Abstract
Accumulating evidence suggests that early adversity is linked to methylation of the glucocorticoid receptor gene NR3C1, which is a key regulator of the hypothalamic-pituitary-adrenal (HPA) axis. Yet no prior work has considered the contribution of methylation of NR3C1 to emerging behavior problems and psychopathology in childhood. The current study examined links between methylation of NR3C1 and behavior problems in preschoolers. Data were drawn from a sample of preschoolers with early adversity (n=171). Children ranged in age from 3 to 5 years, were racially and ethnically diverse, and nearly all qualified for public assistance. Seventy-one children had child welfare documentation of moderate-severe maltreatment in the past six months. Structured record review and interviews in the home were used to assess early adversity. Parents reported on child internalizing and externalizing behavior problems. Methylation of NR3C1 at exons 1D, 1F, and 1H were measured via sodium bisulfite pyrosequencing from saliva DNA. Methylation of NR3C1 at exons 1D and 1F was positively associated with internalizing (r = .21, p < .01 and r = .23, p < .01 respectively), but not externalizing, behavior problems. Furthermore, NR3C1 methylation mediated effects of early adversity on internalizing behavior problems. These results suggest that methylation of NR3C1 contributes to psychopathology in young children, and NR3C1 methylation from saliva DNA is salient to behavioral outcomes.
Keywords: methylation, glucocorticoid receptor, internalizing problems, preschool, adversity
Childhood adversity is associated with the development of behavior problems as well as mood, anxiety, and substance use disorders in both childhood and adulthood (Benjet, Borges, & Medina-Mora, 2010; Green et al., 2010; Slopen, Koenen, & Kubzansky, 2014). Yet the mechanisms underlying links between early adversity and psychopathology are not fully understood. One probable pathway by which early childhood experiences influence the development of later psychopathology is through modification of hypothalamic-pituitary-adrenal (HPA) axis functioning (Heim & Binder, 2012; Tyrka, Burgers, Philip, Price, & Carpenter, 2013). The HPA axis regulates the neuroendocrine response to stress and has been implicated as an important component in the development of psychopathology in studies of both animals and humans (Tyrka et al., 2013; Buitelaar 2013). Emerging evidence suggests that lifetime experiences contribute to epigenetic modifications of key regulatory elements in the HPA-axis, potentially leading to psychopathology and associated behaviors. Understanding mechanisms by which early-adversity modifies HPA-axis functioning is central to elucidating risk underlying the development of psychopathology.
The glucocorticoid receptor, encoded by the gene NR3C1 is a key nuclear hormone receptor which acts as a regulator of the stress response. Glucocorticoids, signaling through the glucocorticoid receptor, have widespread effects on the response to acute and chronic stress via the coordinated regulation of systems, including autonomic, metabolic, and immune system function (Dallman et al., 2004; McEwen, 2012), and serve the critical role of mediating negative feedback regulation of the HPA axis (Jacobson, 2005). Animal and human studies link impairments in glucocorticoid signaling with disorders such as MDD and PTSD. Specifically, alterations in the number of glucocorticoid receptors and their function in the brain and in peripheral cells such as leukocytes have been found in adults with psychopathology (Barden 2004; Yehuda et al., 2010; van Zuiden et al., 2011). Thus, epigenetic regulation of NR3C1 gene expression is of particular interest given its potential role in HPA-mediated psychopathology.
Promoter DNA methylation is a well-established epigenetic regulator of gene expression (Moore, Le, & Fan, 2013). In rodents, methylation of NR3C1 is responsive to many forms of early life stress both prenatally (Lillycrop et al., 2013; Szyf, 2013) and postnatally (Witzmann, Turner, Meriaux, Meijer, & Muller, 2012; Kundakovic, Lim, Gudsnuk, & Champagne, 2013). Low levels of maternal care in rodents result in greater methylation of the promoter region of the hippocampal glucocorticoid receptor gene (exon 17 of the NR3C1 promoter), which interferes with binding of the transcription factor nerve growth factor inducible protein A (NGFI-A), resulting in reduced NR3C1 gene expression (Kosten & Nielsen, 2014; Weaver et al., 2004).
In humans, prenatal stressors such as maternal depression and anxiety, intimate partner violence, and maternal exposure to stressors have also been associated with NR3C1 methylation (Conradt, Lester, Appleton, Armstrong, & Marsit, 2013; Hompes et al., 2013; Mulligan, D’Errico, Stees, & Hughes, 2012; Oberlander et al., 2008; Radtke et al., 2011). Likewise, adults who retrospectively report a history of childhood maltreatment, early parental death, and childhood trauma show associations with NR3C1 gene methylation (Perroud et al., 2011; Tyrka, Price, Marsit, Walters, & Carpenter, 2012). These links have also been demonstrated in postmortem brain from adult suicides (McGowan et al., 2009; Labonte et al., 2012) as well as patients who suffer from bulimia nervosa, depression, or bipolar disorder (Melas et al., 2013; Steiger, Labonte, Groleau, Turecki, & Israel, 2013). And very recent work from our group (Tyrka et al., 2015) and others (Romens, McDonald, Svaren, & Pollak, 2015) demonstrate links between maltreatment and methylation of NR3C1. In turn, methylation of NR3C1 is associated with cortisol reactivity in both infancy (Oberlender et al., 2008; Stroud et al., 2014) and adulthood (Tyrka et al., 2012; Edelman et al., 2012), suggesting that methylation of the glucocorticoid receptor gene may have a functional effect on the HPA axis.
