Abstract
Ubiquitin E3 ligases of the RING and HECT families are distinct not only in their catalytic mechanisms but also in targeting substrates. Now it seems that one heterodimeric complex can target substrates to both types of E3 ligase.
Protein ubiquitylation has a broad and critical role in regulating a wide range of cellular processes. The addition of Lys 48-linked polyubiquitin chains to specific substrate proteins regulates timely degradation by the 26S proteasome. In addition, like other covalent modifications, ubiquitylation can modulate the function of a substrate by causing a conformational change. Ubiquitylation begins with the ATP-dependent activation of ubiquitin by the E1 enzyme, and is followed by the subsequent transfer of ubiquitin to one of a small family of E2 ubiquitin-conjugating enzymes; finally, an E3 ubiquitin ligase is responsible for recognizing a specific substrate and promoting ubiquitin ligation. More than 1,000 distinct E3 ligases are predicted to exist, either as individual proteins or multi-subunit complexes, in mammalian cells.
There are two major families of E3 ligases distinguished by their active domains: the HECT family (‘homologous to the E6-AP carboxy terminus’) and the RING family (first recognized in the human ‘really interesting new gene product’)1,2. The HECT domain mediates interaction with the cognate E2 and, through an evolutionarily conserved cysteine residue, forms a thioester linkage with ubiquitin. Human cells contain as many as 28 HECT proteins and most, if not all, are believed to function as E3 ligases. Unlike the HECT domain, the RING domain promotes a direct transfer of ubiquitin from the E2 to the substrate without forming an intermediate with ubiquitin. Human cells express more than 450 RING proteins, and E3 ligase activity has been experimentally demonstrated for many of them. In addition, although not containing a RING domain themselves, members of the evolutionarily conserved cullin family can bind a small RING protein, either ROC1 or ROC2 (also known as Rbx). A remarkable feature of cullin proteins is that the amino-terminal sequence in each of the six classical human cullin family members interacts selectively with a different motif such as an F-box, a SOCS box, a BTB domain and a WD40 repeat. These common motifs are present in many proteins, suggesting the potential assembly of as many as 300–500 distinct cullin–RING ligase (CRL) complexes in vivo3, making cullins the largest subfamily of E3 ligases.
Not only do HECT and CRL E3 ligases use different catalytic mechanisms in catalysing the transfer of ubiquitin from E2 to the substrate, they are also thought to have unique means of assembly, regulation and substrate targeting. On page 409 of this issue, Maddika and Chen4 identify and characterize a novel E3 ligase that uses DYRK2 as a scaffold for the assembly of a HECT E3 complex and a heterodimeric complex consisting of DDB1 and VPRBP for recruiting substrate. This finding is particularly unexpected because DYRK2 is a protein kinase and DDB1 is established as a key adaptor protein for recruiting substrate to the Cul4–RING ligases (CRL4s)5–8.
DYRK2 is a member of evolutionarily conserved dual-specificity tyrosine (Y)-regulated kinases, whose function has been broadly linked to DNA repair, cell proliferation, differentiation and apoptosis. Maddika and Chen identify a novel DYRK2 complex that contains EDD, DDB1 and VPRBP. EDD (E3 identified by differential display) is a large protein containing multiple domains linked to ubiquitylation, including an N-terminal ubiquitin associated (UBA) domain, a UBR box (a motif important for the targeting of N-end rule substrates) and a C-terminal HECT domain. No known substrate has previously been identified for EDD. DDB1 (damaged-DNA-binding protein) serves as a key linker to bridge a subset of WD40-containing proteins to Cul4–RING ligases5–8. As many as one-third of the 300 WD40 proteins found in human cells could interact with DDB1 (ref.s 5). VPRBP, a WD40-containing protein that binds DDB1, was initially identified as the human HIV Vpr-binding protein. The significance of VPRBP–Vpr interaction remains unclear, especially whether Vpr, like E6, hijacks a VPRBP complex or exploits normal substrate ubiquitylation to benefit HIV propagation. So far, only one candidate substrate, the cytoplasmic localized neurofibromatosis type 2 (NF2) tumor suppressor gene product, Merlin, has been reported to be targeted by VPRBP to the DDB1–Cul4–ROC1 ligase for degradation9. However, there are reasons to believe that VPRBP may target additional proteins, because VPRBP can bind to chromatin and is required for normal DNA replication, and genetic disruption of VPRBP causes early embryonic lethality in mouse and various developmental defects in plants10,11.
In Caenorhabditis elegans, the DYRK2 homolog MBK-2 phosphorylates and regulates the meiotic protein, MEI-1/katanin, the catalytic subunit of the microtubule-severing AAA ATPase complex. Maddika and Chen4 therefore tested whether mammalian katanin was a substrate for the newly identified DYRK2 E3 complex, referred to as EDVP (EDD–DDB1–VPRBP). In vitro binding and in vivo ubiquitylation assays demonstrated that katanin associates with and is polyubiquitylated by the EDVP E3 ligase complex. VPRBP binds directly to, and is required for, bringing katanin to the EDVP E3 ligase; notably, no Cul4 or ROC1 is detected in the complex. Silencing individual components of EDVP, but not Cul4A and Cul4B, severely impaired katanin polyubiquitylation. Maddika and Chen show that DYRK2 acts as a scaffold to assemble the complex components, but this scaffold function does not rely on its kinase activity. However, phosphorylation by DYRK2 is required for subsequent katanin polyubiquitylation: coexpression of either a catalytically inactive DYRK2 or a triple phospho-mutant of katanin inhibits katanin polyubiquitylation. Supporting the physiological relevance of this ubiquitylation, ectopic expression of katanin causes mitotic defects (as determined by the increase in cells with 4N DNA content and positive for phopho-histone H3) that can be largely alleviated by co-expression with wild-type, but not kinase-dead, DYRK2. Knocking down either DYRK2 or EDD causes katanin accumulation and a similar increase in G2/M cells, which can be rescued by simultaneous silencing of katanin. Hence, the EDVP E3 complex is capable of phosphorylation and subsequent ubiquitylation of its substrate.
