Abstract
The ability to encode and retrieve spatial and temporal contextual details of episodic memories (context memory) begins to decline at midlife. In the current study, event-related fMRI was used to investigate the neural correlates of context memory decline in healthy middle aged adults (MA) compared with young adults (YA). Participants were scanned while performing easy and hard versions of spatial and temporal context memory tasks. Scans were obtained at encoding and retrieval. Significant reductions in context memory retrieval accuracy were observed in MA, compared with YA. The fMRI results revealed that overall, both groups exhibited similar patterns of brain activity in parahippocampal cortex, ventral occipito-temporal regions and prefrontal cortex (PFC) during encoding. In contrast, at retrieval, there were group differences in ventral occipito-temporal and PFC activity, due to these regions being more activated in MA, compared with YA. Furthermore, only in YA, increased encoding activity in ventrolateral PFC, and increased retrieval activity in occipital cortex, predicted increased retrieval accuracy. In MA, increased retrieval activity in anterior PFC predicted increased retrieval accuracy. These results suggest that there are changes in PFC contributions to context memory at midlife.
Keywords: aging, compensation, context memory, middle age adults, prefrontal cortex
Introduction
Healthy aging is associated with reductions in episodic memory. Older adults aged 60 years and above show greater reductions in retrieving spatial and temporal contextual details about past events (context memory) versus simply recognizing whether or not an such an item/event was previously encountered (recognition memory) (Cabeza et al. 2000; Rajah and McIntosh 2008; Rajah, Languay et al. 2010). This is likely because context memory tasks place greater demands on both medial temporal lobe (MTL)-related processes, such as associative/relational encoding (Davachi 2006; Shimamura and Wickens 2009), and prefrontal cortex (PFC)-related processes, such as strategic organization and monitoring (Dobbins et al. 2004; Badre and Wagner 2007; Rajah et al. 2008; Shing et al. 2010), compared with recognition memory tasks. In fact, prior studies have shown that age-related declines in context memory are associated with changes in hippocampus and PFC structure and function (Kukolja et al. 2009; Rajah, Kromas et al. 2010; Spaniol and Grady 2010; Maillet and Rajah 2011; Rajah et al. 2011; Dulas and Duarte 2012). Although previous research has compared age-related differences in the neural correlates of context memory in extreme age groups, relatively little is known regarding the behavioral performance and neural correlates of context memory in middle aged adults (MA). Given that behavioral reductions in context memory tasks are established by the age of 60 years, it is likely that context memory decline emerges earlier in adulthood at midlife.
Cansino et al. have conducted 2 studies using event-related potentials (ERPs) to examine young (YA) (mean age = 22.9 years), MA (mean age = 52 years), and older adults (mean age = 72.4 years) during encoding (Cansino, Trejo-Morales, and Hernandez-Ramos 2010) and retrieval (Cansino et al. 2012) of spatial context information associated with objects. MAs' ability to retrieve spatial contextual details fell mid-way between that of YA and older adults (Cansino et al. 2012). During successful spatial context encoding, the mean amplitude of a sustained positive waveform at posterior cortical sites was found to be greater in MA and older adults compared with YA. At retrieval, Cansino et al. (2012) reported that the distributed pattern of frontal activity during successful context retrieval differed between age groups (Cansino et al. 2012). These results point to the likelihood of frontal and posterior cortical involvement in context memory reductions at midlife. However, given the imaging modality employed, the spatial localization of these effects remains unclear.
Few fMRI studies have investigated episodic memory across the adult lifespan and have examined brain activity in MA during episodic encoding and/or retrieval (Grady et al. 2006; Kennedy et al. 2012; Park et al. 2013; Cansino et al. 2015). Several of these studies employed recognition, not context, memory paradigms wherein performance was matched between YA and MA. Kennedy et al. (2012) used an event-related fMRI design to examine increases and decreases in brain activity during successful encoding of scene stimuli as a function of increasing age. Grady et al. (2006) investigated changes in brain activity during the encoding and retrieval of line drawings and words as a function of increasing age using a blocked fMRI paradigm. fMRI data from both encoding and retrieval were analyzed together, across stimulus types, in the study by Grady et al. (2006). Despite differences in study design, both studies reported increased activity in midline cortical regions, including medial PFC, and decreased activity in ventral visual processing regions, with increasing age. Neither of these studies reported significant changes in MTL function with increasing age. Grady et al. (2006) also reported age-related reductions in lateral PFC activity, whereas Kennedy et al. (2012) reported increased lateral PFC activity with increasing age.
While these 2 previous studies examined age-related differences in brain activation across the lifespan (i.e., in participants aged 20s to 80s), Park et al. (2013) directly compared fMRI activity in YA versus MA, and in MA versus older adults during the successful encoding of spatial scenes. They found that decreased encoding-related activity in ventral visual cortex was apparent by midlife, but that increased encoding-related activity in medial PFC only emerged later in life. Park et al. (2013) also reported similar levels of medial temporal and lateral PFC activity during successful encoding across YA, MA, and older adults. Thus, Park et al. (2013) did not observe changes in PFC activity in MA versus YA. Therefore, previous fMRI studies of episodic memory, in which performance was matched between MA and YA have consistently reported no changes in MTL function and differences in ventral visual cortex function by midlife. However, there has been less consistency in the PFC results reported across studies (Grady et al. 2006; Kennedy et al. 2012; Park et al. 2013).
fMRI studies of episodic memory that have compared PFC activity in YA and older adults have generally reported age-related changes in PFC function. For example, in fMRI studies of episodic memory in which older adults performed significantly worse than younger adults, age-related decreases in PFC activity have been observed (Duarte et al. 2008; Rajah, Languay et al. 2010). In contrast, in studies in which performance was matched between age groups, age-related increases in PFC activity have been reported (Cabeza et al. 2002; Morcom et al. 2007; Davis et al. 2008). There have been several hypotheses put forth to explain the observed age-related change in PFC function (see Maillet and Rajah 2013 for review); however, the Compensation-Related Utilization of Neural Circuits Hypothesis (CRUNCH; Cappell et al. (2010)) and the Scaffolding Theory of Aging and Cognition (STAC; Park and Reuter-Lorenz (2009)) directly address the association between age-related change in PFC activity and task performance. Both theories argue that age-related increases in PFC activity reflect neural compensation for increased neural inefficiency within the PFC, and decreased neural function in posterior cortical regions. Additionally, these models argue that this compensation has limits. Specifically, due to reduced processing efficiency, older adults over-recruit PFC in a compensatory manner at lower difficulty levels. However, older adults may reach processing limits faster than YA. Therefore, at higher levels of task difficulty, one may observe age-related decreases in PFC activity and impaired behavioral performance in older adults (Reuter-Lorenz and Cappell 2008).
Given the fMRI findings showing changes in PFC function during episodic memory in older versus younger adults, it is surprising that prior fMRI studies of episodic memory have not consistently reported changes in PFC function at midlife. One possibility is that by using item recognition tasks in which performance was matched between MA and YA, prior studies were only able to identify functional changes that were apparent when middle aged subjects' episodic memory abilities were sufficient to meet task demands. However, it is possible that additional functional changes at midlife may be identified if one used more challenging episodic memory tasks, such as context memory tasks, which are known to place greater demands on ventral visual (Cansino et al. 2002), medial temporal (Davachi et al. 2003; Kukolja et al. 2009), and PFC function (Slotnick et al. 2003; Rajah et al. 2008; Dulas and Duarte 2012) compared with item recognition.
In the current study, we used event-related fMRI to investigate the neural correlates of successful context memory encoding and retrieval in healthy MA and YA. Subjects were tested on 2 types of context memory tasks to identify task-general effects in the neural correlates of episodic memory: spatial (left/right) context memory and temporal (recency) context memory. Subjects performed easy (low encoding load) and difficult (high encoding load) versions of each task type. Task difficulty was modulated within task type to differentiate between age effects, performance effects, and age-by-performance interactions. Multivariate partial least squares (PLS) (McIntosh et al. 2004; McIntosh and Lobaugh 2004) analysis was used to examine main effects of task, age group, and task difficulty on brain activity during successful context encoding and retrieval, and to examine interactions among these variables. In addition, we used linear regression to examine if ventral visual cortex, MTL, and/or PFC activity predicted retrieval accuracy in YA and/or MA (Davis et al. 2008; Cappell et al. 2010). Based on prior findings, we predicted that MA would show reduced accuracy in spatial and temporal context memory relative to younger adults (Cansino et al. 2012) and that reductions in performance would be linked to changes in ventral occipito-temporal activity (Small et al. 2002; Park et al. 2013; Vuoksimaa et al. 2013) and prefrontal activity (Grady et al. 2006; Cansino et al. 2012).
Materials and Methods
Participants
Thirty-four YA (age range 20–35 years, mean age 26.38 years, mean education 16.50 years, 21 females) and 28 MA (age range 40–56 years, mean age 47.96 years, mean education 15.75 years, 20 females) participated in the study. All subjects were healthy at the time of testing and had no history of neurological or psychological illness. All subjects were right-handed as measured by the Edinburgh Inventory for Handedness (Oldfield 1971).
All participants completed 2 sessions that took place on 2 separate days. The first session involved filling out a medical questionnaire, undergoing a neuropsychological assessment, having their blood pressure measured by a nurse, participating in a practice session of the fMRI task, and donating a blood sample for assessment of cholesterol levels. Session 2 involved undergoing fMRI scanning. During Session 1, we administered the following battery of neuropsychological tests to screen out individuals suffering from psychiatric symptoms and dementia, and to obtain measures of memory and language function: the Mini-International Neuropsychiatric Interview (M.I.N.I.) [inclusion cutoff score ≤2, (Sheehan et al. 1998)], Mini-Mental Status Exam [MMSE, exclusion cutoff score <27], (Folstein et al. 1975)], the Beck Depression Inventory (BDI) [inclusion cutoff <15 (Beck 1987)], the California Verbal Learning Task (CVLT) [exclusion cutoff determined per case using age and education (Norman et al. 2000)], the American National Adult Reading Test (NART) [inclusion cutoff ≤2.5 SD (Spreen and Strauss 1997)]. Additional medical exclusion criteria included having a history of or current diagnosis of diabetes, untreated cataracts and glaucoma, and a current diagnosis of high cholesterol levels and/or high blood pressure left untreated in past 6 months. Moreover, anyone with a first-degree relative who had been diagnosed with Alzheimer's disease, or other neurodegenerative disorder, was excluded from the study. All subjects performed a practice session of the fMRI tasks (described below) in a mock MRI scanner. Only those subjects who met all the cutoff criteria and performed above chance on the practice session in Session 1 were invited to participate in the fMRI scanning Session 2. Session 2 occurred within 1 week of Session 1.
One-way between-group analyses of variance (ANOVAs) were conducted on mean years of education and all neuropsychological measures to determine if there were significant group differences on any of these measures (significance threshold P < 0.05) using SPSS for Windows (Version 17.0). All participants signed a consent form approved by ethics board at the Faculty of Medicine, McGill University.
Task Stimuli
The stimuli were black-and-white photographs of age variant human faces, which were cropped from the neck upwards and rated as either neutral or pleasant by 2 independent raters. The stimuli have been used in prior fMRI studies of memory function conducted by our laboratory (Rajah et al. 2008; Rajah, Languay et al. 2010) and details about the stimuli can be found in Rajah et al. (2008, 2010). Unique stimuli were used for each memory task, and each stimulus list, per task, was balanced for age and sex.
Behavioral Methods
Subjects were told that they would be participating in a computer-based memory experiment for nonfamous faces. Subjects participated in 12 fMRI scanning runs while they performed easy and hard versions of spatial and temporal context memory tasks. Both spatial and temporal tasks were used to determine if there were task-general and task-specific neural correlates of spatial and temporal context memory in both age groups. The difficulty manipulation was added to enable the discrimination of functional changes associated with performance main effects, and age by performance interactions. E-Prime (Psychology Software Tools, Inc.; Pittsburgh, PA, USA) was used to present the behavioral protocol and collect accuracy and reaction time (RT; ms).
Each run consisted of 3 experimental blocks: 1 hard spatial or 1 hard temporal context memory task (depending on run), 1 easy spatial context memory task and 1 easy temporal context memory task. Each run was approximately 9 min long. In total, each subject performed 6 hard spatial tasks, 6 hard temporal tasks, 12 easy spatial tasks and 12 easy temporal tasks, for a total of 36 tasks. The task order was counter-balanced within run and run order was counter-balanced across subjects.
Encoding Blocks
A 9-s instruction screen was presented prior to encoding in order to inform the subjects to memorize either the spatial location or the temporal order (depending on the task) of face stimuli. The instruction screen also informed subjects of whether they would see 6 encoding stimuli (easy tasks) or 12 encoding stimuli (hard tasks). Thus, the difficulty manipulation was related to increased encoding load during hard > easy tasks. [Due to programming issues, 8 young adults and 2 middle aged adults were not informed whether the upcoming memory task was an “easy” (6-faces) or “hard” (12-faces) task, whereas all other subjects were informed of this during the encoding instructions. We ran behavioral analyses as outlined in the Behavioral Data Analysis Section to determine if this altered the memory performance of these subjects compared to the remaining sample and found no significant differences in behavioral performance between groups (task main effect and all interactions >0.195). Additionally, inclusion of these subjects did not alter the significance of any latent variables (LVs) identified in fMRI PLS data analysis. Thus, we included data from all participants in the analyses reported in this paper] Face stimuli were presented one-by-one either to the left or right of a fixation cross on the screen. Each encoding stimulus was presented for 2 s, with a variable ITI (2.2–8.8 s, mean ITI = 4.94 s). During encoding, subjects also had to rate each face as “pleasant” or “neutral.” The neutral/pleasantness rating was incorporated because a previous study revealed improved memory for faces encoded using social-emotional evaluations (Grady 2002).
