Article first published online 22 April 2016.
Key Words: narrow spectrum kinase inhibitor, TOP1210, ulcerative colitis
Abstract
Background:
Kinases are key mediators of inflammation, highlighting the potential of kinase inhibitors as treatments for inflammatory disorders. Selective kinase inhibitors, however, have proved disappointing, particularly in the treatment of rheumatoid arthritis and inflammatory bowel disease. Consequently, to improve efficacy, attention has turned to multikinase inhibition.
Methods:
The activity of a narrow spectrum kinase inhibitor, TOP1210, has been compared with selective kinase inhibitors (BIRB-796, dasatinib and BAY-61-3606) in a range of kinase assays, inflammatory cell assays, and in inflamed biopsies from patients with ulcerative colitis (UC). Effects on recombinant P38α, Src, and Syk kinase activities were assessed using Z-lyte assays (Invitrogen, Paisley, United Kingdom). Anti-inflammatory effects were assessed by measurement of proinflammatory cytokine release from peripheral blood mononuclear cells, primary macrophages, HT29 cells, inflamed colonic UC biopsies, and myofibroblasts isolated from inflamed colonic UC mucosa.
Results:
TOP1210 potently inhibits P38α, Src, and Syk kinase activities. Similarly, TOP1210 demonstrates potent inhibitory activity against proinflammatory cytokine release in each of the cellular assays and the inflamed colonic UC biopsies and myofibroblasts isolated from inflamed colonic UC mucosa. Generally, the selective kinase inhibitors showed limited and weaker activity in the cellular assays compared with the broad inhibitory profile of TOP1210. However, combination of the selective inhibitors led to improved efficacy and potency in both cellular and UC biopsy assays.
Conclusions:
Targeted, multikinase inhibition with TOP1210 leads to a broad efficacy profile in both the innate and adaptive immune responses, with significant advantages over existing selective kinase approaches, and potentially offers a much improved therapeutic benefit in inflammatory bowel disease.
Protein kinases are enzymes that catalyze the transfer of a phosphate group from adenosine triphosphate (ATP) to a serine, threonine, or tyrosine residue in their substrates. The protein kinase inhibitor imatinib was first approved for clinical use in 20011; since then, protein kinases have become increasingly attractive therapeutic targets, with over 150 drugs in clinical trials and 28 approved by the Food and Drug Administration.2 Currently, most kinase inhibitors have been approved for use in clinical oncology. However, many of the major classes of immune cell receptors, including T-cell receptor, B-cell receptor, natural killer–cell receptor, and Fc receptors, depend on protein kinases to transduce intracellular signals,3 highlighting the therapeutic potential of kinase inhibitors to treat inflammatory diseases.
Inflammatory bowel disease (IBD) is an umbrella term primarily used to describe two conditions: Crohn's disease and ulcerative colitis (UC). Both diseases are characterized by recurrent intestinal inflammation and epithelial injury believed to be initiated by an inappropriate response to the gut microbiota.4,5 Interplay between luminal antigens, infiltrating leukocytes, the epithelium, and stromal cells results in excess production of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8.6 Cytokine production by these cells is controlled by a diverse array of intracellular kinases. P38α, a member of the P38 mitogen-activated protein kinase (MAPK) family, has been reported to be the most important MAPK isoform in inflammatory cells in the mucosa of patients with IBD.7,8 Being a downstream kinase, P38α is a point of convergence for multiple inflammatory signaling pathways and therefore has garnered much interest as a therapeutic target. In a phase III clinical trial, treatment of patients with active Crohn's disease with BIRB-796, a selective P38 MAPK inhibitor, was associated with a dose-dependent decrease in serum C-reactive protein after 1 week. However, compared to placebo, no clinical efficacy endpoints were reached at any time point in the study.9 One explanation of the lack of efficacy of BIRB-796 may be redundancy in the signaling pathways, overriding the need for P38α in the inflammatory cascade. Potentially, this could be avoided by targeting more upstream signaling nodes and possibly several different targets simultaneously.10 Src family kinase (SFK) members Lck and Fyn are among the first upstream kinases to be engaged upon T-cell receptor activation, and spleen tyrosine kinase (Syk) is engaged early in B-cell receptor and Fc receptor signaling.11 Neither SFK nor Syk has been a clinical target for IBD; however, a selective Syk inhibitor, fostamatinib, has been shown to decrease mucosal damage in a mouse acetic acid–induced colitis model,12 and the key role T cells have in IBD pathogenesis indicates merit in targeting SFK members. Furthermore, fostamatinib is a particularly promising treatment of chronic lymphocytic leukemia13; however, it achieved only limited efficacy in a phase III rheumatoid arthritis trial,14 indicating that for inflammatory disorders of more complex pathophysiology, single target inhibition may not be sufficient for clinical efficacy.
Polypharmacology is emerging as the next paradigm of drug discovery with a belief that targeting multiple signaling nodes with a single drug may lead to improved efficacy, particularly in multifaceted diseases such as IBD and rheumatoid arthritis.15 Initially, it was believed that a high degree of kinase selectivity would be critical for an acceptable safety profile with concerns that systemic multikinase inhibition might increase the risk of an adverse safety profile. This has, however, been addressed through the proposal of topical delivery of kinase inhibitors.16,17 For example, pan-Janus kinase inhibitors are in preclinical development as inhaled therapy for severe asthma.18
A class of narrow spectrum kinase inhibitors (NSKI) has been developed, which target P38α, SFK, and Syk. Targeting these kinases addresses the narrow window of activity achieved with truly selective inhibitors and at the same time avoids potential signaling redundancy as observed when targeting only downstream signaling nodes. To et al19 recently described the superior effects of an NSKI, RV1088, over selective kinase inhibitors in rheumatoid arthritis synovial membrane cells. In this study, we investigated how another NSKI, TOP1210, compares to selective kinase inhibitors, both in terms of breadth of activity and pharmacology (efficacy and potency), across different inflammatory cell types, in selected human cell assays and in tissue explants from patients with UC.
