Abstract
Background
Haematological malignancies are malignant neoplasms of the myeloid or lymphatic cell lines including leukaemia, lymphoma and myeloma. In order to manage physical and psychological aspects of the disease and its treatment, complementary therapies like yoga are coming increasingly into focus. However, the effectiveness of yoga practice for people suffering from haematological malignancies remains unclear.
Objectives
To assess the effects of yoga practice in addition to standard cancer treatment for people with haematological malignancies.
Search methods
Our search strategy included the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1950 to 4th February 2014), databases of ongoing trials (controlled‐trials.com; clinicaltrials.gov), conference proceedings of the American Society of Clinical Oncology, the American Society of Hematology, the European Haematology Association, the European Congress for Integrative Medicine, and Global Advances in Health and Medicine. We handsearched references of these studies from identified trials and relevant review articles. Two review authors independently screened the search results.
Selection criteria
We included randomised controlled trials (RCTs) of yoga in addition to standard care for haematological malignancies compared with standard care only. We did not restrict this to any specific style of yoga.
Data collection and analysis
Two review authors independently extracted data for eligible studies and assessed the risk of bias according to predefined criteria. We evaluated distress, fatigue, anxiety, depression and quality of sleep. Further outcomes we planned to assess were health‐related quality of life (HRQoL), overall survival (OS) and adverse events (AE), but data on these were not available.
Main results
Our search strategies led to 149 potentially relevant references, but only a single small study met our inclusion criteria. The included study was published as a full text article and investigated the feasibility and effect of Tibetan Yoga additional to standard care (N = 20; 1 person dropped out before attending any classes and no data were collected) compared to standard care only (N = 19). The study included people with all stages of Hodgkin and non‐Hodgkin's lymphoma, with and without current cancer treatment. The mean age was 51 years.
We judged the overall risk of bias as high as we found a high risk for performance, detection and attrition bias. Additionally, potential outcome reporting bias could not be completely ruled out. Following the recommendations of GRADE, we judged the overall quality of the body of evidence for all predefined outcomes as 'very low', due to the methodical limitations and the very small sample size.
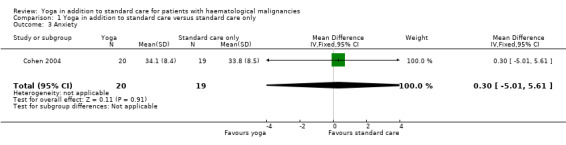
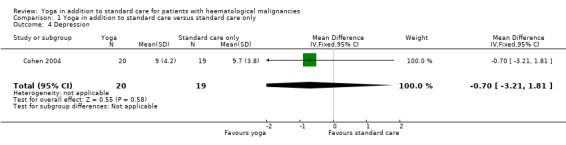
The influence of yoga on HRQoL and OS was not reported. There is no evidence that yoga in addition to standard care compared with standard care only can improve distress in people with haematological malignancies (mean difference (MD) ‐0.30, 95% confidence interval (CI) ‐5.55 to 4.95; P = 0.91). Similarly, there is no evidence of a difference between either group for fatigue (MD 0.00, 95% CI ‐0.94 to 0.94; P = 1.00), anxiety (MD 0.30, 95% CI ‐5.01 to 5.61; P = 0.91) or depression (MD ‐0.70, 95% CI ‐3.21 to 1.81; P = 0.58).
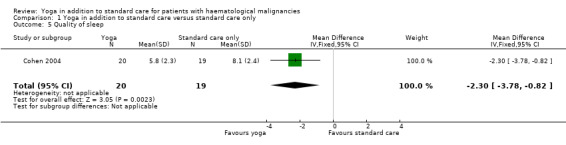
There is very low quality evidence that yoga improves the overall quality of sleep (MD ‐2.30, 95% CI ‐3.78 to ‐0.82; P = 0.002). The yoga groups' total score for the Pittsburgh Sleep Quality Index (PSQI) was 5.8 (± 2.3 SD) and better than the total score (8.1 (± 2.4 SD)) of the control group. A PSQI total score of 0 to 5 indicates good sleep whereas PSQI total score 6 to 21 points towards significant sleep disturbances. The occurrence of AEs was not reported.
Authors' conclusions
The currently available data provide little information about the effectiveness of yoga interventions for people suffering from haematological malignancies. The finding that yoga may be beneficial for the patients' quality of sleep is based on a very small body of evidence. Therefore, the role of yoga as an additional therapy for haematological malignancies remains unclear. Further high‐quality randomised controlled trials with larger numbers of participants are needed to make a definitive statement.
Plain language summary
Yoga in addition to standard care for people with blood or lymph node cancer
Background
Blood and lymph node cancers are referred to as haematological malignancies. These are types of cancer that affect the blood, bone marrow and other parts of the lymphatic system. The most common ones are lymphomas, leukaemias and myelomas. Depending on the kind of cancer and how far it has spread, there are a lot of different options to manage the disease. Usually chemotherapy, radiotherapy or a combination of both is used to treat the disease. If the cancer is widespread, a transplantation of the patients’ own bone marrow cells combined with an aggressive chemotherapy can be a treatment option.
People with cancer most commonly experience high emotional distress, anxiety, fatigue, depression and sleeping problems. These symptoms can persist, even when the treatment has ended. In search of ways to manage and cope with such conditions, more and more patients are turning to complementary and alternative therapies.
Yoga
Yoga, originating in a thousand year old Indian tradition, is becoming more and more popular. There are hundreds of different styles of yoga, but in the Western world, yoga training mostly consists of three main elements: physical postures, breathing exercises and meditation. An increasing number of cancer patients use yoga as an additional way to improve their well‐being. However, a systematic evaluation about the effects of yoga in the management of haematological malignancies is still missing.
Objectives
We reviewed the evidence about the effects of yoga on people with haematological malignancies. We also considered overall survival, distress, fatigue, depression, anxiety, sleep quality and adverse events as important outcomes. We compared people suffering from haematological malignancies treated with yoga and standard cancer care with those treated with standard cancer care only.
Findings
We included a single trial with 39 participants in the review (20 in the yoga group and 19 in the control group). The trial looked at a seven‐week Tibetan Yoga program in a group of people with Hodgkin and non‐Hodgkin's lymphoma. The average age was 51 years. The trial involved patients who were currently receiving anti‐cancer therapy as well as patients who were not receiving active therapy. The trial found insufficient data to make a judgement about the efficacy of yoga on distress, fatigue, depression and anxiety compared with patients not practicing yoga. Yoga can improve the patients' quality of sleep. The trial gave no information about health‐related quality of life, overall survival or adverse events.
On the basis of the GRADE criteria, we judged the overall quality of evidence for yoga concerning the outcomes distress, fatigue, anxiety, depression and quality of sleep as 'very low'.
Conclusion
There are not enough data to say how effective yoga is in the management of haematological malignancies. Therefore, the role of yoga for haematological malignancies remains unclear. Further large, high‐quality randomised controlled trials are needed.
The evidence is up‐to‐date as of 4 February 2014.
Summary of findings
Summary of findings for the main comparison. Yoga in addition to standard care versus standard care only for haematological malignancies.
| Yoga in addition to standard care versus standard care only for haematological malignancies | ||||||
|
Patient or population: patients with haematological malignancies Intervention: yoga in addition to standard Comparison: standard care only | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of patients (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Yoga in addition to standard care versus standard care only | |||||
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | The study provided no data with regard to this outcome. | |
|
Distress
Scale from: 0 to 75. Higher scores indicate a more severe impact of distress |
The mean score of distress in the control groups was 10 | The mean score of distress in the intervention groups was 0.3 lower (5.55 lower to 4.95 higher) |
MD ‐0.30 (95% CI ‐5.55 to 4.95) |
39 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
Fatigue
Scale from: 0 to 10. Higher scores indicate a higher level of fatigue |
The mean level of fatigue in the control groups was 3 | The mean level of fatigue in the intervention groups was 0 higher (0.94 lower to 0.94 higher) |
MD 0.00 (95% CI ‐0.94 to 0.94) |
39 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
Anxiety
Scale from: 20 to 80. Higher scores indicate higher levels of state anxiety |
The mean state anxiety in the control groups was 34 | The mean state anxiety in the intervention groups was 0.3 higher (5.01 lower to 5.61 higher) |
MD 0.30 (95% CI ‐5.01 to 5.61) |
39 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
Depression
Scale from: 0 to 60. Higher scores indicate more depressive symptoms |
The mean level of depression in the control groups was 10 | The mean level of depression in the intervention groups was 0.7 lower (3.21 lower to 1.81 higher) |
MD ‐0.70 (95% CI ‐3.21 to 1.81) |
39 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
|
Quality of sleep
Scale from: 0 to 21. Higher scores indicate a lower quality of sleep |
The mean quality of sleep in the control groups was 8 | The mean quality of sleep in the intervention groups was 2.3 lower (3.78 to 0.82 lower) | MD ‐2.30 (95% CI ‐3.78 to ‐0.82) | 39 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Adverse events | See comment | See comment | Not estimable | 39 (1 study) | See comment | The study provided no data with regard to this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Serious imprecision due to very small sample size; therefore, we downgraded two points.
2 Study participants and personnel administering the intervention were not blinded; also risk of attrition and detection bias and possible outcome reporting bias.
Background
Description of the condition
Haematological malignancies are malignant neoplasms of the myeloid or lymphatic cell lines and include lymphomas, leukaemia, myeloma, myelodysplastic syndromes and myeloproliferative diseases. Haematological malignancies can affect bone marrow, blood cells, lymph nodes and other parts of the lymphatic system.
In the United States of America there were 149,990 new cases of leukaemia, lymphoma and myeloma expected in 2013. This constitutes about 9% of all new diagnosed cancer cases in the USA. The combined age‐adjusted incidence rates for leukaemia (12.8), lymphoma (22.5) and myeloma (5.9) including all various subcategories add up to 41.2 new cases of haematological malignancies per 100,000 men and women per year (Facts 2013; Howlader 2013). Due to the different types, subtypes and the initial stage of the haematological neoplastic disease, the clinical course varies widely. Accordingly, therapy can range from "watchful waiting" to radiotherapy and intensive systematic chemotherapy, including bone marrow transplantation.
Various alternative or additional treatment options are available for people suffering from haematological malignancies, such as immunotherapy or newer classes of drugs such as tyrosine kinase or histone deacetylase inhibitors. Additionally, supportive and palliative care is also available in order to manage psychosocial and physical aspects of the disease and its treatments (Facts 2013; NCCN 2012). Like cancer patients in general, people with haematological malignancies often have to deal with severe side effects of their cancer treatment, substantially affecting their health‐related quality of life (HRQoL) as well as evoking psychological distress and anxiety (Luebbert 2001; Mitchell 2011). In order to manage the effects of their illness, many cancer patients use complementary and alternative medicine (CAM) in addition to standard care. This can include yoga, which is one of the most commonly used complementary therapies (Barnes 2008; Mao 2007; Saydah 2006).
