Abstract
Numerous studies on carcinoma have revealed that the expression level of HOXB7 in cancerous tissues was significantly higher than that in noncancerous tissues. Elevated expression of HOXB7 is associated with the susceptibility to lymph node metastasis and distant metastasis in various tumors. In this study, a meta-analysis was performed to involve majority of relevant articles and explore the association of HOXB7 expression level with metastasis in cancer patients. Literature retrieval was conducted by searching in a number of electronic databases (up to December 1, 2015). The meta-analysis was conducted with RevMan 5.3 software and Stata SE12.0. A total of 1,532 patients with carcinoma from 14 studies were included in analysis. The results of meta-analysis demonstrated that lymph node metastasis was observed more frequently in the patients group with high expression level of HOXB7 than in the patients group with low expression level of HOXB7 (odds ratio =2.17, 95% CI: 1.74–2.71, P<0.00001, fixed-effects model). In addition, a similar result was observed in the association between HOXB7 expression and distant metastasis; the odds ratio was 1.77 (95% CI: 1.09–2.88, P=0.02, fixed-effects model). This meta-analysis demonstrated that the overexpression of HOXB7 was significantly associated with metastasis in cancer patients, which may be served as a common molecular marker for indicating cancer metastasis.
Keywords: homeobox gene, HOXB7, carcinoma, metastasis, meta-analysis
Introduction
Nowadays, cancer is a leading cause of mortality. According to a recent survey, 8.2 million people die from cancer each year and 14.1 million new cases were diagnosed with cancer worldwide.1 A majority of the cancer cases can ultimately develop metastases, which included lymph node metastases (LNM) and distant metastases (DM). The occurrence of metastasis was a critical indicator for survival, which indicated poor prognosis in most cancers.2,3 In addition, the treatment measures were also determined by whether there was metastasis or not. Until now, the precise mechanism on metastasis in cancer cells is still unclear. In recent years, molecular biomarkers, as a hotspot in cancer research, have raised a revolution in the prediction and treatment of cancer.4–6 Until now, we still know nothing about the role of HOXB7 in predicting metastases of various cancers, although it may act as a common molecular marker for both LNM and DM.
HOX genes belong to an important component of superfamily of homeobox genes. A large family of transcriptional factors were encoded, and the expression of many downstream target genes was regulated by HOX genes.7,8 Human HOX genes can be divided into two classes. In class I, there were four paralogous clusters of HOX genes, and they were defined as HOXA, HOXB, HOXC, and HOXD, which were arranged in 2, 12, 17, and 7 chromosomes in turn.9–11 Many studies have reported that HOX genes were frequently deregulated in cancers, which could widely make effects on cellular functions, including proliferation, differentiation, and apoptosis.12–15 HOX genes were also involved in tumor initiation and progression.16,17
HOXB7, as one of class I HOX genes, was found to participate in the process of various cancers, such as gastric cancer, breast cancer (BC), and pancreatic carcinoma.18–20 Some studies revealed that HOXB7 played a crucial role in tumorigenesis and was closely related to the viability, invasion, and metastasis of tumor cells.21,22 A number of studies have demonstrated that the expression level of HOXB7 was upregulated in cancerous tissues and was associated with some clinical features, including LNM and DM.23–25 Therefore, in this study, we collected relevant literatures and performed the meta-analysis. It aimed to explore the relationship between the HOXB7 expression and metastasis and further determine whether HOXB7 could be applied as a putative biomarker for indicating metastasis in cancer patients.
Methods
Literatures’ retrieval strategy
For obtaining potentially eligible studies, integrated online literature retrieval was performed against multiple databases, including PubMed, Springer, Google Scholar, China National Knowledge Infrastructure (CNKI), Chongqing VIP Information Network, and Wanfang. The deadline of retrieval period was up to December 1, 2015. The keywords for the search were as follows: “Homeobox B7”, “HOXB7”, “cancer”, “carcinoma”, and “neoplasm”. In addition, other relevant articles were also obtained by manually viewing the reference list.
