Summary
Age-associated minor inflammation: inflammageing may explain human ageing mechanism(s). Our previous study reported a significant increase in the serum level of highly sensitive C-reactive protein (hsCRP) with normal ageing and the patients with Werner syndrome (WS). To further study the minor inflammatory condition associated with ageing, another possible ageing biomarker: matrix metalloproteinase-9 (MMP9) was examined in the sera from 217 normal Japanese individuals aged between 1 and 100 years and 41 mutation-proven Japanese WS aged between 32 and 70 years. MMP9 was assayed by ELISA. The serum level of MMP9 was elevated significantly (p < 0.001) with normal ageing from both sexes as hsCRP. In contrast to normal ageing, the serum MMP9 level in WS decreased significantly with calendar age (p < 0.05). The MMP9 level (ng/mL) in WS (147.2 ± 28.5) was not significantly different in comparison with those from age-matched normal adult population aged between 25 and 70 years (109.1 ± 9.4), nor normal elderly population aged between 71 and 100 years (179.9 ± 16.1). Although both normal ageing and WS were associated with minor inflammation, the inflammatory parameters such as serum MMP9 and hsCRP changed differently between normal ageing and WS. The WS-specific chronic inflammation including skin ulcer and diabetes mellitus may contribute the different behavior of both ageing biomarkers from normal ageing.
Keywords: Aging, C-reactive protein (CRP), inflammageing, matrix metalloproteinase-9 (MMP9), Werner syndrome
1. Introduction
The significant contribution of low-grade, and systemic inflammation to normal ageing: inflammaging has been proposed to explain pathophysiology of human ageing and age-associated diseases (1,2). The minimal, but significant elevation of highly sensitive C-reactive protein (hsCRP) has been frequently reported in the normal elderly population that may follow the ageing-related conditions including skin atrophy, cataract, arthritis, osteoporosis, hypogonadism, metabolic syndrome, obesity, diabetes mellitus (DM), immune dysfunctions, sarcopenia, cancer, atherosclerosis, cognitive decline and finally death (1–4).
Most Japanese studies on the chronological changes of inflammatory conditions in normal ageing indicated a similar age-associated increase of low grade inflammation to the western studies, though the level of inflammation assessed by hsCRP was several times lower than that in the western populations (3,5).
C-reactive protein (CRP): the representative acute phase protein of pentraxin family, primarily produced by the hepatocytes was also produced by smooth muscle cells and adipocytes (6). Matrix metalloproteinases (MMPs) produced by macrophages, T helper type 2 lymphocytes, fibroblasts, smooth muscle cells and endothelial cells belong to a family of catalytic enzymes for the long-lived extracellular matrix proteins including collagen, gelatin, fibronectin, laminin, elastin and proteoglycans (7,8). MMPs, especially MMP2 (72-kDa gelatinase A) and MMP9 (92-kDa gelatinase B) both in the circulation and the affected tissues have been reported to increase with inflammation, normal ageing and age-associated diseases like atherosclerosis, coronary artery disease and DM in response to interleukin (IL)-13 (6–9).
Elastin fragment degraded by MMP9 produces a large amount of pro-inflammatory cytokines leading to chronic inflammation (10). A strong relationship between serum levels of MMP9 and the process of inflammation characterized by hsCRP has been shown in acute coronary syndromes (9,11).
Werner syndrome (WS; MIM#27770), the representative progeroid syndrome, has been extensively studied as the natural model of human ageing (12). The patients with WS show a wide variety of ageing-associated clinical manifestations such as gray hair/alopecia, hoarseness, cataracts, skin atrophy, skin hyper-/hypo-pigmentation, sarcopenia, DM, hypogonadism, hyperlipidemia, atherosclerosis, osteoporosis, and malignancy at a relatively early stage of their life followed by death at around 50 y.o. due to atherosclerosis-related diseases or malignancy (13). Surprisingly, majority of the WS patients are of Japanese origin, probably because of the relatively high frequency of consanguineous marriage in the rural area of Japan and the extremely high frequency of heterozygous careers in Japan (12). We have reported the inflammatory conditions observed in WS in a series of publications (12).
The aim of this study was to clarify the contribution of minor inflammation to ageing by investigating serum MMP9 levels using the serum samples from apparently normal Japanese volunteers and the mutation-proven Japanese WS patients.
