Abstract
Antifreeze proteins (AFPs) noncolligatively depress the nonequilibrium freezing point of a solution and produce a difference between the melting and freezing points termed thermal hysteresis (TH). Some low-molecular-mass solutes can affect the TH values. The TH enhancement effects of selected polyhydroxy compounds including polyols and carbohydrates on an AFP from the beetle Dendroides canadensis were systematically investigated using differential scanning calorimetry (DSC). The number of hydroxyl groups dominates the molar enhancement effectiveness of polyhydroxy compounds having one to five hydroxyl groups. However, the above rule does not apply for polyhydroxy compounds having more than five hydroxyl groups. The most efficient polyhydroxy enhancer identified is trehalose. In a combination of enhancers the strongest enhancer plays the major role in determining the TH enhancement. Mechanistic insights into identification of highly efficient AFP enhancers are discussed.
Keywords: Antifreeze protein, Thermal hysteresis activity, Polyhydroxy compounds, Antifreeze protein activity enhancement, Differential scanning calorimetry
1. Introduction
Antifreeze proteins (AFPs) have been found in many organisms such as fish [1], insects [2,3], bacteria [4], and plants [5] where they function to enhance the organism’s resistance to freezing injury in winter. AFPs depress the nonequilibrium freezing point of the solution by binding to ice and inhibiting its growth without altering the melting point and the produced difference between the freezing and melting points is termed thermal hysteresis (TH) [6–8]. According to the adsorption-inhibition mechanism of AFP action [9], AFPs adsorb onto the ice crystal surface at preferred growth sites via one or more interactions including hydrophobic, van der Waals interactions [10–12], and/or hydrogen bonding [13–15] and the ice crystal growth process is thereby halted by the Kelvin effect [9].
Five types of AFPs with unrelated structures have been identified in fish [16,17], and at least three types of insect AFPs have been structurally characterized so far [18–21]. The TH activities of beetle AFPs are usually higher than those of fish AFPs [18,22]. The AFPs from the fire-colored beetle Dendroides canadensis (DAFP) and the meal-worm Tenebrio molitor (TmAFP), representing a type of insect AFP, are extensively disulfide bonded right-handed β-helical proteins with repeat structures [19,23]. Similar to TmAFPs, DAFPs have nearly identical 12- or 13-amino acid repeats with a size of 7.3–16.2 kDa [18,24]. The cleavage of the disulfide bonds results in the loss of the antifreeze activities of DAFPs and TmAFPs.
AFP-producing insects usually adapt to subzero temperature by freeze avoidance. In contrast to freeze-tolerant insects, freeze-avoiding insects must lower the freezing point and/or the supercooling point of their body fluids, which can be implemented through the removal of ice nucleators, the production of AFPs as well as the production of low-molecular-mass antifreezes which are mainly polyhydroxy compounds (e.g., glycerol, sorbitol, and glucose) [22,25–28]. The various polyhydroxy compounds in the freeze-avoiding beetles can be produced in multimolar concentrations and act through colligative means to cause freezing and supercooling depression and/or TH enhancement of AFPs [25,28].
In the presence of enhancers, the TH activities of beetle AFPs are further enhanced [8,29–33. The TH of an AFP solution thus depends ] on the intrinsic activity and the concentration of the specific AFP, and the existence of TH enhancers [29,31,32,34]. These TH enhancement substances are either low-molecular-mass molecules or certain proteins, including other AFPs [29–32]. Some low-molecular-mass enhancers, such as glycerol, do play a physiological role in enhancing the TH activity of AFPs in D. canadensis hemolymph [35]. Recently, polycarboxylates were reported as a class of efficient TH enhancers for DAFP-1, though they are not present at sufficiently high concentrations in the insects to act as natural enhancers [33].
Here we investigated the TH activities of a purified DAFP isoform, DAFP-1 (the most prevalent DAFP in D. canadensis hemolymph), in the presence of selected polyhydroxy compounds including polyols and carbohydrates as potential low-molecular-mass TH enhancers using differential scanning calorimetry (DSC). The intentions of the study were to explore a relatively large series of polyhydroxy compounds as potential TH enhancers and to investigate the effects of hydroxyl groups and other modifications in polyhydroxy molecules on their TH enhancement abilities. Some general properties of low-molecular-weight polyhydroxy enhancers are derived.
2. Materials and methods
2.1. Materials
All the chemicals except for 2-dexy-D-erthro-pentose and ally α-D-glucopyranoside (GLYCON Biochemicals, Germany) were purchased from Sigma Chemical Co. (St. Louis, MO) and used without additional purification. All solutions were prepared using Milli-Q water produced from a Synergy water system (Millipore Co.) with a minimum resistivity of 18 MΩ·cm. The samples were filtered through a 0.1 μm filter before use.