Description of the intervention
Yoga is a mind‐body exercise with its origin in Indian philosophy rooting in an over 4000 year‐old tradition (Innes 2005). The word yoga comes from the Sanskrit word "yuj", meaning yoke or union, describing the union between mind, body and spirit. It was originally designed as a method of discipline and attitudes to help people reach spiritual enlightenment through the eight limbs of yoga practice. These eight limbs contain ethical principles for living (yamas and niyamas), physical postures (asanas), breathing practices to regulate the breath and balance life energy (pranayama), meditative practices (pratyahara, dharana, and dyana) and spiritual enlightenment (samadhi) (Bower 2005; Collins 1998; NCCAM 2012). In Western civilisation yoga is considered as a form of CAM (Alexander 2008). The National Center for Complementary and Alternative Medicine defines CAM as "a variety of techniques designed to enhance the mind's capacity to affect bodily function and symptoms" (NCCAM 2012).
Although there are many different styles of yoga, in Western civilisation the focus of yoga practice is on the physical postures and breathing practices not necessarily connected to religious beliefs or a strong spiritual way of life (Collins 1998). In recent publications, yoga has shown positive effects on a variety of specific health conditions including persistent pain (Wren 2011), asthma (Bidwell 2012), cardiovascular disease (Raub 2002), irritable bowel syndrome (Kuttner 2006), metabolic syndrome (Innes 2005), diabetes (Pandey 2011), psychiatric disorders such as depression and anxiety (Cabral 2011), and cancer (Banerjee 2007; Bower 2012; Culos‐Reed 2006).
Cancer patients often have to deal with severe side effects and psychological distress during their cancer treatment, substantially affecting their HRQoL. Among the most common symptoms of cancer and its treatment are pain, depression and fatigue, which may even persist after the treatment has ended (Lin 2011). In the past decade, chemotherapy and several new drugs have helped to increase the rates of blood cancer cure and remission. Therefore, the HRQoL and the absence of side effects during and after the therapy have become more and more important (Howlader 2013). Yoga practice, solely used in addition to standard cancer therapy, is considered to have positive effects on the HRQoL and cancer‐related side effects (Woodyard 2011). Most studies investigating these effects have focused on breast cancer. The findings, that yoga practice is feasible for cancer patients and shows positive effects on anxiety, emotional functioning, cancer‐related distress and cancer‐related symptoms like fatigue, seem promising for an implementation in the management of haematological malignancies (Banasik 2011; Bower 2005; Culos‐Reed 2006; Danhauer 2009; Moadel 2007; Raghavendra 2007; Rao 2009; Vadiraja 2009).
How the intervention might work
Even though the mechanisms of how yoga affects the body are not entirely understood, there are different studies supporting the theory that yoga benefits physical and mental health via down‐regulation of the hypothalamic pituitary adrenal axis and the sympathetic nervous system activity (Ross 2010). It is assumed that yoga practice reduces the allostatic load (the 'wear and tear on the body') in stress response systems and restores optimal homeostasis by increasing the activity of the inhibitory neurotransmitter gamma amino‐butyric acid (GABA) and the parasympathetic nervous system (Streeter 2012). In Western culture, yoga is mostly associated with asana, the physical practice of yoga. It is regarded as a gentle form of physical activity or exercise (Hagins 2007). Physical exercise in people with haematological malignancies has shown beneficial effects on various outcomes like fitness and quality of life (Coleman 2008; Courneya 2009; Mello 2003; Moyer‐Mileur 2009; Persoon 2013; Thorsen 2005).
Why it is important to do this review
Yoga practice has shown considerable therapeutic potential for the complementary treatment of people with solid cancers without having severe adverse effects. However, there is only little information on yoga and its effects in people with haematological malignancies. Therefore, it is important to perform this review in order to systematically collect and appraise the available evidence and determine the effects of yoga on haematological malignancies. To our knowledge, there has been no previous comprehensive systematic review of yoga interventions for people suffering from haematological malignancies.
Objectives
To assess the effects of yoga practice in addition to standard cancer treatment for people with haematological malignancies.
Methods
Criteria for considering studies for this review
Types of studies
We prepared the review according to the Cochrane protocol (Felbel 2012). We only considered randomised controlled trials (RCTs). We included both full‐text and abstract publications if sufficient information was available on study design, characteristics of participants, interventions and outcomes.
Types of participants
We included trials on people with confirmed diagnosis of haematological malignancies. We did not apply age, gender or ethnicity restrictions. We considered all subtypes and stages of haematological malignancies, including newly diagnosed patients and those with relapsed or drug resistant disease. If trials had consisted of mixed populations with different conditions or types of cancers, we would have used data from the haematological malignancy subgroups. If subgroup data for these participants had not been available (after contacting the authors of the trial), we would have excluded the trial if less than 80% of participants had haematological malignancies. However, in the trial included in this review, only people with lymphoma were randomised.
Types of interventions
We included RCTs investigating the effects of yoga in addition to standard care compared with standard care only. We included any form of yoga (i.e. Ashtanga, Bikram, Iyengar, Kundalini, Therapeutic, Tibetan and Power yoga, as well as every other form of yoga). We only included studies using yoga as the main intervention. We only considered studies with a minimum duration of four weeks and a frequency of at least an hour of yoga practice per week.
Anticancer treatment such as chemotherapy, radiotherapy or monoclonal antibodies should have been given according to the underlying disease. Supportive care given either as necessary or following a fixed schedule was allowed. It must have been intended for participants in both groups to receive identical care.
Types of outcome measures
Primary outcomes
We planned to evaluate HRQoL as primary efficacy endpoint, but no data were available. The HRQoL had to be measured with reliable and valid instruments like the Functional Assessment of Cancer Therapy‐General (FACT‐G) (King 2014), the Short Form Health Survey (SF‐36) (Ware 1992) or disease specific questionnaires like the EORTC‐QLQ‐MY24 for people with myeloma (Osborne 2012).
Secondary outcomes
We analysed the following as secondary outcomes.
Distress.
Fatigue.
Anxiety.
Depression.
Quality of sleep.
We planned to analyse the following as secondary outcomes, but no data were available.
Overall survival (OS). We defined OS as the time interval from random treatment assignment onto a study to death from any cause or to the last follow‐up.
Adverse events (AE) if strictly yoga‐related or classified as Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or higher (CTCAE 2009).
Search methods for identification of studies
Electronic searches
We adapted the search strategies as suggested in chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We did not apply any language restriction to reduce possible language bias.
We searched the following databases and sources:
-
Databases of medical literature:
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 1), search strategy see Appendix 1;
Ovid MEDLINE (1950 to 4th February 2014), search strategy see Appendix 2.
-
Conference proceedings of annual meetings of the following societies, if not included in CENTRAL:
American Society of Hematology (ASH) (2005 to 2013);
American Society of Clinical Oncology (ASCO) (2005 to 2013);
European Haematology Association (EHA) (2005 to 2013);
European Congress for Integrative Medicine (ECIM) (2011 to 2013);
Global Advances in Health and Medicine (2011 to 2014).
-
Electronic search in databases of ongoing trials:
Meta‐register of controlled trials: http://www.controlled‐trials.com/mrct/ ;
Meta‐register of controlled trials: http://clinicaltrials.gov/ .
Searching other resources
We handsearched references of all identified trials, relevant review articles and current treatment guidelines for further literature.
Data collection and analysis
Selection of studies
As proposed in chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), two review authors (SF, NS) independently screened the results of the search strategies for eligibility for this review by reading the abstracts. In the case of disagreement we obtained the full text publication. If no consensus had been reached, we would have asked a third review author to give us her or his opinion, but this procedure was not necessary.
We documented the study selection process in a flow chart as recommended in the PRISMA statement (Moher 2009) showing the total numbers of retrieved references and the numbers of included and excluded studies (see Figure 1).
1.
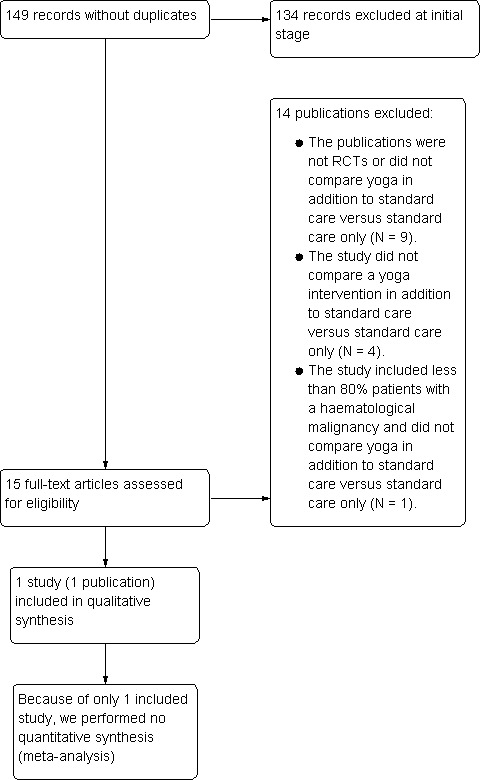
Study flow diagram.
Data extraction and management
Two review authors (SF, NS) independently extracted the data according to the guidelines described in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If it were required, we would have contacted authors of individual studies for additional information. We used a standardised data extraction form containing the following items:
General information: author, title, source, publication date, country, language, duplicate publications.
'Risk of bias' assessment: sequence generation, allocation concealment, blinding (participants, personnel, outcome assessors), incomplete outcome data, selective outcome reporting, other sources of bias.
Study characteristics: trial design, aims, setting and dates, source of participants, inclusion/exclusion criteria, comparability of groups, subgroup analyses, statistical methods, power calculation, treatment cross‐overs, compliance with assigned treatment, length of follow‐up, time point of randomisation.
Participant characteristics: underlying disease, stage of disease, histological subtype, additional diagnoses, age, gender, ethnicity, number of participants recruited/allocated/evaluated, participants lost to follow‐up, type of treatment (multi‐agent chemotherapy, intensity of regimen, number of cycles), additional radiotherapy.
Interventions: type, duration and intensity of the yoga intervention, standard care, duration of follow‐up.
Outcomes: HRQoL, OS, distress, fatigue, anxiety, depression, quality of sleep, and AEs.
We used both full text versions and abstracts including additional information (for example slides) of eligible studies to retrieve the data. We would have extracted trials reported in more than one publication on one form only. If these sources had not provided sufficient information, we had planned to contact the authors for additional details, however, this was not necessary.
Assessment of risk of bias in included studies
To assess the risk of bias, we used a validity assessment form containing the items as suggested in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Sequence generation.
Allocation concealment.
Blinding (patients, personnel, outcome assessors).
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
For every criterion, we made a judgement using one of three categories.
'Low risk': if the criterion was adequately fulfilled in the study, i.e. the study was at a low risk of bias for the given criterion.
'High risk': if the criterion was not fulfilled in the study, i.e. the study was at high risk of bias for the given criterion.
'Unclear': if the study report did not provide sufficient information to allow for a judgement of 'Yes' or 'No' or if the risk of bias was unknown for one of the criteria listed above.