Inclusion and exclusion criteria
Inclusion criteria for the articles were as follows: 1) the role of HOXB7 in the development of human cancer was investigated; 2) related clinicopathologic parameters were described; 3) the expression level of HOXB7 in primary cancerous tissue was measured; and 4) patients were grouped according to the expression level of HOXB7.
Exclusion criteria for the articles were as follows: 1) duplicate publications; 2) studies without valuable data; and 3) reviews, letters, case reports, and expert opinions.
Date extraction
The data and information from all eligible studies were independently extracted by two investigators (YX O and GP Z). The following data and information were collected from each study: author, publication year, country, race, cancer type, total number of patients, number of high HOXB7 expression group and low HOXB7 expression group, number of patients with LNM and DM in each group, and the criteria of high HOXB7 expression. If there were disagreements, a consensus was reached by a third investigator (CQ).
Statistical methods
The present meta-analysis was conducted using RevMan5.3 software and Stata SE12.0. The heterogeneity among eligible studies was determined with the chi-square-based Q-test and I2 statistics; a P-value for Q-test <0.05 and I2-value >50% were considered severe heterogeneity. The random-effects model was applied in studies with a significant heterogeneity (PQ≤0.05, I2≥50%); otherwise, the fixed-effects model was adopted (PQ>0.05, I2<50%). Potential publication bias was assessed with a funnel plot, and sensitivity analysis was also performed to ensure the reliability of results. The P-value <0.05 was considered statistically significant.
Result
Studies’ characteristics
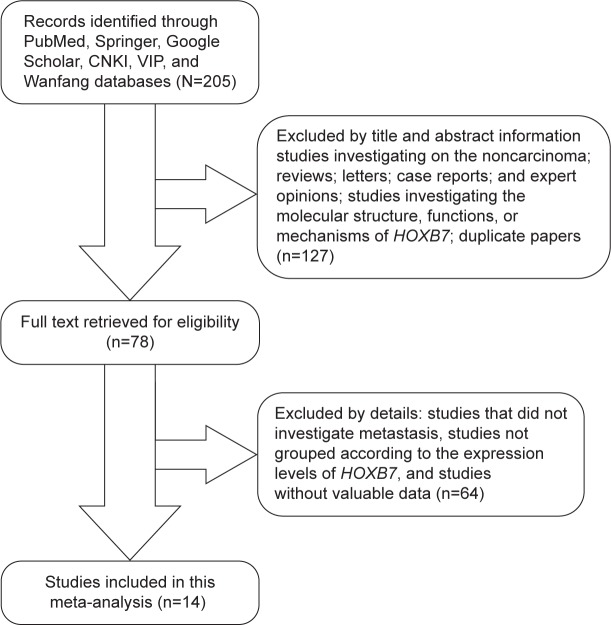
The process of literature retrieval is shown in detail in Figure 1. A total of 14 studies were finally identified to be eligible according to the criteria for selection.23–36 A total of 1,532 patients were included in the current meta-analysis, and the mean sample size of patients was 109.4 (range: 35–280). Among the 14 studies, 12 were from the People’s Republic of China, two were from Brazil, and one was from the USA. Eight different cancer types were evaluated in this meta-analysis, with two BC, three esophagus cancer, one gastric cancer, one lung cancer, two pancreatic cancer, two colorectal cancer, two oral cancer, and one malignant ovarian germ cell tumor. All cancerous specimens were well preserved before RNA extraction. The diagnoses of LNM and DM were all based on pathology.
Figure 1.
A flowchart presenting the steps of literature retrieval and selection.
Abbreviations: CKNI, China National Knowledge Infrastructure; VIP, Chongqing VIP Information Network.
