Abstract
The tumor microenvironment is abundant with exosomes that are secreted by the cancer cells themselves. Exosomes are nanosized, organelle-like membranous structures that are increasingly being recognized as major contributors in the progression of malignant neoplasms. A critical element in melanoma progression is its propensity to metastasize, but little is known about how melanoma cell-derived exosomes modulate the microenvironment to optimize conditions for tumor progression and metastasis. Here, we provide evidence that melanoma cell-derived exosomes promote phenotype switching in primary melanocytes through paracrine/autocrine signaling. We found that the mitogen-activated protein kinase (MAPK) signaling pathway was activated during the exosome-mediated epithelial-to-mesenchymal transition (EMT)-resembling process, which promotes metastasis. Let-7i, an miRNA modulator of EMT, was also involved in this process. We further defined two other miRNA modulators of EMT (miR-191 and let-7a) in serum exosomes for differentiating stage I melanoma patients from non-melanoma subjects. These results provide the first strong molecular evidence that melanoma cell-derived exosomes promote the EMT-resembling process in the tumor microenvironment. Thus, novel strategies targeting EMT and modulating the tumor microenvironment may emerge as important approaches for the treatment of metastatic melanoma.
Keywords: Melanoma, Exosomes, Tumor progression, Epithelial-to-mesenchymal transition, Paracrine/autocrine, Microenvironment
1. Introduction
Exosomes (40–100 nm) are organelle-like lipid-bound membranous structures shed from cell membranes into interstitial spaces and body fluids during tumor development [1]. The microenvironment of a growing tumor is enriched in exosomes that are secreted by cancer cells. Cancer cells manipulate their microenvironment to optimize conditions for growth and metastasis in multiple ways [1–3]. One of the pathways to communicate with neighboring nonneoplastic cells is through paracrine/autocrine signaling mediated by cancer exosomes [1–3]. Intercellular cross-talk between neighboring cells and tumor cells via exosomes in the extracellular environment plays a crucial role in the establishment of mesenchymal status, cancer development, and metastasis [1, 4].
The course of metastasis involves multiple steps, including epithelial-to-mesenchymal transition (EMT) [2, 5]. Melanoma is a melanocyte-originated malignant disease with a high propensity to metastasize. Although melanocytes do not belong to the epithelial lineage, and the term “EMT” may not formally be attributed to melanoma, primary melanocytes do express Ecadherin, which is required for their contact with keratinocytes in the basal layer of the epidermis [6]. Therefore, the EMT-resembling process still contributes to the progression and metastasis of melanoma. In contrast to the majority of epithelial tissues, normal melanocytes express EMT-inducing transcription factors. This is considered an intrinsic factor predisposing melanoma to high metastatic propensity [6]. However, there is little known about how tumor-derived exosomes induce plasticity as EMT in the tumor microenvironment. A better understanding of how tumor-derived exosomes contribute to the manipulation of the tumor microenvironment may present a novel therapeutic modality to target melanoma progression. With this in mind, we pursued the mechanisms of melanoma cell-derived exosomes on cancer growth and progression in the tumor microenvironment. We further explored the possibility of using serum exosomes as potential molecular identifiers in melanoma patients. We demonstrate that melanoma cell-derived exosomes can promote phenotype switching in primary melanocytes through paracrine/autocrine signaling. The mitogen-activated protein kinase (MAPK) signaling pathway and one of the miRNA modulators of EMT (let-7i) were found to be involved in this process. Furthermore, we identified two miRNA modulators of EMT (miR-191 and let-7a) in the serum exosomes of patients with melanoma. Our results suggest that melanoma cells can modulate the microenvironment to maximize its metastatic potential through the EMT-resembling process via self-secreting exosomes. Accordingly, miRNA modulators of EMT in serum exosomes may be used as potential biomarkers or targets for melanoma. Our work may shift research emphasis from genetic changes in melanoma cells to the microenvironment control of cell plasticity by exosomes shed from melanoma cells themselves.
2. Materials and methods
A detailed description of materials and methods is provided in the supplementary text. These data include cell culture conditions, real-time imaging of exosome interaction with melanocytes, RNA isolation, mRNA and miRNA expression by semi-quantitative reverse transcription–PCR, transfection of miRNA, Western blotting, immunofluorescence staining, in vitro migration/invasion assay, wound healing in vitro assay, and RT2 profiler PCR array for MAP kinase signaling pathway.
2.1. Cell lines and culture reagents
Two primary human epidermal melanocytes, HEMa-LP and NHEM-c cells, were purchased from Life Technologies (Carlsbad, CA) and PromoCell (Heidelberg, Germany), respectively. The two human malignant melanoma cell lines, A375 and SK-MEL-28, and the two human lung cancer cell lines, A549 and H1299, were purchased from American Type Culture Collection (Rockville, MD).
2.2. Isolation of exosomes
Exosomes were purified from cell culture supernatants by differential centrifugation as previously described [7, 8]. Briefly, culture medium was collected and centrifuged at 400 × g for 10 min to remove whole cells. The supernatant was then centrifuged at 15,000 × g for 20 min to remove debris. This concentrated material was then ultracentrifuged at 100,000 × g for 90 min to generate the exosome pellet. The pellet was resuspended and washed twice with phosphate buffered saline (PBS). The quantity of the exosomes was determined using a Nanodrop ND-1000 spectrophotometer at 420 nm (Thermo Fisher Scientific, Pittsburgh, PA) [9].
Isolation of exosomes from blood samples was performed using ExoQuick precipitation (System Biosciences, Mountain View, CA). Briefly, 1 ml of serum was centrifuged at 13,000 × g for 15 min to remove debris. The supernatant was mixed with 250 µl of ExoQuick and refrigerated overnight. The ExoQuick/supernatant mixture was then centrifuged at 12,000 × g for 5 min at 4°C, which produced the exosome pellet for RNA isolation.
2.3. Human specimens
De-identified human blood samples from the Louisville Cooperative Tissue Biorepository were used. This study was approved by the University of Louisville Institutional Review Board. All samples were acquired after subjects had provided written informed consent. One ml of serum samples were obtained from each patient diagnosed with stage I melanoma (n2=21) prior to any initial treatment. Age- and sex-matched samples from non-melanoma subjects were selected as controls (n1=20). Control subjects were those who were excluded from any inflammation diseases or immune diseases that may cause high levels of serum exosomes. The patients’ demographics are shown in the Table 1.
Table 1.
Demographics of stage I melanoma patients and non-melanoma subjects
| Non-melanoma subjects (n1 =20) |
Stage I melanoma patients (n2 =21) |
|
|---|---|---|
| Age at diagnosis | ||
| Mean (SD) | 56 (15) | 53 (12) |
| Median (range) | 58 (28–75) | 52 (31–70) |
| Gender | ||
| Male | 10 | 9 |
| Female | 10 | 12 |
2.4. Statistical analysis
Data were representative images or given as mean ± SD. The difference between groups was analyzed by ANOVA or a two-tailed Student t-test as applicable. A p-value of < 0.05 was considered to be statistically significant. By fitting the logistic regression model, we also estimated the AUC (the area under ROC [Receiver Operating Characteristic] curve) for each miRNA in serum exosomes using SAS procedure (Proc Logistic). All calculations were performed with SAS statistical software (SAS Institute Inc., Cary, NC).