In between the encoding and retrieval phases, the subject performed a 1-min long alphabetizing task to prevent rehearsal of encoding stimuli. Subjects were presented with 5 word pairs (5 s/word pair) and were asked to indicate which word came first alphabetically. ITIs were varied between each pair.
Retrieval Blocks
A 9-s instruction screen was presented prior to each retrieval block to inform the subjects of whether they had to select the face that had been presented on the left or right (in the spatial task) or most or least recently (in the temporal task). Thus, the instruction (e.g., Select the face that was presented on the left) was the same for all events within a retrieval block. The instruction varied across different retrieval blocks. During the retrieval block, subjects were presented with 3 retrieval events in the easy tasks and 6 retrieval events in the hard tasks. Each retrieval event consisted of 2 black-and-white photographs from the preceding encoding list. The stimuli were presented vertically (one on top and one on bottom of a central fixation cross) to prevent perceptual bias effects as encoding stimuli were presented horizontally. We randomized the temporal “distance” between the retrieval items. Each retrieval stimulus was presented for 6 s, with variable ITI (2.2–8.8 s, mean ITI = 4.94 s). All motor responses were made with the subjects' right (dominant) hand.
Behavioral Data Analysis
SPSS for Windows (version 17.0) was used to conduct a between-group repeated-measures task (2: temporal, spatial) × difficulty (2: easy, hard) ANOVA to examine main effects and group-by-task interactions (significance threshold P < 0.05). Accuracy and reaction time were compared between YA and MA. Independent t-tests and paired t-tests were performed on the relevant independent variables when needed to clarify any significant interaction effects.
fMRI Data Acquisition
Scanning of subjects was performed in a 3-T Siemens Trio scanner at the Douglas Brain Imaging Center. Subjects were asked to lie in a supine position in the MRI scanner while wearing a standard head coil. At the start of the experiment, T1-weighted structural volumes were acquired using a 5-min gradient echo (GRE) ADNI (Alzheimer's Disease Neuroimaging Initiative) sequence [TR = 2300 ms, TE = 2.98 ms, flip angle 9°, 176 1 mm sagittal slices, 1 × 1 × 1 mm voxels, field of view (FOV) 256 mm2]. BOLD (functional) images were acquired using a single-shot T2-weighted gradient EPI pulse sequence (TR = 2000 ms, TE = 30 ms, FOV = 256 mm2, matrix size = 64 × 64, in-plane resolution = 4 × 4 mm) while subjects conducted the aforementioned behavioral tasks. Each whole-brain acquisition consisted of 32 oblique slices of 4.0 mm thickness with no gap, and was acquired along the anterior-posterior commissural plane. A mixed rapid event-related experimental design was used.
Visual stimuli were generated by a PC computer and were back-projected onto a screen placed in the scanner bore, which was made visible to participants by a mirror mounted within the standard head coil. E-Prime presentation software (Psychology Software Tools, Inc.; Pittsburgh, PA, USA) was used to run the experimental protocol and collect behavioral data. Participants requiring correction for visual acuity wore plastic optical corrective glasses. A fiber-optic 4-button response box was used to perform experimental tasks.
Functional Image Processing and Analysis
Images were reconstructed from raw k-space and were converted to ANALYZE format and subsequent image processing was conducted using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm) run with MATLAB (www.mathworks.com) on a Linux platform. Images from the first 10 s of each run were discarded to control for field in-homogeneities. ArtRepair (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html) was used to correct for slice and volume artifacts. Functional images were spatially realigned to the first image acquired, to correct for movement artifact, using a 6-parameter rigid body spatial transform and a least squares approach. Subjects with head motion >4 mm are typically discarded from the analysis; however, we did not have any such subjects in the current sample. Individual subjects' functional images were spatially normalized to the MNI EPI-template available in SPM8 at 4 × 4 × 4 mm cubic voxel resolution. Images were then smoothed using 8-mm full-width half maximum (FWHM) isotropic Gaussian kernel, to minimize inter-participant anatomic variability (Friston 2004).
Multivariate fMRI Data Analysis
Multivariate spatio-temporal PLS (McIntosh et al. 2004) was conducted on fMRI data with PLSGUI software (http://www.rotman-baycrest.on.ca/index.php?section=84). For all analyses, only the data from correct encoding and retrieval events were analyzed. All subjects had a minimum of 14 correct events per event type included in the analysis. PLS was used to assess task- and age-related similarities and differences in event-related brain activity, using a set of prespecified contrasts (see Table 1). The fMRI data for both groups was stored in a between-group data matrix or “datamat” and represented event-related data for following 8 events: 1) correct spatial context encoding—easy task events (eSE), 2) correct temporal context encoding—easy task events (eTE), 3) correct spatial context encoding—hard task events (eSH), 4) correct temporal context encoding—hard task events (eTH), 5) correct spatial context retrieval—easy task events (rSE), 6) correct temporal context retrieval—easy task events (rTE), 7) correct spatial context retrieval—hard task events (rSH), 8) correct temporal context retrieval—hard task events (rTH). The rows of the datamat represent the mean event-related activity for each of the aforementioned event types, stacked by age group (YA first, then MA). In the present study, there were 62 subjects (34 YA and 28 MA) and 8 event types, for a total of 496 rows. The columns in the datamat represent the signal from each voxel at each time lag. Each time lag contains data for a 2 s period, with the first time lag coinciding with event onset. In this study, 8 time lags were included, thereby including activation spanning 16 s after event onset to encapsulate the entire breadth of the hemodynamic response function. The signal was zeroed at event onset, and expressed as a percentage deviation from this baseline in subsequent time lags.
Table 1.
Contrasts included in the nonrotated PLS
| Contrast number | Contrast | Event types |
|---|---|---|
| Group similarities | ||
| 1 | Encoding > retrieval main effect | eSE, eSH, eTE, eTH > rSE, rTE, rSH, rTH |
| 2 | Spatial > temporal encoding main effect | eSE, eSH > eTE, eTH |
| 3 | Spatial > temporal retrieval main effect | rSE, rSH > rTE, rTH |
| 4 | Easy > hard encoding main effect | eSE, eTE > eSH, eTH |
| 5 | Easy > hard retrieval main effect | rSE, rSE > rSH, rTH |
| Group differences | ||
| 6 | Encoding > retrieval group interaction | Young: eSE, eSH, eTE, eTH > rSE, rSH, rTE, rTH Middle aged: rSE, rTE, rSH, rTH > eSE, eTE, eSH, eTH |
| 7 | Spatial > temporal encoding, group interaction | Young: eSE, eSH > eTE, eTH Middle aged: eTE, eTH > eSE, eSH |
| 8 | Spatial > temporal retrieval, group interaction | Young: rSE, rSH > rTE, rTH; Middle aged: rTE, rTH > rSE, rSH |
| 9 | Easy > hard encoding, group interaction | Young: eSE, eTE > eSH, eTH; Middle aged: eSH, eTH > eSE, eSE |
| 10 | Easy > hard retrieval, group interaction | Young: rSE, rTE > rSH, rTH; Middle aged: rSH, rTH > rSE, rSE |
| 11 | Young > middle aged group main effect | All event types in young > All event types in middle aged |
Note: eSE, encoding spatial easy; eSH, encoding spatial hard; eTE, encoding temporal easy; eTH, encoding temporal hard; rSE, retrieval spatial easy; rSH, retrieval spatial hard; rTE, retrieval temporal easy; rTH, retrieval temporal hard.
The dot product of the between-group data matrix and the contrast matrix was calculated. These contrasts examined group main effects, event-type (task) main effects, difficulty level main effects, and group-by-task and difficulty interactions at encoding and retrieval (see Table 1). This resulting dot-product matrix was then subjected to singular value decomposition, yielding a set of 11 LVs, each containing a matrix of voxel saliences and task saliences, which represent the 11 aforementioned contrasts from Table 1, and their associated singular values. Voxel saliences represent the weighted contribution of each voxel, at each time lag for each of the 11 contrasts. Voxel saliences can have either a positive weight, reflecting a positive relation to the given contrast, or a negative weight, reflecting a negative relation to the given contrast. The singular value indicates the strength of the association between activity in the brain voxels and each contrast of interest. Significance of PLS LVs was based on permutation tests (P < 0.05, 1000 permutations) on the singular values. The stability of each voxel's contribution to a latent variable was assessed with bootstrapping (bootstrap ratio = ±3.28, P < 0.001, 1000 iterations; minimum cluster size = 10). To determine at which time lags the task differences in a given LV were strongest, we also computed temporal brain scores for each task in each significant LV. Peak coordinates are only reported from time lags at which task differences were maximal. These peak coordinates were converted to Talairach space using the icbm2tal transform (Lancaster et al. 2007) as implemented in GingerAle 2.3 (Eickhoff et al. 2009). Since our acquisition incompletely acquired the cerebellum, peak coordinates from this region are not reported. The Talairach and Tournoux atlas (Talairach and Tounoux 1988) was used to identify the Brodmann area (BA) localizations of significant activations.
To illustrate the age-related changes in brain activation in our tasks, we extracted the mean, baseline corrected, percent signal change from the following a regions of interest (ROIs) from significant LVs: occipito-temporal, medial temporal and prefrontal cortices. We chose to focus on occipito-temporal and PFC regions because prior studies have reported age-related changes in these regions at midlife (Grady et al. 2006; Park et al. 2013), and we also examined MTL activation since this region is generally implicated in age-related changes in memory function (Van Petten 2004; Sperling 2007; Park and Reuter-Lorenz 2009; Spaniol et al. 2009). Activation was extracted from a 1 mm sphere centered on the peak coordinates found in these ROIs from specific contrast and plotted to show patterns of activation across tasks and age groups. In addition, we conducted exploratory post hoc group (2: young, middle age) × task (2: temporal, spatial) × difficulty (2: easy, hard) repeated-measures ANOVAs for activity in these ROIs. This was done to verify the PLS results and also to investigate if some ROIs exhibited additional effects, beyond the one defined by the PLS results. Thus, the results from these post hoc ANOVAs are exploratory and not confirmatory (Bender and Lange 2001). Only the highest order significant post hoc results are presented (e.g., if there was a significant difficulty effect and a task × difficulty interaction, only the interaction effect will be presented).
Linear Regression Analysis—Predicting Accuracy from Brain Activity
An additional goal of this study was to determine if activity in ventral visual, medial temporal, and/or prefrontal cortices predicted task accuracy in YA and MA during spatial easy (SE), spatial hard (SH), temporal easy (TE), and temporal hard (TH) tasks, respectively. We used SPSS to conduct backward elimination regression models (P-value for inclusion = 0.05; P-value for exclusion = 0.10) to achieve this goal. We constructed regression models for each context memory task in which the dependent variable was the mean retrieval accuracy for the given task. The predictor variables included age (in years), and the mean baseline corrected activity during lags 2–4 for 1-mm sphere surrounding ROIs identified from the significant PLS results. The predictor variables were the same for all models tested.
We conducted descriptive analyses of all predictor variables selected to identify extreme datapoints (>3 SD), which reflected extreme activation levels for a given predictor ROI in a specific subject, during a specific task. All extreme datapoints were removed before the regression analyses were conducted. For each task, we used 2 different approaches to identify significant predictors: 1) First, we tested across age-group regression models in which data from both age groups were included in the same model, and age was also included as a continuous variable predictor. This approach allowed us to identify brain areas in which activity predicts memory performance across age. 2) Second, we tested within age-group regression models in which the data from each group were split. In these models, age was still included as a predictor to account for the within group variance in age. This approach does not assume that there is continuity in the neural correlates of context memory from young adulthood to middle age, and allowed us to identify unique predictors of memory performance within each age group. Therefore, in total there were 12 models tested: 4 models (SE, SH, TE, TH) which included both age groups, 4 models that included only YA, 4 models for MA only.
To address concerns about potential multicollinearity among predictor variables included in our regression analyses, we ensured that the variance inflation factors (VIF) listed for the full model, prior to the start of the backward elimination process, did not exceed 10 for any predictor entered in the model (Mason and Perreault 1991; O'Brien 2007). If, for a specific model, a predictor variable had a VIF >10 in the full model, we investigated if including this variable biased which model was identified as being most significant by re-running the regression excluding this variable. If exclusion altered the significant model identified, we report the most significant model identified after the removal suspect variable(s) and state which variables were removed due to concerns with multicollinearity in the Results section.
If there were competing significant models we used the R-change and F-statistic change (P > 0.05) for assessing goodness-of-fit and for determining which of the competing models from the step-wise elimination process best predicted accuracy. If there was no change in F-statistic probability (P > 0.05) from removing a predictor variable, this indicated that the removed variable did not add any predictive value to the model, and it was acceptable to remove it from the model. We report β-values for all predictors in the reduced model that best predicts accuracy during SE, SH, TE, and TH tasks in relevant tables, but only discuss significant predictors (t-statistic P < 0.05) in the Results section.
Results
Behavior
Neurospsychological Tests
Table 2 displays group means for years of education and each of the administered neuropsychological tests in YA and MA. One-way between-group ANOVAs indicated that there was a significant group difference in the CVLT long-form free recall test [F1,60 = 5.64, P = 0.02]. No other significant group differences were found.
Table 2.
Group means for education and neuropsychological tests
| Group | Education (years) | MMSE | BDI | NART | LFCVLT | LCRCVLT | RGCVLT |
|---|---|---|---|---|---|---|---|
| Young adults | |||||||
| Mean | 16.50 | 29.76 | 3.56 | 41.26 | 13.76* | 13.97 | 15.41 |
| SE | 0.25 | 0.09 | 0.65 | 0.89 | 0.29 | 0.28 | 0.13 |
| Middle aged adults | |||||||
| Mean | 15.75 | 29.50 | 4.29 | 41.27 | 12.40* | 13.05 | 15.32 |
| SE | 0.35 | 0.14 | 0.83 | 1.20 | 0.47 | 0.41 | 0.15 |
Notes: This table presents the group means and standard errors (SE) for education and other neuropsychological measures taken.