Measures of treatment effect
We calculated continuous outcomes as mean differences. For binary outcomes we would have calculated risk ratios (RR) with 95% confidence intervals (CI). For time‐to‐event outcomes, we would have extracted hazard ratios (HR) from published data according to Parmar and Tierney (Parmar 1998; Tierney 2007). If we had been in the position to include more than one trial in this review, we would have assessed the heterogeneity of treatment effects between trials using a Chi2 test with a significance level at P < 0.1. The I² statistic would have been used to quantify possible heterogeneity (Higgins 2011c).
Dealing with missing data
As suggested in chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011d), there are many potential sources of missing data which had to be taken into account: at study level, at outcome level and at summary data level. If we had considered important data as missing, we would have contacted the original investigators to request missing data. If data were still missing, we would have made explicit our assumptions for any methods used; for example, that the data were assumed to be missing at random or that missing values were assumed to have a particular value, such as a poor outcome. We would have imputed missing data for participants who were lost to follow‐up after randomisation (dichotomous data) assuming poor outcome (worst case scenario) for missing individuals. We would have performed sensitivity analysis to assess how robust results were to reasonable changes in the assumptions that were made. We would have addressed the potential impact of missing data on the findings of the review in the discussion.
Assessment of heterogeneity
As our analysis contained only one study, we did not conduct a meta‐analysis. Had a meta‐analysis been possible, we would have assessed heterogeneity of treatment effects between trials by using a Chi² test with a significance level at P < 0.1 as described in chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). In that case, we would have used the I² statistic to quantify possible heterogeneity (I² > 30% moderate heterogeneity, I² > 50% substantial heterogeneity and I² > 75% considerable heterogeneity). We would have explored potential causes of heterogeneity by sensitivity and subgroup analyses wherever possible.
Assessment of reporting biases
In a meta‐analysis with at least 10 trials we would have explored potential publication bias by generating a funnel plot and statistically testing this by conducting a linear regression test as proposed in chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). We would have considered a P value of less than 0.1 significant for this test. On the basis of a single trial included, we did not perform these steps.
Data synthesis
If we had found more than a single trial meeting the inclusion criteria and had we considered the data sufficiently similar to be combined, we would have pooled results by conducting meta‐analyses using the random‐effects model, while we would have used the fixed‐effect model as a sensitivity analysis. If there had been more trials but they were clinically too heterogeneous to combine (e.g. various types of diseases), we would have performed meta‐analyses by subgroups only without calculating an overall estimate. If different tools were used to evaluate HRQoL fatigue, anxiety, depression or quality of sleep, we would have calculated standardised mean differences to determine effect sizes. We performed analyses according to the recommendations in chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c) and used the Cochrane statistical package Review Manager 5 for analysis (RevMan 2011).
We created a 'Summary of findings' table on relative and absolute risks in each group with the help of GRADEpro software. We summarised the evidence of HRQoL, distress, fatigue, anxiety, depression, quality of sleep and adverse effects in this Table 1.
Subgroup analysis and investigation of heterogeneity
Due to the fact that we were only able to include a single trial, it was not possible to explore heterogeneity for different subgroups. If we had found more trials suitable for inclusion into this review, we would have taken the following parameters into consideration for subgroup analyses, as these parameters are known to cause heterogeneity.
Age (< 18 years, 18 to 40 years, 41 to 60 years, > 60 years).
Entity of underlying disease (indolent non‐Hodgkin's lymphoma, aggressive non‐Hodgkin's lymphoma, acute leukaemia, chronic myeloid leukaemia, multiple myeloma, chronic lymphocytic leukaemia, Hodgkin lymphoma).
Therapy of underlying disease (first‐line, relapse therapy).
Type/duration/intensity of yoga intervention.
Sensitivity analysis
If we had performed a meta‐analysis, we would have based sensitivity analysis on:
quality components with regard to low and high risk of bias;
fixed‐effect modelling.
Results
Description of studies
Only one RCT was found eligible for inclusion in this review.
Results of the search
The search strategy was run on 30th May 2012 and again on 4th February 2014. Our literature search resulted in 149 possibly relevant references through electronic database searches and handsearching. Of these, 134 were excluded at the initial stage of screening because they were duplicates or did not fulfill our predefined inclusion criteria. The remaining 15 publications were retrieved as full‐text publications or abstract publications for detailed evaluation. Finally, one full‐text publication (one trial) with 39 participants was formally included in this systematic review. The overall number of references screened, identified, selected, excluded and included is documented according to PRISMA in the flow diagram (Figure 1).
Included studies
Only one trial with a total of 39 participants fulfilled our inclusion criteria (Cohen 2004). We extracted data from the full text publication of this trial. The recruitment period was not reported. The trial was published first as a full text publication in April 2004. Characteristics of the included trial are summarised in the Characteristics of included studies table.
Design
The included trial was a two‐armed RCT using a wait‐list control design. The trial was primarily designed to assess the feasibility of a Tibetan Yoga intervention in people with lymphoma. In both the intervention group and the control group, there were participants currently receiving chemotherapy as well as participants currently not receiving treatment during the trial.
Sample sizes
The included trial randomised 39 participants.
Setting
The study was conducted at The University of Texas, M. D. Anderson Cancer Center in the United States of America.
Participants
To be included into the trial the participants had to be 18 years or older and be able to read and speak English. Only people with a confirmed diagnosis of lymphoma who were either receiving chemotherapy or had received it within the past 12 months were included. The current or received chemotherapy had to be either a regimen with combined cyclophosphamide, doxorubicin, vincristine and prednisone or regimens with the same drug classes to control for the more severe side effects. The average participant age in both the Tibetan Yoga and the control group was 51 years. The majority of participants, 63.2% (N = 24), had a diagnosis of non‐Hodgkin's lymphoma, while 36.8% (N = 14) had Hodgkin lymphoma. Four participants (21.1%) in each group received current cancer treatment while 15 participants per group (78.9%) were not receiving current cancer treatment. The distribution across the disease stages was not statistically significantly different (Tibetan Yoga group: Stage I, 22%; Stage II, 39%; Stage III, 17%; Stage IV, 22%. Control group: Stage I, 22%; Stage II, 33%; Stage III, 12%; Stage IV, 33% according to Ann Arbor Criteria).
Interventions
Cohen 2004 compared the effects of a seven weekly Tibetan Yoga intervention in addition to standard care with a control group receiving standard care only. The Tibetan Yoga intervention was held once per week. The average duration of the individual sessions was not reported. Every session was supervised by an experienced Tibetan Yoga instructor. The participants received printed materials covering a new area of the program after each class and were encouraged to continue practicing at home at least once a day. In case of a missed yoga session, the opportunity to make up for this by attending another session was offered. After the last session the participants received an audiotape guiding them through all learned techniques. Outcomes of the intervention were measured at baseline and one week, one month and three months after the last yoga session. With the exception of the follow‐up assessments, the control group had no contact with the research personnel but were offered to attend the Tibetan Yoga program after the last follow‐up. To what extent this opportunity was used is not reported.
Outcomes
The included trial was designed as a feasibility study and did not prespecify a primary outcome. The trial evaluated distress, fatigue, anxiety, depression and quality of sleep as outcomes. Adverse events were not reported.
The author reported to have used mixed‐model regression analyses to assess the impact of the Tibetan Yoga intervention relative to the control group. They regressed follow‐up assessments on group, time to follow‐up assessment, the corresponding baseline measure and participant characteristics used in the minimisation‐adaptive assignment procedure. The author stated that there were no statistically significant group‐by‐time interactions for any outcome. Therefore, they reported all outcomes as average intervention effects across all follow‐up time points and adjusted for co‐variables.
Funding
The trial was supported in part by a grant from the Bruce S. Gelb Foundation.
Conflict of interest
No potential conflict of interest of the authors was mentioned.
Excluded studies
We identified 14 additional publications as potentially relevant for this review. However, after obtaining the full text articles, we excluded the publications for the following reasons: 13 publications were not reporting on RCTs or did not compare yoga interventions with standard care only (Adamsen 2006; Buffart 2012; Feng 2004; Fonteyn 2005; Habermann 2009; Jarden 2013; Kelly 2009; Kvillemo 2011; Phipps 2012; Shvarts 2013; Silva 2011; van Haren 2013; Williams 2006) and one trial included less than 80% participants with haematological malignancies and focused only on breathing techniques (Dhruva 2012). For more details see the Characteristics of excluded studies table.
Risk of bias in included studies
Overall we judged the risk of bias of the included trial as high.
For detailed information see the 'Risk of bias' table for the included trial and for an overview of the results, please see Figure 2.
2.
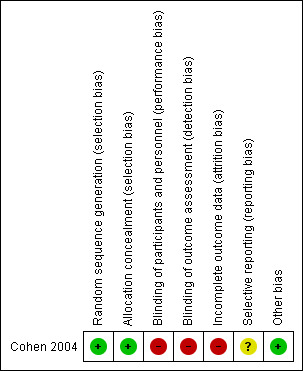
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The study was reported as randomised through the use of minimisation. Potential study participants had to fill out a set of questionnaires. Subsequently, they were assigned either to the intervention or the control group based on the initial questionnaires. According to the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011b) minimisation used for a random sequence generation even implemented without a random element is considered to be equivalent to being random. Therefore, we judged the risk for allocation sequence bias as 'low'. The allocation process is reported to be concealed from all investigators because relevant information for the allocation process was entered into a computer program. The group assignment was performed consecutively by the computer program. Therefore, we judged the risk of selection bias as 'low'.
Blinding
In the included trial neither participants nor personnel such as the yoga instructor were blinded. This was to be expected since the nature of the intervention makes blinding extremely difficult. Outcome assessors were also not reported to be blinded. Consequently, we judged the risk for performance bias as 'high'.
Incomplete outcome data
The included trial was published as a full text article. One participant of the intervention group dropped out of the study before attending any yoga session. The reason for the dropout was not reported. Reasoning that this dropout equalised the group size of both intervention and control group, the study authors decided to collect no follow‐up data for this participant. Although no other dropouts were reported, only 30 out of 38 participants who completed the intervention according to protocol also completed at least one follow‐up. Therefore, we judged the risk for attrition bias as 'high'.
Selective reporting
There was no study protocol for the included trial in http://www.controlled‐trials.com/mrct/ available.
The study authors planned their trial as a feasibility study and hypothesised that yoga had an effect on multiple outcomes.Therefore, they prespecified no primary outcome. All preplanned secondary outcomes as mentioned in the full text article are reported. However, the article mentions some unspecified administered measures assessing some proposed mediators of the benefits of yoga program which were not reported in the publication. For those reasons the risk for reporting bias was judged as 'unclear'.
Other potential sources of bias
The study appeared to be free of other sources of bias and was, therefore, judged as having a 'low' risk. However, it is important to note that there were some differences in the baseline values between the yoga and control groups.
