Abstract
Pompe disease is an inherited neuromuscular disorder that affects respiratory function and leads to dependence on external ventilatory support. We studied the activation of the diaphragm using bilateral phrenic magnetic stimulation and hypothesized that diaphragm compound muscle action potential (CMAP) amplitude and evoked transdiaphragmatic pressure (Twitch PDI) would correlate to disease severity. Eight patients with late onset Pompe disease (LOPD, aged 14–48 years) and four healthy control subjects completed the tests. Maximal Twitch PDI responses were progressively reduced in patients with LOPD compared to control subjects (1.4 – 17.1 cm H2O, p<0.001) and correlated to voluntary functional tests (p<0.05). Additionally, CMAP amplitude (mA) was lower in the patients who used nighttime or fulltime ventilatory support, when compared to controls and patients who used no ventilatory support (p<0.005). However, the normalized (%peak) Twitch PDI and CMAP responses were similar between patients and controls. This suggests a loss of functional phrenic motor units in patients, with normal recruitment of remaining motor units.
Keywords: Pompe, diaphragm, ventilator, stimulation, neuromuscular
Introduction
Pompe disease is a rare neuromuscular disorder characterized by a defect in the gene that encodes acid alpha-glucosidase (GAA), the enzyme responsible for lysosomal breakdown of glycogen (Reviewed in (Fuller et al., 2013)). As a result, glycogen accumulates in the tissues of patients, resulting in altered neuromuscular structure and function. Neural accumulation of glycogen has been observed from post-mortem patient specimens of the cervical cord (DeRuisseau et al., 2009). Additionally, abnormal spontaneous EMG activity (Corti et al., 2015) and impaired NM transmission may be present (Kassardjian et al., 2015). However, there is limited information on both the electrophysiological responses to maximal phrenic stimulation and clinical estimates of ventilatory motor function in patients with Pompe disease.
In the late-onset form of Pompe, patients may retain ambulation into adulthood, yet preferential diaphragm weakness is a prevalent and serious feature that elevates the risk for respiratory failure (Pellegrini et al., 2005). Specifically, the capacity to generate tidal volume gradually decreases with respiratory muscle weakness (Fuller et al., 2013; Mah et al., 2010). Existing evidence from the murine model of Pompe disease and patients suggests that the changes in the phrenic neuromotor drive and motor output of the diaphragm over time may distinguish milder from more severe ventilatory dysfunction (Fuller et al., 2013).
Using twitch transdiaphragmatic pressure (Twitch PDI) as an index of diaphragm muscle function and the evoked compound muscle action potential (CMAP) responses to reflect neuromuscular activity, we sought to evaluate the neuromuscular properties that contribute to phrenic motor dysfunction in late onset Pompe disease. The hypothesis was that Twitch PDI and CMAP responses to phrenic stimulation would be significantly lower in patients than in unaffected, age-matched control subjects, in accordance to their functional requirements for external ventilatory assist.
2.0 Material and Methods
2.1 Subjects
Individuals were eligible to participate if they were at least 12 years of age, medically stable, and either had a confirmed diagnosis of late onset Pompe disease or were an unaffected control subject. Subjects were ineligible if they had a cardiac pacemaker or metal implants in the head or chest, other than dental fillings. The University of Florida Institutional Review Board approved the study procedures, and written informed consent was obtained for participation.
2.2 Clinical Respiratory Muscle Tests
After a minimum 15-minute rest, seated forced vital capacity and maximal inspiratory pressure were tested in accordance to ATS/ERS criteria. Three consistent maximal efforts were typically achieved within 5–6 trials, and of these, the best effort was recorded.
2.3 Magnetic Stimulation of the Phrenic Nerves
The right and left phrenic nerves were stimulated simultaneously with custom 43mm double coils powered by two stimulation units (Magstim 200, UK), using the anterolateral approach described by Mills (Mills et al., 1996). Subjects swallowed two solid state pressure transducers (Millar, USA) and esophageal and gastric placement was confirmed. Additionally, sEMG electrodes (Medi-Trace 200 Series Electrodes, Covidien) were placed in the configuration described by Verin (Verin et al., 2002). Respiratory bands at the chest and abdomen recorded the breathing pattern. After catheter placement, subjects rested a minimum of 10 minutes, then twitch simulations were administered at least 30 seconds apart. Five satisfactory stimulations were obtained at end-exhalation, at 40, 50, 60, 70, 80, 90, and 100% of stimulator output. Magnetic stimulation has been utilized extensively to evaluate human motoneuron excitability and found to elicit consistent EMG responses within a session (Martin et al., 2009).