MMSE, mini-mental status examination; BDI, Beck Depression Inventory; NART, American National Adult Reading Test; LFCVLT, CVLT, long-from free recall; LCRCVLT, CVLT, long-form category-assisted recall; RGCVLT, CVLT, long-term recognition.
*Significant group differences.
fMRI Tasks
Group mean accuracy (percent correct) and reaction time (RT; ms) are shown in Table 3.
Table 3.
Mean retrieval reaction time (RT) and accuracy in scanned tasks
| Group | Spatial easy | Spatial hard | Temporal easy | Temporal hard |
|---|---|---|---|---|
| Young adults | ||||
| Mean RT (ms) | 2248.29 (80.15) | 2378.72 (78.53) | 2630.27 (89.14) | 2790.35 (95.23) |
| Mean accuracy | 0.88 (0.01) | 0.87* (0.02) | 0.76* (0.02) | 0.68* (0.02) |
| Middle aged adults | ||||
| Mean RT (ms) | 2590.67 (99.79) | 2703.23 (88.87) | 2959.38 (95.15) | 3083.78 (100.29) |
| Mean accuracy | 0.85 (0.02) | 0.79* (0.03) | 0.66* (0.03) | 0.57* (0.02) |
Note: Accuracy values are shown as proportion correct per task type with standard error (SE). Reaction time values are shown in milliseconds (ms) per task type with SE.
*Significant group differences in mean accuracy.
Accuracy
The group (2: young, middle age) × task (2: temporal, spatial) × difficulty (2: easy, hard) repeated-measures ANOVA for retrieval accuracy revealed significant main effects for task [F1,60 = 318.36 P < 0.001], difficulty [F1,60 = 52.33 P < 0.001], and group [F1,59 = 11.28 P = 0.001]. In addition, significant task × difficulty [F1,60 = 10.13 P = 0.002] and group × task [F1,60 = 6.15 P = 0.016] interaction effects were found.
To clarify the task × difficulty interaction, we conducted post hoc paired sample t-tests to compare accuracy in easy > hard versions within task type, collapsed across groups. The results indicate that collapsed across group there was a significant difficulty effect in both tasks, but that the effect was larger for temporal > spatial tasks (accuracy during SE > SH: t(1,61) = 3.17, P = 0.002; accuracy during TE > TH (1,61) = 6.42, P < 0.001). However, Table 2 indicates that the difficulty effect in spatial tasks was likely driven by MA since the mean score during SE and SH tasks was similar in YA (SE mean accuracy = 0.88 and SH mean accuracy = 0.87). To verify this, we conducted post hoc within group paired samples t-tests to test for the effect of difficulty within task type. These results confirm that, in YA, there was no significant difference in accuracy between SE versus SH tasks (t < 1), but there was a significant difference in accuracy between TE versus TH tasks (t(1,33) = 4.86, P < 0.001). In MA, there were significant differences in task difficulty for both task types (SE vs. SH, t(1,27) = 3.89, P = 0.001; TE vs. TH, t(1,27) = 4.16, P < 0.001).
To clarify the group × task interaction, we conducted post hoc independent samples t-tests to determine if there were group differences during SE, SH, TE, and TH tasks, respectively. There was no significant group difference in retrieval accuracy during the SE task (t(1,60) = 1.34, P > 0.05), but there were significant group differences in retrieval accuracy for all other tasks (SH, t(1,60) = 2.68, P = 0.01; TE, t(1,60) = 2.98, P = 0.004; TH, t(1,60) = 3.93, P < 0.001).
Reaction time (RT, ms)
The group (2: young, middle age) × task (2: temporal, spatial) × difficulty (2: easy, hard) repeated-measures ANOVA for correct retrieval RT revealed significant main effects of task [F1,60 = 67.31 P < 0.001] and difficulty [F1,60 = 23.47 P < 0.001]. Participants responded more quickly on the spatial > temporal tasks and on easy > hard tasks, respectively. No other significant main effects or interactions were observed.
fMRI Results
Between-Group PLS Results
Five latent variables (LVs) were significant based on permutation testing: 1) LV 1: main effect of encoding > retrieval (P < 0.001; 46% cross-block variance), 2) LV 4: main effect of easy > hard encoding (P < 0.035, 6% cross-block variance), 3) LV 5: main effect of easy > hard retrieval (P < 0.001, 11% cross-block variance), 4) LV 6: interaction of group-by-encoding > retrieval (P < 0.034; 6% cross-block variance), and 5) LV 10: interaction of group-by easy > hard retrieval (P < 0.003; 8% cross-block variance). The whole-brain PLS results for each LV are presented in Tables 4–8 and Figures 1 and 2. Temporal brain scores indicated that for each LV, task differences were maximal in time lags 2–4 (4–8 s poststimulus onset). Therefore, we only report peak coordinates from these lags in Tables 4–8. In addition, when a peak coordinate was found in more than one time lag, we only report it once, at the time lag where the bootstrap ratio was maximal. In the following sections, we present the results for each significant LV.
Table 4.
Local maxima for LV 1: encoding versus retrieval main effect
| Temporal lag | Bootstrap ratio | Spatial extent | Talairach coordinates |
HEM | Gyral location | Brodmann area | Significant post hoc ANOVA results | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Encoding > retrieval | |||||||||
| Left hemisphere | |||||||||
| 2–4 | 12.70 | 2841 | −9 | −80 | −7 | Left | Lingual gyrus | 18*# | E: task × difficulty R: group × difficulty |
| 2, 3 | 9.11 | 1512 | −8 | 45 | 23 | Left | Medial frontal gyrus | 9*# | E: none R: group × task × difficulty |
| 2 | 8.76 | 1839 | −57 | −37 | 21 | Left | Insula | 13 | |
| 4 | 8.18 | 557 | −60 | −42 | −4 | Left | Middle temporal gyrus | 21*# | E: task × difficulty R: none |
| 2, 4 | 7.51 | 283 | −8 | 29 | 0 | Left | Anterior cingulate | 24 | |
| 2 | 7.38 | 121 | −6 | −53 | 68 | Left | Postcentral gyrus | 7 | |
| 4 | 4.94 | 112 | −35 | 21 | 45 | Left | Middle frontal gyrus | 6/8 | |
| 2, 4 | 4.49 | 32 | −20 | −8 | 28 | Left | Caudate | ||
| 4 | 4.43 | 12 | −49 | 25 | −1 | Left | Inferior frontal gyrus | 47*# | E: difficulty R: none |
| 2 | 4.40 | 15 | −38 | 10 | −35 | Left | Anterior temporal gyrus | 38*# | E: difficulty R: none |
| 2 | 4.01 | 10 | −5 | −16 | 35 | Left | Cingulate gyrus | 24 | |
| 4 | 3.81 | 11 | −35 | 37 | 29 | Left | Superior frontal gyrus | 9*# | E: task × difficulty R: group × task |
| Right hemisphere | |||||||||
| 2–4 | 10.29 | 1177 | 54 | −46 | 30 | Right | Supramarginal gyrus | 40 | |
| 2, 4 | 6.50 | 46 | 40 | 17 | −37 | Right | Anterior temporal gyrus | 38*# | E:group × task × difficulty R: none |
| 4 | 5.84 | 93 | 17 | −4 | 29 | Right | Caudate | ||
| 4 | 5.28 | 99 | 55 | −20 | −4 | Right | Middle temporal gyrus | 21*# | E: none R: difficulty |
| 4 | 4.50 | 17 | 40 | −39 | −2 | Right | Parahippocampal gyrus*# | E: task × difficulty R: none |
|
| 3 | 4.02 | 10 | 51 | 36 | 5 | Right | Inferior frontal gyrus | 45*# | E: difficulty R: task |
| 4 | 3.98 | 18 | 58 | −5 | 30 | Right | Precentral gyrus | 6 | |
| 4 | 3.90 | 29 | 2 | −28 | 38 | Right | Cingulate gyrus | 31 | |
| Retrieval > encoding | |||||||||
| Left hemisphere | |||||||||
| 2–4 | −10.50 | 1156 | −38 (−40 |
−52 −82 |
−23 −3)a |
Left | Cerebellum extending to lateral occipital | Culmen/BA 18 | |
| 2, 4 | −9.14 | 60 | −27 | 21 | 2 | Left | Claustrum | ||
| 2, 3 | −8.82 | 164 | −38 | 3 | 29 | Left | Precentral gyrus | 6 | |
| 2 | −7.69 | 99 | −38 | 7 | 30 | Left | Inferior frontal gyrus | 6/44 | |
| 4 | −6.03 | 39 | −38 | −6 | 10 | Left | Insula | 13 | |
| 2–4 | −5.89 | 53 | −38 | 51 | 8 | Left | Middle frontal gyrus | 10*# | E: task × difficulty, R: group × difficulty |
| Right hemisphere | |||||||||
| 4 | −14.30 | 1509 | 40 | −76 | −9 | Right | Fusiform gyrus | 19*# | E: task × difficulty R: group × difficulty and task × difficulty |
| 2, 4 | −9.75 | 465 | 29 | 21 | 3 | Right | Claustrum | ||
| 4 | −7.44 | 223 | 6 | 9 | 48 | Right | Superior frontal gyrus | 6 | |
| 4 | −6.96 | 149 | 10 | −13 | 7 | Right | Thalamus | ||
| 2 | −5.88 | 201 | 10 | 9 | 5 | Right | Caudate | ||
| 2 | −5.02 | 44 | 39 | 18 | 28 | Right | Middle frontal gyrus | 9*# | E: task × difficulty R: group × difficulty and task × difficulty |
Notes: Temporal lag represents the time after event onset, when a cluster of voxels exhibited a contrast effect of interest. The bootstrap ratio threshold was set to ±>3.28, and identified dominant and stable activation clusters. The spatial extent refers to the total number of voxels included in the voxel cluster (threshold = 10). The stereotaxic coordinates are measured in millimeters, and gyral location and Brodmann areas (BAs) were determined by referring to Talairach and Tournoux (1988). The last column presents the highest order significant effect (P < 0.05) observed from the ROI-based post hoc group × task × difficulty repeated-measures ANOVAs. Regions marked with * were ROIs for which mean activity was extracted and plotted in a bar graph. Regions marked with # were ROIS included in the brain–behavior regression analyses. E, significant effects for encoding activity; R, significant effects for retrieval activity; HEM, cerebral hemisphere in which the activation occurred.
aThis peak coordinate was obtained at BSR = 7.6 (P < 0.00001) at which the large ROI extending from of left culmen to lateral occipital was broken up into smaller peaks, including this one in the left lateral occipital.
Table 8.
Local maxima for LV 10: easy versus hard retrieval events, group interaction
| Temporal lag | Bootstrap ratio | Spatial extent | Talairach coordinates |
HEM | Gyral location | Brodmann area | Significant post hoc ANOVA results | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Young easy > hard, MA hard > easy | |||||||||
| Left hemisphere | |||||||||
| 3 | 4.33 | 23 | −23 | 24 | 10 | Left | Claustrum | ||
| 3 | 4.28 | 16 | −20 | 4 | 66 | Left | Superior frontal gyrus | 6 | |
| 2 | 3.99 | 15 | −34 | 24 | 10 | Left | Insula | 13 | |
| 2 | 3.96 | 42 | −35 | −83 | −8 | Left | Middle occipital gyrus | 18*# | R: group × difficulty and task × difficulty |
| 2 | 3.90 | 21 | −12 | −17 | 6 | Left | Thalamus | ||
| 3 | 3.72 | 12 | −45 | −56 | −16 | Left | Fusiform gyrus | 37* | R: group × difficulty |
| Right hemisphere | |||||||||
| 3, 4 | 4.99 | 21 | 47 | −18 | −22 | Right | Inferior temporal gyrus | 20* | R: group × difficulty |
| 2, 3 | 4.99 | 253 | 36 | −79 | −13 | Right | Middle occipital/fusiform gyrus | 18/19* | R: group × difficulty and task × difficulty |
| 2–4 | 4.84 | 33 | 18 | 40 | −2 | Right | Middle and medial frontal gyrus | 10*# | R: group × difficulty |
| 2 | 3.94 | 61 | 6 | −13 | 3 | Right | Thalamus | ||
| 3 | 3.89 | 10 | 10 | −2 | 4 | Right | Lentiform nucleus | ||
| 3 | 3.86 | 10 | 66 | −43 | −2 | Right | Middle temporal gyrus | 21* | R: group × difficulty |
| 3 | 3.84 | 11 | 36 | 37 | −6 | Right | Inferior frontal gyrus | 47* | R: group × difficulty |
Note: Temporal lag represents the time after event onset, when a cluster of voxels exhibited a contrast effect of interest. The bootstrap ratio threshold was set to ±>3.28, and identified dominant and stable activation clusters. The spatial extent refers to the total number of voxels included in the voxel cluster (threshold = 10).The stereotaxic coordinates are measured in millimeters, and gyral location and Brodmann areas (BAs) were determined by referring to Talairach and Tournoux (1988). The last column presents the highest order significant effect (P < 0.05) observed from the ROI-based post hoc group × task × difficulty repeated-measures ANOVAs. R, significant effects for retrieval activity; HEM, cerebral hemisphere in which the activation occurred. Regions marked with # were ROIs for which: 1) mean activity was extracted and plotted in a bar graph and 2) were ROIS included in the brain–behavior regression analyses.