2.2. DAFP-1 preparation
E. coli Origami B cells harboring pET32b-DAFP-1 were grown in LB media supplemented with kanamycin (15 μg/mL) and ampicillin (50 μg/mL). IPTG (0.5 mM) was added to the culture to induce a high level expression of DAFP-1 when OD600 reached 0.6. The cells were harvested by centrifugation at 4 °C. The cells were disrupted mechanically by two passes through a French press (Thermo Fisher). The crude protein was purified using Ni-NTA agarose (Qiagen). The C-terminal tag (containing the hexahistidine) was cleaved using enterokinase (New England Biolabs) and removed using Ni-NTA agarose. The cleaved protein was then purified by ÄKTA Purifier 10 (GE Healthcare) using a Sephacryl S-100 gel filtration column (GE Healthcare). The identity of the purified protein was confirmed using Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry at Stanford Protein and Nucleic Acid Biotechnology Facility (expected 8968.8; observed 8969.4). The protein concentration was determined by UV–Vis spectroscopy (Varian) using the absorption at 280 nm [36]. The concentration of DAFP-1 was 450 μM in all the samples.
2.3. Thermal hysteresis characterization
The TH activities of AFPs are commonly investigated by direct microscopic observation methods including nanoliter osmometer and capillary methods [35,37]. Differential scanning calorimetry (DSC) has also been utilized as a reliable method to study the TH activities of various AFPs [38–43]. The TH of DAFP-1 alone and DAFP-1 in the presence of the selected polyhydroxy compounds were measured as described previously [33] using a DSC 823e (Mettler-Toledo) equipped with an HSS7 high sensitivity sensor and an intracooler chiller. Each combination of DAFP-1 and enhancer was prepared three times and a given sample was measured at least twice. The standard deviations were 0.02 °C.
3. Results
3.1. Effects of the number of hydroxyl groups (n=1–5) in acyclic polyols on DAFP-1 activity
To investigate the effects of the number of hydroxyl groups in polyols on the TH activity of DAFP-1, the TH activity of DAFP-1 in the presence of several acyclic polyols with varying numbers of hydroxyl groups (methanol, ethanol, ethylene glycol, glycerol, threitol and adonitol) was measured using DSC. The structures of the selected polyhydroxy compounds are listed in Fig. 1. The molar enhancement effectiveness order, adonitol (5a)>threitol (4a)>glycerol (3a)>ethylene glycerol (2a) ~1,3-propanediol (2b)>methanol (1a) ~ethanol (1b), is observed for the selected acyclic polyols (Fig. 2). The numbers in parenthesis represent the number of hydroxyl groups in the polyhydroxy compounds and the letters differentiate the polyhydroxy compounds with the same number of hydroxyl groups (Fig. 1). The enhancing ability appears to increase with increasing numbers of hydroxyl groups in the acyclic polyols (n =1–5). Additionally, it seems that the above trend is not significantly affected by increasing carbon chain length. For example, methanol and ethanol have similar TH enhancing ability and both contain one hydroxyl group, although their carbon chain lengths have a 2-fold difference. Likewise, the carbon chain lengths of ethylene glycol (2a) and 1,3-propanediol (2b) are different, the TH enhancing abilities of the two compounds are nearly identical (Fig. 2).
Fig. 1.
The structures of the selected polyhydroxy compounds tested as potential enhancers for DAFP-1. The numbers and the letters indicate the number of hydroxyl groups in the compounds and different compounds with the same number of hydroxyl groups.
Fig. 2.
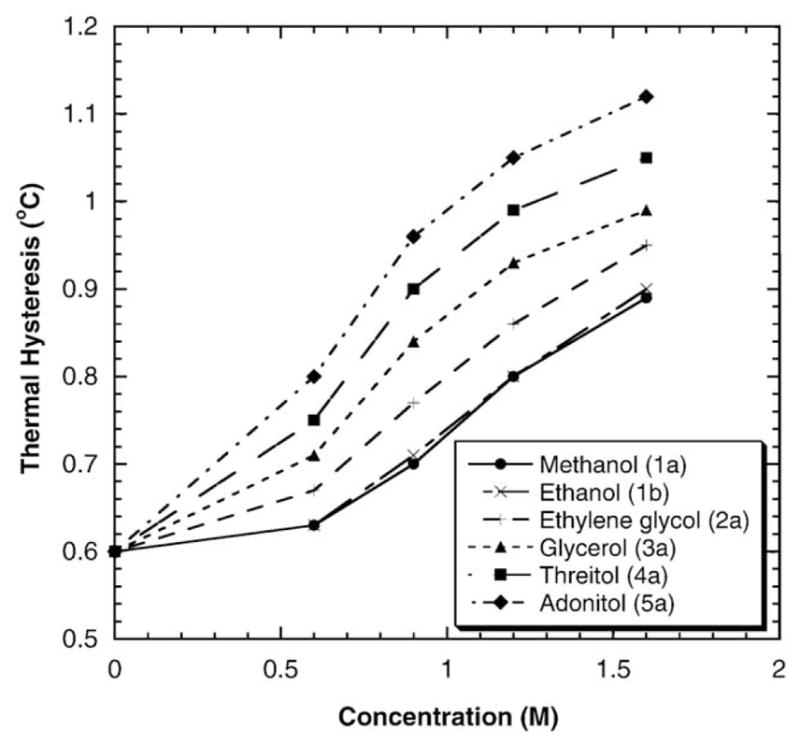
The TH activity of DAFP-1 in the presence of polyols with 1–5 hydroxyl groups.
