Abstract
OBJECTIVES: Evidence suggests palivizumab may be beneficial for respiratory syncytial virus (RSV) infection in pediatric patients, although it is only approved by the US Food and Drug Administration for RSV prophylaxis. The objective of this study is to compare outcomes among pediatric patients with RSV infection who received intravenous palivizumab and standard of care versus standard of care alone.
METHODS: This is a retrospective, single-center cohort study conducted between November 2003 and October 2013. Pediatric patients with active RSV infection treated with intravenous (IV) palivizumab after initiation of mechanical ventilation were matched 1:1 to a control selected from ventilated patients who received standard of care. The primary end point evaluated the duration of mechanical ventilation between groups. Secondary end points included hospital length of stay, intensive care unit length of stay, duration of respiratory support over baseline, time to RSV microbiologic cure, duration of antibiotic therapy, and in-hospital mortality.
RESULTS: A total of 22 patients with a median age of 3 months were included in the study. Patients in the treatment group received a median of 2 doses of IV palivizumab, with a mean dose of 14.2 mg/kg. All patients received bronchodilators and corticosteroids, with the exception of 1 patient in the control group, and only 1 treatment group patient received IV ribavirin. Duration of mechanical ventilation was longer in the treatment group (18.9 ± 9.5 vs. 14.3 ± 9.3 days; p = 0.26). No statistically significant differences were observed between groups for any of the secondary end points.
CONCLUSIONS: Pediatric patients who received IV palivizumab in addition to standard of care for treatment of RSV infection following initiation of mechanical ventilation experienced similar outcomes to those who received standard of care alone. Further studies are necessary to evaluate the potential benefit of IV palivizumab in addition to current standard of care.
INDEX TERMS: mechanical ventilation, palivizumab, respiratory syncytial virus, standard of care
INTRODUCTION
Respiratory syncytial virus (RSV) is a major cause of morbidity and mortality in the pediatric population and is the leading etiology of lower respiratory tract infections in children younger than 1 year.1 Each year about 2.1 million children in the United States who are younger than 5 years will require medical attention for RSV. Globally it is estimated that RSV causes 34 million acute lower respiratory tract infections and 3.4 million hospitalizations in the same population each year.2,3 Mortality associated with RSV has decreased significantly in the past decade, as a recent study estimated death rates as low as 4 per 10,000 hospital admissions in those with a primary diagnosis of RSV.4 Risk factors for acquiring severe RSV infection in those younger than 2 years include premature birth (<35 weeks of gestation), underlying lung/airway disease, congenital heart disease, weight < 5 kg, low socioeconomic status, neuromuscular disease, in utero exposure to secondhand smoke, and immune system compromise.1 Other risk factors identified by the American Academy of Pediatrics (AAP) include regular child care attendance or having one or more siblings or other children younger than 5 years living permanently in the child's household.5
According to the Centers for Disease Control and Prevention, most infants become infected with RSV prior to the age of 2 years, with only a small percentage developing severe infection.6 Typical management includes supportive care measures, such as maintaining adequate hydration and oxygenation along with nasopharyngeal suctioning for symptom relief.1,7,8 Severe RSV infection is not clearly defined; however, the AAP clinical practice guideline for the diagnosis and management of bronchiolitis, which includes RSV, defines severe disease as the presence of “signs and symptoms associated with poor feeding and respiratory distress characterized by tachypnea, nasal flaring, and hypoxemia.”7 Additionally, severe disease can be typified by persistently increasing respiratory effort, apnea, or the need for mechanical ventilation. In patients who develop severe RSV infection, pharmacologic treatment options may be considered. Currently, ribavirin is the only medication approved by the US Food and Drug Administration (FDA) for RSV treatment, yet the AAP recommends against routine ribavirin use. However, ribavirin may be considered in those with severe RSV infection or risk factors for severe infection, most notably immune system compromise and hemodynamically significant cardiopulmonary disease.5,7
Ribavirin (Virazole, Valeant, Bridgewater, NJ) is not often used clinically, for several reasons. Placebo-controlled studies have produced conflicting evidence regarding the effects of ribavirin on outcomes such as days of mechanical ventilation, hospital length of stay (LOS), and intensive care unit (ICU) LOS.9–11 Most of the available studies in RSV treatment report the use of aerosolized ribavirin. There are concerns with this formulation because of its prolonged administration time and risk for sudden deterioration of respiratory function in mechanically ventilated patients, for which there is a black box warning.12 In addition to high cost, there is also the potential for toxicity due to its teratogenic and carcinogenic effects.13,14 Because of the limitations with ribavirin, palivizumab (Synagis, MedImmune, Gaithersburg, MD) has been considered as an alternative therapy for RSV infection.
