Abstract
Podocytes are unique, highly specialized, terminally differentiated cells that are integral components of the kidney glomerular filtration barrier. Podocytes are vulnerable to a variety of injuries and in response they undergo a series of changes ranging from hypertrophy, autophagy, dedifferentiation, mesenchymal transition and detachment to apoptosis, depending on the nature and extent of the insult. Emerging evidence indicates that Wnt/β-catenin signalling has a central role in mediating podocyte dysfunction and proteinuria. Wnts are induced and β-catenin is activated in podocytes in various proteinuric kidney diseases. Genetic or pharmacologic activation of β-catenin is sufficient to impair podocyte integrity and causes proteinuria in healthy mice, whereas podocyte-specific ablation of β-catenin protects against proteinuria after kidney injury. Mechanistically, Wnt/β-catenin controls the expression of several key mediators implicated in podocytopathies, including Snail1, the renin–angiotensin system and matrix metalloproteinase 7. Wnt/β-catenin also negatively regulates Wilms tumour protein, a crucial transcription factor that safeguards podocyte integrity. Targeted inhibition of Wnt/β-catenin signalling preserves podocyte integrity and ameliorates proteinuria in animal models. This Review highlights advances in our understanding of the pathomechanisms of Wnt/β-catenin signalling in mediating podocyte injury, and describes the therapeutic potential of targeting this pathway for the treatment of proteinuric kidney disease.
Introduction
Proteinuria often occurs in the early stages of many forms of primary glomerular diseases.1 The presence of excess proteins in the urine is not only a surrogate marker of kidney damage but also contributes to the development and progression of chronic kidney disease (CKD).1,2 A large body of evidence indicates that proteinuria in most circumstances results from defective glomerular filtration and represents a clinical manifestation of structural and functional impairments to the glomerular filtration barrier.2,3
The glomerular filtration barrier consists of fenestrated endothelium, the glomerular basement membrane (GBM) and epithelial podocyte foot processes. Although the GBM and endothelium are important in establishing size-selective filtration, extensive studies demonstrate that podocyte injury and/or dysfunction has a fundamental role in the pathogenesis of proteinuria in the vast majority of patients with CKD.1,2,4-7 Podocytes are highly specialized, terminally differentiated visceral epithelial cells that reside on the GBM outside the glomerular capillaries.8 Podocyte foot processes interdigitate with their neighbouring counterparts and form a filtration slit that is connected by a slit diaphragm.9 Several proteins associated with the slit diaphragm, such as nephrin and podocin, are of critical importance in establishing the glomerular filtration barrier. Mutations or deletion of the genes that encode these proteins are associated with the development of proteinuria in animal models and in patients with congenital nephrotic syndrome.3 However, mutations in slit diaphragm-associated proteins are extremely rare in most common forms of CKD such as diabetic nephropathy. In this context, a better understanding of the pathogenesis of podocyte injury and/or dysfunction that occurs in the vast majority of patients with acquired CKDs is essential to develop an effective therapy for proteinuria.
Over the past 6 years, mounting evidence has suggested a critical role for dysregulated Wnt/β-catenin signalling in promoting podocyte injury and/or dysfunction and in contributing to the pathogenesis of proteinuria.10-12 Studies indicate that activation of Wnt/β-catenin signalling causes podocyte dedifferentiation and mesenchymal transition, which impairs podocyte integrity and disrupts the glomerular filtration barrier, leading to proteinuria. In this Review, we discuss the regulation, function and mechanisms of Wnt/β-catenin signalling in mediating podocyte injury. We further propose that targeting this signalling pathway might provide a novel and effective strategy for the treatment of proteinuric CKD.
Podocyte responses after injury
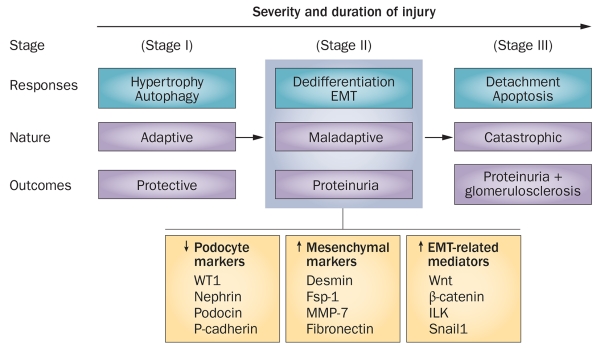
Podocytes are vulnerable to a variety of injurious stimuli such as hyperglycaemia, adriamycin, puromycin, transforming growth factor β (TGF-β) and angiotensin II.13-17 In response to these injurious stimuli, podocytes can undergo a spectrum of changes that may involve hypertrophy, autophagy, dedifferentiation, mesenchymal transition, mitotic catastrophe, detachment and apoptosis.18-21 Not all of these responses, however, lead to a defect in glomerular filtration and result in proteinuria. Depending on the severity and duration of the injury, podocyte responses can be classified into three stages: adaptive, maladaptive and catastrophic,19 each with vastly different consequences (Figure 1).
Figure 1.
The spectrum of podocyte responses after injury. Podocytes respond to injurious stimuli in different ways, depending on the severity and duration of the injury. These responses include hypertrophy, autophagy, dedifferentiation, EMT, detachment and apoptosis. The nature and consequences of these responses are quite different. The processes of hypertrophy and autophagy (stage I) are adaptive and protective. By contrast, the processes of EMT and dedifferentiation (stage II) are maladaptive and can lead to proteinuria, whereas the processes of detachment and apoptosis (stage III) are catastrophic and lead to proteinuria and glomerulosclerosis. The maladaptive changes of podocytes after injury are consistent with the characteristic features of EMT, in which podocytes lose their podocyte-specific markers and gain markers of mesenchymal cells. Abbreviations: EMT, epithelial-to-mesenchymal transition; Fsp-1, fibroblast-specific protein 1; ILK, integrin-linked kinase; MMP-7, matrix metalloproteinase-7; WT1, Wilms tumour protein.
The adaptive response
Podocytes initially respond to an injury by under going hypertrophy and/or autophagy. These responses are adaptive and/or protective attempts to prevent the catastrophic consequence of injury. Although podocytes are terminally differentiated cells with little proliferative capacity, they can undergo hypertrophy by increasing their size to compensate for loss of function. In a rat model of diphtheria toxin-induced podocyte depletion, loss of up to 20% of podocytes caused only transient proteinuria with no effect on renal function,22 suggesting that the glomerular filtration barrier endures temporary impairment after podocyte depletion, but can be quickly repaired and restored to full functionality, presumably by increasing the size of the remaining podocytes.
An increasing number of studies also show that podocytes have a high basal level of autophagy, a lysosomal-mediated self-degradation process that clears unwanted or dysfunctional cellular components under normal physiological conditions.21 Podocyte injury promotes auto phagy,16 whereas inhibition of autophagy induces podocyte apoptosis and aggravates glomerular disease and ageing-related nephropathy.23-25 Induction of auto phagy could therefore be a protective response to safeguard podocytes and minimize damage after injury.
The maladaptive response
In the event of progressive or severe injury, podocytes undergo dedifferentiation and mesenchymal transition, resulting in loss of highly specialized podocyte features, such as podocyte-specific markers, and the acquisition of new mesenchymal markers. These phenotypic changes are consistent with the process of epithelial-to-mesenchymal transition (EMT), which has a critical role in organ development, cancer metastasis and tissue fibrogenesis.19,26 Despite their unique morphology, podocytes are generally considered to be specialized epithelia that possess unique subsets of epithelial proteins. Following injury, such as that which occurs following treatment with TGF-β1, podocytes lose expression of nephrin, podocin, ZO-1 and P-cadherin, and gain new markers, such as desmin, fibroblast-specific protein 1 (Fsp1), matrix metalloproteinase 7 (MMP-7), and fibronectin.27-32 Concurrently, numerous regulators of EMT, such as Wnt/β-catenin, integrin-linked kinase (ILK) and Snail1, are upregulated specifically in podocytes (Figure 1).10,17,33 These in vitro changes, indicative of podocytes undergoing EMT, are also evident in vivo in mice overexpressing TGF-β134 and in mice with adriamycin-induced nephropathy,35 as well as in kidney biopsy samples from patients with proteinuric CKD, such as focal segmental glomerulosclerosis (FSGS) and diabetic nephropathy.10,36
It should be pointed out that whether the maladaptive changes that occur in podocytes after injury can be called EMT is hotly debated.27,37 Unlike tubular epithelial cells, podocytes undergo ‘physiologic EMT’ under normal non-pathologic conditions, by expressing vimentin and displaying a pericyte-like morphology.8 In this context, the maladaptive changes of podocytes seen after injury could be regarded as ‘pathologic EMT’, a process in which podocytes lose their specialized proteins and gain additional mesenchymal features. Some investigators refer to these maladaptive changes as ‘podocyte dedifferentiation’, while others use the term ‘podocyte activation’.37-41 These terms do not, however, accurately reflect the full picture of podocyte phenotypic alterations after injury, as ‘dedifferentiation’ often indicates a cell reverting to its precursor state whereas ‘activation’ usually describes the cellular activities initiated as a response to external stimuli. Regardless of the terms used, a broad consensus suggests that the maladaptive changes associated with podocyte injury will inevitably lead to destruction of the glomerular filtration barrier and result in the onset of proteinuria (Figure 1).