There were two studies that reported on the association between the expression of HOXB7 mRNA and LNM,23,29 and the rest 12 articles have paid attention on the association of HOXB7 protein expression and metastases. Two detection methods (reverse transcription polymerase chain reaction and immunohistochemical staining) were applied to determine the expression levels of HOXB7 in cancerous tissues. The criteria of high HOXB7 expression in all included studies are shown in Table 1. All studies were divided into two groups (high HOXB7 expression group and low HOXB7 expression group). For unifying the result for further analysis, positive expression of HOXB7 was regarded as high expression and negative expression of HOXB7 was classified into low expression group.
Table 1.
The basic information and data of all included studies in the meta-analysis
| Author (year) | Country | Race | Cancer type | Total number | HOXB7 expression
|
Detection method | High expression | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High LNM | High DM | High | Low LNM | Low DM | Low | |||||||
| Zhu et al23 (2015) | People’s Republic of China | Asian | BC | 48 | 18 | – | 33 | 3 | – | 15 | RT-PCR | HOTAIR/GAPDH ≥1.0 |
| Li et al24 (2015) | People’s Republic of China | Asian | EC | 280 | 81 | – | 185 | 26 | – | 95 | IHC | Positive cells >25% |
| Tu et al25 (2015) | People’s Republic of China | Asian | GC | 96 | 47 | 4 | 66 | 16 | 1 | 30 | IHC | Sum of the intensity and extent scores ≥2 |
| Yuan et al26 (2014) | People’s Republic of China | Asian | LC | 75 | 36 | 3 | 57 | 5 | 2 | 18 | IHC | Sum of the intensity and extent scores ≥3 |
| Long et al27 (2014) | People’s Republic of China | Asian | EC | 76 | 26 | 3 | 41 | 21 | 3 | 35 | IHC | Sum of the intensity and extent scores ≥2 |
| Zhang et al28 (2014) | People’s Republic of China | Asian | PC | 44 | 17 | – | 29 | 3 | – | 15 | IHC | Sum of the intensity and extent scores ≥2 |
| Xie et al29 (2013) | People’s Republic of China | Asian | EC | 179 | 63 | – | 115 | 25 | – | 64 | RT-PCR | Above the cutoff value (0.25) |
| Nguyen et al30 (2013) | USA | Caucasian | PC | 145 | 35 | – | 55 | 41 | – | 90 | IHC | Histoscore >110 |
| Wang et al31 (2013) | People’s Republic of China | Asian | CC | 73 | 21 | 11 | 47 | 9 | 1 | 26 | IHC | Sum of the intensity and extent scores ≥2 |
| Bitu et al32 (2012) | Brazil | Mixed | OC | 115 | 31 | – | 55 | 20 | – | 60 | IHC | Positive cells ≥31% |
| Liao et al33 (2011) | People’s Republic of China | Asian | CRC | 224 | 59 | 46 | 121 | 38 | 26 | 103 | IHC | Intensity score ≥2 with positive cells ≥50% |
| De Souza Setubal Destro et al34 (2010) | Brazil | Mixed | OC | 35 | 14 | – | 19 | 7 | – | 16 | IHC | Positive cells >38.2% |
| Ding35 (2010) | People’s Republic of China | Asian | MOGC | 85 | 3 | – | 24 | 0 | – | 61 | IHC | Product of the intensity and extent scores ≥8 |
| Chen et al36 (2009) | People’s Republic of China | Asian | BC | 57 | 25 | – | 41 | 5 | – | 16 | IHC | Sum of the intensity and extent scores ≥3 |
Note: The dashes represent no data.
Abbreviations: BC, breast cancer; CC, colon cancer; CRC, colorectal cancer; DM, distant metastases; EC, esophagus cancer; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GC, gastric cancer; IHC, immunohistochemistry; LC, lung cancer; LNM, lymph node metastases; MOGC, malignant ovarian germ cell tumor; OC, oral cancer; PC, pancreatic cancer; RT-PCR, reverse transcription polymerase chain reaction.