3.2. Effects of polyhydroxy compounds with the same number of hydroxyl groups (n=2–5) on DAFP-1 activity
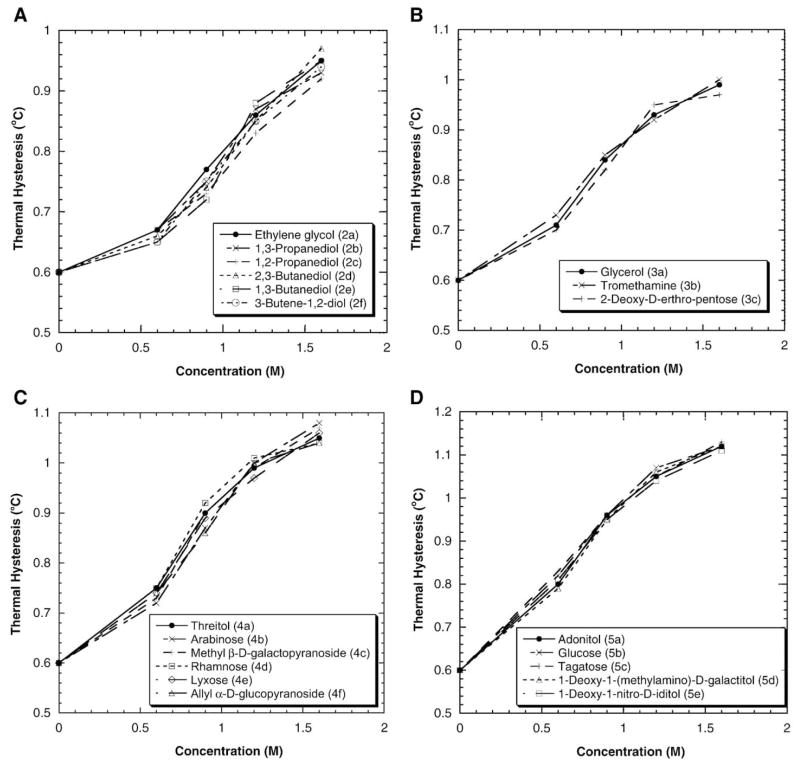
The effects of diol, triol, tetraol, and pentaol and their derivatives on DAFP-1 activity are shown in Fig. 3A–D. Shown in Fig. 3A, the TH enhancement abilities of diols and their derivatives (Fig. 1, compounds 2a–2f), i.e., ethylene glycol (2a), 1,3-propanediol (2b), 1,2-propanediol (2c), 2,3-butanediol (2d), 1,3-butanediol (2e), and 3-butene-1,2-diol (2f), are very similar. The position of the hydroxyl groups and the modification of the carbon chains, such as variation in carbon chain length and double bond inclusion, seem to have little effect on the TH enhancement ability of the molecules.
Fig. 3.
The TH activity of DAFP-1 in the presence of (A) diol derivatives, (B) triol derivatives, (C) tetraol derivatives, and (D) pentol derivatives.
Similar results are observed for triol, tetraol, pentaol and their derivatives. Glycerol (3a), tromethamine (3b), and 2-deoxy-D-erthro-pentose (3c) have three hydroxyl groups and show similar TH enhancement effects on DAFP-1 activity (Fig. 3B). Likewise, the TH enhancement abilities of tetraols and the derivatives threitol (4a), arabinose (4b), methyl β-D-galactopyranoside (4c), rhamnose (4d), lyxose (4e), and ally α-D-glucopyranoside (4f) are nearly identical (Fig. 3C). Pentol and its derivatives adonitol (5a), glucose (5b), tagatose (5c), 1-deoxy-1-(methylamino)-D-galactitol (5d), and 1-deoxy-1-nitro-D-iditol (5e) have nearly the same TH enhancement ability (Fig. 3D). It is apparent that the extent of enhancement ability of acyclic polyols, cyclitols, and carbohydrates containing the same number of hydroxyl groups (n=1–5) is similar and is not affected appreciably by other modifications (i.e., methylation, amination, and nitration).