3. Results
3.1. Communication of melanoma cell-derived exosomes with adjacent primary melanocytes
To assess how the melanoma cell-derived exosomes communicate with the surrounding cells, we labeled the A375 melanoma cell-derived exosomes with PKH26 (red fluorescence dye) and NHEM-c primary melanocytes with PKH67 (green fluorescence dye), respectively, and tracked their communications. Within 16 h of co-culturing, A375 melanoma cell-derived exosomes (red-labeled) were initially present around primary melanocytes (green-labeled) (Fig. 1A). As time elapsed, the red-labeled exosomes merged with the membrane of the green-labeled primary melanocytes and changed in color from red or green to orange (Fig. 1 A–D). A live video of melanoma cell-derived exosomes interacting with the nearby melanocytes was also recorded (https://youtu.be/jeEH4iFqRLw). This result suggests that melanoma cell-derived exosomes can actively communicate with the nearby melanocytes.
Figure 1. A375 melanoma cell-derived exosomes communicate with NHEM-c primary melanocytes.
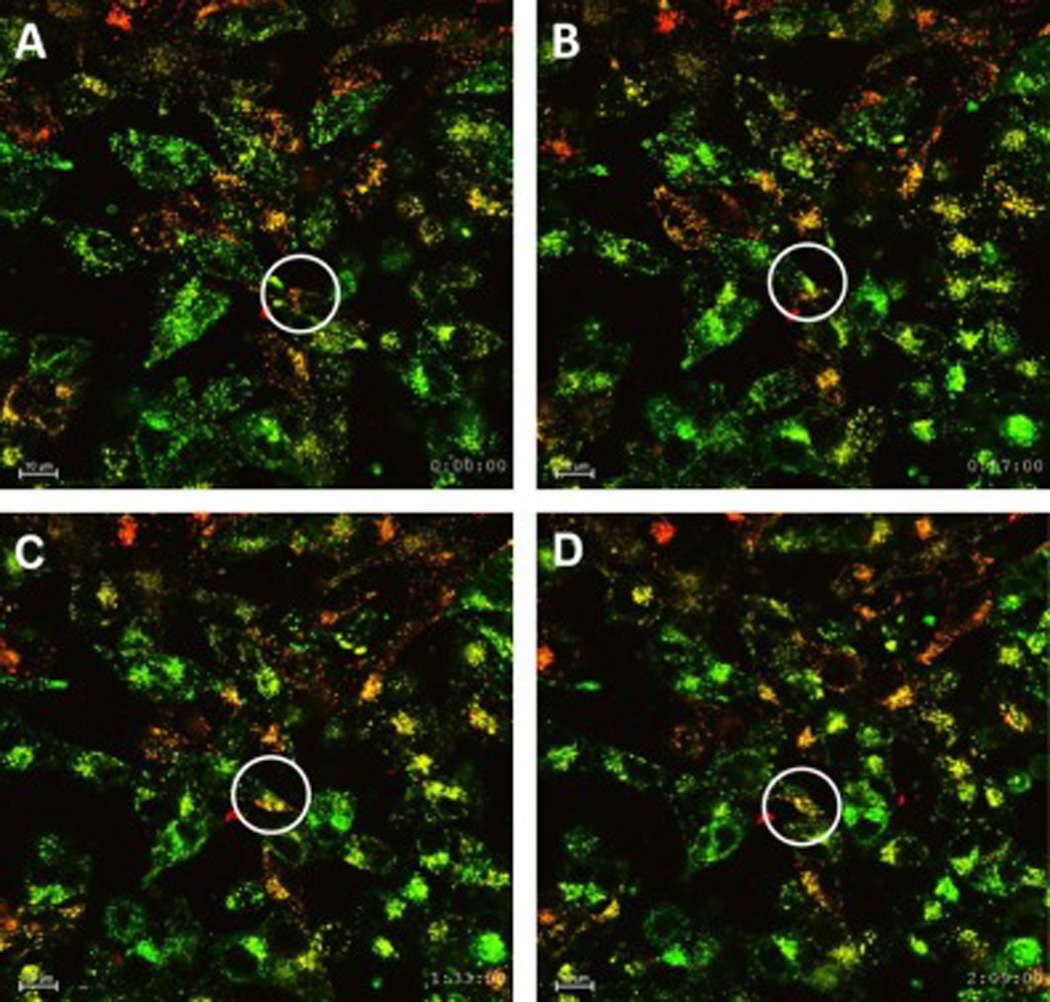
NHEM-c primary melanocytes (1 × 106) were labeled with PKH67green fluorescence dye and seeded into a 35-mm FluoroDish. A375 melanoma cell-derived exosomes (100 µl) were labeled with PKH26 red fluorescence dye and added into the dish. Exosomes were determined with OD420 reading of 0.05. After 16 h of co-culturing with melanocytes and melanoma cell-derived exosomes, live cell images were obtained every 3 min for 3 consecutive h. Communication of exosomes with cells is indicated by circles at designated time points (×600 magnification, scale bar: 10 µm). Figure 1 A–D shows results as time elapsed: Time=0 (A); Time=57 min (B); Time=1 h 33 min (C); Time=2 h 9 min (D).
3.2. Melanoma cell-derived exosomes promoted invasion and migration of primary melanocytes
Based on the above observations, we were interested in exploring the effects of the interaction between the melanoma cell-derived exosomes and the recipient cells. Since tumor-derived exosomes can actively transport their molecules into the cells, we hypothesized whether tumor cell-derived exosomes could activate neighboring primary melanocytes and elicit reciprocal tumor-promoting functions from these cells. To evaluate this, we first conducted wound healing in vitro assay studies to assess cell motility after melanoma cell-derived exosomes were added into the media of the primary melanocytes. The migration rate of the HEMa-LP cells and NHEM-c cells was significantly increased after A375 or SK-MEL-28 cellderived melanoma exosomes were added (Fig. 2A).
Figure 2. Tumor cell-derived exosomes facilitate the migration (A, D) and invasion (B, C) of melanocytes.
