Abstract
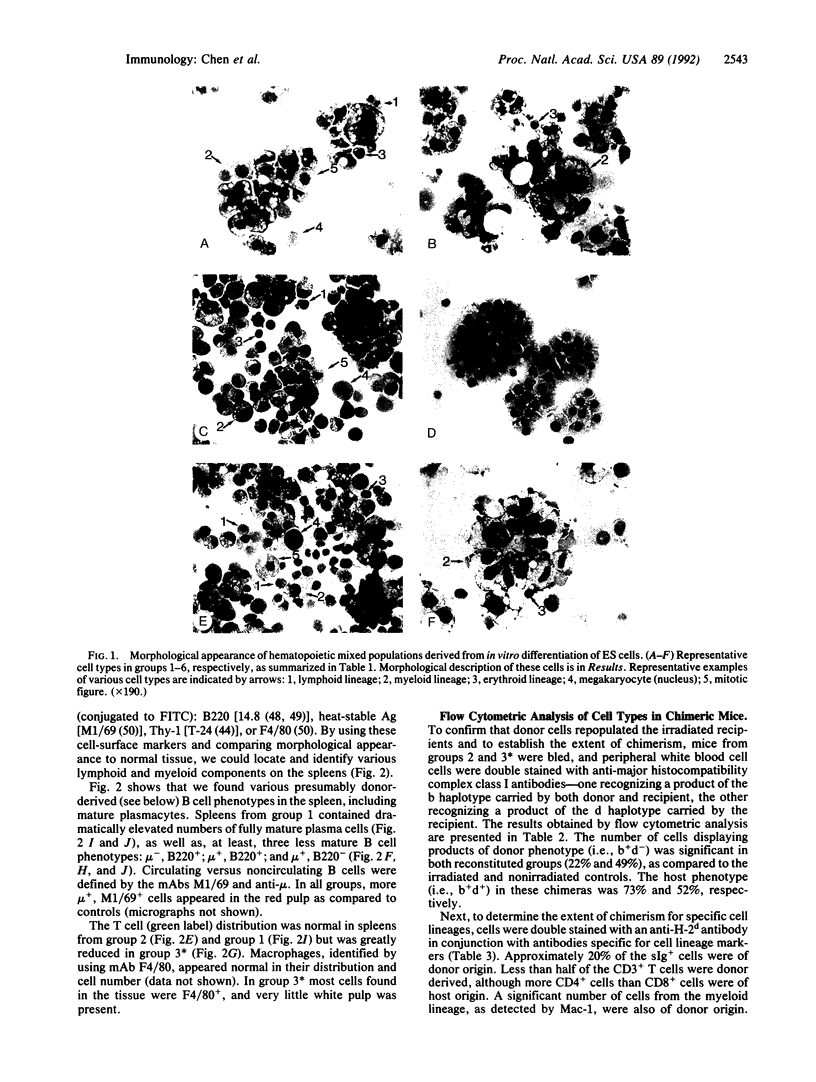
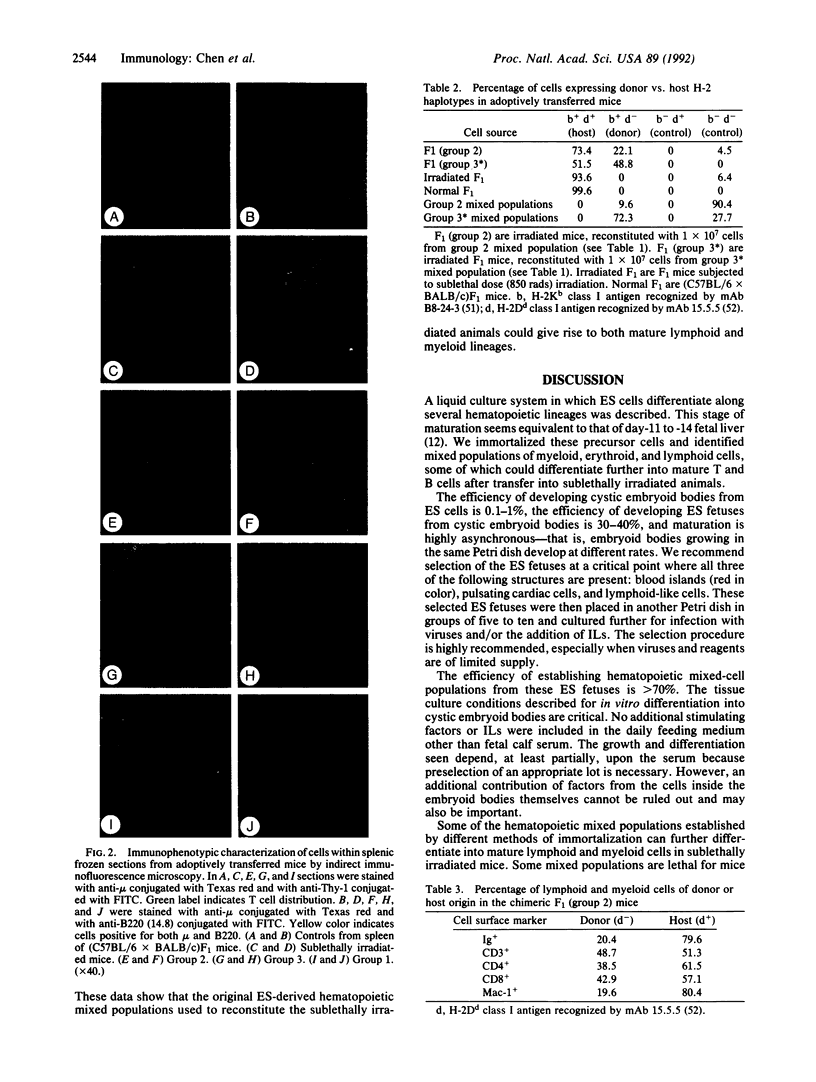
Mouse embryonic stem (ES) cells have the potential to differentiate into embryoid bodies in vitro and mimic normal embryonic development. The "ES fetus" is a specific development at a late stage seen under our culture conditions. We have established several mixed populations from ES fetuses by using combinations of retroviruses carrying different oncogenes (v-abl, v-raf, c-myc), interleukins 2 and 3, and Con A. Six groups of mixed populations were characterized by immunophenotyping. For some groups, transfer of cells into sublethally irradiated mice resulted in the development of macrophages, mature T and B lymphocytes, and plasma cells of donor origin. Thus, these mixed populations may contain immortalized precursors of hematopoietic lineages. These mixed populations should be valuable for defining hematopoietic stem cells and their committed progenitors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Clynes R., Wax J., Stanton L. W., Smith-Gill S., Potter M., Marcu K. B. Rapid induction of IgM-secreting murine plasmacytomas by pristane and an immunoglobulin heavy-chain promoter/enhancer-driven c-myc/v-Ha-ras retrovirus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6067–6071. doi: 10.1073/pnas.85.16.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Mulvaney D., Coutinho A., Cazenave P. A. A novel cell surface molecule on early B-lineage cells. Nature. 1986 Jun 5;321(6070):616–618. doi: 10.1038/321616a0. [DOI] [PubMed] [Google Scholar]
- Cudennec C. A., Thiery J. P., Le Douarin N. M. In vitro induction of adult erythropoiesis in early mouse yolk sac. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2412–2416. doi: 10.1073/pnas.78.4.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara T. M., Efstratiadis A., Robertson E. J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990 May 3;345(6270):78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- Dennert G., Hyman R., Lesley J., Trowbridge I. S. Effects of cytotoxic monoclonal antibody specific for T200 glycoprotein on functional lymphoid cell populations. Cell Immunol. 1980 Aug 1;53(2):350–364. doi: 10.1016/0008-8749(80)90335-4. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Lajtha L. G. Proliferation of haemopoietic stem cells in vitro. Br J Haematol. 1974 Dec;28(4):525–530. doi: 10.1111/j.1365-2141.1974.tb06671.x. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Doetschman T., Gregg R. G., Maeda N., Hooper M. L., Melton D. W., Thompson S., Smithies O. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987 Dec 10;330(6148):576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- Gossler A., Doetschman T., Korn R., Serfling E., Kemler R. Transgenesis by means of blastocyst-derived embryonic stem cell lines. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9065–9069. doi: 10.1073/pnas.83.23.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran W. L., Poenie M., Kimura J., Tsien R., Weiss A., Allison J. P. Expression and function of the CD3-antigen receptor on murine CD4+8+ thymocytes. Nature. 1987 Nov 12;330(6144):170–173. doi: 10.1038/330170a0. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Nature of cells forming erythroid colonies in agar after stimulation by spleen conditioned medium. J Cell Physiol. 1978 Mar;94(3):243–252. doi: 10.1002/jcp.1040940302. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Watanabe T., Sun L., Scheid M. P. Antigens displayed on murine B lymphocyte precursors. J Immunol. 1981 Dec;127(6):2262–2268. [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Watanabe T., Sun L., Scheid M. P. Antigens displayed on murine B lymphocyte precursors. J Immunol. 1981 Dec;127(6):2262–2268. [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Smithies O. Inactivating the beta 2-microglobulin locus in mouse embryonic stem cells by homologous recombination. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8932–8935. doi: 10.1073/pnas.86.22.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosco M. H., Tew J. G., Szakal A. K. Antigenic phenotyping of isolated and in situ rodent follicular dendritic cells (FDC) with emphasis on the ultrastructural demonstration of Ia antigens. Anat Rec. 1986 Jul;215(3):201-13, 219-25. doi: 10.1002/ar.1092150303. [DOI] [PubMed] [Google Scholar]
- Labastie M. C., Thiery J. P., Le Douarin N. M. Mouse yolk sac and intraembryonic tissues produce factors able to elicit differentiation of erythroid burst-forming units and colony-forming units, respectively. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1453–1456. doi: 10.1073/pnas.81.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKearn J. P., Baum C., Davie J. M. Cell surface antigens expressed by subsets of pre-B cells and B cells. J Immunol. 1984 Jan;132(1):332–339. [PubMed] [Google Scholar]
- Melchers F., Abramczuk J. Murine embryonic blood between day 10 and 13 of gestation as a source of immature precursor B cells. Eur J Immunol. 1980 Oct;10(10):763–767. doi: 10.1002/eji.1830101007. [DOI] [PubMed] [Google Scholar]
- Melchers F., Strasser A., Bauer S. R., Kudo A., Thalmann P., Rolink A. Cellular stages and molecular steps of murine B-cell development. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):183–189. doi: 10.1101/sqb.1989.054.01.023. [DOI] [PubMed] [Google Scholar]