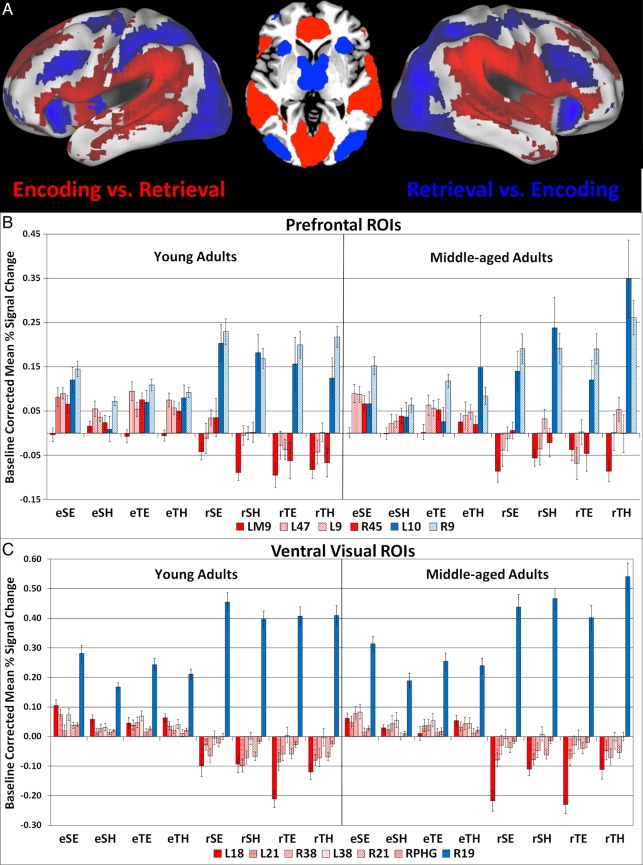
Figure 1.
(A) The singular image for Contrast 1, Encoding > Retrieval, at a bootstrap ratio of ±3.28, (P < 0.001), which reflects reliable activations at time lags 2–4. Red regions were activated to a greater extent at Encoding > Retrieval, while blue regions showed the opposite effect. (B) Bar graph representing mean activation with standard error bars in regions of interest in the prefrontal cortex in this contrast. (C) Bar graph representing mean activation with standard error bars in ventral visual and PHC regions of interest in this contrast. Regions are identified by their hemisphere and Brodmann area. L, left; R, right; eSE, easy spatial encoding; eSH, hard spatial encoding; eTE, easy temporal encoding; eTH, hard temporal encoding; rSE, easy spatial retrieval; rSH, hard spatial retrieval; rTE, easy temporal retrieval; rTH, hard temporal retrieval.
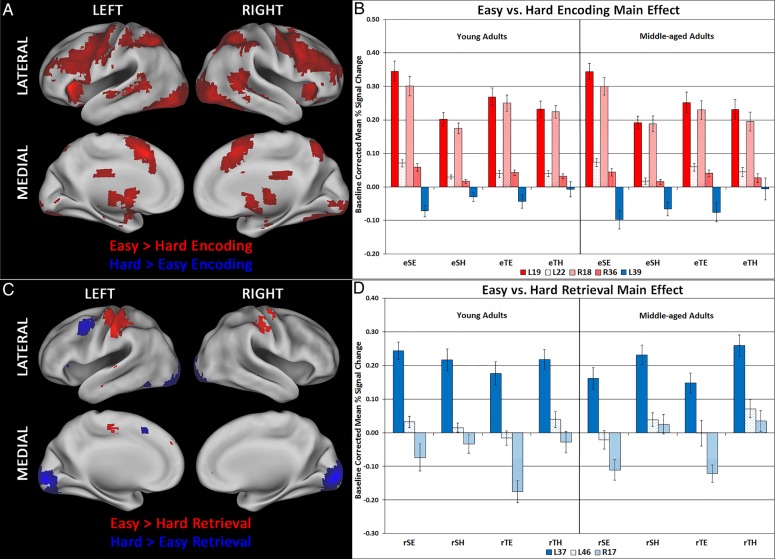
Figure 2.
(A) The singular image for Contrast 4, Easy > Hard Encoding, at a bootstrap ratio of ±3.28 (P < 0.001), which reflects reliable activations at time lags 2–4. Red regions were activated to a greater extent during easy > hard encoding. A single small region (BA39) showed the opposite effect; this region did not display using Caret software. (B) Bar graph representing mean activation with standard error bars in regions of interest in this contrast. (C) The singular image for Contrast 5, Easy > Hard Retrieval, at a bootstrap ratio of ±3.28 (P < 0.001), which reflects reliable activations at time lags 2–4. Red regions were activated to a greater extent in Easy > Hard Retrieval, while blue regions showed the opposite effect. (D) Bar graph representing mean activation with standard error bars in regions of interest in this contrast. Regions are identified by their hemisphere and Brodmann area. L, left; R, right; eSE, easy spatial encoding; eSH, hard spatial encoding; eTE, easy temporal encoding; eTH, hard temporal encoding; rSE, easy spatial retrieval; rSH, hard spatial retrieval; rTE, easy temporal retrieval; rTH, hard temporal retrieval.
LV 1: encoding versus retrieval events, main effect
Figure 1A and Table 4 present the whole-brain PLS results for LV 1: encoding versus retrieval main effect. This contrast identified regions that were differentially activated during all encoding events (eSE, eSH, eTE, and eTH) compared with all retrieval events (rSE, rSH, rTE, and rTH), in both age groups. During encoding, compared with retrieval, there was greater activation in several regions in both age groups including: left dorsomedial PFC (BA 9), bilateral ventrolateral PFC (VLPFC, left BA 47 and right BA 45), left dorsolateral PFC (DLPFC, left BA 9), left medial occipital cortex (BA18), bilateral anterior temporal gyrus (BA 38), bilateral middle temporal gyrus (BA21), and right parahippocampal cortex (PHC). During retrieval, compared with encoding, there was increased activity in bilateral regions of lateral occipital/fusiform cortex (left BA 18, right BA 19), left anterior PFC (APFC, BA 10), and right DLPFC (BA 9) in both age groups.
Given our interest in occipito-temporal, medial temporal, and prefrontal cortices (see Materials and Methods), we extracted the baseline corrected, mean percent signal change for these ROIs (marked by asterisks in Table 4) and plotted these activation profiles in Figure 1B (prefrontal ROIs) and Figure 1C (ventral visual and medial temporal ROIs). These plots indicated that although all the brain regions identified in LV1 exhibited a main effect of encoding versus retrieval, several of the brain regions also appeared to be modulated by other aspects of the study design. To examine this further, we conducted exploratory post hoc group (2: young, middle age) × task (2: temporal, spatial) × difficulty (2: easy, hard) repeated-measures ANOVAs for encoding activity and for retrieval activity, separately, for the ROIs depicted in Figure 1B,C. The results from these post hoc analyses are presented in the last column of Table 4. Only the highest order significant effects identified for encoding activity, and for retrieval activity, are presented.
LV 1: Post Hoc ANOVAs of Encoding Activity in ROIs
During encoding, the post hoc analyses indicated that there was a significant group × task × difficulty interaction in right anterior temporal cortex activity (BA 38). Within group pair-wise comparisons indicated this effect was due to there being more activity in this region during TE, compared with SE tasks, in YA (P < 0.001), but there being no other significant pair-wise effects in this region in either age group. In addition, there were significant task × difficulty interactions in encoding activity in several LV1 ROIs, such as: left APFC (BA 10), bilateral DLPFC (BA 9), right PHC, left middle temporal cortex (BA 21), right fusiform gyrus (BA 19), and medial occipital cortex (BA 18). There was greater activity in these regions during SE, compared with SH, encoding tasks, but similar levels of activity during TE and TH encoding tasks in both age groups.
In 3 LV1 ROIs, there were significant difficulty main effects in encoding activity; bilateral VLPFC and left anterior temporal cortex (BA 38). Activity in these regions was greater during easy spatial and temporal encoding tasks, compared with hard spatial and temporal encoding tasks, in both age groups.
LV 1: Post Hoc ANOVAs of Retrieval Activity in ROIs
The post hoc ANOVAs indicate that there were significant group × difficulty interactions in retrieval activity in the following LV1 ROIs: left APFC (BA 10), right DLPFC (BA 9), right fusiform gyrus (BA 19), and left medial occipital cortex (BA 18). In addition, there were significant task × difficulty interactions in retrieval activity in right DLPFC and right fusiform gyrus. Although these regions exhibited similar post hoc effects, the underlying pattern of activity in each of these regions was different. For instance, in the case of left APFC and right fusiform gyrus, these effects were due to activity in these regions being greater during hard compared with easy spatial and temporal context memory tasks in MA alone. In contrast, in YA, activity in these 2 regions was relatively the same across all retrieval tasks. In the case of left medial occipital cortex (BA 18), the significant group × difficulty interaction was because there was reduced activity in this region, relative to baseline (increased de-activation) during easy retrieval tasks, compared with hard retrieval tasks, in MA; and similar levels of activity in this region across retrieval tasks YA, with the exception of TE retrieval tasks.
There was a significant group × task effect in retrieval activity in left DLPFC (BA 9) because there was increased activity in this region during hard, compared with easy, retrieval tasks in MA; and increased activity in this region during SE tasks, compared with TE tasks, in YA. In addition, there was a significant group × task × difficulty interaction in left medial PFC (BA 9) activity. This was because there was an opposite task × difficulty activation pattern in this region for YA versus MA (see Fig. 1B). In YA activity in left dorsomedial PFC was lower relative to baseline during SH and TE retrieval tasks, compared with SE and TH retrieval tasks. In MA activity in this region was lower relative to baseline during SE and TH retrieval tasks, compared with SH and TE retrieval tasks.
In summary, LV1 ROIs were differentially activated during context encoding versus context retrieval. In addition, the exploratory post hoc ANOVAs also indicate that these ROIs exhibited other experiment effects. These observations are consistent with the observation that many of the LV1 ROIs were also identified in subsequent LVs.
LV4: Easy versus Hard Encoding Events, Main Effect
Figure 2A and Table 5 present the whole-brain PLS results for LV4: group similarities in brain activity during easy, compared with hard, encoding events. There was more activity in a variety of brain regions during easy, compared with hard, encoding tasks in both age groups, including: bilateral middle occipital cortex activity (right BA 18, left BA 19), left middle/anterior temporal cortex (BA 21/22) and right PHC (BA 36). We extracted the mean activity for regions marked with asterisks in Table 5, and plotted these values in Figure 2B. The activation profiles indicate there was increased deactivation in left middle temporal cortex (BA 39) during easy, compared with hard, encoding tasks in both age groups. Figure 2B also suggests that the easy versus hard encoding activity effect in many regions was driven by the spatial task. We conducted exploratory post hoc group × task × difficulty repeated-measures ANOVAs to examine this. The results from these analyses are presented in the last column of Table 5.
Table 5.
Local maxima for LV 4: easy versus hard encoding main effect
| Temporal lag | Bootstrap ratio | Spatial extent | Talairach coordinates |
HEM | Gyral location | Brodmann area | Significant post hoc ANOVA results | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Easy > hard encoding | |||||||||
| Left hemisphere | |||||||||
| 4 | 7.93 | 737 | −38 | −79 | −15 | Left | Fusiform gyrus | 19* | E: task × difficulty |
| 4 | 7.00 | 1322 | −46 | −10 | 53 | Left | Precentral gyrus | 4 | |
| 2, 3 | 6.50 | 675 | −27 | 20 | 9 | Left | Claustrum | ||
| 2, 3 | 5.95 | 163 | −9 | 13 | 45 | Left | Anterior cingulate | 32 | |
| 2–4 | 5.20 | 107 | −49 | −43 | −1 | Left | Middle/anterior temporal gyrus | 21/22* | E: task × difficulty |
| 3 | 4.91 | 176 | −31 | −51 | 42 | Left | Inferior parietal lobule | 40 | |
| 2 | 4.83 | 225 | −28 | −62 | 41 | Left | Superior parietal lobule | 7 | |
| 2 | 4.27 | 28 | −46 | −18 | 59 | Left | Postcentral gyrus | 3 | |
| Right hemisphere | |||||||||
| 3, 4 | 8.28 | 645 | 25 | −87 | −11 | Right | Inferior occipital gyrus | 18* | E: task × difficulty |
| 3, 4 | 6.56 | 1325 | 43 | −3 | 55 | Right | Precentral gyrus | 6 | |
| 2 | 6.31 | 214 | 18 | 10 | −2 | Right | Lentiform nucleus | ||
| 4 | 5.89 | 94 | 43 | −27 | −8 | Right | Parahippocampal gyrus | 36* | E: task × difficulty |
| 2–4 | 5.70 | 205 | 28 | −58 | 42 | Right | Superior parietal lobule | 7 | |
| 2 | 5.44 | 351 | 39 | 7 | 27 | Right | Inferior frontal gyrus | 6/44 | |
| 3 | 5.37 | 193 | 29 | 21 | 3 | Right | Claustrum | ||
| 3 | 4.55 | 44 | 2 | −30 | 27 | Right | Cingulate gyrus | 23 | |
| Hard > easy encoding | |||||||||
| Left hemisphere | |||||||||
| 4 | −3.78 | 13 | −46 | −75 | 29 | Left | Middle temporal gyrus | 39* | E: difficulty |
Note: Temporal lag represents the time after event onset, when a cluster of voxels exhibited a contrast effect of interest. The bootstrap ratio threshold was set to ±>3.28, and identified dominant and stable activation clusters. The spatial extent refers to the total number of voxels included in the voxel cluster (threshold = 10).The stereotaxic coordinates are measured in millimeters, and gyral location and Brodmann areas (BAs) were determined by referring to Talairach and Tournoux (1988). The last column presents the highest order significant effect (P < 0.05) observed from the ROI-based post hoc group × task × difficulty repeated-measures ANOVAs. Regions marked with * were ROIs for which mean activity was extracted and plotted in a bar graph. E, significant effects for encoding activity. HEM, cerebral hemisphere in which the activation occurred.