2.4 Data Analysis
EMG was sampled at 10 kHz, band-pass filtered at 3–1000 Hz, and respiratory parameters sampled at 1 kHz (PowerLab S30-16, ADInstruments). Data were analyzed off-line using Lab Chart Pro v7.2 (ADInstruments, Colorado Springs). For each intensity of stimulation, the three stimulations that yielded the highest Pdi were averaged and reported. These three stimulations were used for subsequent analysis of the CMAP. The right and left CMAP were similar and thus were averaged together. The Twitch PDI (cm H2O) and peak-to-peak CMAP amplitude (mA) responses were also normalized to the percentage of the value achieved at 100% stimulator output (%peak). At each stimulation intensity, the Twitch PDI and peak-to-peak CMAP amplitudes (both absolute and %peak) were averaged to construct recruitment curves. The effects of stimulator output settings and group assignment were contrasted with two-way ANOVA and Tukey’s post-hoc tests. Group differences in the onset latency and duration of the CMAP at 100% stimulator output were calculated with independent t-tests. Strength of association between the evoked and voluntary maneuvers was evaluated with Pearson’s correlation. Statistical analysis was completed using GraphPad Prism 5.0, using a significance threshold of p<0.05. The mean and standard deviation of the data are reported.
3.0 Results
3.1 Sample
Eight patients with later onset Pompe Disease and four control subjects volunteered to participate. Patients had diverse ages, ambulatory status, and ventilatory function (Table 1). The mean age of the patients (34.2 ±13.3 years) was matched to the control group (32.5 ±8.3 years, p=0.917), and body mass index did not differ between the patients (22.7 ± 5.2 kg/m2) and controls (22.4 ± 2.3 kg/m2, p=0.85). Three patients reported they did not use ventilatory assistance (“no assist”), three reported the use of overnight non-invasive support (“nighttime assist”), and two subjects reported the routine use of non-invasive support overnight plus at least six hours of daytime support (“full-time assist”). All of the patients who did not use daytime ventilatory support remained ambulatory in the community, with one exception (P3). This patient previously used nighttime support, but discontinued the therapy prior to the study
Table 1.
Characteristics of the sample
| Subject | Sex | Age (years) | Age at diagnosis (years) | Max. Inspiratory Pressure (cm H2O) | Forced Vital Capacity (% predicted) | Ambulation | Ventilatory support |
|---|---|---|---|---|---|---|---|
| Pompe | |||||||
| P1 | Female | 14 | 10 | 62 | 87 | Community | None |
| P2 | Female | 18 | 14 | 49 | 86 | Community | None |
| P3 | Female | 38 | 35 | 52 | 60 | Household | None |
| P4 | Female | 41 | 39 | 52 | 79 | Community | Nighttime |
| P5 | Female | 46 | 40 | 50 | 89 | Community | Nighttime |
| P6 | Female | 25 | 24 | 30 | 23 | Community | Nighttime |
| P7 | Male | 48 | 35 | 13 | 10 | Non-Ambulatory | Fulltime |
| P8 | Female | 43 | 30 | 20 | 15 | Non-Ambulatory | Fulltime |
|
| |||||||
| Average | 34.2 ± 13.3 | 38.4 ± 11.4 | 41 ± 18 | 56 ± 34 | |||
|
| |||||||
| Controls | |||||||
| C1 | Female | 42 | - | 85 | 90 | - | - |
| C2 | Male | 35 | - | 125 | 96 | - | - |
| C3 | Female | 31 | - | 80 | 89 | - | - |
| C4 | Female | 23 | - | 105 | 107 | - | - |
|
| |||||||
| Average | 32.5 ± 8.3 | 98 ± 21 | 96 ± 9 | ||||
3.2. Voluntary tests
The mean forced vital capacity of the patients was 56 (±34) % of expected values for age, gender, height, and ethnicity. In contrast, control subjects achieved 95 (±9) % of the expected FVC. MIP averaged 40.1 (±17.1) cm H2O in the patients, and 98.8 (±20.6) cm H2O in the control subjects.