METHODS
Kinase Assays
A commercial 384-well fluorescence resonance energy transfer (FRET)-based kinase assay for Src, P38α, and Syk kinases was used to measure inhibitory activity of compounds. Compound or vehicle (dimethyl sulfoxide [DMSO], 1% v/v) was incubated with kinase of interest (P38α, 20 ng/mL; Src, 750 ng/mL; or Syk, 500 ng/mL) for 2 hours. Z-lyte peptide (Invitrogen, Paisley, United Kingdom), selective for an individual kinase, was added (Ser threonine 4 peptide for P38α, Tyr2 peptide for Src and Syk). ATP, 10 μM, 200 μM, or 15 μM, for P38α Src and Syk, respectively, was then added. In addition, inactive MAPK-activated protein kinase (MAPKAP)2 (180 ng/mL) was added to the P38α reaction mix. After 1 hour incubation, development reagent was added followed by another 1 hour incubation. The reaction was terminated and read using a fluorescence microplate reader.
Patients and Samples
Peripheral venous blood from healthy volunteers was collected by venepuncture and anticoagulated with ethylenediaminetetraacetic acid (EDTA). Perendoscopic biopsies or surgical specimens were taken from macroscopically inflamed colonic areas of patients with UC. Diagnosis of UC was made according to clinical and histologic criteria and confirmed by endoscopy. Each patient or volunteer who took part in the study was recruited after appropriate local ethics committee approval, and informed consent was obtained in all cases.
Preparation of Human Peripheral Blood Mononuclear Cells
Blood was diluted (1:1) with phosphate-buffered saline (PBS)-containing EDTA (2 mM) before overlaying on Lymphoprep. The sample was centrifuged (1200 × g, 20 min), and the resultant “buffy coat” containing the peripheral blood mononuclear cells (PBMCs) was collected. After further centrifugation (780 × g, 10 min) and washing (×2) in PBS containing EDTA (2 mM), the final cell pellet was resuspended in 10 mL PBS/EDTA (2 mM). Cells were counted and the final concentration was adjusted to 1 × 106 cells per mL by resuspension in RPMI 1640 culture media containing heat inactivated fetal bovine serum (FBS, 10% v/v), penicillin/streptomycin, and HEPES (25 mM).
Cellular Assays
Lipopolysaccharide Stimulation of PBMCs
PBMCs (200 μL) were incubated (2 hours, 37°C, 5% CO2) with test compound (0.1–1000 ng/mL) or vehicle (DMSO, 0.5% v/v). Lipopolysaccharide (LPS, 1 ng/mL) was added, and after 24-hour incubation (37°C, 5% CO2), plates were centrifuged (780 × g, 2 min) and supernatants were collected for analysis of IL-8 levels.
CD3/CD28 Stimulation of PBMCs
PBMCs (200 μL) were added to anti-CD3 (clone OKT3, 0.6 μg/mL) and anti-CD28 (clone CD28.2, 12 μg/mL) coated 96-well plates. Test compound (0.1–1000 ng/mL) or vehicle (DMSO, 0.5% v/v) was added. After 72-hour incubation (37°C, 5% CO2), plates were centrifuged (780 × g, 2 min) before collection of supernatants for analysis of IL-2 and interferon (IFN)-γ levels.
LPS Activation of Monocyte-derived Macrophages
CD14+ cells were isolated from human PBMCs by positive selection using magnetic beads. Cells were resuspended in RPMI containing 10% FBS and cultured (37°C, 5% CO2) in the presence of human recombinant granulocyte-macrophage colony-stimulating factor (100 ng/mL) for 12 to 14 days. Cells were harvested and resuspended (2 × 105 cells per mL), dispensed into 96-well plates (100 μL/well) and allowed to equilibrate (2 hours). Test compound (0.1–1000 ng/mL) or vehicle (DMSO 0.5% v/v) was incubated with cells (2 h) before stimulation with LPS (10 ng/mL) for 24 hours. Supernatants were collected for IL-8 and TNF-α analysis.
IL-8 Production by HT29 Cells
HT29 cells, a colonic epithelial cell line, were seeded (2 × 105 cells per well) from established cultures onto 96-well plates in culture media (McCoy's 5a containing 10% FBS) and allowed to adhere for 24 hours. Test compound (0.1–1000 ng/mL) or vehicle (DMSO, 0.5% v/v) was added for 2 hours (37°C, 5% CO2) before stimulation by addition of recombinant human IL-1β (5 ng/mL). After further 24 hours (37°C, 5% CO2), plates were centrifuged (195 × g, 5 min) and supernatants were collected for measurement of IL-8.
In separate experiments, the potential cytotoxic effects of compounds were assessed by incubation (37°C, 24 h) of compound or vehicle with HT29 cells seeded as above. Viability of cells was assessed by addition of Presto blue and reading fluorescence (560 nm/590 nm) after 10 to 30 minutes. At the chosen concentrations of test compounds used in these studies, no significant decrease in viability was detected (data not shown).
Myofibroblast Isolation and Culture
Intestinal myofibroblasts were isolated from inflamed resected UC mucosa. The mucosa was dissected from the submucosa with a scalpel and cut into 1 mm square pieces. These were then cultured at 37°C in a humidified CO2 incubator in D-MEM medium, supplemented with 20% FBS, 100-U/mL penicillin, 100 μg/mL streptomycin, 50 μg/mL gentamicin, and 1 μg/mL amphotericin. Established colonies of myofibroblasts were seeded into 25 cm2 culture flasks and cultured in DMEM medium, supplemented with 20% FBS and antibiotics. At confluence, the cells were passaged using trypsin-EDTA in a 1:2 to 1:3 split ratios. Cells were grown to at least passage 4 before use. Subconfluent monolayers of myofibroblasts were seeded (5 × 104 cells/well) into 12-well plates and cultured overnight at 37°C, 5% CO2 before being stimulated with 20 ng/mL recombinant human TNF-α and either vehicle (DMSO, 0.5% v/v) or test compound (TOP1210, 0.000001–1 μg/mL; BIRB-796, 0.01–10 μg/mL; dasatinib, 0.01–10 μg/mL; or BAY-61-3606, 0.01–10 μg/mL). After 24 hour culture, supernatants were collected for measurement of IL-6 and IL-8 by enzyme-linked immunosorbent assay (ELISA). Results were expressed as mean percent inhibition ± SEM compared with TNF-α + DMSO.
