infection; the chemokine RANTES is involved in leukocyte recruitment, and type I interferon (IFN) is involved in viral clearance but also contributes to the ‘flu-like’ symptoms of dengue disease (Charo and Ransohoff, 2006; Rothman, 2011). As we showed previously, DENV2 does not induce type I IFN, and transiently induces proinflammatory cytokines and chemokines (Fig. S6C) (Aguirre et al., 2012; Rodriguez-Madoz et al., 2010a). In contrast, DENV4 induced higher levels of chemokines and proinflammatory cytokines (Fig. 7E, S6C), perhaps due to intrinsic viral differences or due to higher replication levels (Fig. 7C). JG40 dose-dependently reduced the induction of IFN-α, TNF-α, RANTES, IL-1 β and IL-6 in DENV4-infected MDDCs (Fig. 7E). However, macrophage inflammatory protein 1β (MIP-1β), involved in leukocyte recruitment (Charo and Ransohoff, 2006), was unaffected (Fig. S6C), indicating that JG40 does not grossly alter the ability of DCs to induce and secrete proteins. In principle, the effects of JG40 on cytokine and chemokine production may stem from the inhibitor's effects on viral replication, and the resultant reduction in immunostimulatory pathogen-associated molecular patterns (PAMPs). However, when we directly stimulated MDDCs with the dsRNA mimic poly(I:C) without viral infection, JG40 still reduced TNF-α and RANTES induction, while IFN- α was unaffected (Fig. 7F, S6D). Thus, JG40 directly modulates the production of a subset of cytokines independently of its effects on viral replication.
Discussion
Due to their small genomes, RNA viruses rely on the host machinery to support their life cycle, providing a window into fundamental cellular processes. Here we examined the role of the complex Hsp70 chaperone network, central to protein homeostasis, in DENV replication. We find that distinct Hsp70 isoforms and their DnaJ cofactors orchestrate the proteostatic control of specific steps in the DENV life cycle. Distinct steps in DENV replication require different DNAJ Hsp70-cofactors localized to distinct cellular compartments. Our data illustrate how a non-specific chaperone like Hsp70 acquires functional and subcellular specificity through the action of DNAJ cofactors. Furthermore, compounds targeting Hsp70 appear to be promising antivirals, for several reasons. Firstly, due to the dependence on Hsp70 at various steps of the viral life cycle, DENV cannot escape these drugs. Secondly, because these compounds allosterically modulate Hsp70 rather than fully block its activity, they exhibit negligible toxicity at concentrations that completely block virus production. This favorable therapeutic window likely arises from the chaperone-hyperdependence of the virus. Most excitingly, these compounds are effective against different DENV serotypes and diverse flaviviruses. Importantly, they also decrease the virally-induced release of inflammatory cytokines that contribute to severe dengue disease. The effectiveness of antivirals is severely limited by the emergence and spread of resistance (Lipsitch et al., 2007). However, so far no viral resistance has been observed for three unrelated viruses treated with inhibitors of two different chaperones, Hsp90 and Hsp70. This unique property of targeting viral proteostasis may close a gap in antiviral drug development.
Multiple DNAJs facilitate distinct steps of the DENV replication cycle
The Hsp70 network has become progressively diversified in evolution (Powers and Balch, 2013). Our work on one simple “organism” that uses the protein folding machinery provides insight into the division of labor and connectivity of the many DNAJs and Hsp70s in human cells. It is striking how many DNAJs are involved in viral replication. Nine DNAJs have strong effects upon depletion, while others, such as DNAJC25 or DNAJC13, have smaller but reproducible effects. As we observed strong effects with single gene depletions, there must be limited redundancy among these DNAJ isoforms. Some DNAJs, e.g. DNAJB11, were reorganized upon infection (Fig. 6) while others, e.g. DNAJA2, were not (data not shown). Future studies should clarify whether DENV actively remodels chaperone localization or whether the high levels of viral substrates concentrate their binding factors.
Our data indicate a role for Hsp70 and a subset of DNAJs in viral entry, and a direct role for Hsp70 and DNAJB11 in viral RNA synthesis and for Hsp70 and DNAJB6 in virion production. Hsp70 is not only required for folding and assembly but also for function of the active replication complex. Interestingly, DNAJB6 is important in the regulation of protein aggregation diseases (Hageman et al., 2010), suggesting this chaperone may function to regulate protein assembly processes. While these DNAJs have a direct role in viral proteostasis, at least some DNAJs may participate in host processes required by DENV.