Emerging evidence also suggests that methylation of NR3C1 is linked to behavior. In rodents, methylation of NR3C1 is associated with increased anxiety behaviors (Kosten, Huang, & Nielsen, 2014). In human neonates, methylation of NR3C1 is linked with neurobehavior following delivery (Bromer, Marsit, Armstrong, Padbury, & Lester, 2013; Conradt et al., 2013). For example, methylation of NR3C1 in placenta DNA is linked with neonate attention, quality of movement, hypotonia, and lethergy (Bromer et al., 2013; Conradt et al., 2013), and among newborns of mothers who reported depression in pregnancy, placental NR3C1 methylation is associated with deficits in self-regulation in response to a physical exam (Conradt et al., 2013). In adults, MDD, borderline personality disorder, and PTSD have also been associated with NR3C1 gene methylation (Dammann et al., 2011; Na et al., 2014; Yehuda et al., 2014).
Taken together, prior work with animals, human neonates, and adults suggest that NR3C1 gene methylation may be associated with behavioral indicators of risk, yet no previous work has considered links between NR3C1 methylation and the development of behavior problems or emerging psychopathology in childhood. In recent work from our group with the current sample of preschoolers, we demonstrated that early adversity was positively correlated with NR3C1 promoter methylation and that individual stress measures were significantly associated with a several CpG sites in these regions (Tyrka et al., 2015). It is possible that methylation of NR3C1 is a pathway by which early adversity contributes to behavior problems in young children.
Collectively, prior research suggests that NR3C1 may be a key mechanism underlying links between early adversity and the development of psychopathology. Evidence from studies with adults support this perspective, yet no prior research has considered these associations in childhood. Internalizing and externalizing behavior problems are early indicators of risk in young children, and have been linked with behavior problems and mood and anxiety disorders in later childhood and adolescence (Pihlakoski et al., 2006; Roza, Hofstra, van der Ende & Verhulst, 2003). The current study examined links between methylation of three alternate first exons in the promoter of NR3C1 and emerging behavior problems in preschoolers. The majority of prior research examining methylation of NR3C1 in humans has focused on methylation at exon 1F (see Turecki & Meaney, in press for a review), we extend this research and consider methylation at exons1D and 1H given emerging work suggesting that methylation at these regions is sensitive to stress exposure and regulates gene expression (e.g. Moore et al., 2012; Labonte et al., 2012; Hompes et al., 2013). These alternative first exons lead to tissue specific expression of the glucocorticoid receptor, and subsequently tissue specific control of NR3C1 expression (Moore et al., 2012). Given that research with these exons is still emerging, more work is needed to understand their links with behavioral outcomes. Finally, we examined the possibility that NR3C1 mediates effects of early adversity. We hypothesized that methylation of exons1D, 1F, and 1H of the NR3C1 gene would be associated with a greater risk of behavior problems, and that methylation of NR3C1 would mediate effects of early adversity.
Method
One-hundred and seventy-one families residing in Rhode Island enrolled in this study. One child from each family was included in the study. Children ranged in age from 3 to 5 years (M = 50.3 months; SD = 8.5 months), were racially and ethnically diverse (39 White non-Hispanic, 82 Hispanic, 25 Black, 25 other races), and 82 were male. Most caregivers (n=162) were biological mothers. Thirty-three caregivers had less than a high school degree, 68 completed high school, 53 had some post-secondary education, and 17 had a bachelor’s degree. One-hundred caregivers were unemployed and 154 of the families qualified for public assistance. Seventy-one children (41.5%) had substantiated cases of moderate to severe child maltreatment within the past six months as described below.
Procedure
Families with a maltreated child were identified from the local child welfare agency and an emergency maltreatment assessment service via record review. Families of children with no indicated case of maltreatment within the past six months were recruited at a pediatric medical clinic during a well-child visit as well as at childcare centers. Based on review of available medical records and parent report, children with a chronic illness, medication use, obesity, and failure-to-thrive were excluded. Those with acute illness or medication use were included no less than 2 weeks following resolution of illness and medication use.
Families completed two home visits and questionnaires between the visits. The first home visit, during which caregivers completed interviews on child stress exposure and a saliva sample for DNA isolation was collected from the children, is the focus of the current report.
Measures
Child maltreatment status
All families consented to examination of Rhode Island child welfare records to determine maltreatment status. Trained research staff coded the records using the System for Coding Subtype and Severity of Maltreatment in Child Protective Records (Barnett, Manly, & Cicchetti, 1993). Five maltreatment subtypes and severity scores ranging from 1 (least severe) to 5 (most severe) were derived. Children with a case of moderate to severe levels of maltreatment (score of 3–5) within the last six months were considered as part of the maltreated group (n=71). Nine children had substantiated cases of physical abuse, 15 sexual abuse, 9 physical neglect/failure to provide, 19 physical neglect/lack of supervision, and 47 emotional maltreatment. Three of the maltreated children were removed from the home and were in the care of their maternal grandmother. The comparison group included children who had never had a substantiated case of maltreatment. In addition five children had an episode of moderate maltreatment that occurred at least 18 months prior to participation. Results demonstrated the same pattern of effects whether these children were in the maltreatment or comparison group, therefore they were included in the comparison group.
Contextual stress interview
Caregivers completed a semi-structured interview developed in our laboratory to assess the child’s experience of contextual stressors in the past month and in the child’s lifetime. Categories were: death of a caregiver, separation from a caregiver, frequent change of residence or homelessness (defined as more than 1 change of residence per year, residing in a shelter, or temporarily living with a friend or relative), inadequate food or clothing (defined as not enough money to purchase food or clothing for the family, must rely on a community food pantry to feed children), and other events including witnessing neighborhood violence or parental arrest. Interviews were conducted and scored by trained clinical social workers and PhD level psychologists. The project coordinator reviewed each interview to ensure compliance to the scoring protocol. Each domain was scored positive if at least one episode occurred, and domains were summed to determine the number of stressors the child experienced in the past month and their lifetime. Possible scores ranged from 0 (no stressors) to 5 (stressors in all five domains) for each summary scale. Past month stressors ranged from 0 to 3, with mean of 0.63 and SD of 0.85, and lifetime stressors ranged from 0 to 4 with mean of 1.37 and SD of 1.24.