Despite the lack of FDA approval for treatment of RSV infection, palivizumab is an agent of interest because of its ease of administration, safety profile, and proposed efficacy.15 Three studies describing the use of a one-time intravenous (IV) dose of palivizumab 15 mg/kg have been conducted in pediatric patients 2 years or younger. Results showed high survival rates as well as reductions in hospital days attributable to RSV, reductions in hospital days requiring supplemental oxygen, and decreases in RSV concentrations in respiratory tract secretions.16–18 However, the utility of palivizumab in RSV infection remains unclear because of limitations of the available evidence, which include concomitant use of ribavirin and the lack of comparator control groups. The purpose of this study is to determine the effects of IV palivizumab plus the standard of care versus standard of care alone in the treatment of RSV infection in those requiring mechanical ventilation.
MATERIALS AND METHODS
This was a retrospective, single-center cohort study of pediatric patients hospitalized for RSV infection between October 2003 and November 2013. Patients in the treatment group were identified through a health care network–wide IV palivizumab use report. Control group patients were identified via the International Classification of Diseases, Ninth Revision diagnosis codes for RSV (079.6, 466.11, 480.1) and mechanical ventilation (93.90, 96.7). Patients were included in the study if they received IV palivizumab and/or standard of care following initiation of mechanical ventilation; were 25 years or younger; and had a positive RSV diagnostic test result by rapid antigen, direct fluorescent antibody (DFA), or polymerase chain reaction (PCR). The treatment group received 1 to 3 doses of IV palivizumab 15 mg/kg. Dosing was based on previous IV palivizumab studies16–19 and is similar to intramuscular (IM) palivizumab dosing in RSV prophylaxis. Doses were infused intravenously for 5 to 30 minutes and administered in 24-hour intervals when more than 1 dose was given. Standard of care was defined as the receipt of IV fluids and oxygen supplementation, bronchodilators or corticosteroids, and/or ribavirin.1 Patients who received IM palivizumab for treatment of active RSV infection were excluded. Patients in the treatment group who met inclusion criteria were matched 1:1 to a control based on sex, age on admission, and admission date.
The primary end point was the duration of mechanical ventilation after exposure to treatment or control. Secondary end points included hospital LOS, ICU, LOS, duration of respiratory support over baseline, time to RSV microbiologic cure, duration of antibiotic therapy, and in-hospital mortality. Time to RSV microbiologic cure was defined as the time from first RSV positive diagnostic test result to first negative RSV diagnostic test result.
The study was approved by the local Institutional Review Board. Because of the retrospective nature of the study, written informed consent was not required and was subsequently waived. A sample size of 44 patients per group was required to detect a 2-day difference in duration of mechanical ventilation between groups, assuming 80% power and a duration of mechanical ventilation of 8 days in the control group.9 Continuous variables were analyzed using a Student t-test or Mann-Whitney U test upon determination of parametric assumptions using the Shapiro-Wilk test. Nominal data were analyzed using a Fischer exact test or χ2 test, as appropriate. An a priori alpha was set at 0.05 for statistical significance.
RESULTS
A total of 41 patients in the hospital network received at least one dose of IV palivizumab during the study period and were evaluated for inclusion. Of these patients, 30 were excluded for the following reasons: no RSV-positive diagnostic test result (n = 24); not mechanically ventilated at the time of IV palivizumab initiation (n = 4); and receipt of at least 1 dose of IM palivizumab for treatment in addition to IV palivizumab (n = 2). The remaining 11 patients met inclusion criteria and were matched 1:1 to a control. Baseline demographics are listed in Table 1. A total of 64% of patients (n = 14) were male. Median age on admission was about 3 months, with an age range from 19 days to 12 years.