Therapeutic potential of Hsp70 inhibitors as pan-flavivirus antivirals
The Hsp70 inhibitor used here, JG40, caused over four logs reduction in DENV2 and DENV4 replication in MDDCs, with negligible toxicity to host cells. Since MDDCs closely model human physiology and disease, these reductions may more accurately reflect the dependence of DENV on the cellular protein folding machinery during human infection than cell lines. In addition, JG40 suppressed the production of proinflammatory cytokines and chemokines, suggesting that Hsp70 inhibitors might benefit patients both by directly inhibiting DENV replication, and by reducing the production of cytokines contributing to severe dengue disease. The comparison of JG40 with the specific NS5 inhibitor 2′CMA is informative for designing antiviral strategies. While 2′CMA is more specific and potent in inhibiting vRNA production, the infected cell makes sufficient precursors to continue new virus production long after inhibitor addition. In contrast, JG40 simultaneously inhibits replication of viral RNA and packaging of viral particles, increasing its effectiveness. One exciting finding is that JG40 is effective against a diverse set of mosquito- and tick-borne flaviviruses that cause severe human disease (Fig. 7, S6). Our experimental design in primary human cells only measured post-entry effects, thus these compounds might be even more effective in vivo as viral entry would additionally be targeted. Future research should further compare the chaperone requirements of different flaviviruses at all stages of their life cycles.
Hsp70 inhibitors may represent a novel class of broadly-acting antivirals to treat diverse flavivirus infections for which there are no specific approved treatments. Considering their pan-flavivirus specificity alongside the lack of observed resistance, such compounds could become an important tool for reducing the worldwide human disease burden caused by these diverse viruses.
Summary of Experimental Procedures
Infectious cDNA clone pD2/IC-30P-A of DENV2 (strain 16681) [from CDC (Butrapet et al., 2000)] was used to in vitro transcribe vRNA that was electroporated into Huh7 cells to produce infectious DENV2. Kunjin virus (KUNV) and Langat virus (LGTV) were kindly provided by Dr. Jean Lim (Icahn School of Medicine at Mount Sinai, NY USA). DENV and yellow fever virus (YFV) stocks were prepared in Huh7 and C6/36 cells; KUNV and LGTV stocks were prepared on Vero cells. Virus titrations were carried out using the methylcellulose overlay method as described in Extended Experimental Procedures.
Cells were maintained at 37°C (Huh7) or 32°C (C6/36). MDDCs were generated from healthy human blood donors (New York Blood Center, NY, USA) as described (Rodriguez-Madoz et al., 2010). qRT-PCR quantification of purified RNA was performed using primers specified in Table S1. Transcripts were normalized to GAPDH or Rps11 mRNA (human) or 18S rRNA (mosquito). Viral genome equivalents were quantified using +ssRNA virus-specific standards. Each experiment was independently performed at least three times and each time was performed in triplicate.
Extracellular secreted cytokines were quantified using a customized multiplex ELISA (Millipore, Billerica, MA USA) as per manufacturer's instructions.
Plasmid transfections, immunoprecipitations, immunoblots, isolation of DENV replication complexes and in vitro DENV replication assay and other biochemical analyses were performed as described in the Extended Experimental Procedures. For immunofluorescence, cells cultured on glass slides were incubated with the relevant primary and secondary antibodies and imaged by confocal microscope and stochastic reconstruction microscopy (STORM) as described in Extended Experimental Procedures.
Statistical significance was determined using two-tailed Student's t test analysis for samples of equal variance, with sequentially rejective Bonferroni correction where appropriate. Significance levels are as stated in the figure legends.
Supplementary Material
Acknowledgments
We thank L. Nguyen for technical assistance, Drs. P. Dolan and A. Gamarnik for helpful discussions. This work was supported by a contract from DARPA Prophecy to RA, JF and A F-S; NIH grants R01AI073450 to AF-S and R01NS059690 to J.E.G; Wellcome Trust grant 096062 to KM and Fellowships from the Naito Foundation and Uehara Memorial Foundation to ST.
Footnotes
Author Contributions: ST and JF conceived the project; ST, RA and JF designed and interpreted experiments in tissue culture. ST performed all experiments in cultured cells. KM and AF-S designed and interpreted MDDC experiments; DB-R isolated and differentiated MDDCs. XL, JNR and JEG synthesized and characterized Hsp70 inhibitors. ST, KM, RA, AF-S and JF wrote the manuscript. All authors contributed to preparation of the manuscript.
Author information: The authors declare no competing financial interest.