Association between HOXB7 expression level and LNM
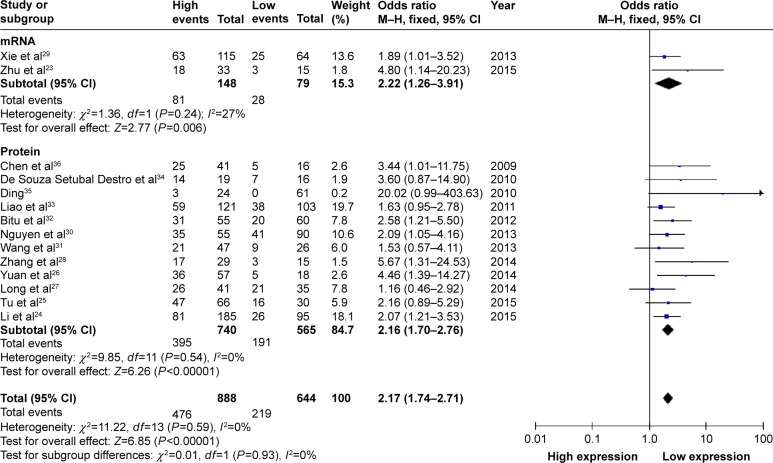
All 14 studies provided the number of patients with LNM based on different HOXB7 expression levels in a total of 1,532 patients. The fixed-effects model was adopted as there was limited heterogeneity across studies (I2=0%, PQ=0.59). The odds ratio (OR), expressed as high HOXB7 expression group versus low HOXB7 expression group, was 2.17 (95% CI: 1.74–2.71, P<0.00001; Figure 2). In the subgroup analysis, the result showed that there was a significant association between the expression level of HOXB7 mRNA and LNM (OR =2.22, 95% CI: 1.26–3.91, P=0.006). For the association between the expression level of HOXB7 protein and LNM, the OR, expressed as high HOXB7 protein expression group versus low HOXB7 protein expression group, was 2.16 (95% CI: 1.70–2.76, P<0.00001). From the analysis results, when the LNM incidence of cancers was compared between the two groups, we found that there was a significant difference in the LNM incidence between the high and low expression groups. This result demonstrated that cancer patients determined with high HOXB7 expression (including mRNA and protein) in cancerous tissues were more prone to developing LNM.
Figure 2.
A forest plot for the association between HOXB7 expression levels with LNM.
Abbreviations: df, degrees of freedom; LNM, lymph node metastases; M–H, Mantel–Haenszel test.
Association between HOXB7 expression level and DM
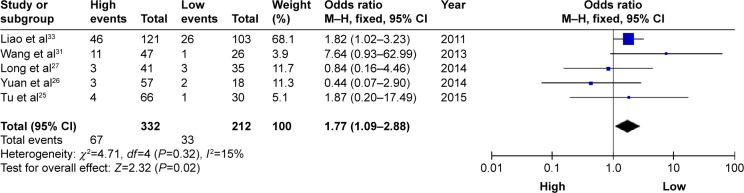
Five studies reported the number of patients with DM based on different HOXB7 protein expression levels in a total of 544 patients. There was no significant heterogeneity among the studies (I2=15%, PQ=0.32); thus, the fixed-effects model was adopted. Analysis showed a pooled OR =1.77 (95% CI: 1.09–2.88, P=0.02; Figure 3). Compared with the low HOXB7 expression group, the DM rate was significantly increased in the high HOXB7 expression group. The result showed that patients with high HOXB7 protein expression level in tumor tissues may indicate an increased risk of developing DM.
Figure 3.
A forest plot for the association between HOXB7 expression levels with DM.
Abbreviations: df, degrees of freedom; DM, distant metastases; M–H, Mantel–Haenszel test.
In addition, sensitivity analysis and assessment of publication bias were not performed due to the relatively small heterogeneity across studies in distant metastasis and limited number of included studies.
Publication bias
For meta-analysis of the association between HOXB7 expression level and LNM, the funnel plot was slightly asymmetrical (Figure 4), and then, the trim and fill method was applied to test for publication bias. The results showed that there was no significant publication bias across these studies.
Figure 4.