3.3. Evoked responses
3.3.1. Entire Sample
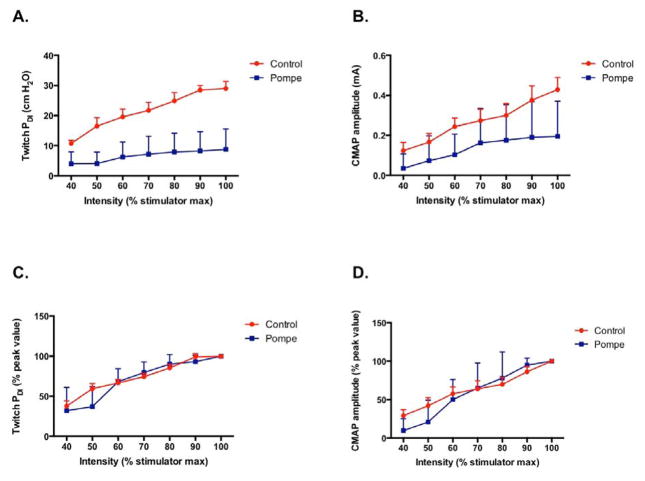
Absolute Twitch PDI output was lower in patients at every level of stimulation output, when compared to unaffected controls (p<0.001, Fig 1A). Likewise the absolute peak to peak CMAP amplitude was lower in patients (p<0.001, Fig 1B) and appeared to be more variable. In all subjects, the PDI and CMAP both increased significantly as the stimulator output settings were raised (p<0.001). Although the evoked responses were clearly reduced in the patient sample (Fig. 1A–B), the normalized Twitch PDI and CMAP recruitment (i.e., expressed as %peak) was indistinguishable between patients and controls (NS, Figs 1C and 1D).
Figure 1.
3.3.2 Phrenic neuromuscular function stratified by level of respiratory support
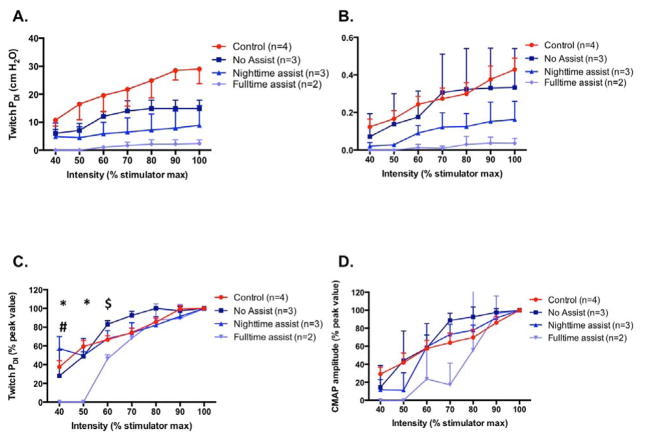
We separately compared stimulation responses between the no assist patients, the nighttime-assist patients, fulltime-assist patients, and the controls. A significant group effect was observed for both the absolute Twitch PDI (p<0.001) and CMAP amplitude (p<0.001) responses. Compared to the controls, Twitch PDI was significantly lower in no-assist patients, and continued to significantly worsen as the level of ventilatory assist increased (Fig 2A). In addition, the CMAP of nighttime- and fulltime-assist patients was significantly smaller than the no-assist patients and healthy controls (Fig 2B). There was a significant interaction between stimulation output and %peak Twitch PDI (p<0.001). In fulltime assist, Twitch PDI at 40% and 50%peak was lower than all of the other groups, and 60%peak Twitch PDI was lower than control subjects (Fig 2C). In nighttime assist, 40%peak Twitch PDI was lower than no-assist patients (Fig 2C). There was a significant group main effect for %peak CMAP (p<0.001), where responses were significantly lower in fulltime-assist users than the other patients or controls (Fig 2D). In general, Twitch PDI and CMAP responses of the full-time assist patients were severely diminished and could not be consistently detected at stimulation intensities <80% of maximum output.
Figure 2.
3.3.3. Timing of Evoked Responses
At the highest stimulator output, the onset latency of the CMAP response did not differ between the patients (6.9 ±1.5 msec) and controls (6.1 ±2.6 msec, p=0.54). While CMAP duration varied among the patients (42.8 ±20.1 msec), it did not significantly differ from the controls (35.2 ±6.2 msec, p=0.48).