Table 1.
Baseline Demographics and Patient Characteristics
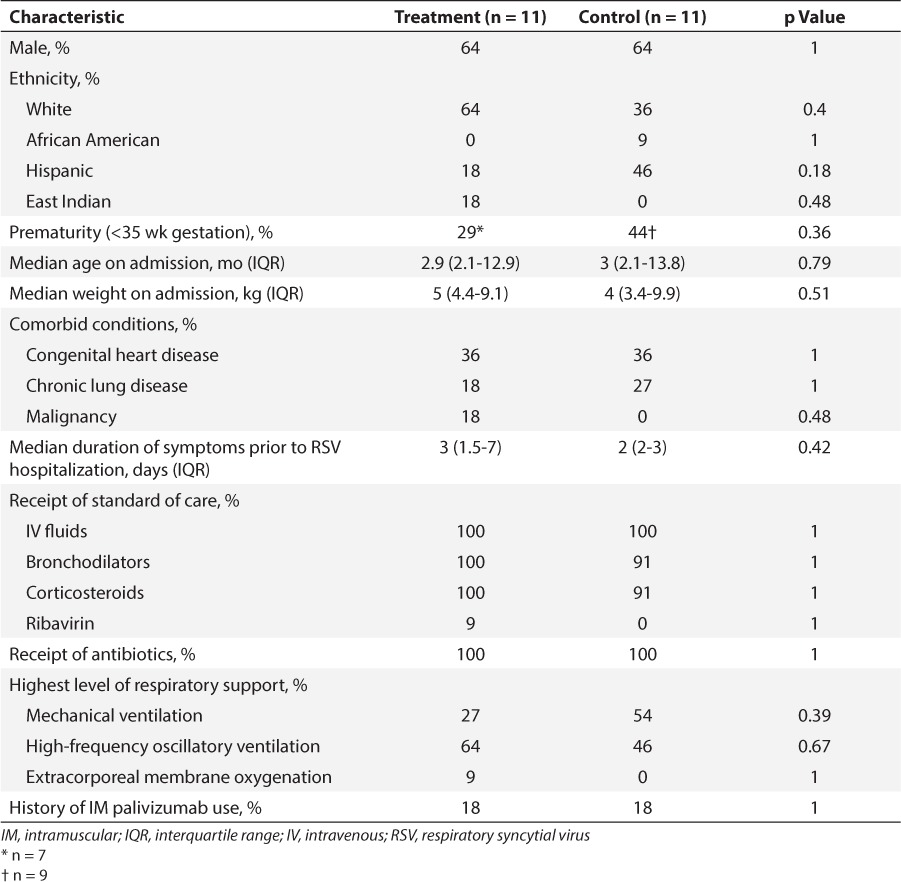
On admission, patients presented with a 1- to 7-day history of respiratory distress, including cough, congestion, increased work of breathing, decreased oral intake, and/or fever. High-risk comorbid conditions were similar between groups. All but 1 control group patient received IV fluids, bronchodilators, and corticosteroids. One patient in the treatment group received IV ribavirin following IV palivizumab treatment. Empiric antibiotic therapy was administered to all study patients. More patients in the treatment group required a higher level of ventilatory support versus control, with 1 patient requiring extracorporeal membrane oxygenation (ECMO; 73% vs. 46%; p = 0.39). Of the 4 patients who had received IM palivizumab for RSV prophylaxis (n = 2, treatment; n = 2, control), 1 from each group received prophylaxis in the previous RSV season. The other control group patient received IM palivizumab in the same season as the hospitalization for RSV, albeit 2 months prior to admission. The date of receipt of prophylactic palivizumab for the remaining treatment group patient is unknown.