Organ Culture
Inflamed biopsies from patients with UC were placed (one biopsy per well) in 24-well plates in 300 μL serum-free HL-1 medium supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin, and cultured at 37°C, 5% CO2 with either vehicle (DMSO, 0.5% v/v) or test compound (TOP1210, 0.001–1 μg/mL; 1 μg/mL BIRB-796; 1 μg/mL dasatinib; or 1 μg/mL BAY-61-3606), or selective kinase inhibitors in combination. After 24 hour culture, supernatants of mucosal biopsies were collected for measurement of IL-1β, IL-6, and IL-8 by ELISA. Results were expressed as mean percent inhibition ± SEM compared with DMSO.
Enzyme-linked Immunosorbent Assay
All cytokines were measured using commercial ELISA kits according to manufacturers' instructions.
Materials and Reagents: TOP1210, BIRB-796, Dasatinib, BAY61-3606
TOP1210, 3-((4-((4-(3-(3-(tert-butyl)-1-(p-tolyl)-1H-pyrazol-5-yl)ureido)naphthalen-1 yl)oxy)pyrimidin-2-yl)amino)-5-ethynyl-N-(2-(2-(2-methoxyethoxy)ethoxy)ethyl)benzamide, was prepared employing published procedures.20 Dasatinib was supplied by LC Laboratories (Woburn, MA), and BIRB-796 was obtained from Ontario Chemicals Inc. (Ontario, Canada).
Z-lyte assay materials and heat-inactivated FBS were obtained from Invitrogen (Paisley, United Kingdom). Lymphoprep was obtained from Alere Ltd. (Stockport, Cheshire, United Kingdom). Anti-CD3 and anti-CD28 antibodies were supplied by eBioscience (Hatfield, Hertfordshire, United Kingdom) and BD Bioscience (Cowley, Oxford, United Kingdom), respectively. ELISA kits were obtained from R&D systems (Abingdon, United Kingdom). All other reagents were obtained from Sigma-Aldrich (Gillingham, Dorset, United Kingdom).
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA) using the one-way analysis of variance followed by the Dunnett's multiple-comparisons test or the Kruskal–Wallis test followed by the Dunn's multiple-comparisons test. A level of P < 0.05 was considered statistically significant.
RESULTS
Inhibition of Key Kinases
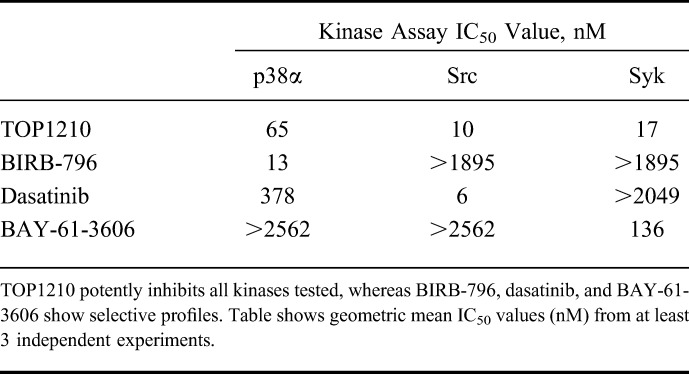
The inhibitory activity of TOP1210 and selective kinase inhibitors was assessed in an ATP-dependent substrate phosphorylation assay (Table 1). The selective kinase inhibitors chosen for this study were BIRB-796 (p38 MAPK inhibitor), dasatinib (SFK inhibitor), and BAY-61-3606 (Syk inhibitor). Inhibition of Src was considered to be representative of effects on SFK because of the high level of homology within this kinase family. TOP1210 treatment achieved potent, concentration-related inhibition of p38α, Src, and Syk kinase activities with IC50 values of 65, 10, and 17 nM, respectively. In contrast, BIRB-796 and BAY-61-3606 only inhibited their respective kinase targets. Dasatinib potently inhibited Src kinase activity (IC50, 6 nM) but was also a weak inhibitor of p38α (IC50, 378 nM). TOP1210 potency was comparable to (IC50, within 5-fold) or, in the case of BAY-61-3606, greater than that of the selective kinase inhibitors at their respective target kinase.
TABLE 1.
Inhibitory Effects of Selective Kinase Inhibitors and the NSKI TOP1210 on p38α, Src, or Syk Kinase Activity in a Biochemical Z-lyte Based Assay
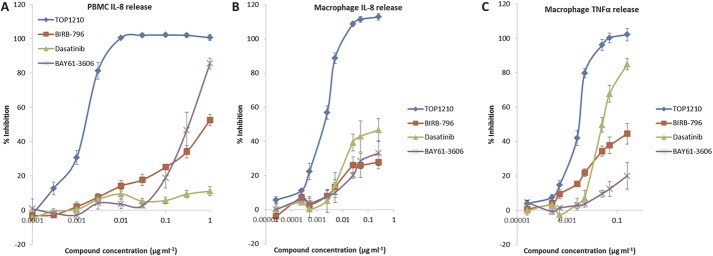
Effect of TOP1210 and Selective Kinase Inhibitors on Innate, Adaptive, and Epithelial Cellular Responses
Mucosal inflammation involves the interplay of innate and adaptive immune mechanisms with the epithelium. As a model of innate immunity, PBMCs were stimulated with LPS, leading to IL-8 release (15,658 ± 1500 pg/mL, mean ± SEM). TOP1210 achieved concentration-dependent (0.1–1000 ng/mL) and maximal (100%) inhibition of IL-8 release (Fig. 1A) with an IC50 value of 1.9 nM (Table 2). In contrast, both BIRB-796 and dasatinib failed to achieve 50% inhibition at any concentration up to the maximum tested (1 μg/mL). BAY-61-3606 achieved a maximum of 83% inhibition but with a potency (IC50 607 nM, Table 2) some 300-fold weaker than TOP1210. A similar profile was observed in LPS-stimulated primary human macrophages with TOP1210 demonstrating superior activity over the selective inhibitors, achieving potent, maximal inhibition of IL-8 (Fig. 1B) and TNF-α release (Fig. 1C) with IC50 values of 2.2 and 3.3 nM, respectively (Table 2). BIRB-796 and BAY-61-3606 failed to achieve 50% inhibition of either IL-8 or TNF-α at any concentration up to the maximum tested (250 ng/mL). Dasatinib achieved 87% inhibition of TNF-α release but was approximately 30-fold weaker (IC50, 52 nM, Table 2) than TOP1210 and achieved less than 50% inhibition of IL-8 release.