The catastrophic response
Prolonged and/or severe injury induces podocyte detachment and apoptosis, resulting in podocyte depletion, which exacerbates proteinuria and leads to glomerulosclerosis. In view of the very limited ability of podocytes to proliferate, podocyte loss, either via detachment or by apoptosis, will have catastrophic consequences (Figure 1). Podocytes adhere to the GBM only by their foot processes. Under pathophysiologic conditions they are constantly challenged by mechanical stressors, such as glomerular hypertension and hyperfiltration,42 which render the podocytes susceptible to loss by detachment from the GBM. Indeed, numbers of viable podocytes are increased in the urine in rat models of glomerular disease.43 Foot process effacement, which is often seen after glomerular injury, has been proposed to represent a protective attempt of podocytes to escape detachment.44 Notably, several proteins related to EMT, such as Wnt/β-catenin, ILK, Snail1 and Fsp1, promote cell motility, which facilitates podocyte detachment.17,36,45 Interestingly, 91% of urinary synaptopodin-positive podocytes in patients with diabetic nephropathy express Fsp1,36 suggesting that podocyte detachment is one of the ultimate consequences of the maladaptive response to injury.
Another mechanism that can lead to podocyte depletion is so-called mitotic catastrophe, a previously unrecognized form of podocyte loss.46,47 Terminally differentiated podocytes are not able to simultaneously complete mitosis and maintain their 3D actin cytoskeleton, owing to their unique morphology and ease of detachment from the GBM. After injury, podocytes may be prompted to proliferate under the influence of mitotic signals such as Wnt/β-catenin or its downstream target cyclin D1. When podocyte mitosis is attempted, however, cytokinesis cannot be completed efficiently, presumably because rearrangement of the actin cytoskeleton disrupts the integrity of podocyte foot processes, leading to aberrant podocyte mitosis, detachment and death, in a process known as mitotic catastrophe. The attempt of the mature podocyte to undergo mitosis could therefore accelerate podocyte loss and promote proteinuria.
The importance of podocyte depletion in proteinuria has been elegantly illustrated in a rat model of diphtheria toxin-induced podocyte depletion.22 In this model, loss of 21–40% of podocytes causes persistent proteinuria, whereas depletion of >40% of podocytes results in heavy proteinuria and glomerulosclerosis.22 Without any doubt, reduction of podocyte numbers in otherwise healthy kidneys induces proteinuria in experimental animal models.22,48-51 The extent of podocyte loss not only controls the onset of proteinuria, but also determines whether glomerular injury progresses to glomerulosclerosis (Figure 1).22
Podocyte depletion, however, is not required for proteinuria to occur in most circumstances. Extensive studies indicate that proteinuria typically precedes podocyte depletion, suggesting that podocyte loss, albeit sufficient, is not necessary for the onset of proteinuria. Experimental data show that podocyte apoptosis is an extremely rare event in common forms of proteinuric CKD, such as FSGS and diabetic nephropathy.14 Even in adriamycin-induced nephropathy—a classic model of FSGS characterized by podocyte depletion—podocyte apoptosis is negligible.35 It should be stressed that estimating podocyte number by staining for podocyte-specific markers such as Wilms tumour protein (WT1) and nephrin is not reliable and tends to underestimate podocyte density, because dedifferentiated podocytes lose expression of these markers.35 In this context, it is conceivable that podocyte dysfunction resulting from the maladaptive response to injury, rather than podocyte loss per se, might be the primary cause of proteinuria in most circumstances.
Of note, different podocytes in a given glomerulus can respond to the same injury in different ways. In other words, podocyte responses are not a synchronized event. Podocytes in the same setting can therefore undergo adaptive, maladaptive and catastrophic responses; however, as the severity and duration of the injury increase, the primacy of the podocyte response as a group will shift from an adaptive to catastrophic pathway (Figure 1).
Wnt/β-catenin and podocyte dysfunction
Podocyte EMT, the maladaptive response after injury, leads to impaired integrity of the glomerular filtration barrier and proteinuria. In this context, identification of the key mediators that regulate podocyte EMT is of utmost importance to elucidate the mechanism underlying proteinuria and for the design of effective therapeutic strategies. Emerging data suggest a crucial role for Wnt/β-catenin signalling—a developmental signalling pathway that is vital for cell fate determination during development52—in the pathogenesis of proteinuria.53-55
The Wnt/β-catenin signal cascade has an essential role in organogenesis, tissue homeostasis and human disease.56-59 Wnts are a family of 19 distinct secretory proteins. Upon binding to their cell membrane receptor, Frizzled, and co-receptors, low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6), Wnts induce a series of downstream signalling events involving Disheveled, axin, adenomatosis polyposis coli (APC) and glycogen synthase kinase (GSK)-3β, resulting in dephosphorylation of β-catenin. This series of events stabilizes and activates β-catenin, stimulating its translocation to the nuclei, where it binds to T cell factor (TCF)/lymphoid enhancer-binding factor (LEF) to promote the transcription of Wnt target genes.57 Wnt/β-catenin signalling is tightly regulated by secreted antagonists of Wnt signalling, such as soluble Frizzled-related protein (sFRP), Wnt inhibitory factor (WIF) and Dickkopf (DKK) proteins.56,57 Klotho, an anti-ageing protein that is highly expressed in kidney tubular epithelia, is also an endogenous Wnt antagonist.60
Mounting evidence indicates that Wnt/β-catenin signalling has a pivotal role in promoting podocyte injury and dysfunction, thereby contributing to the patho genesis of proteinuria. Wnt/β-catenin signalling is activated in a wide variety of animal models of proteinuric kidney diseases induced by hyperglycaemia, adriamycin, subtotal renal ablation, chronic angiotensin II infusion, protein overload and HIV.10-12,15,61-64 Both Wnt and β-catenin are specifically activated in glomerular podocytes from patients with FSGS and diabetic nephro pathy, supporting the clinical relevance of this signalling pathway to the pathogenesis of human proteinuric kidney dis orders.10,11 Of note, activation of Wnt/β-catenin signalling clearly precedes the onset of proteinuria in mouse model of adriamycin-induced nephropathy, suggesting a causative role for Wnt/β-catenin in proteinuria.10 In vivo expression of Wnt1 consistently aggravates podocyte injury and proteinuria in adriamycin-induced nephro pathy,10 and activation of Wnt/β-catenin signalling also mediates TGF-β1-induced podocytopathy and proteinuria in vivo.34 In cultured podocytes in vitro, activation of β-catenin represses nephrin expression, indicating that this signalling pathway specifically disrupts the slit diaphragm of the glomerular filter.10
Genetic approaches to induce either the gain or loss of Wnt/β-catenin signalling have unambiguously confirmed a crucial role for this pathway in mediating podocytopathy and proteinuria. Mice with podocyte-specific expression of stabilized β-catenin develop albuminuria and exhibit increased susceptibility to glomerular injury,11 suggesting that podocyte-specific activation of β-catenin signalling is sufficient to cause proteinuria in vivo. Similarly, pharmacologic activation of β-catenin in podocytes by administration of the GSK-3β inhibitor lithium chloride triggers foot process effacement and onset of proteinuria.10 Furthermore, two independent groups have reported that mice with podocyte-specific ablation of β-catenin are protected against albuminuria following adriamycin-induced injury.10,12 In addition, blockade of Wnt/β-catenin with the Wnt antagonist DKK1 reduces proteinuria and podocyte lesions after administration of adriamycin or angiotensin II, and in a mouse model of HIV-associated nephropathy (HIVAN).10,15,64 These observations undoubtedly underscore a role for Wnt/β-catenin signalling in mediating podocyte dysfunction and proteinuria.