Traumatic life events
The Diagnostic Infant and Preschool Assessment (Scheeringa & Haslett, 2010) interview was conducted with caregivers to assess child experiences of traumatic life events. Interviews were conducted by trained clinical social workers and PhD level psychologists, reviewed in a group supervision format, and scored based upon group consensus. Traumatic events in each domain were dichotomized (no trauma versus ≥ 1 trauma), then summed to create a scale for number of types of traumas experienced in the child’s lifetime. Physical and sexual abuse were not included because they were assessed as maltreatment (above). Possible scores ranged from 0 to 8, and in the present sample ranged from 0 to 4 with mean of 0.95 and SD of 1.04.
Adversity Composite
The number of types of maltreatment experienced, the number of lifetime contextual stressors, and the number of traumatic life events were summed to create an adversity composite. Associations among adversity variables used to create the composite are displayed in Table 1. Possible scores ranged from 0 to 21, and in the sample ranged from 0 to 9 with a mean of 2.97 and a SD of 2.48.
Table 1.
Associations Among Independent Adversity Variables
| 1 | 2 | 3 | |
|---|---|---|---|
| 1. Maltreatment Status | -- | -- | -- |
| 2. Past Month Stress | .29*** | -- | -- |
| 3. Lifetime Stress | .44*** | .68*** | -- |
| 4. Number of Traumas | .35*** | .30*** | .52*** |
Note:
p < .001.
Behavior Problems
Caregivers complete the Child Behavior Checklist for Ages 1.5 to 5 (CBCL; Achenbach & Rescorla, 2000) to assess internalizing and externalizing behavior problems. For each of the 100 behaviors, parents assessed their children on a 3-point scale from 0 (Not True) to 2 (Very True). T scores were used for data analysis. Possible scores range from 0 to 100, and in the sample ranged from 29 to 74 for Internalizing Behaviors (M = 51.29, SD = 8.37) and from 28 to 97 for Externalizing Behaviors (M = 46.82, SD = 11.56). The CBCL is a reliable and valid measure with strong test-retest reliability (r = .90 and .87 for internalizing and externalizing scales respectively) as well as discriminant validity between children who were and were not referred for behavioral health services (Achenbach & Rescorla, 2000).
GR Methylation
Saliva samples were obtained using the Oragene DISCOVER kits (OGR-575) for Assisted Collections (DNA Genotek, Kanata, Ontario, Canada), and DNA was isolated following the manufacturer’s instructions. Sodium bisulfite modification was performed with 500 ng of DNA using the EZ DNA methylation Kit (Zymo Research, Irvine, CA, USA). For DNA methylation detection, bisulfite pyrosequencing was employed in three locations within the NR3C1 region: promoter of exon 1D, promoter of exon 1F (3 assays) and promoter of exon 1H (Figure 1). PyroMark Assay Design software version 2.0.1.15 (Qiagen) was used to design the pyrosequencing assays. Amplification PCR and sequencing primers (Integrated DNA Technologies, Inc, Coralville, IA) and the genomic locations of the assays are provided in Table 2. The PyroMark PCR kit and forward and reverse primers were used to amplify specific regions of NR3C1 promoter. Amplification cycling conditions were as follows: 94°C for 15 min followed by 45 cycles of 94°C for 1 min, 60°C for 1 min and 72°C for 1 min with a final extension of 10 min at 72°C. Five forward pyrosequencing assays covering a total of 36 CpG loci were performed in triplicate using the PyroMark MD (Qiagen). Non-CpG cytosines within assays served as internal controls to verify bisulfite DNA modification efficiency. Each run included a non-template control. Percent DNA methylation at each CpG locus was quantified with the PyroMark CpG software, version 1.0.11 (Qiagen). All procedures were performed following manufacturer’s protocols. Sodium bisulfite-modified, fully-methylated referent positive control and fully-unmethylated (whole genome amplified) negative control DNA (Qiagen, Valencia CA) was examined with each batch. DNA that was not sodium bisulfite-modified served as a control for non-specific amplification. Methylation quantification was performed using the Pyromark Software (Qiagen).
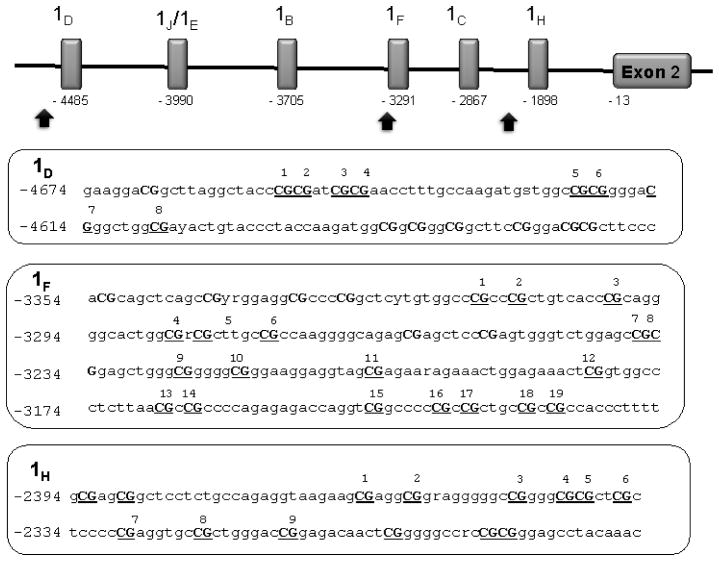
Figure 1. Schematic diagram of noncoding alternate first exons in the NR3C1 promoter region.
