Abstract
In vivo, cells reside in a complex environment regulating their fate and function. Most of this complexity is lacking in standard in vitro models, leading to readouts falling short of predicting the actual in vivo situation. The use of engineering tools, combined with deep biological knowledge, leads to the development and use of bioreactors providing biologically sound niches. Such bioreactors offer new tools for biological research, and are now also entering the field of cancer research. Here we present the development and validation of a modular bioreactor system providing: (i) high throughput analyses, (ii) a range of biological conditions, (iii) high degree of control, and (iv) application of physiological stimuli to the cultured samples. The bioreactor was used to engineer a three-dimensional (3D) tissue model of cancer, where the effects of mechanical stimulation on the tumor phenotype were evaluated. Mechanical stimuli applied to the engineered tumor model activated the mechanotransduction machinery and resulted in measurable changes of mRNA levels towards a more aggressive tumor phenotype.
I. INTRODUCTION
Throughout its history, the main focus of Tissue Engineering (TE) has been to repair or replace damaged tissues using engineering systems designed by following the developmental principles [1]. A more recent application of TE is to create disease models for drug screening and modeling of disease, including cancer [2, 3]. TE constructs are cultured in vitro with a high degree of control over the culture parameters, using cells, scaffolds and bioreactors designed to mimic tissue specific environments..
Mechanical forces, either generated by cell contractions or from external sources, have strong effects on cell differentiation, growth and survival, playing key roles in the development of many organs, such as bone, cartilage or lung [4]. Recent studies suggest that the increased pressure due to tissue stiffening in the tumor microenvironment leads to an invasive cancer cell phenotype [5]. Still, the design and development of advanced devices that can simulate the microenvironment sensed by tumor cells within the body, remains an uncharted territory. We developed a modular bioreactor platform designed to support tissue constructs in a wide range of operating conditions, and capable of supporting multi-parametric stimulation and data acquisition. This platform is specifically designed for a broad spectrum of applications, from tissue engineering to drug discovery and disease modeling. In this study, we investigated the effects of compression loading using our previously developed TE model of bone cancer [6] using this novel platform.
II. Materials and Methods
A. Bioreactor platform
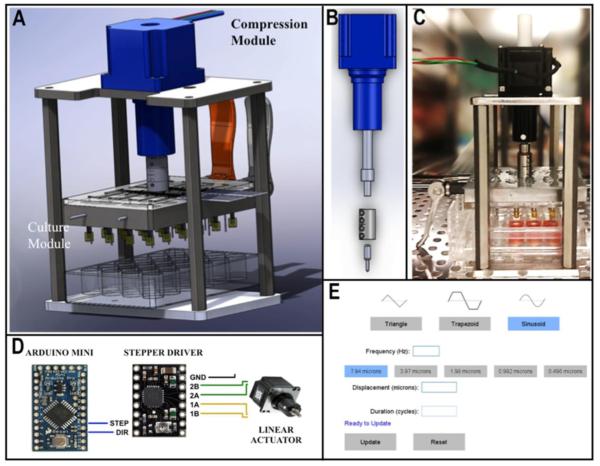
The bioreactor shown in Fig. 1 was developed to promote mass transport and induce mechanical stimuli within the engineered tissues specimens under dynamic, unconfined compression applied periodically during tissue cultivation. The device is compatible with a standard cell culture 24-well-plate, so that 24 specimens could be subjected simultaneously to dynamic compressive loading. A linear actuator and a stepper motor were used to produce displacements of different magnitudes, frequencies and waveforms. A linear variable differential transformer (LVDT) measured the real displacement of the actuator in response to the applied displacement.
Figure 1. Overview of the bioreactor system.
(A) Digitalized image of the entire device. (B) Linear actuator. (C) Image of the assembled bioreactor platform inside the incubator. (D) Microcontroller and stepper actuator. (E) Snapshot of the user interface.
The loading control system consisted of an Arduino Pro Mini and an A4988 stepper motor driver IC. In order to maintain the cell viability, the culture chamber was filled with culture medium and all experiments were conducted in the bioreactor placed within a 37°C/5% CO2 incubator.