FIGURE 1.
TOP1210 is a potent inhibitor of the innate immune responses in LPS-stimulated human PBMCs and macrophages. TOP1210 inhibits IL-8 release by LPS-stimulated PBMCs (A) and macrophages (B), and also LPS-stimulated TNF-α release by macrophages (C), with greater potency than any of the selective kinases tested. Generally, compared to TOP1210, BIRB-796, dasatinib, and BAY61-3606 have weak potency and efficacy in both PBMCs and macrophages. Graphs represent means of at least 3 independent experiments ± SEM.
TABLE 2.
Effect of TOP1210 and Selective Kinase Inhibitors on Innate (LPS Stimulation of PBMCs or Primary Macrophages), Adaptive (Anti-CD3/Anti-CD28 Stimulation of PBMCs), and Epithelial (IL-1β Stimulation of HT29 Cells) Cellular Response Assays
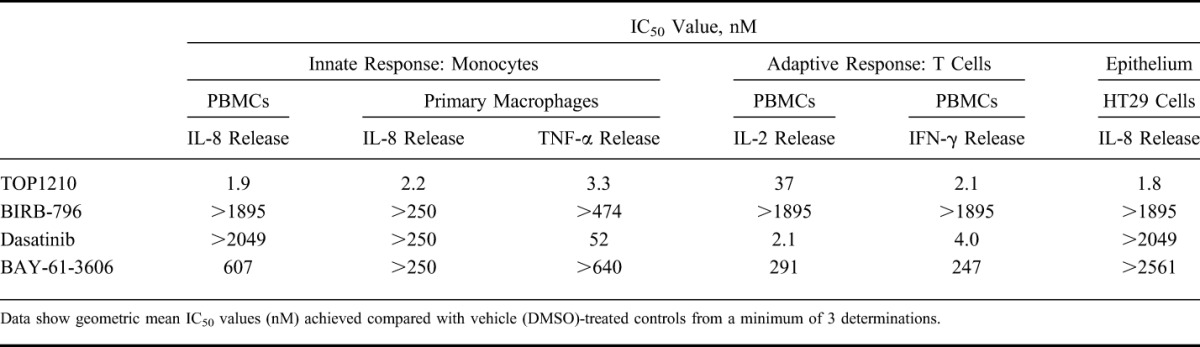
To model the adaptive immune response, PBMCs were stimulated with anti-CD3 and anti-CD28 to activate the T-cell population. This stimulation led to release of IFN-γ (16,146 ± 5926 pg/mL, mean ± SEM) and IL-2 (39,742 ± 9652 pg/mL, mean ± SEM). TOP1210 achieved maximal inhibition of IFN-γ release (Fig. 2A) with an IC50 of 2.1 nM (Table 2). As expected, the SFK selective inhibitor, dasatinib, was also a potent inhibitor of IFN-γ release with similar potency (IC50, 4.0 nM, Table 2) to that of TOP1210. BIRB-796 was inactive in the assay, and BAY-61-3606, although achieving maximal efficacy, was 120-fold weaker (IC50, 247 nM) than TOP1210. In the IL-2 release assay, a very similar profile was observed with the selective kinase inhibitors (Fig. 2B). TOP1210, however, was 18-fold weaker in potency as an inhibitor of IL-2 release (IC50, 37 nM, Table 2) compared with IFN-γ release.
FIGURE 2.
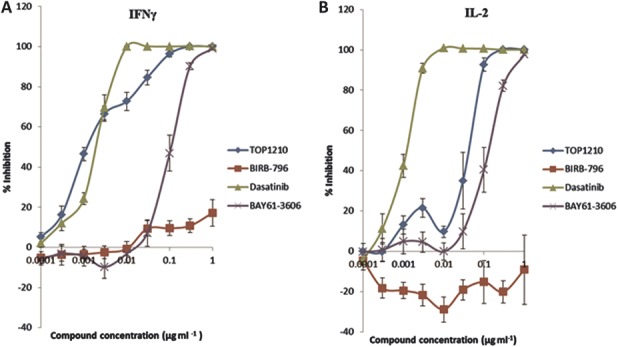
TOP1210 is a potent inhibitor of an adaptive immune response in anti-CD3/anti-CD28–stimulated T-cell release of IFN-γ (A) and IL-2 (B). Both TOP1210 and dasatinib inhibit IFN-γ release with similar potency, whereas TOP1210 is approximately 15-fold weaker than dasatinib as an inhibitor of IL-2 release. BAY61-3606 demonstrates weaker inhibition, whereas BIRB-796 has little-to-no effect. Graphs represent means of at least 3 independent experiments ± SEM.
Inflammation of the epithelium is a hallmark of many mucosal disorders. Because of the difficulty of culturing primary human colonic cells, HT29 cells, a human intestinal epithelial cell line, has been used as a model of human colonic epithelium. Stimulation of these cells with IL-1β leads to IL-8 release levels of 1736 ± 93 pg/mL (mean ± SEM). TOP1210 potently (IC50, 1.8 nM, Table 2) inhibited IL-8 release with maximal efficacy (Fig. 3). In contrast, all three selective kinase inhibitors were only weakly active in the assay with none achieving greater than 50% inhibition.
FIGURE 3.