A total of 45% of patients (n = 5) in the treatment group received a one-time dose of IV palivizumab. A total of 4 patients received 2 IV palivizumab doses, and 2 patients were given 3 doses. On average, treatment group patients received 14.2 ± 1.5 mg/kg per dose. Prior to receipt of the first IV palivizumab dose, patients in the treatment group were mechanically ventilated for a median of 3 days (interquartile range [IQR], 1.5–6.5 days).
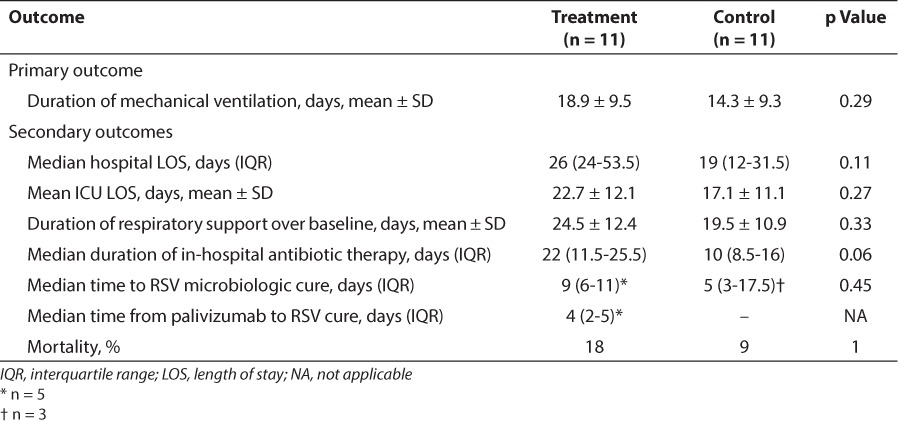
The duration of mechanical ventilation was longer in the treatment group compared with the control group (18.9 ± 9.5 vs. 14.3 ± 9.3 days; p = 0.26). Secondary end points, including hospital LOS, ICU LOS, duration of respiratory support over baseline, duration of antibiotic therapy, and time to RSV microbiologic cure, were not significantly different when the treatment and control groups were compared. In-hospital mortality was no different between groups, although there was one additional death in the treatment group versus the control group (Table 2).
Table 2.
Primary and Secondary Outcomes
Initial RSV diagnostic test results for both the treatment and control groups are listed in Table 3. A rapid antigen test was obtained for all patients included in the study. More patients in the treatment group received DFA or PCR tests. One patient in each group also tested positive for influenza A; another treatment group patient tested positive for influenza A and B, and parainfluenza 3; and a control group patient tested positive for parainfluenza 2. Blood cultures were positive in 1 patient in each group (Pseudomonas aeruginosa in the treatment group; Staphylococcus aureus in the control group). One patient in the treatment group had a positive urine culture (extended-spectrum beta-lactamase–producing Escherichia coli). The bacterial organisms recovered from respiratory cultures are depicted in the Figure.
Table 3.
Initial Respiratory Syncytial Virus Diagnostic Test Results
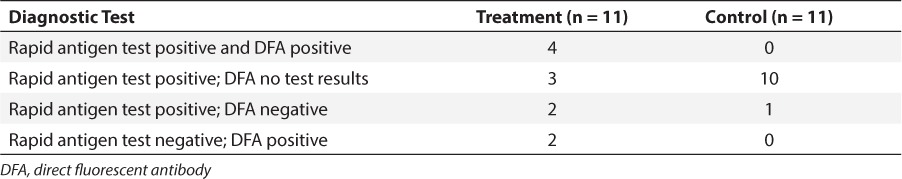
Figure.
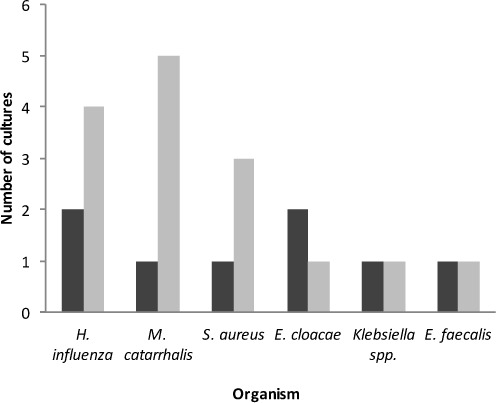
Positive respiratory culture results.
