Abstract
Chronic obstructive pulmonary disease (COPD) is a common, complex and heterogeneous condition which is responsible for considerable and growing morbidity, mortality and healthcare expense worldwide. In order to decipher the complexity of COPD, it is imperative that we identify groups of patients with similar clinical characteristics, prognosis and/or therapeutic needs, so called clinical phenotypes. This strategy is logical for research but it may be of limited clinical value because clinical phenotypes may overlap in the same patient and the same clinical phenotype could result from different biological mechanisms. With the goal of matching assessment to treatment choices, the most recent iteration of GOLD reorganized treatment objectives into two categories (improving symptoms, i.e., dyspnoea and health status, and decreasing future risk, as predicted by FEV1 level and exacerbations history), thus moving closer to individualized medicine using currently available bronchodilators and anti-inflammatory medications. Yet, future therapeutic options are likely to include targeting endotypes which reflect subtypes of patients defined by a distinct pathophysiological mechanism. Specific biomarkers of these endotypes would be particularly useful for clinical practice, especially when clinical phenotype alone is insufficient to identify the underlying endotype. Currently, a limited series of potential COPD endotypes and biomarkers have been suggested. We will gain empiric knowledge from proof of concept trials in COPD with emerging drugs that target specific inflammatory pathways. In each instance, specific endotyping and biomarker efforts will likely be required for success of these trials, since the pathways are likely to be operative in only a subset of patients. Network analysis of human diseases offers the possibility of a better understanding of disease patho-biologic complexity while facilitating the development of new therapeutic alternatives and, importantly, a reclassification of complex diseases. All these development should pave the way towards personalized treatment of COPD in the clinic.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, complex and heterogeneous condition which is responsible for considerable and growing morbidity, mortality and healthcare expenses worldwide (1). Within this context complexity relates to many different components with non-linear dynamic interactions, whereas heterogeneity implies that not all of these components are present in all patients at any given time point and/or at different time points in the same patient (2). To address this complexity and heterogeneity it is imperative to identify groups of patients with similar clinical characteristics, prognosis and/or therapeutic needs, so called clinical phenotypes (3). This strategy is logical for research as it may facilitate a more homogeneous selection of patients in whom to decipher the complexity of COPD. On the other hand, it may be of limited clinical value because, first, clinical phenotypes may overlap in the same patient and, second, the same clinical phenotype could result from different biological mechanisms (i.e. there can be “etiologic heterogeneity”). Although the evolution of therapeutic approaches has increasingly attempted to address these complexities, to date the majority of therapeutic options belong to a limited number of pharmacological classes, i.e. bronchodilators (short-acting and long-acting β2 agonists (SABA and LABA) and corresponding antimuscarinic agents (SAMA and LAMA)) and inhaled corticosteroids (ICS).
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) was established in 1998 to improve the diagnosis, management and prevention of COPD. A challenge for GOLD has been to provide recommendations for the correct use of the available therapies while positioning recommendations flexibly to allow the use of future, innovative therapeutic approaches that have the potential to target a personalized medicine approach. Another challenge faced by GOLD and other guideline or strategy documents, is the current paucity of evidence on how clinicians can identify patients who are more likely to benefit from available COPD treatments. This underlines the need for new treatment approaches in conjunction with refining the way treatment indications are determined. Finally, in most patients COPD is associated with other chronic diseases as part of a chronic comorbid condition that should be addressed globally. This may decrease the likelihood that treatments targeting only the COPD component will change the natural history of the patient’s disease.
The current review addresses the strengths and limitations, including gaps in the evidence, of the approaches taken by GOLD to move towards personalized medicine. We also address potential future approaches to position emerging therapies that will likely target specific biological pathways. As such, we also review the concept of an endotype, “a subtype of a (clinical) condition defined by a distinct pathophysiological mechanism, ” (4) as it may relate to future COPD therapy, and the role of biomarkers in marking endotypes and directing therapy.
Search strategy and selection criteria
To inform this brief review of endotypes and targeted therapies in COPD, we utilized several approaches. We searched the Cochrane Library, MEDLINE, and EMBASE. Search terms included “COPD AND (clinical trial OR effectiveness OR systematic review), one year. For the preceding years of this search we used the bibliography cited in the GOLD document (GOLD search strategy is COPD, filters: human, all adult, items with abstracts, clinical trial or systematic review. In addition we used a search strategy of “COPD” OR “Chronic Obstructive Pulmonary Disease" OR "Emphysema" OR "Chronic Bronchitis" AND "LABA" OR “LAMA” OR “LABA/LAMA” or “ICS” OR “ICS/LABA.” Finally we used the search “COPD” OR “Chronic Obstructive Pulmonary Disease" OR "Emphysema" OR "Chronic Bronchitis" AND "Endotype" OR “Molecular Phenotype” OR “Biomarker-directed” OR “Biomarker-driven” OR “Targeted Therapy.” Since some of these terms have only recently entered the medical vocabulary, we then supplemented this search strategy by pursuing relevant references in the associated bibliographies.
Positioning current therapeutic options – the GOLD (r)evolution
The GOLD 2001 and 2006 reports used the degree of airflow limitation alone (as assessed by the FEV1 value) to assess disease severity. Bronchodilators were recommended as treatments to improve lung function and reduce symptoms in all patients, with ICS reserved for patients with severe and very severe airflow limitation suffering with repeated exacerbations. In 2011, the GOLD document acknowledged that using FEV1 alone to assess disease severity was an overly simplistic approach (1), as FEV1 is often a poor predictor of the degree of symptoms, health status impairment and risk of exacerbations (5). Treatment objectives were re-organized into two categories: relieving current symptoms and reducing the risk of future adverse health events. A well-known 4-quadrant assessment system that characterizes patients into broad phenotypic categories, based on symptoms and risk of exacerbations (as assessed by FEV1 and the history of exacerbations in the last year) was introduced with the goal of matching assessment to treatment choices, thus moving “COPD treatment towards individualized medicine – matching the patient’s therapy more closely to his or her needs.” This evolution was a major step forward in the strategy for COPD management, and other groups have proposed conceptually similar approaches, although with several variations (6, 7).
Current GOLD treatment propositions
For all GOLD groups, short acting bronchodilators are recommended for symptom relief. These drugs may be sufficient in GOLD A patients. When patients exhibit more symptomatic limitation (GOLD B) long acting bronchodilators are recommended as maintenance. In patients at greater risk of an exacerbation (GOLD C and D) the first choice pharmacotherapy includes ICS/LABA combinations or LAMA, as both of these drug classes reduce exacerbation rates and improve lung function and health status (1). LABA/LAMA combinations are suggested as an option for GOLD B, C and D patients, as there is evidence that these drugs are more effective than long acting bronchodilator monotherapy, although most of these studies were not designed to capture effects on exacerbations (8) or were performed in patients who do not have a history of frequent exacerbations (9). Roflumilast, a phosphodiesterase 4 inhibitor, is positioned to prevent exacerbations in GOLD C and D patients with a chronic bronchitic phenotype, a novel example of phenotypically driven therapy (3, 10).