2. Materials and Methods
2.1. Study population
A total of 217 normal serum samples (M = 91, F = 126) between 1 to 100 years old were the same sera as were studied in the previous study (5). The normal individuals, enjoying the usual daily life at home or nursing home, had neither apparent inflammatory diseases including infection, cancer, lymphoproliferative disorders, DM, Alzheimer disease, autoimmune diseases and arthritis at the time of serum sampling, nor history of cardio-/cerebro-vascular accidents. Exclusion protocol for elderly individuals met the SENIEUR criteria (14).
Serum samples were also obtained from untreated 41 mutation-proven WS (M = 24, F = 17; between 32 and 70 years old); a part of “Goto collection of Werner syndrome” (http://www.brc.riken.jp/lab/cell/english/index_gmc.shtml). As indicated in Table 1, nine patients with WS were free from skin ulecers SU[SU(−)], while 32 had SU[SU(+)]. Twenty four had DM [DM(+)], but 17 did not [DM(−)]. WS patients were sub-grouped into 1) SU(+)DM(+) (n = 20), 2) SU(+)DM(−) (n = 12), 3) SU(−)DM(+) (n = 4) and 4) SU(−)DM(−) (n = 5). For statistical comparison with WS, normal individuals were divided into two groups according to their age: normal adult aged between 25 and 70 years (NA, n = 86) and normal elderly aged between 71 and 100 years (NE, n = 85).
Table 1. Clinical characteristics in Werner syndrome patients.
| Subgroups | SU | DM | ID | Age | Sex | MMP-9 (ng/mL) | hsCRP (ug/mL) |
|---|---|---|---|---|---|---|---|
| 1 | + | + | WS12901 | 32 | F | 134 | 2.61 |
| 1 | + | + | WS57201 | 37 | M | 351 | 22.8 |
| 1 | + | + | WS19201 | 38 | M | 82.2 | 7.03 |
| 1 | + | + | WS56301 | 39 | M | 284 | 0.79 |
| 1 | + | + | WS5501 | 40 | F | 10.2 | 11.1 |
| 1 | + | + | WS57801 | 41 | M | 122 | 1.04 |
| 1 | + | + | WS51301 | 42 | M | 252 | 17.4 |
| 1 | + | + | WS19201 | 44 | M | 124 | 18.7 |
| 1 | + | + | WS4705 | 45 | F | 224.3 | 42.4 |
| 1 | + | + | WS6301 | 46 | M | 184 | 27.2 |
| 1 | + | + | WS53601 | 46 | M | 24 | 16.3 |
| 1 | + | + | WS0101 | 47 | M | 49.3 | 15 |
| 1 | + | + | WS58501 | 51 | M | 414 | 4.02 |
| 1 | + | + | WS58301 | 53 | M | 357 | 3.21 |
| 1 | + | + | WS4704 | 54 | M | 32.1 | 3.42 |
| 1 | + | + | WS17201 | 54 | F | 198.5 | 2.26 |
| 1 | + | + | WS0801 | 55 | F | 167 | 8.99 |
| 1 | + | + | WS54801 | 57 | M | 127 | 5.88 |
| 1 | + | + | WS56201 | 70 | M | 85.8 | 10.3 |
| 1 | + | + | WS1801 | 70 | M | 235.5 | 28.3 |
| 2 | + | − | WS6103 | 32 | M | 2 | 1.62 |
| 2 | + | − | WS6104 | 32 | M | 867.6 | 25 |
| 2 | + | − | WS14501 | 35 | M | 129 | 4.55 |
| 2 | + | − | WS51601 | 36 | F | 222 | 0.98 |
| 2 | + | − | WS53101 | 38 | F | 294 | 22.9 |
| 2 | + | − | WS53901 | 43 | F | 38.4 | 1.28 |
| 2 | + | − | WS53801 | 46 | F | 103 | 0.98 |
| 2 | + | − | WS2101 | 50 | F | 30.8 | 8.66 |
| 2 | + | − | WS55801 | 53 | F | 45.4 | 18.2 |
| 2 | + | − | WS52901 | 54 | F | 45.8 | 10.8 |
| 2 | + | − | WS54001 | 57 | F | 70.3 | 7.04 |
| 2 | + | − | WS4701 | 59 | F | 33.6 | 11.8 |
| 3 | − | + | WS58701 | 35 | M | 108 | 2.93 |
| 3 | − | + | WS57701 | 38 | F | 197 | 2.07 |
| 3 | − | + | WS57401 | 41 | M | 0 | 26.9 |
| 3 | − | + | WS4401 | 41 | M | 30 | 24.9 |
| 4 | − | − | WS5801 | 43 | M | 2.9 | 1.28 |
| 4 | − | − | WS0402 | 47 | M | 19.5 | 1.15 |
| 4 | − | − | WS7501 | 48 | M | 66.7 | 22.3 |
| 4 | − | − | WS0401 | 49 | F | 52.9 | 4.76 |
| 4 | − | − | WS10501 | 52 | F | 16.7 | 3.86 |
Note: 32 had skin ulcers (SU) and 24 DM at blood sampling. Subgroups: 1) SU(+)DM(+); n = 20, 2) SU(+)DM(−); n = 12, 3) SU(−)DM(+); n 4) SU(−)DM(−); n = 5. SU: skin ulcer, DM: diabetes mellitus, MMP9: matrix metalloproteinase 9, hsCRP: high sensitivity CRP
All of the individuals provided written informed consent for this study, which was approved by the Ethics Committee of Toin University of Yokohama. All of the samples were stored at −80°C until use.