Of note, β-catenin can be activated by signals other than Wnt. One upstream regulator of β-catenin is ILK, which is itself upregulated by a variety of injurious stimuli such as adriamycin, puromycin, high glucose levels, TGF-β1 and angiotensin II.17,65 Although controversy exists as to whether ILK is a true kinase,66,67 upregulation of ILK is reported to lead to GSK-3β phosphorylation and β-catenin stabilization in many cell types including podocytes. ILK is also upregulated in glomerular podocytes in mouse models and in biopsy samples from patients with proteinuric CKD, and its inhibitor ameliorates proteinuria in mice with adriamycin-induced injury.17
Targets and mechanisms of Wnt/β-catenin
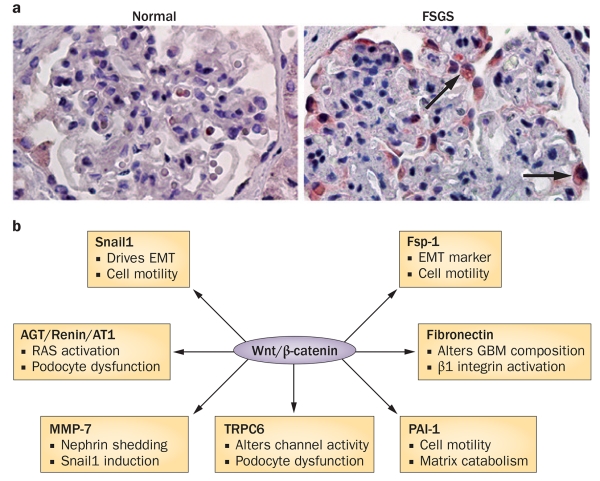
Wnt/β-catenin signalling elicits its biological activities by inducing the expression of target genes, such as those that encode Snail1, Fsp-1 and MMP-7. Active β-catenin translocates to the nucleus, binds to TCF/LEF transcription factors and recruits co-activators, including CREB-binding protein (CBP) or its closely related protein p300, to form a transcriptionally active complex that drives the expression of target genes. Over the past 6 years, novel transcriptional targets of Wnt/β-catenin have been identified in podocytes (Figure 2). Improved understanding of the role of these targets will help to explain how activation of Wnt/β-catenin signalling leads to podocyte dysfunction.
Figure 2.
Wnt/β-catenin signalling is activated in the injured kidney and exerts its effects by inducing transcription of target genes. a | Immunohistochemical staining shows podocyte-specific induction of Wnt1 protein (arrows) in a kidney biopsy sample from a patient with FSGS. b | Targets of Wnt/β-catenin that are involved in pathways of podocyte injury and/or dysfunction. Target genes include those that encode Snail1, components of the RAS, MMP-7, TRPC6, Fsp-1, PAI-1 and fibronectin, with various effects on other pathways and cell function. Abbreviations: AGT, angiotensinogen; AT1, angiotensin II type 1 receptor; EMT, epithelial-to-mesenchymal transition; FSGS, focal segmental glomerulosclerosis; Fsp1, fibroblast-specific protein 1; GBM, glomerular basement membrane; MMP-7, matrix metalloproteinase 7; PAI-1, plasminogen activator inhibitor-1; RAS, renin–angiotensin system; TRPC6, transient receptor potential channel 6. Permission for part a obtained from the American Society of Nephrology © Dai, C. et al. J. Am. Soc. Nephrol. 20, 1997-2008 (2009).
Snail1
Activation of Wnt/β-catenin in glomerular podocytes induces the expression of Snail1, a zinc finger transcription repressor that has a key role in driving EMT.68-71 During embryologic development, Snail1 is downregulated when mesenchymal cells differentiate into epithelium.71 Snail1 is well known for its ability to disrupt epithelial cell-cell adhesion by repressing expression of E-cadherin,19,26,71 and also induces inhibitor of differenti ation 1 (Id1), a transcription antagonist that has a critical role in facilitating EMT and renal inflammation.72
Two distinct mechanisms account for the induction of Snail1 by Wnt/β-catenin in podocytes.34 Forced expression of constitutively active β-catenin induces Snail1 mRNA expression, suggesting that Snail1 is transcriptional target of Wnt signalling.10,73 In addition, Snail1 is also regulated post-translationally by GSK-3β,74 the kinase that governs β-catenin stability. Therefore, inhib ition of GSK-3β by Wnt signalling simultaneously stabilizes both β-catenin and Snail1 proteins. These findings suggest that Wnt/β-cateinin signalling possesses an extraordinary ability to induce both Snail1 mRNA and Snail1 protein expression via dual mechanisms in podocytes.
Snail1 acts as a transcriptional repressor and specifically inhibits the expression of nephrin, a key protein associated with the podocyte slit diaphragm.10,33,75,76 In vitro studies demonstrate that Snail1 binds E-box motifs in a specific segment of Nphs1, the gene that encodes nephrin. The binding of Snail1 to Nphs1 represses the transcription of Nphs1 mRNA and down-regulates levels of nephrin protein.33 These effects trigger podocyte dedifferentiation and EMT—maladaptive changes that impair integrity of the glomerular filtration barrier.29,31,32,77 Whether Snail1 can also regulate other proteins associated with the slit diaphragm besides nephrin remains to be investigated. Nevertheless, the induction of Snail1 by Wnt/β-catenin could contribute to the mechanism by which hyperactive Wnt signalling impairs podocyte function and perturbs the integrity of the slit diaphragm under pathological conditions.
The renin–angiotensin system
The renin–angiotensin system also has a critical role in regulating podocyte behaviour, glomerular hypertension and kidney function. Although the renin–angiotensin system is generally considered to be a hormonal system in which the expression of its different components are regulated in multiple distinct organs such as the liver, kidney and lung, local activation of the intrarenal renin–angiotensin system is important in the induction of proteinuria and kidney damage.78 Podocytes express many components of the renin–angiotensin system including angiotensinogen, renin, angiotensin II type 1 receptor and the renin receptor.79,80 Crucial functions of podocytes, such as cytoskeletal organization, contraction, autophagy and apoptosis are regulated by angiotensin II, the principal effector of the renin–angiotensin system.81-84 Blockade of the renin–angiotensin system with angiotensin-converting enzyme (ACE) inhibitors or AT1-receptor blockers (ARBs) is effective, albeit insufficient, in reducing proteinuria in patients with CKD.85,86
A 2015 study demonstrated that multiple genes involved in the renin–angiotensin system, including genes that encode angiotensinogen, renin, ACE, and angiotensin II type-1 and type-2 receptors, possess TCF/LEF binding sites in their promoter regions, and that Wnt/β-catenin directly controls their expression in vitro and in vivo.79 In cultured podocytes, β-catenin induces expression of genes that encode angiotensinogen, renin and angiotensin II type-1 receptor, indicating that these genes are novel targets of canonical Wnt signalling.79 Such a link between Wnt/β-catenin signalling and activation of the renin–angiotensin system holds immense promise for future therapeutic applications, as targeting Wnt/β-catenin signalling should, in theory, achieve greater thera peutic efficacy than current renin–angiotensin system blockade. Indeed, either transient or delayed treatment with ICG-001, a small-molecule inhibitor that blocks β-catenin-mediated gene transcription,73,87,88 reversed established proteinuria and kidney damage in a mouse model of adriamycin-induced nephropathy.79
The discovery of a link between Wnt/β-catenin signalling and the renin–angiotensin system provides novel insights into the mechanisms by which Wnt signalling causes podocytopathy, proteinuria and kidney damage. Angiotensin II stimulates the generation of reactive oxygen species, induces expression of TGF-β and activates NF-κB in kidney cells,82,85,89,90 implying that Wnt/β-catenin signalling could exert broad actions in the pathogenesis of proteinuric kidney disease. Notably, a study has shown that angiotensin II induces Wnt1 expression and activates β-catenin in podocytes and that DKK1 protects against proteinuria initiated by angiotensin II infusion.15 Therefore, Wnt/β-catenin and the renin–angiotensin system can mutually stimulate each other in podocytes, creating a vicious cycle that leads to progressive proteinuria.