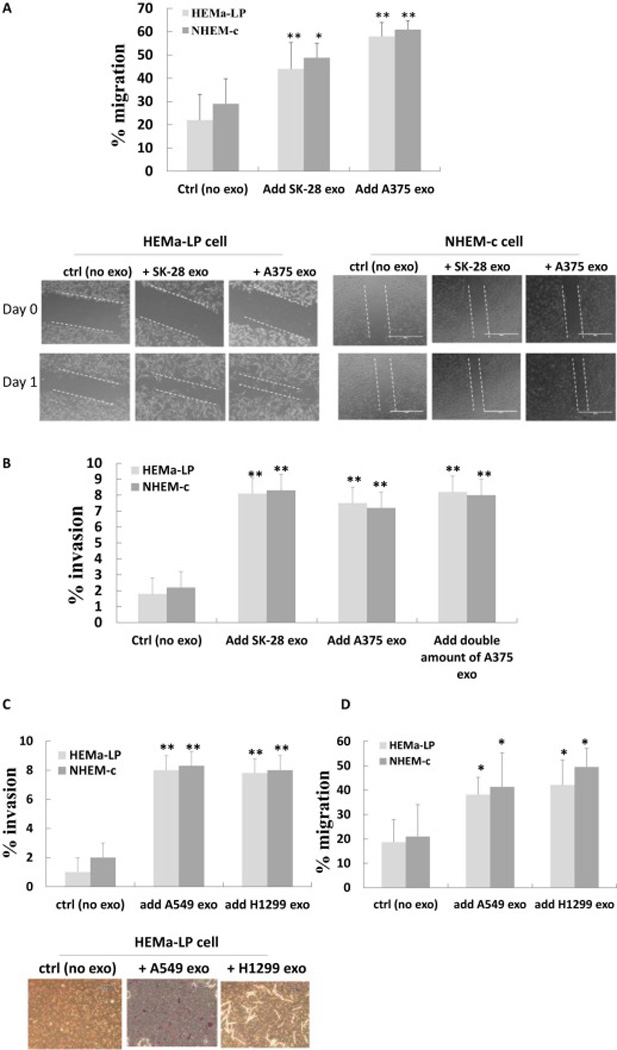
(A, D) Wound healing in vitro assay of HEMa-LP and NHEM-c cells after co-culturing with tumor-cell derived exosomes. HEMa-LP or NHEM-c cells (8×104) were seeded in 24-well plates. Melanoma cell-derived exosomes (100 µl) (A) or lung cancer cell-derived exosomes (100 µl) (D) were added in each well the next day. Exosomes were determined with an OD420 reading of 0.05. Serum-free media was used as a control (no exosomes). Wounds were created and photographed at time zero (t=0 h) and at 24 h after adding tumor cell-derived exosomes (t=24 h). Quantification of the migration rate is shown in the upper panel of A and in D (* p<0.05, ** p<0.01, n=3). Migration rate of the HEMa-LP cells and NHEM-c cells significantly increased after adding A375 or SK-MEL-28-derived melanoma exosomes (A) and A549 or H1299-derived lung cancer exosomes (D) in those cells. The photographed images are shown in the lower panel of A (×100 magnification, scale bar: 1000 µm and refers to all panels). (B, C) Invasion assay of HEMa-LP and NHEM-c cells after co-culturing with tumor-cell derived exosomes. HEMa-LP cells or NHEM-c cells (2.8×104) were plated in control chambers and invasion chambers in 24-well plates. A375 or SK-MEL-28-derived melanoma exosomes (50 ul) (B) and A549 or H1299-derived lung cancer exosomes (50 ul) (C) were added to each chamber every other day as designated vs controls (no exosomes). Exosomes were determined with an OD420 reading of 0.05. After 6 days of co-culturing of melanocytes with tumor cell-derived exosomes, quantification of the percent invasion of HEMa-LP or NHEM-c cells was calculated (* p<0.05, ** p<0.01, n=3). The percent invasion of HEMa-LP cells or NHEM-c cells after co-culturing with tumor-cell derived exosomes significantly increased compared to the control melanocytes without adding melanoma cell-derived exosomes. Lower panel of C shows photographed images (×200 magnification, scale bar: 600 µm and refers to all panels) (ctrl: control, exo: exosome).
We then conducted invasion assay studies to further assess the behaviors of primary melanocytes after co-culturing with the melanoma cell-derived exosomes. After incubation of A375 or SK-MEL-28 cell-derived exosomes with HEMa-LP or NHEM-c cells for 6 days, the results showed that the percentage of invasion of HEMa-LP cells or NHEM-c cells was significantly higher than those of the control melanocytes without melanoma cell-derived exosomes added (Fig. 2B). Interestingly, when the amount of melanoma cell-derived exosomes was doubled, the invasion ability of the primary melanocytes increased, but it was not doubled (Fig. 2B). One possibility for this result could be that, once the invasion ability of the primary melanocytes was activated by tumor cell-derived exosomes, the invasion ability of the primary melanocytes could not increase proportionately with the amount of the tumor cell-derived exosomes. We further assessed whether the increased invasion ability of primary melanocytes promoted by the melanoma cell-derived exosomes was tumor cell-specific. The results showed that the percentage of invasion of HEMa-LP cells and NHEM-c cells was also increased after coculturing with A549 or H1299 lung cancer cell-derived exosomes for 6 days (Fig. 2C). Similarly, the migration rate of the HEMa-LP cells and NHEM-c cells was significantly increased after A549 or H1299 lung cancer cell-derived exosomes were added (Fig. 2D).
Taken together, these results suggest that melanoma cell-derived exosomes may facilitate tumor expansion and progression by favoring the invasion and migration of the surrounding primary melanocytes. The lung cancer cell-derived exosomes have similar effects in promoting primary melanocytes’ migration and invasion, implying that the increased cell motility and invasion abilities promoted by tumor cell-derived exosomes are not cell line-specific.
3.3. Changes of EMT markers in primary melanocytes promoted by melanoma cell-derived exosomes
After observing the functional changes of the primary melanocytes advanced by melanoma cell-derived exosomes, we were interested in learning more about the mechanisms that promote those changes. Since EMT has been implicated as a cellular process during tumorigenesis that facilitates tumor cell invasion and metastasis, we speculated that the increased migration and invasion abilities in melanocytes following the addition of the tumor cell-derived exosomes were concomitant with the acquisition of an EMT-like state. To better understand this, we first studied the expression of EMT markers by real-time RT-PCR. After co-culturing with A375 or SK-MEL-28-derived exosomes, the epithelial marker E-cadherin was significantly downregulated, while the mesenchymal marker vimentin was upregulated considerably in HEMa-LP and NHEM-c cells (Fig. 3A). Another mesenchymal marker, N-cadherin, was barely detected in both of the primary melanocytes by real-time RT-PCR (data not shown). Western blot analysis confirmed the same trend of changes in the epithelial marker E-cadherin and the mesenchymal marker vimentin in HEMa-LP cells and NHEM-c cells after co-culturing with melanoma cell-derived exosomes (Fig. 3B). Results of immunofluorescence staining of the mesenchymal marker vimentin also showed its stronger expression in HEMa-LP and NHEM-c cells after A375 or SK-MEL-28-derived exosomes were added compared with the control cells without melanoma cell-derived exosomes added (Fig. 3C). The expression level changes of E cadherin and vimentin in melanocytes were consistent with the increased migration and invasion abilities induced by the tumor cell-derived exosomes.
Figure 3. Acquisition of an EMT-like state in primary melanocytes promoted by melanoma cellderived exosomes.