Strengths and weaknesses of GOLD propositions
The overall concept of matching more closely the clinical assessment of an individual patient to specific treatment options is attractive and a first step towards personalized therapy: “high symptom” patients (groups B and D) require more bronchodilators, while “high risk” patients (groups C and D) may require anti-inflammatory therapy. However, some GOLD treatment propositions may be criticized for not being strictly evidence-based (11), which is largely due to the paucity of evidence on treatment effects in different subgroups of patients. For example, more studies are required to investigate the effects of LABA/LAMA combinations in patients with a history of exacerbations (12). Additionally, triple therapy (ICS/LABA plus LAMA) is a treatment option for group D patients, and is frequently prescribed in clinical practice, but the evidence for this regime preventing exacerbations is limited (13). Group C and D patients are heterogeneous regarding the future risk, depending on the way they qualified for these categories (low FEV1, history of exacerbations, or both). It would thus be logical to restrict the use of ICS/LABA to those who have a history of exacerbations with or without severe airflow limitation, long-acting bronchodilator(s) being preferred for those with low FEV1 and no exacerbation history (14). Furthermore, while results of the ECLIPSE study identified a stable clinical phenotype of patients with ≥ 2 exacerbations each year (supporting GOLD’s threshold defining patients at risk) (15), ICS/LABA combinations have been studied predominantly in populations with an annual exacerbation rate nearer 1; thus, the corresponding GOLD treatment positioning does not quite match the evidence generated by randomized clinical trials (RCTs). Indeed, no therapeutic trial published before the 2011 GOLD document and only a few published after used selection criteria strictly matching the current GOLD classification (16).
Recent evidence from clinical trials: supporting, enriching or challenging current treatment propositions?
How should inhaled corticosteroids be used in COPD?
Studies have demonstrated inconsistent effects of ICS on the rate of FEV1 decline (17), so the main focus of use for these drugs is to prevent exacerbations. ICS are generally used as part of fixed-dose combinations (FDCs) with LABAs in order to maximize the clinical benefits. Historically, many clinical trials assessing these FDC have included patients with FEV1<50% predicted and a history of exacerbations (18, 19). In contrast, the TORCH study included patients with FEV<60% predicted (pre-BD) to study fluticasone propionate/salmeterol (20), and patients with FEV1<70% predicted (post-BD) were used to study the effect of fluticasone furoate/vilanterol (21); in both cases these ICS/LABA combinations had a greater effect than the LABA alone on exacerbation rates. This suggests that for patients with a history of exacerbations, the level of airflow limitation is less important in predicting benefit from ICS. Furthermore, fluticasone furoate/vilanterol was studied using ICS doses of 50, 100 and 200 µg, addressing for the first time the lack of evidence on dose-response relations regarding long-term ICS effects (21). The dose-response curve was relatively flat in terms of exacerbations, suggesting that high ICS doses are not required to achieve optimal benefit in COPD. However, it must be noted that the number of side effects of interest (i.e., pneumonia and bone fractures) was not decreased at the lower dosages.
ICS withdrawal in patients receiving long-acting bronchodilators
In 2011, a systematic review concluded that ICS withdrawal was not associated with important deterioration in overall patient outcomes, but that this result could be influenced by the definition of exacerbations and concomitant treatments (22). In 2014, results of the WISDOM trial were reported (23). This randomized controlled trial included patients with severe airflow limitation and a history of exacerbations despite often being on ICS treatment (70% were taking ICS treatment at screening), who received triple therapy during a run in period before being randomized into the ICS withdrawal and control (ICS maintained) groups. In the withdrawal group, the dose of fluticasone propionate was progressively reduced (1000 µg/day, 500 µg/day, 200 µg/day) every 6 weeks before the drug was eventually stopped. There was no difference in the occurrence of exacerbations between the two groups, but there was a difference between groups of 40 ml loss of FEV1 over the 40 weeks following complete withdrawal. Despite extensive subgroup analyses (24) the study did not identify patients at increased withdrawal-associated risk of exacerbations, but it should be noted that the overall exacerbation rate in both arms was relatively low (approximately 0.5 exacerbations / patient / year), which probably made it difficult to detect a treatment difference in the context of a low rate of events and, as occurs in many clinical trials, WISDOM was not powered for such subgroup analysis. Nevertheless, the loss of lung function after ICS withdrawal, also reported by two other previous studies (25, 26), suggests some benefit for ICS in these patients. Likewise, these results also suggest that low-dose ICS may be sufficient in some patients.
When to use combination treatments
Comparisons between ICS/LABA FDCs and LAMAs did not reveal any consistent difference in terms of exacerbation rate (27). In general, combination treatments including ICS/LABA, LABA/LAMA and ICS/LABA+LAMA have been found to be superior to bronchodilator monotherapy treatments at least for some clinical endpoints (27–32). However, the magnitude of the overall difference between combination treatments and monotherapy is often limited and less than the expected additive effect of the components of the combination. Furthermore, most studies of LABA/LAMA combination inhalers have included patients that were previously treated with at least one long acting bronchodilator monotherapy. Thus, it would seem logical to restrict the use of combination treatments to patients with dyspnoea and/or exacerbations persisting despite previous long acting bronchodilator monotherapy. GOLD does not include such a step-by-step approach, but this may be what many clinicians expect from therapeutic guidance, provided that the rules and criteria guiding choices are simple enough and evidence-based. Combining precision and simplicity, as a prerequisite for successful guidelines implementation, remains a major challenge for personalized medicine (33).
Future therapeutic directions
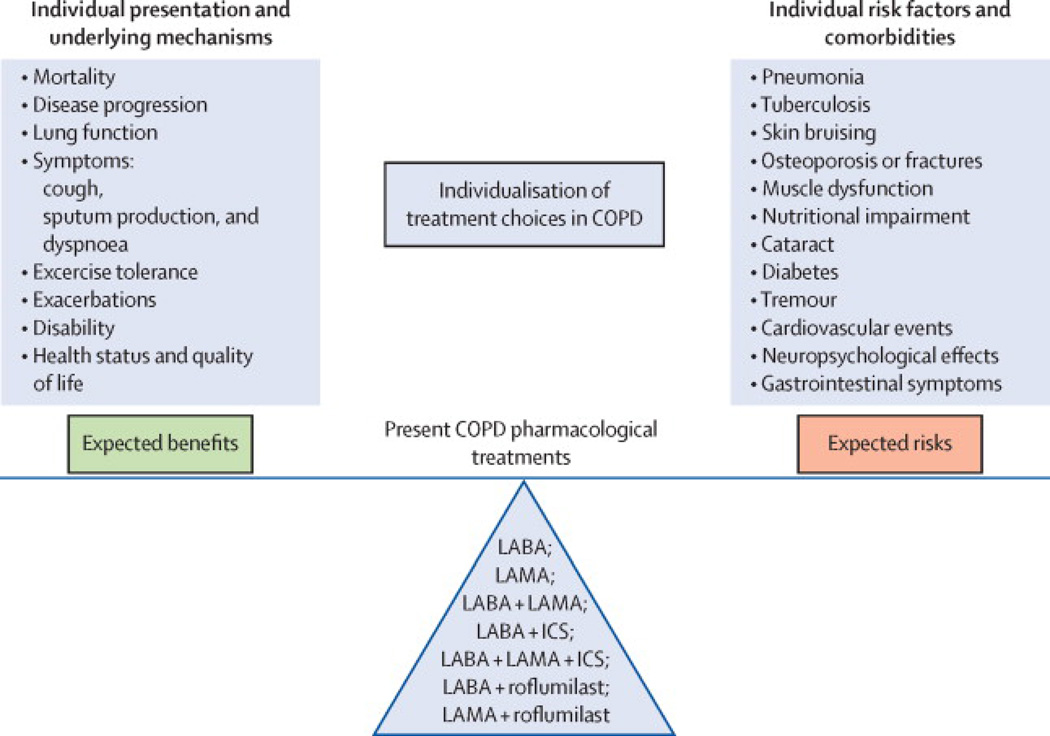
Disappointingly, the overall magnitude of clinical benefit of the different pharmacological treatment options in COPD patients remains somewhat limited. Although symptomatic benefits and reductions in exacerbation rates can be achieved with currently available treatments, the effects on the lung function decline and mortality in long term studies have been disappointing (20, 34). This can be at least in part explained by the presence of some degree of irreversible morphological abnormalities including emphysema and loss of small airways (35). Various inflammatory mechanisms involved in COPD do not respond well to corticosteroids, thus also limiting treatment effects (36). Furthermore, the response to corticosteroids appears to differ based on inflammatory cells involved, the presence of eosinophils suggesting greater likelihood of response (37). Since morphological changes and biological features are markedly heterogeneous among COPD populations (38, 39), the sensitivity to pharmacological treatments will vary between patients. These observations suggest that (i) individualizing the currently available treatments based on more in depth individual characterization of pathophysiology may optimize efficacy, and (ii) developing new treatments targeting specific mechanisms involved in subgroups of patients may be an effective strategy. Additionally, we need to adopt a benefit-risk approach for therapeutics (33) rather than a purely efficacy-based reasoning (Figure 1). Regarding long-acting bronchodilators, the risk of cardio-vascular events has been the topic of several studies with somehow contradictory results depending on study designs and populations’ characteristics (40–43). Regarding inhaled corticosteroids (ICS), there are growing concerns related to the risk of pneumonia and systemic side-effects (44). Yet, these risks should be balanced with an apparent reduction of a composite outcome of death and COPD hospitalization recently described in patients received LABA and ICS vs. those receiving LABA alone (45). Since these data come from an observational database study, they warrant further confirmation.