2.2. Determination of MMP9 and hsCRP
The concentration of MMP9 (ng/mL) in the sera was determined by specific sandwich ELISA using a Human MMP9 ELISA kit (Fuji Chemical Industries, Toyama, Japan) as described before (15). hsCRP was assayed by Circulex high-sensitivity CRP ELISA kit (Cyclex Co., Nagano, Japan) (5).
2.3. Statistical Analysis
The multiple liner regression model was used to examine relationship between aging and serum level of MMP9 with adjustment of sex effect on the serum levels and to examine relationship among WS, NE and NA. We coded one for male and zero for female in Sex variable.
Differences of serum levels of MMP9 between two groups (NE vs. NA, WS vs. NA, WS vs. NE or subgroups defined by the presence/absence of SU or DM, respectively) were tested by two-sample t-test with unequal variances. Multiple regression analyses were performed to explain the relationship between age and serum levels of MMP9 with adjustment of sex effect on the serum levels and to examine relationship among WS, NE and NA. These models were selected based on the Akaike's information Criterion (AIC) (16). Statistical Language R (17) was used for these analyses. p values < 0.05 were considered to be statistically significant.
3. Results
3.1. Charcteristics of MMP9 in normal individuals
Using a non-linear regression model, statistically significant temporal effect of age on the serum level of MMP9 was observed (p < 0.001) with adjustment of sex effect on the serum levels as indicated in Figure 1.
Figure 1.
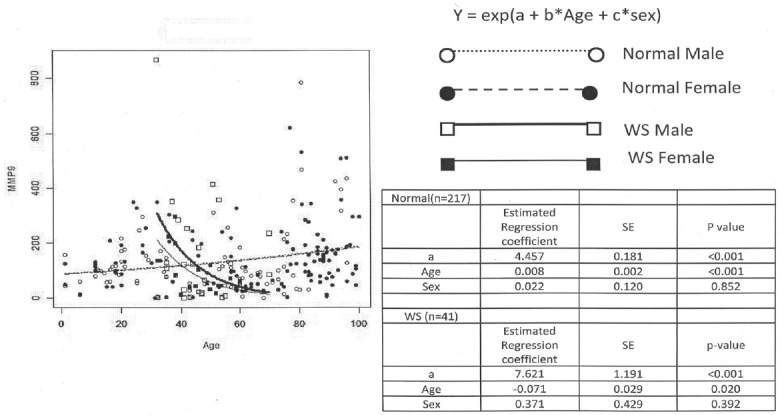
Serum MMP9 in normal ageing and Werner syndrome from both sexes. Non-linear regression model used in this analysis was expressed as MMP9 = exp (a + b*Age + c*Sex), where a, b, and c are estimated regression coefficients. Estimated regression coefficient and p values for age and sex are inserted in the right table next to the figure for comparison. ○: Normal Male (dotted line; n = 91), ●: Normal Female (dotted line; n = 126), □: WS Male (solid line; n = 24), ■: WS Female (solid line; n = 17).