- Nishikawa S. I., Hayashi S. I., Ogawa M., Kunisada T., Nishikawa S., Sudo T., Nakauchi H., Suda T. Control of cell growth and differentiation during early B-cell development by stromal cell molecules. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):171–174. doi: 10.1101/sqb.1989.054.01.021. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Nishikawa S., Ikuta K., Yamamura F., Naito M., Takahashi K., Nishikawa S. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO J. 1988 May;7(5):1337–1343. doi: 10.1002/j.1460-2075.1988.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J. J., Raff M. C., Cooper M. D. Studies on the generation of B lymphocytes in the mouse embryo. Eur J Immunol. 1976 Jul;5(7):468–473. doi: 10.1002/eji.1830050708. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Moore M. A., Lee G. The fate of fetal and adult B-cell progenitors grafted into immunodeficient CBA/N mice. J Exp Med. 1979 Sep 19;150(3):548–563. doi: 10.1084/jem.150.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi M., Pierani A., Boglia A., De Libero G., Mori L., Marini V., Ricciardi-Castagnoli P. Generation of new oncogenic murine retroviruses by cotransfection of cloned AKR and MH2 proviruses. Oncogene. 1989 Feb;4(2):223–230. [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P. L., Goff S. P., Robertson E. J. Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science. 1989 Nov 10;246(4931):799–803. doi: 10.1126/science.2554496. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Strasser A. PB76: a novel surface glycoprotein preferentially expressed on mouse pre-B cells and plasma cells detected by the monoclonal antibody G-5-2. Eur J Immunol. 1988 Nov;18(11):1803–1810. doi: 10.1002/eji.1830181123. [DOI] [PubMed] [Google Scholar]
- Thompson S., Clarke A. R., Pow A. M., Hooper M. L., Melton D. W. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell. 1989 Jan 27;56(2):313–321. doi: 10.1016/0092-8674(89)90905-7. [DOI] [PubMed] [Google Scholar]
- Troppmair J., Potter M., Wax J. S., Rapp U. R. An altered v-raf is required in addition to v-myc in J3V1 virus for acceleration of murine plasmacytomagenesis. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9941–9945. doi: 10.1073/pnas.86.24.9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Wong P. M., Clarke B. J., Carr D. H., Chui D. H. Adult hemoglobins are synthesized in erythroid colonies in vitro derived from murine circulating hemopoietic progenitor cells during embryonic development. Proc Natl Acad Sci U S A. 1982 May;79(9):2952–2956. doi: 10.1073/pnas.79.9.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Li E., Sajjadi F., Subramani S., Jaenisch R. Germ-line transmission of a disrupted beta 2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 1989 Nov 23;342(6248):435–438. doi: 10.1038/342435a0. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]