▪ = treatment;  = control
= control
DISCUSSION
Results of this retrospective cohort study revealed no difference in outcomes when IV palivizumab was added to standard of care for the treatment of RSV infection following initiation of mechanical ventilation in the pediatric population. To our knowledge, this is the first study to compare the effects of IV palivizumab in addition to standard of care versus standard of care alone for RSV treatment.
Because of the lack of high-quality evidence, there is no recommended dose or dosing schedule for IV palivizumab in the treatment of RSV. The dosing strategies used in the present study were dictated by pediatric infectious disease and/or critical care physician's preference. Although most of the literature describes the use of a single 15 mg/kg dose of IV palivizumab,16–18 6 of 11 patients (55%) in the treatment group received more than 1 dose of IV palivizumab, up to a maximum of 3 doses. The only other study to incorporate the use of repeat doses of IV palivizumab in mechanically ventilated patients involved 2 immunocompromised patients. Because of progressive deterioration and respiratory distress, a second dose was given within 5 days of the initial dose. One patient was successfully extubated shortly after the second dose of IV palivizumab and survived, whereas the other died 13 days after initial diagnosis of RSV infection.18 Upon review of decisions to administer 1 dose versus multiple doses of IV palivizumab in the present study, repeat doses were ordered based on the presence of ongoing respiratory depression or worsening pulmonary status, as well as RSV quantitative DFA test results.
Patients were mechanically ventilated for a median of 3 days prior to receipt of the first IV palivizumab dose, which is relatively consistent with previous literature describing the administration of IV palivizumab within 24 to 48 hours of intubation.16–18 Malley et al17 studied outcomes and effects of IV palivizumab on RSV concentrations in secretions of mechanically ventilated pediatric patients hospitalized with RSV infection. A total of 17 patients received a one-time dose of IV palivizumab, resulting in a mean hospital LOS of 14.5 days, duration of mechanical ventilation of 8.8 days, and duration of supplemental oxygen of 12.3 days; all of these are much shorter durations than those described in the present study. However, this is likely due to the exclusion of high-risk patient populations, including those with immunodeficiency, those with hemodynamically significant cardiac abnormalities, those requiring home oxygen therapy, and those receiving a higher level of ventilatory support while hospitalized.17 In contrast, Chávez-Bueno et al18 described the use of IV palivizumab in 31 high-risk pediatric patients, which more accurately depicts the population of the present study. Still, only 5 patients (16%) required mechanical ventilation, including 1 patient who received ECMO. Two patients were immunocompromised and died within 3 weeks of RSV diagnosis. Of the 3 mechanically ventilated patients who survived, the median hospital LOS was 26.3 days after RSV diagnosis, more comparable to results of the present study. Yet 13 patients (59%) in the present study required a higher level of mechanical ventilation, a much greater percentage compared with previous literature.
Although not originally anticipated, only 1 patient in the present study received ribavirin as part of the RSV treatment regimen. Two of the three current IV palivizumab studies excluded patients who received ribavirin for treatment of current RSV illness.16,17 However, Chávez-Bueno et al18 described a majority of patients (80%) who received concomitant IV or inhaled ribavirin, an important difference from the present study. We described use of IV ribavirin in a 12-year-old male with a past medical history significant for acute lymphoblastic leukemia, who was receiving chemotherapy at the time of RSV hospitalization. Four days after receiving a third IV palivizumab dose for RSV treatment, the patient was placed on ECMO in light of his rapid deterioration of respiratory status and immunosuppressed state. It was at that time that FDA approval was received for compassionate use of IV ribavirin to help combat the patient's viral load until his immune system recovered. The patient received a 33 mg/kg loading dose of IV ribavirin infused for 30 minutes, followed by 16 mg/kg every 6 hours for 4 days before discontinuation of therapy prematurely because of the development of hemolytic anemia, the principal toxicity of ribavirin. The patient died 4 days later, 16 days following initial RSV diagnosis.