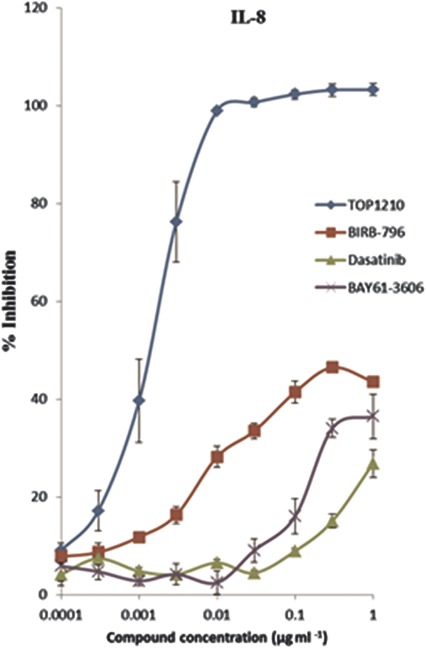
TOP1210 is a potent inhibitor of IL-1β–stimulated IL-8 release from HT29 cells. Selective kinase inhibitors achieve less than 50% inhibition and are weak inhibitors of IL-8 release from HT29 cells. Graph represents means of at least 3 independent experiments ± SEM.
In a propidium iodide, flow cytometry–based assay, TOP1210 was shown not to effect cell viability at any of the concentrations tested in the cellular assays (data not shown).
In summary, TOP1210 was potent and highly efficacious across all cellular models of innate, adaptive, and epithelial responses. In contrast, the selective kinase inhibitors of P38α, SFK, and Syk were only active in some assays and for the most part much less potent than TOP1210.
Effect of Selective Kinase Inhibitor Combination on IL-8 Release by HT29 Cells
BIRB-796 (0.1–1000 ng/mL) produced concentration-dependent inhibition of IL-8 secretion from IL-1β–stimulated HT29 epithelial cells (Fig. 4A) with a maximum inhibition of 33%. Dasatinib and BAY-61-3606 when tested alone had a similar inhibitory profile, achieving maximal inhibitions at 1000 ng/mL of 22% and 17%, respectively (Fig. 4A). Neither dasatinib nor BAY-61-3606 had any significant inhibitory effect at 100 ng/mL (Fig. 4A). In combination with BIRB-796, however, both 100-ng/mL dasatinib and 100 ng/mL BAY-61-3606 induced a leftward shift of the BIRB-796 concentration effect curve and increased the maximum inhibition to approximately 50% (Fig. 4B). A triple combination of BIRB-796, 100 ng/mL dasatinib, and 100 ng/mL BAY-61-3606 induced a further leftward shift and increased the maximum inhibition to 63% (Fig. 4B). A more than additive effect is illustrated in Fig. 4C, where the combination of noninhibitory concentrations of BIRB-796 (1 ng/mL), dasatinib (100 ng/mL), and BAY-61-3606 (100 ng/mL) results in 30% inhibition of IL-8 secretion.
FIGURE 4.
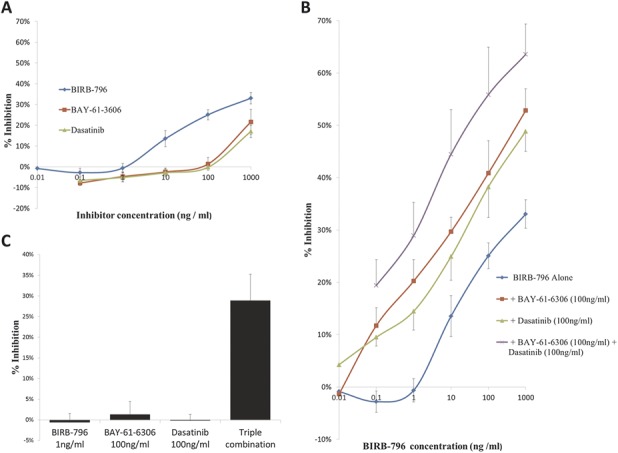
Combination of selective kinase inhibitors leads to more than additive inhibitory effects in reducing IL-8 secretion from IL-1β–stimulated HT29 cells. BIRB-796, BAY-61-3606, and dasatinib individually induce concentration-dependent inhibition of IL-8 secretion but with limited efficacy (A). BIRB-796 in combination with BAY-61-3606 (100 ng/mL) and/or dasatinib (100 ng/mL) produces concentration-dependent inhibition of IL-8 secretion with improved efficacy and potency (B). Compared to the lack of effect of the same concentrations when used individually, BIRB-796 (1 ng/mL)/BAY-61-3606 (100 ng/mL)/dasatinib (100 ng/mL) combination treatment is effective in reducing IL-8 concentration in a more than additive manner (C). Graphs represent means of at least 3 independent experiments ± SEM.
Effect of TOP1210 and Selective Kinase Inhibitors on Proinflammatory Cytokine Release by UC Myofibroblasts
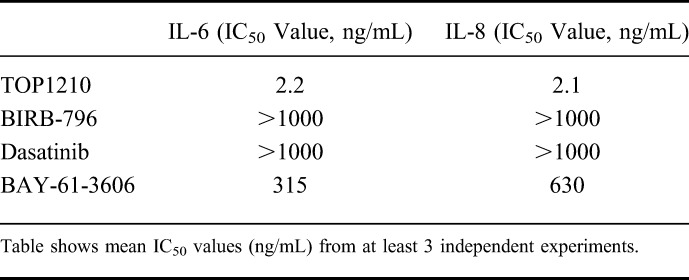
Myofibroblasts isolated from inflamed UC mucosa were used to compare the anti-inflammatory activity of TOP1210 to that of selective kinase inhibitors. Vehicle (DMSO) treated myofibroblasts, after TNF-α stimulation, released IL-6 and IL-8 levels of 14,740 ± 6518 pg/mL and 49,363 ± 15,694 pg/mL (means ± SEM), respectively. TOP1210 achieved concentration-dependent inhibition of both IL-6 (IC50, 2.2 ng/mL) and IL-8 (IC50, 2.1 ng/mL) production by UC myofibroblasts (Fig. 5, Table 3). BIRB-796 required 1 μg/mL to significantly reduce both IL-6 and IL-8 release with a maximum inhibition of only 70% achieved. Moreover, dasatinib (0.1–10 μg/mL) significantly reduced IL-6 release in a concentration-dependent manner by approximately 36% to 60%, and 10 μg/mL dasatinib significantly inhibited IL-8 by approximately 70%. Finally, BAY-61-3606 was the most active of the selective kinase inhibitors in this assay, significantly reducing both IL-6 and IL-8 release with efficacy of over 90%, however, at a weaker potency compared with TOP1210. Although the selective kinase inhibitors reduced both IL-6 and IL-8 release from UC myofibroblasts, in all cases, they were significantly less potent than TOP1210 (Table 3), and in most cases, maximum efficacy was much reduced compared with TOP1210 (Fig. 5).