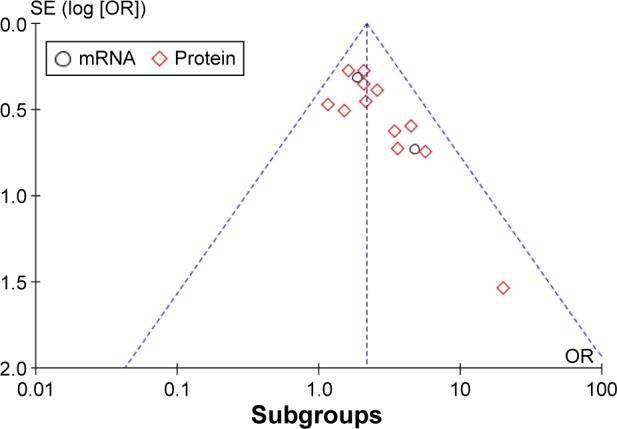
A funnel plot analysis of potential publication bias.
Abbreviations: SE, standard error; OR, odds ratio.
Sensitivity analysis
For meta-analysis of the association between HOXB7 expression level and LNM, sensitivity analysis was performed by deleting each study in turn from the pooled analysis. It aimed to test the influence of the removed data set on the overall ORs. The result was not significantly influenced by the exclusion of each study, suggesting that the result of synthetic analysis was robust.
Discussion
As we all know, cancer is a severe threat to human health, and there are millions of people who die from cancer every year. The LNM is the most common metastasis pathway in most cancers, and distant metastasis often occurs in the later stages of cancer. LNM and DM are positively significant for diagnosis in tumor–node–metastasis staging and treatment for cancer patients, as well as are important indicators for predicting prognosis. Thereby, further discovery of new molecular markers to predict metastasis for cancer is still essential and full of clinical significance.
A growing number of researches have showed that the expression of HOXB7 was higher in cancerous tissues compared with paired noncancerous tissues. There were associations between HOXB7 expression and certain clinical characteristics of cancer patients. The patients with high expression levels of HOXB7 had an increased risk of metastasis as well as a poor overall survival.26–28 Furthermore, the overexpression of HOXB7 was closely related to the aggressive behavior of tumor cells. However, the exact mechanism of how HOXB7 makes effects on promoting tumor cell invasion and metastasis has not been clear and is still in research stage. Wu et al37 found that tumor invasion in BC could be promoted by HOXB7 through Ras/Rho pathway activation after upregulating basic fibroblast growth factor (bFGF), which was a known transcriptional target of HOXB7. In the malignant melanoma, it has demonstrated that miR-196a was a central regulator of HOXB7 expression, which played an important role in melanoma progression.38 Recently, a study by Liu et al39 showed that the migration and invasion of BC cells may be increased by overexpression of HOXB7, and this result could be reversed by knockdown of transforming growth factor (TGF)β2 or pharmacologic inhibition of TGFβ signaling. It suggested that HOXB7 may make effects on promoting tumor malignant progression through the activation of TGFβ signaling pathway.42–44 HOXB7 was also found to promote the migration and metastasis in lung adenocarcinoma through activation of the TGFβ/SMAD3 signaling.40 Chile et al41 reported that HOXB7 was also involved in cell proliferation and viability. Cell cycle arrest and apoptosis could be induced by the knockdown of HOXB7 in pancreatic ductal adenocarcinomas, while decreased protein level could significantly lead to the increased apoptosis rate. HOXB7 may be a promising target for future cancer therapies.
Conclusion
This meta-analysis has explored the relation between HOXB7 expression levels with LNM and DM for carcinoma. From the results of this current meta-analysis, we found that the occurrence probability of LNM and DM was higher in cancer patients with high HOXB7 expression, comparing with that of those with low HOXB7 expression. Nevertheless, we also found that there were some limitations in this meta-analysis. Firstly, the included number of cancer patients has been still relatively small, hence larger and better design studies would be necessary to confirm the obtained results. Besides, most patients included in this meta-analysis have been Asian, the patients of other races accounted for a small percentage. Additionally, potential publication bias may exist, although no significant publication bias was observed based on trim and fill method and the sensitivity analysis have also showed the results were robust. Furthermore, the criterion of high HOXB7 expression was varied in these studies. Therefore, more larger size, multicenter, and higher quality studies are needed for further research, based on a unified criterion for classifying HOXB7 expression groups.