3.3. Effects of the number of hydroxyl groups (n>5) in polyhydroxy compounds on DAFP-1 activity
Fig. 4 shows the TH enhancement effects of sorbitol (6a) and several disaccharides having eight hydroxyl groups on DAFP-1 activity. The order of TH enhancing effectiveness is: trehalose (8a)>maltose (8b)>lactose (8c)>sorbitol (6a) ~sucrose (8d) at 0.50 M and trehalose (8a)>maltose (8b)>sorbitol (6a)>sucrose (8d) at 0.80–1.60 M. Apparently, the trend that the enhancing ability increases with increasing number of hydroxyl groups in the polyols observed in Fig. 2 is not held for polyhydroxy compounds having more than five –OH groups. In addition, more complicated concentration dependent effects are observed. The TH enhancement abilities of the common disaccharides trehalose, maltose, lactose, and sucrose, are quite different although they all contain eight –OH groups. Trehalose is the most efficient one tested. In contrast to the other disaccharides tested in this study, sucrose has low TH enhancement efficiency. The TH enhancement abilities of sucrose and sorbitol are nearly identical at 0.50 M. However, at higher concentrations (i.e., 0.8, 1.2 and 1.6 M), sorbitol exhibits stronger enhancement efficiency than sucrose. The results of sorbitol and sucrose reported here are consistent with the previous study [32].
Fig. 4.
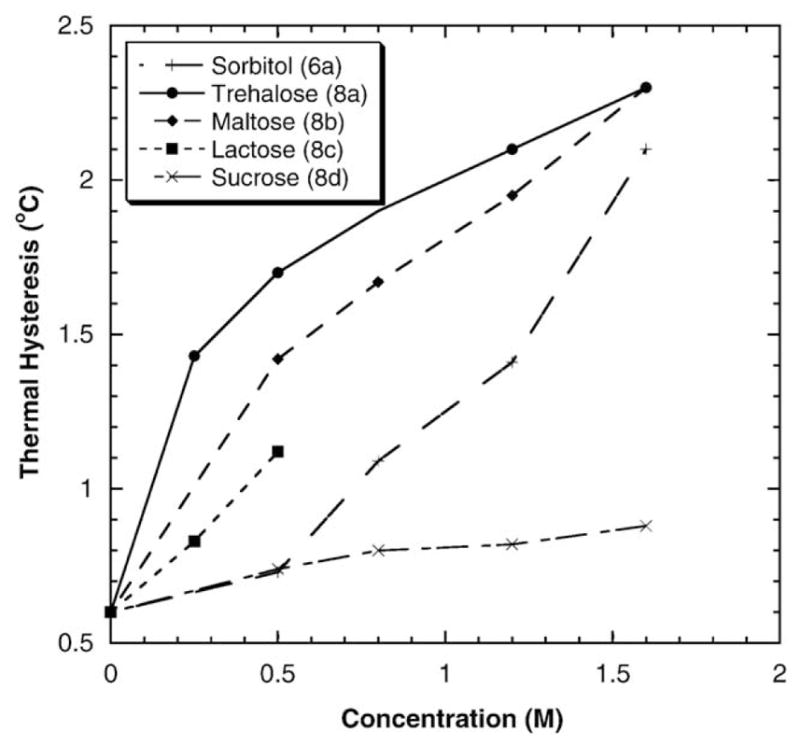
The TH activity of DAFP-1 in the presence of polyhydroxy compounds with more than five hydroxyl groups.
3.4. Effects of the combinations of polyhydroxy compounds on DAFP-1 activity
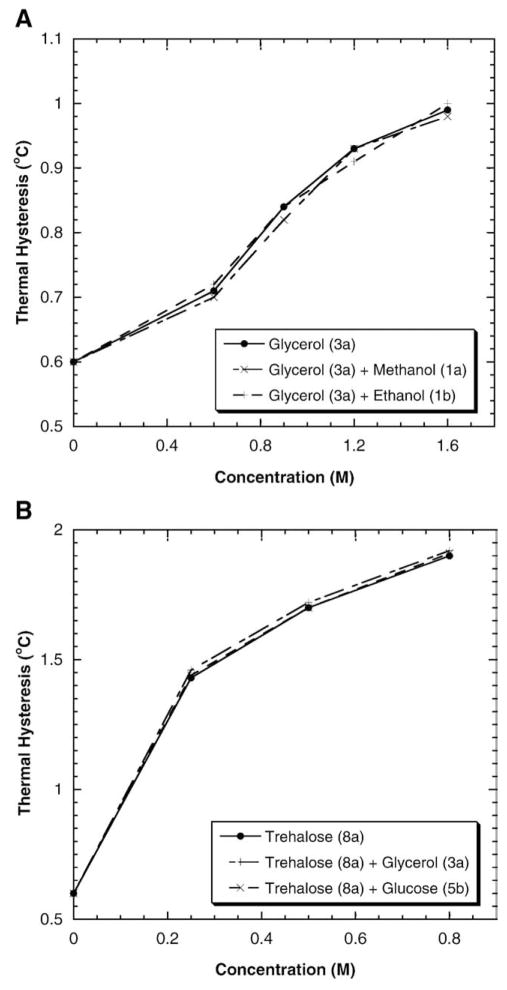
The enhancement effects of four pairs of polyhydroxy compound combinations, i.e., glycerol and methanol, glycerol and ethanol, trehalose and glycerol, and trehalose and glucose on DAFP-1 activity were shown in Fig. 5A and B. There is no further enhancement on the TH activity of DAFP-1 solutions in the presence of the combinations of a stronger enhancer and a weaker enhancer compared to the effect of the stronger enhancer alone in the system within the experimental error: glycerol~glycerol+methanol~glycerol+ethanol (Fig. 5A) and trehalose~trehalose+glycerol~trehalose+glucose (Fig. 5B). For example, the TH value of DAFP-1 solution in the presence of 0.50 M glycerol and 0.50 M methanol is nearly identical to that in the presence of 0.50 M glycerol alone (Fig. 5A) and the TH value of DAFP-1 solution in the presence of 0.50 M trehalose and 0.50 M glucose is very close to that in the presence of 0.50 M trehalose alone (Fig. 5B). Similar results were observed for other combinations of the polyhydroxy enhancers (data not shown). The results demonstrate that a stronger enhancer plays a significant role in the presence of combinations of similar enhancers and there may be a competitive process between a stronger and a weaker enhancer in the system though the mechanism is still unclear. Our limited data here also suggest that the viscosities of the solutions have little effect on the TH values at least for these systems.
Fig. 5.