Figure 1.
Considering the benefit-risk balance and its individual determinants when personalizing COPD treatment choices. When deciding which pharmacological treatment option the clinician will prescribe to a given patient, he/she has to consider (i) expected benefits (left), which are determined by individual presentation and underlying mechanisms and (ii) possible risks (right), which depend on individual risk factors and comorbidities.
Identification of responders or patients at increased risk of side effects
While some patient characteristics clearly influence treatment choice for invasive approaches such as lung volume reduction surgery (46), most large trials investigating COPD pharmacological treatments have not crisply identified predictors of responders or non-responders, or patients at increased risk of side-effects. Importantly, the usual way of analysing data from randomized, controlled, clinical trials (RCTs) is to compare mean effects between treatment arms, which may lead to miss patient who go in a direction opposite to the majority. An example of this is the identification of a subgroup of COPD patients with persistent systemic inflammation using network analysis that was not identified using the more conventional group mean comparison (47). Furthermore, most RCTs do not fully reflect real-life populations and contexts (48), and sample sizes are too limited for extensive and powerful subgroup analyses. An exception isroflumilast, which was shown to be especially effective when used with long-acting bronchodilators in patients with symptoms of chronic bronchitis, FEV1<50% predicted and a history of exacerbations (49). Similarly, azithromycin was found to be more effective at reducing exacerbations in older patients with milder disease who have stopped smoking (50). In terms of side-effects, a greater increase in the risk of pneumonia related to ICS was found in current smokers, and patients with prior pneumonia, body mass index <25 kg/m2 and severe airflow limitation (51). Beyond intrinsic patients’ characteristics, environmental factors may also influence the benefit-risk ratio of ICS, as recently suggested for the ICS-associated risk of tuberculosis, which is greater in countries with high incidence of tuberculosis (52).
Further progress in the identification of specific characteristics associated with response or adverse effects from treatment can be provided by several approaches: (i) post-hoc exploratory analyses of available therapeutic trials; (ii) observational cohort studies (retrospective, e.g., using databases, or prospective), especially with a comparative effectiveness design; (iii) pragmatic randomized controlled trials; and (iv) novel analytical strategies, such as cluster and network analysis (48); and, (v) large, long-term, “classical” RCTs. All these studies should be performed in carefully selected and extensively characterized patients. In addition to precise clinical characterization (including physiology and imaging), biomarkers will most likely be of major interest both to identify target patients and to assess treatment effects. Given the complex relationships between different biological levels (genomics, epigenetics, proteomics, metabolomics, cell physiology, inflammation, and repair mechanisms, among others), their identification is likely to come from multi-level, dynamic network analyses (47, 53). In fact, studies of gene signatures that used this analytical approach have already generated attractive hypotheses regarding mechanisms and predictors of steroid response independent of the clinical phenotype (54, 55). All these avenues of research have been very recently highlighted in an ATS/ERS statement on “research questions in COPD”.
The potential impact of endotyping and biomarker-directed approaches to future therapeutic decision making
Current thinking with respect to COPD endotypes
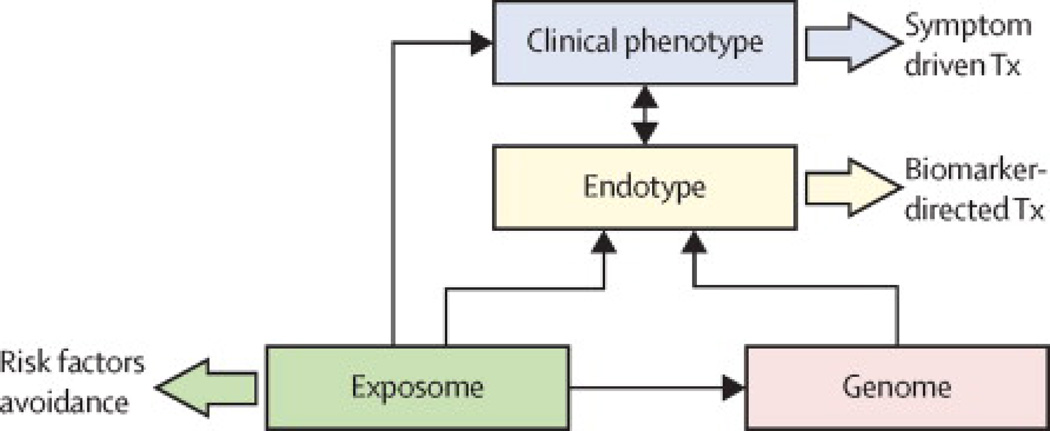
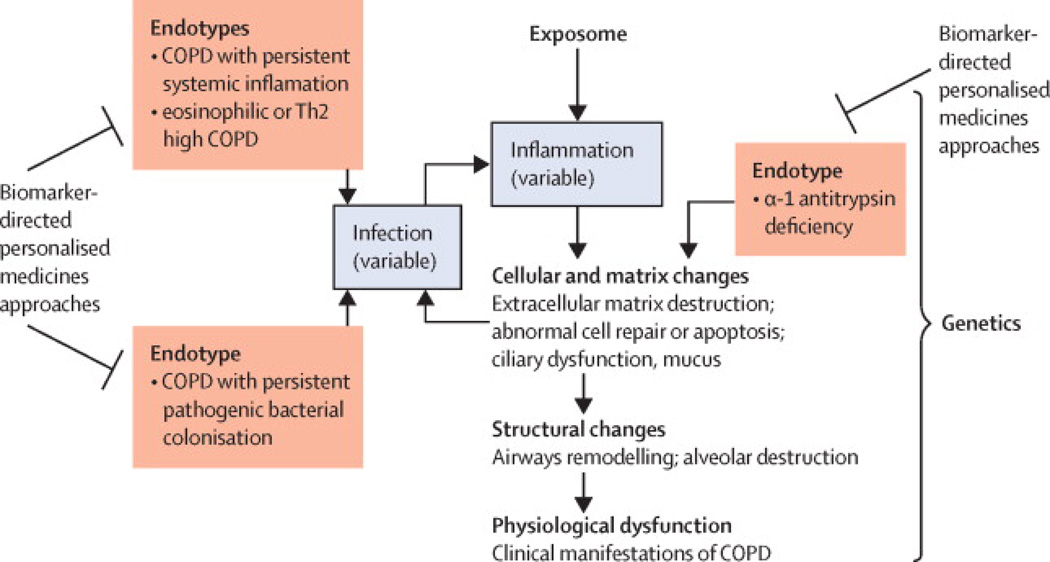
As discussed above, an endotype is a subtype of a (clinical) condition defined by a distinct pathophysiological mechanism, while a clinical phenotype is a “single or combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes (symptoms, exacerbations, response to therapy, rate of disease progression, or death) (3)” (Figure 2). Historically, studies aiming at identifying and characterizing sub-populations of COPD patients have been directed at (clinical) phenotyping. Using various statistical techniques to explore cohorts and link cross-sectional characteristics together and to longitudinal outcomes, some phenotypes have been reproducibly identified: they include patients with metabolic and cardio-vascular comorbidities, patients with severe airflow limitation occurring at an early age, frequent exacerbators, patients with predominant emphysema versus predominant airways disease (56, 57). Some but not all these phenotypes have been linked to specific biological mechanisms (endotypes), while many can actually correspond to several endotypes (e.g., frequent exacerbators, patients with cardiovascular comorbidities). As noted previously, linking endotypes to clinical phenotypes and to endotype-specific biomarkers will be crucial, since phenotypes and biomarkers are more accessible to clinicians than endotypes. Therefore, the formal identification of an endotype implies the recognition of several shared features including clinical characteristics, biomarkers, physiology, genetics, histopathology, epidemiology, and treatment response (4). One well-recognized subset of COPD, alpha-1 antitrypsin deficiency (see below), already meets all of these criteria for an endotype. Other potential COPD endotypes that we identify below only partially fulfil these criteria, although we believe that they are worthy of further investigation as “potential” endotypes. Three of the potential endotypes of COPD that we refer to below are based on markers of inflammation or airway colonization with pathogenic bacteria. Both of these processes can contribute to disease progression in COPD through persistent activation of the immune response (e.g. neutrophilic inflammation in response to bacterial colonization), the production of factors that injure lung cells or the extracellular matrix (ECM) (e.g. neutrophil elastase), structural changes that are a consequence of cellular and ECM injury (e.g. emphysema) and the physiological dysfunction that we recognize as COPD (e.g. loss of elastic recoil and consequent airflow obstruction) (Figure 3).