There were significant task × difficulty interactions in encoding activity in bilateral occipital cortex, left middle/anterior temporal cortex, and right PHC. This was due to there being greater activity in these regions during SE, compared with SH, encoding tasks, but similar activity in these regions during TE and TH encoding, in both age groups. These results are consistent with the effects observed for similar peaks from LV 1 at encoding.
LV 5: Easy versus Hard Retrieval Events, Main Effect
Figure 2C and Table 6 present the whole-brain PLS results for LV 5: group similarities in brain activity during easy, compared with hard, retrieval events. There was more activity in left fusiform gyrus (BA37), right cuneus (BA17), and left middle frontal gyrus (BA46) during hard, compared with easy, retrieval tasks in both age groups. To further explore the activity patterns in these ROIs, we extracted the baseline corrected, mean percent signal change in these ROIs (marked by asterisks in Table 6) and plotted them in Figure 2D. We also conducted post hoc group × task × difficulty repeated-measures ANOVAs on these ROIs. The results are presented in the last column of Table 6.
Table 6.
Local maxima for LV 5: easy versus hard retrieval main effect
| Temporal lag | Bootstrap ratio | Spatial extent | Talairach coordinates |
HEM | Gyral location | Brodmann area | Significant post hoc ANOVA results | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Easy > hard | |||||||||
| Left hemisphere | |||||||||
| 3 | 5.42 | 197 | −35 | −26 | 59 | Left | Precentral gyrus | 4 | |
| 2, 4 | 5.19 | 81 | −46 | −29 | 58 | Left | Postcentral gyrus | 2 | |
| 3 | 4.02 | 10 | −9 | −21 | 45 | Left | Medial frontal gyrus | 6 | |
| 3 | 3.92 | 11 | −12 | 40 | 40 | Left | Superior frontal gyrus | 8 | |
| Right hemisphere | |||||||||
| 2 | 4.13 | 71 | 43 | −32 | 42 | Right | Inferior parietal lobule | 40 | |
| Hard > easy | |||||||||
| Left hemisphere | |||||||||
| 4 | −5.41 | 112 | −39 | 2 | 43 | Left | Middle frontal gyrus | 6 | |
| 3 | −3.84 | 11 | −45 | −56 | −16 | Left | Fusiform gyrus | 37* | R: group × difficulty and group × task |
| 3 | −3.62 | 10 | −31 | 39 | 15 | Left | Middle frontal gyrus | 46* | R: difficulty |
| Right hemisphere | |||||||||
| 2–4 | −9.97 | 758 | 13 | −92 | 3 | Right | Cuneus | 17* | R: task × difficulty |
Note: Temporal lag represents the time after event onset, when a cluster of voxels exhibited a contrast effect of interest. The bootstrap ratio threshold was set to ±>3.28, and identified dominant and stable activation clusters. The spatial extent refers to the total number of voxels included in the voxel cluster (threshold = 10).The stereotaxic coordinates are measured in millimeters, and gyral location and Brodmann areas (BAs) were determined by referring to Talairach and Tournoux (1988). The last column presents the highest order significant effect (P < 0.05) observed from the ROI-based post hoc group × task × difficulty repeated-measures ANOVAs. Regions marked with * were ROIs for which mean activity was extracted and plotted in a bar graph. R, significant effects for retrieval activity; HEM, cerebral hemisphere in which the activation occurred.
Although the statistical effects identified for right fusiform gyrus (BA 37) in LV 5 differ slightly from those observed for a similar peak in LV 1 [Table 4: right fusiform gyrus (BA 19)], the activation patterns are similar in both ROIs. For example, the post hoc ANOVAs identified significant post hoc group × difficulty interactions in retrieval activity for both right fusiform gyrus ROIs. This was due to there being more activity in right fusiform gyrus during hard, compared with easy, context retrieval tasks in MA, whereas activity in right fusiform gyrus was relatively the same across all context retrieval tasks in YA (see Figs 1C and 2D). The post hoc ANOVAs also indicated there was a significant task × difficulty effect in right cuneus during retrieval. This was due to both age groups having increased deactivation in this region during easy, compared with hard, context retrieval tasks. These results are consistent with the effects observed for similar peaks from LV 1 at retrieval.
LV 6: Encoding versus Retrieval Events, Group Interaction Effect
Table 7 presents the whole-brain PLS results for LV 6, which identified group differences in brain activity during successful encoding, compared with retrieval. Since this LV did not identify significant effects in our ROIs, we do not discuss it further.
Table 7.
Local maxima for LV6: encoding versus retrieval, group interaction
| Temporal lag | Bootstrap ratio | Spatial extent | Talairach coordinates |
HEM | Gyral location | Brodmann area | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Young encoding > retrieval, MA retrieval > encoding | ||||||||
| Left hemisphere | ||||||||
| 4 | 4.35 | 13 | −12 | 37 | −3 | Left | Anterior cingulate | 24 |
| 2 | 3.69 | 14 | −9 | −11 | 18 | Left | Thalamus | |
| Right hemisphere | ||||||||
| 2 | 3.87 | 36 | 24 | −75 | 55 | Right | Precuneus | 7 |
| Young retrieval > encoding, MA encoding > retrieval | ||||||||
| Left hemisphere | ||||||||
| 2 | −3.74 | 13 | −39 | −22 | 59 | Left | Precentral gyrus | 4 |
Note: Temporal lag represents the time after event onset, when a cluster of voxels exhibited a contrast effect of interest. The bootstrap ratio threshold was set to ±>3.28, and identified dominant and stable activation clusters. The spatial extent refers to the total number of voxels included in the voxel cluster (threshold = 10). The stereotaxic coordinates are measured in millimeters, and gyral location and Brodmann areas (BAs) were determined by referring to Talairach and Tournoux (1988). HEM, cerebral hemisphere in which the activation occurred.
LV 10: Easy > Hard Retrieval Events, Group Interaction
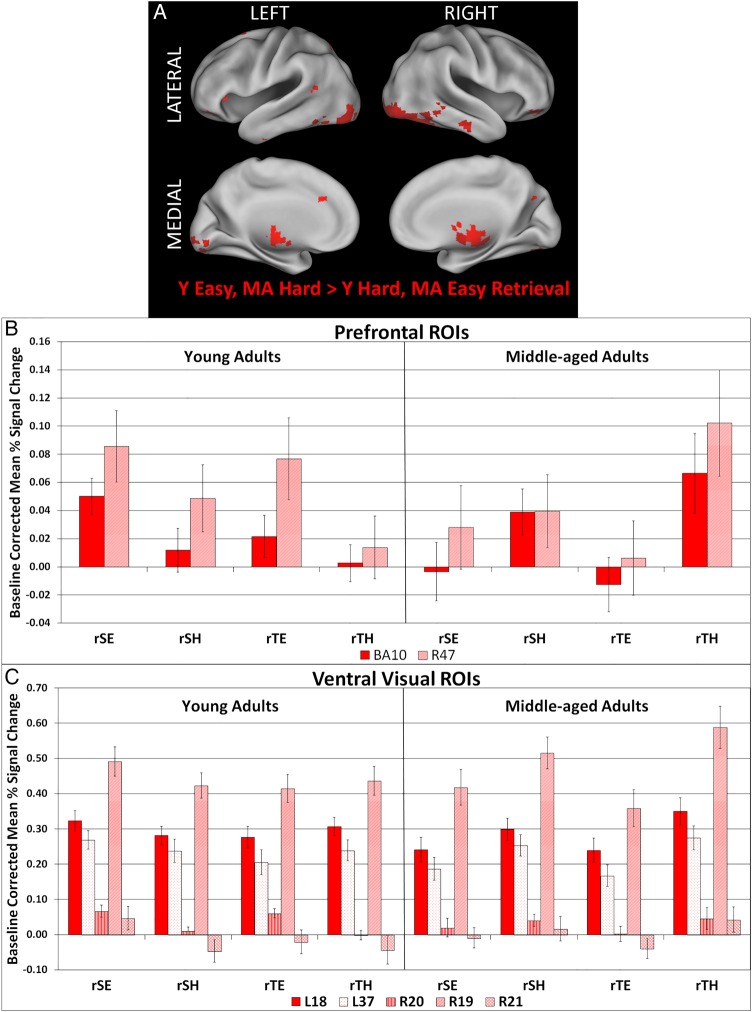
Figure 3A and Table 8 present the whole-brain PLS results for LV 10, which identified group differences in brain activity during easy, compared with hard, retrieval events. Positive saliences represent regions where MA exhibited increased activation during hard, compared with easy, retrieval events, and YA exhibited the inverse effect. There were no significant negative saliences for this LV at the thresholds used.
Figure 3.
(A) The singular image for Contrast 10, group-by-easy > hard retrieval interaction, at a bootstrap ratio of ±3.28 (P < 0.001), which reflects reliable activations at time lags 2–4. (B,C) Bar graphs representing mean activation with standard error bars in regions of interest (ROIs) that exhibited an encoding > retrieval interaction in young > middle aged adults. Regions are identified by their hemisphere and Brodmann area. L, left; R, right; eSE, easy spatial encoding; eSH, hard spatial encoding; eTE, easy temporal encoding; eTH, hard temporal encoding; rSE, easy spatial retrieval; rSH, hard spatial retrieval; rTE, easy temporal retrieval; rTH, hard temporal retrieval.
We extracted the baseline corrected, mean percent signal change in a subset of ROIs identified in this LV (marked by asterisks in Table 8) and plotted these activation profiles in Figure 3B (PFC ROIs) and 3C (ventral visual ROIs). Figure 3B shows that there was increased activity in right medial APFC (BA 10) and right VLPFC (BA 47) during easy, compared with hard, retrieval tasks in YA, and increased activity in these regions during hard, compared with easy, retrieval tasks in MA. Figure 3C indicates that a similar pattern of activity was observed in right inferior (BA 20) and middle (BA 21) temporal cortices. In contrast, activity in left fusiform cortex (BA 37), left middle occipital cortex (left BA 18), and right fusiform cortex (BA 19) was similar across all retrieval tasks in YA, but was greater during hard, compared with easy, context retrieval tasks in MA. Post hoc ANOVAs were conducted and the results are presented in the last column of Table 8. The post hoc ANOVAs corroborate the LV effect and identify significant group × difficulty interaction effects for all ROIs.
Linear Regressions
Predictor variables for the regression analyses were primarily from LV1 since this LV best encapsulated the encoding and retrieval activations common to both age groups and because it explained most of the variance in the data (46% cross-block variance). We also included some regions from LV 10 to balance the hemispheric representation of brain regions included in the model, where possible. Although some ROIs from LV4 and LV5 were similar to those seen in LV1, the peak BSR for these overlapping areas was larger for the coordinates identified in LV1. Moreover, the post hoc ANOVAs indicated that there was overlap in the activation patterns observed in LV1 and LV4 and 5. Thus, only including peaks for LV1 represented effects in these regions identified in subsequent LVs.
For ROIs selected from LV1, we used either the encoding activity, the retrieval activity, or the sum of activity in encoding and retrieval (encoding + retrieval activity), as predictor values, based on the effects, each ROI illustrated in the post hoc ANOVAs presented above. For example, if a region only exhibited significant post hoc effects during encoding, we included its activity during encoding as a predictor in each model. If a region only exhibited significant post hoc effects during retrieval, we included its activity during retrieval as a predictor in each model. However, if a region exhibited both encoding- and retrieval-related effects, we calculated the sum of encoding + retrieval activity and used it as a predictor in each model. This was done to reduce multicollinearity arising from including both encoding and retrieval activity from the same region and same LV.
There were a total of 16 predictor variables included in each of the models: age (in years), the sum of encoding + retrieval activity in left medial occipital cortex from LV1 (LV1, left medial BA 18), retrieval activity in left middle occipital cortex from LV10 (LV10, left lateral BA 18), the sum of encoding + retrieval activity in right middle occipital cortex from LV1 (LV1, right BA 19), encoding activity in left BA 21 from LV1 (LV1, left BA 21), retrieval activity in right BA 21 from LV1 (LV1, right BA 21), encoding activity in bilateral anterior temporal cortex (LV1, bilateral BA 38), encoding activity in right PHC (LV 1, right PHC), encoding in left VLPFC from LV1 (LV1, left BA 47), the sum of encoding + retrieval activity in right VLPFC from LV1 (LV1, right BA 45), the sum of encoding + retrieval activity in left DLPFC from LV1 (LV1, left lateral BA 9), the sum of encoding + retrieval activity in right DLPFC from LV1 (LV1, right BA 9), the sum of encoding + retrieval activity in left APFC from LV1 (LV1, left BA 10), and retrieval activity in left medial PFC (LV1, left medial BA 9) and right APFC from LV10 (LV10, right BA 10). The coordinates for these ROIs are marked with # in Tables 4 and 8. Mean activity in these ROIs were also plotted in Figures 1–3.
The descriptive analyses of the ROIs identified the following extreme datapoints in ROI activity which were removed prior to running the regression models: 1) SE models: 3 extreme datapoints in YA and one extreme datapoint in MA were identified for activity during SE tasks; 2) SH models: 3 extreme datapoints in YA and 3 extreme datapoint in MA were identified for activity during SH tasks; 3) TE models: 3 extreme datapoints in YA were identified for activity during TE tasks; 4) TH models: one extreme datapoint in YA and 4 extreme datapoints in MA were identified for activity during TH tasks. The extreme datapoints identified across models were not consistently from the same subjects. Thus, there were no outlier subjects on the whole. These extreme datapoints were removed to prevent bias in the regression results.
Table 9 presents the significant across age-group backward step-wise regression results for the models predicting SE, SH, TE, and TH retrieval accuracy. Tables 10 and 11 present the most significant within group backward step-wise regression results for SE, SH, TE, and TH retrieval accuracy models for YA and MA, respectively. Predictors with significant t-values are marked with an asterisk in the table. Below we summarize the results observed for each task type by reviewing the significant predictors (t-statistic P < 0.05) identified in across and within group analyses.