Matrix metalloproteinase 7
MMP-7 is the major downstream target of Wnt/β-catenin signalling in the injured kidney.91 MMP-7, also known as matrilysin, is a secreted zinc and calcium-dependent endopeptidase that degrades a broad range of extracellular matrix substrates such as type IV collagen, laminin, fibronectin and entactin.92 MMP-7 also cleaves additional substrates such as cell-associated Fas ligand, promotes the release of tumour necrosis factor, and mediates E-cadherin ectodomain shedding. Expression of MMP-7 is transcriptionally regulated by Wnt/β-catenin in glomerular podocytes and kidney tubular cells in vitro.91 Expression of MMP-7 is induced in the injured kidney, and levels of MMP-7 correlated closely with renal Wnt/β-catenin signalling activity in various models of CKD and in kidney biopsy samples from patients with CKD.91 In fact, we have proposed that urinary MMP-7 levels could serve as a surrogate marker of renal Wnt/β-catenin activity and severity of kidney dysfunction in CKD.91
Of particular interest, we found that MMP-7 proteolytically degrades nephrin in an ex vivo glomerular mini-organ culture, suggesting that it can cause ectodomain shedding of nephrin from the podocyte (Y. Liu, unpublished work). The proteolytic effect of MMP-7 is dependent on its enzymatic activity. MMP-7 also induces expression of Snail1 in podocytes—an effect that is possibly secondary to nephrin shedding. Mice with genetic ablation of MMP-7 are protected against podocyte injury and proteinuria after injection of adriamycin (Y. Liu, unpublished work). MMP-7 could therefore represent another important downstream effector of Wnt/β-catenin signalling that mediates its pathogenic actions in proteinuric CKD.
TRPC6
Wnt/β-catenin signalling might also cause podocyte dysfunction by inducing expression of the canonical transient receptor potential cation channel 6 (TRPC6), a calcium channel that is expressed in podocytes.84 Mutations in TRPC6 are associated with familial forms of nephrotic syndrome and FSGS,84,93-95 and TRPC6 expression is increased in acquired glomerular disease.84 Transgenic mice with podocyte-specific overexpression of TRPC6 develop albuminuria,95 whereas TRPC6-deficient mice are resistant to proteinuria after chronic infusion of angiotensin.94 High glucose levels upregulate TRPC6 expression in podocytes in a Wnt/β-catenin-dependent fashion, whereas DKK1 blocks induction of TRPC6 expression.96 The induction of TRPC6 could therefore be one explanation as to how Wnt/β-catenin signalling causes podocyte injury and proteinuria.96
Other targets
The expression of several other genes that could be implicated in podocyte dysfunction is also controlled by Wnt/β-catenin signalling. Studies show that plasminogen activator inhibitor-1 (PAI-1) and fibronectin are down-stream targets of Wnt/β-catenin, as is Fsp1 (also known as S100A4).97,98 PAI-1, fibronectin and Fsp1 are induced in podocytes in patients and animal models of proteinuric kidney disease.35,36 Fibronectin, a major interstitial matrix component, is mainly produced by mesenchymal cells such as fibroblasts. Increased fibronectin production by maladapted podocytes would alter the molecular composition of the GBM, thereby further perturbing podocyte behaviour. The induction of Fsp1 in podocytes in response to Wnt/β-catenin signalling is particularly intriguing because this protein often serves as a mesenchymal marker in EMT studies. Functionally, both PAI-1 and Fsp1 promote cell motility, consistent with the view that increased podocyte motility is associated with proteinuria.13,99 Interestingly, a study showed that 86% of podocytes in the urine sediment of patients with proteinuric kidney disease express Fsp1,36 suggesting that detachment could be the ultimate fate of podocytes following the phenotypic alterations mediated by Wnt/β-catenin signalling.
The most studied targets of Wnt/β-catenin are cyclin D1 and Myc, two proteins involved in cell cycle regulation and cell proliferation.56,59 Whether these proteins are also induced in glomerular podocytes in diseased kidneys is poorly understood. The possibility exists that after induction of cyclin D1 and undergoing cell division, podocytes detach and/or undergo apoptosis due to the process of mitotic catastrophe. This scenario would make the in vivo detection of cyclin D1 induction in podocytes extremely difficult. Clearly, this area deserves further investigation.
Interplay between Wnt/β-catenin and WT1
The phenotype, morphology and function of mature podocytes in adult kidneys are controlled by their unique transcription programmes. WT1 is a key transcription factor with a fundamental role in maintaining the differentiated state of podocytes. WT1 is exclusively expressed in glomerular podocytes in adult kidneys.100 As a zinc finger transcription factor, WT1 can bind to the transcriptional co-activator CBP and drive the expression of a host of podocyte-specific genes, such as nephrin, podocin and podocalyxin.35,101-104 WT1 is essential for normal embryonic kidney development, and genetic deletion and mutations in WT1 cause severe kidney disorders characterized by glomerular lesions, proteinuria and FSGS.105-107
Loss of WT1 is a hallmark of proteinuric kidney diseases; however, the mediators and mechanisms responsible for WT1 depletion in diseased kidneys are largely unknown. One report indicated involvement of micro-RNA-193a in WT1 depletion;108 however, a 2015 study showed that downregulation of WT1 is mediated by Wnt/β-catenin signalling.35 In mice with adriamycin-induced nephropathy, loss of Wt1 does not result from reduced expression of Wt1 mRNA or a loss of W1-expressing podocytes,35 rather, it is mainly caused by an increase in the degradation of Wt1 protein through the ubiquitin-mediated, proteasome-dependent pathway.35 In agreement with this finding, activation of Wnt/β-catenin signalling in cultured podocytes had no effect on Wt1 mRNA expression but markedly suppressed Wt1 protein levels. This downregulation of Wt1 protein by Wnt/β-catenin is blocked in the presence of proteasome inhibitor.35 Although the molecular details as to how Wnt/β-catenin signalling leads to WT1 degradation remain elusive, Wnt/β-catenin most likely mediates expression of a WT1-specific ubiquitin ligase or some other target gene, which in turn induces WT1 degradation. Regardless of the mechanisms involved, these new findings suggest that Wnt/β-catenin targets a key transcription factor within podocytes for degradation, thereby leading to podocyte dysfunction.
Of note, loss of WT1 would, in turn, further amplify the transcriptional activity of β-catenin in the injured podocytes, as WT1 is a negative inhibitor of Wnt/β-catenin signalling.109-112 The mutual antagonistic activities of WT1 and β-catenin are most likely mediated by competitive binding to their common transcriptional co-activator CBP.35,110 As both WT1 and β-catenin require CBP to efficiently stimulate expression of their target genes, activation of either WT1 or β-catenin is predicted to compete for a limited pool of CBP within podocytes. In this regard, Wnt/β-catenin-mediated degradation of WT1 will further augment the action of β-catenin by liberating CBP from WT1 binding. Wnt/β-catenin activation and loss of WT1 would therefore form a vicious feed-forward cycle, leading to exacerbated podocyte injury.35
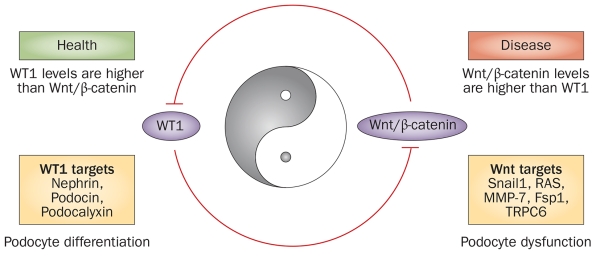
Emerging evidence suggests that Wnt/β-catenin and WT1 are two master regulators with opposing roles in podocyte biology. WT1 seems to predominantly drive and maintain podocyte differentiation by promoting the expression of podocyte-specific genes such as nephrin, podocin and podocalyxin, as well as by inhibiting Wnt/β-catenin signalling. Conversely, Wnt/β-catenin signalling orchestrates podocyte dedifferentiation by inducing Snail1, MMP-7, TRPC6 and Fsp1, as well as by activating the renin–angiotensin system and by triggering ubiquitin-mediated degradation of WT1. We therefore see Wnt/β-catenin and WT1 as functioning in a Yin and Yang relationship, antagonizing each other to control podocyte differentiation and dedifferentiation programmes (Figure 3).35 Taking the above findings together, it is conceivable that the reciprocal interplay between Wnt/β-catenin and WT1 dictates the state of podocyte health and disease in vivo.35
Figure 3.