FIGURE 5.
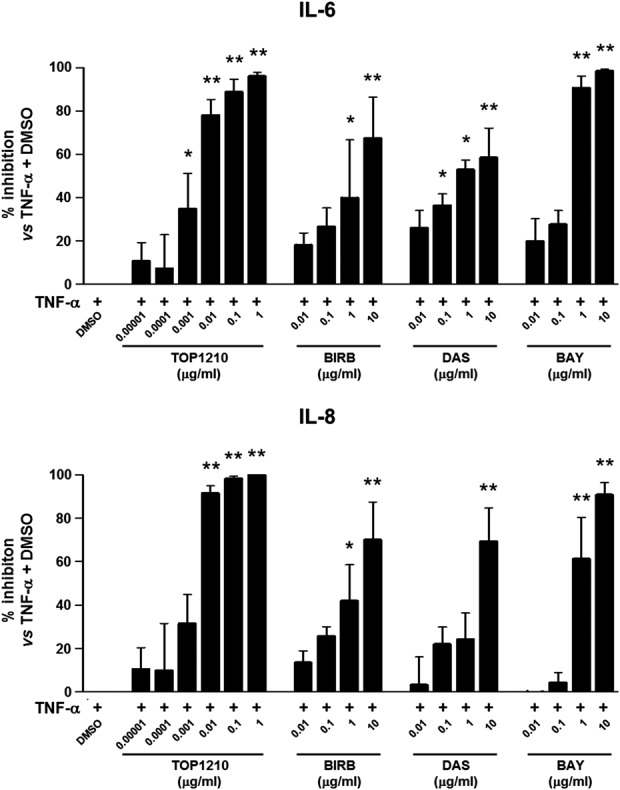
TOP1210 is more potent than selective kinase inhibitors in downregulating proinflammatory cytokine release from TNF-α–stimulated UC mucosal myofibroblasts. Effect of different concentrations of TOP1210, BIRB-796 (BIRB), dasatinib (DAS), or BAY-61-3606 (BAY) on the release of IL 6 and IL-8, expressed as mean percent inhibition compared with stimulated control (DMSO; 20-ng/mL recombinant human TNF-α + DMSO, 0.5% v/v). Bars represent mean ± SEM of at least 3 independent experiments. *P < 0.01 versus stimulated control; **P < 0.001 versus stimulated control.
TABLE 3.
Inhibitory Effects of the NSKI TOP1210 and the Selective Kinase Inhibitors on Proinflammatory Cytokine Release by UC Myofibroblasts
Effect of TOP1210 and Selective Kinase Inhibitors Alone or in Combination with Proinflammatory Cytokine Release by UC Biopsies
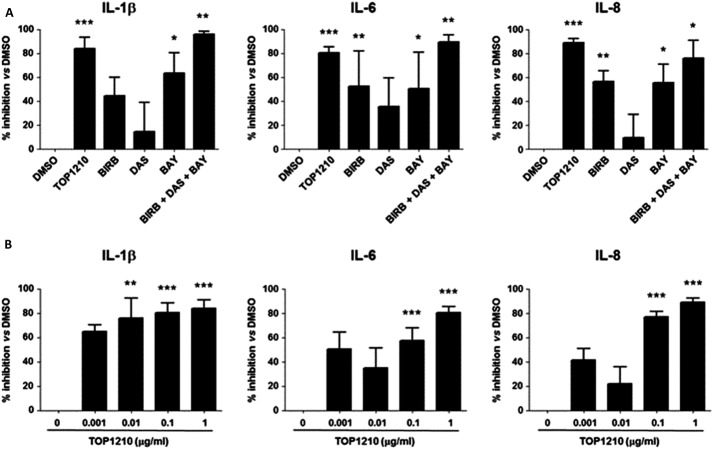
Biopsies from patients with IBD can be used as an inflammatory model of disease21 and have been shown here to spontaneously release high levels of proinflammatory cytokines (IL-1β, 459 ± 124 pg/mL; IL-6, 18,428 ± 4847 pg/mL; and IL-8 67,155 ± 13,377 pg/mL [means ± SEM]). TOP1210 and selective kinase inhibitors at 1 μg/mL were profiled in an organ culture assay using inflamed colonic biopsies from patients with UC. TOP1210 significantly inhibited IL-1β, IL-6, and IL-8 release from inflamed UC biopsies by 80% to 90% (Fig. 6A, B). BAY-61-3606 and BIRB-796 also significantly inhibited release of all three cytokines (50%–65% inhibition), but with a reduced effect compared with TOP1210. Dasatinib did not have any significant effect on IL-1β, IL-6, and IL-8 release from the UC biopsies. When the three selective kinase inhibitors were combined, the level of inhibition produced against all 3 cytokines was similar to that for TOP1210 (Fig. 6A). Moreover, TOP1210 inhibited IL-1β, IL-6, and IL-8 release by UC biopsies in a concentration-dependent manner (Fig. 6B).
FIGURE 6.
TOP1210 is superior to selective kinase inhibitors alone in downregulating proinflammatory cytokine release from UC biopsies. TOP1210 activity is comparable to a combination of all three selective inhibitors and reduces proinflammatory cytokine release from UC biopsies in a concentration-dependent manner. Effect of 1 μg/mL TOP1210, 1 μg/mL BIRB-796 (BIRB), 1 μg/mL dasatinib (DAS), and 1 μg/mL BAY-61-3606 (BAY), or the combination of the 3 selective kinase inhibitors (A) or TOP1210 (0.001–1 μg/mL) (B) on the release of IL-1β, IL-6, and IL-8, expressed as mean percent inhibition compared with control (DMSO; DMSO, 0.5% v/v), in organ culture supernatants of inflamed colonic biopsies from patients with UC. Bars represent mean ± SEM of at least 3 independent experiments. *P < 0.05 versus DMSO; **P < 0.005 versus DMSO; ***P < 0.0005 versus DMSO.