Figure 2.
Diagram depicting the inter-relationships (small arrows) between the ‘exposome’ (a term that describes the “totality of human environmental exposures, from conception onwards” (102)), the genetic background of the individual (Genome), the Endotype (biological networks that enable and restrict reactions) and the final clinical expression of the disease (Clinical Phenotype). Large arrows indicate different therapeutic strategies. For further explanation, see text.
Figure 3.
Our current understanding of potential endotypes of COPD. Depicted are the relationships between inflammation, cellular changes, structural changes and physiological dysfunction in COPD, and the role that chronic infection can play in perpetuating inflammation. Superimposed are potential endotypes of COPD (in red text) that relate to subtypes of inflammation, the presence of colonization with pathogenic bacteria and the absence of a mechanism protective against extracellular matrix destruction (alpha-1 antitrypsin deficiency).
Alpha-1 antitrypsin deficiency
Alpha-1 antitrypsin (A1AT) deficiency meets all of the criteria for an endotype of COPD. It has known genetic underpinnings, distinct clinical characteristics, characteristic histopathology, distinct epidemiology, and a mechanism-directed treatment approach that is guided by biomarkers (serum A1AT level, A1AT protein phenotyping and A1AT genotyping) (58). However, alpha-1 antitrypsin deficiency is a relatively unique endotype of COPD in that it is a Mendelian disorder. Other endotypes of COPD, as they are identified, are likely to be complex diseases in which predisposition depends on a number of genes and in which developmental and environmental influences are more prominent.
COPD with persistent systemic inflammation
A subgroup of COPD patients with persistently elevated inflammatory biomarker levels in the blood (white blood cells (WBC) count, C-reactive protein (CRP), interleukin (IL)-6 and fibrinogen) has significantly increased all-cause mortality and exacerbation frequency (59). However, it is not yet clear whether this persistent systemic inflammation can be treated pharmacologically and which biomarkers would be most relevant to targeting of specific treatment(s).
Eosinophilic/Th2-high COPD
There is accumulating evidence that this subgroup of patients with COPD who are marked by sputum and/or blood eosinophilia may respond to corticosteroids and possibly to blockers of cytokines produced by T-helper type 2 (Th2) cells. (60). For example, the presence of elevated sputum eosinophils in stable COPD was associated with improvement in symptoms, post-bronchodilator FEV1 and shuttle walk (a test of functional capacity) in a crossover, randomized trial of systemic corticosteroids (61). Another study confirmed that sputum eosinophilia predicts benefit with systemic corticosteroids (62), and other studies showed that this predictive relationship extends to inhaled corticosteroids as well (62–64). Thus, sputum eosinophils are a biomarker that may be useful in future decision-making relevant to the targeted use of inhaled corticosteroid in COPD. It is possible that blood eosinophils could be a useful surrogate for sputum eosinophils, especially if the blood eosinophilia is persistent. Persistent blood eosinophilia (>2%) was present in 37% of COPD subjects in the ECLIPSE study (65), a longitudinal observational study of COPD. In addition, post-hoc analyses of a recent randomized trial of benralizumab (an anti-interleukin-5 [IL-5] receptor alpha monoclonal antibody) in COPD patients with sputum eosinophilia, suggested a response in a subgroup with elevated blood eosinophil levels (defined as either ≥200 cells/µl or ≥300 cells/µl) (66). As in asthma, several Th2 cytokines could drive inflammation in eosinophilic/Th2 high COPD. Since these Th2 cytokines (IL-5, IL-4 and IL-13) are difficult to measure directly, specific biomarkers of these cytokines could be valuable. Recently, Christenson et al identified molecular biomarkers of ACOS that may be useful in distinguishing the effects of Th2 cytokines and could point to blood biomarkers that might be clinically useful in addition to or as an alternative to eosinophilia (54).
COPD with bacterial colonization
Bacterial colonization is common in COPD, is thought to drive inflammation and risk for exacerbation (67), and characterizes an important subset of patients with stable COPD. Thus COPD with bacterial colonization represents a clinical phenotype and, insofar as this colonization contributes to the biological mechanisms that perpetuate COPD, could be considered an endotype. One new therapy that reduces the risk for exacerbation in COPD is an antibiotic, azithromycin (50, 68). Whether this benefit is due to the antibacterial effect of azithromycin on bacterial colonization or more direct anti-inflammatory effects is uncertain, but biomarkers of bacterial colonization in COPD could be valuable for targeting azithromycin or other emerging antibacterial approaches. While procalcitonin has been mostly assessed as a marker of bacterial infection rather than colonization (69, 70), the “E-nose”, a method for measurement of volatile organic compounds (VOC) in exhaled breath, could help identify colonized patients (71). The beneficial effect of azithromycin (reduction in risk for exacerbation) is accompanied by a reduction in plasma sTNFrII levels which also suggests a possible surrogate outcome measure (72). Ultimately, since different bacteria can elicit different host responses (73–75), optimal endotyping of COPD based on bacterial colonization may depend on methods that are specific for pathogenic species.
Biological sub-types of COPD exacerbations
Exacerbations of COPD are associated with a clinically relevant negative impact in both the short term (morbidity, mortality and increased cost) and the long term (by accelerating decline in lung function) (76, 77). Optimized strategies to prevent and treat them better are therefore an important medical need. To maximize the benefit of corticosteroids and antibiotics in COPD exacerbations, and to develop new therapeutic alternatives, it would be valuable to have biomarkers that identify subtypes of exacerbations that respond to specific therapies. Bafadel et al recently described 4 subtypes of exacerbations defined by distinct biomarker profiles (sputum IL-1β, serum CXCL10 and blood eosinophils) which they postulated reflect distinct underlying biology (bacterial, viral, eosinophilic and pauci-inflammatory, respectively) (78). Randomized trial data and a meta-analysis suggest that blood eosinophil levels could be used to target use of oral corticosteroids for the acute treatment of exacerbations (79, 80). Somewhat weaker data suggest that procalcitonin and CRP could guide antibiotic use (70, 81).