Supplemental Information: Supplemental Information includes 1 Supplemental Table; 6 Supplemental Figures and an Extended Experimental Procedures section and can be found online at X.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, Bernal-Rubio D, Shabman RS, Simon V, Rodriguez-Madoz JR, et al. DENV Inhibits Type I IFN Production in Infected Cells by Cleaving Human STING. PLoS Pathog. 2012:e1002934. doi: 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte-Sengupta S, Sirohi D, Kuhn RJ. Coupling of replication and assembly in flaviviruses. Current opinion in virology. 2014;9:134–142. doi: 10.1016/j.coviro.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimon VA, Gillies AT, Rauch JN, Gestwicki JE. Hsp70 protein complexes as drug targets. Current pharmaceutical design. 2013;19:404–417. doi: 10.2174/138161213804143699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balinsky CA, Schmeisser H, Ganesan S, Singh K, Pierson TC, Zoon KC. Nucleolin interacts with the dengue virus capsid protein and plays a role in formation of infectious virus particles. Journal of virology. 2013;87:13094–13106. doi: 10.1128/JVI.00704-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butrapet S, Huang CY, Pierro DJ, Bhamarapravati N, Gubler DJ, Kinney RM. Attenuation markers of a candidate dengue type 2 vaccine virus, strain 16681 (PDK-53), are defined by mutations in the 5′ noncoding region and nonstructural proteins 1 and 3. Journal of virology. 2000;74:3011–3019. doi: 10.1128/jvi.74.7.3011-3019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. The New England Journal of Medicine. 2006:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Three decades of antiviral drugs. Nat Rev Drug Discov. 2007;6:941–941. [Google Scholar]
- Dowd KA, Mukherjee S, Kuhn RJ, Pierson TC. Combined effects of the structural heterogeneity and dynamics of flaviviruses on antibody recognition. Journal of virology. 2014;88:11726–11737. doi: 10.1128/JVI.01140-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller R, Andino R, Frydman J. Hsp90 inhibitors exhibit resistance-free antiviral activity against respiratory syncytial virus. PloS one. 2013;8:e56762. doi: 10.1371/journal.pone.0056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller R, Taguwa S, Frydman J. Broad action of Hsp90 as a host chaperone required for viral replication. Biochimica et biophysica acta. 2012;1823:698–706. doi: 10.1016/j.bbamcr.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller R, Vignuzzi M, Andino R, Frydman J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes & development. 2007;21:195–205. doi: 10.1101/gad.1505307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clinical microbiology reviews. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J, Rujano MA, van Waarde MA, Kakkar V, Dirks RP, Govorukhina N, Oosterveld-Hut HM, Lubsen NH, Kampinga HH. A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Molecular cell. 2010;37:355–369. doi: 10.1016/j.molcel.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- Hannemann H, Sung PY, Chiu HC, Yousuf A, Bird J, Lim SP, Davidson AD. Serotype-specific differences in dengue virus non-structural protein 5 nuclear localization. The Journal of biological chemistry. 2013;288:22621–22635. doi: 10.1074/jbc.M113.481382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Junjhon J, Pennington JG, Edwards TJ, Perera R, Lanman J, Kuhn RJ. Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. Journal of virology. 2014;88:4687–4697. doi: 10.1128/JVI.00118-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nature reviews Molecular cell biology. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauring AS, Frydman J, Andino R. The role of mutational robustness in RNA virus evolution. Nature reviews Microbiology. 2013;11:327–336. doi: 10.1038/nrmicro3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Colvin T, Rauch JN, Acosta-Alvear D, Kampmann M, Dunyak B, Hann B, Aftab BT, Murnane M, Cho M, et al. Validation of the Hsp70-Bag3 Protein-Protein Interaction as a Potential Therapeutic Target in Cancer. Molecular cancer therapeutics. 2015 doi: 10.1158/1535-7163.MCT-14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Srinivasan SR, Connarn J, Ahmad A, Young ZT, Kabza AM, Zuiderweg ER, Sun D, Gestwicki JE. Analogs of the Allosteric Heat Shock Protein 70 (Hsp70) Inhibitor, MKT-077, as Anti-Cancer Agents. ACS medicinal chemistry letters. 2013;4 doi: 10.1021/ml400204n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SP, Wang QY, Noble CG, Chen YL, Dong H, Zou B, Yokokawa F, Nilar S, Smith P, Beer D, et al. Ten years of dengue drug discovery: progress and prospects. Antiviral research. 2013;100:500–519. doi: 10.1016/j.antiviral.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Limjindaporn T, Wongwiwat W, Noisakran S, Srisawat C, Netsawang J, Puttikhunt C, Kasinrerk W, Avirutnan P, Thiemmeca S, Sriburi R, et al. Interaction of dengue virus envelope protein with endoplasmic reticulum-resident chaperones facilitates dengue virus production. Biochemical and biophysical research communications. 2009;379:196–200. doi: 10.1016/j.bbrc.2008.12.070. [DOI] [PubMed] [Google Scholar]