This study raised two interesting questions whose resolution may shed new light on mechanisms of ubiquitylation and substrate targeting. First, how does DYRK2-mediated phosphorylation of substrate katanin contribute to subsequent ubiquitylation by EDD? Substrate phosphorylation is known to have a key function in the initial recognition by some E3s, as best documented for several substrates whose phosphorylation triggers the binding with specific F-box proteins and subsequent ubiquitylation by the SCF/CRL1 complex. Unlike phosphorylation-dependent binding between substrate and the F-box, there is no evidence that DYRK2-mediated phosphorylation is required for katanin to bind with VprBPDDB1. However, the phospho-mutant katanin cannot be efficiently ubiquitinated. Similarly, the catalytic mutant DYRK2 does not seem to have any defect in assembling EDD, DDB1 and VPRBP but fails to promote katanin polyubiquitylation. Could phosphorylation have a function in orienting the substrate towards or closer to the ubiquitin-linked catalytic Cys in the HECT domain of EDD? It has been deduced from structural analysis of several E3s that the distance between the active Cys residue either in the E2 bound to the RING finger or in the HECT domain is too far away for transfer of ubiquitin to the substrate. For example, the Cys in the active site of E2is 41 Å away from the active site in the HECT domain in E6AP, and 50 Å away from the nearest amino-acid F-box protein in the SCF/CRL1 complex12,13.
Second, how does the DDB1–VPRBP heterodimer determine which substrate is targeted to which E3? Among the estimated 90-plus DWD (DDB1-binding WD40) proteins, VPRBP is unique in that it is a particularly large protein that is abundantly expressed in many cell types and, like DDB1, has an essential function for cell growth and embryo development. Do these properties make the DDB1–VPRBP heterodimer a unique complex in recruiting different substrates to different E3 ligases? Are there other DWD proteins, in addition to VPRBP, that are also capable of shuttling between both families of E3 ligases? DYRK2 was not detected by several previous proteomic screens of proteins associated with DDB1 and VPRBP, suggesting that we may still be underestimating the reach of adaptor proteins and substrate receptor complexes in targeting substrate proteins for ubiquitylation. We have already seen that individual F-box proteins can target multiple substrates to specific CRLs. For example, the SKP2 and β-TrCP F-box proteins have each been linked to the ubiquitylation of nearly 30 proteins14. These current findings demonstrate even more versatility in targeting substrates for ubiquitylation than previously realized, and indicate the potential to expand the repertoire of specific protein substrates ubiquitylated by E3 ligases.
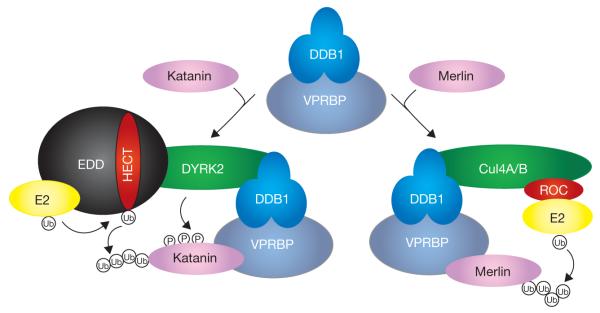
Figure 1.
DDB1–VPRBP targets substrates to distinct E3 ubiquitin ligase complexes. The DDB1–VPRBP heterodimer can target different substrate to a DYRK2–HECT or a Cul4–ROC1 E3 ligase complex. DYRK2 is required for assembly of the E3 complex and for phosphorylation of its substrate katanin, but not for the initial binding of katanin with VPRBP. Ub, ubiquitin.
References
- 1.Huibregtse JM, Scheffner M, Beaudenon S, Howley PM. Proc. Natl Acad. Sci. USA. 1995;92:2563–2567. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovering R, et al. Proc. Natl Acad. Sci. USA. 1993;90:21112–22116. [Google Scholar]
- 3.Petroski MD, Deshaies RJ. Nature Rev. Mol. Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 4.Maddika S, Chen J. Nature Cell Biol. 2009;11:409–419. doi: 10.1038/ncb1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He YJ, McCall CM, Hu J, Zeng Y, Xiong Y. Genes Dev. 2006;20:2949–2954. doi: 10.1101/gad.1483206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higa LA, et al. Nature Cell Biol. 2006;8:1277–1283. doi: 10.1038/ncb1490. [DOI] [PubMed] [Google Scholar]
- 7.Angers S, et al. Nature. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- 8.Jin J, Arias EE, Chen J, Harper JW, Walter JC. Mol. Cell. 2006;23:709–721. doi: 10.1016/j.molcel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Chen J. Oncogene. 2008;27:4056–4064. doi: 10.1038/onc.2008.44. [DOI] [PubMed] [Google Scholar]
- 10.McCall CM, et al. Mol. Cell. Biol. 2008;28:5621–5633. doi: 10.1128/MCB.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, et al. Plant Cell. 2008;20:1437–1455. doi: 10.1105/tpc.108.058891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L, et al. Science. 1999;286:1321–1326. doi: 10.1126/science.286.5443.1321. [DOI] [PubMed] [Google Scholar]
- 13.Zheng N, et al. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 14.Frescas D, Pagano M. Nature Rev. Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]