Non-linear regression model expressed as MMP9 = exp (a + b*Age + c*Sex) was selected based on the AIC, where a, b and c are estimated regression coefficients. The serum level of MMP9 significantly increased 1.008 (= exp (0.008)) times a year, as shown in the coefficient of Age in the right table of Figure 1. Neither significant gender difference of serum level of MMP9 was observed concerning to the age-associated increase in MMP9 level as indicated in the right table of Figure 1, nor concerning to the mean ± SE level of MMP9 (ng/mL) throughout all ages between male (127.6 ± 12.7; n = 91, solid line for regression model) and female (143.8 ± 10.1; n = 126, dotted line for regression model), as of the result of hsCRP (5).
The serum level of MMP9 in the NE (179.9 ± 16.1; n = 85) was significantly elevated in comparison with the NA (109.1 ± 9.4; n = 86) from both sexes (p < 0.001) as indicated in Table 2.
Table 2. Serum MMP9 in Werner syndrome and normal individuals from different age groups of both sexes.
| Group | Mean | SE | p value |
|---|---|---|---|
| NA (n = 86) | 109.1 | 9.4 | - |
| NE (n = 85) | 179.9 | 16.1 | < 0.001 |
| WS (n = 41) | 142.3 | 24.9 | 0.21 |
Note: Reference group was normal adult 25–70 years old group (NA). The p value between reference group and normal elderly 71–100 years old group (NE) was p < 0.001. The p value between WS and NA or NE were statistically insignificant (p > 0.05).
3.2. Characteristics of MMP9 in WS patients
The serum level of MMP9 in WS patients (142.3 ± 24.9 ng/mL) was insignificantly increased compared with the age-matched NA from both sexes (109.1 ± 9.4), but insignificantly decreased compared with the NE (179.9 ± 16.1) (Table. 2).
The serum levels of MMP9 in WS patients was significantly decreased (p < 0.02) with ageing as indicated in Figure 1.
The serum MMP9 was significantly different between SU(+) (166.9 ± 5.1; n = 32) and SU(−) groups (54.9 ± 7.0; n = 9) (p < 0.001) or DM(+) (158 ± 4.8; n = 24) and DM(−) groups (120 ± 12.2; n = 17) (p < 0.01), respectively. The MMP9 level in Group 1 SU(+)DM(+) subgroup (172.9 ± 26.1; n = 20) was significantly increased compared with that in Group 4 SU(−)DM(−) subgroup (31.7 ± 12; n = 5) (p < 0.001). However, the MMP9 level was not significantly different between Group 1 SU(+)DM(+), Group 2 SU(+)DM(−) and Group 3 SU(−)DM(+) subgroups, respectively. The comparisons between the rest of the subgroup combination were also statistically insignificant as shown in Table 3.
Table 3. Serum MMP9 in Werner syndrome from different subgroups.
| Subgroups | Mean | SE |
p value matrix |
||
|---|---|---|---|---|---|
| Group 2 | Group 3 | Group 4 | |||
| Group 1: SU(+)DM(+) (n = 20) | 172.9 | 26.1 | 0.83 | 0.14 | < 0.001 |
| Group 2: SU(+)DM(−) (n = 12) | 156.8 | 69.3 | - | 0.39 | 0.10 |
| Group 3: SU(−)DM(+) (n = 4) | 83.8 | 44.1 | 0.39 | - | 0.33 |
| Group 4: SU(−)DM(−) (n = 5) | 31.7 | 12.0 | 0.10 | 0.33 | - |
Note: Serum MMP9 in group 1 was significantly elevated (p < 0.001) compared with group4. No significant differences were between the rest of any subgroup combinations. SU: skin ulcer, DM: diabetes mellitus.
4. Discussion
Although CRP is the prototypical acute-phase reactant in man, both serum hsCRP and MMP9 have been proposed as biomarkers of atherosclerosis-associated diseases including coronary heart disease and cerebro-vascular accidents (18–20). CRP can act as pro-inflammatory by inducing the expression of tumor necrosis factor α and IL-1 (21).
However, CRP as a component of the innate immune system can function as a protective machinery against a variety of inflammatory conditions and autoimmunity by activating the classical complement pathway (22), enhancing phagocytosis, and binding to the Fcγreceptors on leukocytes, leading to the anti-inflammatory cytokine production such as IL-10, transforming growth factor β and IL-12 (23–28).
Similarly, MMP9 can degrade gelatin, leading to the activation of potent signals for cell survival and tissue repair on one-hand, but the degraded fragments can also promote the production of pro-inflammatory mediators on the other hand (7). Both CRP and MMP9 may have beneficial and deleterious effects for an organism, coining antagonistic pleiotropy (1,2).