Schematic diagram of noncoding exons in the NR3C1 promoter region. Upper panel: The number below each noncoding exon represents the distance to the translational start located at −13 nucleotides upstream of the start of Exon 2. The arrows represent the regions sequenced in this study, the promoter of exon 1D, promoter and exon 1F and promoter of exon 1H. Lower panel: The boxes contain the genomic sequences analyzed for each region. The analyzed CpG loci are underlined and numbered according to each exon region.
Table 2.
Pyrosequencing Primers and Assay Sequences
| NR3C1 Assay Name (CpG Positions) |
Primers* | Sequence to analyze (converted) Chromosome location |
|---|---|---|
|
1D (8) |
PCR forward GGATAAGAGGTTTGTTGAAAGTTTATT PCR reverse Biotin-ACTCCCCCTACTCTAACAT Forward sequencing 1D AGGAAGGAAGGTTTAGGT |
5:142785061-142785008 TATTYGG/AYGATYGYGAATTTTTGTTAAGATGKTGGTYGYGGGGAYGGGTTGGYGATATTGTATTTTATTAAGATGG |
|
1F 3 pyrosequencing assays (19) |
PCR forward TTTTTTTTTTGAAGTTTTTTT PCR reverse Biotin-CCCCCAACTCCCCAAAAA Forward sequencing F0 GAGGAGTTTAGGTTTTTGTG Forward sequencing F1 GAGTGGTTTGGAGT Forward sequencing F2 AGAAAAGAATTGGAGAAATT |
sequence F0 (CpG 1-6) 5:142783720-142783676 GTTYGTTYGTTGTTATTYGTAGGGGTATTGGYGG/AYGTTTGTYGTTAAGGGGTAGAG sequence F1 (CpG 7–11) 5:142783640-142783588 YGYGGAGTTGGGYGGGGGYGGGAAGGAGGTAGYGAGAAAAGAAATTGGAGAAA sequence F2 (CpG 12–19) 5:142783585-142783501 YGGTGGTTTTTTTAAYGTYGTTTTAGAGAGATTAGGTYGGTTTTYGTYGTTGTYGTYGTTGTTTTTTTTTTTGGGGAGTTGGGGG |
|
1H (9) |
PCR forward AGGGGTTTTTTTTTTATTTATGGAGA PCR reverse Biotin-CCCACTCCCCCAAACTAATAAAAATTTAT Forward sequencing 1H GAGAGGTTTTTTTGTTAGAGGTA |
5:142782749-142782607 AGAAGYGAGGYGGRAGGGGGTYGGGGYGYGTTYGTTTTTTYGAGGTGTYGTTGGGATYGGAGATAA |
Primer sequences are given 5′ to 3′ direction
The percent of alleles that were methylated was used in statistical analyses. Consistent with other studies on NR3C1 methylation in a variety of cell types, and prior work that has used the pyrosequencing approach (Turecki & Meaney, 2014), methylation levels were low across these regions. This is consistent with data indicating that very low basal levels of promoter methylation are characteristic of genes that are highly expressed, and methylation in these regions is tightly coupled with gene transcription (Brenet et al., 2011). For CpG sites in region 1D, percent methylation ranged from 0 to 7.02 with mean of 1.11 and SD of 0.19 across the whole region. Region 1F methylation varied from 0 to 6.39. The mean and SD of the whole region was 1.46 and 0.26. For 1H, the range of methylation was 0 to 6.92, and the mean across the region was 1.23 and SD of 0.22.
Statistical analysis
Associations of demographic characteristics and child behavior problems, and demographic characteristics and mean methylation at the NR3C1 exons were examined using Pearson correlations and t-tests. Associations of methylation and child behavior problems controlling for relevant covariates were examined using partial correlations. We first examined mean methylation at each of the alternate first exons and followed this by consideration of individual CpG sites when significant effects were seen for mean methylation, obviating the need to adjust for multiple comparisons. Models testing NR3C1 as a mediator of effects of adversity on behavior problems were tested using multiple regression and bootstrapping procedures outlined by Preacher and Hayes (2008). First, unconditional models testing associations of the adversity variables and behavior problems were tested. Next, conditional models testing associations of the adversity variables and behavior problems controlling for NR3C1 methylation were tested. The significance of the indirect effect, which corresponds to the drop in the effect of the adversity variable on behavior problems when NR3C1 was included in the model, was determined using 95% bias corrected confidence intervals from 1,000 bootstrap re-samples. Confidence intervals that did not include zero were considered to be statistically significant, and indicated partial mediation.
Results
Preliminary Analyses
Child race and socioeconomic adversity were not associated with behavior problems or mean methylation at any of the NR3C1 first exons. Child age was not associated with behavior problems, but was negatively associated with mean methylation at 1H (p =.042). Child sex was not associated with behavior problems, but males had greater mean methylation at 1F than females (p =.008). Thus, age and sex were included as covariates in hypothesis testing. Furthermore, because internalizing and externalizing behaviors were positively associated with one another (r = .64, p < .001), externalizing behaviors was included as a covariate when testing associations with internalizing behaviors, and vice-versa. Importantly, results were consistent when internalizing and externalizing behaviors were and were not included as covariates in the models.