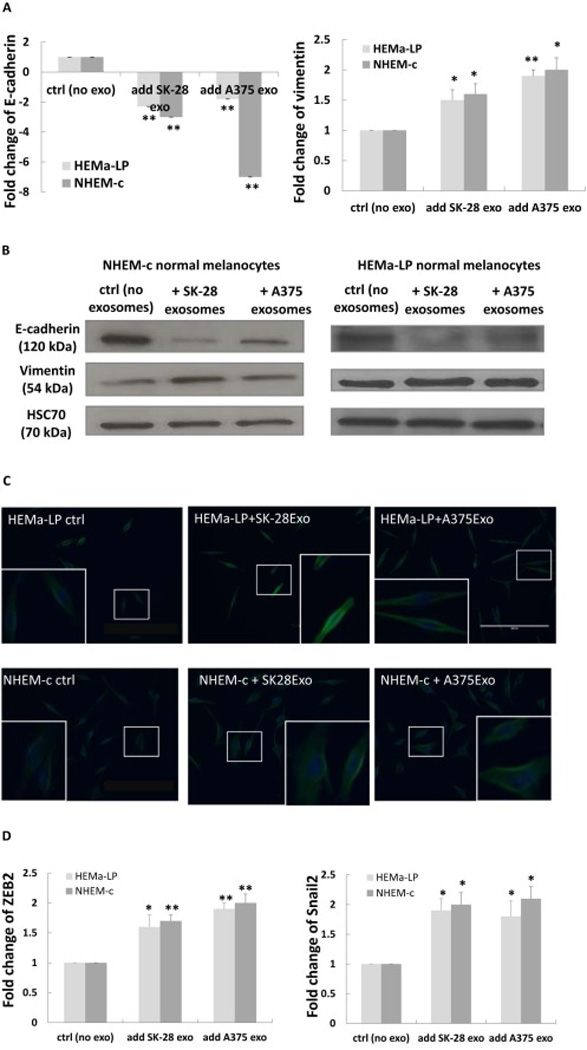
Downregulation of E-cadherin and upregulation of vimentin in primary melanocytes (HEMa-LP and NHEM-c) after taking up melanoma cell-derived exosomes by real-time RT-PCR (A) and by Western blot analysis (B). (A) HEMa-LP or NHEM-c cells (7×105) were seeded in 6-well plates and treated with melanoma cellderived exosomes as described in Fig. 2A. After co-culturing (24 h) of melanocytes with melanoma cell-derived exosomes, total RNA from melanocytes were extracted. Expression levels of designated mRNA were analyzed by real-time RT-PCR (* p<0.05, ** p<0.01, n=3). (B) HEMa-LP or NHEM-c cells (6×105) were seeded in 6-well plates and treated with melanoma cell-derived exosomes every other day as described in Fig. 2B. Cell protein expressions were analyzed using the indicated antibodies after 6 days of co-culturing of primary melanocytes with melanoma cell-derived exosomes. (C) Enhanced vimentin expression after adding melanoma cell-derived exosomes by immunofluorescence staining. HEMa-LP and NHEM-c melanocytes (2×105) were seeded in 12-well plates and treated with melanoma cell-derived exosomes as described in Fig. 2A. After of co-culturing (72 h) of melanocytes with melanoma cell-derived exosomes, cells were fixed and stained for vimentin. The images show overlay of vimentin detected using Alexa Fluor 488 goat anti-rabbit IgG (green) and counter-stained with 4,6-diamidino-2-phenylindole (DAPI) (blue) (X200 magnification, scale bar: 200 µm). The insert represents higher magnification of the boxed area. (D) Upregulation of ZEB2 and Snail 2 by real-time RT-PCR after co-culturing (24 h) of HEMa-LP and NHEM-c primary melanocytes with A375 or SK-MEL-28-derived exosomes (* p<0.05, ** p<0.01, n=3). HEMa-LP or NHEM-c cells were seeded and treated as described in Fig. 2A. Real-time RT-PCR were performed and analyzed as described in Fig. 3A (ctrl: control, exo: exosome).
Transcriptional repressors, such as Snail and ZEB family members, are known to be key regulators in inducing the process of EMT [6, 10, 11]. We determined the expression status of these transcription factors in melanocytes after co-culturing with the melanoma cell-derived exosomes. Since ZEB1 and Snail 1 have very low expression in both of the melanocytes HEMa-LP and NHEM-c (data not shown), we focused on ZEB2 and Snail 2. The results showed that the expression of ZEB2 and Snail 2 in the primary melanocytes was upregulated after co-culturing with the melanoma cell-derived exosomes (Fig. 3D), concomitant with the decreased expression of E-cadherin and increased expression of vimentin. These findings indicated that taking-up of melanoma cell-derived exosomes by melanocytes can lead to the upregulation of ZEB2 and Snail 2 in melanocytes, resulting in downregulated expression of the epithelial marker gene, upregulated expression of the mesenchymal marker gene, and acquisition of the EMT-like phenotype.
Overall, these data show that the melanoma cell-derived exosomes may boost cell migration and invasion through the modulation of the EMT-resembling process.
3.4. Melanoma cell-derived exosome-mediated EMT was regulated by let-7i
The let-7 family has been implicated in the regulation of EMT [12]. We quantitated the expression of let-7i to evaluate whether this miRNA could play a role in melanoma cell-derived exosome-mediated EMT. The expression of let-7i in HEMa-LP and NHEM-c cells was downregulated significantly after co-culturing with A375 or SK-MEL-28 cell-derived exosomes (Fig. 4A). After transfection of let-7i mimic into the HEMa-LP and NHEM-c cells followed by co-culturing with A375 or SK-MEL-28 cell-derived exosomes, the invasion ability of the HEMa-LP and NHEM-c cells was significantly decreased compared with the melanocytes transfected with miRNA mimic negative controls followed by co-culturing with A375 or SK-MEL-28 cellderived exosomes (Fig. 4B). Along with the decreased cell invasion ability, transfection of let-7i mimic followed by co-culturing with A375 or SK-MEL-28 cell-derived exosomes could lead to the re-expression of the epithelial marker E-cadherin and the inhibition of the mesenchymal marker vimentin as compared with control melanocytes. Figure 4C shows results of real-time RT-PCR analysis as evidence of this change. Immunofluorescence staining of the mesenchymal marker vimentin in Figure 4D shows reduced expression in NHEM-c primary melanocytes cocultured with A375 or SK-MEL-28 cell-derived exosomes after transfection of the let-7i mimic versus transfection of miRNA mimic negative control (Fig. 4D). These results suggest that let-7i could regulate the invasion ability of the primary melanocytes driven by the melanoma cellderived exosomes through the modulation of the expression of EMT markers.
Figure 4. Melanoma cell-derived exosome-mediated EMT in primary melanocytes was regulated by let-7i.