Effects of interventions
See: Table 1
The only trial eligible for inclusion in this review used a traditional taught form of Tibetan Yoga in a weekly session format (Cohen 2004). The trial compared a Tibetan Yoga intervention in addition to standard care with standard care only.
The purpose of the trial was to assess whether yoga was feasible for people suffering from lymphoma and the negative effects of its treatment. Furthermore, Cohen et al. tried to investigate the effects of the Tibetan Yoga intervention on distress, fatigue, anxiety, depression and quality of sleep. Outcomes were measured at baseline and one week, one month and three months after the last yoga session. Even though 39 participants were included into the trial and only one person dropped out, outcome data were only available for 30 participants (16 in the yoga group and 14 in the control group). The P values and 95% confidence intervals are presented in relation to the group comparisons for the follow‐up data. The follow‐up adjustment scores represent least‐square means that were adjusted for the baseline value of the outcome measure and the information used for minimisation (state anxiety, age, gender, treatment status and type of cancer, e.g. Hodgkin or non‐Hodgkin's lymphoma).
Primary outcomes
Health‐related Quality of Life (HRQoL)
This outcome was not evaluated in the included trial.
Secondary outcomes
Distress
The included trial used theImpact of Events Scale (IES) to assess the participants' subjective level of stress. The IES is a validated and well established questionnaire designed to measure a person's cognitive responses to stressful events (Sundin 2002; Sundin 2003) and has been used in various cancer populations (Bleiker 2000; Brown 2003; Chen 2005; Kangas 2002). The IES is a 15‐item self reported scale that provides separate scores for the two subscales Intrusive thoughts and Avoidance plus a total score. The score for the total scale ranges from 0 to 75 with scores greater than 25 suggesting a moderate to severe impact of distress (Horowitz 1979).
Cohen et al. found no statistically significant differences between the two groups. The yoga group showed no statistically significant improvement in comparison to the control group. The yoga groups' mean total score was 10.2 (± 8.2 standard deviation (SD)). The wait‐list control group showed a mean total score of 10.5 (± 8.5 SD) (mean difference (MD) ‐0.30, 95% confidence interval (CI) ‐5.55 to 4.95; P = 0.91). These scores represent the average effects across all follow‐up time points (Cohen 2004).
Fatigue
The Brief Fatigue Inventory (BFI) is an established and validated questionnaire to assess fatigue severity in clinical settings. It is often used in cancer populations and has shown satisfactory psychometric properties in this concern (Catania 2013; Mendoza 1999; Mystakidou 2008; Radbruch 2003). The BFI contains nine items providing a total score ranging from 0 to 10. The usual fatigue severity is defined as none (0 points), mild (1 to 3 points), moderate (4 to 7 points) and severe (8 to 10 points) (Chang 2007).
There were no statistically significant differences between the yoga and control groups. Both groups showed an identical mean total score of 3.1 (± 1.5 SD) representing the average intervention effect across all follow‐up time points (MD 0.00, 95% CI ‐0.94 to 0.94; P = 1.00) (Cohen 2004).
Anxiety
In order to evaluate anxiety, Cohen et al. used the validated Spielberger State Trait Anxiety Inventory (STAI). The STAI is often used for the assessment of anxiety in cancer populations and distinguishes state and trait anxiety in two separate scales (Heim 1993; Klein 2011; Stark 2002). Each scale contains 20 separate questions for assessing either state or trait anxiety. Cohen et al. reported to have only used the inventory for state anxiety to assess the participants' current level of anxiety. Each of the two scales ranges from 20 to 80 with higher scores indicating greater anxiety (Spielberger 1970). Scores from 39 to 55 and higher are suggested as cut‐off points to detect clinically significant symptoms in the state anxiety scale. However, up to now no validated cut‐off scores exist for cancer patients (Julian 2011).
In the trial, the two study groups showed no statistically significant differences. The yoga group showed a mean total score of 34.1 (± 8.4 SD) and the wait‐list control group a mean total score of 33.8 (± 8.5 SD) (MD 0.30, 95% CI ‐5.01 to 5.61; P = 0.91). These scores represent the average effects across all follow‐up time points (Cohen 2004).
Depression
To measure depression, the Centers for Epidemiologic Studies‐Depression (CES‐D), was used by Cohen et al. It is a validated questionnaire, commonly used to evaluate depression in cancer patients and has presented satisfactory psychometric properties for breast cancer patients (Hann 1999).The questionnaire contains 20‐items and is focused on the affective components of depression. The score ranges from 0 to 60 with higher scores indicating greater depressive symptoms (Radloff 1977). A score of 16 or greater hints at a risk of clinical depression.
There were no statistically significant differences between the yoga and control groups. The yoga group showed a mean total score of 9.0 (± 4.2 SD) and the control group a mean total score of 9.7 (± 3.8 SD) (MD ‐0.70, 95% CI ‐3.21 to 1.81; P = 0.58). These scores represent the average intervention effect across all follow‐up time points (Cohen 2004).
Quality of sleep
Cohen et al. used the validated Pittsburgh Sleep Quality Index (PSQI) to measure quality of sleep. The PSQI is a self‐rated questionnaire containing 19 items. In psychometric evaluations the PSQI was found to be a reliable and valid instrument for the assessment of sleep quality in cancer patients (Beck 2004; Carpenter 1998; Kotronoulas 2011). It assesses the quality of sleep and sleep disturbances over one month in seven subscales (Subjective sleep quality, Sleep latency, Sleep duration, Habitual sleep efficiency, Sleep disturbances, Use of sleeping medications andDaytime dysfunction) providing a total score from 0 to 21. Total PSQI scores greater than 5 were found to provide a good measure to divide poor sleepers (PSQI total score 6 to 21) from good sleepers (PSQI total score 0 to 5) and hint at significant sleep disturbances (Buysse 1989).
In the included trial, yoga practise led to a significantly better quality of sleep in comparison to standard care only. The yoga group's mean total score representing the average intervention effect across all follow‐up time points was 5.8 (± 2.3 SD). The mean total score of the control group was 8.1 (± 2.4 SD) (MD ‐2.30, 95% CI ‐3.78 to ‐0.82; P = 0.002). The yoga practitioners showed statistically significant better results for the PSQI total score, as well as for the four subscales Subjective sleep quality, Sleep latency, Sleep duration andUse of sleeping medications. The yoga arm showed no statistically significant differences for the subscales Sleep efficiency, Sleep disturbances andDaytime dysfunction (Cohen 2004).
Subscale results
For the results of the seven subscales of the PSQI please see Table 3.
1. Subscale outcomes for the Pittsburgh Sleep Quality Index (PSQI).
| Subscale | Effect estimate | P value |
| Sleep quality | MD ‐0.41; 95% CI ‐0.75 to ‐0.07 | 0.02 |
| Sleep latency | MD ‐0.58; 95% CI ‐1.02 to ‐0.14 | 0.009 |
| Sleep duration | MD ‐0.46; 95% CI ‐0.86 to ‐0.06 | 0.02 |
| Sleep efficacy | MD ‐0.08; 95% CI ‐0.50 to 0.34 | 0.71 |
| Sleep disturbances | MD ‐0.10; 95% CI ‐0.34 to 0.14 | 0.42 |
| Sleeping medications | MD ‐0.73; 95% CI ‐1.30 to ‐0.16 | 0.01 |
| Daytime dysfunction | MD 0.03; 95% CI ‐0.36 to 0.42 | 0.88 |
MD = mean difference; CI = confidence interval
Overall survival (OS)
The included trial was primarily designed as a feasibility study and did not provide any information about the participants' overall survival. Therefore, no conclusion can be drawn as to whether or not a yoga intervention could favourably influence the overall survival of people with lymphoma.
Adverse events
The included trial did not report any adverse events. Since yoga interventions are known for their relative lack of serious adverse events, especially when they are focused on visualisation, breathing and mindfulness instead of on demanding physical postures, it is likely that no major adverse advents related to the intervention occurred during the trial. However, it cannot be ruled out that minor yoga‐related adverse events may have occurred but were not reported.
Discussion
Summary of main results
The following findings emerge from this systematic review, evaluating the role of yoga in addition to standard care compared with standard care only for the management of haematological malignancies. The single trial that met our inclusion criteria evaluated the feasibility, psychological adjustment and sleep quality in a group of 39 patients with Hodgkin and non‐Hodgkin's lymphoma treated by a Tibetan Yoga intervention.
Trials that investigate the effects of yoga interventions for people with haematological malignancies or include people with haematological malignancies are still very rare, despite the increasing scientific interest about yoga.
Yoga interventions seem to be feasible for people suffering from haematological malignancies, especially when they are primarily focused on meditation and breathing techniques instead of demanding physical postures.
There is no evidence for differences between yoga in addition to standard care and standard care only for the endpoints distress, fatigue, anxiety or depression.
There is some evidence that yoga interventions in addition to standard care may improve the quality of sleep up to a three month follow‐up.
There are no data for HRQoL, OS and AEs.
Overall completeness and applicability of evidence
This systematic review included only a single study. The included study is an RCT using minimisation for randomisation. The study enrolled only a total of 39 patients suffering from lymphoma. Other haematological malignancies were not considered. The trial included participants undergoing treatment or having completed treatment within the previous 12 months. The chosen style of yoga was Tibetan Yoga, a gentle form of yoga less commonly used in the Western civilisation. The trial was conducted in the United States of America. Even though we did not apply language limitations for our search, we were only able to include one paper published in the English language. Therefore, we might have missed some non‐translated trials from India or the Asian region, where the practice of yoga is more common than in Western countries.
In terms of applicability, the majority of participants were female (63.2%). The mean participant age was 51 years. Ethnicity or further socio‐demographic information was not provided. In order to enable future comparison between trials investigating haematological malignancies and yoga, a detailed description of the study population would be useful. The included trial measured the effect of Tibetan Yoga at the end of the intervention with follow‐up of three months. Thus, how sustainable the observed improvement in sleep quality is remains unclear.
The included study used only self report questionnaires to assess outcomes. While this may reduce the risk of bias through an unblinded outcome assessor, it may be susceptible to response bias. Furthermore, because of the present lack of understanding about the mechanisms of how yoga interventions might work, it is not possible to estimate if the benefits can be attributed directly to the Tibetan Yoga intervention program or to some nonspecific associated elements such as received care and attention. Due to the small sample size which reduces the validity of findings by itself, we did not perform a subgroup analysis. Therefore, we are not able to predict whether the observed improvement of sleep quality might affect all participants or just a small subgroup to a larger extent.
Quality of the evidence
Results of this systematic review should be interpreted with caution owing to the risk of bias. The quality of the one trial included was at high risk of bias and the number of 39 patients included into this trial was very small. The trial was at a high risk of performance bias because of lack of blinding of the participants and the personnel administering the intervention. This is a common problem in trials investigating yoga and comparable interventions, since blinding for participants and instructors demands a great amount of effort. Additionally, the trial was at a high risk of detection bias because of unblinded outcome assessors. The trial reported only outcomes for 30 out of 38 participants who completed the study and was, therefore, at a high risk of attrition bias.