Associations of NR3C1 Methylation and Internalizing Behavior Problems
Region 1D
Table 3 displays associations of behavior problems and mean methylation at region 1D and the other first exons. Mean methylation across exon 1D was positively associated with internalizing behavior problems. Internalizing behavior problems were also associated with methylation of individual CpG sites at exon 1D. Examination of the individual CpG sites revealed significant effects for internalizing behaviors at CpG 3 (r = .16, p = .045), CpG 4 (r = .25, p = .001), and CpG 6 (r = .22, p = .004). A trend level association of internalizing behaviors and methylation of 1F was observed at CpG 7 (r = .15, p = .052).
Table 3.
Associations of Internalizing and Externalizing Behavior Problems With Mean Methylation at Alternate First Exons
| Internalizing Behaviors | Externalizing Behaviors | |
|---|---|---|
| Methylation of Exon 1D | r = .21** | r = −.12 |
| Methylation of Exon 1F | r = .23** | r = −.09 |
| Methylation of Exon 1H | r = .05 | r = −.03 |
Notes:
p < .05.
p < .01.
Child sex and age were included as covariates when testing associations of internalizing and externalizing behavior with alternate first exons. Externalizing behaviors were included as a covariate when testing associations with internalizing behaviors, and internalizing behaviors were included as a covariate when testing associations with externalizing behaviors.
Region 1F
Internalizing behaviors were also positively associated with mean methylation at exon 1F (Table 3). Examination of the individual CpG sites revealed significant effects for internalizing behaviors at CpG 3 (r = .26, p = .001), CpG 4 (r = .17, p = .032), CpG 9 (r = .16, p = .035), CpG 13 (r = .22, p = .005), CpG 14 (r = .20, p = .010) and CpG 19 (r = .16, p = .036). Trend level associations of internalizing behaviors and methylation of 1F were observed at CpG 5 (r = .15, p = .058), CpG 6 (r = .14, p = .085), and CpG 18 (r = .15, p = .055).
Region 1H
There were no associations of internalizing behaviors with mean methylation at exon 1H (Table 3). Likewise, there were no significant associations of internalizing behaviors and methylation at exon 1H at any of the individual CpG sites. Thus, methylation at exon 1H was considered no further.
Adversity, NR3C1 Methylation, and Internalizing Behavior Problems
Results of the unconditional models testing associations of the adversity variables and internalizing behavior problems are displayed in the top row of Table 4. All five adversity variables including the adversity composite, maltreatment status, past month stress, lifetime stress, and the number of traumatic life events were positively associated with internalizing behavior problems. Consistent with our previously published work with the current sample of preschoolers (Tyrka et al., 2015), the adversity composite was positively associated with greater mean methylation at exons 1D (r = .16, p = .038) and 1F (r = .16, p = .043, respectively). Likewise, childhood maltreatment was associated with greater mean methylation at exon 1D (F = 7.95, p = .005), past month stress was associated with greater mean methylation at exon 1F (r = .17, p = .026), and lifetime stress was associated with greater mean methylation at exons 1D (r = .16, p = .039) and 1F (r = .18, p = .017, respectively). Traumatic life events was not associated with mean methylation at any of the alternate first exons.
Table 4.
General Linear Models and Bootstrapping Results Testing Effects of Adversity Variables on Internalizing Behavior Problems
| Adversity Composite B(SE) | Maltreatment Status B(SE) | Past Month Stress B(SE) | Lifetime Stress B(SE) | Number of Traumas B(SE) | |
|---|---|---|---|---|---|
| Unconditional Models | |||||
| Adversity variable | .63** (.19) | 2.24* (.98) | 1.48** (.57) | 1.22** (.39) | 1.05* (.47) |
| Conditional Models | |||||
| Adversity variable | .50* (.19) | 1.66t (.97) | 1.15* (.56) | .93* (.39) | .92* (.45) |
| Methylation of Exon 1D | 5.22* (2.46) | 5.25* (2.52) | 5.80* (2.46) | 5.31* (2.46) | 5.88* (2.46) |
| Methylation of Exon 1F | 4.44* (1.83) | 4.97** (1.84) | 4.48* (1.85) | 4.36* (1.85) | 4.88** (1.83) |
| Indirect Effects | |||||
| B(SE) | .14 (.07) | .56 (.33) | .32 (.19) | .29 (.13) | .15 (.15) |
| Confidence Interval | .03, .31 | .04, 1.35 | .05, .84 | .10, .66 | −.17, .44 |
Notes:
p < .10.
p < .05.
p < .01.
Child sex, age, and externalizing behaviors were included in all models as covariates.
Confidence Interval = 95% bias corrected confidence interval (CI) for the significance of the indirect effect; if CI does not include zero the indirect effect is considered statistically significant and is displayed in bold. Significant indirect effects represent adversity variables which are partially mediated by methylation of Exons 1D and 1F.
Results of the conditional models testing associations of the adversity variables and internalizing behavior problems controlling for the variance associated with NR3C1 methylation at exons 1D and 1F are also displayed in Table 4. All five adversity variables became less strongly associated with internalizing behavior problems when methylation at exons 1D and 1F were included in the models. Examination of indirect effects of the adversity variables on internalizing behavior problems through methylation at exons 1D and 1F (displayed at the bottom of Table 4), revealed significant indirect effects of the adversity composite, maltreatment status, past month stress, and lifetime stress. Thus, methylation of NR3C1 mediated effects of these four adversity variables on internalizing problems. In contrast, there was not a significant indirect effect of the number of traumatic life events.
Associations of NR3C1 Methylation and Externalizing Behavior Problems
In contrast to results for internalizing behavior problems, externalizing behavior problems were not associated with mean methylation across exon 1D, 1F, or 1H.