Footnotes
Author contributions
H-L Luo and P-Q Zhu were involved in the design of this meta-analysis and revision of this manuscript; they have also given final approval for submission. Y-X Ou, G-P Zhang, and C Qiu were involved in publication collection. F-T Liu was involved in data analysis of the study and manuscript writing. He has also assisted in the design of this work. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2014;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cho JH, Lee YS, Sun DI, et al. Prognostic impact of lymph node micrometastasis in oral and oropharyngeal squamous cell carcinomas. Head Neck. 2015 Dec 17; doi: 10.1002/hed.24314. Epub. [DOI] [PubMed] [Google Scholar]
- 3.Li P, Wu F, Zhao H, et al. Analysis of the factors affecting lymph node metastasis and the prognosis of rectal neuroendocrine tumors. Int J Clin Exp Pathol. 2015;8(10):13331–13338. [PMC free article] [PubMed] [Google Scholar]
- 4.Mirzaei H, Gholamin S, Shahidsales S, et al. MicroRNAs as potential diagnostic and prognostic biomarkers in melanoma. Eur J Cancer. 2015;53:25–32. doi: 10.1016/j.ejca.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Gao J, Cao R, Mu H. Long non-coding RNA UCA1 may be a novel diagnostic and predictive biomarker in plasma for early gastric cancer. Int J Clin Exp Pathol. 2015;8(10):12936–12942. [PMC free article] [PubMed] [Google Scholar]
- 6.Treilleux I, Arnedos M, Cropet C, et al. Translational studies within the TAMRAD randomized GINECO trial: evidence for mTORC1 activation marker as a predictive factor for everolimus efficacy in advanced breast cancer. Ann Oncol. 2015;26(1):120–125. doi: 10.1093/annonc/mdu497. [DOI] [PubMed] [Google Scholar]
- 7.Cantile M, Schiavo G, Terracciano L, Cillo C. Homeobox genes in normal and abnormal vasculogenesis. Nutr Metab Cardiovasc Dis. 2008;18(10):651–658. doi: 10.1016/j.numecd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Samuel S, Naora H. Homeobox gene expression in cancer: insights from developmental regulation and deregulation. Eur J Cancer. 2005;41(16):2428–2437. doi: 10.1016/j.ejca.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Sukumar S. Role of homeobox genes in normal mammary gland development and breast tumorigenesis. J Mammary Gland Biol Neoplasia. 2003;8(2):159–175. doi: 10.1023/a:1025996707117. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Sukumar S. HOX genes: emerging stars in cancer. Cancer Biol Ther. 2003;2(5):524–525. doi: 10.4161/cbt.2.5.525. [DOI] [PubMed] [Google Scholar]
- 11.Cillo C, Faiella A, Cantile M, Boncinelli E. Homeobox genes and cancer. Exp Cell Res. 1999;248(1):1–9. doi: 10.1006/excr.1999.4451. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Liu G, Shen D, et al. HOXA1 enhances the cell proliferation, invasion and metastasis of prostate cancer cells. Oncol Rep. 2015;34(3):1203–1210. doi: 10.3892/or.2015.4085. [DOI] [PubMed] [Google Scholar]
- 13.Seifert A, Werheid DF, Knapp SM, Tobiasch E. Role of Hox genes in stem cell differentiation. World J Stem Cells. 2015;7(3):583–595. doi: 10.4252/wjsc.v7.i3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domsch K, Papagiannouli F, Lohmann I. The HOX-apoptosis regulatory interplay in development and disease. Curr Top Dev Biol. 2015;114:121–158. doi: 10.1016/bs.ctdb.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Platais C, Hakami F, Darda L, Lambert DW, Morgan R, Hunter KD. The role of HOX genes in head and neck squamous cell carcinoma. J Oral Pathol Med. 2015 Dec 14; doi: 10.1111/jop.12388. Epub. [DOI] [PubMed] [Google Scholar]