Comorbidities
Comorbidities such as cardiovascular disease or sarcopenia (muscle wasting) are highly prevalent in COPD and impact negatively its clinical course and prognosis (82). Yet, not all COPD patients suffer them or, if they do, present the same pattern. Recent analysis indicates that certain comorbidities share molecular pathways and may constitute shared therapeutic targets (83, 84). Therefore, efforts to understand the biology of clinical phenotypes of COPD characterized by specific constellations of comorbidities may ultimately lead to the identification of specific endotypes of COPD characterized by specific biomarkers and treatment responses.
Lung cancer
Lung cancer is highly prevalent among COPD patients and constitutes one of the main causes of death in this population, especially in patients with mild to moderate airflow limitation (85). Smoking is the main risk factor for both COPD and lung cancer, but not all smokers develop COPD or cancer (1, 86). Yet, those who develop COPD, particularly if emphysema is present, have a much higher risk of developing lung cancer than those without COPD (87, 88), suggesting a synergistic effect between COPD (and emphysema) and lung cancer. The molecular mechanisms linking COPD and lung cancer are unclear, but accumulating evidence suggests that the chronic inflammatory response that characterizes COPD is likely to play a key pathogenic role (89). It follows that a better understanding of the complex molecular networks that characterize such response is essential to design effective chemopreventive and immune-therapeutic strategies.
Future directions
We will gain empiric knowledge from proof of concept trials in COPD with emerging drugs that target specific inflammatory pathways (monoclonal antibodies against IL-5, 4, 6, 13, 17 and IL1β for example) (90). Yet, there are at least two reasons for caution as we approach these clinical trials. First, it is possible that a given endotype might be relevant for only a small subset of the population. If so, these trials will have to consider either enrolling only subjects who are likely to respond to the therapy based on a given biomarker, or enrolling “all-comers” with stratification based on biomarker levels. The latter approach has the advantage that it allows assessment of response in the “biomarker negative” group. Second, an endotype or biomarker-directed therapy could be relatively weak if it targets a pathway that only represents one of several contributing pathways to COPD in a given patient. However, current experience in other respiratory diseases, such as lung cancer (EGFR), asthma (Th2 high, based on periostin) and even in COPD (steroids for eosinophilic COPD and augmentation therapy for A1AT) argue that the benefits of targeted therapy can be of sufficient magnitude to make them clinically relevant. Another important future direction is the longitudinal study of biomarker-defined subgroups to better understand the stability of these subgroupings. Finally, it is important to consider that the subgroups of COPD patients defined by these biomarkers may themselves be extremely complex since they include non-linear, dynamic epigenetic interactions between multiple spatial and time scales mediated by nonlinear biological networks that enable, filter, condition and buffer them (91). Systems biology and Network medicine offer an integrative, multi-level, dynamic approach for the understanding and eventual therapeutic modification of these complex molecular, functional, clinical and environmental networks (47, 92, 93). Recent studies have used this strategy (39, 94, 95). Network analysis of human diseases not only offers the possibility of a better understanding of disease patho-biologic complexity of different disease subtypes (endotypes), but facilitates also the development of new therapeutic alternatives and, importantly, a reclassification of complex diseases (33, 96, 97). In essence, all these development should pave the way towards personalized medicine, also known as P4 medicine or precision medicine (2, 98, 99). In this context, the precision medicine initiative launched very recently by President Barak Obama indicates how to progress towards better health (100). In the meantime, the concept of a “control panel” for COPD (101) that identifies “treatable clinical traits” could represent an appropriate way forward to facilitate the implementation of a more personalized treatment of COPD in the clinic (2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Prescott G. Woodruff, Division of Pulmonary, Critical Care, Sleep and Allergy. Department of Medicine and Cardiovascular Research Institute, University of California, San Francisco, USA.
Alvar Agusti, Thorax Institute, Hospital Clinic, IDIBAPS, University of Barcelona, CIBERES, Spain.
Nicolas Roche, Cochin Hospital Group, Assistance Publique H00F4;pitaux de Paris, University Paris Descartes (EA2511), Paris, France.
Dave Singh, University Of Manchester, University Hospital Of South Manchester Foundations Trust, Manchester, M23 9QZ, UK.
Fernando J. Martinez, Weill Cornell Medical College, New York, New York, USA; University of Michigan Health System, Ann Arbor, MI, USA.
References
- 1.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Agusti A. The path to personalised medicine in COPD. Thorax. 2014;69:857–864. doi: 10.1136/thoraxjnl-2014-205507. [DOI] [PubMed] [Google Scholar]
- 3.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, Make BJ, Rabe KF, Rennard SI, Sciurba FC, Silverman EK, Vestbo J, Washko GR, Wouters EF, Martinez FJ. Chronic obstructive pulmonary disease phenotypes: the future of COPD. American Journal of Respiratory and Critical Care Medicine. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF, Jr, Wardlaw AJ, Wenzel SE, Greenberger PA. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. The Journal of Allergy and Clinical Immunology. 2011;127:355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, MacNee W, Miller BE, Rennard S, Silverman EK, Tal-Singer R, Wouters E, Yates JC, Vestbo J Evaluation of, COPD Longitudinally to Identify Predictive Surrogate Endpoints investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respiratory Research. 2010;11:122. [Google Scholar]
- 6.Miravitlles M, Soler-Cataluna JJ, Calle M, Molina J, Almagro P, Quintano JA, Trigueros JA, Pinera P, Simon A, Riesco JA, Ancochea J, Soriano JB. A new approach to grading and treating COPD based on clinical phenotypes: summary of the Spanish COPD guidelines (GesEPOC) Primary Care Respiratory Journal : Journal of the General Practice Airways Group. 2013;22:117–121. doi: 10.4104/pcrj.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koblizek V, Chlumsky J, Zindr V, Neumannova K, Zatloukal J, Zak J, Sedlak V, Kocianova J, Zatloukal J, Hejduk K, Pracharova S, Czech P Czech Pneumological Phthisiological Society. Chronic Obstructive Pulmonary Disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented care. BiomedicalPpapers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia. 2013;157:189–201. doi: 10.5507/bp.2013.039. [DOI] [PubMed] [Google Scholar]
- 8.Tashkin DP, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009;6:17–25. doi: 10.1080/15412550902724073. [DOI] [PubMed] [Google Scholar]
- 9.Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D'Andrea P, Chen H, Banerji D. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. The Lancet Respiratory Medicine. 2013;1:51–60. doi: 10.1016/S2213-2600(12)70052-8. [DOI] [PubMed] [Google Scholar]
- 10.Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(14)62410-7. [DOI] [PubMed] [Google Scholar]
- 11.Lin FJ, Lee TA, Wong PS, Pickard AS. Evaluation of changes in guidelines for medication management of stable chronic obstructive pulmonary disease. Journal of Evaluation in Clinical Practice. 2013;19:953–960. doi: 10.1111/j.1365-2753.2012.01892.x. [DOI] [PubMed] [Google Scholar]