B. Tissue engineered model of tumor
The protocols for the formation of the tissue engineered Ewing Sarcoma model, the induction of osteogenic differentiation of human mesenchymal stem cells (hMSC) and the fabrication of the bone scaffolds were as in our previous studies [6]. Briefly, each bone scaffold was seeded with 1.5 106 hMSCs (passage 3) and cultured in 6 mL of osteogenic medium for 4 weeks. Medium was changed biweekly. After 4 weeks, bone tissue constructs were bisected; one half was infused with aggregates of Ewing’s sarcoma cells (3 spheroids per scaffold) (this group was termed TE-ES) and the other half of the bone tissue construct was used as a control (this group was termed TEbone).
C. Validation of the bioreactor
Validation was carried out to confirm the spatial and temporal accuracy of platen movement in the absence of tissue specimen, and determine whether or not the tissue cultures can be maintained over prolonged periods of time (several weeks). To monitor the displacement of the lid, we used an electronic dial indicator (Mitutoyo Electronic Indicators, resolution ±3 μm) mounted on the bioreactor. Different magnitudes of displacement were tested for compressive loading applied using three different waveforms: triangular, trapezoid and sinusoidal. Frequency was held constant at 0.1 Hz in all groups.
D. Finite element analysis of stress field resulting from mechanical stimulation of trabecular bone
Finite element models of scaffolds undergoing uniaxial unconfined compression were created using COMSOL MULTIPHYSICS software (COMSOL, Burlington, MA). In the dynamic, unconfined compression experiment conducted in this study, a trabecular bone cylinder (thickness h0 = 2 mm and radius r0 = 2 mm) was placed between two frictionless impermeable platens and subjected to a sinusoidal displacement with a magnitude of 0.7% strain and a frequency of 1 Hz. The scaffold was assumed to be linearly elastic [7], with the Young Modulus (50 MPa), density (434 kg/m3) and Poisson ratio (0.3) according to our previously published study [8]. A Quasi-static analysis was performed to solve the time-dependent changes in force and displacement.
E. Stimulation protocol
After the construct preparation, tissue specimens in the loading group were cultured for 24 hours in the bioreactor and subjected to 3 cycles of loading. The first cycle consisted of 0.7% of strain (for a 2 mm thick scaffold, it corresponds to the 14 μm displacement amplitude), applied using a sinusoidal waveform, 1 Hz frequency for 1800 loading cycles (30 min of stimulation). The samples were stimulated right after placement into the bioreactor, and then again twice a day after an overnight rest. The same stimulation protocol was applied to TE-bone constructs that served as control samples. The samples were harvested for analysis.
F. Quantitative real-time PCR (qRT-PCR)
Total RNA was obtained using Trizol (Life Technologies) following the manufacturer’s instructions. RNA preparations (2 mg) were treated with “Ready-to-go youprime first-strand beads” (GE Healthcare) to generate cDNA. Quantitative real-time PCR was performed using DNA Master SYBR Green I mix (Applied Biosystems). mRNA expression levels were quantified applying the DCt method, DCt ¼ (Ct of gene of interest e Ct of Actin). GFP primers were selected as previously reported [9]. Other qRT-PCR primer sequences were obtained from the PrimerBank database (http://pga.mgh.harvard.edu/primerbank/).
G. Histology and Immunohistochemistry (IHC)
TE-ES and TE-bone models were fixed in 10% formalin, embedded in paraffin, sectioned at 5 μm). The sections were then stained for Phalloidin (Alexa Fluor® 488 Phalloidin).
III. Results and Discussion
It is well known that mechanical stimuli in the bone microenvironment are important for bone homeostasis and growth [10, 11], and for cancer progression and metastasis [12, 13]. Here, we developed a compression bioreactor to improve bone cancer modeling in vitro with physiological-like mechanical stimuli. The bioreactor can support different culture and stimulation conditions, for analyzing up to 24 independent samples in parallel, providing an efficient fluid-exchange system suitable for drug studies (Fig.1 A-C).
A. Bioreactor validation
We assessed the accuracy of platen displacement measured by the LVDT sensor. Different magnitudes of displacement were configured for each pattern of compressive loading using three waveforms (triangular, trapezoid and sinusoidal), at constant frequencies of 0.1 and 1 Hz. The applied and measured displacements were correlated to each other. For all waveforms, the measured accuracy was ~90%,.The coefficient of determination (R2) was >0.99 for all waveforms, confirming a high correlation between the outcomes and the predicted values.