Also in contrast to current literature, all but one patient included in the present study received corticosteroids and bronchodilators as part of RSV treatment. Malley et al17 described use of corticosteroids in only 60% of patients, whereas receipt of other standard of care measures, excluding ribavirin use, was not documented in any of the three currently available IV palivizumab studies.16–18 These points highlight key additions to the literature because no study to date has analyzed the use of IV palivizumab in addition to standard of care without the use of concomitant ribavirin, nor has any study included a complete description of the use of standard of care. However, it is important to note that the most recent AAP guidelines recommend against the use of corticosteroids, bronchodilators, and nasopharyngeal suctioning because of the lack of evidence of significant benefit and increased risk for adverse events associated with these interventions.8
In addition to the above-mentioned therapies, treatment guidelines for RSV also recommend against the use of antibiotics unless there is a strong suspicion for secondary bacterial infection.8 Current literature suggests a low incidence of bacterial infection in patients presenting with RSV infection. However, real-world data show that up to 75% of patients are given empiric antibiotic therapy.20,21 According to the AAP, antibiotics continue to be used in this setting because of young age, presence of fever, and concern for undetected bacterial infection at presentation.7,8 This is reflected in the present study, because all included patients received empiric antibiotic therapy upon hospital admission. Treatment group patients received a longer course of antibiotic therapy versus control (p = 0.06), although this trend coincides with other secondary outcome durations in the treatment group. Original research of IV palivizumab for RSV treatment has inconsistently documented the presence of positive bacterial cultures, whereas no studies to date have reported receipt of antibiotics.16–18 Therefore, this study provides a clearer picture of antibiotic use in the setting of RSV infection in current clinical practice.
Diagnosis of RSV infection in the present study was made via collection of a nasal swab, nasopharyngeal wash, or tracheal aspirate specimen during the typical RSV season (i.e. October–March). Most treatment group patients (73%) had tracheal aspirate specimens used for diagnosis, whereas control patients received a diagnosis of RSV infection by rapid antigen using a nasal swab or nasopharyngeal wash. This is important because tracheal aspirate specimens positive for RSV are evidence of lower respiratory tract illness, with the potential to cause more severe RSV infection compared with upper respiratory tract specimens.17,22 Although specimen collection and diagnostic testing procedure varied, all patients received an initial rapid antigen test, most likely because of its advantages of short turnaround time, simple interpretation, and relatively low cost. Important disadvantages include a wide range of specificity (75%–100%) and sensitivity (59%–97%). Direct fluorescent antibody testing yields a quantitative result (i.e., 1+, 2+, 3+, 4+), which describes the patient's RSV inoculum. This diagnostic assay has much higher sensitivity (93%–98%) and specificity (92%–97%) compared with the rapid antigen test. However, the most sensitive and specific diagnosis of RSV is via PCR testing, which has the added advantage of simultaneous identification of other respiratory viruses. Disadvantages of this diagnostic test are higher costs and, specific to the present study site, a longer turnaround time.22 Although not used as the initial diagnostic test for any patients in this study, PCR results were obtained for 3 treatment group patients, all after receipt of at least 1 dose of IV palivizumab. The results confirmed previous DFA results but contradicted one of the rapid antigen tests. Because of the superior specificity and sensitivity of PCR and DFA for RSV, future studies should consider consistent use of PCR and/or DFA to more accurately give a diagnosis for patients with RSV infection.
There are several limitations of the present study inherent to a retrospective design. The limited sample size provides high probability of a type II error; therefore, the potential benefit of IV palivizumab cannot be excluded. Second, there was no validated severity of illness scoring tool used in this study, comparable to previous literature. Treatment group patients received a higher level of ventilatory support and experienced a longer duration of mechanical ventilation, antibiotic therapy, and time to RSV microbiologic cure versus control group patients, possibly indicating severity of illness differences between groups. However, once patients received the first dose of IV palivizumab, time to documented RSV microbiologic cure was fairly rapid (4 days [IQR, 2–5 days]). Perhaps if IV palivizumab was given earlier in the treatment course, duration of mechanical ventilation and time to RSV microbiologic cure may have been shorter.