Table 9.
Across age-group regression results
| Model | Predictor | Standardized β | T statistic |
|---|---|---|---|
| Spatial easy accuracy [F6,51 = 7.20, P < 0.001; adjusted R2 = 0.40] | |||
| LV1, left medial BA 18 (sum E + R) | +0.30 | 2.68* | |
| LV1, right BA 38 (E) | −0.29 | −2.55* | |
| LV1, left BA 47 (E) | +0.19 | 1.70 | |
| LV1, right BA 45 (sum E + R) | −0.34 | −2.94* | |
| LV1, left lateral BA 9 (sum E + R) | +0.36 | 3.05* | |
| LV10, right BA 10 (R) | +0.19 | 1.70 | |
| Spatial hard accuracy [F3,50 = 9.48, P < 0.001; adjusted R2 = 0.32] | |||
| Age | −0.41 | −3.54* | |
| LV10, left lateral BA 18 (R) | −0.19 | −1.69 | |
| LV1, left BA 47 (E) | +0.33 | 2.86* | |
| Temporal easy accuracy [F4,54 = 6.26, P < 0.001; adjusted R2 = 0.27] | |||
| Age | −0.43 | −3.82* | |
| LV1, left BA 21 (E) | −0.26 | −1.83 | |
| LV1, right PHC (E) | +0.31 | 2.18* | |
| LV1, left lateral BA 9 (sum E + R) | +0.33 | 2.81* | |
| Temporal hard accuracy [F1,55 = 20.10, P < 0.001; adjusted R2 = 0.25] | |||
| Age | −0.52 | −4.48* | |
Note: This table presents the significant models resulting from a backward elimination step-wise regression analysis to identify predictors of retrieval accuracy on spatial and temporal context memory tasks under easy (low-load) and hard (high-load) conditions across both age groups. LV, the significant latent variable from the PLS results from which the ROI was selected from. In parentheses beside each predictor, we identify whether the mean activity included for each ROI reflected task-specific encoding (E) activity, retrieval activity (R), or the sum of activity from encoding and retrieval (sum E + R). Refer to Tables 4–8 for specific coordinates, and details of each ROI.
BA, Brodmann area.
*Refers to t-values that were significant at P < 0.05.
Table 10.
Within group regression results for young adults
| Model | Predictor | Standardized β | T statistic |
|---|---|---|---|
| Spatial easy accuracy [F2,28 = 4.77, P = 0.02; adjusted R2 = 0.20] | |||
| LV1, left BA 47 (E) | +0.48 | 2.78* | |
| LV1, right BA 45 (sum E + R) | −0.37 | −2.16* | |
| Spatial hard accuracy [F4,24 = 6.76, P = 0.001; adjusted R2 = 0.45] | |||
| Age | −0.37 | −2.56* | |
| LV1, left BA 47 (E) | +0.51 | 3.11* | |
| LV1, left BA 10 (sum E + R) | −0.30 | −1.82 | |
| LV10, right BA 10 (R) | −0.45 | −3.11* | |
| Temporal easy accuracy [F4,25 = 5.58, P = 0.002; adjusted R2 = 0.39] | |||
| LV10, left lateral BA 18 (R) | +0.35 | 2.21* | |
| LV1, left BA 21 (E) | −0.58 | −2.66* | |
| LV1, right PHC (E) | +0.86 | 3.98* | |
| LV1, left lateral BA 9 (sum E + R) | +0.45 | 2.69* | |
| Temporal hard accuracy [F7,24 = 3.18, P = 0.02; adjusted R2 = 0.33] | |||
| LV10, left lateral BA 18 (R) | +0.52 | 2.73* | |
| LV1, left BA 21 (E) | −0.50 | −2.66* | |
| LV1, right BA 21 (R) | +0.56 | 3.01* | |
| LV1, left BA 38 (E) | −0.56 | −2.97* | |
| LV1, left BA 47 (E) | +0.66 | 3.08* | |
| LV1, right BA 45 (sum E + R) | −0.46 | −2.62* | |
| LV1, right BA 9 (sum E + R) | −0.40 | −2.23* | |
Note: This table presents the significant models resulting from a backward step-wise regression analysis to identify predictors of retrieval accuracy on spatial and temporal context memory tasks under easy (low-load) and hard (high-load) conditions across both age groups. LV, the significant latent variable from the PLS results from which the ROI was selected from. In parentheses beside each predictor, we identify whether the mean activity included for each ROI reflected task-specific encoding (E) activity, retrieval activity (R), or the sum of activity from encoding and retrieval (sum E + R). Refer to Tables 4–8 for specific coordinates, and details of each ROI.
BA, Brodmann area.
*Refers to t-values that were significant at P < 0.05.
Table 11.
Within group regression results for middle aged adults
| Model | Predictor | Standardized β | T statistic |
|---|---|---|---|
| Spatial easy accuracy [F9,17 = 9.56, P < 0.001; adjusted R2 = 0.75] | |||
| Age | −0.25 | −2.02 | |
| LV1, left medial BA 18 (sum E + R) | +0.35 | 3.02* | |
| LV10, left lateral BA 18 (R) | −0.25 | −1.89 | |
| LV1, left BA 21 (E) | −0.30 | −2.48* | |
| LV1, left BA 38 (E) | +0.34 | 1.87 | |
| LV1, right BA 38 (E) | −0.39 | −2.03 | |
| LV1, left lateral BA 9 (sum E + R) | +0.70 | 4.59* | |
| LV1, left BA 10 (sum E + R) | −0.32 | −2.07* | |
| LV10, right BA 10 (R) | +0.49 | 3.52* | |
| Spatial hard accuracy [F6,18 = 4.60, P = 0.005; adjusted R2 = 0.47] | |||
| Age | −0.63 | −3.73* | |
| LV10, left lateral BA 18 (R) | −0.40 | −2.42* | |
| LV1, left BA 21 (E) | −0.40 | −2.46* | |
| LV1, left BA 38 (E) | +0.38 | 2.29* | |
| LV1, left BA 47 (E) | +0.35 | 2.05 | |
| LV1, left BA 10 (sum E + R) | +0.40 | 2.34* | |
| Temporal easy accuracy [F5,22 = 5.21, P = 0.003; adjusted R2 = 0.44] | |||
| Age | −0.45 | −2.78* | |
| LV1, right BA 19 (sum E + R) | −0.33 | −2.18* | |
| LV1, right PHC (E) | +0.48 | 2.76* | |
| LV1, left BA 47 (E) | −0.50 | −2.96* | |
| LV10, right BA 10 (R) | +0.35 | 2.29* | |
| Temporal hard accuracy [F7,17 = 11.18, P < 0.001; adjusted R2 = 0.75] | |||
| Age | −0.67 | −5.88* | |
| LV10, left lateral BA 18 (R) | −0.60 | −5.23* | |
| LV1, right BA 19 (sum E + R) | −0.25 | −2.23* | |
| LV1, left BA 38 (E) | +0.46 | 4.02* | |
| LV1, right BA 45 (sum E + R) | −0.24 | 1.98 | |
| LV1, right BA 9 (sum E + R) | +0.24 | 2.01 | |
| LV1, left medial BA 9 (R) | +0.44 | 3.72* | |
Note: This table presents the significant models resulting from a backward step-wise regression analysis to identify predictors of retrieval accuracy on spatial and temporal context memory tasks under easy (low-load) and hard (high-load) conditions across both age groups. LV, the significant latent variable from the PLS results from which the ROI was selected from. In parentheses beside each predictor, we identify whether the mean activity included for each ROI reflected task-specific encoding (E) activity, retrieval activity (R), or the sum of activity from encoding and retrieval (sum E + R). Refer to Tables 4–8 for specific coordinates, and details of each ROI.
BA, Brodmann area.
*Refers to t-values that were significant at P < 0.05.
Summary
The regression analyses indicate that, by conducting across age-group analyses alone, few predictors of retrieval accuracy were identified for tasks. This suggests that there were few ROIs that similarly predicted task accuracy across age. However, by conducting within group analyses, we identified different brain regions that significantly predicted retrieval accuracy across tasks in YA and MA. In YA, increased retrieval accuracy on both spatial tasks and on the TH task was supported by increased left VLPFC activity at encoding. In addition, increased retrieval accuracy on both temporal tasks was predicted by increased retrieval activity in left middle occipital cortex (BA 18) in YA. A significant positive association between increased left VLPFC activity during either encoding or retrieval and retrieval accuracy was not observed for any task in MA. Furthermore, in MA retrieval accuracy on SH and TH tasks was “negatively” associated with increased retrieval activity in left middle occipital cortex. Thus, there were age-related differences in the brain–behavior associations involving encoding activity in left VLPFC and retrieval activity in left middle occipital cortex across tasks.
In addition, for MA, increased encoding activity in left anterior temporal cortex (BA 38) predicted increased retrieval accuracy during SE, SH, and TH tasks; similar to the pattern observed for left VLPFC in YA. However, these associations were only significant during SH and TH tasks. In contrast, in YA increased encoding activity in left anterior temporal cortex was a negative predictor of TH retrieval accuracy. Also, in the case of MA, increased activity in several PFC regions, (other than left VLPFC) was positively associated with retrieval accuracy. For example, activity in either left and/or right APFC positively predicted SE, SH, and TE retrieval accuracy, and reduced deactivation in left medial PFC predicted TH retrieval accuracy. In contrast, in YA, increased activity in other PFC regions was negatively linked to retrieval accuracy across tasks, with the exceptions of left VLPFC and left DLPFC.
In addition to the overall pattern of group differences in brain–behavior associations, there were 2 patterns of group similarity in brain–behavior associations: 1) in both age groups, increased right PHC activity during TE tasks was positively associated with retrieval accuracy; and 2) in both age groups, increased encoding activity in left DLPFC supported retrieval accuracy. However, in the case of left DLPFC, the positive association was significant during the easiest, low-load, SE task in MA, and in YA this association was only significant during the more difficult (based on lower accuracy), TE task.
Discussion
In the current study, event-related fMRI was used to identify the functional brain changes associated with the onset of context memory decline at midlife. We tested subjects on easy (low encoding load) and hard (high encoding load) versions of spatial and temporal context memory tasks. We found that MA did not exhibit slower RT compared with YA during either spatial or temporal context memory tasks. It has been shown that older adults (aged 60 years and above) exhibit slower RT compared with YA on a variety of episodic memory tasks (Salthouse 1996; Verhaeghen and Salthouse 1997) and that this age-related slowing may be due to changes in the white matter integrity of the frontal lobes (Bucur et al. 2008). The fact that MAs' RT was not significantly different from that of YA in the current study, suggests that the white matter integrity of the frontal lobes may remain intact from young adulthood to midlife (Lebel et al. 2012).
Our behavioral results indicated that MA exhibited lower retrieval accuracy on SH, TE, and TH tasks compared with YA. Performance was matched between groups on SE tasks. Our findings with regards to the spatial context memory task are consistent with prior work. For example, Cansino et al. (2010, 2012) have also reported spatial context memory deficits in MA compared with YA on tasks that had a high encoding load of a 120 images. Also, in a working memory study in which young and older adults were required to maintain either 4, 5, or 7 letters, no significant age differences in retrieval accuracy were reported during the 4 and 5 load conditions, but a significant age difference in retrieval accuracy was observed during the 7 load condition (Cappell et al. 2010). Taken together these findings indicate that in MA, spatial context memory remains intact when there are few encoding stimuli (i.e., SE tasks); but as the encoding load increases to 12 (i.e., SH tasks), spatial context memory deficits become apparent. Therefore, stimulus load at encoding has a significant impact on spatial context memory abilities in MA.
Our behavioral results also indicated that there was a significant decline in temporal context memory in MA versus YA. In fact, both age groups had lower retrieval accuracy during temporal context memory tasks compared with spatial context memory tasks. This finding is consistent with prior studies that have reported poorer performance on temporal versus spatial context memory tasks in older and in younger adults (Parkin et al. 1995; Rajah, Languay et al. 2010). Temporal context memory tasks are generally thought to be more challenging than spatial context memory tasks because they place greater demands on working memory and relational processes that are thought to be mediated by the PFC (Rajah, Languay et al. 2010; Crane et al. 2011). Therefore, the current study was the first, to our knowledge, to show that deficits in temporal context memory arise as early as midlife and may reflect changes in PFC function.
Our event-related fMRI results revealed 3 results: 1) there were no significant group differences in brain activity during either spatial or temporal context encoding, 2) there were group differences in brain–behavior associations at encoding in left VLPFC and left anterior temporal cortex, and 3) there were group differences in both brain activity and in brain–behavior associations during context retrieval, which primarily involved PFC and ventral occipito-temporal regions. Surprisingly, the PLS results did not identify a LV that reflected significant task differences in brain activity, even though the behavioral results identified significant task, group × task and task × difficulty effects for accuracy. However, the exploratory ROI-based post hoc ANOVAs indicated that several ROIs exhibited significant task-related and group-related patterns of interactions in encoding, and/or retrieval activity. In addition, the brain–behavior regression analyses indicate that there were group differences in region-specific contributions to retrieval accuracy across tasks. In the following sections, we discuss group similarities and differences in fMRI activity and brain–behavior associations during successful context encoding and successful context retrieval.
Group Similarities and Differences at Encoding
The PLS results indicated there were no significant age-group differences in brain activity at encoding in YA versus MA during successful encoding of spatial and temporal contextual details. Both age groups increased activity in a distributed set of brain regions during successful encoding, compared with retrieval, which included: medial occipital cortex, right PHC, bilateral middle and anterior temporal cortex, bilateral VLPFC, and left DLPFC. However, we observed group differences in brain–behavioral associations between encoding activity in some PFC and ventral temporal regions, and retrieval accuracy. In the following subsections, we discuss our findings at encoding in greater detail for our regions of interest: PHC, ventral occipito-temporal cortices, and PFC.