The interplay between Wnt/β-catenin signalling and WT1 dictates podocyte health and disease. Wnt/β-catenin and WT1, acting in a Yin–Yang relationship, antagonize each other. These master regulators have opposing roles in podocyte biology; their ratio dictates the state of podocyte health (differentiation) and disease (dysfunction) in vivo. Abbreviations: Fsp-1, fibroblast-specific protein 1; MMP-7, matrix metalloproteinase 7; RAS, renin–angiotensin system; TRPC6, transient receptor potential channel 6; WT1, Wilms tumour protein.
Strategies to target Wnt/β-catenin
Given the importance of Wnt/β-catenin signalling in podocyte injury and proteinuria, it is not difficult to speculate that Wnt/β-catenin blockade could provide a novel strategy for therapeutic intervention in proteinuric CKD. Over the past 6 years, a variety of strategies to block this signalling pathway have been investigated in proteinuric CKD, with quite promising findings from preclinical studies (Table 1).
Table 1.
Strategies to target Wnt/β-catenin signalling in proteinuric kidney diseases
| Agent | Mechanisms of action | Disease model | Outcome | References |
|---|---|---|---|---|
| Klotho | Binds and sequesters Wnts | ADR nephropathy | Reduced proteinuria | Zhou et al.60 |
| Remnant kidney | Reduced proteinuria | Y. Liu, unpublished work | ||
| DKK1 | Binds and Inhibits LRP5/6 | ADR nephropathy | Reduced proteinuria | Dai et al.10 |
| Ang II infusion | Reduced proteinuria | Jiang et al.15 | ||
| HIVAN | Reduced proteinuria | Shkreli et al.64 | ||
| DKD | Increased proteinuria | Kato et al.11 | ||
| VDR agonist | Binds and sequesters β-catenin | ADR nephropathy | Reduced proteinuria | He et al.61 |
| ICG-001 | Binds CBP and disrupts β-catenin/CBP interaction |
ADR nephropathy | Reduced proteinuria | Zhou et al.79 |
| Remnant kidney | Reduced proteinuria | Y. Liu, unpublished work | ||
| Ang II infusion | Reduced proteinuria | Y. Liu, unpublished work |
Abbreviations: ADR, adriamycin; Ang II, angiotensin II; CBP, CREB-binding protein; DKD, diabetic kidney disease; DKK1, Dickkopf 1; HIVAN, HIV-associated nephropathy; LRP-5/6, low-density lipoprotein receptor-related proteins 5 and 6; VDR, vitamin D receptor.
Binding and sequestering Wnts
As many Wnt ligands are induced in the diseased kidney,10,113 therapeutic strategies to target individual Wnts are neither practical nor efficient. This theory is supported by a genetic study in which knockout of Wnt4 in mice had no impact on kidney disease progression.114 A strategy to block the actions of the majority of, if not all, Wnt ligands might therefore be necessary to effectively protect podocytes against injury and ameliorate proteinuria.
Klotho is an anti-ageing protein that is highly expressed in the kidney tubular epithelium under normal physiologic conditions.60 A 2013 study demonstrated that Klotho binds and sequesters Wnt ligands, thereby acting as an endogenous antagonist of Wnt signalling.60 Similar to findings in aged kidneys, kidneys from patients with CKD are in a state of Klotho deficiency.115-119 Notably, the kidney is the principal organ that contributes to Klotho levels in the circulation,120 and therefore depletion of Klotho in the diseased kidney would release suppression of Wnt/β-catenin signalling in many tissues including the glomerulus, leading to increased podocyte injury and proteinuria. In vitro, Klotho binds to multiple Wnts such as Wnt1, Wnt4 and Wnt7a and blocks Wnt-triggered activation of β-catenin. Supplementation of exogenous secreted Klotho by gene delivery in vivo preserves podocyte integrity and reduces proteinuria in mice with adriamycin-induced nephropathy.60 The therapeutic efficacy of exogenous Klotho has also been demonstrated in a remnant kidney model after subtotal renal ablation (Y. Liu, unpublished work). Exogenous Klotho, by virtue of its ability to bind and sequester Wnt ligands might, therefore, hold promise as a therapeutic agent for proteinuric kidney disease.
Blocking co-receptor activation
The canonical Wnt signalling pathway requires activation of the Wnt co-receptors LRP-5/6. DKK1, a secreted protein with two cysteine rich regions, interacts with LRP-5/6 and blocks their activation59 and has been used in a number of studies to disrupt canonical Wnt signalling. In mouse model of adriamycin-induced nephropathy, injections of DKK1 protected against podocyte dysfunction and proteinuria.10 DKK1 is also effective in reducing angiotensin II-induced podocytopathy and proteinuria in mice.15 The kidneys of humans and mice with HIVAN exhibit activation of Wnt signalling, and systemic expression of DKK1 in a mouse model of HIVAN results in marked normalization of podocytes, characterized by re-expression of differentiation markers and improved filtration barrier function.64
Controversy exists, however, regarding the exact role of DKK1 in podocyte injury and proteinuria. Kato and colleagues have reported that mice with podocyte-specific expression of DKK1 show increased susceptibility to diabetic kidney disease.11 The reason for this discrepancy remains unknown. One possibility is that DKK1 might have functions other than inhibiting canonical Wnt signalling. Consistent with this possibility, a 2013 study showed that DKK1 blocks myofibroblast activation by a mechanism that is independent of β-catenin inhibition.121
Sequestering and inhibiting β-catenin
The canonical pathway of all Wnt ligands converges on β-catenin activation, rendering β-catenin as an ideal target for therapeutic intervention. Podocyte-specific and tubule-specific knockout of the gene that encodes β-catenin does not cause any overt abnormality,10,12,122 suggesting that the β-catenin protein is functionally dispensable in the kidney under normal physiological conditions. These studies also imply that inhibition of β-catenin might have no or few adverse effects and could be an effective approach for the treatment of proteinuric CKD.
Studies show that following its activation by agonists such as paricalcitol, the vitamin D receptor (VDR) translocates to the nucleus, where it physically interacts with nuclear β-catenin and sequesters its ability to activate gene transcription.61 This observation is consistent with many studies showing that VDR activators are renoprotective in models of proteinuric CKD.123-125 In glomerular diseases, activation of VDR inhibits expression Wnt/β-catenin target genes such as Snail1 and TRPC6, prevents podocyte injury and reduces proteinuria.61,125 The VDR-mediated sequestration of β-catenin might therefore contribute to the renal protection of vitamin D analogues in proteinuric CKD.
Disrupting β-catenin–CBP interactions
As mentioned above, upon activation by Wnt, β-catenin translocates to the nucleus where it binds to the TCF/LEF family of transcription factors and recruits the coactivators CBP or p300. ICG-001, a β-turn bicyclic peptidomimetic, is a unique small-molecule that selectively binds to CBP and disrupts the interaction of β-catenin with CBP.73,87,88 Although CBP and p300 are considered to be indistinguishable in terms of their abilities to promote gene expression, evidence indicates that differential recruitment of the co-activators in fact determines the selective expression of subsets of Wnt target genes.87 β-catenin–p300 signalling is instrumental in initiating cellular differentiation, whereas β-catenin–CBP-driven gene transcription is critical for inducing EMT by controlling a battery of genes such as those that encode Snail1, MMP-7, fibronectin and Fsp1. By selectively disrupting the interaction of β-catenin with CBP, ICG-001 can inhibit the expression of these genes with promising outcomes.
Administration of ICG-001 can restore podocyte integrity and reduces proteinuria in mice with established kidney disease caused by adriamycin.79 Interestingly, even transient treatment with ICG-001 for 2 weeks is sufficient to block activation of the renin–angiotensin system and reduce proteinuria and kidney damage in this model.79 ICG-001 is also effective in protecting against podocyte injury and development of proteinuria in rat models of CKD induced by chronic infusion of angiotensin II or by subtotal renal ablation (Y. Liu, unpublished work). These preclinical results suggest that targeting Wnt/β-catenin by small-molecule inhibitors such as ICG-001 might be an effective treatment option for proteinuric CKD.
Clinical trials of targeting Wnt/β-catenin
Although the preclinical studies described above show promise for the use of targeting Wnt/β-catenin signalling in proteinuric kidney disease, clinical validation of this approach is required. Trials to assess the safety and efficacy of specific antagonists of Wnt/β-catenin for human proteinuric kidney diseases have not yet been initiated. Interestingly, a 2010 study showed that addition of paricalcitol to standard renin–angiotensin system blockade therapy reduces albuminuria in patients with diabetic nephropathy;126 however, whether the effect of paricalcitol was mediated by the VDR-mediated sequestration of β-catenin remains unknown.