Discussion
To our knowledge the current study is the first to test the association of methylation of the glucocorticoid receptor gene NR3C1 and internalizing and externalizing behavior problems in children. Additionally, this work is the first to demonstrate an association of behavior problems and NR3C1 promoter methylation in saliva DNA. Results suggest that methylation of exons 1D and 1F is associated with internalizing behavior problems among preschoolers, and partially mediates effects of early adversity on internalizing problems. Associations of specific CpG sites at exons 1D and 1F and internalizing problems were also observed. These results add to accumulating knowledge of the mechanisms by which early adversity “gets under the skin” and contributes to developmental trajectories of risk and resilience throughout childhood and adulthood. Furthermore, results underscore the role of the HPA stress response system in the development of psychopathology, including both biological and behavioral indicators of risk, among young children.
These results are consistent with previous findings that methylation of NR3C1 in placenta DNA is linked with various indicators of neurobehavior among neonates including attention, quality of movement, hypotonia, and lethergy (Bromer et al., 2013; Conradt et al., 2013), as well as self-regulation, but only among mothers who reported depression in pregnancy (Conradt et al., 2013). Our findings are also consistent with prior findings linking methylation of NR3C1 with MDD, PTSD, and borderline personality disorder in adulthood (Dammann et al., 2011; Na et al., 2014; Yehuda et al., 2014). We extend this literature by demonstrating associations between methylation of NR3C1 and behavioral outcomes in early childhood, and provide evidence that NR3C1 methylation is a mechanism linking early adversity to internalizing behavior problems. Internalizing behaviors in early childhood are associated with behavior problems and mood and anxiety disorders in later childhood and adolescence (Pihlakoski et al., 2006; Roza et al., 2003). Thus, methylation of NR3C1 in early childhood may be an early indicator of risk for later psychopathology.
The significant effects of methylation of NR3C1 in the current study were observed in relation to internalizing behavior problems, but not externalizing behavior problems. Prior research examining links between HPA functioning indexed by cortisol and externalizing psychopathology have demonstrated inconsistent results with relatively small effect sizes (for a meta-analysis see Alink et al., 2008). Some argue that links between HPA functioning and externalizing behaviors are more likely to be observed under conditions of stress (e.g. cortisol reactivity) rather than contexts that are not physiologically evocative (van Goozen, Fairchild, Snoek, & Harold, 2007). More research is necessary to understand if methylation of NR3C1 is relevant to externalizing behaviors in later childhood and adolescence.
The current study considered methylation at exons 1D and 1H in addition to methylation at exon 1F, the regulatory region studied in most prior research. The precise role of alternative first exons in gene transcription in various tissues is unclear, but there is evidence that they are involved in the methylation-responsive and tissue-specific control of gene expression (Moore et al; also Labonte et al.; Brenet et al). Internalizing behavior was associated with methylation at exons 1D and 1F, but not exon 1H. It is notable that two studies that found effects at 1H in association with maltreatment or psychopathology revealed lower levels of methylation at this exon in contrast to higher levels of methylation at other exons in the promoter of this gene (Steiger et al., 2013; Labonte et al., 2012), and that methylation at 1H was linked with greater levels of gene expression (Labonte et al., 2012). Future research is necessary to better understand methylation at alternative first exons of NR3C1 and their role in the development of psychopathology across the lifespan.
In the current study, associations of internalizing behavior problems and NR3C1 promoter methylation were observed in saliva DNA. To our knowledge, the current study is the first to demonstrate an association of methylation of NR3C1 in saliva DNA and a behavioral outcome in childhood. Collectively with evidence suggesting that saliva DNA may originate from blood leukocytes (Endler, Greinix, Winkler, Mitterbauer, & Mannhalter, 1999; Thiede, Prange-Krex, Freiberg-Richter, Bornhauser, & Ehninger, 2000), this work suggests that methylation of NR3C1 in saliva is a biomarker relevant to behavioral outcomes. Importantly, the preschoolers in our sample were unable to provide saliva via passive drool, and a standardized method to collect pooled saliva using a sponge was used. Therefore, the samples likely contained a mixture of epithelial cell DNA and DNA that originated from leukocytes, and it is possible that the proportion of epithelial and leukocytes influenced our results. It is also possible that the proportion of leukocytes vary as a function of behavioral phenotype, however, prior work suggests that NR3C1 methylation does not differ according to leukocyte cell type (Talens et al., 2010). Findings in the current study linking methylation of saliva DNA to behavioral outcomes provides support for the validity of this approach. Given difficulties obtaining parental consent and young children’s assent to collect blood, particularly in vulnerable populations, the current study has important implications for future work with children.
This study has a number of strengths including a diverse and low-income sample of preschoolers with and without maltreatment, detailed measurement of early adversity, a conservative approach to hypothesis testing by first examining mean methylation across each exon of NR3C1 then methylation at each individual CpG site, and inclusion of three alternate first exons in the NR3C1 promoter. There are also some limitations of the current work which pose important directions for future research. Children with chronic illness or who received medications were excluded from participating in the study, and the sample overall had low levels of internalizing behavior problems. The overall levels of methylation observed in this study, although consistent with prior work (Turecki & Meaney, in press; Bromer et al., 2013; Oberlander et al., 2008), were also low. We did not measure gene expression, so we cannot determine whether these are functional effects, but low levels of promoter methylation are characteristic of genes that are highly expressed (Brenet et al., 2011; Moore et al., 2012). Our findings are consistent with the limited prior research on NR3C1 and behavioral outcomes in neonates and in adults, and extend this work to a high-risk group of preschool-aged children. Future work is needed to determine the longer term outcomes of these associations. Our behavioral outcomes of interest, internalizing and externalizing behavior problems, were assessed using parent report. Future research should utilize observational assessments of child behavior to determine if associations of NR3C1 methylation and child behavior outcomes are robust when independent observers rate child behavior. In addition, longitudinal research in this domain is an important next step. Numerous factors likely influence the plasticity of gene methylation in response to environmental signals; this is likely specific to the particular gene and gene region, developmental stage, type and severity of exposure, and cell- and tissue-type. Future research in children should draw upon multiple assessments of stress, NR3C1 methylation, and child behavior over time to understand the time course and other determinants of these effects. Finally, child welfare records in the state of Rhode Island were coded to determine child maltreatment status, yet it is possible that some children had undocumented maltreatment, or documented cases of maltreatment in other states. Our assessment of traumatic life events and other contextual stressors was an attempt to address this limitation and capture those children who experienced undocumented maltreatment.