Right Parahippocampal Cortex
The PLS results indicated both groups exhibited greater right PHC activity during encoding, compared with retrieval, and during easy, compared with hard, encoding tasks. The observation of greater right PHC activity during easy, compared with hard, encoding tasks, may be related to the greater retrieval success observed during easy tasks. This interpretation is supported by the findings of a positive association between increased encoding activity in right PHC and increased context retrieval accuracy during TE tasks in both age groups.
Prior work has supported a role for the PHC in context memory (Davachi et al. 2003; Martin et al. 2013; Wang et al. 2013) and visuospatial processing (Epstein and Kanwisher 1998; Maguire et al. 1998). Bar et al. (2008) have suggested that the PHC may be important for making contextual associations across domains (Bar et al. 2008; Aminoff et al. 2013). Consistent with this hypothesis they reported increased left PHC activity during the perception of famous, compared with novel, faces, wherein famous faces elicited more contextual associations than novel faces (Bar et al. 2008). Therefore, our findings suggest that right PHC activity support spatial and temporal context encoding of face stimuli; and that this function is preserved at midlife. This conclusion is consistent with prior studies that have also not observed changes in medial temporal function at midlife (Park et al. 2013; Cansino et al. 2015).
The Prefrontal Cortex
In the current study, we observed no significant age-group differences in PFC activity during encoding. Both groups had increased activity in bilateral VLPFC and left DLPFC activity during successful context encoding, compared with retrieval. This is consistent with the results reported by Park et al. (2013). In that fMRI study, no significant differences in PFC activity were reported between MA and YA during the successful encoding of scene stimuli. Instead, age-related changes in PFC encoding activity were observed later in adulthood, between older adults and MA.
In an ERP study of spatial context encoding for pictures of colored objects, Cansino et al. (2010) reported similar amplitude effects in right lateral frontal positivity in YA, MA, and older adults during successful spatial context encoding. Yet, the onset of this waveform was progressively delayed from young adulthood to midlife, and to older age. This implies that there may be subtle changes in the timing of PFC involvement at encoding that are apparent by midlife.
Given the imaging modality employed in the current study, we were unable to ascertain if there were age-related delays in the onset of lateral PFC activity at encoding. However, our linear regression results support the hypothesis that there may be underlying changes in PFC function during context encoding from young adulthood to midlife, despite there being no significant group differences in PFC activity at encoding. Specifically, in YA only, we found that increased encoding activity in left VLPFC (BA 47) predicted retrieval accuracy on SE, SH, and TH tasks. During TE tasks, retrieval accuracy was positively predicted by increased encoding and retrieval activity in left DLPFC. Left lateral PFC activation has been consistently observed during episodic encoding tasks (Fletcher et al. 1998; Dove et al. 2006; Spaniol et al. 2009; Dulas and Duarte 2011). Encoding activity in left VLPFC is thought to reflect this regions' role in mediating goal-directed, item-specific, semantic retrieval, which is important when using a categorical verbal/semantic strategy during episodic encoding (Habib et al. 2003; Badre and Wagner 2007). In contrast, left DLPFC activity at encoding is thought to reflect this regions' role in mediating relational encoding strategies across stimuli (Murray and Ranganath 2007; Long et al. 2010). Based on these findings, we hypothesize that left VLPFC activity in the current study reflected the implementation of an item-specific semantic encoding strategy that directly benefitted SE, SH, and TH retrieval accuracy in YA. This interpretation is consistent with the idea that subjects likely used a categorical left versus right semantic label during encoding, which would have benefitted spatial context memory tasks. In contrast, using a relational encoding strategy would have supported the successful encoding of the relative temporal order of stimuli during temporal context memory tasks, and is consistent with the observed positive association left DLPFC activity and TE retrieval accuracy. It was surprising that activity in left VLPFC, not DLPFC, predicted TH retrieval accuracy. We speculate that this may be due to YA having difficulty in implementing a relational strategy across 12 stimuli in the TH task, and thus reverting back to using a semantic encoding strategy, and left VLPFC function, to support performance on TH tasks. However, this interpretation is debatable.
In contrast to YA, there were no significant positive brain–behavior associations involving left VLPFC in MA. In fact, increased left VLPFC activity was related to poorer TE retrieval accuracy in MA. Instead, a positive brain–behavior association was seen between left DLPFC activity and SE retrieval accuracy in MA, whereas in YA, left DLPFC activity was found to positively predict retrieval accuracy on the more difficult (based on retrieval accuracy) TE retrieval task. Therefore, our results indicate that, even though activity levels in left VLPFC and left DLPFC remained the same between YA and MA, by midlife there may be underlying changes in the functional impact of these brain regions on context retrieval accuracy, which were identified in the regression results.
Based on these observations, we hypothesize that at midlife there are deficits in left VLPFC function at encoding, which MA successfully compensate for by increasing left DLPFC activity during SE tasks—the only tasks where there were no significant group differences in retrieval accuracy. This interpretation is consistent with prior studies that have reported reductions in left VLPFC activity and deficits in associative memory retrieval in older adults, compared with YA (Logan et al. 2002; Sperling 2007; Addis et al. 2014). Our results also suggest that by middle age, adults recruit more anterior DLPFC regions at lower levels of task difficulty compared with YA, during successful context encoding; perhaps, to compensate for the aforementioned changes in VLPFC regions (Davis et al. 2008; Park and Reuter-Lorenz 2009; Dennis and Cabeza 2012). However, with progressive increases in task demands, our PLS and regression results indicated that MAs' performance on SH, TE, and TH tasks was associated with increased activation in left anterior temporal cortex at encoding, and/or with increased activation of right APFC, and increased de-activation of left medial PFC, at retrieval (discussed below).
Ventral Occipital and Temporal Cortices
In the current study, we observed group similarities in ventral occipital and temporal cortex activation at encoding. There was increased activity in medial occipital and bilateral middle and anterior temporal cortices during encoding, compared with retrieval. In addition, there was increased activity in right lateral and left fusiform cortices during easy, compared with hard, encoding tasks in both age groups. These results are in contrast to the decreased occipito-temporal cortex activity reported in prior fMRI studies of episodic encoding across the adult lifespan (Grady et al. 2006; Kennedy et al. 2012). However, in the episodic encoding study by Park et al. (2013), both similarities and differences in ventral occipital-temporal activations were reported across YA, MA, and older adults. Specifically, Park et al. (2013) reported group similarities in left inferior occipital-temporal cortices and in right middle occipital-temporal cortices during the successful encoding of scene stimuli. However, decreased encoding activity in bilateral fusiform cortex was reported in MA, compared with YA. We observed similar levels of encoding activations in a various ventral occipital-temporal regions in YA and MA. Discrepancies between our current activation findings, and those of prior studies, may be related to the fact that the stimuli used in earlier experiments were more diverse than ours. Grady et al. (2006) employed drawing and words from different semantic categories, and Kennedy et al. (2012) and Park et al. (2013) used colorful pictures of diverse outdoor scenes (Kennedy et al. 2012; Park et al. 2013). In addition, prior studies employed item recognition tasks in which performance was matched. In contrast, in the current study, we used a perceptually homogenous set of stimuli, black-and-white photographs of faces, and employed context memory tasks, in which there were group differences in retrieval accuracy. Therefore, our study likely placed higher demands on visual processing, which may have led to greater equivalence in ventral visual cortex activity in both age groups at encoding.
Despite the overall group similarities in occipital and temporal cortex activity at encoding, we observed a group difference in the brain–behavior associations between encoding activity in left anterior temporal cortex (BA 38) and context retrieval accuracy. In MA, increased encoding activity in left anterior temporal cortex positively predicted subsequent retrieval accuracy on SE, SH, and TH tasks. This pattern was not observed in YA. In fact, in YA increased encoding activity in left anterior temporal cortex activity was negatively associated with TH retrieval accuracy. Interestingly, the pattern of brain–behavior associations reported in MA for left anterior temporal cortex parallels the pattern of brain–behavioral association reported in YA for left VLPFC, which was discussed in the section above.
The anterior temporal cortex is thought to be important for making specific semantic category decisions (Rogers et al. 2006). For example, in a recent fMRI study of visual face discrimination, it was found that activity in bilateral fusiform cortex reflected the general detection of face stimuli, whereas activity in anterior inferior temporal cortex was important for discriminating between individual faces (Kriegeskorte et al. 2007). Given the parallel patterns of brain–behavior association observed for left anterior temporal cortex and left VLPFC between age groups, it is possible that due to changes in left VLPFC function at encoding, MA relied more on left anterior temporal cortex to support the utilization of semantic processes during context encoding.
Group Similarities and Differences at Retrieval
To our knowledge, ours is the first fMRI study to report age-related similarities and differences in brain activity between MA and YA during episodic memory retrieval. The first LV from our PLS results indicated that there was increased activity in bilateral lateral occipital, right DLPFC and left APFC during successful context retrieval, compared with encoding, in both age groups. However, the post hoc ANOVAs, subsequent LV 10, and regression results indicated that overall, there were marked group differences in activity, and in brain–behavior association, during successful context retrieval. These differences in brain activity and brain–behavior associations were primarily observed in ventral occipito-temporal and prefrontal cortices. In the following subsections, we discuss our findings in these 2 regions of interest in greater detail.
Prefrontal Cortex
YA and MA had increased activation in right DLPFC and left APFC during successful context retrieval compared with encoding. However, the post hoc analyses indicated MA had more activity in these regions during hard, compared with easy, retrieval tasks; whereas, in YA, activity in these regions was not modulated by task difficulty at retrieval. We also observed more activity in right APFC and right VLPFC activity during hard, compared with easy, retrieval tasks in MA. Moreover, our regression results indicated that, in MA, increased retrieval activity in APFC supported SE and TE retrieval accuracy, and increased encoding + retrieval activity in left APFC supported SH retrieval accuracy.
We have previously reported a positive brain–behavior association between right APFC activity and temporal context retrieval in older adults (Rajah, Languay et al. 2010). More recently, Lighthall et al. (2014) have reported increased APFC activity in older versus YA during delayed retrieval, compared with immediate retrieval, of contextual information for previously encoded objects. Moreover, only in older adults was activity in this region associated with task performance (Lighthall et al. 2014).
More anterior aspects of the lateral PFC have been hypothesized to be important for implementing progressively more abstract relational strategies during memory tasks; whereas, more posterior aspects of the lateral PFC are hypothesized to be important for implementing more concrete response/perception based strategies (Christoff et al. 2001; Dobbins et al. 2002; Koechlin et al. 2003; Badre and D'Esposito 2007; Barbalat et al. 2009). Therefore, our current findings expand upon earlier studies comparing older versus YA, and show that by midlife adults over-recruit more anterior portions of the PFC during hard versus easy context memory tasks, compared with YA. This may reflect greater reliance on more abstract retrieval strategies by midlife, perhaps due to changes in encoding strategies and changes in ventral visual function at retrieval (discussed below).
Ventral Occipital and Temporal Cortices
Increased activity in left middle occipital and right fusiform cortex was observed in both age groups during successful context retrieval. However, MA over-recruited these regions during hard context retrieval tasks compared with YA. In addition, there were group differences in brain–behavior associations involving left middle occipital and right fusiform cortex.
Middle occipital and fusiform activity has been observed during a perceptual matching task for face stimuli (Grady et al. 1994) and activation of these regions at retrieval in both YA and MA may reflect the utilization of a visually based retrieval strategy. In YA, there was a positive association between left middle occipital cortex retrieval activity and TE and TH retrieval accuracy. This suggests that using a visually based retrieval strategy benefitted temporal context retrieval in YA, even though retrieval accuracy on these tasks was lower than on the spatial context tasks.
In contrast, in MA, increased left middle occipital cortex activity was found to negatively predict retrieval accuracy on SE, SH, and TH tasks. Also, in MA, increased encoding and retrieval activity in right fusiform cortex was negatively associated with retrieval accuracy on TE and TH tasks. Moreover, there was increased retrieval activity in both of these regions during hard, compared with easy, retrieval tasks in MA. These observations suggest that MA used a visually based retrieval strategy more during hard > easy context memory tasks, but this was not beneficial to their task performance.
One possibility is that the over-recruitment of left middle occipital cortex and right fusiform cortex at retrieval in MA may reflect neural inefficiency and functional deficits in these regions (Park et al. 2004; Morcom et al. 2007). This implies that there are visual processing deficits at retrieval at midlife. This interpretation is consistent with studies that have reported linear decreases in white matter integrity (fractional anisotropy) in occipital and temporal cortices across the adult lifespan (Kennedy and Raz 2009), and age-related changes in ventral occipito-temporal activity at midlife (Park et al. 2013). It is also consistent with the observed negative brain–behavior association involving retrieval activity in these regions and context retrieval accuracy in MA only. However, given that there were no age-related differences in ventral occipital and temporal cortex activity at encoding in the current study, it is debatable if MA have neural inefficiency or deficits in ventral visual processing. Future examination of the three-way association between context memory performance, functional activation, and structural integrity of ventral occipito-temporal cortex would help to shed light on this possible explanation of our current findings.
Conclusions
This was the first fMRI study to examine the neural correlates of spatial and temporal context encoding and retrieval in YA and MA. The current study design afforded us the ability to directly examine how brain activity, at either encoding and/or retrieval, was related to memory performance across context memory tasks that varied in difficulty, as defined by retrieval accuracy. Surprisingly, we did not observe significant age-group differences in brain activity at encoding. However, we observed group differences in retrieval activity and in our regression results.