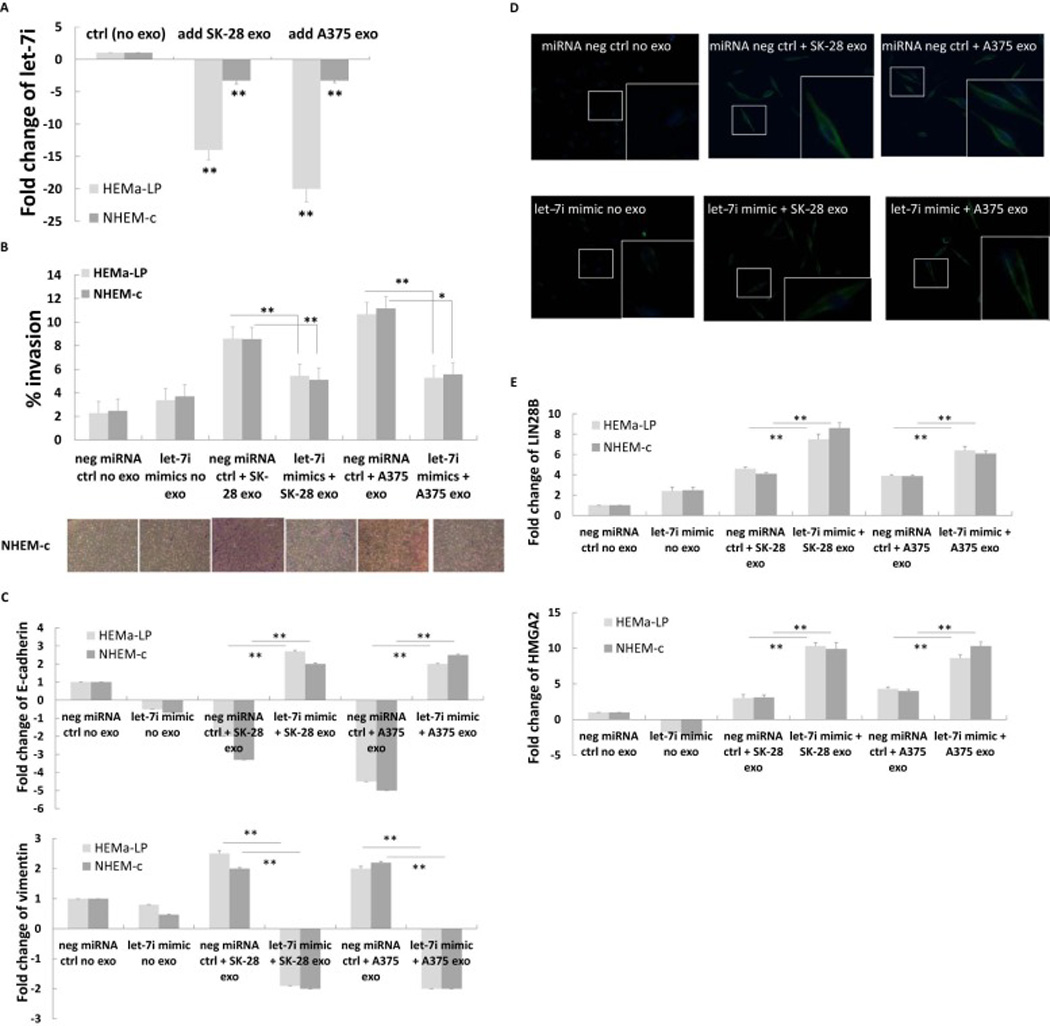
(A) Downregulation of let-7i in primary melanocytes after co-culturing with melanoma cell-derived exosomes by real time RT-PCR. HEMa-LP or NHEM-c cells (7×105) were seeded in 6-well plates and treated with melanoma cell-derived exosomes as described in Fig. 2A. After co-culturing (24 h) of HEMa-LP and NHEM-c primary melanocytes with A375 or SK-MEL-28-derived exosomes, total RNA from melanocytes were extracted. Total RNA (10 ng) was converted into cDNA by miRNA reverse transcription kit and used to perform miRNA real time RT-PCR (** p<0.01, n=3). (B) Decreased invasion abilities of HEMa-LP and NHEM-c cells after transfection of let-7i mimic followed by co-culturing with A375 or SK-MEL-28 cell-derived exosomes vs transfection of miRNA mimic negative control followed by co-culturing with A375 or SK-MEL-28 cell-derived exosomes by invasion assay. HEMa-LP cells or NHEM-c cells (2.8×104) were plated in control chambers and invasion chambers in 24-well plates. Cells were transfected with either let-7i mimic or miRNA mimic negative control the next day. After 8 h of transfection, 50 ul of melanoma cell-derived exosomes determined with an OD420 reading of 0.05 were added to each chamber as designated. Serum-free media was used as a control (no exosomes). After HEMa-LP or NHEM-c cells were co-cultured 5 days with A375 or SK-MEL-28 cell-derived exosomes, quantification of the percent invasion of HEMa-LP or NHEM-c cells was calculated (* p<0.05, ** p<0.01, n=3). The photographed images are shown in the lower panel (x200 magnification, scale bar: 600 µm and refers to all panels). (C) Transfection of let-7i mimic followed by co-culturing with A375 or SK-MEL-28 cell-derived exosomes resulted in the re-expression of the epithelial marker E-cadherin and the inhibition of the mesenchymal marker vimentin by real-time RT-PCR. NHEM-c or HEMa-LP cells (7×105) were seeded in 6-well plates. Transfection of the miRNA mimic and co-culturing of the HEMa-LP and NHEM-c melanocytes with melanoma cell-derived exosomes were performed as described in B. Cells were harvested for real-time RT-PCR analysis after co-culturing (24 h) with melanoma exosomes (** p<0.01, n=3). (D) Decreased vimentin expression in primary melanocytes after transfection of let-7i mimic followed by adding melanoma cell-derived exosomes by immunofluorescence staining. NHEM-c melanocytes (2×105) were seeded in 12-well plates. Transfection of the let-7i miRNA mimic and co-culturing of the NHEM-c melanocytes with melanoma cell-derived exosomes were performed as described in B. Cells were fixed and stained for vimentin after 24 h of co-culturing of NHEM-c melanocytes with A375 or SK-MEL-28 cell-derived exosomes. The images show overlay of vimentin detected using Alexa Fluor 488 goat anti-rabbit IgG (green) and counter-stained with 4,6-diamidino-2-phenylindole (DAPI) (blue) (X200 magnification). The insert represents higher magnification of the boxed area (neg ctrl: negative control, exo: exosome). (E) Upregulation of LIN28B and HMGA2 by real-time RT-PCR after transfection of let-7i mimic followed by co-culturing with A375 or SK-MEL-28 cell-derived exosomes. NHEM-c or HEMa-LP cells (7×105) were seeded in 6-well plates. Transfection of the miRNA mimic and co-culturing of the HEMa-LP and NHEM-c melanocytes with melanoma cell-derived exosomes were performed as described in B. Cells were harvested for real-time RT-PCR analysis after co-culturing (24 h) with melanoma exosomes (** p<0.01, n=3).
LIN28B and HMGA2 are the two specific targets of let-7i that are likely to contribute to EMT [13, 14]. We assessed whether let-7i mimic transfection would cause changes in the two target genes and regulate the process of the melanoma cell-derived exosome-mediated EMT. The results showed that transfection of let-7i mimic followed by co-culturing with A375 or SK-MEL-28 cell-derived exosomes could lead to the significant upregulation of LIN28B and HMGA2 as compared with the transfection of control miRNA followed by co-culturing with A375 or SK-MEL-28 cell-derived exosomes (Fig. 4E). These results imply that let-7i may regulate the process of melanoma cell-derived exosome-mediated EMT through LIN28B and HMGA2.
3.5. MAPK pathway was involved in melanoma cell-derived exosome-mediated EMT
Studies have demonstrated that activation of the MAPK pathway leads to the induction of EMT, resulting in cell invasion [15]. We assessed whether the MAPK pathway was involved in the melanoma cell-derived exosome-mediated EMT-resembling process. The results of the MAPK RT2 profiler PCR array showed a significant induction of several members of the mitogen-activated protein kinase kinase kinase (MKKK) (Fig. 5A), mitogen-activated protein kinase kinase (MKK) (Fig. 5B), and mitogen-activated protein kinases (MAPK) (Fig. 5C) in NHEM-c cells followed by co-culturing for 24 h with SK-MEL-28-derived exosomes vs NHEM-c control cells without exosomes added. We selected one kinase from each of the MKKK, MKK, and MAPK groups and conducted RT-PCR studies to confirm the data from the RT2 profiler PCR array. Activation of major MAP kinases, such as MAP3K4 (from MKKK group), MAP2K5 (from MKK group), and MAPK13 (from MAPK group) was validated in HEMa-LP and NHEMc cells after co-culturing with A375 or SK-MEL-28-derived exosomes (Fig. 5D, 5E). These results suggest that activation of the MAPK pathway is likely to have a crucial role in the signaling cascade, leading to the activation of the melanoma cell-derived exosome-mediated EMT-resembling process and resulting in melanoma invasion.
Figure 5. Activation of the MAPK signaling pathway in melanoma cell-derived exosomemediated EMT.