In consideration of the high risk of bias of the included trial and the very small number of participants included (N = 39) we judged the overall quality of evidence as 'very low'. For further details please see the Table 1 and Figure 2.
Potential biases in the review process
The primary limitation of this systematic review is the very small number of eligible randomised controlled trials that met our inclusion criteria. Although we performed an extensive search of the literature with no language restrictions, it is possible that we might have missed Asian or Indian RCTs that were unpublished, ongoing or published as dissertation only. Therefore, publication bias and language bias cannot be excluded.
Agreements and disagreements with other studies or reviews
To our knowledge this is the first comprehensive systematic review focusing on yoga interventions for people with haematologic malignancies. There are several recent systematic reviews evaluating the effectiveness of yoga for people with solid cancers (Boehm 2012; Buffart 2012; Cramer 2012; Lin 2011; Zhang 2012). For the most part, these reviews focused on breast cancer. All these reviews evaluated yoga as a feasible intervention for cancer patients that is not associated with serious adverse events.
Lin 2011 reported that yoga group interventions for cancer patients compared to wait‐list control groups or supportive therapy groups showed statistically significantly greater improvements in psychological health, including anxiety, depression, distress and stress, but only a small positive effect on HRQoL. The meta‐analysis found no statistically significant improvements in overall effects for physical health. A positive effect of yoga on fatigue was not observed. Comparable results regarding fatigue were found by Boehm 2012. Investigating the effect of yoga on fatigue, they included multiple diseases such as cancer, multiple sclerosis, chronic pancreatitis, fibromyalgia, asthma and healthy populations into their meta‐analysis and observed only a small positive effect on fatigue that was even smaller in cancer populations. Summarising 12 RCTs with breast cancer patients and one with patients with lymphoma (Cohen 2004) in a meta analysis, Buffart 2012 found yoga to be a feasible intervention for cancer patients and survivors. They observed a small effect on functional well‐being and moderate to large effects for psychosocial outcomes in patients with breast cancer. The effects of yoga on physical function and sleep in breast cancer patients were only small and not statistically significant. Zhang 2012 found a mild HRQoL‐improvement for women with breast cancer and observed only a slight improvement for psychologic function outcomes, such as anxiety, depression, distress and sleep, which were not statistically significant. Cramer 2012 investigated the effects of yoga on HRQoL and psychological health in breast cancer patients and survivors. They found evidence for a moderate short‐term effect on global HRQoL along with small short‐term effects on the functional, social and spiritual quality of life. However, the observed short‐term effects on HRQoL could not be clearly attributed to the yoga intervention rather than bias. Owing to its positive risk profile, they concluded that yoga can be recommended as an intervention to improve psychological health during breast cancer treatment, but evidence for longer‐term effects of yoga was still missing. Most of the authors criticised the fact that the majority of the included RCTs were of lower quality and generally contained only small numbers of participants.
Authors' conclusions
Implications for practice.
No reliable conclusions can be drawn at present regarding the efficacy of yoga as a treatment for haematological malignancies. A lack of relevant quantitative research was identified. There is some evidence that yoga interventions in addition to standard care may improve the quality of sleep in people with lymphoma. However, this finding must be interpreted cautiously since these data originated only from a single small study.
Implications for research.
More high‐quality trials with larger numbers of participants are needed to evaluate potential favourable or unfavourable effects of yoga for people suffering from haematological malignancies. To achieve this goal, multicentre studies in different countries should be considered. Studies should emphasise good methodical quality, longer follow‐up periods and include patient relevant outcomes such as adverse events, quality of life and overall survival.
Yoga can be considered as a many‐faceted intervention consisting of different elements like physical postures, meditation and breathing techniques. These elements may influence patients and outcomes in different ways. Since the effects and mechanisms of yoga interventions are not fully scientifically understood, researchers would be well advised to agree on a standardised set of well validated instruments for outcome assessment. In order to enable better conclusions and make future trials more comparable, a detailed description of intervention procedures and yoga postures in forthcoming studies is recommended. In regard to the many different styles of yoga it could be useful to either concentrate on the most popular styles of yoga or to focus on particular aspects of the interventions such as physical postures.
Due to the nature of a yoga intervention, blinding of participants is problematic. Sham procedures may be an option to enable a better differentiation between the effects of the yoga intervention and the influence of given attention to the participants. Even if this is not possible, the blinding of outcome assessors is crucial to reduce detection bias. This is noteworthy since the trial included in this review did not report blinding of outcome assessors.
Available safety data for yoga practiced by cancer patients suggest that yoga is not associated with serious adverse events if it is practiced cautiously and supervised by qualified yoga instructors. However, even if no adverse events were observed, most studies fail to report the presence or absence of them. Therefore, future studies should place more value on the reporting of adverse events and the reasons for withdrawals and study dropouts.
Acknowledgements
We would like to thank Kathrin Bauer, Oliver Blank, Sabine Kluge, Michaela Rancea and Andrea Will of the Cochrane Haematological Malignancies Group (CHMG) Editorial Base.
Appendices
Appendix 1. CENTRAL search strategy
| ID | Search |
| #1 | MeSH descriptor Hematologic Diseases explode all trees |
| #2 | MeSH descriptor Hematologic Neoplasms explode all trees |
| #3 | (hematolog* NEAR/1 malignan*) or (hematolog* NEAR/1 neoplas*) or (haematolog* NEAR/1 malignan*) or (haematolog* NEAR/1 neoplas*) |
| #4 | MeSH descriptor Bone Marrow Diseases explode all trees |
| #5 | MeSH descriptor Lymphoma explode all trees |
| #6 | MeSH descriptor Leukemia explode all trees |
| #7 | (hogkin* or hodkin* or hodgin*):ti,ab,kw |
| #8 | lymphogranulomato* |
| #9 | lymphom* |
| #10 | histiocy* |
| #11 | granulom* |
| #12 | non‐hodgkin* |
| #13 | nonhodgkin* |
| #14 | reticulosis |
| #15 | reticulosarcom* |
| #16 | (burkitt* NEAR/ (lymph* or tumo*)) |
| #17 | brill‐symmer* |
| #18 | plasm**ytom* |
| #19 | myelom* |
| #20 | sezary |
| #21 | leuk*em* |
| #22 | myelodysplas* |
| #23 | aplast* an*em* |
| #24 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23) |
| #25 | MeSH descriptor Yoga explode all trees |
| #26 | MeSH descriptor Relaxation Therapy explode all trees |
| #27 | MeSH descriptor Meditation explode all trees |
| #28 | yoga* |
| #29 | relaxation* |
| #30 | meditat* |
| #31 | asana* |
| #32 | pranayama* |
| #33 | yogic* |
| #34 | (#25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33) |
| #35 | (#24 AND #34) |
Update 04.02.2014
| ID | Search |
| #1 | MeSH descriptor: [Hematologic Diseases] explode all trees |
| #2 | MeSH descriptor: [Hematologic Neoplasms] explode all trees |
| #3 | (hematolog* near/1 malignan*) OR (hematolog* near/1 neoplas*) OR (haematolog* near/1 malignan*) OR (haematolog* near/1 neoplas*) |
| #4 | MeSH descriptor: [Bone Marrow Diseases] explode all trees |
| #5 | MeSH descriptor: [Lymphoma] explode all trees |
| #6 | MeSH descriptor: [Leukemia] explode all trees |
| #7 | (hogkin* or hodkin* or hodgin*):ti,ab,kw |
| #8 | lymphogranulomato* |
| #9 | lymphom* |
| #10 | histiocy* |
| #11 | granulom* |
| #12 | non‐hodgkin* |
| #13 | nonhodgkin* |
| #14 | reticulosis |
| #15 | reticulosarcom* |
| #16 | (burkitt* NEAR/ (lymph* or tumo*)) |
| #17 | brill‐symmer* |
| #18 | plasm**ytom* |
| #19 | myelom* |
| #20 | sezary |
| #21 | leuk*em* |
| #22 | myelodysplas* |
| #23 | aplast* an*em* |
| #24 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 |
| #25 | MeSH descriptor: [Yoga] explode all trees |
| #26 | MeSH descriptor: [Relaxation Therapy] explode all trees |
| #27 | MeSH descriptor: [Meditation] explode all trees |
| #28 | yoga* |
| #29 | relaxation* |
| #30 | meditat* |
| #31 | asana* |
| #32 | pranayama* |
| #33 | yogic* |
| #34 | #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 |
| #35 | #24 and #34 |
| #36 | #35 from 2013 to 2014, in Trials |
Appendix 2. MEDLINE search strategy
| # | Searches |
| 1 | YOGA/ |
| 2 | RELAXATION TECHNIQUES/ |
| 3 | MEDITATION/ |
| 4 | yoga$.tw,kf,ot. |
| 5 | relaxation$.tw,kf,ot. |
| 6 | meditat$.tw,kf,ot. |
| 7 | asana$.tw,kf,ot. |
| 8 | pranayama$.tw,kf,ot. |
| 9 | yogic$.tw,kf,ot. |
| 10 | or/1‐9 |
| 11 | HEMATOLOGIC DISEASES/ |
| 12 | exp HEMATOLOGIC NEOPLASMS/ |
| 13 | (hematolog$ adj1 malignan$).tw,kf,ot. |
| 14 | (hematolog$ adj1 neoplas$).tw,kf,ot. |
| 15 | (haematolog$ adj1 malignan$).tw,kf,ot. |
| 16 | (haematolog$ adj1 neoplas$).tw,kf,ot. |
| 17 | exp BONE MARROW DISEASES/ |
| 18 | exp LYMPHOMA/ |
| 19 | exp LEUKEMIA/ |
| 20 | hodgkin$.tw,kf,ot. |
| 21 | lymphogranulomato$.tw,kf,ot. |
| 22 | lymphom$.tw,kf,ot. |
| 23 | histiocy$.tw,kf,ot. |
| 24 | granulom$.tw,kf,ot. |
| 25 | non‐hodgkin$.tw,kf,ot. |
| 26 | nonhodgkin$.tw,kf,ot. |
| 27 | reticulosis.tw,kf,ot. |
| 28 | reticulosarcom$.tw,kf,ot. |
| 29 | (burkitt$ adj (lymph$ or tumo?r$)).tw,kf,ot. |
| 30 | lymphosarcom$.tw,kf,ot. |
| 31 | brill‐symmer$.tw,kf,ot. |
| 32 | plasm##ytom$.tw,kf,ot. |
| 33 | myelom$.tw,kf,ot. |
| 34 | sezary.tw,kf,ot. |
| 35 | leuk?em$.tw,kf,ot. |