- 16.Hamid AR, Hoogland AM, Smit F, et al. The role of HOXC6 in prostate cancer development. Prostate. 2015;75(16):1868–1876. doi: 10.1002/pros.23065. [DOI] [PubMed] [Google Scholar]
- 17.Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10(5):361–371. doi: 10.1038/nrc2826. [DOI] [PubMed] [Google Scholar]
- 18.Cai JQ, Xu XW, Mou YP, Chen K, Pan Y, Wu D. Upregulation of HOXB7 promotes the tumorigenesis and progression of gastric cancer and correlates with clinical characteristics. Tumour Biol. 2015 Aug 26; doi: 10.1007/s13277-015-3948-3. Epub. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Lee JS, Liang X, et al. Hoxb7 inhibits transgenic HER-2/neu-induced mouse mammary tumor onset but promotes progression and lung metastasis. Cancer Res. 2008;68(10):3637–3644. doi: 10.1158/0008-5472.CAN-07-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chile T, Fortes MA, Corrêa-Giannella ML, et al. HOXB7 mRNA is overexpressed in pancreatic ductal adenocarcinomas and its knockdown induces cell cycle arrest and apoptosis. BMC Cancer. 2013;13:451. doi: 10.1186/1471-2407-13-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma R, Zhang D, Hu PC, Li Q, Lin CY. HOXB7-S3 inhibits the proliferation and invasion of MCF-7 human breast cancer cells. Mol Med Rep. 2015;12(4):4901–4908. doi: 10.3892/mmr.2015.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kokonoe M, Jun-Ichi H, Minoru T, et al. Aberrant expression of HOX genes in human invasive breast carcinoma. Oncol Rep. 2005;13(4):673–679. [PubMed] [Google Scholar]
- 23.Zhu FX, Ma R, Lin CY, et al. Expression of homeobox B7(HOBX7) and its clinical significance in breast cancers. Med J Wuhan Univ. 2015;36(1):58–61. Chinese. [Google Scholar]
- 24.Li H, Shen LY, Yan WP, et al. Deregulated HOXB7 expression predicts poor prognosis of patients with esophageal squamous cell carcinoma and regulates cancer cell proliferation in vitro and in vivo. PLoS One. 2015;10(6):e0130551. doi: 10.1371/journal.pone.0130551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu W, Zhu X, Han Y, Wen Y, Qiu G, Zhou C. Overexpression of HOXB7 is associated with a poor prognosis in patients with gastric cancer. Oncol Lett. 2015;10(5):2967–2973. doi: 10.3892/ol.2015.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan W, Zhang X, Xu Y, Li S, Hu Y, Wu S. Role of HOXB7 in regulation of progression and metastasis of human lung adenocarcinoma. Mol Carcinog. 2014;53(1):49–57. doi: 10.1002/mc.21947. [DOI] [PubMed] [Google Scholar]
- 27.Long QY, Zhou J, Zhang XL, Cao JH. HOXB7 predicts poor clinical outcome in patients with advanced esophageal squamous cell cancer. Asian Pac J Cancer Prev. 2014;15(4):1563–1566. doi: 10.7314/apjcp.2014.15.4.1563. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Zheng S, Du Y, Wang Y, Zang W, Zhao G. Levels of HOXB7 and miR-337 in pancreatic ductal adenocarcinoma patients. Diagn Pathol. 2014;9(2):1–7. doi: 10.1186/1746-1596-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie X, Zhang SS, Wen J, et al. Prognostic value of HOXB7 mRNA expression in human oesophageal squamous cell cancer. Biomarkers. 2013;18(4):297–303. doi: 10.3109/1354750X.2013.773380. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen Kovochich A, Arensman M, Lay AR, et al. HOXB7 promotes invasion and predicts survival in pancreatic adenocarcinoma. Cancer. 2013;119(3):529–539. doi: 10.1002/cncr.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang DQ, Yan DW, Tang HM, et al. Expression of homeobox B7 gene in colon carcinoma and its clinicopathological significance. Chin J Exp Surg. 2013;30(3):438–440. in Chinese. [Google Scholar]