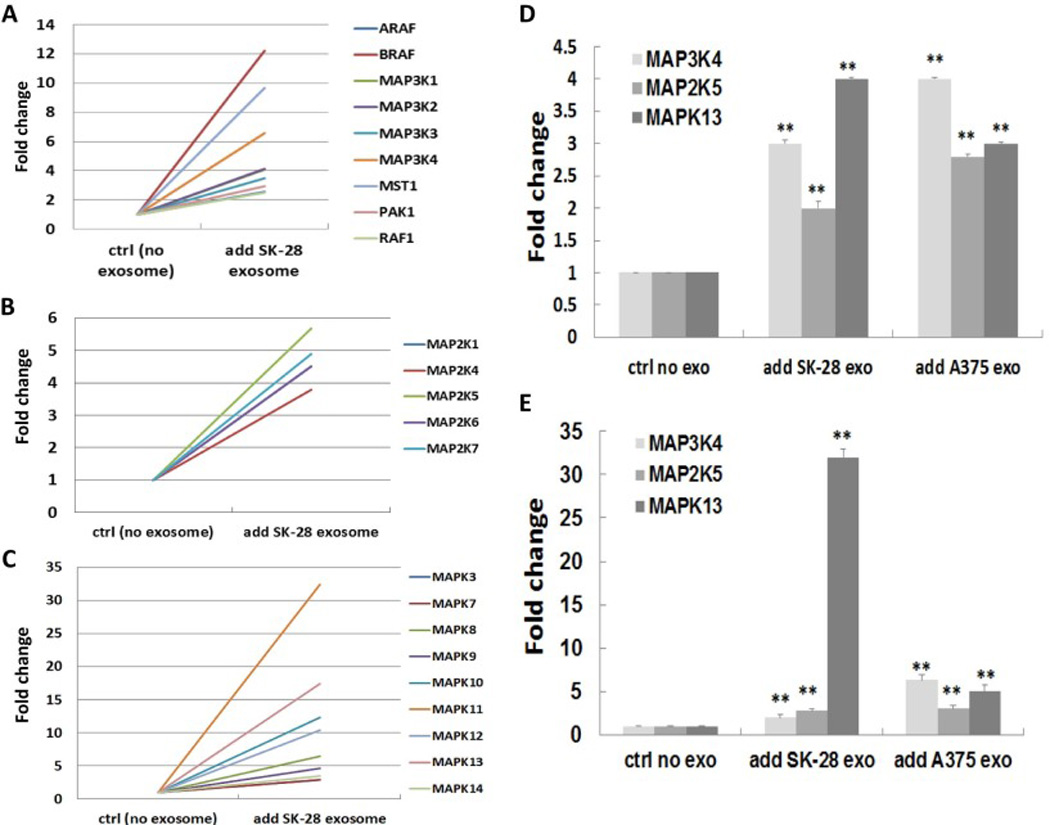
Activation of the genes in the MKKK group (A), MKK group (B), and MAPK group (C) in NHEM-c cells after co-culturing with SK-MEL-28 cell-derived exosomes by RT2 profiler PCR array analysis. NHEM-c cells (7×105) were seeded in 6-well plate. SK-MEL-28 cell-derived exosomes (100 ul) determined with an OD420 reading of 0.05 were added to the well. Serum-free media was used as a control (no exosomes). After 24 h of co-culturing, NHEM-c cells were harvested for RNA extraction and RT2 profiler PCR array analysis. (D, E) Upregulation of the major MAPK pathway molecules in HEMa-LP (D) and NHEM-c cells (E) after co-culturing with melanoma cell-derived exosomes was confirmed by real-time RT-PCR. HEMa-LP and NHEM-c cells (7×105) were seeded in 6-well plates and treated as described in Fig. 2A. After co-culturing (24 h) of HEMa-LP and NHEM-c cells with melanoma cell-derived exosomes, total RNA from cells were extracted and analyzed by real-time RT-PCR as described in Fig. 3A (** p<0.01, n=3) (ctrl: control, exo: exosome).
3.6. Differential expression of the miRNA EMT regulators in melanoma patients versus nonmelanoma subjects
miRNAs are the most abundant and most active cargoes in exosomes. Therefore, we investigated whether the EMT-related miRNAs in serum exosomes were dysregulated in melanoma patients, and whether they could serve as potential biomarkers in identifying early stage (stage I) melanoma patients. We compared the expression levels of several exosome miRNAs of EMT regulators between stage I melanoma patients and non-melanoma subjects. The results showed that miR-191 and let-7a were upregulated 3.6- and 4.1-fold, respectively, in stage I melanoma patients as compared with non-melanoma subjects (Table 2). The calculated AUC for miR-191 and let-7a was 0.699 and 0.763, respectively (Table 2). Another two EMT-related miRNAs, miR-23a and let-7i, were upregulated 3.96- and 5.1-fold, respectively, in stage I melanoma patients as compared with non-melanoma subjects, but without statistical significance (Table 2). These results suggested that miR-191 and let-7a were differentially expressed in stage I melanoma patients as compared with non-melanoma subjects. These EMT-related miRNAs might be potential identifiers for distinguishing stage I melanoma patients from non-melanoma subjects.
Table 2.
Differentially expressed exosome miRNAs in stage I melanoma patients compared to non-melanoma subjects
| miRNAs | Fold change | P value | AUC |
|---|---|---|---|
| let-7a | 4.1 | 0.014 | 0.763 |
| miR-191 | 3.6 | 0.030 | 0.699 |
| miR-23a | 3.96 | 0.206 | 0.668 |
| let-7i | 5.1 | 0.267 | 0.643 |
4. Discussion
Although genetic changes are indisputable causes of melanoma formation, the key role of the skin microenvironment is becoming more and more acknowledged. Progression of melanoma is dependent on cross-talk between tumor cells and the adjacent microenvironment, which is enriched in exosomes constantly secreted by cancer cells. In our study, we first demonstrated that melanoma cell-derived exosomes actively interact with normal melanocytes. This provided a prerequisite for the subsequent functional effects of melanoma cell-derived exosomes on the recipient cells in the microenvironment. The phenomenon of the fusion of fibroblasts with melanoma cell-derived exosomes was also observed by other researchers [3, 16]. Cancer-derived exosomes can also strongly chemoattract endothelial cells in several different types of cancer [17–20]. These facts support the idea that tumor-derived exosomes may take an active part in the remodeling of the microenvironment through its close intercommunication with the adjacent cells. After interacting with the melanoma cell-derived exosomes, we perceived increased migration and invasion abilities in the primary melanocytes. Moreover, the enhanced migration and invasion abilities were also facilitated by lung cancer cell-derived exosomes. These results suggest that different types of tumor cell-derived exosomes may carry similar oncogenic molecules that can transfer to the recipient normal cells and promote pre-oncogenic transformation in the normal cells. Tumor-derived exosomes function both as tumor messengers in the intercellular cross-talk in the microenvironment and as a contributor to tumor growth and progression. Therefore, targeting the tumor exosome-mediated cancer progression may be applicable for the treatment of various types of cancer.