The TH activity of DAFP-1 in the presence of polyhydroxy compound combinations. (A) glycerol and a weaker enhancer (methanol or ethanol); (B) trehalose and a weaker enhancer (glycerol or glucose).
4. Discussion
In contrast to polycarboxylates shown previously to enhance DAFP activity [33], polyhydroxy compounds are often found at high concentrations in the hemolymph of overwintering insects [22,25,26,44]. The polyhydroxy compounds identified in many winter insects are quite varied and may be composed of various acyclic polyols, cyclitols, and carbohydrates. Several polyhydroxy compounds tested here (e.g., glycerol, sorbitol, glucose, maltose, and trehalose) have been identified at high concentrations in some species of freeze-avoiding insects [26]. Glycerol is the only reported low-molecular-weight molecule whose concentration is high enough in D. canadensis hemolymph to function physiologically as an enhancer [32,35]. In freezing-tolerant insects, high concentrations of polyhydroxy compounds (e.g., glycerol and trehalose) are also identified and function as cryoprotectants (e.g., lessening the extent of cell dehydration and protecting biomolecular structures during freezing). Therefore, the systematic investigation of low-molecular-weight polyhydroxy compounds as potential enhancers for AFPs is important to provide further insight into identification of highly efficient AFP enhancers.
The relationship between the structures of polyhydroxy compounds and their TH enhancement ability is systematically investigated in this study. The selected polyhydroxy compounds do not have TH themselves, but they can enhance the TH activity of DAFPs. The present results demonstrate that the number of hydroxyl groups plays a major role in determining the TH enhancement effects of polyhydroxy compounds on DAFP-1 but the position of the hydroxyl groups, the length of the carbon chains, or the modifications of the carbon chains have little effect on the TH enhancement abilities of these molecules. However, the polyhydroxy molecules having more than five hydroxyl groups show complex results. Trehalose, a well-known natural cryoprotectant, is the most efficient TH enhancer among the tested polyhydroxy enhancers in this work. Our results on the TH activity of DAFP-1 in the presence of the combinations of a stronger and a weaker enhancer indicated that the weaker enhancer plays little role in the combinations and a competitive process may occur. This phenomenon has also been reported for the polycarboxylate TH enhancers of DAFP-1 recently [33].
Though various AFP enhancer molecules have been identified, our understanding of AFP enhancers remains lacking [29–32,45,46]. Hypotheses have been proposed for the mechanism of protein enhancers and low-molecular-mass enhancers of beetle AFPs. Protein enhancers usually form larger protein complexes with beetle AFP molecules and increase the TH activities of beetle AFPs by blocking larger surface areas of ice. As a low-molecular-mass enhancer, glycerol has been reported to promote the interactions of certain DAFPs with other DAFPs and with other protein enhancers and thereby further the enhancement [30,31].