B. Theoretical prediction of mechanical events within the TE tumor model
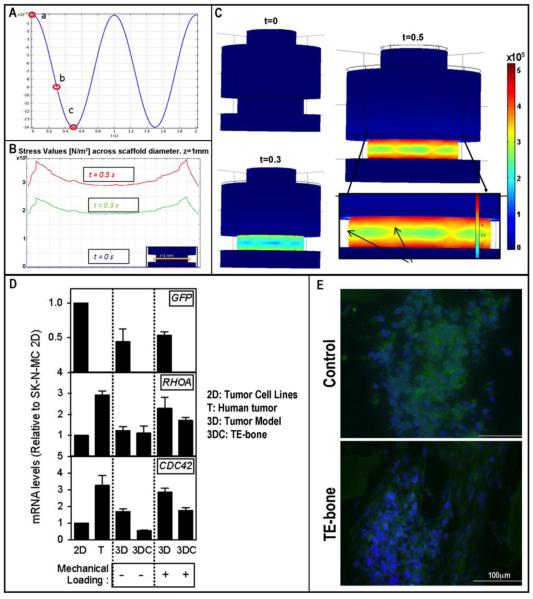
The stress field within the TE model in response to unidirectional compression was evaluated using finite element analysis. The motion waveform was a sine wave with 14 μm displacement amplitude (0.7% of strain within a 2×4 mm scaffold), at 1 Hz. The stresses within the construct were in the range of 2.5×105 − 5.0×105 [Pa], depending on the position within the plug (Fig.2 A-C).
Figure 2. Results summary.
A. Simulated loading waveform; a, b and c highlight the time points further analyzed in panels B and C. B. Finite element analysis of stress values on cross section of the TE model at t=0, 0.3 and 0.5 seconds. Values are in N/m2. C. Finite element analysis of stress field resulting from dynamic compressive stimulation. Arrows in magnified insert highlight small radial deformations due to scaffold compression. D. Re-expression of RHOA (Top) and CDC42 (Bottom). E. Immunostainings for Phalloidin. Nuclei are counterstained with DAPI.
C. Cytoskeletal remodeling in TE tumor model
One of the main features of the TE model of tumor we previously described [6] is that the 3D tissue environment, induced the re-expression of genes related to focal adhesion and cancer progression. Among these genes, CDC42 showed higher expression in tumor samples harvested from patients than in tumor cell lines. CDC42 and RHOA are known to be involved into the mechanotransduction machinery [14-17]. By regulating f-actin polymerization, stress fibers play an important role in several cellular functions including cell morphology and migration. In order to analyze the expression of CDC42 and RHOA, a qRT-PCR was performed, comparing the stimulated TE tumor model and the TE bone to the respective counterparts that were not stimulated, and to the tumors harvested from patients. The expression was normalized to the expression of the same genes in the SK-N-MC cell lines (Fig.2D). The expression of RHOA was similar in the TE tumor and the TE bone model. However, in the stimulated group, the fold-change was >2 for the TE tumor. Finally, the expression of CDC42 was almost 2-fold for the not stimulated TE tumor, and <1 for the static TE bone. In contrast, the fold change of the same gene in the TE tumor after the stimulation was 3 and for the TE bone was 2. The induction of CDC42 is related to the Rho family of GTPases. Inhibition of some Rho pathway members through therapeutic compounds was successfully applied in preclinical studies, suggesting that CDC42 could be a potential candidate for ES therapy. Taken together, these data show that biophysical stimuli applied to the TE model activate the mechanotransduction machinery, which in turn modulates the mRNA expression levels.
Focal adhesion genes, such as CDC42 and RHOA, have been previously linked to cell morphology and motility. It is known that these genes play a key role in the polymerization of the globular actin into filamentous actin, commonly referred as stress fibers [18]. In particular, the polymerization of actin leads to a reorganization of the cell’s cytoskeleton and to changes of its morphology. When stimulated for three times during 24 hours, we could detect groups of small rounded cells surrounded by elongated and highly oriented cells (Fig. 2E). A previous study [5] showed that increased pressure due to tissue stiffening during tumor progression results in more aggressive behavior leading to morphology changes and migration.