Several obstacles to performing clinical trials of Wnt/β-catenin inhibitors in patients with CKD exist, including the need for more preclinical data, concerns with regard to the safety profile of such an approach, and a shortage of funding. Given the crucial role of Wnt/β-catenin signalling in the homeostasis of many organs, such as the intestine and haematopoietic system, potential adverse effects of targeting this signalling pathway would require careful consideration for any trial with vigilant monitoring of patients over the long term. Several Phase I and Phase II clinical trials using agents that target Wnt/β-catenin have now been initiated in the oncology field;87,127 these studies will provide important insights into the adverse effect profile of this approach.
Conclusions
Podocytes have a unique position in the glomerulus and are essential in establishing and maintaining an intact glomerular filtration barrier. These terminally differentiated cells are vulnerable to a variety of injuries and often undergo adaptive, maladaptive or catastrophic responses after insults (Figure 1). In the past few years, considerable advances have been made in identifying and delineating the signalling pathways that lead to podocyte dysfunction and proteinuria. Mounting experimental data demonstrate a pivotal role for Wnt/β-catenin signalling in triggering podocyte dysfunction in a variety of acquired forms of proteinuric CKD. By characterizing its target genes and pathways we now have much better understanding than ever before as to how hyperactive Wnt/β-catenin affects podocyte integrity. This knowledge has translated into several novel therapeutic strategies that have been assessed in the preclinical setting (Table 1).
The challenges that lie ahead to fully elucidate the biologic actions of Wnt/β-catenin in podocytes remain enormous, however. At this point, almost nothing is known about the relative contribution of each individual Wnt protein in podocyte biology and pathology. Given that 19 different Wnts are present in mammalian system and that many of them are upregulated in proteinuric CKD, defining the exact role of the different Wnts in proteinuria represents a daunting task. Furthermore, while canonical Wnt signalling has a clearly defined role in podocyte dysfunction, the potential effects of non-canonical Wnt pathways need to be carefully elucidated. Another area that deserves further investigation is the complex interplay between Wnt/β-catenin signalling and other traditional culprits in proteinuric CKD such as TGF-β and angiotensin II. Nevertheless, in view of the promising preclinical data in animal models obtained thus far, we are cautiously optimistic, and excited, by the prospect that targeting this pathway might offer a novel and effective therapy for the millions of patients worldwide with proteinuric kidney disease.
Key points.
-
■
Podocytes are susceptible to various glomerular injuries and undergo a series of adaptive, maladaptive or catastrophic responses, depending on the severity and duration of the insult
-
■
Wnt/β-catenin signalling is activated in glomerular podocytes in a wide variety of proteinuric kidney diseases; gain or loss-of-function studies have confirmed a role for Wnt/β-catenin signalling in mediating podocyte dysfunction and proteinuria
-
■
Wnt/β-catenin controls the transcription of a battery of target genes such as Snail1, MMP-7 and Fsp1, and mediates podocyte dedifferentiation and mesenchymal transition, thereby inducing podocytopathy and proteinuria
-
■
Wnt/β-catenin suppresses Wilms tumour protein, a key transcription factor that safeguards podocyte integrity through ubiquitin-mediated, proteasome-dependent protein degradation
-
■
Targeted inhibition of Wnt/β-catenin signalling by a variety of approaches preserves podocyte integrity, reduces proteinuria and ameliorates kidney damage
Acknowledgements
We apologize to our colleagues whose important findings could not be cited in this article due to space limitations. Reviews were often cited at the expense of original work. Our own work described in this Review was supported by the National Basic Research Program of China Grant 2012CB517700, National Science Foundation of China Grants 81130011 and 81370839, and NIH Grants DK064005 and DK091239.
Footnotes
Author contributions
Both authors contributed equally to researching the data for the article and to discussions of the content. Y.L. wrote the article. Both authors reviewed and/or edited the manuscript before submission.
Competing interests
The authors declare no competing interests.
References
- 1.Reiser J, Sever S. Podocyte biology and pathogenesis of kidney disease. Annu. Rev. Med. 2013;64:357–366. doi: 10.1146/annurev-med-050311-163340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas MC. Pathogenesis and progression of proteinuria. Contrib. Nephrol. 2011;170:48–56. doi: 10.1159/000324943. [DOI] [PubMed] [Google Scholar]
- 3.Grahammer F, Schell C, Huber TB. Molecular understanding of the slit diaphragm. Pediatr. Nephrol. 2013;28:1957–1962. doi: 10.1007/s00467-012-2375-6. [DOI] [PubMed] [Google Scholar]
- 4.Brinkkoetter PT, Ising C, Benzing T. The role of the podocyte in albumin filtration. Nat. Rev. Nephrol. 2013;9:328–336. doi: 10.1038/nrneph.2013.78. [DOI] [PubMed] [Google Scholar]
- 5.Mathieson PW. The podocyte as a target for therapies—new and old. Nat. Rev. Nephrol. 2012;8:52–56. doi: 10.1038/nrneph.2011.171. [DOI] [PubMed] [Google Scholar]
- 6.Menzel S, Moeller MJ. Role of the podocyte in proteinuria. Pediatr. Nephrol. 2011;26:1775–1780. doi: 10.1007/s00467-010-1725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat. Rev. Nephrol. 2009;5:463–468. doi: 10.1038/nrneph.2009.108. [DOI] [PubMed] [Google Scholar]
- 8.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu. Rev. Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grahammer F, Schell C, Huber TB. The podocyte slit diaphragm—from a thin grey line to a complex signalling hub. Nat. Rev. Nephrol. 2013;9:587–598. doi: 10.1038/nrneph.2013.169. [DOI] [PubMed] [Google Scholar]
- 10.Dai C, et al. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato H, et al. Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J. Biol. Chem. 2011;286:26003–26015. doi: 10.1074/jbc.M111.223164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heikkila E, et al. β-catenin mediates adriamycin-induced albuminuria and podocyte injury in the adult mouse kidneys. Nephrol. Dial. Transplant. 2010;25:2437–2446. doi: 10.1093/ndt/gfq076. [DOI] [PubMed] [Google Scholar]
- 13.Herman-Edelstein M, Weinstein T, Gafter U. TGFβ1-dependent podocyte dysfunction. Curr. Opin. Nephrol. Hypertens. 2013;22:93–99. doi: 10.1097/MNH.0b013e32835b4870. [DOI] [PubMed] [Google Scholar]
- 14.Dai C, Saleem MA, Holzman LB, Mathieson P, Liu Y. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int. 2010;77:962–973. doi: 10.1038/ki.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang L, et al. Calmodulin-dependent protein kinase II/cAMP response element-binding protein/Wnt/β-catenin signaling cascade regulates angiotensin II-induced podocyte injury and albuminuria. J. Biol. Chem. 2013;288:23368–23379. doi: 10.1074/jbc.M113.460394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma T, et al. High glucose induces autophagy in podocytes. Exp. Cell Res. 2013;319:779–789. doi: 10.1016/j.yexcr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang YS, et al. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 2010;78:363–373. doi: 10.1038/ki.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 2010;21:212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng C, et al. Podocyte autophagic activity plays a protective role in renal injury and delays the progression of podocytopathies. J. Pathol. 2014;234:203–213. doi: 10.1002/path.4382. [DOI] [PubMed] [Google Scholar]
- 21.Hartleben B, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wharram BL, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 23.Kume S, Yamahara K, Yasuda M, Maegawa H, Koya D. Autophagy: emerging therapeutic target for diabetic nephropathy. Semin. Nephrol. 2014;34:9–16. doi: 10.1016/j.semnephrol.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Hartleben B, Wanner N, Huber TB. Autophagy in glomerular health and disease. Semin. Nephrol. 2014;34:42–52. doi: 10.1016/j.semnephrol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Fang L, et al. Autophagy inhibition induces podocyte apoptosis by activating the proapoptotic pathway of endoplasmic reticulum stress. Exp. Cell Res. 2014;322:290–301. doi: 10.1016/j.yexcr.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am. J. Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, et al. Epithelial-to-mesenchymal transition in podocytes mediated by activation of NADPH oxidase in hyperhomocysteinemia. Pflugers Arch. 2011;462:455–467. doi: 10.1007/s00424-011-0981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boini KM, et al. Implication of CD38 gene in podocyte epithelial-to-mesenchymal transition and glomerular sclerosis. J. Cell. Mol. Med. 2012;16:1674–1685. doi: 10.1111/j.1582-4934.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai HY, et al. The roles of connective tissue growth factor and integrin-linked kinase in high glucose-induced phenotypic alterations of podocytes. J. Cell Biochem. 2012;113:293–301. doi: 10.1002/jcb.23355. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, et al. Mesangial medium from IgA nephropathy patients induces podocyte epithelial-to-mesenchymal transition through activation of the phosphatidyl inositol-3-kinase/Akt signaling pathway. Cell Physiol. Biochem. 2012;29:743–752. doi: 10.1159/000170949. [DOI] [PubMed] [Google Scholar]