EMT has implications on cancer progression by triggering the loss of cell–cell adhesion to facilitate tumor cell invasion [4, 21, 22]. The transition from melanocyte to melanoma involves a series of genetic and environmental changes, the primary of which is the loss of E-cadherin expression [5]. In our study, we observed that the expression level of E-cadherin was reduced while the mesenchymal marker vimentin was elevated in primary melanocytes after co-culturing with melanoma cell-derived exosomes. The expression levels of ZEB2 and Snail 2 were also significantly increased in melanocytes during this course. This is consistent with the acquired EMT characteristics of the melanocytes after communication with the tumor-derived exosomes. These data support the fact that tumor-derived exosomes can drive the phenotype switching of the normal melanocytes through autocrine/paracrine signaling and may contribute to the expansion and progression of melanoma. Similar to our findings, melanoma exosomes were shown to educate bone marrow progenitor cells toward a pro-metastatic phenotype [23]. Tumor-derived exosomes can trigger the phenotypic conversion of progenitor smooth muscle cells to tumor-promoting cells [24]. Breast cancer and prostate cancer-derived exosomes can induce a myofibroblastic phenotype [25, 26]. Tumor-tropic patient-derived mesenchymal stem cells primed with prostate cancer cell-derived exosomes can undergo genetic instability, oncogenic transformation, and develop prostate tumors in vivo [27]. These results imply that several different kinds of normal cells in the tumor microenvironment may be educated via tumor-derived exosomes. This may be one of the mechanisms underlying the switch of normal cells into cancer-supporting cells, and therefore, contributing to cancer progression.
Regulation of mRNA by miRNA prompted us to investigate whether some miRNAs could play a role in the process of EMT driven by melanoma cell-derived exosomes. miR-200 and let-7 are the largest two miRNA families that contribute to the pathogenesis and progression of several human malignancies [28–31]. However, miR-200a had low expression in the two melanocytes that we tested (data not shown). Additionally, in our serum exosome samples from human subjects, the expression level of miR-200a had large variations (data not shown). Therefore, we focused on the let-7 family in this study. We found that after co-culturing with melanoma cell-derived exosomes, the melanocytes showed decreased expression of let-7i, which is consistent with the cell’s acquired EMT-like characteristics. Re-expression of let-7i in melanocytes showed reversal of EMT characteristics, as documented by increased expression of E-cadherin and decreased expression of vimentin, and concomitant with the decreased invasion ability facilitated by melanoma cell-derived exosomes. LIN28B and HMGA2 are the two major targets related to EMT, as confirmed by three microRNA databases: TargetScanHuman 7.0 (www.targetscan.org), miRDB (www.mirdb.org), and www.microRNA.org. Let-7, LIN28B, and HMGA2 have been reported to form an axis to adjust the EMT process [13, 14, 32]. In our current study, we also noticed the significant upregulation of LIN28B and HMGA2 after transfection of let-7i mimic followed by co-culturing with A375 or SK-MEL-28 cell-derived exosomes vs controls. These results suggest that let-7i may participate in the regulation of an EMT-like process driven by the melanoma-derived exosomes through the two target genes, LIN28B and HMGA2. Certainly, further investigation is needed to understand how let-7i and its targets, LIN28B and HMGA2, form a circuit to adjust the melanoma cell-derived exosome-mediated EMT process. Re-expression of let-7i could be useful for intervening in the pre-metastatic status induced by the melanoma-derived exosomes. Indeed, targeting let-7i in the EMT process has been a strategy for treatment of tumors [33].
There are several pathways (ie, MAPK, NF-kappa B, and PI3K/AKT) that are constitutively hyperactivated in melanoma and contribute to EMT [34–37]. In the current study, we have demonstrated that communication through melanoma-derived exosomes induced the activation of numerous molecules in the MAPK signaling pathway, such as MAP3K4, MAP2K5, and MAPK13, in the primary melanocytes. This result is consistent with the previous findings that the MAPK pathway is involved in the induction of EMT in melanoma, breast cancer, prostate cancer, and many other types of cancer [36, 37]. MAPK signaling may contribute to regulating the EMT-like process driven by the melanoma cell-derived exosomes. Therefore, targeting the MAPK pathway is expected to provide a novel approach for inhibition of the melanoma progression and metastasis through adjusting the EMT process in the microenvironment. Indeed, several MAPK inhibitors have been shown effective for the treatment of melanoma [15].
Exosomes are now widely accepted to mirror and bind to the cells from which they arise. This provides the rationale for using tumor-derived exosomes as biomarkers or drug targets. Based on our current finding that miRNAs regulate the process of EMT, which is driven by the melanoma cell-derived exosomes, it is logical to speculate that miRNA regulators of EMT in the melanoma cell-derived exosomes could be useful to support the diagnosis and treatment of melanoma in the clinical setting. Except for miR-200a and let-7i, which were involved in the EMT process as we discussed above, miR-191 is another onco-miR that modulates EMT. Inhibition of miR-191 expression has been reported to block the EMT process and decrease the cell’s migratory capacity and neoplastic properties [38]. Studies also show that miR-23a can regulate EMT through targeting E-cadherin [39, 40]. Let-7a is also involved in cancer cell migration, invasion, and EMT through its specific protein targets, such as LIN28B and HMGA2 [13, 14]. In this current study, even though miR-23a and let-7a showed high-fold changes in serum exosomes isolated from stage I melanoma patients versus non-melanoma subjects, there were no significant differences of miR-23a and let-7a between these two subject groups. However, let-7a and miR-191 were upregulated significantly in these two subject groups. These two miRNA regulators of EMT may serve as potential identifiers for distinguishing melanoma from non-melanoma subjects. We acknowledge the limitations of the small cohorts used in this pilot study. Identification of additional signatures, including miR-23a and let-7a, in a larger sample size is clearly of interest. Additionally, exploration of the dysregulation of these miRNAs in melanoma progression is warranted.
In conclusion, our results provide the first strong molecular evidence that melanoma cellderived exosomes may promote the EMT-resembling process through autocrine/paracrine signaling, creating a tumor-supporting microenvironment. Novel strategies targeting the EMT process and modulating the tumor microenvironment may emerge as important approaches for the prevention of tumor progression and/or treatment of metastatic melanoma. Furthermore, tumor-derived exosomes harboring EMT regulators may serve as molecular identifiers or future drug targets for melanoma.
Supplementary Material
Highlights.
Melanoma cell-derived exosomes promote phenotype switching in primary melanocytes.
MAPK pathway and let-7i were involved in exosome-mediated EMT.
EMT-related miRNAs (miR-191& let-7a) in exosomes may be used for melanoma diagnosis.
Targeting exosome-mediated EMT may be an approach to prevent tumor progression.
Acknowledgments
This work was supported by University of Louisville School of Medicine Collaborative Matching Grant (H. Hao), University of Louisville Clinical and Translational Science Pilot Grant program Innovative Award (K.M. McMasters), and Melanoma Research Foundation Established Investigator Award (K.M. McMasters). S. Barry and D. Kmetz were supported by National Cancer Institute grant R25-CA-134283. The authors thank Mrs. Margaret Abby for expert assistance with manuscript preparation. We thank Dr. Venkatakrishna R Jala at the Department of Microbiology and Immunology for his assistance with the real-time imaging of exosomes and melanocytes. We thank Dr. Andrei Smolenkov for his coordination with the clinical samples.