- 32.Bitu CC, Carrera M, Lopes MA, Kowalski LP, Soares FA, Coletta RD. HOXB7 expression is a prognostic factor for oral squamous cell carcinoma. Histopathology. 2012;60(4):662–665. doi: 10.1111/j.1365-2559.2011.04102.x. [DOI] [PubMed] [Google Scholar]
- 33.Liao WT, Jiang D, Yuan J, et al. HOXB7 as a prognostic factor and mediator of colorectal cancer progression. Clin Cancer Res. 2011;17(11):3569–3578. doi: 10.1158/1078-0432.CCR-10-2533. [DOI] [PubMed] [Google Scholar]
- 34.De Souza Setubal Destro MF, Bitu CC, Zecchin KG, et al. Overexpression of HOXB7 homeobox gene in oral cancer induces cellular proliferation and is associated with poor prognosis. Int J Oncol. 2010;36(1):141–149. [PubMed] [Google Scholar]
- 35.Ding H. Master’s Thesis. Sun Yat-Sen University; Guangzhou: 2010. The study of the expression of HOXB7 gene and its clinical significance in malignant ovarian germ-cell tumor. [Google Scholar]
- 36.Chen ZH, LV GM, Ji TH. Expression of homeobox B7 in breast cancers and its clinical significance. China Trop Med. 2009;9(9):1697–1806. in Chinese. [Google Scholar]
- 37.Wu X, Chen H, Parker B, et al. HOXB7, a homeodomain protein, is overexpressed in breast cancer and confers epithelial-mesenchymal transition. Cancer Res. 2006;66(19):9527–9534. doi: 10.1158/0008-5472.CAN-05-4470. [DOI] [PubMed] [Google Scholar]
- 38.Braig S, Mueller DW, Rothhammer T, Bosserhoff AK. MicroRNA miR-196a is a central regulator of HOX-B7 and BMP4 expression in malignant melanoma. Cell Mol Life Sci. 2010;67(20):3535–3548. doi: 10.1007/s00018-010-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S, Jin K, Hui Y, et al. HOXB7 promotes malignant progression by activating the TGFβ signaling pathway. Cancer Res. 2015;75(4):709–719. doi: 10.1158/0008-5472.CAN-14-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuang L, Li WH, Li K, Mao Y, Gao CL, Zhang C. Hoxb7 promotes growth and metastasis of lung adenocarcinoma cells through regulation of the tgf-β/smad3 signaling. J Biol Regul Homeost Agents. 2015;29(3):601–608. [PubMed] [Google Scholar]
- 41.Chile T, Brentani HP, Maria DA, et al. HOXB7 mRNA is overexpressed in pancreatic ductal adenocarcinomas and its knockdown induces cell cycle arrest and apoptosis. BMC Cancer. 2013;13(1):1–12. doi: 10.1186/1471-2407-13-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bohlius J, Schmidlin KC, Schwarzer G, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373(9674):1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 43.Yan S, Jiao X, Li K, Li W, Zou H. The impact of IGF-1R expression on the outcomes of patients with breast cancer: a meta-analysis. Onco Targets Ther. 2015;8:279–287. doi: 10.2147/OTT.S74774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen YY, Wang LW, Wang SY, et al. Meta-analysis of postoperative adjuvant chemotherapy without radiotherapy in early stage non-small cell lung cancer. Onco Targets Ther. 2015;8:2033–2043. doi: 10.2147/OTT.S88700. [DOI] [PMC free article] [PubMed] [Google Scholar]