In the present study, we have reported significantly increasing levels of serum MMP9 with normal ageing, resulting from the sum of tissue degradation and tissue repair. However, the level of serum MMP9 in Japanese population was several times lower than that in the western populations, the situation being similar as of hsCRP as already reported (5). The sharp contrast of serum hsCRP and MMP9 levels between Japanese and the western populations is not still clear, but has been presumed by the difference in diet, lifestyle and body mass index (3,7).
WS has been nominated as a human model of natural ageing, ageing twice faster than normal (12). Although the serum level of hsCRP in WS was several times higher than the normal control and tended to increase with calendar age, another inflammation biomarker: MMP9 in WS was significantly decreased with ageing and neither significantly changed compared with age-matched NA, nor NE.
The contrasting profile of hsCRP and MMP9 between normal ageing and WS may suggest that i) the ageing in WS may not progress in accordance with normal calendar ageing, but progress in the genetically-determined unique fashion as the patients with WS age faster than normal, and age prematurely even at a relatively younger stage; ii) as was described in the Introduction, the inflammatory proteins: hsCRP and MMP9 are produced by different cell types, repectively and WS patients with SU and DM produced more MMP9, but not hsCRP by hidden inflammation/infection than those without; iii) the limited number of the subgroups in WS may affect the statistics, though our Goto collection is unquestionably the world largest (12).
We have shown the ageing-associated increasing level of inflammation markers such as hsCRP and MMP9 by using serum samples from carefully selected normal Japanese ages between 1 and 100 y.o. from both sexes. There was no gender difference concerning to the degree and the trend of inflammation.
As shown in the previous publication on WS (5), we did not find a significant difference between SU(+) DM(+) group and SU(−)DM(−) group concerning to the serum level of hsCRP. However, we have found a significant difference between SU(+)DM(+) group and SU(−)DM(−) group in the present MMP9 study. As hsCRP and MMP9 may change differently in response to inflammation possibly induced by SU and DM, we should bear the different behavior of hsCRP and MMP9 as aging markers in mind.
Further study may clarify the effect of mild inflammation:inflammageing on the process of normal ageing and the patients with progeroid syndrome such as WS.
5. Conclusion
Although minor inflammation evaluated by MMP9 may be associated with normal aging and the patients with WS, the serum levels of MMP9 and hsCRP may change differently between two conditions.
Acknowledgements
The work was supported by the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (#24590902). We would like to thank Ms. T. Watanabe at Wayoen Nursing Home, Drs. S. Hayashi at Fukui General Hospital and T. Ogino at Kyoritsu Ogino Hospital for collecting serum samples from healthy elderly individuals.
References
- 1. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000; 908:244-254. [DOI] [PubMed] [Google Scholar]
- 2. Goto M. Inflammaging (inflammation+aging):A driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? BioSci Trends. 2008; 2:218-230. [PubMed] [Google Scholar]
- 3. Arima H, Kubo M, Yonemoto K, Doi Y, Ninomiya T, Tanizaki Y, Hata J, Matsumura K, Iida M, Kiyohara Y. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese:the Hisayama Study. Arteriroscler Thromb Vasc Biol. 2008; 28:1385-1391. [DOI] [PubMed] [Google Scholar]
- 4. Balkwill F, Coussens LM. An inflammatory link. Nature 2004; 431:405-406. [DOI] [PubMed] [Google Scholar]
- 5. Goto M, Sugimoto K, Hayashi S, Ogino T, Sugimoto M, Furuichi Y, Matsuura M, Ishikawa Y, Iwaki-Egawa S, Watanabe Y. Aging-associated inflammation in healthy Japanese individuals and patients with Werner syndrome. Exp Gerontol 2012; 47:936-939. [DOI] [PubMed] [Google Scholar]
- 6. Verma S, Szmitko PE, Ridker PM. C-reactive protein comes of age. Nat Clin Pract Cardiovasc Med. 2005; 2:29-36. [DOI] [PubMed] [Google Scholar]