Abbreviations
- AUC
area under ROC (Receiver Operating Characteristic) curve
- EMT
epithelial-to-mesenchymal transition
- HEMa-LP
human epidermal melanocytes adult-lightly pigmented
- MAP
mitogen-activated protein
- MAPK
mitogen-activated protein kinase
- MKK
mitogen-activated protein kinase kinase
- MKKK
mitogen-activated protein kinase kinase kinase
- NHEM-c
normal human epidermal melanocytes
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PI3K/AKT
phosphoinositide 3-kinase/protein kinase B
- ROC
receiver operating characteristic
- RT-PCR
reverse transcription polymerase chain reaction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am. J. Pathol. 2014;184:28–41. doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greening DW, Gopal SK, Mathias RA, Liu L, Sheng J, Zhu HJ, et al. Emerging roles of exosomes during epithelial–mesenchymal transition and cancer progression. Semin. Cell Dev. Biol. 2015;40:60–71. doi: 10.1016/j.semcdb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Hood JL, Pan H, Lanza GM, Wickline SA. Paracrine induction of endothelium by tumor microvesicles. Consortium for Translational Research in Advanced Imaging and Nanomedicine (C-TRAIN) Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vella LJ. The emerging role of exosomes in epithelial-mesenchymal-transition in cancer. Front. Oncol. 2014;4:361. doi: 10.3389/fonc.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JT, Herlyn M. Microenvironmental influences in melanoma progression. J. Cell Biochem. 2007;101:862–872. doi: 10.1002/jcb.21204. PMID: 17171636. [DOI] [PubMed] [Google Scholar]
- 6.Caramel J, Papadogeorgakis E, Hill L, Browne GJ, Richard G G, Wierinckx A, et al. A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell. 2013;24:466–480. doi: 10.1016/j.ccr.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Bronisz A, Wang Y, Nowicki MO, Peruzzi P, Ansari KI, Ogawa D, et al. Extracellular vesicles modulate the glioblastoma microenvironment via a tumor suppression signaling network directed by miR-1. Cancer Res. 2014;74:738–750. doi: 10.1158/0008-5472.CAN-13-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. PMID: 8642258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao D, Ohlendorf J, Chen Y, Taylor DD, Rai SN, Waigel S, et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One. 2012;7:e46874. doi: 10.1371/journal.pone.0046874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith BN, Bhowmick NA. Role of EMT in metastasis and therapy resistance. J. Clin. Med. 2016;5:E17. doi: 10.3390/jcm5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad A, Maitah MY, Ginnebaugh KR, Li Y, Bao B, Gadgeel SM, et al. Inhibition of Hedgehog signaling sensitizes NSCLC cells to standard therapies through modulation of EMT-regulating miRNAs. J. Hematol. Oncol. 2013;6:77. doi: 10.1186/1756-8722-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu A, Wu K, Li J, Mo Y, Lin Y, Wang Y, et al. Let-7a inhibits migration, invasion and epithelial-mesenchymal transition by targeting HMGA2 in nasopharyngeal carcinoma. J. Transl. Med. 2015;13:105. doi: 10.1186/s12967-015-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Li H, Feng J, Cui X, Huang W, Li Y et al. Lin28 induces epithelial-to-mesenchymal transition and stemness via downregulation of let-7a in breast cancer cells. PLoS One. 2013;8:e83083. doi: 10.1371/journal.pone.0083083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuzillet C, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, Raymond E. MEK in cancer and cancer therapy. Pharmacol. Ther. 2014;141:160–171. doi: 10.1016/j.pharmthera.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhao XP, Wang M, Song Y, Song K, Yan TL, Wang L, et al. Membrane microvesicles as mediators for melanoma-fibroblasts communication: Roles of the VCAM-1/VLA-4 axis and the ERK1/2 signal pathway. Cancer Lett. 2015;360:125–133. doi: 10.1016/j.canlet.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Millimaggi D, Mari M, D’Ascenzo S, Carosa E, Jannini EA, Zucker S, et al. Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia. 2007;9:349–57. doi: 10.1593/neo.07133. PMID: 17460779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 19.Taverna S, Flugy A, Saieva L, Kohn EC, Santoro A, Meraviglia S, et al. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int. J. Cancer. 2012;130:2033–2043. doi: 10.1002/ijc.26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Zhu XJ, Zeng C, Wu PH, Wang HX, Chen ZC, et al. Microvesicles secreted from human multiple myeloma cells promote angiogenesis. Acta Pharmacol. Sin. 2014;35:230–238. doi: 10.1038/aps.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keshamouni VG, Michailidis G, Grasso CS, Anthwal S, Strahler JR, Walker A, et al. Differential protein expression profiling by iTRAQ-2DLC-MS/MS of lung cancer cells undergoing epithelial-mesenchymal transition reveals a migratory/invasive phenotype. J. Proteome Res. 2006;5:1143–1154. doi: 10.1021/pr050455t. PMID: 16674103. [DOI] [PubMed] [Google Scholar]
- 22.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. PMID: 16951136. [DOI] [PubMed] [Google Scholar]
- 23.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atay S, Banskota S, Crow J, Sethi G, Rink L, Godwin AK. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc. Natl. Acad. Sci. USA. 2014;111:711–716. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 26.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int. J. Oncol. 2012;40:130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 27.Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32:983–997. doi: 10.1002/stem.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 29.Koutsaki M, Spandidos DA, Zaravinos A. Epithelial-mesenchymal transition-associated miRNAs in ovarian carcinoma, with highlight on the miR-200 family: prognostic value and prospective role in ovarian cancer therapeutics. Cancer Lett. 2014;351:173–181. doi: 10.1016/j.canlet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Feng X, Wang Z, Fillmore R, Xi Y. MiR-200, a new star miRNA in human cancer. Cancer Lett. 2014;344:166–173. doi: 10.1016/j.canlet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–852. doi: 10.4161/cc.8.6.7907. PMID: 19221491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alam M, Ahmad R, Rajabi H, Kufe D. MUC1-C Induces the LIN28B→LET-7→HMGA2 Axis to Regulate Self-Renewal in NSCLC. Mol Cancer Res. 2015;13:449–460. doi: 10.1158/1541-7786.MCR-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang N, Kaur S, Volinia S, Greshock J, Lassus H, Hasegawa K, et al. MicroRNA microarray identifies let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res. 2008;68:10307–10314. doi: 10.1158/0008-5472.CAN-08-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier F, Schittek B, Busch S, Garbe C, Smalley K, Satyamoorthy K, et al. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front Biosci. 2005;10:2986–3001. doi: 10.2741/1755. PMID: 15970553. [DOI] [PubMed] [Google Scholar]
- 35.Weiss MB, Abel EV, Mayberry MM, Basile KJ, Berger AC, Aplin AE. TWIST1 is an ERK1/2 effector that promotes invasion and regulates MMP-1 expression in human melanoma cells. Cancer Res. 2012;72:6382–6392. doi: 10.1158/0008-5472.CAN-12-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. PMID: 17496923. [DOI] [PubMed] [Google Scholar]
- 37.Lin K, Baritaki S, Militelo L, Malaponte G, Bevelacqua Y, Bonavida B. The Role of B-RAF mutations in melanoma and the induction of EMT via dysregulation of the NF-k B/Snail/RKIP/PTEN Circuit. Genes Cancer. 2010;1:409–420. doi: 10.1177/1947601910373795. PMID: 20827424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y, et al. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol. Carcinog. 2015;54:E148–E161. doi: 10.1002/mc.22221. [DOI] [PubMed] [Google Scholar]
- 39.Zheng H, Li W, Wang Y, Xie T, Cai Y, Wang Z, et al. miR-23a inhibits E-cadherin expression and is regulated by AP-1 and NFAT4 complex during Fas-induced EMT in gastrointestinal cancer. Carcinogenesis. 2014;35:173–183. doi: 10.1093/carcin/bgt274. [DOI] [PubMed] [Google Scholar]
- 40.Cao M, Seike M, Soeno C, Mizutani H, Kitamura K, Minegishi Y, et al. MiR-23a regulates TGF-β-induced epithelial-mesenchymal transition by targeting E-cadherin in lung cancer cells. Int. J. Oncol. 2012;41:869–875. doi: 10.3892/ijo.2012.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