- Lindenbach BD, TH, Rice CM. Flaviviridae: the viruses and their replication. Fields Virology. (5th) 2007:1101–1152. [Google Scholar]
- Lipsitch M, Cohen T, Murray M, Levin BR. Antiviral resistance and the control of pandemic influenza. PLoS medicine. 2007;4:e15. doi: 10.1371/journal.pmed.0040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP. Recruitment of Hsp70 chaperones: a crucial part of viral survival strategies. Reviews of physiology, biochemistry and pharmacology. 2005;153:1–46. doi: 10.1007/s10254-004-0025-5. [DOI] [PubMed] [Google Scholar]
- Nagy PD, Pogany J. The dependence of viral RNA replication on co-opted host factors. Nature reviews Microbiology. 2012;10:137–149. doi: 10.1038/nrmicro2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L, Tatu U. Molecular chaperones in pathogen virulence: emerging new targets for therapy. Cell host & microbe. 2008;4:519–527. doi: 10.1016/j.chom.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MC, Flaherty KM, McKay DB. Lysine 71 of the chaperone protein Hsc70 Is essential for ATP hydrolysis. The Journal of biological chemistry. 1996;271:15874–15878. doi: 10.1074/jbc.271.27.15874. [DOI] [PubMed] [Google Scholar]
- Pagni S, Fernandez-Sesma A. Evasion of the human innate immune system by dengue virus. Immunol Res. 2012:152–159. doi: 10.1007/s12026-012-8334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R, Kuhn RJ. Structural proteomics of dengue virus. Current opinion in microbiology. 2008;11:369–377. doi: 10.1016/j.mib.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers ET, Balch WE. Diversity in the origins of proteostasis networks--a driver for protein function in evolution. Nature reviews Molecular cell biology. 2013;14:237–248. doi: 10.1038/nrm3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Madoz JR, Belicha-Villanueva A, Bernal-Rubio D, Ashour J, Ayllon J, Fernandez-Sesma A. Inhibition of the type I interferon response in human dendritic cells by dengue virus infection requires a catalytically active NS2B3 complex. Journal of virology. 2010;84:9760–9774. doi: 10.1128/JVI.01051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nature reviews Immunology. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- Samsa MM, Mondotte JA, Iglesias NG, Assuncao-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS pathogens. 2009;5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MA, Diamond MS, Harris E. Dendritic cells in dengue virus infection: targets of virus replication and mediators of immunity. Frontiers in immunology. 2014;5:647. doi: 10.3389/fimmu.2014.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard DS, Undurraga EA, Betancourt-Cravioto M, Guzman MG, Halstead SB, Harris E, Mudin RN, Murray KO, Tapia-Conyer R, Gubler DJ. Approaches to refining estimates of global burden and economics of dengue. PLoS neglected tropical diseases. 2014;8:e3306. doi: 10.1371/journal.pntd.0003306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KC, Bengoechea R, Harms MB, Weihl CC, True HL. Myopathy-causing mutations in an HSP40 chaperone disrupt processing of specific client conformers. J Biol Chem. 2014;289:21120–21130. doi: 10.1074/jbc.M114.572461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nature reviews Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AM, Miyata Y, Klinedinst S, Peng HM, Chua JP, Komiyama T, Li X, Morishima Y, Merry DE, Pratt WB, et al. Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nature chemical biology. 2013;9:112–118. doi: 10.1038/nchembio.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell host & microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DS, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, Foloppe N, Francis GL, Graham CJ, Howes R, et al. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. Journal of medicinal chemistry. 2009;52:1510–1513. doi: 10.1021/jm801627a. [DOI] [PubMed] [Google Scholar]
- Ye J, Chen Z, Zhang B, Miao H, Zohaib A, Xu Q, Chen H, Cao S. Heat shock protein 70 is associated with replicase complex of Japanese encephalitis virus and positively regulates viral genome replication. PloS one. 2013;8:e75188. doi: 10.1371/journal.pone.0075188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Sperzel L, Nurnberger C, Bredenbeek PJ, Lubick KJ, Best SM, Stoyanov CT, Law LM, Yuan Z, Rice CM, et al. Identification and characterization of the host protein DNAJC14 as a broadly active flavivirus replication modulator. PLoS pathogens. 2011;7:e1001255. doi: 10.1371/journal.ppat.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