IV. Conclusions
Improved tumor modeling can be achieved by introducing physiological and biophysical stimuli commonly present in bone microenvironment. To this end, we developed a novel bioreactor system that easily adapts to different culture and stimulation conditions, is easy to assemble and supports 24 independent samples. Cultivation of the human tumors in this bioreactor under physiological stimulation induced the polarization and orientation of the cells, leading to a more aggressive tumor phenotype. More experiment and analysis are necessary to better understand and describe the effects of such stimuli on the TE-ES. Further optimization and biological validation of our bioreactor will lead to the establishment of an enabling technology for broader applicability cancer research, in the form of a bioengineering platform for studies of cancer biology and high-throughput screening in vitro.
Acknowledgment
The authors thank Nina Tandon for her help with the development of the control system.
Footnotes
Research supported by NIH (EB002520) and NYSTEM (C028119).
Contributor Information
A. Marturano-Kruik, Columbia University, Department of Biomedical Engineering, New York NY 10032, am3934@columbia.edu
K. Yeager, Columbia University, Department of Biomedical Engineering, New York NY 10032, ky2161@columbia.edu
D. Bach, Cooper Union. Department of Mechanical Engineering, New York NY 10003, bach@cooper.edu
A. Villasante, Columbia University, Department of Biomedical Engineering, New York NY 10032, av2499@columbia.edu
E. Cimetta, Columbia University, Department of Biomedical Engineering, New York NY 10032.
G. Vunjak-Novakovic, Columbia University, Department of Biomedical Engineering, New York NY 10032.
References
- [1].Langer R, Vacanti JP, Vacanti CA, Atala A, Freed LE, Vunjak-Novakovic G. Tissue engineering: biomedical applications. Tissue Eng. 1995;1:151–61. doi: 10.1089/ten.1995.1.151. [DOI] [PubMed] [Google Scholar]
- [2].Villasante A, Vunjak-Novakovic G. Tissue-engineered models of human tumors for cancer research. Expert Opin Drug Discov. 2015 Mar;10:257–68. doi: 10.1517/17460441.2015.1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Villasante A, Vunjak-Novakovic G. Bioengineered tumors. Bioengineered. 2015 Jan 23;:1–4. doi: 10.1080/21655979.2015.1011039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003 Aug;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- [5].Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, Jain RK, Munn LL. Mechanical compression drives cancer cells toward invasive phenotype. Proc Natl Acad Sci U S A. 2012 Jan 17;109:911–6. doi: 10.1073/pnas.1118910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Villasante A, Marturano-Kruik A, Vunjak-Novakovic G. Bioengineered human tumor within a bone niche. Biomaterials. 2014 Jul;35:5785–94. doi: 10.1016/j.biomaterials.2014.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Keaveny TM, Guo XE, Wachtel EF, McMahon TA, Hayes WC. Trabecular bone exhibits fully linear elastic behavior and yields at low strains. J Biomech. 1994 Sep;27:1127–36. doi: 10.1016/0021-9290(94)90053-1. [DOI] [PubMed] [Google Scholar]
- [8].Marcos-Campos I, Marolt D, Petridis P, Bhumiratana S, Schmidt D, Vunjak-Novakovic G. Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials. 2012 Nov;33:8329–42. doi: 10.1016/j.biomaterials.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pasque V, Gillich A, Garrett N, Gurdon JB. Histone variant macroH2A confers resistance to nuclear reprogramming. EMBO J. 2011 Jun 15;30:2373–87. doi: 10.1038/emboj.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rauh J, Milan F, Gunther KP, Stiehler M. Bioreactor systems for bone tissue engineering. Tissue Eng Part B Rev. 2011 Aug;17:263–80. doi: 10.1089/ten.TEB.2010.0612. [DOI] [PubMed] [Google Scholar]
- [11].Schulte FA, Ruffoni D, Lambers FM, Christen D, Webster DJ, Kuhn G, Muller R. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS One. 2013;8:e62172. doi: 10.1371/journal.pone.0062172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Craig DH, Basson MD. Biological impact of mechanical stimuli on tumor metastasis. Cell Cycle. 2009 Mar 15;8:828–31. doi: 10.4161/cc.8.6.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Menon S, Beningo KA. Cancer cell invasion is enhanced by applied mechanical stimulation. PLoS One. 2011;6:e17277. doi: 10.1371/journal.pone.0017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006 Aug 25;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [15].Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009 Jan;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- [16].Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005 Sep;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [17].Yu H, Mouw JK, Weaver VM. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 2011 Jan;21:47–56. doi: 10.1016/j.tcb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]