- 32.Lv Z, et al. Rac1/PAK1 signaling promotes epithelial-mesenchymal transition of podocytes in vitro via triggering β-catenin transcriptional activity under high glucose conditions. Int. J. Biochem. Cell Biol. 2013;45:255–264. doi: 10.1016/j.biocel.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Matsui I, et al. Snail, a transcriptional regulator, represses nephrin expression in glomerular epithelial cells of nephrotic rats. Lab. Invest. 2007;87:273–283. doi: 10.1038/labinvest.3700518. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Dai C, Li Y, Liu Y. Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria. Kidney Int. 2011;80:1159–1169. doi: 10.1038/ki.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou L, et al. Mutual antagonism of Wilms’ tumor 1 and β-catenin dictates podocyte health and disease. J. Am. Soc. Nephrol. 2015;26:677–691. doi: 10.1681/ASN.2013101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi Y, et al. Epithelial-mesenchymal transition as an explanation for podocyte depletion in diabetic nephropathy. Am. J. Kidney Dis. 2009;54:653–664. doi: 10.1053/j.ajkd.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 37.May CJ, Saleem M, Welsh GI. Podocyte dedifferentiation: a specialized process for a specialized cell. Front. Endocrinol. 2014;5:148. doi: 10.3389/fendo.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan RJ, Liu Y. Arrestin(g) podocyte injury with endothelin antagonism. J. Am. Soc. Nephrol. 2014;25:423–425. doi: 10.1681/ASN.2013111230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buelli S, et al. β-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J. Am. Soc. Nephrol. 2014;25:523–533. doi: 10.1681/ASN.2013040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J. Clin. Invest. 2014;124:2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herman-Edelstein M, et al. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-β: a model for diabetic podocytopathy. Diabetes. 2011;60:1779–1788. doi: 10.2337/db10-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kriz W, Lemley KV. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J. Am. Soc. Nephrol. 2015;26:258–269. doi: 10.1681/ASN.2014030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu D, et al. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J. Am. Soc. Nephrol. 2005;16:1733–1741. doi: 10.1681/ASN.2005020159. [DOI] [PubMed] [Google Scholar]
- 44.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. The podocyte’s response to stress: the enigma of foot process effacement. Am. J. Physiol. Renal Physiol. 2013;304:F333–F347. doi: 10.1152/ajprenal.00478.2012. [DOI] [PubMed] [Google Scholar]
- 45.Kato H, Susztak K. Repair problems in podocytes: Wnt, Notch, and glomerulosclerosis. Semin. Nephrol. 2012;32:350–356. doi: 10.1016/j.semnephrol.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liapis H, Romagnani P, Anders HJ. New insights into the pathology of podocyte loss: mitotic catastrophe. Am. J. Pathol. 2013;183:1364–1374. doi: 10.1016/j.ajpath.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lasagni L, Lazzeri E, Shankland SJ, Anders HJ, Romagnani P. Podocyte mitosis—a catastrophe. Curr. Mol. Med. 2013;13:13–23. doi: 10.2174/1566524011307010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou LL, et al. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int. 2009;76:1148–1160. doi: 10.1038/ki.2009.322. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y. Advanced oxidation protein products: a causative link between oxidative stress and podocyte depletion. Kidney Int. 2009;76:1125–1127. doi: 10.1038/ki.2009.352. [DOI] [PubMed] [Google Scholar]
- 50.Tharaux PL, Huber TB. How many ways can a podocyte die? Semin. Nephrol. 2012;32:394–404. doi: 10.1016/j.semnephrol.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Tang Y, Howell DN, Ruiz P, Spurney RF. A novel mouse model of podocyte depletion. Nephron Exp. Nephrol. 2012;121:e10–e22. doi: 10.1159/000342369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grinstein M, Yelin R, Herzlinger D, Schultheiss TM. Generation of the podocyte and tubular components of an amniote kidney: timing of specification and a role for Wnt signaling. Development. 2013;140:4565–4573. doi: 10.1242/dev.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan RJ, Zhou D, Zhou L, Liu Y. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int. Suppl. 2014;4:84–90. doi: 10.1038/kisup.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao L, Wang M, Yang S, Liu F, Sun L. A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy. Biomed. Res. Int. 2013;2013:987064. doi: 10.1155/2013/987064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J. Pathol. 2013;229:221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 56.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 58.Saito-Diaz K, et al. The way Wnt works: components and mechanism. Growth Factors. 2013;31:1–31. doi: 10.3109/08977194.2012.752737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 2013;24:771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan RJ, et al. Extracellular superoxide dismutase protects against proteinuric kidney disease. J. Am. Soc. Nephrol. doi: 10.1681/ASN.2014060613. http://dx.doi.org/10.1681/ASN.2014060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou T, et al. Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia. 2012;55:255–266. doi: 10.1007/s00125-011-2314-2. [DOI] [PubMed] [Google Scholar]
- 64.Shkreli M, et al. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat. Med. 2012;18:111–119. doi: 10.1038/nm.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naves MA, et al. Podocyte Wnt/β-catenin pathway is activated by integrin-linked kinase in clinical and experimental focal segmental glomerulosclerosis. J. Nephrol. 2012;25:401–409. doi: 10.5301/jn.5000017. [DOI] [PubMed] [Google Scholar]
- 66.Lange A, et al. Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature. 2009;461:1002–1006. doi: 10.1038/nature08468. [DOI] [PubMed] [Google Scholar]
- 67.Wickstrom SA, Lange A, Montanez E, Fassler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 2010;29:281–291. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, et al. TGF-β-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci. Signal. 2014;7:ra91. doi: 10.1126/scisignal.2005304. [DOI] [PubMed] [Google Scholar]
- 69.Millanes-Romero A, et al. Regulation of heterochromatin transcription by Snail1/LOXL2 during epithelial-to-mesenchymal transition. Mol. Cell. 2013;52:746–757. doi: 10.1016/j.molcel.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 70.Zhang K, et al. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia de Herreros A, Baulida J. Cooperation, amplification, and feed-back in epithelial-mesenchymal transition. Biochim. Biophys. Acta. 2012;1825:223–228. doi: 10.1016/j.bbcan.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, Wen X, Liu Y. Tubular cell dedifferentiation and peritubular inflammation are coupled by the transcription regulator Id1 in renal fibrogenesis. Kidney Int. 2012;81:880–891. doi: 10.1038/ki.2011.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hao S, et al. Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 2011;22:1642–1653. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu ZC, et al. AKT/GSK-3β regulates stability and transcription of snail which is crucial for bFGF-induced epithelial-mesenchymal transition of prostate cancer cells. Biochim. Biophys. Acta. 2014;1840:3096–3105. doi: 10.1016/j.bbagen.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 75.Locatelli M, et al. Shiga toxin promotes podocyte injury in experimental hemolytic uremic syndrome via activation of the alternative pathway of complement. J. Am. Soc. Nephrol. 2014;25:1786–1798. doi: 10.1681/ASN.2013050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, et al. Nephrin preserves podocyte viability and glomerular structure and function in adult kidneys. J. Am. Soc. Nephrol. doi: 10.1681/ASN.2014040405. http://dx.doi.org/10.1681/ASN.2014040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li C, Siragy HM. High glucose induces podocyte injury via enhanced (pro)renin receptor-Wnt-β-catenin-snail signaling pathway. PLoS ONE. 2014;9:e89233. doi: 10.1371/journal.pone.0089233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wennmann DO, Hsu HH, Pavenstadt H. The renin-angiotensin-aldosterone system in podocytes. Semin. Nephrol. 2012;32:377–384. doi: 10.1016/j.semnephrol.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Zhou L, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 2015;26:107–120. doi: 10.1681/ASN.2014010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakoda M, Itoh H, Ichihara A. Podocytes as a target of prorenin in diabetes. Curr. Diabetes Rev. 2010;7:17–21. doi: 10.2174/157339911794273955. [DOI] [PubMed] [Google Scholar]