- 7. Van den Steen PE, Opdenakker G, Wormald MR, Dwek RA, Rudd PM. Matrix remodelling enzymes, the protease cascade and glycosylation. Biochim Biophys Acta 2001; 1528:61-73. [DOI] [PubMed] [Google Scholar]
- 8. Borkakoti N. Structural studies of matrix metalloproteinases. J Mol Med. 2000; 78:261-268. [DOI] [PubMed] [Google Scholar]
- 9. Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WC, Sutherland P, Wilson PWF, Vasan RS. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures. The Framingham heart study. Circulation 2004; 13:28-29. [DOI] [PubMed] [Google Scholar]
- 10. Tayebjee MH, Lip GYH, Blann AD, MacFadyen RJ. Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb Res. 2005; 115:205-210. [DOI] [PubMed] [Google Scholar]
- 11. Bonnema DD, Webb CS, Pennington WR, Stroud RE, Leonardi AE, Clark LL, MacIure CD, Finklea L, Spinale FG, Zile MR. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalliproteinases (TIMPs). J Cardac Fail 2007; 13:530-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goto M, Miller RW. Monograph on Cancer Research, From premature gray hair to helicase-Werner syndrome:Implications for aging and cance, No. 49, Japan Scientific Societies Press & Karger, Tokyo, Japan, 2001; pp 1-163. [Google Scholar]
- 13. Goto M, Miller RW, Ishikawa Y, Sugano H. Excess of rare cancers in Werner's syndrome (adult progeria). Cancer Epidemiol Biomarker Prevent. 1996; 5:239-246. [PubMed] [Google Scholar]
- 14. Ligthart GJ, Corberand JX, Fournier C, Galanaud P, Hijmans W, Kennes B, Muller-Hermelink HK, Steinmann GG. Admission criteria for immunogerontological studies in man:The SENIEUR protocol. Mech Age Dev. 1984; 28:47-55. [DOI] [PubMed] [Google Scholar]
- 15. Iwaki-Egawa S, Watanabe Y, Matsuno H. Correlations between matrix metalloproteinase-9 and adenosine deaminase isozymes in synovial fluid from patients with rheumatoid arthritis. J Rheumatol. 2001; 28:485-489. [PubMed] [Google Scholar]
- 16. Akaike H. Information theory and an extension of the maximum likelihood principle. 2nd International Symposium on Information Theory. 1973; 1:267-281. [Google Scholar]
- 17. Ihaka R. Gentleman R: A Language for Data Analysis and Graphics. J Comp Grap Stat 1996; 5:299-314. [Google Scholar]
- 18. Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006; 119:166.e17-28. [DOI] [PubMed] [Google Scholar]
- 19. Inokubo Y, Hanada H, Ishizaka H, Fukushi T, Kamada T, Okumura K. Plasma levels of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 are increased in the coronary circulation in patients with acute coronary syndrome. Am Heart J. 2001; 141:211-217. [DOI] [PubMed] [Google Scholar]
- 20. Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Cll Cardiol. 1998; 32:368-372. [DOI] [PubMed] [Google Scholar]
- 21. Ballou SP, Lozanski G. Induction of inflammatory cytokine release from human monocytes by C-reactive protein. Cytokine 1992; 4:361-368. [DOI] [PubMed] [Google Scholar]
- 22. Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal polysaccharide and with the choline phosphatides, lectin and sphingomyelin. J Immunol. 1974; 112:2135-2147. [PubMed] [Google Scholar]
- 23. Stein MP, Edberg JC, Kimberly RP, Mangan EK, Bharadwaj D, Mold C, Du Clos TW. C-reactive protein binding to FcgammaRlla on human monocytes and neutrophils is allele-specific. J Clin Invest. 2000; 105:369-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for C-reactive protein on leukocytes is fcgamma receptor ll. J Exp Med. 1999; 190:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mold C, Rodriguez W, Rodic-Polic B, Du Clos TW. C-reactive protein mediates protection from lipopolysaccharide through interactions with Fc gamma R. J Immunol. 2003; 169:7019-7025. [DOI] [PubMed] [Google Scholar]
- 26. Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 2004; 21:643-653. [DOI] [PubMed] [Google Scholar]
- 27. Du Clos TW. C-reactive protein as a regulator of autoimmunity and inflammation. Arthritis Rheum. 2003; 48:1475-1477. [DOI] [PubMed] [Google Scholar]
- 28. Gershov D, Kim S, Brot N, Elkon KB. C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an anti-inflammatory innate immune response: Implications for systemic autoimmunity. J Exp Med. 2000; 192:1353-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]