| 36 | myelodysplas$.tw,kf,ot. |
| 37 | aplast$ an?em$.ti,kf,ot. |
| 38 | or/11‐37 |
| 39 | randomized controlled trial.pt. |
| 40 | controlled clinical trial.pt. |
| 41 | randomi?ed.ab. |
| 42 | placebo.ab. |
| 43 | drug therapy.fs. |
| 44 | randomly.ab. |
| 45 | trial.ab. |
| 46 | groups.ab. |
| 47 | or/39‐46 |
| 48 | humans.sh. |
| 49 | 47 and 48 |
| 50 | 10 and 38 and 49 |
Update April 2013 to February 2014
| # | Searches | Results |
| 1 | YOGA/ | 1433 |
| 2 | RELAXATION TECHNIQUES/ | 5633 |
| 3 | MEDITATION/ | 1430 |
| 4 | yoga$.tw,kf,ot. | 1444 |
| 5 | relaxation$.tw,kf,ot. | 74985 |
| 6 | meditat$.tw,kf,ot. | 2498 |
| 7 | asana$.tw,kf,ot. | 88 |
| 8 | pranayama$.tw,kf,ot. | 95 |
| 9 | yogic$.tw,kf,ot. | 144 |
| 10 | or/1‐9 | 81455 |
| 11 | HEMATOLOGIC DISEASES/ | 12342 |
| 12 | exp HEMATOLOGIC NEOPLASMS/ | 9209 |
| 13 | (hematolog$ adj1 malignan$).tw,kf,ot. | 11374 |
| 14 | (hematolog$ adj1 neoplas$).tw,kf,ot. | 828 |
| 15 | (haematolog$ adj1 malignan$).tw,kf,ot. | 2916 |
| 16 | (haematolog$ adj1 neoplas$).tw,kf,ot. | 180 |
| 17 | exp BONE MARROW DISEASES/ | 70394 |
| 18 | exp LYMPHOMA/ | 141882 |
| 19 | exp LEUKEMIA/ | 192880 |
| 20 | hodgkin$.tw,kf,ot. | 51614 |
| 21 | lymphogranulomato$.tw,kf,ot. | 1963 |
| 22 | lymphom$.tw,kf,ot. | 121956 |
| 23 | histiocy$.tw,kf,ot. | 20497 |
| 24 | granulom$.tw,kf,ot. | 51509 |
| 25 | non‐hodgkin$.tw,kf,ot. | 28507 |
| 26 | nonhodgkin$.tw,kf,ot. | 102 |
| 27 | reticulosis.tw,kf,ot. | 1397 |
| 28 | reticulosarcom$.tw,kf,ot. | 1129 |
| 29 | (burkitt$ adj (lymph$ or tumo?r$)).tw,kf,ot. | 7115 |
| 30 | lymphosarcom$.tw,kf,ot. | 4470 |
| 31 | brill‐symmer$.tw,kf,ot. | 224 |
| 32 | plasm##ytom$.tw,kf,ot. | 6384 |
| 33 | myelom$.tw,kf,ot. | 44344 |
| 34 | sezary.tw,kf,ot. | 1680 |
| 35 | leuk?em$.tw,kf,ot. | 201862 |
| 36 | myelodysplas$.tw,kf,ot. | 13571 |
| 37 | aplast$ an?em$.ti,kf,ot. | 4822 |
| 38 | or/11‐37 | 545476 |
| 39 | randomized controlled trial.pt. | 359892 |
| 40 | controlled clinical trial.pt. | 86935 |
| 41 | randomi?ed.ab | 311358 |
| 42 | randomi?ed.ab. | 141347 |
| 43 | drug therapy.fs. | 1652983 |
| 44 | randomly.ab. | 186582 |
| 45 | trial.ab. | 268535 |
| 46 | groups.ab. | 1204649 |
| 47 | or/39‐46 | 3102203 |
| 48 | humans.sh. | 13101199 |
| 49 | 47 and 48 | 2536609 |
| 50 | 10 and 38 and 49 | 86 |
| 51 | limit 50 to ed=20120524‐20130401 | 5 |
| 52 | limit 50 to ed=20130401‐20140204 | 2 |
Data and analyses
Comparison 1. Yoga in addition to standard care versus standard care only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Distress | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐5.55, 4.95] |
| 2 Fatigue | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.94, 0.94] |
| 3 Anxiety | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐5.01, 5.61] |
| 4 Depression | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐3.21, 1.81] |
| 5 Quality of sleep | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐2.3 [‐3.78, ‐0.82] |
1.1. Analysis.
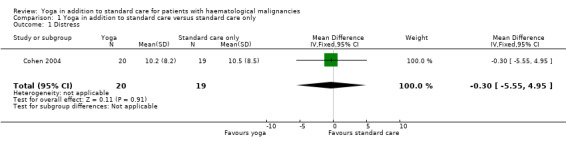
Comparison 1 Yoga in addition to standard care versus standard care only, Outcome 1 Distress.
1.2. Analysis.
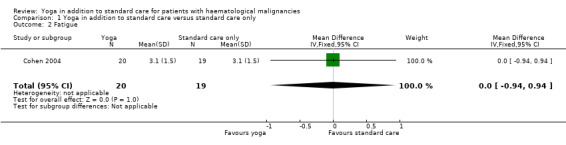
Comparison 1 Yoga in addition to standard care versus standard care only, Outcome 2 Fatigue.
1.3. Analysis.
Comparison 1 Yoga in addition to standard care versus standard care only, Outcome 3 Anxiety.
1.4. Analysis.
Comparison 1 Yoga in addition to standard care versus standard care only, Outcome 4 Depression.
1.5. Analysis.
Comparison 1 Yoga in addition to standard care versus standard care only, Outcome 5 Quality of sleep.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cohen 2004.
| Methods | Study design: RCT. Number of randomised participants: 39. Date of study start: not reported. Date of study end: not reported. Length of intervention: 7 weeks. Duration of units of yoga intervention: not reported. Randomisation
Recruitment period
Median follow‐up time
|
|
| Participants | Eligibility criteria
Exclusion criteria
Patients (N = 39)
Mean age
Gender
Stage of disease (Ann Arbor Criteria) Intervention group: 7 participants had Hodgkin lymphoma, 15 participants were not actively receiving treatment for their cancer, 4 participants were actively receiving chemotherapy.
Control group: 7 participants had Hodgkin lymphoma, 15 participants were not actively receiving treatment for their cancer, 4 participants were actively receiving chemotherapy.
Country
|
|
| Interventions | Tibetan Yoga group
Control group
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
The occurrence of adverse events was not reported. Outcomes were measured at baseline and 1 week, 1 month and 3 months after the last yoga session. |
|
| Notes | The study was supported in part by a grant from the Bruce S. Gelb Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were assigned to either the Tibetan Yoga group or the wait‐list control group once they had completed the baseline questionnaires. Group assignment was conducted sequentially using minimisation, a form of adaptive assignment (...)” Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: “The allocation process was concealed from all investigators because all the relevant information was entered into a computer program and group assignment was determined by the program.” Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel such as the Tibetan Yoga instructor were unblinded, as common in this kind of intervention. Comment: Not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors is not reported. Comment: Not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: ”One patient in the Tibetan Yoga group dropped out of the study before attending any classes, making the number of patients in that group 19; therefore, we did not collect any follow‐up data for this patient”. Quote: "16 of 19 patients (84%) in the Tibetan Yoga group completed at least one follow‐up“. "14 of 19 patients (74%) in the control group completed at least one follow‐up." Comment: Only one participant is reported as a dropout before the end of the trial but follow‐up data are only given for 30 out of 38 participants who completed the trial. Additionally, the reasoning for the dropout is not reported. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Other measures assessing some proposed mediators of the benefits of the yoga program were administered but are not reported here". Comment: Selective reporting of outcomes is unlikely but cannot be excluded. |
| Other bias | Low risk | The study was supported in part by a grant from Bruce S. Gelb Foundation Comment: The study appears to be free of other sources of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamsen 2006 | This study was excluded as it was not an RCT and used a multidimensional exercise intervention instead of yoga as the main intervention. |
| Buffart 2012 | This publication was excluded as it was not an RCT. |
| Dhruva 2012 | This study was excluded because less than 80% of the included participants had a haematological malignancy and the study focused only on pranayama (breathing techniques). |
| Feng 2004 | This study was excluded as it was not an RCT. |
| Fonteyn 2005 | This study was excluded as it was not an RCT and used mindfulness meditation instead of yoga as the main intervention. |
| Habermann 2009 | This study was excluded as it was not an RCT and did not compare a yoga intervention in addition to standard care with standard care only. |
| Jarden 2013 | This study was excluded as it focused on aerobic progression and did not compare a yoga intervention in addition to standard care with standard care only. |
| Kelly 2009 | This study was excluded as it was not an RCT and did not compare a yoga intervention in addition to standard care with standard care only. |
| Kvillemo 2011 | This study was excluded as it was not an RCT and used a mindfulness stress‐reduction training program instead of yoga as the main intervention. |
| Phipps 2012 | This study was excluded as it did not compare a yoga intervention in addition to standard care with standard care only. |
| Shvarts 2013 | This study was excluded as it did not compare a yoga intervention in addition to standard care with standard care only. |
| Silva 2011 | This publication was excluded as it was not an RCT and did not compare a yoga intervention in addition to standard care with standard care only. |
| van Haren 2013 | This publication was excluded as it was not an RCT and did not compare a yoga intervention in addition to standard care with standard care only. |
| Williams 2006 | This study was excluded as it was not an RCT and did not compare a yoga intervention in addition to standard care with standard care only. |
Differences between protocol and review
We have specified the primary outcome from QoL to HRQoL and defined specific scales. This will be relevant for future updates as neither QoL nor HRQoL have been measured in the included trial.
We added distress as a secondary outcome, as it represents one scale of quality of life.
We removed overall survival from the list of outcomes that were planned to be included in the 'Summary of findings' table, as it has not been mentioned in the included trial and to report less than eight outcomes.
We searched additional conference proceedings for complementary medicine (ECIM and Global Advances in Health and Medicine) and ongoing trials (http://clinicaltrials.gov/).
We calculated continuous outcomes as mean differences, as we included only one trial. If more trials will be included in potential future updates, using different scales to measure one outcome, we will calculate continuous outcomes as standardised mean differences.
Contributions of authors
Steffen Felbel: development and writing of protocol, search strategy
Joerg J Meerpohl: clinical and methodological input
Andreas Engert: clinical expertise and advice
Ina Monsef: development of the search strategy
Nicole Skoetz: statistical and methodological expertise and advice
Sources of support
Internal sources
-
University of Cologne, Germany.
Department I of Internal Medicine
External sources
-
NCCAM, USA.
This work was partially funded by Grant Number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM). The contents of this systematic review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or the National Institutes of Health.
Declarations of interest
None known.