3.4. Associations between Evoked and Voluntary Responses
Maximal Twitch PDI was significantly and positively correlated with FVC (r=0.862, p<0.01) and MIP (r=0.768, p<0.05). Additionally, the correlation between FVC and MIP was highly significant (r=0.935, p<0.005). Maximal CMAP amplitude did not correlate with Twitch PDI or any of the clinical tests.
4.0. Discussion
This experiment revealed several novel findings regarding evoked diaphragm motor responses in later-onset Pompe disease. First, significant decreases in Twitch PDI among all patients indicated diaphragm muscle fiber dysfunction was present even in independently breathing patients with mild clinical symptoms. Second, the preservation of CMAP amplitude distinguished independently-breathing patients from those with clinically significant hypoventilation who required nighttime or daytime support.
Progressive increases in stimulation intensity elicited similar rates of increase in CMAP and Twitch PDI in the pooled patient sample and controls, when expressed as %peak response. The normalized data from the %peak recruitment curve indicates that the functioning diaphragm motor units in the Pompe patients were recruited in a normal manner. However, the clearly reduced raw CMAP and Twitch PDI output is suggestive of a reduced number of functional diaphragm motor units and/or loss of phrenic motoneurons, a finding supported by studies in murine Pompe (DeRuisseau et al., 2009). These findings differ somewhat from the normalized m-wave recruitment defects recorded in the tibialis anterior muscle of ambulatory adults with Pompe, where the amplitude and rate of increase in m-wave stimulation trended lower in patients than in healthy controls (Corti et al., 2015). It was concluded that patients had fewer available motor units in the tibialis anterior and recruited the remaining motor units more slowly than unaffected controls. We speculate that differences in duty cycle, fiber composition, and susceptibility to atrophy between the diaphragm and tibialis anterior muscles may have contributed to these distinctions in motor unit recruitment.
When the patients were stratified according to ventilator use, other differences emerged. Independently breathing patients had clinically significant reductions in absolute Twitch PDI (Mills et al., 1996), yet retained a robust CMAP response to stimulation that was similar to the control subjects. These findings are in agreement with a proposed model of the critical periods leading to ventilatory failure in Pompe (Fuller et al., 2013). In this conceptual model, both neural and muscle tissue are susceptible to glycogen accumulation, but in milder dysfunction, impaired respiratory muscle function can be initially met by compensatory increases in neural drive. The strong correlations between Twitch PDI (Prigent et al., 2012), but not CMAP, and clinical estimates of respiratory muscle strength, point to a reconfiguration of control to preserve independent ventilation. A reorganization of respiratory output to engage accessory ventilatory muscles can also mitigate sleep hypoventilation (Bennett et al., 2004).
With disease progression, neural compensation is inadequate to overcome ventilatory muscle contractile dysfunction, and external support will be required to maintain ventilation. In patients who required fulltime assistance, %peak Twitch PDI and CMAP responses were profoundly dampened and not consistently achieved at the lowest levels of stimulation. In other words, patients with severe Pompe disease and daytime hypoventilation had an impaired ability to recruit the remaining functional motor units with progressively greater levels of stimulation.
There are recognizable limitations to the study design. The sample size was restricted by the rarity of Pompe disease, and confirmatory studies are needed in a larger patient sample. While surface EMG responses to magnetic stimulation are consistent (Martin et al., 2009), they yield less precision than esophageal or intramuscular electrodes, and therefore interpretations of absolute CMAP amplitude must be interpreted with caution. Pulmonary function tests revealed a restrictive pattern of pulmonary dysfunction in patients, which was consistent with their diagnosis of neuromuscular disease. Further study should also examine the influences of lung volume and diffusion capacity on the evoked neuromuscular responses.
In conclusion, these findings illustrate diaphragm pressure generation was reduced in all patients, even those with relatively mild clinical symptoms. Distinctions in phrenic neuromuscular function, as measured by CMAP amplitude, were noted between independently breathing patients and those who used external ventilatory support. This suggests that neural/mixed neuromuscular involvement yielded clinically significant changes in ventilator reliance. Normalized recruitment was similar in patients and healthy controls, indicating that a fewer number of motor units rather than an inability to activate motor units as a potential etiology. It may be useful to compare mechanisms of ventilatory insufficiency in other neuromuscular disease populations, and further studies of the effects of therapeutic interventions on phrenic motor dysfunction are needed for the future.