It is reasonable to assume that at least some of the enhancers reported in this work may stimulate the interactions among DAFP-1 molecules on the ice surface and a larger surface area of the ice may thus be blocked by the protein complexes of two or more DAFP-1 molecules. It should be noted that relatively high concentrations of polyhydroxy enhancers may be required to stimulate the interactions, otherwise the extent of the enhancement is negligible [31]. Upon freezing the polyhydroxy enhancer molecules may stimulate the interactions among DAFP-1 molecules by hydrogen bonding to residues such as Glu, His and Arg in DAFP-1 [47]. Such interactions may not exist in liquid water since the hydrogen bonds between DAFP-1 and carbohydrate enhancer molecules can be highly interrupted by water molecules. There are two Glu, one His and four Arg residues in DAFP-1, which may hydrogen bond to seven carbohydrate molecules on the ice surface [31]. The ice surface coverage can be roughly estimated by the molecular mass of DAFP-1 and enhancer complex. However, the estimated enhancement effect of DAFP-1 and enhancer complex is generally smaller than the theoretical effect from the Kelvin effect (Wang and Wen, unpublished data). Possible explanations could be: (1) the above estimated model is too simple. Other possible contributions to the TH enhancement phenomenon by some polyhydroxy compounds may be due to fine changes in AFP structures and/or AFP-ice/water interactions. (2) The Kelvin effect itself is a simple mechanism which has its own limitations [9]. Other mechanisms, such as changes in solubility of AFPs at the ice–water interfacial region [48], were also proposed for AFPs. (3) Another possible mechanism by which these polyhydroxy compounds function as TH enhancers is that the presence of polyhydroxy compounds decreases the free water in the system. Polyhydroxy compounds may form hydrogen bonding networks with water molecules. As the concentration of polyhydroxy molecules rises, the effective concentration of free water decreases and the percentage of the free water molecules joining to the ice surface thus becomes less. At this point, neither the actual mechanism for DAFP is fully understood nor the mechanism(s) of the enhancement effect. All of these possibilities (1–3), plus others, may play a role.
The DAFP isoforms (i.e., DAFP-1, DAFP-2, and DAFP-4) have very similar structures and antifreeze activities [31]. TmAFPs and DAFPs are similar in structure and activity [18]. Therefore, it would not be surprising to see some or all of the above polyhydroxy compounds may also function as enhancers for other DAFP isoforms and/or TmAFPs. In addition, it would be interesting to learn whether these compounds could enhance the TH activity of DAFP isoform combinations.
Acknowledgments
X. Wen thanks the support from California State University at Los Angeles, the NIH-RIMI program (P20 MD001824-01), and CSUPERB.
Abbreviations
- AFP
antifreeze protein
- TH
thermal hysteresis
- DSC
differential scanning calorimetry
- DAFP
Dendroides canadensis antifreeze protein
References
- 1.DeVries AL, Wohlschlag DE. Freezing resistance in some Antarctic fishes. Science. 1969;163:1073–1075. doi: 10.1126/science.163.3871.1073. [DOI] [PubMed] [Google Scholar]
- 2.Duman JG. The role of macromolecular antifreeze in the darkling beetle, Meracantha contracta. J Comp Physiol B Biochem Syst Environ Physiol B. 1977;115:279–286. [Google Scholar]
- 3.Tomchaney AP, Morris JP, Kang SH, Duman JG. Purification, composition, and physical properties of a thermal hysteresis “antifreeze” protein from larvae of the beetle Tenebrio molitor. Biochemistry. 1982;21:716–721. doi: 10.1021/bi00533a020. [DOI] [PubMed] [Google Scholar]
- 4.Duman JG, Olsen TM. Thermal hysteresis protein activity in bacteria, fungi, and phylogenetically diverse plants. Cryobiology. 1993;30:322–328. [Google Scholar]
- 5.Urrutia ME, Duman JG, Knight CA. Plant thermal hysteresis proteins. Biochim Biophys Acta. 1992;1121:199–206. doi: 10.1016/0167-4838(92)90355-h. [DOI] [PubMed] [Google Scholar]
- 6.DeVries AL. Glycoproteins as biological antifreeze agents in Antarctic fishes. Science. 1971;172:1152–1155. doi: 10.1126/science.172.3988.1152. [DOI] [PubMed] [Google Scholar]