Our regression results indicated that there were age differences in brain–behavior associations at encoding, despite the fact that activation patterns were the same between age groups at encoding. Specifically, we observed changes in how encoding activity in VLPFC and left anterior temporal cortex related to subsequent context retrieval. In YA, encoding activity in left VLPFC predicted retrieval accuracy in a variety of context memory tasks. This was not observed in MA. Instead, in that age cohort, encoding activity in left anterior temporal cortex predicted retrieval accuracy in a variety of context memory asks, in a manner that paralleled the pattern observed for left VLPFC in YA.
We hypothesize that the aforementioned changes in brain–behavior associations at encoding in MA impacted the patterns of brain activity and brain–behavior associations observed at retrieval; and ultimately had a negative impact on context memory in MA. Specifically, we hypothesize that there are retrieval-related changes in left middle occipital and right fusiform function at midlife, and that the observed over-recruitment of PFC in MA at retrieval may reflect attempted functional compensation for these changes in visual function at retrieval, and for the encoding-related changes in VLPFC function (Rajah and D'Esposito 2005). This hypothesis is supported by the observation that in MA, increased ventral visual activity at retrieval reliance was not beneficial to retrieval accuracy, but increased DLPFC and APFC activity was. In YA, the opposite pattern was observed: increased retrieval activity in PFC was negatively associated with retrieval accuracy, but increased retrieval activity in left middle occipital cortex was positively associated with temporal context retrieval. This pattern of results is consistent with the posterior-to-anterior shift in aging model (Davis et al. 2008).
Our results were also consistent with the predictions of the CRUNCH/STAC models (Park and Reuter-Lorenz 2009; Cappell et al. 2010). The regression analyses indicated that, in MA, compared with YA, activity in more PFC and occipital and temporal cortex regions directly predicted performance SE tasks—the task in which performance was matched between age groups. Moreover, during hard tasks, MA increased activity in various PFC regions at retrieval, and this recruitment was compensatory. Yet, MA performed significantly worse than YA on these harder context memory tasks. This indicates that there are limits to compensation, and that these limits are present at midlife.
It is important to note that our regression analyses indicated that age was a significant predictor of context retrieval, particularly in the middle aged group. This implies that there were additional neural and/or physiological factors, not accounted for in our regression models, which correlated with age, and impacted context memory performance in MA. Future studies should aim to broaden our understanding of the neural changes that arise at midlife, and how they are modulated with increasing age during this adult critical period. Such research will help us gain insight into the initial changes in brain function that are associated with the onset of memory decline and advance our current models of health aging and memory function. Ultimately, this knowledge will aid the development of future therapies aimed at improving memory function in healthy adults, at an earlier age where intervention may be more promising.
Funding
This work was supported by CIHR Operating Grant #MOP126105 and Alzheimer's Society of Canada Research Grant # 1435 awarded to M.N.R. Thank you to K. Borja, J. Lam, A.Swierkot, and L. Wallace who helped with subject recruitment and testing. Thank you to M. Binns for his advice on the regression analyses.
Notes
Conflict of Interest: None declared.
References
- Addis DR, Giovanello KS, Vu MA, Schacter DL. 2014. Age-related changes in prefrontal and hippocampal contributions to relational encoding. Neuroimage. 84:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E, Kveraga K, Bar M. 2013. The role of the parahippocampal cortex in cognition. Trends Cogn Sci. 17(8):379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. 2007. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 19(12):2082–2099. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. 2007. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 45(13):2883–2901. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Ishai A. 2008. Famous faces activate contextual associations in the parahippocampal cortex. Cereb Cortex. 18(6):1233–1238. [DOI] [PubMed] [Google Scholar]
- Barbalat G, Chambon V, Franck N, Koechlin E, Farrer C. 2009. Organization of cognitive control within the lateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry. 66(4):377–386. [DOI] [PubMed] [Google Scholar]
- Beck AT. 1987. Beck depression inventroy. TX: The Psychological Corporation. [Google Scholar]
- Bender R, Lange S. 2001. Adjusting for multiple testing—when and how? J Clin Epidemiol. 54(4):343–349. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. 2008. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 29(7):1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. 2000. Age-related differences in neural activity during item and temporal-order memory retrieval: a positron emission tomography study. J Cogn Neurosci. 12(1):197–206. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. 2002. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 17(3):1394–1402. [DOI] [PubMed] [Google Scholar]
- Cansino S, Estrada-Manilla C, Trejo-Morales P, Pasaye-Alcaraz EH, Aguilar-Castaneda E, Salgado-Lujambio P, Sosa-Ortiz AL. 2015. fMRI subsequent source memory effects in young, middle-aged and old adults. Behav Brain Res. 280:24–35. [DOI] [PubMed] [Google Scholar]
- Cansino S, Hernandez-Ramos E, Trejo-Morales P. 2012. Neural correlates of source memory retrieval in young, middle-aged and elderly adults. Biol Psychol. 90(1):33–49. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. 2002. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 12(10):1048–1056. [DOI] [PubMed] [Google Scholar]
- Cansino S, Trejo-Morales P, Hernandez-Ramos E. 2010. Age-related changes in neural activity during source memory encoding in young, middle-aged and elderly adults. Neuropsychologia. 48(9):2537–2549. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. 2010. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 46(4):462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. 2001. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 14(5):1136–1149. [DOI] [PubMed] [Google Scholar]
- Crane D, Maillet D, Floden D, Valiquette L, Rajah MN. 2011. Similarities in the patterns of prefrontal cortex activity during spatial and temporal context memory retrieval after equating for task structure and performance. Neuroimage. 54(2):1549–1564. [DOI] [PubMed] [Google Scholar]
- Davachi L. 2006. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 16(6):693–700. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. 2003. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 100(4):2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. 2008. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 18(5):1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. 2012. Frontal lobes and aging: deterioration and compensation. In: Stuss D, Knight R, editors. Principles of frontal lobe function, 2nd ed New York: Oxford University Press. [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. 2002. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 35(5):989–996. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Simons JS, Schacter DL. 2004. fMRI evidence for separable and lateralized prefrontal memory monitoring processes. J Cogn Neurosci. 16(6):908–920. [DOI] [PubMed] [Google Scholar]
- Dove A, Brett M, Cusack R, Owen AM. 2006. Dissociable contributions of the mid-ventrolateral frontal cortex and the medial temporal lobe system to human memory. Neuroimage. 31(4):1790–1801. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. 2008. The effects of aging on the neural correlates of subjective and objective recollection. Cereb Cortex. 18(9):2169–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. 2011. The effects of aging on material-independent and material-dependent neural correlates of contextual binding. Neuroimage. 57(3):1192–1204. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. 2012. The effects of aging on material-independent and material-dependent neural correlates of source memory retrieval. Cereb Cortex. 22(1):37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. 2009. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 30(9):2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. 1998. A cortical representation of the local visual environment. Nature. 392(6676):598–601. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. 1998. The functional roles of prefrontal cortex in episodic memory. I. Encoding. Brain. 121(Pt 7):1239–1248. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12(3):189–198. [DOI] [PubMed] [Google Scholar]
- Friston K. 2004. Experimental design and statistical parametric mapping. In Frackowiak RS, Friston K, Frith CD, Dolan RJ, Price CJ, Zeki S, Ashburner J, Penny W editors Human Brain Function. 2nd ed. London: Elsevier Academic Press; p. 599–634. [Google Scholar]
- Grady CL. 2002. Age-related differences in face processing: a meta-analysis of three functional neuroimaging experiments. Can J Exp Psychol. 56(3):208–220. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Wagner E, Haxby JV. 1994. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 14(3 Pt 2):1450–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. 2006. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 18(2):227–241. [DOI] [PubMed] [Google Scholar]
- Habib R, Nyberg L, Tulving E. 2003. Hemispheric asymmetries of memory: the HERA model revisited. Trends Cogn Sci. 7(6):241–245. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. 2009. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 1297:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Devous MD Sr, Hebrank AC, Bischof GN, Park DC. 2012. Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. Neuroimage. 62(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. 2003. The architecture of cognitive control in the human prefrontal cortex. Science. 302(5648):1181–1185. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Formisano E, Sorger B, Goebel R. 2007. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci USA. 104(51):20600–20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Wilms M, Mirzazade S, Fink GR. 2009. Ageing-related changes of neural activity associated with spatial contextual memory. Neurobiol Aging. 30(4):630–645. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. 2007. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 28(11):1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 60(1):340–352. [DOI] [PubMed] [Google Scholar]
- Lighthall NR, Huettel SA, Cabeza R. 2014. Functional compensation in the ventromedial prefrontal cortex improves memory-dependent decisions in older adults. J Neurosci. 34(47):15648–15657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. 2002. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 33(5):827–840. [DOI] [PubMed] [Google Scholar]
- Long NM, Oztekin I, Badre D. 2010. Separable prefrontal cortex contributions to free recall. J Neurosci. 30(33):10967–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Burgess N, Donnett JG, O'Keefe J. 1998. Knowing where things are parahippocampal involvement in encoding object locations in virtual large-scale space. J Cogn Neurosci. 10(1):61–76. [DOI] [PubMed] [Google Scholar]
- Maillet D, Rajah MN. 2011. Age-related changes in the three-way correlation between anterior hippocampus volume, whole-brain patterns of encoding activity and subsequent context retrieval. Brain Res. 1420:68–79. [DOI] [PubMed] [Google Scholar]
- Maillet D, Rajah MN. 2013. Association between prefrontal activity and volume change in prefrontal and medial temporal lobes in aging and dementia: a review. Ageing Res Rev. 12(2):479–489. [DOI] [PubMed] [Google Scholar]
- Martin CB, McLean DA, O'Neil EB, Kohler S. 2013. Distinct familiarity-based response patterns for faces and buildings in perirhinal and parahippocampal cortex. J Neurosci. 33(26):10915–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CH, Perreault WD. 1991. Collinearity, power, and interpretation of multiple regression analysis. J Marketing Res. 28(3):268–280. [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. 2004. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 23(2):764–775. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ. 2004. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 23(Suppl 1):S250–S263. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. 2007. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb Cortex. 17(11):2491–2506. [DOI] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C. 2007. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J Neurosci. 27(20):5515–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Evans JD, Miller WS, Heaton RK. 2000. Demographically corrected norms for the California Verbal Learning Test. J Clin Exp Neuropsychol. 22(1):80–94. [DOI] [PubMed] [Google Scholar]
- O'Brien RM. 2007. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 41(5):673–690. [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9(1):97–113. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. 2004. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA. 101(35):13091–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. 2009. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 60:173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kennedy KM, Rodrigue KM, Hebrank A, Park DC. 2013. An fMRI study of episodic encoding across the lifespan: changes in subsequent memory effects are evident by middle-age. Neuropsychologia. 51(3):448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM, Hunkin NM. 1995. Relationships between normal aging, frontal lobe function, and memory for temporal and spatial information. Neuropsychology. 9(3):304–312. [Google Scholar]
- Rajah MN, Ames B, D'Esposito M. 2008. Prefrontal contributions to domain-general executive control processes during temporal context retrieval. Neuropsychologia. 46(4):1088–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. 2005. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 128(Pt 9):1964–1983. [DOI] [PubMed] [Google Scholar]
- Rajah MN, Kromas M, Han JE, Pruessner JC. 2010. Group differences in anterior hippocampal volume and in the retrieval of spatial and temporal context memory in healthy young versus older adults. Neuropsychologia. 48(14):4020–4030. [DOI] [PubMed] [Google Scholar]
- Rajah MN, Languay R, Grady CL. 2011. Age-related changes in right middle frontal gyrus volumes correlate with altered episodic retrieval activity. J Neurosci. 31:17941–17954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, Languay R, Valiquette L. 2010. Age-related changes in prefrontal cortex activity are associated with behavioural deficits in both temporal and spatial context memory retrieval in older adults. Cortex. 46(4):535–549. [DOI] [PubMed] [Google Scholar]
- Rajah MN, McIntosh AR. 2008. Age-related differences in brain activity during verbal recency memory. Brain Res. 1199:111–125. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. 2008. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 17(3):177–182. [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, Price CJ. 2006. Anterior temporal cortex and semantic memory: reconciling findings from neuropsychology and functional imaging. Cogn Affect Behav Neurosci. 6(3):201–213. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. 1996. The processing-speed theory of adult age differences in cognition. Psychol Rev. 103(3):403–428. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 59(Suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- Shimamura AP, Wickens TD. 2009. Superadditive memory strength for item and source recognition: the role of hierarchical relational binding in the medial temporal lobe. Psychol Rev. 116(1):1–19. [DOI] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Brehmer Y, Muller V, Li SC, Lindenberger U. 2010. Episodic memory across the lifespan: the contributions of associative and strategic components. Neurosci Biobehav Rev. 34(7):1080–1091. [DOI] [PubMed] [Google Scholar]
- Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. 2002. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol. 51(3):290–295. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J Jr. 2003. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 17(1):75–82. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. 2009. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 47(8–9):1765–1779. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Grady C. 2010. Aging and the neural correlates of source memory: over-recruitment and functional reorganization. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R. 2007. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann N Y Acad Sci. 1097:146–155. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. 1997. A compendium of neuropsychological tests: administration, norms, and commentary. New York: Oxford University Press. [Google Scholar]
- Talairach J, Tournoux P. 1988. Co-planar stereotaxic atlas of the human brain (M. Rayport, Trans.). New York: Thieme Medical Publishers, Inc. [Google Scholar]
- Van Petten C. 2004. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 42(10):1394–1413. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. 1997. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychol Bull. 122(3):231–249. [DOI] [PubMed] [Google Scholar]
- Vuoksimaa E, Panizzon MS, Chen CH, Eyler LT, Fennema-Notestine C, Fiecas MJ, Fischl B, Franz CE, Grant MD, Jak AJ et al. 2013. Cognitive reserve moderates the association between hippocampal volume and episodic memory in middle age. Neuropsychologia. 51(6):1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Yonelinas AP, Ranganath C. 2013. Dissociable neural correlates of item and context retrieval in the medial temporal lobes. Behav Brain Res. 254:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]