Another important limitation identified by the authors involves the variability of IV palivizumab dosing in the treatment group. The number of palivizumab doses administered could not be controlled for because of the retrospective nature of the study. Also, it was difficult to fully assess and compare outcomes between those who received one dose versus multiple doses because of the limited number of patients who received more than one dose. Future studies must incorporate a consistent dosing strategy to more accurately measure the effects of IV palivizumab.
The time to RSV microbiologic cure could not be accurately assessed in most patients, because repeat testing was only ordered in 36% of patients (n = 8) and was also not done at standardized times. Although microbiologic clearance of RSV has not been documented in previous literature and may not consistently correlate with complete clinical cure, achieving a more rapid viral clearance may lead to a shorter duration of mechanical ventilation or respiratory support, as well as hospital or ICU LOS. Therefore, measurement of RSV microbiologic cure adds a potential impactful end point for future RSV literature.
Limited information was available regarding receipt of IM palivizumab for RSV prophylaxis prior to hospitalization, because only the date of last known receipt was recorded from retrospective chart review when available. Therefore, it is unknown exactly how many patients were given IM palivizumab prophylaxis and it is also unclear whether the drug was administered on a consistent monthly basis up to a maximum of 5 doses during RSV season, as recommended by the AAP.5,23
Additionally, the variation in diagnostic procedures used possibly led to inappropriate inclusion or exclusion of patients. Because of the wide range of sensitivity and specificity of rapid antigen tests for RSV, there was a possibility of false-negative results and exclusion of patients with true RSV infection who may have benefited from the receipt of IV palivizumab. Conversely, a negative DFA result with a positive rapid antigen test may represent a false-positive result due to the consistently higher sensitivity of the DFA test. This may have led to inappropriate use of IV palivizumab in patients who did not actually have an active RSV infection, also contributing to unnecessary medication costs. Despite the variation in diagnostic procedure, this is the only study to date to incorporate the use of PCR in RSV diagnosis and study inclusion.
Lastly, patients with documented positive influenza or parainfluenza tests, as well as those with positive bacterial cultures, were not excluded from the study. One could argue that although these patients tested positive for RSV also, outcomes could have been more affected by the presence of influenza, parainfluenza, or bacterial infections compared with patients with RSV only. However, all but 2 patients (91%) included in the study had at least 1 positive diagnostic viral test or bacterial culture in addition to RSV.
In conclusion, results of this retrospective cohort showed no significant differences in outcomes with IV palivizumab in addition to current standard of care versus standard of care alone in the treatment of RSV infection in mechanically ventilated pediatric patients. Because of the small sample size, lack of study power, and identified limitations, a definitive conclusion regarding the benefit, or lack thereof, of IV palivizumab in the RSV treatment setting cannot be fully supported by this study. However, the ability to compare the effects of IV palivizumab in addition to standard of care without concomitant ribavirin versus standard of care alone addresses major gaps in RSV treatment literature. Future IV palivizumab research must include a larger sample size and control for severity of illness differences between treatment groups, while incorporating consistent palivizumab dosing strategies and diagnostic procedures. More data are needed to fully evaluate the potential benefit of IV palivizumab in the treatment of RSV infection in the pediatric population.
Acknowledgments
The Introduction and Materials and Methods sections were presented in poster form at the American Society of Health-System Pharmacists Midyear Clinical Meeting in Orlando, Florida, on December 11, 2013. The study was presented in platform presentation form at the Annual Alcáldé Southwest Leadership Conference Seminar in Houston, Texas, on April 10, 2014, and in poster form at the Annual University of Texas Celebrating Pharmacy Research Excellence Day in Austin, Texas, on April 22, 2014. This study was also presented in poster form at the Annual Pediatric Pharmacy Advocacy Group Meeting in Nashville, Tennessee, on May 2, 2014.