- 7.Barrett J. Thermal hysteresis proteins. Int J Biochem Cell Biol. 2001;33:105–117. doi: 10.1016/s1357-2725(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 8.Duman JG, Wu DW, Olsen TM, Urrutia ME, Tursman D. Thermal-hysteresis proteins. In: Steponkus PL, editor. Advances in Low-Temperature Biology. Vol. 2. JAI Press; London: 1993. pp. 131–182. [Google Scholar]
- 9.Raymond JA, DeVries AL. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci U S A. 1977;74:2589–2593. doi: 10.1073/pnas.74.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao H, Houston ME, Hodges RS, Kay CM, Sykes BD, Loewen MC, Davies PL, Sonnichsen FD. A diminished role for hydrogen bonds in antifreeze protein binding to ice. Biochemistry. 1997;36:14652–14660. doi: 10.1021/bi970817d. [DOI] [PubMed] [Google Scholar]
- 11.Haymet ADJ, Ward LG, Harding MM, Knight CA. Valine substituted winter flounder ‘antifreeze’: preservation of ice growth hysteresis. FEBS Lett. 1998;430:301–306. doi: 10.1016/s0014-5793(98)00652-8. [DOI] [PubMed] [Google Scholar]
- 12.Jia ZC, Davies PL. Antifreeze proteins: an unusual receptor-ligand interaction. Trends Biochem Sci. 2002;27:101–106. doi: 10.1016/s0968-0004(01)02028-x. [DOI] [PubMed] [Google Scholar]
- 13.Sicheri F, Yang DSC. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature. 1995;375:427–431. doi: 10.1038/375427a0. [DOI] [PubMed] [Google Scholar]
- 14.Cheng A, Merz KM. Ice-binding mechanism of winter flounder antifreeze proteins. Biophys J. 1997;73:2851–2873. doi: 10.1016/S0006-3495(97)78315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou KC. Energy-optimized structure of antifreeze protein and its binding mechanism. J Mol Biol. 1992;223:509–517. doi: 10.1016/0022-2836(92)90666-8. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher GL, Hew CL, Davies PL. Antifreeze proteins of teleost fishes. Annu Rev Physiol. 2001;63:359–390. doi: 10.1146/annurev.physiol.63.1.359. [DOI] [PubMed] [Google Scholar]
- 17.Harding MM, Anderberg PI, Haymet ADJ. ‘Antifreeze’ glycoproteins from polar fish. Eur J Biochem. 2003;270:1381–1392. doi: 10.1046/j.1432-1033.2003.03488.x. [DOI] [PubMed] [Google Scholar]
- 18.Graether SP, Sykes BD. Cold survival in freeze-intolerant insects. The structure and function of beta-helical antifreeze proteins. Eur J Biochem. 2004;271:3285–3296. doi: 10.1111/j.1432-1033.2004.04256.x. [DOI] [PubMed] [Google Scholar]
- 19.Li N, Chibber BAK, Castellino FJ, Duman JG. Mapping of disulfide bridges in antifreeze proteins from overwintering larvae of the beetle Dendroides canadensis. Biochemistry. 1998;37:6343–6350. doi: 10.1021/bi972853i. [DOI] [PubMed] [Google Scholar]
- 20.Graham LA, Davies PL. Glycine-rich antifreeze proteins from snow fleas. Science. 2005;310:461. doi: 10.1126/science.1115145. [DOI] [PubMed] [Google Scholar]
- 21.Graether SP, Kuiper MJ, Gagne SM, Walker VK, Jia Z, Sykes BD, Davies PL. β-helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature. 2000;406:325–328. doi: 10.1038/35018610. [DOI] [PubMed] [Google Scholar]
- 22.Duman JG. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu Rev Physiol. 2001;63:327–357. doi: 10.1146/annurev.physiol.63.1.327. [DOI] [PubMed] [Google Scholar]
- 23.Graham LA, Liou YC, Walker VK, Davies PL. Hyperactive antifreeze protein from beetles. Nature. 1997;388:727–728. doi: 10.1038/41908. [DOI] [PubMed] [Google Scholar]
- 24.Liou YC, Thibault P, Walker VK, Davies PL, Graham LA. A complex family of highly heterogeneous and internally repetitive hyperactive antifreeze proteins from the beetle Tenebrio molitor. Biochemistry. 1999;38:11415–11424. doi: 10.1021/bi990613s. [DOI] [PubMed] [Google Scholar]
- 25.Zachariassen KE. The role of polyols and nucleating agents in cold-hardy beetles. J Comp Physiol B Biochem Syst Environ Physiol. 1980;140:227–234. [Google Scholar]
- 26.Sømme L. Supercooling and winter survival in terrestrial arthropods. Comp Biochem Physiol A Physiol. 1982;73:519–543. [Google Scholar]
- 27.Duman JG, Wu DW, Xu L, Tursman D, Olsen TM. Adaptations of insects to subzero temperatures. Q Rev Biol. 1991;66:387–410. [Google Scholar]
- 28.Zachariassen KE. Physiology of cold tolerance in insects. Physiol Rev. 1985;65:799–832. doi: 10.1152/physrev.1985.65.4.799. [DOI] [PubMed] [Google Scholar]
- 29.Wu DW, Duman JG, Xu L. Enhancement of insect antifreeze protein activity by antibodies. Biochim Biophys Acta. 1991;1076:416–420. doi: 10.1016/0167-4838(91)90485-i. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Duman JG. A thaumatin-like protein from larvae of the beetle Dendroides canadensis enhances the activity of antifreeze proteins. Biochemistry. 2006;45:1278–1284. doi: 10.1021/bi051680r. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Duman JG. Antifreeze proteins of the beetle Dendroides canadensis enhance one another’s activities. Biochemistry. 2005;44:10305–10312. doi: 10.1021/bi050728y. [DOI] [PubMed] [Google Scholar]