- 12.Frampton JE. QVA149 (indacaterol/glycopyrronium fixed-dose combination): a review of its use in patients with chronic obstructive pulmonary disease. Drugs. 2014;74:465–488. doi: 10.1007/s40265-014-0194-8. [DOI] [PubMed] [Google Scholar]
- 13.Karner C, Cates CJ. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. The Cochrane Database of Systematic Reviews. 2011:CD008532. doi: 10.1002/14651858.CD008532.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agusti A, Fabbri LM. Inhaled steroids in COPD: when should they be used? The Lancet Respiratory Medicine. 2014;2:869–871. doi: 10.1016/S2213-2600(14)70227-9. [DOI] [PubMed] [Google Scholar]
- 15.Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. The New England Journal of Medicine. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 16.Rossi A, van der Molen T, del Olmo R, Papi A, Wehbe L, Quinn M, Lu C, Young D, Cameron R, Bucchioni E, Altman P. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. The European Respiratory Journal. 2014;44:1548–1556. doi: 10.1183/09031936.00126814. [DOI] [PubMed] [Google Scholar]
- 17.Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. The Cochrane Database of Systematic Reviews. 2012;7:CD002991. doi: 10.1002/14651858.CD002991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wedzicha JA, Singh D, Vestbo J, Paggiaro PL, Jones PW, Bonnet-Gonod F, Cohuet G, Corradi M, Vezzoli S, Petruzzelli S, Agusti A Forward Investigators. Extra fine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respiratory Medicine. 2014;108:1153–1162. doi: 10.1016/j.rmed.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, Peterson S, Olsson H. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. The European Respiratory Journal. 2003;21:74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 20.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. The New England Journal of Medicine. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 21.Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, Wachtel A, Martinez FJ, Barnhart F, Sanford L, Lettis S, Crim C, Calverley PM. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. The Lancet Respiratory Medicine. 2013;1:210–223. doi: 10.1016/S2213-2600(13)70040-7. [DOI] [PubMed] [Google Scholar]
- 22.Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD--a systematic review and comment on trial methodology. Respiratory Research. 2011;12:107. doi: 10.1186/1465-9921-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, Towse L, Finnigan H, Dahl R, Decramer M, Chanez P, Wouters EF, Calverley PM, Investigators W. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. The New England Journal of Medicine. 2014;371:1285–1294. doi: 10.1056/NEJMoa1407154. [DOI] [PubMed] [Google Scholar]
- 24.Magnussen H, Tetzlaff K, Calverley PM. Inhaled glucocorticoids and COPD exacerbations. The New England Journal of Medicine. 2015;372:93–94. doi: 10.1056/NEJMc1413308. [DOI] [PubMed] [Google Scholar]
- 25.van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. American Journal of Respiratory and Critical Care Medicine. 2002;166:1358–1363. doi: 10.1164/rccm.200206-512OC. [DOI] [PubMed] [Google Scholar]
- 26.Wouters EF, Postma DS, Fokkens B, Hop WC, Prins J, Kuipers AF, Pasma HR, Hensing CA, Creutzberg EC Cosmic Study Group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax. 2005;60:480–487. doi: 10.1136/thx.2004.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long-acting beta2-agonist versus tiotropium for chronic obstructive pulmonary disease. The Cochrane Database of Systematic Reviews. 2013;5:CD007891. doi: 10.1002/14651858.CD007891.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, Balter M, O'Donnell D, McIvor A, Sharma S, Bishop G, Anthony J, Cowie R, Field S, Hirsch A, Hernandez P, Rivington R, Road J, Hoffstein V, Hodder R, Marciniuk D, McCormack D, Fox G, Cox G, Prins HB, Ford G, Bleskie D, Doucette S, Mayers I, Chapman K, Zamel N, FitzGerald M Canadian Thoracic Society/Canadian Respiratory Clinical Research Consortium. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine. 2007;146:545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 29.Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA Inspire Investigators. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. American Journal of Respiratory and Critical Care Medicine. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 30.Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. The Cochrane Database of Systematic Reviews. 2012;9:CD006829. doi: 10.1002/14651858.CD006829.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. The Cochrane Database of Systematic Reviews. 2013;8:CD006826. doi: 10.1002/14651858.CD006826.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. The Cochrane Database of Systematic Reviews. 2014;3:CD010844. doi: 10.1002/14651858.CD010844.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agusti A, Anto JM, Auffray C, Barbe F, Barreiro E, Dorca J, Escarrabill J, Faner R, Furlong LI, Garcia-Aymerich J, Gea J, Lindmark B, Monso E, Plaza V, Puhan MA, Roca J, Ruiz-Manzano J, Sampietro-Colom L, Sanz F, Serrano L, Sharpe J, Sibila O, Silverman EK, Sterk PJ, Sznajder JI. Personalized Respiratory Medicine: Exploring the Horizon, Addressing the Issues. Summary of a BRN-AJRCCM Workshop Held in Barcelona on June 12, 2014. American Journal of Respiratory and Critical Care Medicine. 2015;191:391–401. doi: 10.1164/rccm.201410-1935PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M Uplift Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. The New England Journal of Medicine. 2008;359:1543–1554. [Google Scholar]
- 35.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Pare PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. The New England Journal of Medicine. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 37.Chanez P, Vignola AM, O'Shaugnessy T, Enander I, Li D, Jeffery PK, Bousquet J. Corticosteroid reversibility in COPD is related to features of asthma. American Journal of Respiratory and Critical Care Medicine. 1997;155:1529–1534. doi: 10.1164/ajrccm.155.5.9154853. [DOI] [PubMed] [Google Scholar]
- 38.Caramori G, Adcock IM, Di Stefano A, Chung KF. Cytokine inhibition in the treatment of COPD. International Journal of Chronic Obstructive Pulmonary Disease. 2014;9:397–412. doi: 10.2147/COPD.S42544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castaldi PJ, Dy J, Ross J, Chang Y, Washko GR, Curran-Everett D, Williams A, Lynch DA, Make BJ, Crapo JD, Bowler RP, Regan EA, Hokanson JE, Kinney GL, Han MK, Soler X, Ramsdell JW, Barr RG, Foreman M, van Beek E, Casaburi R, Criner GJ, Lutz SM, Rennard SI, Santorico S, Sciurba FC, DeMeo DL, Hersh CP, Silverman EK, Cho MH. Cluster analysis in the COPD Gene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax. 2014;69:415–422. doi: 10.1136/thoraxjnl-2013-203601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, Dusser D, Joseph E, Kattenbeck S, Koenen-Bergmann M, Pledger G, Calverley P Tiospir Investigators. Tiotropium Respimat inhaler and the risk of death in COPD. The New England Journal of Medicine. 2013;369:1491–1501. doi: 10.1056/NEJMoa1303342. [DOI] [PubMed] [Google Scholar]
- 41.Verhamme KM, Afonso A, Romio S, Stricker BC, Brusselle GG, Sturkenboom MC. Use of tiotropium Respimat Soft Mist Inhaler versus HandiHaler and mortality in patients with COPD. The European Respiratory Journal. 2013;42:606–615. doi: 10.1183/09031936.00005813. [DOI] [PubMed] [Google Scholar]
- 42.Gershon A, Croxford R, Calzavara A, To T, Stanbrook MB, Upshur R, Stukel TA. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Internal Medicine. 2013;173:1175–1185. doi: 10.1001/jamainternmed.2013.1016. [DOI] [PubMed] [Google Scholar]
- 43.Groenwold RH, de Vries F, de Boer A, Pestman WR, Rutten FH, Hoes AW, Klungel OH. Balance measures for propensity score methods: a clinical example on beta-agonist use and the risk of myocardial infarction. Pharmacoepidemiology and Drug Safety. 2011;20:1130–1137. doi: 10.1002/pds.2251. [DOI] [PubMed] [Google Scholar]