Associations of specific CpG sites at exons 1D and 1F and internalizing problems were observed. The transcription factor binding site database Transfac reports that the regions identified with differential methylation associated with internalizing behaviors in this study, including exon 1F site 3 and exon 1D site 6, contain transcription factor binding sites related to transcription initiation and activation of RNA Pol II, suggesting these may be important regions in transcriptional control. Furthermore, exon 1F site 9 is a NgF1A binding site, and is known to be functional. More directed mechanistic studies are necessary to fully elucidate the effect of methylation of these specific sites. Gene expression data would allow determination of whether the levels of methylation observed in the current study are functional.
This is the first study to demonstrate an association of methylation of NR3C1 and behavioral symptoms in childhood. Additionally, this work is the first to demonstrate that methylation of NR3C1 is a mediator of the association of early adversity and internalizing problems. These results suggest that methylation of NR3C1 has an important role in HPA-mediated psychopathology, and is an early indicator of emerging psychopathology. Furthermore, the current study suggests that methylation of NR3C1 in saliva DNA is relevant to behavioral outcomes in early childhood, posing exciting avenues for future research with children from which collection of blood and other tissues may not be feasible.
Acknowledgments
This research was supported by grant R01 MH083704 awarded to the last author from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIMH. We thank the many research assistants who contributed to this project including Rebecca Berger and Ashley Clement. We also thank Hasbro Children’s Hospital, Rhode Island Head Start, and the Rhode Island Department of Children, Youth, and Families for assisting in recruitment of study participants.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- Alink LR, van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental Psychobiology. 2008;50(5):427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. Journal of Psychiatry and Neuroscience. 2004;29(3):185–193. [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: the interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Norwood, NJ: Ablex Publishing Corp; 1993. pp. 7–73. [Google Scholar]
- Benjet C, Borges G, Medina-Mora ME. Chronic childhood adversity and onset of psychopathology during three life stages: childhood, adolescence and adulthood. Journal of Psychiatric Research. 2010;44(11):732–740. doi: 10.1016/j.jpsychires.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Brenet F, Moh M, Funk P, Feierstein E, Viale AJ, Socci ND, Scandura JM. DNA methylation of the first exon is tightly linked to transcriptional silencing. PloS one. 2011;6:e14524. doi: 10.1371/journal.pone.0014524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer C, Marsit CJ, Armstrong DA, Padbury JF, Lester B. Genetic and epigenetic variation of the glucocorticoid receptor (NR3C1) in placenta and infant neurobehavior. Developmental Psychobiology. 2013;55(7):673–683. doi: 10.1002/dev.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitelaar JK. The role of the HPA-axis in understanding psychopathology: cause, consequence, mediator, or moderator? European Child and Adolescent Psychiatry. 2013;22(7):387–389. doi: 10.1007/s00787-013-0441-7. [DOI] [PubMed] [Google Scholar]
- Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ. The roles of DNA methylation of NR3C1 and 11beta-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics. 2013;8(12):1321–1329. doi: 10.4161/epi.26634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids--food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- Dammann G, Teschler S, Haag T, Altmuller F, Tuczek F, Dammann RH. Increased DNA methylation of neuropsychiatric genes occurs in borderline personality disorder. Epigenetics. 2011;6(12):1454–1462. doi: 10.4161/epi.6.12.18363. [DOI] [PubMed] [Google Scholar]
- Edelman S, Shalev I, Uzefovsky F, Israel S, Knafo A, Kremer I, Mankuta D, Kaitz M, Ebstein RP. Epigenetic and genetic factors predict women’s salivary cortisol following a threat to the social self. PLoS One. 2012;7(11):e48597. doi: 10.1371/journal.pone.0048597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler G, Greinix H, Winkler K, Mitterbauer G, Mannhalter C. Genetic fingerprinting in mouthwashes of patients after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;24(1):95–98. doi: 10.1038/sj.bmt.1701815. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental neurology. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hompes T, Izzi B, Gellens E, Morreels M, Fieuws S, Pexsters A, Claes S. Investigating the influence of maternal cortisol and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood. Journal of Psychiatric Research. 2013;47(7):880–891. doi: 10.1016/j.jpsychires.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinology Metabolism Clinics of North America. 2005;34(2):271–292. vii. doi: 10.1016/j.ecl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Huang W, Nielsen DA. Sex and litter effects on anxiety and DNA methylation levels of stress and neurotrophin genes in adolescent rats. Developmental Psychobiology. 2014;56(3):392–406. doi: 10.1002/dev.21106. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Nielsen DA. Litter and sex effects on maternal behavior and DNA methylation of the Nr3c1 exon 1 promoter gene in hippocampus and cerebellum. International Journal of Developmental Neuroscience. 2014;36C:5–12. doi: 10.1016/j.ijdevneu.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Lim S, Gudsnuk K, Champagne FA. Sex-specific and strain dependent effects of early life adversity on behavioral and epigenetic outcomes. Front Psychiatry. 2013;4:78. doi: 10.3389/fpsyt.2013.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, Turecki G. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biological Psychiatry. 2012;72(1):41–48. doi: 10.1016/j.biopsych.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. British Journal of Nutrition. 2007;97(6):1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences USA. 2012;109(Sup 2):17180–10785. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melas PA, Wei Y, Wong CC, Sjoholm LK, Aberg E, Mill J, Schalling M, Forsell Y, Lavebratt C. Genetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversities. International Journal of Neuropsychopharmacology. 2013;16(7):1513–1528. doi: 10.1017/S1461145713000102. [DOI] [PubMed] [Google Scholar]
- Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38(1):23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan CJ, D’Errico NC, Stees J, Hughes DA. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics. 2012;7(8):853–857. doi: 10.4161/epi.21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na KS, Chang HS, Won E, Han KM, Choi S, Tae WS, Ham BJ. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PLoS One. 2014;9(1):e85425. doi: 10.1371/journal.pone.0085425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Perroud N, Paoloni-Giacobino A, Prada P, Olie E, Salzmann A, Nicastro R, Malafosse A. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Translational Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlakoski L, Sourander A, Aromaa M, Rautava P, Helenius H, Sillanpaa M. The continuity of psychopathology from early childhood to preadolescence: a prospective cohort study of 3–12-year-old children. European Child and Adolescent Psychiatry. 2006;15(7):409–417. doi: 10.1007/s00787-006-0548-1. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, Elbert T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Translational Psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romens SE, McDonald J, Svaren J, Pollak SD. Associations between early life stress and gene methylation in children. Child Development. 2014;86:303–309. doi: 10.1111/cdev.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza SJ, Hofstra MB, van der Ende J, Verhulst FC. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: a 14-year follow-up during childhood, adolescence, and young adulthood. The American Journal of Psychiatry. 2003;160(12):2116–2121. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- Scheeringa MS, Haslett N. The reliability and criterion validity of the Diagnostic Infant and Preschool Assessment: a new diagnostic instrument for young children. Child Psychiatry and Human Development. 2010;41(3):299–312. doi: 10.1007/s10578-009-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD. Cumulative adversity in childhood and emergent risk factors for long-term health. The Journal of Pediatrics. 2014;164:631–638. doi: 10.1016/j.jpeds.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Steiger H, Labonte B, Groleau P, Turecki G, Israel M. Methylation of the glucocorticoid receptor gene promoter in bulimic women: associations with borderline personality disorder, suicidality, and exposure to childhood abuse. International Journal of Eating Disorders. 2013;46(3):246–255. doi: 10.1002/eat.22113. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Rodriguez D, McCallum M, Salisbury AL, Phipps MG, Marsit CJ. Maternal smoking during pregnancy and infant stress response: Test of a prenatal programming hypothesis. Psychoneuroendocrinology. 2014 doi: 10.1016/j.psyneuen.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M. The genome- and system-wide response of DNA methylation to early life adversity and its implication on mental health. Canadian Journal of Psychiatry. 2013;58(12):697–704. doi: 10.1177/070674371305801208. [DOI] [PubMed] [Google Scholar]
- Talens RP, Boomsma DI, Tobi EW, Kremer D, Jukema JW, Willemsen G, Heijmans BT. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB Journal. 2010;24(9):3135–3144. doi: 10.1096/fj.09-150490. [DOI] [PubMed] [Google Scholar]
- Thiede C, Prange-Krex G, Freiberg-Richter J, Bornhauser M, Ehninger G. Buccal swabs but not mouthwash samples can be used to obtain pretransplant DNA fingerprints from recipients of allogeneic bone marrow transplants. Bone Marrow Transplant. 2000;25(5):575–577. doi: 10.1038/sj.bmt.1702170. [DOI] [PubMed] [Google Scholar]
- Turecki G, Meaney MJ. Effects of the social environment and stress on glucocorticoid receptor gene methylation: A systematic review. Biological Psychiatry. doi: 10.1016/j.biopsych.2014.11.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Burgers DE, Philip NS, Price LH, Carpenter LL. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatrica Scandinavica. 2013;128(6):434–447. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS One. 2012;7(1):e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Eslinger NM, Marsit CJ, Lesseur C, Armstrong DA, Philip NS, Josefson B, Seifer R. Methylation of exons 1D, 1F, and 1H of the glucocorticoid receptor gene promoter and exposure to adversity in pre-school aged children. Development and Psychopathology. 2015;27:577–585. doi: 10.1017/S0954579415000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goozen S, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Heijnen CJ, Kavelaars A. Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after miltary deployment. American Journal of Psychiatry. 2011;168(1):89–96. doi: 10.1176/appi.ajp.2010.10050706. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Witzmann SR, Turner JD, Meriaux SB, Meijer OC, Muller CP. Epigenetic regulation of the glucocorticoid receptor promoter 1(7) in adult rats. Epigenetics. 2012;7(11):1290–1301. doi: 10.4161/epi.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Bierer LM, Henn-Haase C, Lehrner A, Desarnaud F, Meaney MJ. Lower Methylation of Glucocorticoid Receptor Gene Promoter 1F in Peripheral Blood of Veterans with Posttraumatic Stress Disorder. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology (Berlin) 2010;212(3):405–417. doi: 10.1007/s00213-010-1969-6. [DOI] [PubMed] [Google Scholar]