- 81.Hsu HH, et al. Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J. Mol. Med. 2008;86:1379–1394. doi: 10.1007/s00109-008-0399-y. [DOI] [PubMed] [Google Scholar]
- 82.Marquez E, Riera M, Pascual J, Soler MJ. Renin-angiotensin system within the diabetic podocyte. Am. J. Physiol. Renal Physiol. 2015;308:F1–F10. doi: 10.1152/ajprenal.00531.2013. [DOI] [PubMed] [Google Scholar]
- 83.Ortiz-Melo DI, Spurney RF. Special delivery: podocyte injury promotes renal angiotensin II generation from liver-derived angiotensinogen. Kidney Int. 2014;85:1009–1011. doi: 10.1038/ki.2013.440. [DOI] [PubMed] [Google Scholar]
- 84.Sonneveld R, et al. Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am. J. Pathol. 2014;184:1715–1726. doi: 10.1016/j.ajpath.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 85.Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J. Am. Soc. Nephrol. 2012;23:1917–1928. doi: 10.1681/ASN.2012040390. [DOI] [PubMed] [Google Scholar]
- 86.Lambers Heerspink HJ, de Borst MH, Bakker SJ, Navis GJ. Improving the efficacy of RAAS blockade in patients with chronic kidney disease. Nat. Rev. Nephrol. 2013;9:112–121. doi: 10.1038/nrneph.2012.281. [DOI] [PubMed] [Google Scholar]
- 87.Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henderson WR, Jr, et al. Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl Acad. Sci. USA. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santos PC, Krieger JE, Pereira AC. Renin-angiotensin system, hypertension, and chronic kidney disease: pharmacogenetic implications. J. Pharmacol. Sci. 2012;120:77–88. doi: 10.1254/jphs.12r03cr. [DOI] [PubMed] [Google Scholar]
- 90.Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp. Cell Res. 2012;318:1049–1056. doi: 10.1016/j.yexcr.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He W, et al. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J. Am. Soc. Nephrol. 2012;23:294–304. doi: 10.1681/ASN.2011050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and disease. Am. J. Physiol. Renal Physiol. 2012;302:F1351–F1361. doi: 10.1152/ajprenal.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chiluiza D, Krishna S, Schumacher VA, Schlondorff J. Gain-of-function mutations in transient receptor potential C6 (TRPC6) activate extracellular signal-regulated kinases 1/2 (ERK1/2) J. Biol. Chem. 2013;288:18407–18420. doi: 10.1074/jbc.M113.463059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eckel J, et al. TRPC6 enhances angiotensin II-induced albuminuria. J. Am. Soc. Nephrol. 2011;22:526–535. doi: 10.1681/ASN.2010050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krall P, et al. Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS ONE. 2010;5:e12859. doi: 10.1371/journal.pone.0012859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Z, Xu J, Xu P, Liu S, Yang Z. Wnt/β-catenin signalling pathway mediates high glucose induced cell injury through activation of TRPC6 in podocytes. Cell Prolif. 2013;46:76–85. doi: 10.1111/cpr.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He W, et al. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J. Biol. Chem. 2010;285:24665–24675. doi: 10.1074/jbc.M109.091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sack U, et al. S100A4-induced cell motility and metastasis is restricted by the Wnt/β-catenin pathway inhibitor calcimycin in colon cancer cells. Mol. Biol. Cell. 2011;22:3344–3354. doi: 10.1091/mbc.E10-09-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mundel P, Reiser J. Proteinuria: an enzymatic disease of the podocyte? Kidney Int. 2010;77:571–580. doi: 10.1038/ki.2009.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Venkatareddy M, et al. Estimating podocyte number and density using a single histologic section. J. Am. Soc. Nephrol. 2014;25:1118–1129. doi: 10.1681/ASN.2013080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang D, Li Y, Wu C, Liu Y. PINCH1 is transcriptional regulator in podocytes that interacts with WT1 and represses podocalyxin expression. PLoS ONE. 2011;6:e17048. doi: 10.1371/journal.pone.0017048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dong L, et al. Integration of cistromic and transcriptomic analyses identifies Nphs2, Mafb, and Magi2 as Wilms’ tumor 1 target genes in podocyte differentiation and maintenance. J. Am. Soc. Nephrol. doi: 10.1681/ASN.2014080819. http://dx.doi.org/10.1681/ASN.2014080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schumacher VA, et al. WT1-dependent sulfatase expression maintains the normal glomerular filtration barrier. J. Am. Soc. Nephrol. 2011;22:1286–1296. doi: 10.1681/ASN.2010080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kann M, et al. Genome-wide analysis of Wilms’ tumor 1-controlled gene expression in podocytes reveals key regulatory mechanisms. J. Am. Soc. Nephrol. doi: 10.1681/ASN.2014090940. http://dx.doi.org/10.1681/ASN.2014090940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Benetti E, et al. A novel WT1 gene mutation in a three-generation family with progressive isolated focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2010;5:698–702. doi: 10.2215/CJN.05670809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hall G, et al. A novel missense mutation of Wilms’ tumor 1 causes autosomal dominant FSGS. J. Am. Soc. Nephrol. 2015;26:831–843. doi: 10.1681/ASN.2013101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chau YY, et al. Acute multiple organ failure in adult mice deleted for the developmental regulator WT1. PLoS Genet. 2011;7:e1002404. doi: 10.1371/journal.pgen.1002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gebeshuber CA, et al. Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat. Med. 2013;19:481–487. doi: 10.1038/nm.3142. [DOI] [PubMed] [Google Scholar]
- 109.Chang H, et al. Wt1 negatively regulates β-catenin signaling during testis development. Development. 2008;135:1875–1885. doi: 10.1242/dev.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim MK, et al. An integrated genome screen identifies the Wnt signaling pathway as a major target of WT1. Proc. Natl Acad. Sci. USA. 2009;106:11154–11159. doi: 10.1073/pnas.0901591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Major MB, et al. Wilms tumor suppressor WTX negatively regulates WNT/β-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 112.Kim MS, et al. A novel Wilms tumor 1 (WT1) target gene negatively regulates the WNT signaling pathway. J. Biol. Chem. 2010;285:14585–14593. doi: 10.1074/jbc.M109.094334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He W, et al. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD. Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J. Am. Soc. Nephrol. 2013;24:1399–1412. doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim HR, et al. Circulating α-klotho levels in CKD and relationship to progression. Am. J. Kidney Dis. 2013;61:899–909. doi: 10.1053/j.ajkd.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 116.Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L. Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin. J. Am. Soc. Nephrol. 2013;8:1899–1905. doi: 10.2215/CJN.02700313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu MC, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib. Nephrol. 2013;180:47–63. doi: 10.1159/000346778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guan X, et al. Klotho suppresses renal tubulo-interstitial fibrosis by controlling basic fibroblast growth factor-2 signalling. J. Pathol. 2014;234:560–572. doi: 10.1002/path.4420. [DOI] [PubMed] [Google Scholar]
- 120.Lindberg K, et al. The kidney is the principal organ mediating klotho effects. J. Am. Soc. Nephrol. 2014;25:2169–2175. doi: 10.1681/ASN.2013111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ren S, et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc. Natl Acad. Sci. USA. 2013;110:1440–1445. doi: 10.1073/pnas.1211179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou D, et al. Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury. Kidney Int. 2012;82:537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang X, Song Z, Guo Y, Zhou M. The novel role of TRPC6 in vitamin D ameliorating podocyte injury in STZ-induced diabetic rats. Mol. Cell Biochem. 2015;399:155–165. doi: 10.1007/s11010-014-2242-9. [DOI] [PubMed] [Google Scholar]
- 124.Song Z, Guo Y, Zhou M, Zhang X. The PI3K/p-Akt signaling pathway participates in calcitriol ameliorating podocyte injury in DN rats. Metabolism. 2014;63:1324–1333. doi: 10.1016/j.metabol.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 125.Sonneveld R, et al. Vitamin D down-regulates TRPC6 expression in podocyte injury and proteinuric glomerular disease. Am. J. Pathol. 2013;182:1196–1204. doi: 10.1016/j.ajpath.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 126.de Zeeuw D, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 127.Floege J. Antagonism of canonical Wnt/β-catenin signaling: taking RAS blockade to the next level? J. Am. Soc. Nephrol. 2015;26:3–5. doi: 10.1681/ASN.2014060567. [DOI] [PMC free article] [PubMed] [Google Scholar]