New
References
References to studies included in this review
Cohen 2004 {published data only}
- Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul‐Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer 2004;100(10):2253‐60. [PUBMED: 15139072] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adamsen 2006 {published data only}
- Adamsen L, Quist M, Midtgaard J, Andersen C, Moller T, Knutsen L, et al. The effect of a multidimensional exercise intervention on physical capacity, well‐being and quality of life in cancer patients undergoing chemotherapy. Supportive Care in Cancer 2006;14(2):116‐27. [PUBMED: 16096771] [DOI] [PubMed] [Google Scholar]
Buffart 2012 {published data only}
- Buffart LM, Uffelen JG, Riphagen II, Brug J, Mechelen W, Brown WJ, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta‐analysis of randomized controlled trials. BMC Cancer 2012;12:559. [PUBMED: 23181734] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhruva 2012 {published data only}
- Dhruva A, Miaskowski C, Abrams D, Acree M, Cooper B, Goodman S, et al. Yoga breathing for cancer chemotherapy‐associated symptoms and quality of life: results of a pilot randomized controlled trial. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2012;18(5):473‐9. [PUBMED: 22525009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2004 {published data only}
- Feng AY, Hallmeyer S, Peace D, Wunder J, Warber SL, Wilson M, et al. Integrative tumor board: Recurrent lymphoma. Integrative Cancer Therapies 2004;3(3):238‐56. [PUBMED: 15312265] [DOI] [PubMed] [Google Scholar]
Fonteyn 2005 {published data only}
- Fonteyn M, Bauer‐Wu S. Using qualitative evaluation in a feasibility study to improve and refine a complementary therapy intervention prior to subsequent research. Complementary Therapies in Clinical Practice 2005;11(4):247‐52. [PUBMED: 16290895] [DOI] [PubMed] [Google Scholar]
Habermann 2009 {published data only}
- Habermann TM, Thompson CA, LaPlant BR, Bauer BA, Janney CA, Clark MM, et al. Complementary and alternative medicine use among long‐term lymphoma survivors: a pilot study. American Journal of Hematology 2009;84(12):795‐8. [PUBMED: 19894247] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarden 2013 {published data only}
- Jarden M, Moller T, Kjeldsen L, Birgens H, Christensen JF, Bang Christensen K, et al. Patient Activation through Counseling and Exercise‐‐Acute Leukemia (PACE‐AL)‐‐a randomized controlled trial. BMC Cancer 2013;13:446. [PUBMED: 24083543] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2009 {published data only}
Kvillemo 2011 {published data only}
- Kvillemo P, Branstrom R. Experiences of a mindfulness‐based stress‐reduction intervention among patients with cancer. Cancer Nursing 2011;34(1):24‐31. [PUBMED: 20555256] [DOI] [PubMed] [Google Scholar]
Phipps 2012 {published data only}
- Phipps S, Peasant C, Barrera M, Alderfer MA, Huang Q, Vannatta K. Resilience in children undergoing stem cell transplantation: results of a complementary intervention trial. Pediatrics 2012;129(3):e762‐70. [PUBMED: 22311995] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shvarts 2013 {published data only}
- Shvarts V, Chung S. Perampanel: newly approved, novel antiepileptic medication for partial‐onset seizures. Expert Review of Neurotherapeutics 2013;13(2):131‐4. [PUBMED: 23368799] [DOI] [PubMed] [Google Scholar]
Silva 2011 {published data only}
- Silva S, Teboul JL. Defining the adequate arterial pressure target during septic shock: not a 'micro' issue but the microcirculation can help. Critical Care (London, England) 2011; Vol. 15, issue 6:1004. [PUBMED: 22047945] [DOI] [PMC free article] [PubMed]
van Haren 2013 {published data only}
- Haren IE, Timmerman H, Potting CM, Blijlevens NM, Staal JB, Nijhuis‐van der Sanden MW. Physical exercise for patients undergoing hematopoietic stem cell transplantation: systematic review and meta‐analyses of randomized controlled trials. Physical Therapy 2013;93(4):514‐28. [PUBMED: 23224217] [DOI] [PubMed] [Google Scholar]
Williams 2006 {published data only}
- Williams PD, Piamjariyakul U, Ducey K, Badura J, Boltz KD, Olberding K, et al. Cancer treatment, symptom monitoring, and self‐care in adults: pilot study. Cancer Nursing 2006;29(5):347‐55. [PUBMED: 17006107] [DOI] [PubMed] [Google Scholar]
Additional references
Alexander 2008
- Alexander GK, Taylor AG, Innes KE, Kulbok P, Selfe TK. Contextualizing the effects of yoga therapy on diabetes management: a review of the social determinants of physical activity. Family & Community Health 2008;31(3):228‐39. [PUBMED: 18552604] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banasik 2011
- Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. Journal of the American Academy of Nurse Practitioners 2011;23(3):135‐42. [PUBMED: 21355946] [DOI] [PubMed] [Google Scholar]
Banerjee 2007
- Banerjee B, Vadiraj HS, Ram A, Rao R, Jayapal M, Gopinath KS, et al. Effects of an integrated yoga program in modulating psychological stress and radiation‐induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integrative Cancer Therapies 2007;6(3):242‐50. [PUBMED: 17761637] [DOI] [PubMed] [Google Scholar]
Barnes 2008
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. National Health Statistics Reports 2008;10(12):1‐23. [PUBMED: 19361005] [PubMed] [Google Scholar]
Beck 2004
- Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. Journal of Pain and Symptom Management 2004;27(2):140‐8. [PUBMED: 15157038] [DOI] [PubMed] [Google Scholar]
Bidwell 2012
- Bidwell AJ, Yazel B, Davin D, Fairchild TJ, Kanaley JA. Yoga training improves quality of life in women with asthma. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2012;18(8):749‐55. [PUBMED: 22775424] [DOI] [PubMed] [Google Scholar]
Bleiker 2000
- Bleiker EM, Pouwer F, Ploeg HM, Leer JW, Ader HJ. Psychological distress two years after diagnosis of breast cancer: frequency and prediction. Patient Education and Counseling 2000;40(3):209‐17. [PUBMED: 10838000] [DOI] [PubMed] [Google Scholar]
Boehm 2012
- Boehm K, Ostermann T, Milazzo S, Bussing A. Effects of yoga interventions on fatigue: a meta‐analysis. Evidence‐based complementary and alternative medicine : eCAM 2012;2012:124703. [PUBMED: 22991569] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bower 2005
- Bower JE, Woolery A, Sternlieb B, Garet D. Yoga for cancer patients and survivors. Cancer Control: Journal of the Moffitt Cancer Center 2005;12(3):165‐71. [PUBMED: 16062164] [DOI] [PubMed] [Google Scholar]
Bower 2012
- Bower JE, Garet D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer 2012;118(15):3766‐75. [PUBMED: 22180393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2003
- Brown KW, Levy AR, Rosberger Z, Edgar L. Psychological distress and cancer survival: a follow‐up 10 years after diagnosis. Psychosomatic Medicine 2003;65(4):636‐43. [PUBMED: 12883115] [DOI] [PubMed] [Google Scholar]
Buysse 1989
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28(2):193‐213. [PUBMED: 2748771] [DOI] [PubMed] [Google Scholar]
Cabral 2011
- Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta‐analysis. The Primary Care Companion to CNS Disorders 2011;13(4). [PUBMED: 22132353] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 1998
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research 1998;45(1):5‐13. [PUBMED: 9720850] [DOI] [PubMed] [Google Scholar]
Catania 2013
- Catania G, Bell C, Ottonelli S, Marchetti M, Bryce J, Grossi A, et al. Cancer‐related fatigue in Italian cancer patients: validation of the Italian version of the Brief Fatigue Inventory (BFI). Supportive Care in Cancer 2013;21(2):413‐9. [PUBMED: 22790224] [DOI] [PubMed] [Google Scholar]
Chang 2007
- Chang YJ, Lee JS, Lee CG, Lee WS, Lee KS, Bang SM, et al. Assessment of clinical relevant fatigue level in cancer. Supportive Care in Cancer 2007;15(7):891‐6. [PUBMED: 17318593] [DOI] [PubMed] [Google Scholar]
Chen 2005
- Chen SC, Lai YH, Liao CT, Lin CC. Psychometric testing of the Impact of Event Scale‐Chinese Version (IES‐C) in oral cancer patients in Taiwan. Supportive Care in Cancer 2005;13(7):485‐92. [PUBMED: 15717159] [DOI] [PubMed] [Google Scholar]
Coleman 2008
- Coleman EA, Coon SK, Kennedy RL, Lockhart KD, Stewart CB, Anaissie EJ, et al. Effects of exercise in combination with epoetin alfa during high‐dose chemotherapy and autologous peripheral blood stem cell transplantation for multiple myeloma. Oncology Nursing Forum 2008;35(3):E53‐61. [PUBMED: 18467280] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1998
- Collins C. Yoga: intuition, preventive medicine, and treatment. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1998;27(5):563‐8. [PUBMED: 9773368] [DOI] [PubMed] [Google Scholar]
Courneya 2009
- Courneya KS, Sellar CM, Stevinson C, McNeely ML, Friedenreich CM, Peddle CJ, et al. Moderator effects in a randomized controlled trial of exercise training in lymphoma patients. Cancer Epidemiology, Biomarkers & Prevention 2009;18(10):2600‐7. [PUBMED: 19815635] [DOI] [PubMed] [Google Scholar]
Cramer 2012
- Cramer H, Lange S, Klose P, Paul A, Dobos G. Yoga for breast cancer patients and survivors: a systematic review and meta‐analysis. BMC Cancer 2012;12:412. [PUBMED: 22988934] [DOI] [PMC free article] [PubMed] [Google Scholar]
CTCAE 2009
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events Version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed 26 May 2012).
Culos‐Reed 2006
- Culos‐Reed SN, Carlson LE, Daroux LM, Hately‐Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psycho‐Oncology 2006;15(10):891‐7. [PUBMED: 16374892] [DOI] [PubMed] [Google Scholar]
Danhauer 2009
- Danhauer SC, Mihalko SL, Russell GB, Campbell CR, Felder L, Daley K, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psycho‐Oncology 2009;18(4):360‐8. [PUBMED: 19242916] [DOI] [PMC free article] [PubMed] [Google Scholar]
Facts 2013
- The Leukemia & Lymphoma Society. Facts 2013. http://www.lls.org/resourcecenter/freeeducationmaterials/generalcancer/facts (assessed 13 February 2014).
Felbel 2012
- Felbel S, Meerpohl Joerg J, Monsef I, Engert A, Skoetz N. Yoga for haematological malignancies. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010146] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows 2008.
Hagins 2007
- Hagins M, Moore W, Rundle A. Does practicing Hatha yoga satisfy recommendations for intensity of physical activity which improves and maintains health and cardiovascular fitness?. BMC Complementary and Alternative Medicine 2007;7:40. [PUBMED: 18053143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hann 1999
- Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES‐D). Journal of Psychosomatic Research 1999;46(5):437‐43. [PUBMED: 10404478] [DOI] [PubMed] [Google Scholar]
Heim 1993
- Heim HM, Oei TP. Comparison of prostate cancer patients with and without pain. Pain 1993;53(2):159‐62. [PUBMED: 8336985] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Higgins 2011d
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Horowitz 1979
- Horowitz M, Wilner N, Alvarez W. Impact of events scale: measure of subjective stress. Psychosomatic Medicine 1979;41(3):209‐18. [DOI] [PubMed] [Google Scholar]
Howlader 2013
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975‐2010. http://seer.cancer.gov/csr/1975_2010/ (assessed 13 February 2014).