- 44.Price D, Yawn B, Brusselle G, Rossi A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Primary Care Respiratory Journal : Journal of the General Practice Airways Group. 2013;22:92–100. doi: 10.4104/pcrj.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gershon AS, Campitelli MA, Croxford R, Stanbrook MB, To T, Upshur R, Stephenson AL, Stukel TA. Combination long-acting beta-agonists and inhaled corticosteroids compared with long-acting beta-agonists alone in older adults with chronic obstructive pulmonary disease. JAMA. 2014;312:1114–1121. doi: 10.1001/jama.2014.11432. [DOI] [PubMed] [Google Scholar]
- 46.Criner GJ, Cordova F, Sternberg AL, Martinez FJ. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. American Journal of Respiratory and Critical Care Medicine. 2011;184:881–893. doi: 10.1164/rccm.201103-0455CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diez D, Agusti A, Wheelock CE. Network analysis in the investigation of chronic respiratory diseases. From basics to application. American Journal of Respiratory and Critical Care Medicine. 2014;190:981–988. doi: 10.1164/rccm.201403-0421PP. [DOI] [PubMed] [Google Scholar]
- 48.Roche N, Reddel HK, Agusti A, Bateman ED, Krishnan JA, Martin RJ, Papi A, Postma D, Thomas M, Brusselle G, Israel E, Rand C, Chisholm A, Price D Respiratory Effectiveness Group. Integrating real-life studies in the global therapeutic research framework. The Lancet Respiratory Medicine. 2013;1:e29–e30. doi: 10.1016/S2213-2600(13)70199-1. [DOI] [PubMed] [Google Scholar]
- 49.Rabe KF. Roflumilast for the treatment of chronic obstructive pulmonary disease. Expert Review of Respiratory Medicine. 2010;4:543–555. doi: 10.1586/ers.10.56. [DOI] [PubMed] [Google Scholar]
- 50.Han MK, Tayob N, Murray S, Dransfield MT, Washko G, Scanlon PD, Criner GJ, Casaburi R, Connett J, Lazarus SC, Albert R, Woodruff P, Martinez FJ. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. American Journal of Respiratory and Critical Care Medicine. 2014;189:1503–1508. doi: 10.1164/rccm.201402-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crim C, Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, Wachtel A, Martinez FJ, Barnhart F, Lettis S, Calverley PM. Pneumonia Risk with Inhaled Fluticasone Furoate and Vilanterol Compared with Vilanterol Alone in Patients with COPD. Annals of the American Thoracic Society. 2015;12:27–34. doi: 10.1513/AnnalsATS.201409-413OC. [DOI] [PubMed] [Google Scholar]
- 52.Chung WS, Chen YF, Hsu JC, Yang WT, Chen SC, Chiang JY. Inhaled corticosteroids and the increased risk of pulmonary tuberculosis: a population-based case-control study. International Journal of Clinical Practice. 2014;68:1193–1199. doi: 10.1111/ijcp.12459. [DOI] [PubMed] [Google Scholar]
- 53.Wheelock CE, Goss VM, Balgoma D, Nicholas B, Brandsma J, Skipp PJ, Snowden S, Burg D, D'Amico A, Horvath I, Chaiboonchoe A, Ahmed H, Ballereau S, Rossios C, Chung KF, Montuschi P, Fowler SJ, Adcock IM, Postle AD, Dahlen SE, Rowe A, Sterk PJ, Auffray C, Djukanovic RU Biopred Study Group. Application of 'omics technologies to biomarker discovery in inflammatory lung diseases. The European Respiratory Journal. 2013;42:802–825. doi: 10.1183/09031936.00078812. [DOI] [PubMed] [Google Scholar]
- 54.Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, Lenberg ME, Spira A, Woodruff PG. Asthma-COPD Overlap: Clinical Relevance of Genomic Signatures of Type 2 Inflammation in COPD. American Journal of Respiratory and Critical Care Medicine. 2015 doi: 10.1164/rccm.201408-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van den Berge M, Steiling K, Timens W, Hiemstra PS, Sterk PJ, Heijink IH, Liu G, Alekseyev YO, Lenburg ME, Spira A, Postma DS. Airway gene expression in COPD is dynamic with inhaled corticosteroid treatment and reflects biological pathways associated with disease activity. Thorax. 2014;69:14–23. doi: 10.1136/thoraxjnl-2012-202878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vestbo J, Agusti A, Wouters EF, Bakke P, Calverley PM, Celli B, Coxson H, Crim C, Edwards LD, Locantore N, Lomas DA, MacNee W, Miller B, Rennard SI, Silverman EK, Yates JC, Tal-Singer R Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints investigators. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. American Journal of Respiratory and Critical Care Medicine. 2014;189:1022–1030. doi: 10.1164/rccm.201311-2006PP. [DOI] [PubMed] [Google Scholar]
- 57.Burgel PR, Paillasseur JL, Roche N. Identification of clinical phenotypes using cluster analyses in COPD patients with multiple comorbidities. BioMed Research International. 2014;2014:420134. doi: 10.1155/2014/420134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stockley RA, Turner AM. alpha-1-Antitrypsin deficiency: clinical variability, assessment, and treatment. Trends in Molecular Medicine. 2014;20:105–115. doi: 10.1016/j.molmed.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Agusti A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, Vestbo J, Lomas DA, Calverley PM, Wouters E, Crim C, Yates JC, Silverman EK, Coxson HO, Bakke P, Mayer RJ, Celli B Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints investigators. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PloS one. 2012;7:e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bujarski S, Parulekar AD, Sharafkhaneh A, Hanania NA. The Asthma COPD Overlap Syndrome (ACOS) Current Allergy and Asthma Reports. 2015;15:509. doi: 10.1007/s11882-014-0509-6. [DOI] [PubMed] [Google Scholar]
- 61.Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, Pavord ID. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356:1480–1485. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 62.Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, Monteiro W, Berry M, Parker D, Wardlaw AJ, Pavord ID. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. The European Respiratory Journal. 2007;29:906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 63.Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, Berry M, Parker D, Monteiro W, Pavord ID, Bradding P. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60:193–198. doi: 10.1136/thx.2004.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. The European Respiratory Journal. 2006;27:964–971. doi: 10.1183/09031936.06.00072105. [DOI] [PubMed] [Google Scholar]
- 65.Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. The European Respiratory Journal. 2014;44:1697–1700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 66.Brightling CE, Bleecker ER, Panettieri RA, Jr, Bafadhel M, She D, Ward CK, Xu X, Birrell C, van der Merwe R. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. The Lancet Respiratory Medicine. 2014;2:891–901. doi: 10.1016/S2213-2600(14)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sethi S, Maloney J, Grove L, Wrona C, Berenson CS. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2006;173:991–998. doi: 10.1164/rccm.200509-1525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Make B, Marchetti N, Martinez FJ, Madinger NE, McEvoy C, Niewoehner DE, Porsasz J, Price CS, Reilly J, Scanlon PD, Sciurba FC, Scharf SM, Washko GR, Woodruff PG, Anthonisen NR COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. The New England Journal of Medicine. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bafadhel M, Clark TW, Reid C, Medina MJ, Batham S, Barer MR, Nicholson KG, Brightling CE. Procalcitonin and C-reactive protein in hospitalized adult patients with community-acquired pneumonia or exacerbation of asthma or COPD. Chest. 2011;139:1410–1418. doi: 10.1378/chest.10-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinez FJ, Curtis JL. Procalcitonin-guided antibiotic therapy in COPD exacerbations: closer but not quite there. Chest. 2007;131:1–2. doi: 10.1378/chest.06-2567. [DOI] [PubMed] [Google Scholar]