Highlights.
Transdiaphragmatic pressure (PDI) was lower in all patients, compared to controls.
CMAP amplitude was lower in patients who used external ventilatory support.
PDI but not CMAP correlated to clinical estimates of respiratory muscle function.
The results point to a reconfiguration of phrenic output to preserve ventilation.
Acknowledgments
The authors express their gratitude to the Acid Maltase Deficiency Association for supporting this project. DDF and BJB received support from NIH 2R01HD052682-06A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett JR, Dunroy HM, Corfield DR, Hart N, Simonds AK, Polkey MI, Morrell MJ. Respiratory muscle activity during REM sleep in patients with diaphragm paralysis. Neurology. 2004;62:134–137. doi: 10.1212/01.wnl.0000101719.84675.e0. [DOI] [PubMed] [Google Scholar]
- Corti M, Smith BK, Falk DJ, Lawson LA, Fuller DD, Subramony SH, Byrne BJ, Christou EA. Altered activation of the tibialis anterior in individuals with Pompe disease: Implications for motor unit dysfunction. Muscle Nerve. 2015;51:877–883. doi: 10.1002/mus.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuisseau LR, Fuller DD, Qiu K, DeRuisseau KC, Donnelly WH, Jr, Mah C, Reier PJ, Byrne BJ. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc Natl Acad Sci U S A. 2009;106:9419–9424. doi: 10.1073/pnas.0902534106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, ElMallah MK, Smith BK, Corti M, Lawson LA, Falk DJ, Byrne BJ. The respiratory neuromuscular system in Pompe disease. Respir Physiol Neurobiol. 2013;189:241–249. doi: 10.1016/j.resp.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassardjian CD, Engel AG, Sorenson EJ. Electromyographic findings in 37 patients with adult-onset acid maltase deficiency. Muscle Nerve. 2015;51:759–761. doi: 10.1002/mus.24620. [DOI] [PubMed] [Google Scholar]
- Mah CS, Falk DJ, Germain SA, Kelley JS, Lewis MA, Cloutier DA, DeRuisseau LR, Conlon TJ, Cresawn KO, Fraites TJ, Jr, Campbell-Thompson M, Fuller DD, Byrne BJ. Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease. Mol Ther. 2010;18:502–510. doi: 10.1038/mt.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Hudson AL, Gandevia SC, Taylor JL. Reproducible measurement of human motoneuron excitability with magnetic stimulation of the corticospinal tract. J Neurophysiol. 2009;102:606–613. doi: 10.1152/jn.91348.2008. [DOI] [PubMed] [Google Scholar]
- Mills GH, Kyroussis D, Hamnegard CH, Polkey MI, Green M, Moxham J. Bilateral magnetic stimulation of the phrenic nerves from an anterolateral approach. Am J Respir Crit Care Med. 1996;154:1099–1105. doi: 10.1164/ajrccm.154.4.8887614. [DOI] [PubMed] [Google Scholar]
- Pellegrini N, Laforet P, Orlikowski D, Pellegrini M, Caillaud C, Eymard B, Raphael JC, Lofaso F. Respiratory insufficiency and limb muscle weakness in adults with Pompe’s disease. Eur Respir J. 2005;26:1024–1031. doi: 10.1183/09031936.05.00020005. [DOI] [PubMed] [Google Scholar]
- Prigent H, Orlikowski D, Laforet P, Letilly N, Falaize L, Pellegrini N, Annane D, Raphael JC, Lofaso F. Supine volume drop and diaphragmatic function in adults with Pompe disease. Eur Respir J. 2012;39:1545–1546. doi: 10.1183/09031936.00169011. [DOI] [PubMed] [Google Scholar]
- Verin E, Straus C, Demoule A, Mialon P, Derenne JP, Similowski T. Validation of improved recording site to measure phrenic conduction from surface electrodes in humans. J Appl Physiol (1985) 2002;92:967–974. doi: 10.1152/japplphysiol.00652.2001. [DOI] [PubMed] [Google Scholar]