DISCUSSION
Selective kinase inhibitors to date have been disappointing in the treatment of inflammatory disorders, particularly in rheumatoid arthritis and IBD.9,14 Consequently, attention has turned to multikinase inhibition in an attempt to improve efficacy by targeting a broader range of cell types and cytokines. In this study, TOP1210 has been compared to selective kinase inhibitors (BIRB-796, dasatinib, and BAY-61-3606) in a range of inflammatory cell assays and in inflamed biopsies from patients with UC.
TOP1210 potently inhibits p38α, SFK, and Syk kinases and is comparable or higher in potency than each of the selective inhibitors at their relevant kinase. The limited anti-inflammatory profile of each of the selective kinase inhibitors can, in part, be explained by both the relative expression of their target kinases in selected cell types and the participation of specific kinases in signaling pathways associated with the stimuli used in the cell assay. P38α and the Src family members, Src, Fyn, and Yes, are ubiquitously expressed in a broad range of stromal and hematopoietic cells, whereas the expression of other SFK members (Lck and Hck) and Syk is restricted to hematopoietic cells. It is, perhaps, unsurprising that inhibition of Syk by BAY-61-3606 results in poor efficacy in the innate cell assays given that the LPS stimulation acts through TLR4 signaling pathway, which does not involve Syk. Conversely, although P38α is ubiquitously expressed and involved in most signaling cascades tested, its selective inhibition by BIRB-796 failed to achieve even 50% inhibition in any of the cellular assays. P38α is involved in downstream signaling, and redundancy at this level may explain lack of efficacy, which has indeed been alluded to an explanation of the disappointing results with BIRB-796 in clinical studies.10 In macrophages, the effects of SFK inhibition with dasatinib appeared to be cytokine dependent with relatively potent inhibition of TNF-a achieved compared with the poor activity against IL-8 release. In contrast, the multikinase inhibitory profile of TOP1210 results in potent inhibition of both IL-8 and TNF-α in these innate responses from monocytes and macrophages, respectively. If we consider the adaptive responses, the importance of SFK members Lck and Fyn in T-cell receptor signaling helps to explain the potent and efficacious inhibition achieved with dasatinib. The potent activity of TOP1210 in these T-cell assays is therefore most likely to be directly attributed to its potent SFK inhibition. Collectively, TOP1210 achieves potent inhibition across cellular models of both innate and adaptive immunities, highlighting the broad acting anti-inflammatory potential of the compound compared with selective kinase inhibition. Interestingly, TOP1210 activity is far superior over the individual selective inhibitors suggesting that there is added benefit in simultaneous targeting of P38α, SFK, and Syk, both in efficacy and breadth of effects across cell types particularly in the case of IL-8 release.
Disruption of the epithelium is central to the development of mucosal disorders, particularly in UC. As a consequence of epithelial disruption, soluble mediators are released, for example, IL-8, serving to recruit circulating neutrophils into the site of inflammation. Interestingly, we demonstrate that the selective kinase inhibitors are only weakly active in the IL-8 release assay, yet TOP1210 is highly potent and efficacious. A similar trend was observed in the IL-8 release assay in monocytes, so the disparity between TOP1210 and the selective inhibitors may be a cytokine, rather than cell specific phenomenon. A range of inflammatory cytokines is known to be key mediators of IBD, with IL-1β, IL-6, IL-8, and IL-2 all being elevated in tissues from patients with IBD.6 IL-8, however, seems of particular importance in UC as serum levels of IL-8 correlate with disease activity.22 In this study, we have demonstrated that TOP1210 potently inhibits IL-8 secretion from monocytes, epithelial cells, UC myofibroblasts, and UC biopsies, yet interestingly, the selective kinase inhibitors individually were, at best, only weakly active.
To investigate this further, the IL-8 release assay in HT29 epithelial cells was used to study the effects of selective inhibitor combinations. Individually, the selective kinase inhibitors achieve poor inhibition. However, combination studies demonstrate that the effects of inhibiting p38α with BIRB-796 can be augmented in a more than additive manner by concomitant inhibition of SFK and/or Syk using the appropriate selective kinase inhibitors. The more than additive inhibitory effects are best demonstrated when considering concentrations that, individually, are without inhibitory effect. On combination, these concentrations bring about augmented inhibition, albeit limited (approximately 30%). The more than additive effects of noninhibitory concentrations of dasatinib and BAY-61-3606 result in both increased potency (as seen by leftward shifts of BIRB-796 concentration–effect curve) and improved efficacy of BIRB-796. These combination studies demonstrate that simultaneous inhibition of P38α, SFK, and Syk is a key to achieving highly potent inhibitory effects and suggest that the effects achieved in HT29 cells with TOP1210 are most likely mediated through the inhibition of the optimized targets P38α, SFK, and Syk. In this investigation, IL-1β was used as a stimulus of HT29 cells; however, in UC, a wide range of inflammatory stimuli may be acting on the epithelial cells, with numerous pathways being activated and more kinases being involved. In these circumstances, multikinase inhibition may be even more beneficial.
Although extensive combination experiments could not be performed with UC biopsies because of limited availability of tissue, the data generated with the organ culture model support the more than additive effects observed in the combination studies in HT29 cells. The broad inhibitory actions of TOP1210 across a range of cell types translated to potent and efficacious effects on cytokine release from inflamed UC colonic biopsies. TOP1210 inhibited, in a concentration dependent manner, all the cytokines measured, namely, IL-1β, IL-6, and IL-8, and had superior efficacy compared with the selective kinase inhibitors at equivalent concentrations.