- 71.Sibila O, Garcia-Bellmunt L, Giner J, Merino JL, Suarez-Cuartin G, Torrego A, Solanes I, Castillo D, Valera JL, Cosio BG, Plaza V, Agusti A. Identification of airway bacterial colonization by an electronic nose in Chronic Obstructive Pulmonary Disease. Respiratory Medicine. 2014;108:1608–1614. doi: 10.1016/j.rmed.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Woodruff PG, Chatila W, Connett JE, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Marchetti N, Rogers TJ, Scanlon PD, Sin DD, Voelker H, Wendt C, Albert RK COPD Clinical Research Network. Tumour necrosis factor receptor-75 and risk of COPD exacerbation in the azithromycin trial. The European Respiratory Journal. 2014;43:295–298. doi: 10.1183/09031936.00140613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh R, Mackay AJ, Patel AR, Garcha DS, Kowlessar BS, Brill SE, Donnelly LE, Barnes PJ, Donaldson GC, Wedzicha JA. Inflammatory thresholds and the species-specific effects of colonising bacteria in stable chronic obstructive pulmonary disease. Respiratory Research. 2014;15:114. doi: 10.1186/s12931-014-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. The American Journal of Medicine. 2000;109:288–295. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- 75.Barker BL, Haldar K, Patel H, Pavord ID, Barer MR, Brightling CE, Bafadhel M. Association between pathogens detected using quantitative polymerase chain reaction with airway inflammation in COPD at stable state and exacerbations. Chest. 2015;147:46–55. doi: 10.1378/chest.14-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanner RE, Anthonisen NR, Connett JE Lung Health Study Research Group. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. American Journal of Respiratory and Critical Care Medicine. 2001;164:358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 78.Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, Lindblad K, Patel H, Rugman P, Dodson P, Jenkins M, Saunders M, Newbold P, Green RH, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. American Journal of Respiratory and Critical Care Medicine. 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 79.Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. American Journal of Respiratory and Critical Care Medicine. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bafadhel M, Davies L, Calverley PM, Aaron SD, Brightling CE, Pavord ID. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. The European Respiratory Journal. 2014;44:789–791. doi: 10.1183/09031936.00062614. [DOI] [PubMed] [Google Scholar]
- 81.Stolz D, Christ-Crain M, Morgenthaler NG, Leuppi J, Miedinger D, Bingisser R, Muller C, Struck J, Muller B, Tamm M. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;131:1058–1067. doi: 10.1378/chest.06-2336. [DOI] [PubMed] [Google Scholar]
- 82.Faner R, Cruz T, Lopez-Giraldo A, Agusti A. Network medicine, multimorbidity and the lung in the elderly. The European Respiratory Journal. 2014;44:775–788. doi: 10.1183/09031936.00078714. [DOI] [PubMed] [Google Scholar]
- 83.Grosdidier S, Ferrer A, Faner R, Pinero J, Roca J, Cosio B, Agusti A, Gea J, Sanz F, Furlong LI. Network medicine analysis of COPD multimorbidities. Respiratory Research. 2014;15:111. doi: 10.1186/s12931-014-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nature Reviews Drug Discovery. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 85.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: Role of comorbidities. The European Respiratory Journal. 2006;28:1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 86.Gaga M, Powell CA, Schraufnagel DE, Schonfeld N, Rabe K, Hill NS, Sculier JP ATS ERS Task Force on the Role of the Pulmonologist in the Management of Lung Cancer. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. American Journal of Respiratory and Critical Care Medicine. 2013;188:503–507. doi: 10.1164/rccm.201307-1269ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Torres JP, Marin JM, Casanova C, Cote C, Carrizo S, Cordoba-Lanus E, Baz-Davila R, Zulueta JJ, Aguirre-Jaime A, Saetta M, Cosio MG, Celli BR. Lung cancer in patients with chronic obstructive pulmonary disease-- incidence and predicting factors. American Journal of Respiratory and Critical Care Medicine. 2011;184:913–919. doi: 10.1164/rccm.201103-0430OC. [DOI] [PubMed] [Google Scholar]
- 88.de-Torres JP, Wilson DO, Sanchez-Salcedo P, Weissfeld JL, Berto J, Campo A, Alcaide AB, Garcia-Granero M, Celli BR, Zulueta JJ. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score. American Journal of Respiratory and Critical Care Medicine. 2015;191:285–291. doi: 10.1164/rccm.201407-1210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kadara H, Fujimoto J, Yoo SY, Maki Y, Gower AC, Kabbout M, Garcia MM, Chow CW, Chu Z, Mendoza G, Shen L, Kalhor N, Hong WK, Moran C, Wang J, Spira A, Coombes KR, Wistuba II. Transcriptomic architecture of the adjacent airway field cancerization in non-small cell lung cancer. Journal of the National Cancer Institute. 2014;106 doi: 10.1093/jnci/dju004. dju004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brusselle G, Bracke K. Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Annals of the American Thoracic Society. 2014;11(Suppl 5):S322–S328. doi: 10.1513/AnnalsATS.201403-118AW. [DOI] [PubMed] [Google Scholar]
- 91.Kohl P, Crampin EJ, Quinn TA, Noble D. Systems biology: an approach. Clinical Pharmacology and Therapeutics. 2010;88:25–33. doi: 10.1038/clpt.2010.92. [DOI] [PubMed] [Google Scholar]
- 92.Barabasi AL. Network medicine--from obesity to the "diseasome". The New England Journal of Medicine. 2007;357:404–407. doi: 10.1056/NEJMe078114. [DOI] [PubMed] [Google Scholar]
- 93.Barabasi AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nature Reviews Genetics. 2011;12:56–68. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Menche J, Sharma A, Cho MH, Mayer RJ, Rennard SI, Celli B, Miller BE, Locantore N, Tal-Singer R, Ghosh S, Larminie C, Bradley G, Riley JH, Agusti A, Silverman EK, Barabasi AL. A diVIsive Shuffling Approach (VIStA) for gene expression analysis to identify subtypes in Chronic Obstructive Pulmonary Disease. BMC Systems Biology. 2014;8(Suppl 2):S8. doi: 10.1186/1752-0509-8-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rennard SI, Locantore N, Delafont B, Tal-Singer R, Silverman EK, Vestbo J, Miller BE, Bakke P, Celli B, Calverley PM, Coxson H, Crim C, Edwards LD, Lomas DA, MacNee W, Wouters EF, Yates JC, Coca I, Agusti A on behalf of the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints investigators. Identification of Five COPD Subgroups with Different Prognoses in the ECLIPSE Cohort Using Cluster Analysis. Annals of the American Thoracic Society. 2015 doi: 10.1513/AnnalsATS.201403-125OC. [DOI] [PubMed] [Google Scholar]
- 96.Loscalzo J, Kohane I, Barabasi AL. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Molecular Systems Biology. 2007;3:124. doi: 10.1038/msb4100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vanfleteren LE, Kocks JW, Stone IS, Breyer-Kohansal R, Greulich T, Lacedonia D, Buhl R, Fabbri LM, Pavord ID, Barnes N, Wouters EF, Agusti A. Moving from the Oslerian paradigm to the post-genomic era: are asthma and COPD outdated terms? Thorax. 2014;69:72–79. doi: 10.1136/thoraxjnl-2013-203602. [DOI] [PubMed] [Google Scholar]
- 98.Hood L, Balling R, Auffray C. Revolutionizing medicine in the 21st century through systems approaches. Biotechnology Journal. 2012;7:992–1001. doi: 10.1002/biot.201100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mirnezami R, Nicholson J, Darzi A. Preparing for precision medicine. The New England Journal of Medicine. 2012;366:489–491. doi: 10.1056/NEJMp1114866. [DOI] [PubMed] [Google Scholar]
- 100.Collins FS, Varmus H. A New Initiative on Precision Medicine. The New England Journal of Medicine. 2015 doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Agusti A, MacNee W. The COPD control panel: towards personalised medicine in COPD. Thorax. 2013;68:687–690. doi: 10.1136/thoraxjnl-2012-202772. [DOI] [PubMed] [Google Scholar]
- 102.Vrijheid M. The exposome: a new paradigm to study the impact of environment on health. Thorax. 2014;69:876–878. doi: 10.1136/thoraxjnl-2013-204949. [DOI] [PubMed] [Google Scholar]