Abbreviations:
- AAP
American Academy of Pediatrics
- DFA
direct fluorescent antibody
- ECMO
extracorporeal membrane oxygenation
- FDA
Food and Drug Administration
- ICU
intensive care unit
- IM
intramuscular
- IV
intravenous
- LOS
length of stay
- PCR
polymerase chain reaction
- RSV
respiratory syncytial virus
Footnotes
Disclosure The principal investigator takes full responsibility for the integrity of the study data and accuracy of the data analysis. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Dawson-Caswell M, Muncie HL. Respiratory syncytial virus infection in children. Am Fam Physician. 2011;83(2):141–146. [PubMed] [Google Scholar]
- 2.Hall CB, Weinberg GA, Iwane MK et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair H, Nokes DJ, Gessner BD et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byington CL, Wilkes J, Korgenski K et al. Respiratory syncytial virus-associated mortality in hospitalized infants and young children. Pediatrics. 2015;135(1):e24–e31. doi: 10.1542/peds.2014-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Committee on Infectious Diseases. From the American Academy of Pediatrics: policy statements--modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124(6):1694–1701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Respiratory syncytial virus infection (RSV): frequently asked questions, 2008. http://www.cdc.gov/RSV/about/faq.html. Accessed January 2, 2016.
- 7.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 8.Ralston SL, Lieberthal AS, Meissner HC et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 9.Meert KL. Aerosolized ribavirin in mechanically ventilated children with respiratory syncytial virus lower respiratory tract disease: a prospective, double-blind, randomized trial. Crit Care Med. 1994;22(4):566–572. doi: 10.1097/00003246-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Guerguerian AM, Gauthier M, Lebel MH et al. Ribavirin in ventilated respiratory syncytial virus bronchiolitis: a randomized, placebo-controlled trial. Am J Resp Crit Care Med. 1999;160(3):829–834. doi: 10.1164/ajrccm.160.3.9810013. [DOI] [PubMed] [Google Scholar]
- 11.Hall CB. Respiratory syncytial virus. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th ed. London, UK: Churchill Livingstone; 2009. pp. 2207–2221. [Google Scholar]
- 12.Virazole [prescribing information] Bridgewater, NJ: Valeant; 2013. [Google Scholar]
- 13.Eiland LS. Respiratory syncytial virus: diagnosis, treatment and prevention. J Pediatr Pharmacol Ther. 2009;14(2):75–85. doi: 10.5863/1551-6776-14.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kneyber M, Moll HA, de Groot R. Treatment and prevention of respiratory syncytial virus infection. Eur J Pediatr. 2000;159(6):399–411. doi: 10.1007/s004310051296. [DOI] [PubMed] [Google Scholar]
- 15.Johnson S, Oliver C, Prince G et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176(5):1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 16.Sáez-Llorens X, Moreno MT, Ramilo O et al. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23(8):707–712. doi: 10.1097/01.inf.0000133165.85909.08. [DOI] [PubMed] [Google Scholar]
- 17.Malley R, DeVincenzo J, Ramilo O et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178(6):1555–1561. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 18.Chávez-Bueno S, Mejías A, Merryman RA et al. Intravenous palivizumab and ribavirin combination for respiratory syncytial virus disease in high-risk pediatric patients. Pediatr Infect Dis J. 2007;26(12):1089–1093. doi: 10.1097/INF.0b013e3181343b7e. [DOI] [PubMed] [Google Scholar]
- 19.Synagis [prescribing information] Gaithersburg, MD: MedImmune, Inc; 2013. [Google Scholar]
- 20.Purcell K, Fergie J. Driscoll children's hospital respiratory syncytial virus database: risk factors, treatment and hospital course in 3308 infants and young children, 1991 to 2002. Pediatr Infect Dis J. 2004;23(5):418–423. doi: 10.1097/01.inf.0000126273.27123.33. [DOI] [PubMed] [Google Scholar]
- 21.Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322–324. doi: 10.1001/archpedi.156.4.322. [DOI] [PubMed] [Google Scholar]
- 22.Henrickson KJ, Hall CB. Diagnostic assays for respiratory syncytial virus disease. Pediatr Infect Dis J. 2007;26(11):S36–S40. doi: 10.1097/INF.0b013e318157da6f. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Pediatrics Committee on Infectious Diseases and Bronchiolitis Guidelines Committee. Policy statement: updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):620–638. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]