UC is a complex disease characterized by recurrent episodes of colonic inflammation involving immune and nonimmune cells, and also the colonic epithelium.23 In addition to a T-cell response, the inflammatory process in UC involves gross epithelial cell changes, and macrophage and neutrophil infiltration driven by an array of inflammatory cytokines.5 Many approaches targeting a single cytokine or cell type seem to have limited efficacy, or development has been stopped because of toxic effects.6 Conversely, a therapeutic approach that targets multiple cellular pathways and mechanisms is more likely to be effective.24 These studies have shown that the NSKI TOP1210 leads to a broad profile of inflammatory cell inhibition with greatly enhanced effects over those of selective kinases. Recently, tofacitinib, an oral pan-Janus kinase inhibitor, has been effective in a clinical trial in UC.25,26 Primarily targeting T cells, this has shown promise but has generally been limited in dose because of toxic side effects, in the same manner as other systemic kinase inhibitor approaches. Nevertheless, there may be opportunities to capitalize on the promise shown, for example, by further optimizing targets or by restricting systemic exposure through topical administration.
In summary, selective kinase inhibitors demonstrate limited efficacy and potency and individually have activity only in selected cell types. In contrast, TOP1210, through multikinase inhibition, demonstrates potent, efficacious, and broad inhibitory activity in UC tissues and across a range of cell types including epithelial cells, innate, and adaptive immune cells. These studies suggest that the inhibition of multiple kinases, either though combined selective kinase inhibitors or by NSKI TOP1210, results in more than additive anti-inflammatory effects. The broad efficacy profile of TOP1210 offers significant advantages over existing selective kinase approaches and potentially offers a much improved therapeutic benefit in IBD.
ACKNOWLEDGMENTS
The authors thank Sygnature Discovery Limited for synthesis of TOP1210.
Footnotes
P. Biancheri has received research funding from Topivert Pharma Ltd. M. R. Foster, M. C. T. Fyfe, S. Sirohi, Y. Solanke, A. Rowley, S. Webber, and C. A. Walshe are all employees and shareholders of Topivert Pharma Ltd. T. T. MacDonald is a shareholder and paid consultant of Topivert Pharma Ltd. E. Wood has no conflict of interest to disclose.
P. Biancheri and M. R. Foster have contributed equally to this article.
REFERENCES
- 1.Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. Chemother Res Pract. 2014;2014:357027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu P, Nielsen TE, Clausen MH. Small-molecule kinase inhibitors: an analysis of FDA-approved drugs. Drug Discov Today. 2016;21:5–10. [DOI] [PubMed] [Google Scholar]
- 3.Samelson L. Immunoreceptor signaling. Cold Spring Harb Perspec Biol. 2011;3:a011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–1605. [DOI] [PubMed] [Google Scholar]
- 5.Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet. 2012;380:1606–1619. [DOI] [PubMed] [Google Scholar]
- 6.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. [DOI] [PubMed] [Google Scholar]
- 7.Docena G, Rovedatti L, Kruidenier L, et al. Down-regulation of p38 mitogen-activated protein kinase activation and proinflammatory cytokine production by mitogen-activated protein kinase inhibitors in inflammatory bowel disease. Clin Exp Immunol. 2010;162:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNamee EN, Collins CB, Lebsack MD, et al. Cell-specific inhibition of p38alpha as a therapeutic strategy for inflammatory bowel disease. Gastroenterology. 2010;138:1237–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber S, Feagan B, D'Haens G, et al. ; BIRB 796 Study Group. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:325–334. [DOI] [PubMed] [Google Scholar]
- 10.Hammaker D, Firestein GS. “Go upstream, young man”: lessons learned from the p38 saga. Ann Rheum Dis. 2010;69:i77–i82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signalling cross talk. Cold Spring Harb Perspect Biol. 2011;3:a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Can G, Ayvaz S, Can H, et al. The Syk inhibitor fostamatinib decreases the severity of colonic mucosal damage in a rodent model of colitis. J Crohns Colitis. 2015;9:907–917. [DOI] [PubMed] [Google Scholar]
- 13.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinblatt ME, Genovese MC, Ho M, et al. Effects of fostamatinib, an oral spleen tyrosine kinase inhibitor, in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheumatol. 2014;66:3255–3264. [DOI] [PubMed] [Google Scholar]
- 15.Mencher SK, Wang LG. Promiscuous drugs compared to selective drugs (promiscuity can be a virtue). BMC Clin Pharmcol. 2005;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chopra P, Kanoje V, Semwal A, et al. Therapeutic potential of inhaled p38 mitogen-activated protein kinase inhibitors for inflammatory pulmonary diseases. Expert Opin Investig Drugs. 2008;17:1411–1425. [DOI] [PubMed] [Google Scholar]
- 17.Barnes P. In: Rogers TJ, Criner GJ, Cornwall WD, et al., eds. Smoking and Lung Inflammation: Basic, Pre-clinical and Clinical Research Advances. New York: Springer Science and Business Media; 2013:191–207. [Google Scholar]
- 18.Wiegman CH, Russell KE, Seiffert J, et al. The selective Pan-Janus kinase (JAK) inhibitor VR588 demonstrates potent anti-inflammatory activity in a murine chronic house dust mite (HDM) model of asthma. Am J Respir Crit Care Med. 2015;191:A6435. [Google Scholar]
- 19.To WS, Aungier SR, Cartwright AJ, et al. Potent anti-inflammatory effects of the narrow spectrum kinase inhibitor RV1088 on rheumatoid arthritis synovial membrane cells. Br J Pharmacol. 2015;172:3805–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fyfe MCT, inventor; “Kinase inhibitors”. International Patent Publication, assignee. WO 2014/140582. September 18, 2014. [Google Scholar]
- 21.Rovedatti L, Kudo T, Biancheri P, et al. Differential regulation of interleukin 17 and interferon γ production in inflammatory bowel disease. Gut. 2009;58:1629–1636. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Perálvarez ML, García-Sánchez V, Villar-Pastor CM, et al. Role of serum cytokine profile in ulcerative colitis assessment. Inflamm Bowel Dis. 2012;18:1864–1871. [DOI] [PubMed] [Google Scholar]
- 23.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald TT. Inside the microbial and immune labyrinth: totally gutted. Nat Med. 2010;16:1194–1195. [DOI] [PubMed] [Google Scholar]
- 25.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. [DOI] [PubMed] [Google Scholar]
- 26.Panés J, Su C, Bushmakin AG, et al. Randomized trial of tofacitinib in active ulcerative colitis: analysis of efficacy based on patient-reported outcomes. BMC Gastroenterol. 2015;15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]