- 32.Li N, Andorfer C, Duman J. Enhancement of insect antifreeze protein activity by solutes of low molecular mass. J Exp Biol. 1998;201:2243–2251. doi: 10.1242/jeb.201.15.2243. [DOI] [PubMed] [Google Scholar]
- 33.Amornwittawat N, Wang S, Duman JG, Wen X. Polycarboxylates enhance beetle antifreeze protein activity. Biochim Biophys Acta. 2008;1784:1942–1948. doi: 10.1016/j.bbapap.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans RP, Hobbs RS, Goddard SV, Fletcher GL. The importance of dissolved salts to the in vivo efficacy of antifreeze proteins. Comp Biochem Physiol Part A Mol Integr Physiol. 2007;148:556–561. doi: 10.1016/j.cbpa.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Duman JG, Serianni AS. The role of endogenous antifreeze protein enhancers in the hemolymph thermal hysteresis activity of the beetle Dendroides canadensis. J Insect Physiol. 2002;48:103–111. doi: 10.1016/s0022-1910(01)00150-0. [DOI] [PubMed] [Google Scholar]
- 36.Gill SC, von Hippel PH. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 37.DeVries AL. Antifreeze glycopeptides and peptides: interactions with ice and water. In: Packer L, editor. Methods in Enzymology, Vol. 127, Biomembranes Part O: Protons and water: Structure and Translocation. Academic Press; Orlando, FL: 1986. pp. 293–303. [DOI] [PubMed] [Google Scholar]
- 38.Hansen TN, Baust JG. Differential scanning calorimetric analysis of antifreeze protein activity in the common mealworm, Tenebrio molitor. Biochim Biophys Acta. 1988;957:217–221. doi: 10.1016/0167-4838(88)90275-0. [DOI] [PubMed] [Google Scholar]
- 39.Hansen TN, Baust JG. Differential scanning calorimetric analysis of Tenebrio molitor antifreeze protein activity. Cryobiology. 1989;26:383–388. doi: 10.1016/0167-4838(88)90275-0. [DOI] [PubMed] [Google Scholar]
- 40.Lu M, Wang B, Li Z, Fei Y, Wei L, Gao S. Differential scanning calorimetric and circular dichroistic studies on plant antifreeze proteins. J Therm Anal Calorim. 2002;67:689–698. [Google Scholar]
- 41.Wharton DA, Block W. Differential scanning calorimetry studies on an Antarctic nematode (Panagrolaimus davidi) which survives intracellular freezing. Cryobiology. 1997;34:114–121. doi: 10.1006/cryo.1996.1989. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Zhang H, Wang L, Yao H. Validation of antifreeze properties of glutathione based on its thermodynamic characteristics and protection of baker’s yeast during cryopreservation. J Agric Food Chem. 2007;55:4698–4703. doi: 10.1021/jf070387q. [DOI] [PubMed] [Google Scholar]
- 43.Ramlov H, DeVries AL, Wilson PW. Antifreeze glycoproteins from the antarctic fish Dissostichus mawsoni studied by differential scanning calorimetry (DSC) in combination with nanolitre osmometry. Cryoletters. 2005;26:73–84. [PubMed] [Google Scholar]
- 44.Meier P, Zettel J. Cold hardiness in Entomobrya nivalis (Collembola, Entomobryidae): Annual cycle of polyols and antifreeze proteins, and antifreeze triggering by temperature and photoperiod. J Comp Physiol B Biochem Syst Environ Physiol. 1997;167:297–304. [Google Scholar]
- 45.Caple G, Kerr WL, Burcham TS, Osuga DT, Yeh Y, Feeney RE. Superadditive effects in mixtures of fish antifreeze glycoproteins and polyalcohols or surfactants. J Colloid Interface Sci. 1986;111:299–304. [Google Scholar]
- 46.Kerr WL, Burcham TS, Osuga DT, Yeh Y, Feeney RE, Caple G. Synergistic depression of the freezing temperature in solutions of surfactants and antifreeze glycoproteins. Cryoletters. 1985:107–114. [Google Scholar]
- 47.Li G, Gouzy M, Fuhrhop J. Recognition process with amphiphilic carbohydrates in water. In: Meijere AD, Houk KN, Kessler H, Lehn J-M, Ley SV, Schreiber SL, Thiem J, Trost BM, Vögtle F, Yamamoto H, editors. Topics in Current Chemistry, Vol. 218, Host-Guest Chemistry, Mimetic Approaches to Study Carbohydrate Recognition. Springer; Berlin, Heidelberg: 2002. pp. 133–158. [Google Scholar]
- 48.Kristiansen E, Zachariassen KE. The mechanism by which fish antifreeze proteins cause thermal hysteresis. Cryobiology. 2005;51:262–280. doi: 10.1016/j.cryobiol.2005.07.007. [DOI] [PubMed] [Google Scholar]