Innes 2005
- Innes KE, Bourguignon C, Taylor AG. Risk indices associated with the insulin resistance syndrome, cardiovascular disease, and possible protection with yoga: a systematic review. Journal of the American Board of Family Practice 2005;18(6):491‐519. [PUBMED: 16322413] [DOI] [PubMed] [Google Scholar]
Julian 2011
- Julian LJ. Measures of anxiety: State‐Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale‐Anxiety (HADS‐A). Arthritis Care & Research 2011;63 Suppl 11:S467‐72. [PUBMED: 22588767] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kangas 2002
- Kangas M, Henry JL, Bryant RA. Posttraumatic stress disorder following cancer. A conceptual and empirical review. Clinical Psychology Review 2002;22(4):499‐524. [PUBMED: 12094509] [DOI] [PubMed] [Google Scholar]
King 2014
- King MT, Bell ML, Costa D, Butow P, Oh B. The Quality of Life Questionnaire Core 30 (QLQ‐C30) and Functional Assessment of Cancer‐General (FACT‐G) differ in responsiveness, relative efficiency, and therefore required sample size. Journal of Clinical Epidemiology 2014;67(1):100‐7. [PUBMED: 24125895] [DOI] [PubMed] [Google Scholar]
Klein 2011
- Klein D, Mercier M, Abeilard E, Puyraveau M, Danzon A, Dalstein V, et al. Long‐term quality of life after breast cancer: a French registry‐based controlled study. Breast Cancer Research and Treatment 2011;129(1):125‐34. [PUBMED: 21340477] [DOI] [PubMed] [Google Scholar]
Kotronoulas 2011
- Kotronoulas GC, Papadopoulou CN, Papapetrou A, Patiraki E. Psychometric evaluation and feasibility of the Greek Pittsburgh Sleep Quality Index (GR‐PSQI) in patients with cancer receiving chemotherapy. Supportive Care in Cancer 2011;19(11):1831‐40. [PUBMED: 20972588] [DOI] [PubMed] [Google Scholar]
Kuttner 2006
- Kuttner L, Chambers CT, Hardial J, Israel DM, Jacobson K, Evans K. A randomized trial of yoga for adolescents with irritable bowel syndrome. Pain Research & Management 2006;11(4):217‐23. [PUBMED: 17149454] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J (editors). Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Lin 2011
- Lin KY, Hu YT, Chang KJ, Lin HF, Tsauo JY. Effects of yoga on psychological health, quality of life, and physical health of patients with cancer: a meta‐analysis. Evidence‐Based Complementary and Alternative Medicine : eCAM 2011;2011:659876. [PUBMED: 21437197] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luebbert 2001
- Luebbert K, Dahme B, Hasenbring M. The effectiveness of relaxation training in reducing treatment‐related symptoms and improving emotional adjustment in acute non‐surgical cancer treatment: a meta‐analytical review. Psycho‐Oncology 2001;10(6):490‐502. [PUBMED: 11747061] [DOI] [PubMed] [Google Scholar]
Mao 2007
- Mao JJ, Farrar JT, Xie SX, Bowman MA, Armstrong K. Use of complementary and alternative medicine and prayer among a national sample of cancer survivors compared to other populations without cancer. Complementary Therapies in Medicine 2007;15(1):21‐9. [PUBMED: 17352968] [DOI] [PubMed] [Google Scholar]
Mello 2003
- Mello M, Tanaka C, Dulley FL. Effects of an exercise program on muscle performance in patients undergoing allogeneic bone marrow transplantation. Bone Marrow Transplantation 2003;32(7):723‐8. [PUBMED: 13130321] [DOI] [PubMed] [Google Scholar]
Mendoza 1999
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85(5):1186‐96. [PUBMED: 10091805] [DOI] [PubMed] [Google Scholar]
Mitchell 2011
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative‐care settings: a meta‐analysis of 94 interview‐based studies. Lancet Oncology 2011;12(2):160‐74. [PUBMED: 21251875] [DOI] [PubMed] [Google Scholar]
Moadel 2007
- Moadel AB, Shah C, Wylie‐Rosett J, Harris MS, Patel SR, Hall CB, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. Journal of Clinical Oncology 2007;25(28):4387‐95. [PUBMED: 17785709] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [PUBMED: 19631508] [DOI] [PubMed] [Google Scholar]
Moyer‐Mileur 2009
- Moyer‐Mileur LJ, Ransdell L, Bruggers CS. Fitness of children with standard‐risk acute lymphoblastic leukemia during maintenance therapy: response to a home‐based exercise and nutrition program. Journal of Pediatric Hematology/Oncology 2009;31(4):259‐66. [PUBMED: 19346877] [DOI] [PubMed] [Google Scholar]
Mystakidou 2008
- Mystakidou K, Tsilika E, Parpa E, Mendoza TR, Pistevou‐Gombaki K, Vlahos L, et al. Psychometric properties of the brief fatigue inventory in Greek patients with advanced cancer. Journal of Pain and Symptom Management 2008;36(4):367‐73. [PUBMED: 18440770] [DOI] [PubMed] [Google Scholar]
NCCAM 2012
- NCCAM. The National Center for Complementary and Alternative Medicine. http://nccam.nih.gov/health/whatiscam (accessed 20 March 2012).
NCCN 2012
- NCCN. NCCN clinical practice guidelines in oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site (accessed 14 April 2012).
Osborne 2012
- Osborne TR, Ramsenthaler C, Siegert RJ, Edmonds PM, Schey SA, Higginson IJ. What issues matter most to people with multiple myeloma and how well are we measuring them? A systematic review of quality of life tools. European Journal of Haematology 2012;89(6):437‐57. [PUBMED: 22985406] [DOI] [PubMed] [Google Scholar]
Pandey 2011
- Pandey A, Tripathi P, Pandey R, Srivatava R, Goswami S. Alternative therapies useful in the management of diabetes: A systematic review. Journal of Pharmacy & Bioallied Sciences 2011;3(4):504‐12. [PUBMED: 22219583] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] [DOI] [PubMed] [Google Scholar]
Persoon 2013
- Persoon S, Kersten MJ, Weiden K, Buffart LM, Nollet F, Brug J, et al. Effects of exercise in patients treated with stem cell transplantation for a hematologic malignancy: a systematic review and meta‐analysis. Cancer Treatment Reviews 2013;39(6):682‐90. [PUBMED: 23485478] [DOI] [PubMed] [Google Scholar]
Radbruch 2003
- Radbruch L, Sabatowski R, Elsner F, Everts J, Mendoza T, Cleeland C. Validation of the German version of the brief fatigue inventory. Journal of Pain and Symptom Management 2003;25(5):449‐58. [PUBMED: 12727043] [DOI] [PubMed] [Google Scholar]
Radloff 1977
- Radloff LS. The CES‐D scale: a new self‐report depression scale for research in the general population. Applied Psychological Meassurement 1977;1:385‐401. [Google Scholar]
Raghavendra 2007
- Raghavendra RM, Nagarathna R, Nagendra HR, Gopinath KS, Srinath BS, Ravi BD, et al. Effects of an integrated yoga programme on chemotherapy‐induced nausea and emesis in breast cancer patients. European Journal of Cancer Care 2007;16(6):462‐74. [PUBMED: 17944760] [DOI] [PubMed] [Google Scholar]
Rao 2009
- Rao MR, Raghuram N, Nagendra HR, Gopinath KS, Srinath BS, Diwakar RB, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: a randomized controlled trial. Complementary Therapies in Medicine 2009;17(1):1‐8. [PUBMED: 19114222] [DOI] [PubMed] [Google Scholar]
Raub 2002
- Raub JA. Psychophysiologic effects of Hatha Yoga on musculoskeletal and cardiopulmonary function: a literature review. Journal of Alternative and Complementary Medicine 2002;8(6):797‐812. [PUBMED: 12614533] [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ross 2010
- Ross A, Thomas S. The health benefits of yoga and exercise: a review of comparison studies. Journal of Alternative and Complementary Medicine 2010;16(1):3‐12. [PUBMED: 20105062] [DOI] [PubMed] [Google Scholar]
Saydah 2006
- Saydah SH, Eberhardt MS. Use of complementary and alternative medicine among adults with chronic diseases: United States 2002. Journal of Alternative and Complementary Medicine 2006;12(8):805‐12. [PUBMED: 17034287] [DOI] [PubMed] [Google Scholar]
Spielberger 1970
- Spielberger CD, Gorsuch R, Lushene RE. STAI manual for the State‐Trait Anxiety Inventory. Consulting Psychologists Press, 1970. [Google Scholar]
Stark 2002
- Stark D, Kiely M, Smith A, Velikova G, House A, Selby P. Anxiety disorders in cancer patients: their nature, associations, and relation to quality of life. Journal of Clinical Oncology 2002;20(14):3137‐48. [PUBMED: 12118028] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Streeter 2012
- Streeter CC, Gerbarg PL, Saper RB, Ciraulo DA, Brown RP. Effects of yoga on the autonomic nervous system, gamma‐aminobutyric‐acid, and allostasis in epilepsy, depression, and post‐traumatic stress disorder. Medical Hypotheses 2012;78(5):571‐9. [PUBMED: 22365651] [DOI] [PubMed] [Google Scholar]
Sundin 2002
- Sundin EC, Horowitz MJ. Impact of Event Scale: psychometric properties. British Journal of Psychiatry 2002;180:205‐9. [PUBMED: 11872511] [DOI] [PubMed] [Google Scholar]
Sundin 2003
- Sundin EC, Horowitz MJ. Horowitz's Impact of Event Scale evaluation of 20 years of use. Psychosomatic Medicine 2003;65(5):870‐6. [PUBMED: 14508034] [DOI] [PubMed] [Google Scholar]
Thorsen 2005
- Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health‐related quality of life in young and middle‐aged cancer patients shortly after chemotherapy. Journal of Clinical Oncology 2005;23(10):2378‐88. [PUBMED: 15800330] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vadiraja 2009
- Vadiraja HS, Rao MR, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, et al. Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complementary Therapies in Medicine 2009;17(5‐6):274‐80. [PUBMED: 19942107] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PUBMED: 1593914] [PubMed] [Google Scholar]
Woodyard 2011
- Woodyard C. Exploring the therapeutic effects of yoga and its ability to increase quality of life. International Journal of Yoga 2011;4(2):49‐54. [PUBMED: 22022122] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wren 2011
- Wren AA, Wright MA, Carson JW, Keefe FJ. Yoga for persistent pain: new findings and directions for an ancient practice. Pain 2011;152(3):477‐80. [PUBMED: 21247696] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2012
- Zhang J, Yang KH, Tian JH, Wang CM. Effects of yoga on psychologic function and quality of life in women with breast cancer: a meta‐analysis of randomized controlled trials. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2012;18(11):994‐1002. [PUBMED: 22909345] [DOI] [PubMed] [Google Scholar]


